Solid Lipid Nanoparticles Administering Antioxidant Grape Seed-Derived Polyphenol Compounds: A Potential Application in Aquaculture †
Abstract
:1. Introduction
2. Results
2.1. Physico-Chemical Properties of SLN
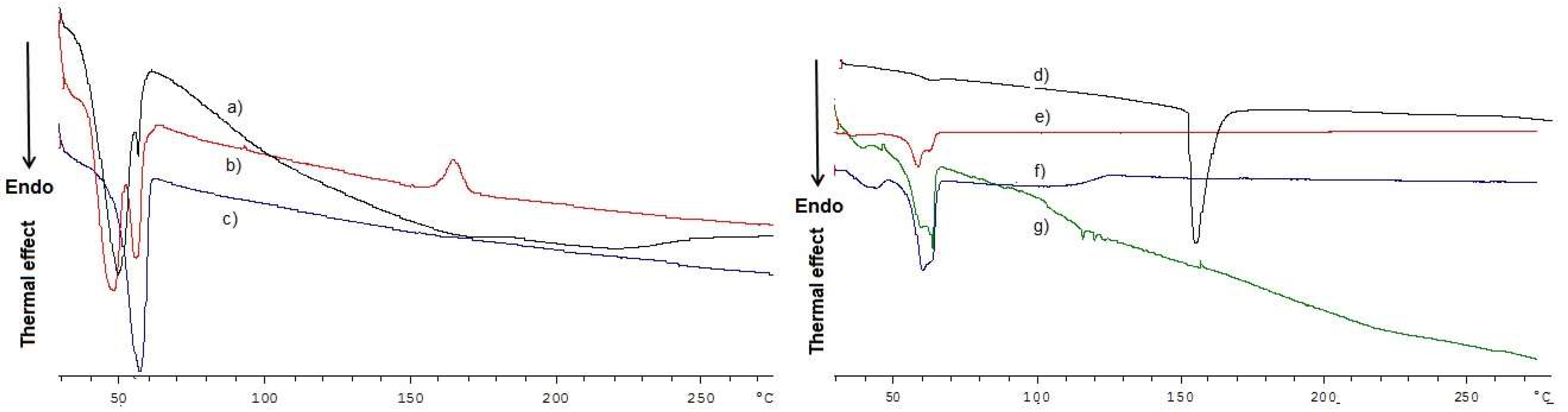
2.2. Solid-State Studies
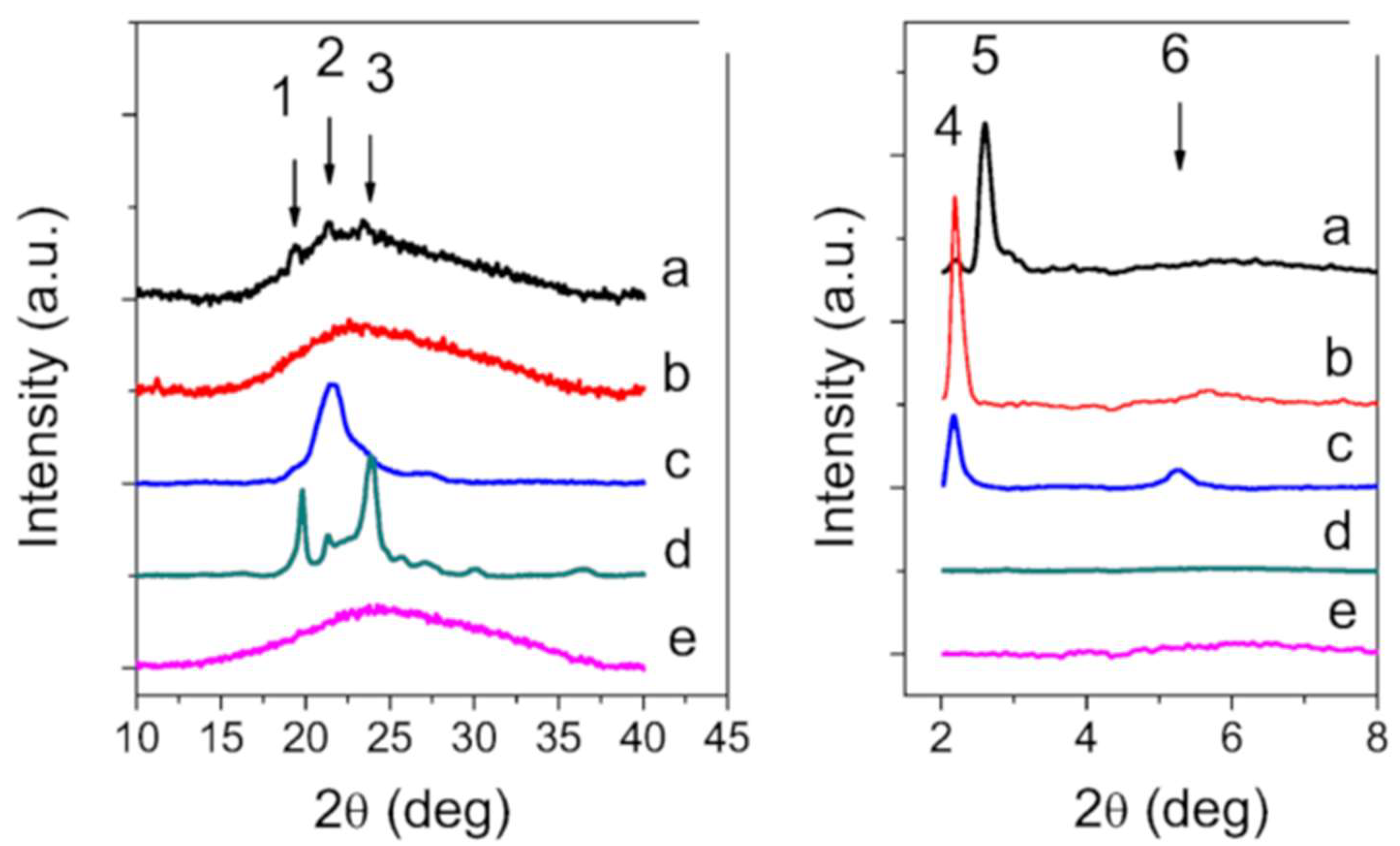
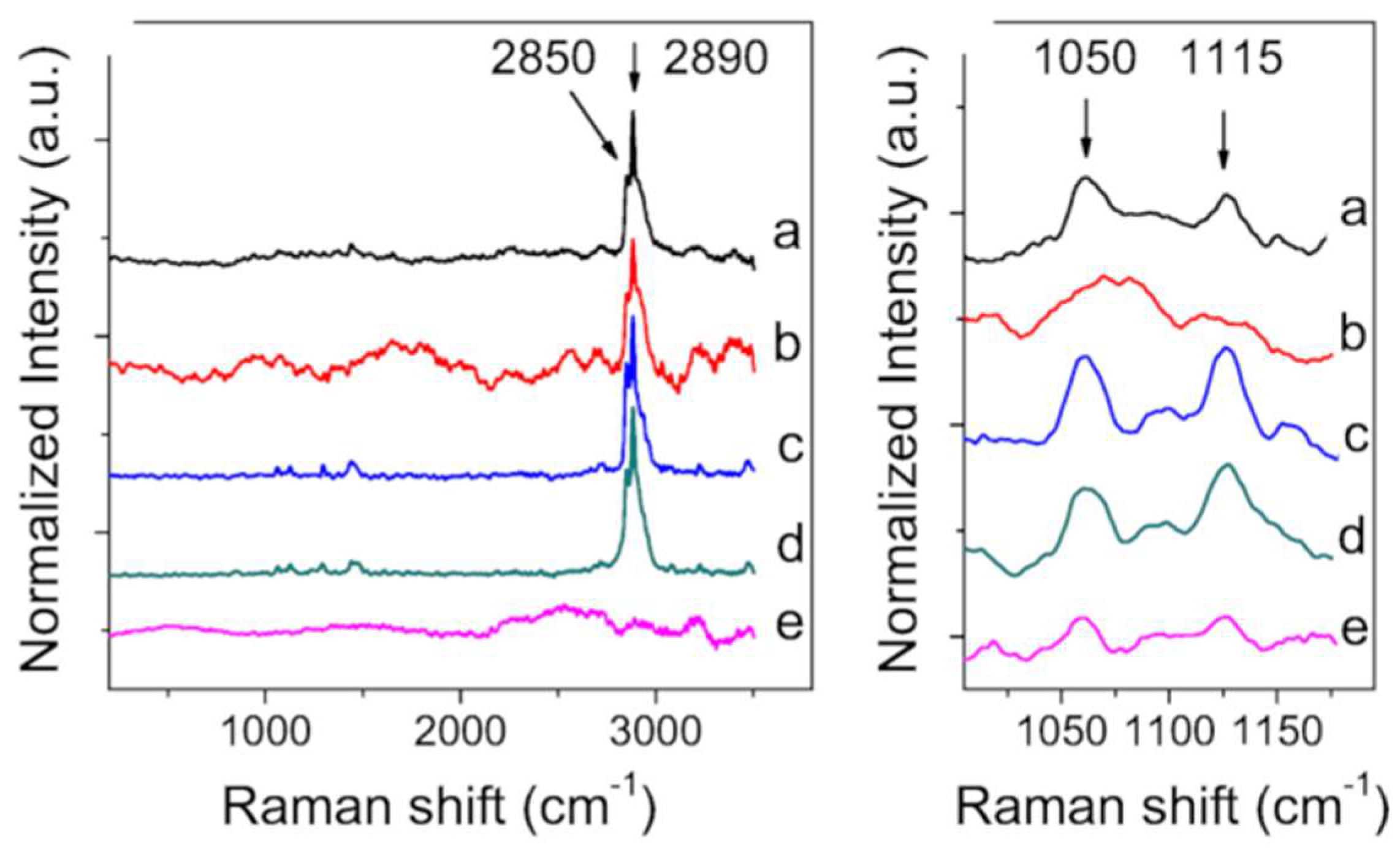
2.3. X-ray Diffraction and Raman Spectroscopy
2.4. Antioxidant Activity of GSE-SLN
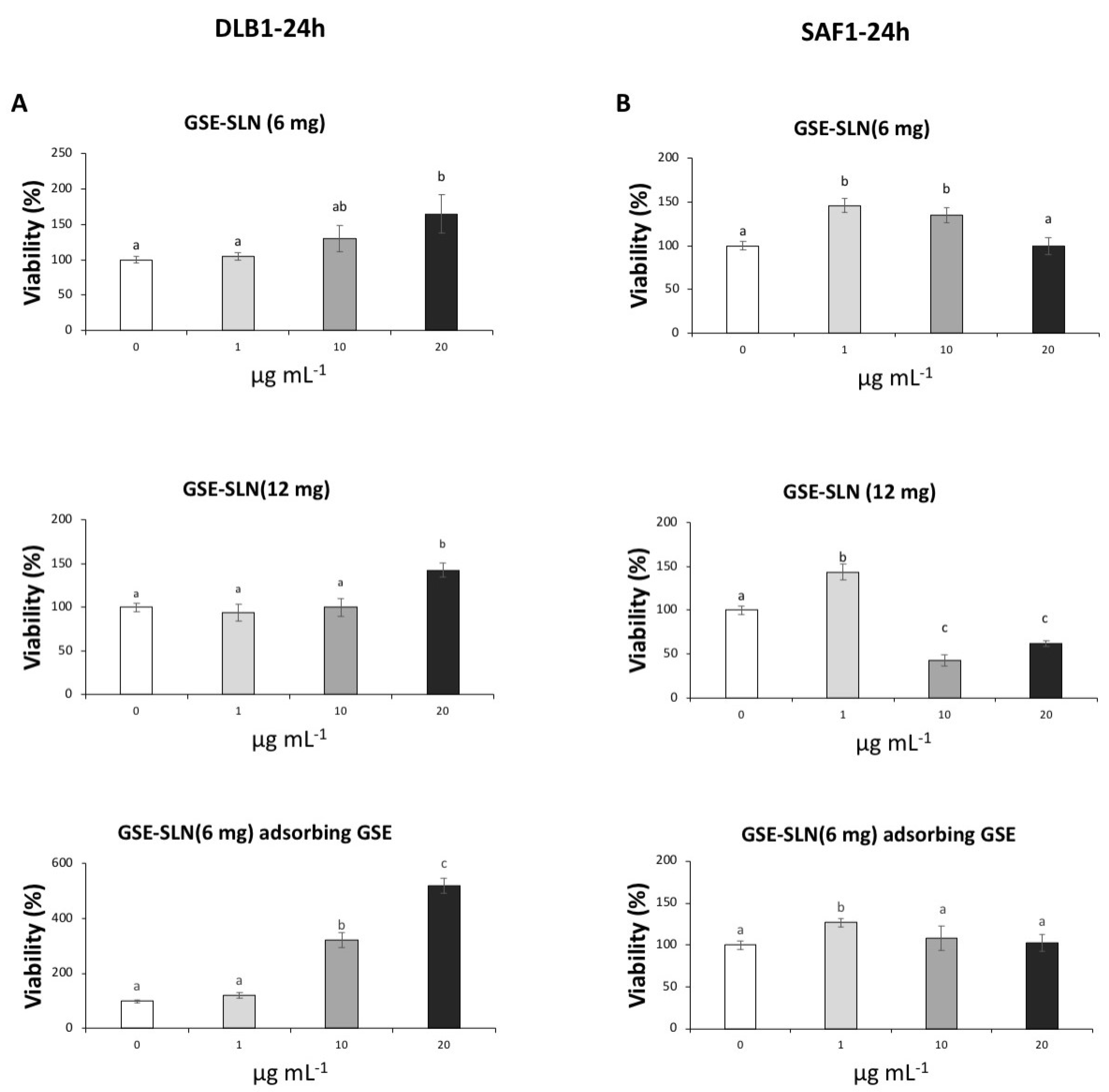
2.5. Viability Assay
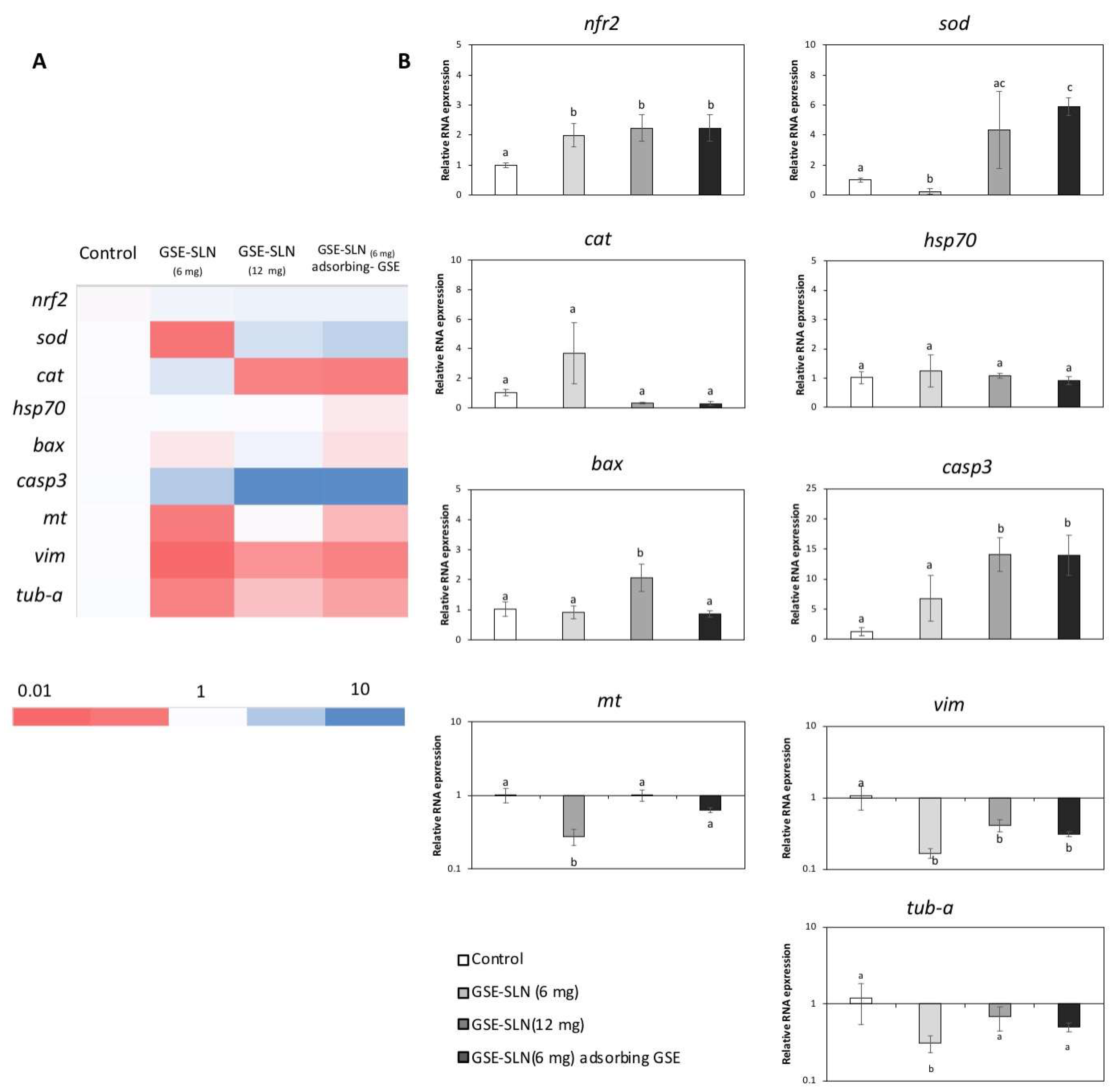
2.6. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of SLN
4.3. Physic-Chemical Characterization of SLN
4.4. Transmission Electron Spectroscopy (TEM)
4.5. Solid State Studies
4.6. Total Antioxidant Activity
4.7. Cell Lines Culture
4.7.1. Cell Monolayers DLB-1
4.7.2. In Vitro Incubation of Fish Cell Lines with Particles
4.8. Viability Assay
4.9. Gene Expression
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Castellani, S.; Trapani, A.; Spagnoletta, A.; di Toma, L.; Magrone, T.; Di Gioia, S.; Mandracchia, D.; Trapani, G.; Jirillo, E.; Conese, M. Nanoparticle delivery of grape seed-derived proanthocyanidins to airway epithelial cells dampens oxidative stress and inflammation. J. Transl. Med. 2018, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Guerra, L.; Corbo, F.; Castellani, S.; Sanna, E.; Capobianco, L.; Monteduro, A.G.; Manno, D.E.; Mandracchia, D.; Di Gioia, S.; et al. Cyto/Biocompatibility of Dopamine Combined with the Antioxidant Grape Seed-Derived Polyphenol Compounds in Solid Lipid Nanoparticles. Molecules 2021, 26, 916. [Google Scholar] [CrossRef]
- Lu, R.H.; Qin, C.B.; Yang, F.; Zhang, W.Y.; Zhang, Y.R.; Yang, G.K.; Yang, L.P.; Meng, X.L.; Yan, X.; Nie, G.X. Grape seed proanthocyanidin extract ameliorates hepatic lipid accumulation and inflammation in grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2020, 46, 1665–1677. [Google Scholar] [CrossRef]
- Cerbaro, A.F.; Rodrigues, V.S.B.; Rigotti, M.; Branco, C.S.; Rech, G.; de Oliveira, D.L.; Salvador, M. Grape seed proanthocyanidins improves mitochondrial function and reduces oxidative stress through an increase in sirtuin 3 expression in EA. hy926 cells in high glucose condition. Mol. Biol. Rep. 2020, 47, 3319–3330. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Puglia, C.; Cortesi, R. New Strategies for the Delivery of Some Natural Anti-oxidants with Therapeutic Properties. Mini Rev. Med. Chem. 2019, 19, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Nastruzzi, C.; Valacchi, G.; Cortesi, R. Lipid nanostructures for antioxidant delivery: A comparative preformulation study. Beilstein J. Nanotechnol. 