Molecular Analysis of the Melanogenesis Inhibitory Effect of Saponins-Rich Fraction of Argania spinosa Leaves Extract
Abstract
1. Introduction
2. Results
2.1. Qualitative Evaluation of Saponins in Argan Polar Fractions
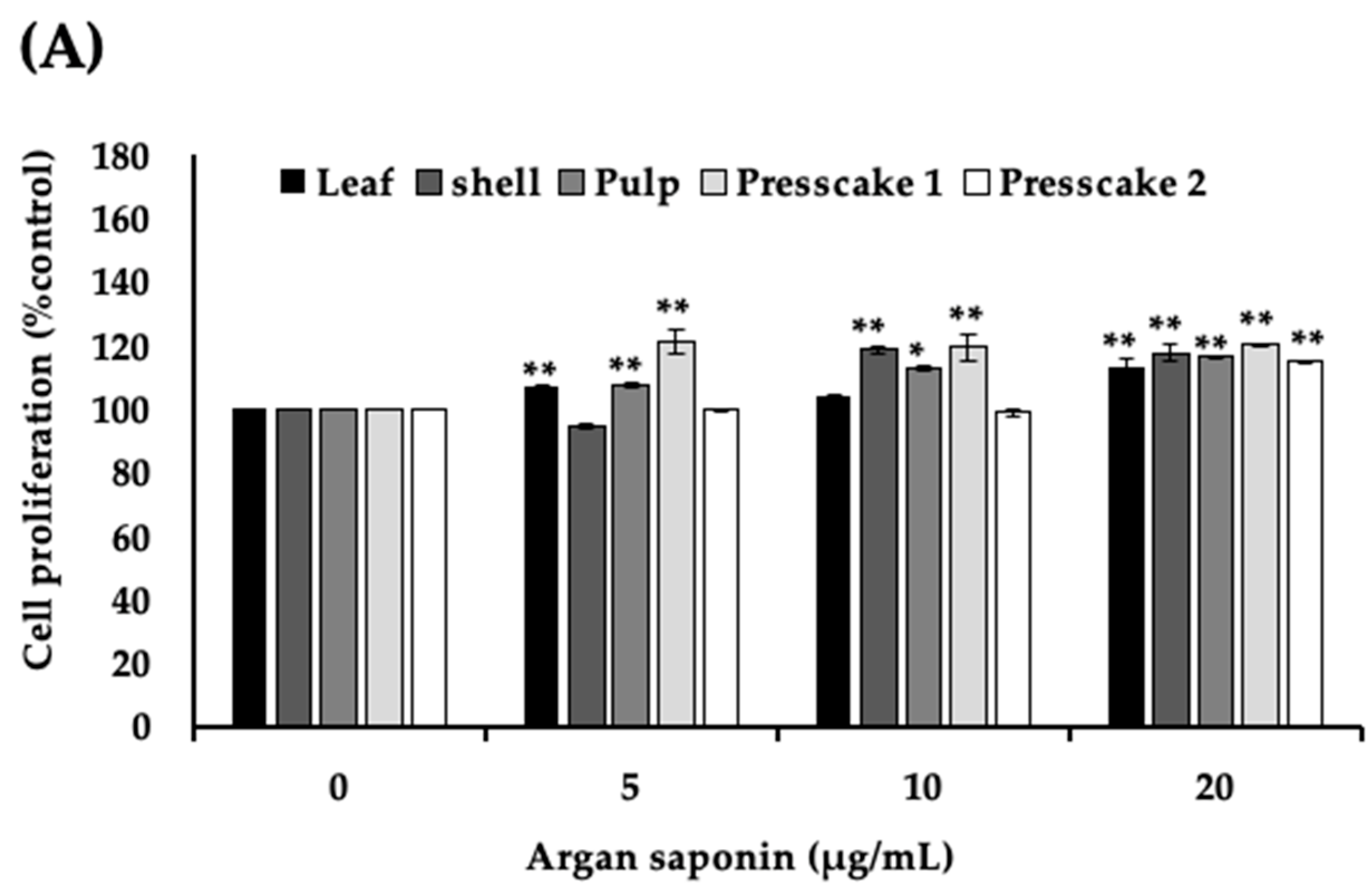
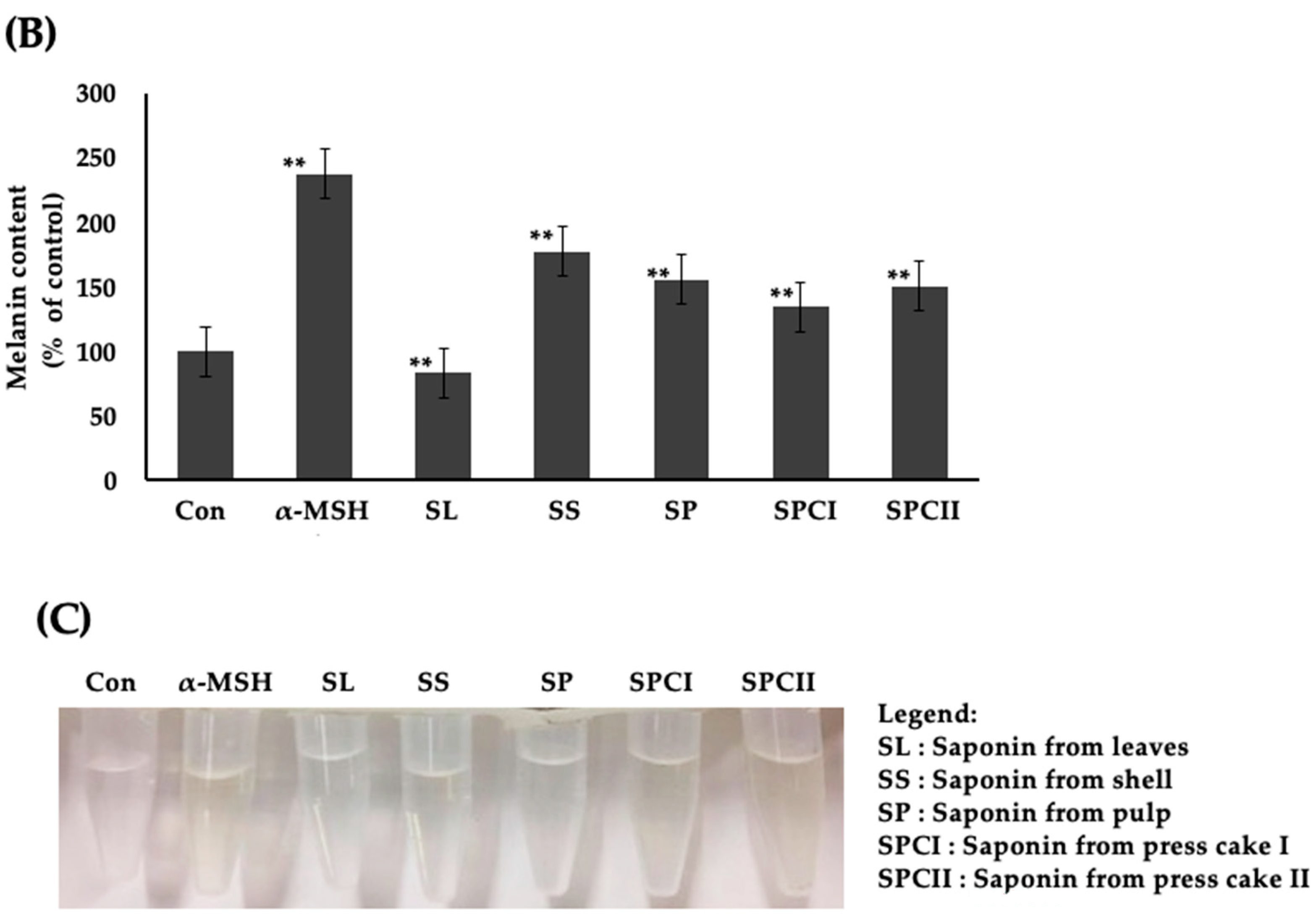
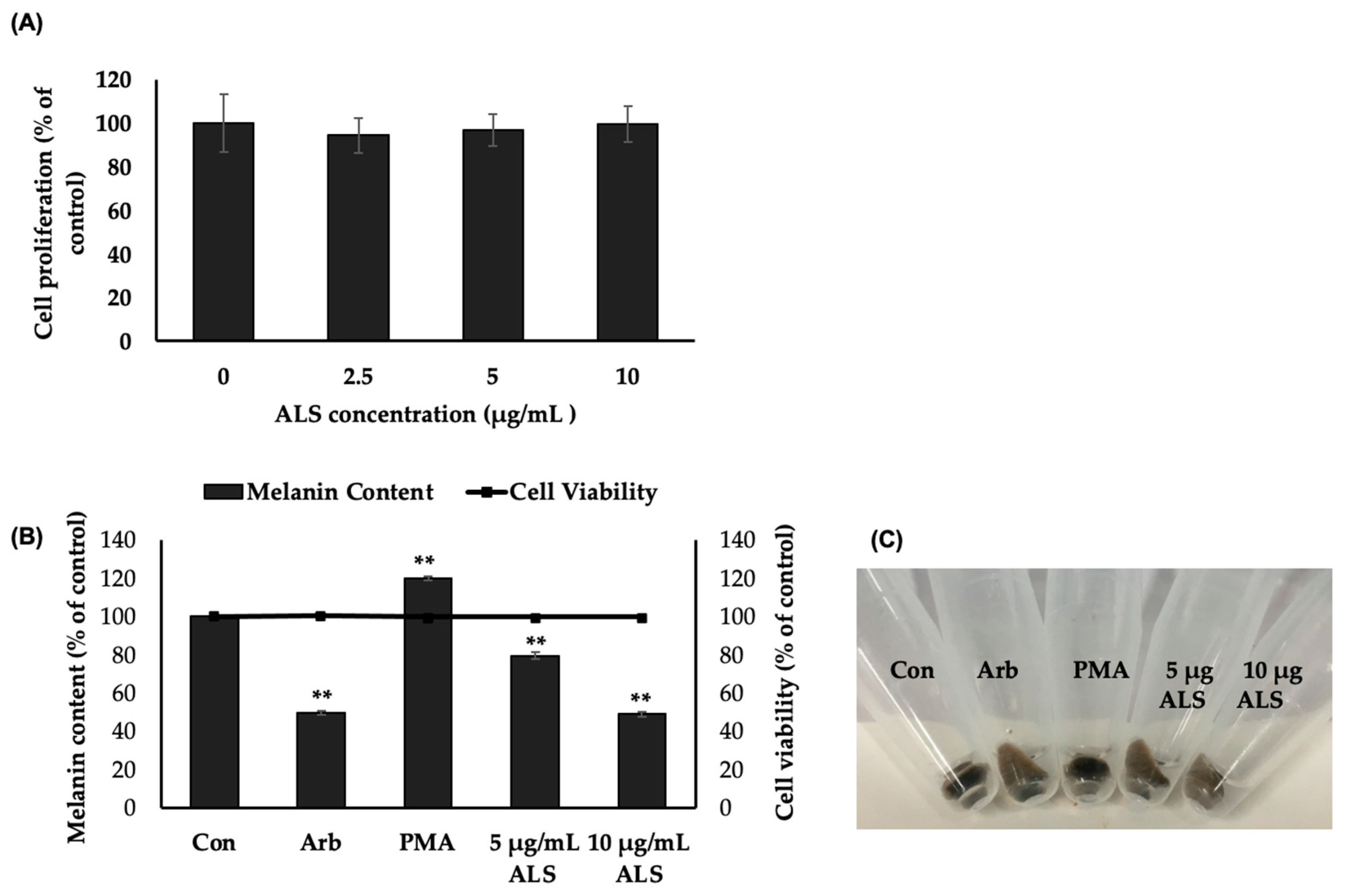
