The Impact of a Graded Maximal Exercise Protocol on Exhaled Volatile Organic Compounds: A Pilot Study
Abstract
:1. Introduction
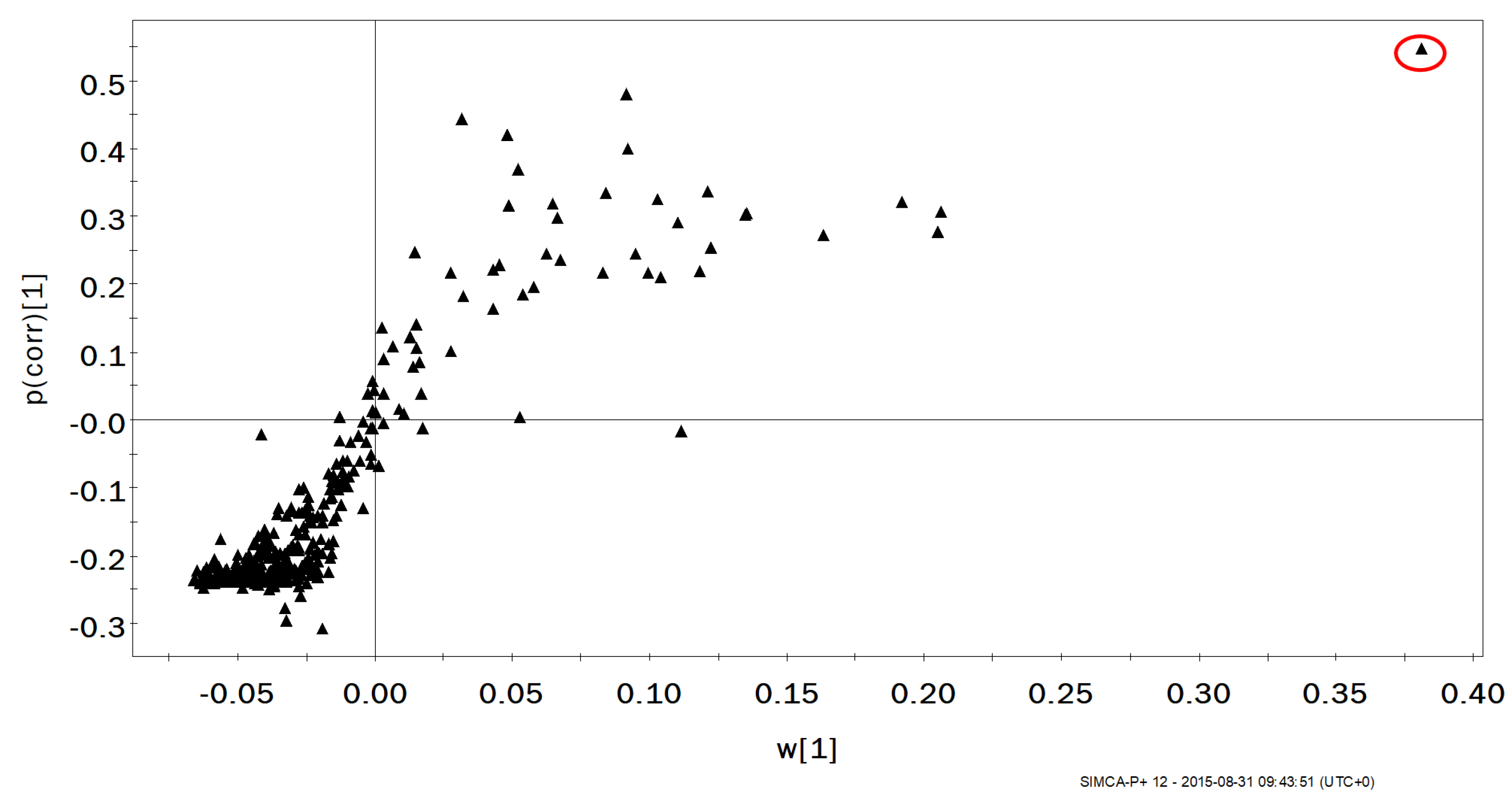
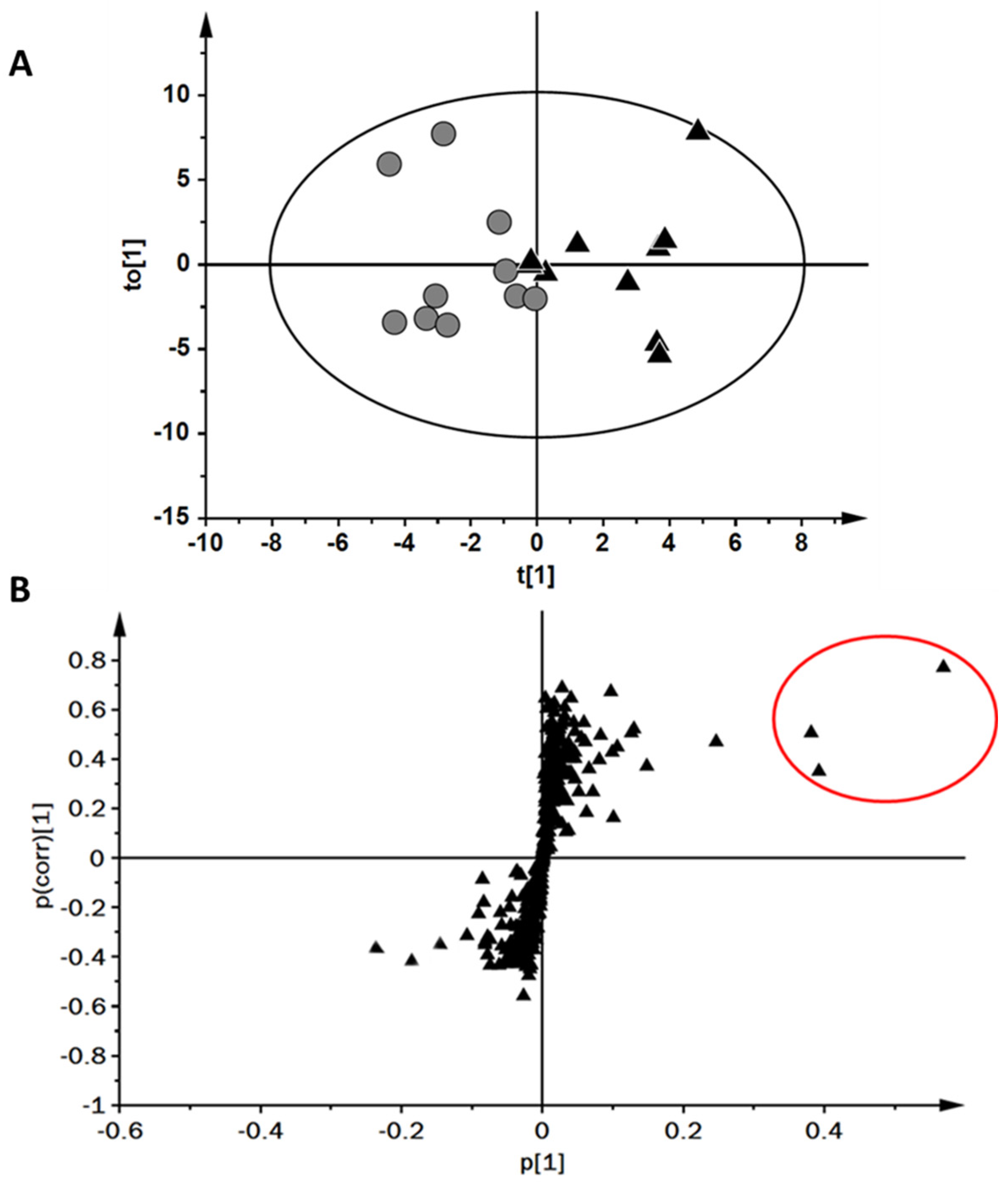
2. Results
2.1. Changes in Exhaled VOCs Following Exercise
2.2. Comparison of Upper and Lower Tertiles of Maximal Oxygen Uptake
3. Discussion
4. Materials and Methods
4.1. Ethical Clearance
4.2. Participant Information
4.3. Experimental Design
4.4. Exercise Protocol
4.5. Exercise Testing
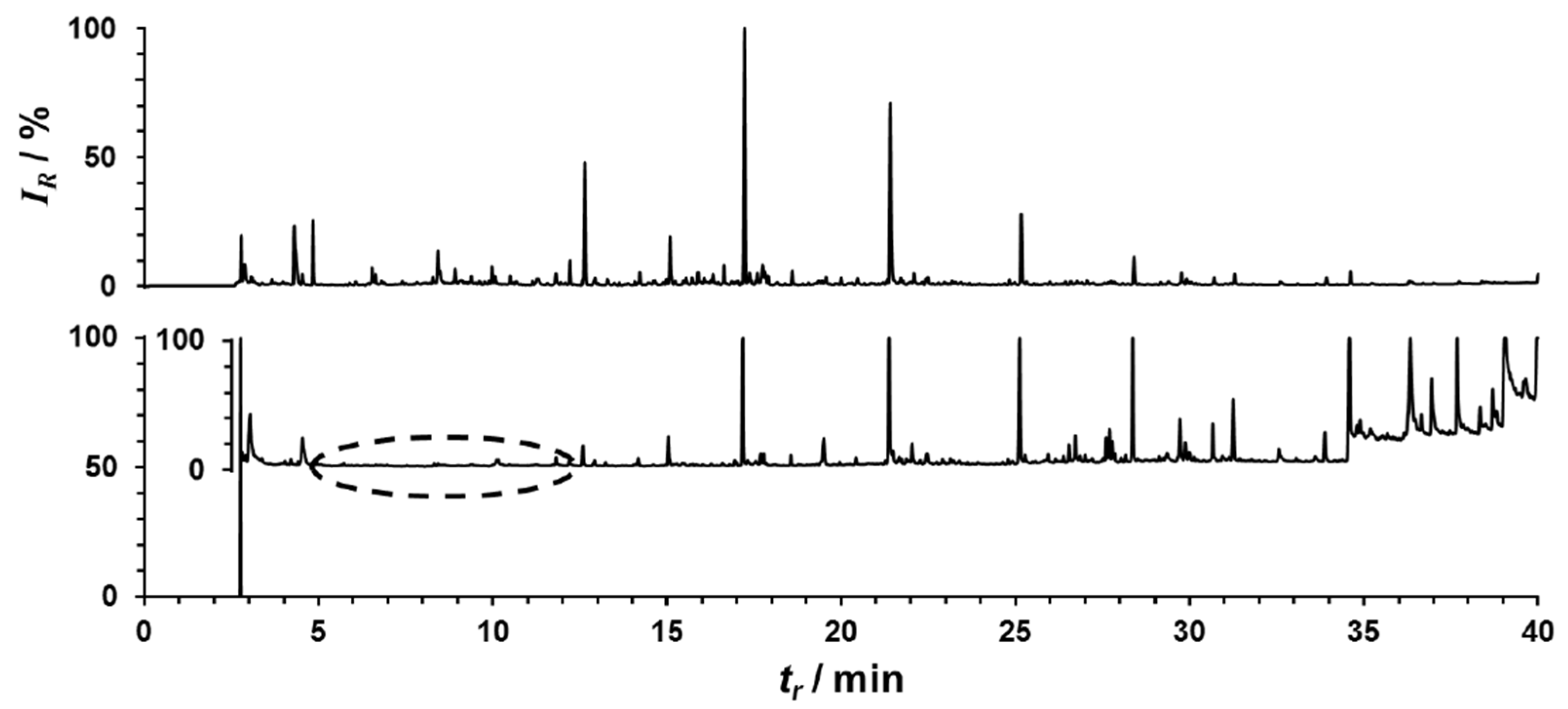
4.6. Exhaled Breath Sampling and Analysis
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Heaney, L.M.; Deighton, K.; Suzuki, T. Non-targeted metabolomics in sport and exercise science. J. Sports Sci. 2019, 37, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Miekisch, W.; Schubert, J.; Buszewski, B.; Ligor, T.; Jezierski, T.; Pleil, J.; Risby, T. Analysis of Exhaled Breath for Disease Detection. Annu. Rev. Anal. Chem. 2014, 7, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Garzinsky, A.; Thomas, A.; Krug, O.; Thevis, M. Probing for the presence of doping agents in exhaled breath using chromatographic-mass spectrometric approaches. Rapid Commun. Mass Spectrom. 2020, 35, e8939. [Google Scholar] [CrossRef] [PubMed]