2019, 10, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Mandracchia, D.; Di Franco, C.; Cordero, H.; Morcillo, P.; Comparelli, R.; Cuesta, A.; Esteban, M.A. In vitro characterization of 6-Coumarin loaded solid lipid nanoparticles and their uptake by immunocompetent fish cells. Colloids Surf. B Biointerfaces 2015, 127, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Tripodo, G.; Mandracchia, D.; Cioffi, N.; Ditaranto, N.; De Leo, V.; Cordero, H.; Esteban, M.A. Glutathione-loaded solid lipid nanoparticles based on Gelucire® 50/13: Spectroscopic characterization and interactions with fish cells. J. Drug Deliv. Sci. Technol. 2018, 47, 359–366. [Google Scholar] [CrossRef]
- Trapani, A.; Tripodo, G.; Mandracchia, D.; Cioffi, N.; Ditaranto, N.; Cerezuela, R.; Esteban, M.A. Glutathione loaded solid lipid nanoparticles: Preparation and in vitro evaluation as delivery systems of the antioxidant peptide to immunocompetent fish cells. J. Cell. Biotechnol. 2016, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mura, P.; Maestrelli, F.; D’Ambrosio, M.; Luceri, C.; Cirri, M. Evaluation and Comparison of Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Vectors to Develop Hydrochlorothiazide Effective and Safe Pediatric Oral Liquid Formulations. Pharmaceutics 2021, 13, 437. [Google Scholar] [CrossRef]
- Nazemiyeh, E.; Eskandani, M.; Sheikhloie, H.; Nazemiyeh, H. Formulation and Physicochemical Characterization of Lycopene-Loaded Solid Lipid Nanoparticles. Adv. Pharm. Bull. 2016, 6, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Vaassen, J.; Bartscher, K.; Breitkreutz, J. Taste masked lipid pellets with enhanced release of hydrophobic active ingredient. Int. J. Pharm. 2012, 429, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Williams, M.A.; Jones, D.S.; Andrews, G.P. Hot-melt extrusion technology and pharmaceutical application. Ther. Deliv. 2012, 3, 787–797. [Google Scholar] [CrossRef]
- El Hadri, M.; Achahbar, A.; El Khamkhami, J.; Khelifa, B.; Faivre, V.; Abbas, O.; Bresson, S. Lyotropic behavior of Gelucire 50/13 by XRD, Raman and IR spectroscopies according to hydration. Chem. Phys. Lipids 2016, 200, 11–23. [Google Scholar] [CrossRef]
- Perteghella, S.; Mandracchia, D.; Torre, M.L.; Tamma, R.; Ribatti, D.; Trapani, A.; Tripodo, G. Anti-angiogenic activity of N,O-carboxymethyl-chitosan surface modified solid lipid nanoparticles for oral delivery of curcumin. J. Drug Deliv. Sci. Technol. 2020, 56, 101494. [Google Scholar] [CrossRef]