2.2. Saponins-Rich Fraction of Argan Leaves, Fruit Pulp, Shell, and Press Cakes Modulated Melanogenesis without Cytotoxicity
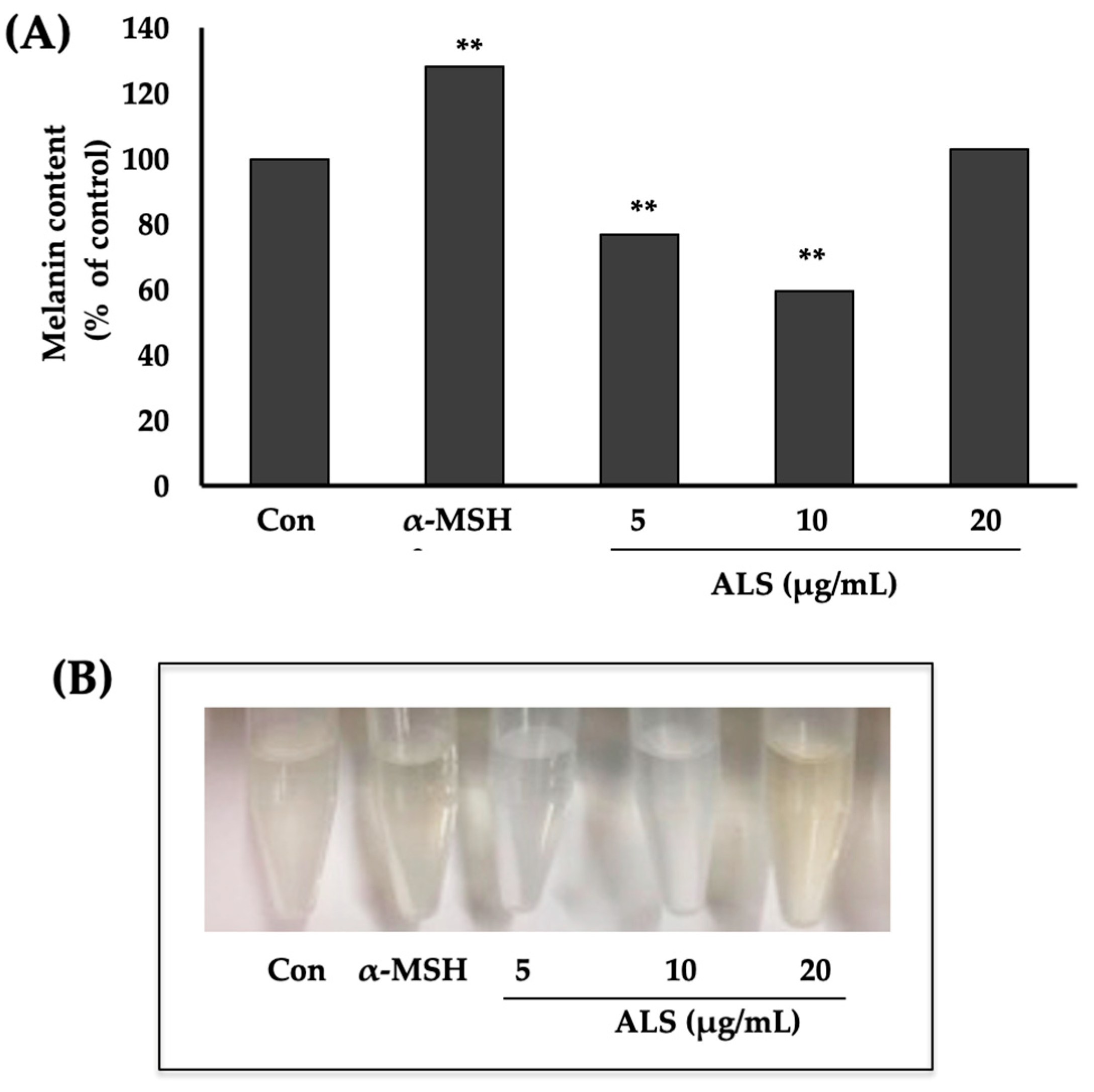