- Heaney, L.M.; Ruszkiewicz, D.M.; Arthur, K.L.; Hadjithekli, A.; Aldcroft, C.; Lindley, M.R.; Thomas, C.P.; Turner, M.A.; Reynolds, J.C. Real-time monitoring of exhaled volatiles using atmospheric pressure chemical ionization on a compact mass spectrometer. Bioanalysis 2016, 8, 1325–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Costello, B.D.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- Basanta, M.; Koimtzis, T.; Singh, D.; Wilson, I.; Thomas, C.L.P. An adaptive breath sampler for use with human subjects with an impaired respiratory function. Analyst 2007, 132, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Liangou, A.; Tasoglou, A.; Huber, H.J.; Wistrom, C.; Brody, K.; Menon, P.G.; Bebekoski, T.; Menschel, K.; Davidson-Fiedler, M.; DeMarco, K.; et al. A method for the identification of COVID-19 biomarkers in human breath using Proton Transfer Reaction Time-of-Flight Mass Spectrometry. EClinicalMedicine 2021, 42, 101207. [Google Scholar] [CrossRef] [PubMed]
- Heaney, L.M.; Lindley, M.R. Translation of exhaled breath volatile analyses to sport and exercise applications. Metabolomics 2017, 13, 139. [Google Scholar] [CrossRef] [Green Version]
- King, J.; Kupferthaler, A.; Unterkofler, K.; Koc, H.; Teschl, S.; Teschl, G.; Miekisch, W.; Schubert, J.; Hinterhuber, H.; Amann, A. Isoprene and acetone concentration profiles during exercise on an ergometer. J. Breath Res. 2009, 3, 027006. [Google Scholar] [CrossRef] [PubMed]