- Souto, E.B.; Mehnert, W.; Müller, R.H. Polymorphic behaviour of Compritol® 888 ATO as bulk lipid and as SLN and NLC. J. Microencapsul 2006, 23, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Cyvin, S.J.; Rymo, L.; Bowie, J.H.; Williams, D.H.; Bunnenberg, E.; Djerassi, C.; Records, R. Classification of glyceride crystal forms. Acta Chem. Scand. 1966, 20, 2255–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himawan, C.; Starov, V.M.; Stapley, A.G. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Bugeat, S.; Perez, J.; Briard-Bion, V.; Pradel, P.; Ferlay, A.; Bourgaux, C.; Lopez, C. Unsaturated fatty acid enriched vs. control milk triacylglycerols:S olid and liquid TAG phases examined by synchrotron radiation X-ray diffraction coupled with DSC. Food Res. Int. 2015, 67, 91–101. [Google Scholar] [CrossRef]
- Takeguchi, S.; Sato, A.; Hondoh, H.; Aoki, M.; Uehara, H.; Ueno, S. Multiple beta Forms of Saturated Monoacid Triacylglycerol Crystals. Molecules 2020, 25, 5086. [Google Scholar] [CrossRef]
- Spiker, R.C.; Levin, I.W. Effect of bilayer curvature on vibrational Raman spectroscopic behavior of phospholipid-water assemblies. Biochim. Biophys. Acta-Biomembr. 1976, 455, 560–575. [Google Scholar] [CrossRef]
- de Lange, M.J.; Bonn, M.; Müller, M. Direct measurement of phase coexistence in DPPC/cholesterol vesicles using Raman spectroscopy. Chem. Phys. Lipids 2007, 146, 76–84. [Google Scholar] [CrossRef]
- Schultz, Z.D.; Levin, I.W. Vibrational spectroscopy of biomembranes. Annu Rev. Anal. Chem. 2011, 4, 343–366. [Google Scholar] [CrossRef]
- Nordgreen, A.; Yúfera, M.; Hamre, K. Evaluation of changes in nutrient composition during production of crosslinked protein microencapsulated diets for marine fish larvae and suspension feeders. Aquaculture 2008, 285, 159–166. [Google Scholar] [CrossRef]
- Bejar, J.; Borrego, J.J.; Alvarez, M.C. A continuous cell line from the cultured marine fish gilt-head seabream (Sparus aurata L.). Aquaculture 1997, 150, 143–153. [Google Scholar] [CrossRef]
- Beltrán, J.M.G.; Espinosa, C.; Guardiola, F.A.; Esteban, M.A. In vitro effects of Origanum vulgare leaf extracts on gilthead seabream (Sparus aurata L.) leucocytes, cytotoxic, bactericidal and antioxidant activities. Fish Shellfish Immunol. 2018, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, Y.; Wang, S.; Xu, S. Resveratrol relieves chlorothalonil-induced apoptosis and necroptosis through miR-15a/Bcl2-A20 axis in fish kidney cells. Fish Shellfish Immunol. 