- Bikov, A.; Lazar, Z.; Schandl, K.; Antus, B.; Losonczy, G.; Horvath, I. Exercise changes volatiles in exhaled breath assessed by an electronic nose. Acta Physiol. Hung. 2011, 98, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Décombaz, J.; Grathwohl, D.; Pollien, P.; Schmitt, J.A.J.; Borrani, F.; Lecoultre, V. Effect of short-duration lipid supplementation on fat oxidation during exercise and cycling performance. Appl. Physiol. Nutr. Metab. 2013, 38, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F.E. Diagnostic potential of breath analysis—focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Kupferthaler, A.; Frauscher, B.; Hackner, H.; Unterkofler, K.; Teschl, G.; Hinterhuber, H.; Amann, A.; Högl, B. Measurement of endogenous acetone and isoprene in exhaled breath during sleep. Physiol. Meas. 2012, 33, 413–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [PubMed]
- Howley, E.T.; Bassett, D.R.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292. [Google Scholar] [CrossRef] [PubMed]
- Heaney, L.M.; Kang, S.; Turner, M.A.; Lindley, M.R.; Thomas, C.L.P. Evidence for alternative exhaled elimination profiles of disinfection by-products and potential markers of airway responses to swimming in a chlorinated pool environment. Indoor Air 2020, 30, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.A.; Guallar-Hoyas, C.; Kent, A.L.; Wilson, I.D.; Thomas, C.L. Comparison