2020, 107, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Catalano, A.; Carocci, A.; Carrieri, A.; Mercurio, A.; Rosato, A.; Mandracchia, D.; Tripodo, G.; Schiavone, B.I.P.; Franchini, C.; et al. Effect of Methyl-beta-Cyclodextrin on the antimicrobial activity of a new series of poorly water-soluble benzothiazoles. Carbohydr. Polym. 2019, 207, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Laquintana, V.; Lopedota, A.; Franco, M.; Latrofa, A.; Talani, G.; Sanna, E.; Trapani, G.; Liso, G. Evaluation of new propofol aqueous solutions for intravenous anesthesia. Int. J. Pharm. 2004, 278, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Partridge, G.J.; Rao, S.; Woolley, L.D.; Pilmer, L.; Lymbery, A.J.; Prestidge, C.A. Bioavailability and palatability of praziquantel incorporated into solid-lipid nanoparticles fed to yellowtail kingfish Seriola lalandi. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 218, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurpel, G.W.H.; Schins, J.M.; Müller, M. Direct Measurement of Chain Order in Single Phospholipid Mono- and Bilayers with Multiplex CARS. J. Phys. Chem. B 2004, 108, 3400–3403. [Google Scholar] [CrossRef]
- Holen, E.; Araujo, P.; Xie, S.; Søfteland, L.; Espe, M. Resveratrol inhibited LPS induced transcription of immune genes and secretion of eicosanoids in Atlantic salmon (Salmo salar), comparing mono-, co- and a novel triple cell culture model of head kidney leukocytes, liver cells and visceral adipocyte tissue. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 224, 108560. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, A.V.; Leal-Tassias, A.; Rodríguez-Sánchez, N.; Gil, M.P.; Martorell, P.; Genovés, S.; Acosta, C.; Burks, D.; Ramón, D.; Mullor, J.L. Use of Medaka Fish as Vertebrate Model to Study the Effect of Cocoa Polyphenols in the Resistance to Oxidative Stress and Life Span Extension. Rejuvenation Res. 2018, 21, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Cristallini, C.; Barbani, N.; Bianchi, S.; Maltinti, S.; Baldassare, A.; Ishak, R.; Onor, M.; Ambrosio, L.; Castelvetro, V.; Cascone, M.G. Assessing two-way interactions between cells and inorganic nanoparticles. J. Mater. Sci. Mater. Med. 2019, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.S.; Seo, J.E.; Kim, M.S.; Kang, M.H.; Oh, D.H.; Jeon, S.O.; Seong Hoon, J.; Choi, Y.W.; Lee, S. A retinyl palmitate-loaded solid lipid nanoparticle system: Effect of surface modification with dicetyl phosphate on skin permeation in vitro and anti-wrinkle effect in vivo. Int. J. Pharm. 2013, 452, 311–320. [Google Scholar] [CrossRef] [PubMed]
- de Souza Guedes, L.; Martinez, R.M.; Bou-Chacra, N.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. An Overview on Topical Administration of Carotenoids and Coenzyme Q10 Loaded in Lipid Nanoparticles. Antioxidants 2021, 10, 1034. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Hariri, R.; Kouhsoltani, M.; Shokri, J.; Javadzadeh, Y.; Hamishehkar, H. Enhanced stability and dermal delivery of hydroquinone using solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2015, 136, 1004–1010. [Google Scholar] [CrossRef]
- Brum, G.; Carbone, T.; Still, E.; Correia, V.; Szulak, K.; Calianese, D.; Best, C.; Cammarata, G.; Higgins, K.; Ji, F.; et al. N-acetylcysteine potentiates doxorubicin-induced ATM and p53 activation in ovarian cancer cells. Int. J. Oncol. 2013, 42, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Dimitriadis, V.K.; Gougoula, C.; Anestis, A.; Pörtner, H.O.; Michaelidis, B. Monitoring the biochemical and cellular responses of marine bivalves during thermal stress by using biomarkers. Mar. Environ. Res. 2012, 73, 70–77. [Google Scholar] [CrossRef]
- Espinosa, C.; Beltrán, J.M.G.; Messina, C.M.; Esteban, M. Effect of Jasonia glutinosa on immune and oxidative status of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2020, 100, 58–69. [Google Scholar] [CrossRef]
- Fazio, A.; Cerezuela, R.; Panuccio, M.R.; Cuesta, A.; Esteban, M.A. In vitro effects of Italian Lavandula multifida L. leaf extracts on gilthead seabream (Sparus aurata) leucocytes and SAF-1 cells. Fish Shellfish Immunol. 2017, 66, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Formigari, A.; Irato, P.; Santon, A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: Biochemical and cytochemical aspects. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 443–459. [Google Scholar] [CrossRef]
- Giudice, A.; Arra, C.; Turco, M.C. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Transcr. Factors 2010, 647, 37–74. [Google Scholar]