of metabolomic profiles obtained using chemical ionization and electron ionization MS in exhaled breath. Bioanalysis 2011, 3, 2731–2738. [Google Scholar] [CrossRef] [PubMed]



| Relative VO2MAX | Absolute VO2MAX | ||||||
|---|---|---|---|---|---|---|---|
| High | Low | p Value | High | Low | p Value | ||
| Age (years) | 23 (3) | 22 (3) | 0.247 | 24 (4) | 22 (2) | 0.796 | |
| Mass (kg) | 72.6 (6.2) | 89.3 (9.1) | <0.0005 | 90.2 (9.6) | 75.7 (9.2) | 0.005 | |
| Height (cm) | 176 (7) | 183 (5) | 0.023 | 184 (6) | 177 (7) | 0.075 | |
| BMI (kg/m2) | 23.5 (1.4) | 26.6 (2.4) | 0.004 | 26.7 (2.0) | 24 (1.5) | 0.004 | |
| Weekly activity | Vigorous (min) | 325 (196) | 230 (113) | 0.353 | 374 (173) | 228 (152) | 0.052 |
| Moderate (min) | 89 (112) | 144 (103) | 0.143 | 174 (155) | 168 (325) | 0.218 | |
| Walking (min) | 225 (99) | 292 (179) | 0.280 | 133 (84) | 331 (145) | 0.002 | |
| Relative VO2MAX (mL(O2)/kg/min) | 51.9 (2.6) | 40.9 (2.8) | <0.0005 | 47.3 (5.9) | 45.0 (5.6) | 0.481 | |
| Absolute VO2MAX (L(O2)/min) | 3.8 (0.4) | 3.6 (0.4) | 0.481 | 4.2 (0.2) | 3.4 (0.1) | <0.0005 | |
| Final stage heart rate (beats/min) | 191 (7) | 186 (9) | 0.277 | 185 (10) | 192 (5) | 0.063 | |
| Room temperature (°C) | 21.1 (1.0) | 21.4 (0.8) | 0.247 | 21.1 (0.9) | 21.2 (1.1) | 0.796 | |