- Heydari, A.R.; Wu, B.; Takahashi, R.; Strong, R.; Richardson, A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol. Cell Biol. 1993, 13, 2909–2918. [Google Scholar] [PubMed] [Green Version]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Messina, C.M.; Pizzo, F.; Santulli, A.; Buselic, I.; Boban, M.; Orhanovic, S.; Mladineo, I. Anisakis pegreffii (Nematoda: Anisakidae) products modulate oxidative stress and apoptosis-related biomarkers in human cell lines. Parasit. Vectors 2016, 9, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Yoshida, M.; Saegusa, Y.; Fukuda, A.; Akama, Y.; Owada, S. Measurement of radical-scavenging ability in hepatic metallothionein of rat using in vivo electron spin resonance spectroscopy. Toxicology 2005, 213, 74–80. [Google Scholar] [CrossRef]
- Yuksel, Y.; Yuksel, R.; Yagmurca, M.; Haltas, H.; Erdamar, H.; Toktas, M.; Ozcan, O. Effects of quercetin on methotrexate-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 2017, 36, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Bichat, F.; Mouawad, R.; Solis-Recendez, G.; Khayat, D.; Bastian, G. Cytoskeleton alteration in MCF7R cells, a multidrug resistant human breast cancer cell line. Anticancer Res. 1997, 17, 3393–3401. [Google Scholar]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapani, A.; Mandracchia, D.; Tripodo, G.; Di Gioia, S.; Castellani, S.; Cioffi, N.; Ditaranto, N.; Esteban, M.A.; Conese, M. Solid lipid nanoparticles made of self-emulsifying lipids for efficient encapsulation of hydrophilic substances. AIP Conf. Proc. 2019, 2145, 020004. [Google Scholar]
- Mandracchia, D.; Trapani, A.; Perteghella, S.; Sorrenti, M.; Catenacci, L.; Torre, M.L.; Trapani, G.; Tripodo, G. pH-sensitive inulin-based nanomicelles for intestinal site-specific and controlled release of celecoxib. Carbohydr. Polym. 2018, 181, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Cometa, S.; Bonifacio, M.A.; Trapani, G.; Di Gioia, S.; Dazzi, L.; De Giglio, E.; Trapani, A. In vitro investigations on dopamine loaded Solid Lipid Nanoparticles. J. Pharm. BioMed. Anal. 2020, 185, 113257. [Google Scholar] [CrossRef]
- Carbone, C.; Caddeo, C.; Grimaudo, M.A.; Manno, D.E.; Serra, A.; Musumeci, T. Ferulic Acid-NLC with Lavandula Essential Oil: A Possible Strategy for Wound-Healing? Nanomaterials 2020, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Trapani, A.; Tripodo, G.; Perrone, M.G.; Giammona, G.; Trapani, G.; Colabufo, N.A. In vitro evaluation of glycol chitosan based formulations as oral delivery systems for efflux pump inhibition. Carbohydr. Polym. 2017, 166, 73–82. [Google Scholar] [CrossRef]
- Tripodo, G.; Trapani, A.; Rosato, A.; Di Franco, C.; Tamma, R.; Trapani, G.; Ribatti, D.; Mandracchia, D. Hydrogels for biomedical applications from glycol chitosan and PEG diglycidyl ether exhibit pro-angiogenic and antibacterial activity. Carbohydr. Polym. 2018, 198, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. Methods to measure the antioxidant activity in plant material. A comparative discussion. Free Radic. Res. 1999, 31, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Béjar, J.; Porta, J.; Borrego, J.J.; Alvarez, M.C. The piscine SAF-1 cell line: Genetic stability and labeling. Mar. Biotechnol. 2005, 7, 389–395. [Google Scholar] [CrossRef]
- Cerezuela, R.; Meseguer, J.; Esteban, M.A. Effects of dietary inulin, Bacillus subtilis and microalgae on intestinal gene expression in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2013, 34, 843–848. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Morcillo, P.; Chaves-Pozo, E.; Meseguer, J.; Esteban, M.Á.; Cuesta, A. Establishment of a new teleost brain cell line (DLB-1) from the European sea bass and its use to study metal toxicology. Toxicol. Vitr. 2017, 38, 91–100. [Google Scholar] [CrossRef]




| Formulation | Size (nm) | PDI | Zeta Potential (mV) | Association Efficiency (AE) (%) |
|---|---|---|---|---|
| Unloaded SLN | 486(±5) | 0.42–0.48 | −32.7(±1.1) | − |
| GSE-SLN(6mg) | 208(±21) ** | 0.44–0.49 | −43.4(±1.8) ** | 49.7(±3.2) |
| GSE-SLN(12mg) | 139(±15) ** | 0.44–0.48 | −25.6(±2.8) ** | 64.9(±1.0) |
| GSE-SLN(6mg)-adsorbing GSE | 283(±32) ** | 0.50–0.59 | −43.0(±1.3) ** | 74.6(±0.2) |
| Sample | I1115/I1050 | I2890/I2850 |
|---|---|---|
| Precirol® ATO5 | 1 | 1.4 |
| Gelucire® 50/13 | 1.02 | 1.67 |
| Pure GSE | 0.968 | 1.51 |
| GSE-SLN(6mg) | 0.867 | 1.83 |
| GSE-SLN(6mg)-adsorbing GSE | 0.562 | 1.84 |
| Formulation | Total Antioxidant Activity (TAA) (eq Asc.) mM/mg Nanoparticles |
|---|---|
| GSE-SLN(6mg) | 1.735 ± 0.327 |
| GSE-SLN(12mg) | 2.202 ± 0.321 |
| GSE-SLN(6mg)-adsorbing GSE | 1.411 ± 0.200 |
| Gene | Accession Number | F/R Primer Sequence (5′–3′) |
|---|---|---|
| nrf-2 | FP335773 | F: GTTCAGTCGGTGCTTTGACA |
| R: CTCTGATGTGCGTCTCTCCA | ||
| sod | AJ937872 | F: CCATGGTAAGAATCATGGCGG |
| R: CGTGGATCACCATGGTTCTG | ||
| cat | FG264808 | F: TTCCCGTCCTTCATTCACTC |
| R: CTCCAGAAGTCCCACACCAT | ||
| hsp70 | EU805481 | F: AATGTTCTGCGCATCATCAA |
| R: GCCTCCACCAAGATCAAAGA | ||
| bax | AM963390 | F: CAACAAGATGGCATCACACC |
| R: TGAACCCGCTCGTATATGAAA | ||
| casp3 | EU722334 | F: CTGATCTGGATGGAGGCATT |
| R: AGTAGTAGCCTGGGGCTGTG | ||
| mt | X97276 | F: ACAAACTGCTCCTGCACCTC |
| R: CAGCTAGTGTCGCACGTCTT | ||
| vim | FM155527 | F: CGCTTACCTGTGAGGTGGAT |
| R: GTGTCTTGGTAACCGCCTGT | ||
| tub-a | AY326430 | F: AAGATGTGAACTCCGCCATC |
| R: CTGGTAGTTGATGCCCACCT | ||