| Total exercise time (min) | 19 (3) | 18 (2) | 0.387 | 20 (2) | 17 (2) | <0.0005 | |
| Room pressure (mmHg) | 764 (7) | 760 (6) | 0.218 | 762 (14) | 764 (6) | 0.353 | |
| (%) | 21.0 (0.1) | 20.9 (0.1) | 0.165 | 21.0 (0.1) | 21.0 (0.1) | 0.631 | |
| (%) | 0.05 (0.01) | 0.04 (0.01) | 0.739 | 0.04 (0.01) | 0.04 (0.01) | 0.971 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heaney, L.M.; Kang, S.; Turner, M.A.; Lindley, M.R.; Thomas, C.L.P. The Impact of a Graded Maximal Exercise Protocol on Exhaled Volatile Organic Compounds: A Pilot Study. Molecules 2022, 27, 370. https://doi.org/10.3390/molecules27020370
Heaney LM, Kang S, Turner MA, Lindley MR, Thomas CLP. The Impact of a Graded Maximal Exercise Protocol on Exhaled Volatile Organic Compounds: A Pilot Study. Molecules. 2022; 27(2):370. https://doi.org/10.3390/molecules27020370
Chicago/Turabian StyleHeaney, Liam M., Shuo Kang, Matthew A. Turner, Martin R. Lindley, and C. L. Paul Thomas. 2022. "The Impact of a Graded Maximal Exercise Protocol on Exhaled Volatile Organic Compounds: A Pilot Study" Molecules 27, no. 2: 370. https://doi.org/10.3390/molecules27020370
APA StyleHeaney, L. M., Kang, S., Turner, M. A., Lindley, M. R., & Thomas, C. L. P. (2022). The Impact of a Graded Maximal Exercise Protocol on Exhaled Volatile Organic Compounds: A Pilot Study. Molecules, 27(2), 370. https://doi.org/10.3390/molecules27020370