| act-β | X89920 | F: GGCACCACACCTTCTACAATG |
| R: GTGGTGGTGAAGCTGTAGCC | ||
| 18S | AM490061 | F: CTTCAACGCTCAGGTCATCAT |
| R: AGTTGGCACCGTTTATGGTC | ||
| Gene | Accession Number | F/R Primer Sequence (5′–3′) |
|---|---|---|
| nrf2 | DLAgn_00051120 | F: AACTAAGCCTCCCCTCACAC |
| R: GTTGTGGTCCATCTCCTCCA | ||
| sod | FJ860004 | F: TGTTGGAGACCTGGGAGATG |
| R: ATTGGGCCTGTGAGAGTGAG | ||
| cat | FJ860003 | F: GAGGTTTGCCTGATGGCTAC |
| R: TGCAGTAGAAACGCTCACCA | ||
| hsp70 | AY423555 | F: CTGCTAAGAATGGCCTGGAG |
| R: CTCGTTGCACTTGTCCAGAA | ||
| bax | FM011848 | F: TGTCGACTCGTCATCAAAGC |
| R: CACATGTTCCCGGAGGTAGT | ||
| casp3 | DQ345773 | F: AATTCACCAGGCTTCAATGC |
| R: CTACGGCAGAGACGACATCA | ||
| mt | AF199014 | F: GCACCACCTGCAAGAAGACT |
| R: AGCTGGTGTCGCACGTCT | ||
| vim | FM018579 | F: AGCGCCAGATTAGAGAGCTG |
| R: GCCATCTCGTCCTTCATGTT | ||
| tub-a | AY326429 | F: ACGAGGCCATCTACGACATC |
| R: GGCCGTTATGGACGAGACTA | ||
| act-β | AJ537421 | F: TCCCTGGAGAAGAGCTACGA |
| R: AGGAAGGAAGGCTGGAAAAG | ||
| 18S | AY831388 | F: TTCCTTTGATCGCTCTTAACG |
| R: TCTGATAAATGCACGCATCC | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trapani, A.; Esteban, M.Á.; Curci, F.; Manno, D.E.; Serra, A.; Fracchiolla, G.; Espinosa-Ruiz, C.; Castellani, S.; Conese, M. Solid Lipid Nanoparticles Administering Antioxidant Grape Seed-Derived Polyphenol Compounds: A Potential Application in Aquaculture. Molecules 2022, 27, 344. https://doi.org/10.3390/molecules27020344
Trapani A, Esteban MÁ, Curci F, Manno DE, Serra A, Fracchiolla G, Espinosa-Ruiz C, Castellani S, Conese M. Solid Lipid Nanoparticles Administering Antioxidant Grape Seed-Derived Polyphenol Compounds: A Potential Application in Aquaculture. Molecules. 2022; 27(2):344. https://doi.org/10.3390/molecules27020344
Chicago/Turabian StyleTrapani, Adriana, María Ángeles Esteban, Francesca Curci, Daniela Erminia Manno, Antonio Serra, Giuseppe Fracchiolla, Cristóbal Espinosa-Ruiz, Stefano Castellani, and Massimo Conese. 2022. "Solid Lipid Nanoparticles Administering Antioxidant Grape Seed-Derived Polyphenol Compounds: A Potential Application in Aquaculture" Molecules 27, no. 2: 344. https://doi.org/10.3390/molecules27020344
APA StyleTrapani, A., Esteban, M. Á., Curci, F., Manno, D. E., Serra, A., Fracchiolla, G., Espinosa-Ruiz, C., Castellani, S., & Conese, M. (2022). Solid Lipid Nanoparticles Administering Antioxidant Grape Seed-Derived Polyphenol Compounds: A Potential Application in Aquaculture. Molecules, 27(2), 344. https://doi.org/10.3390/molecules27020344