2.3. Saponins-Rich Fraction of Argan Leaves (ALS) Extract Inhibits Melanogenesis in B16 Cells
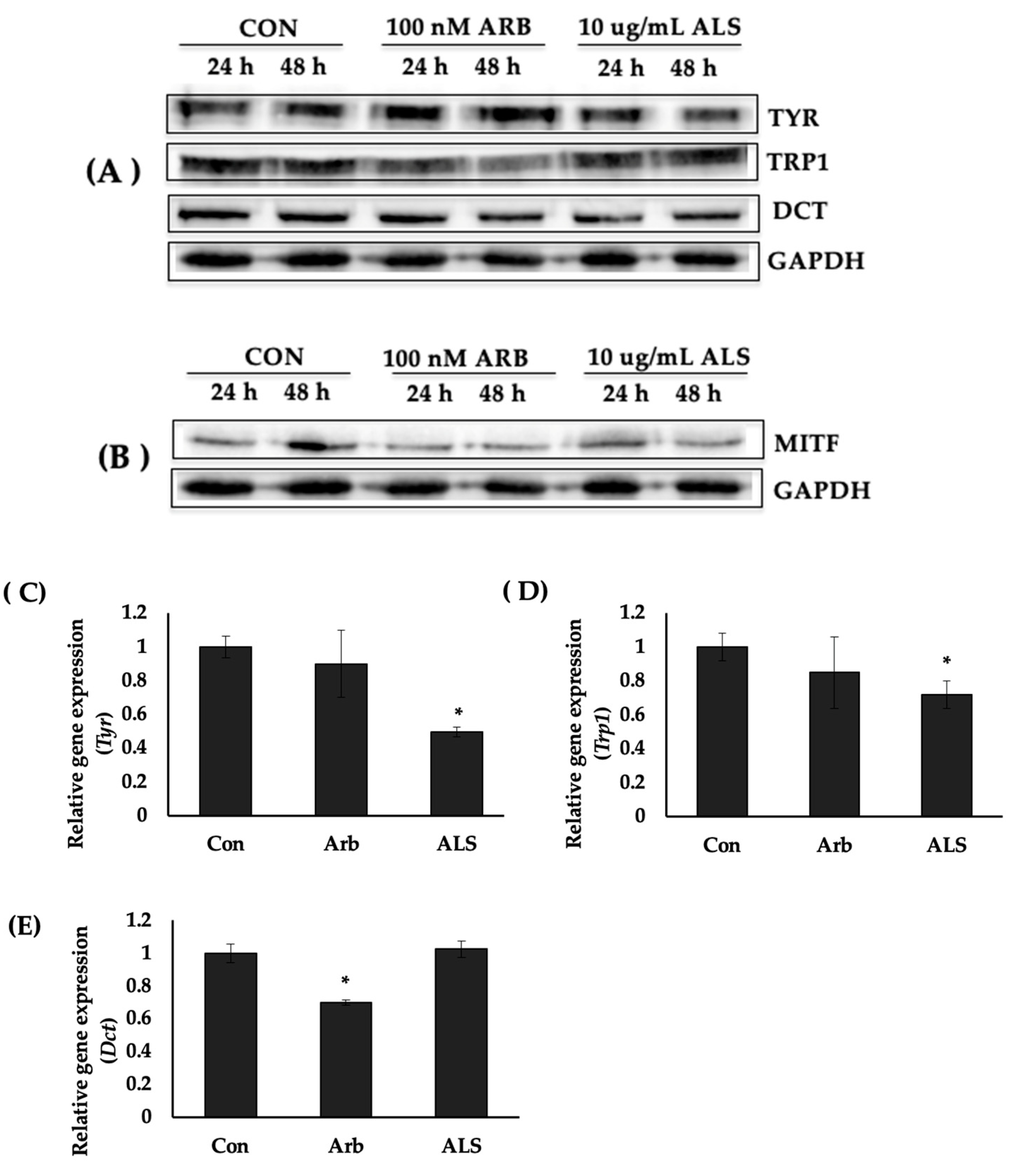
2.4. ALS Decreased the Protein Expression of Melanogenesis Enzymes
2.5. ALS Downregulated the Expression of Tyr and Trp1 Genes
2.6. ALS Inhibited Melanogenesis in Human Epidermal Melanocytes (HEMs)
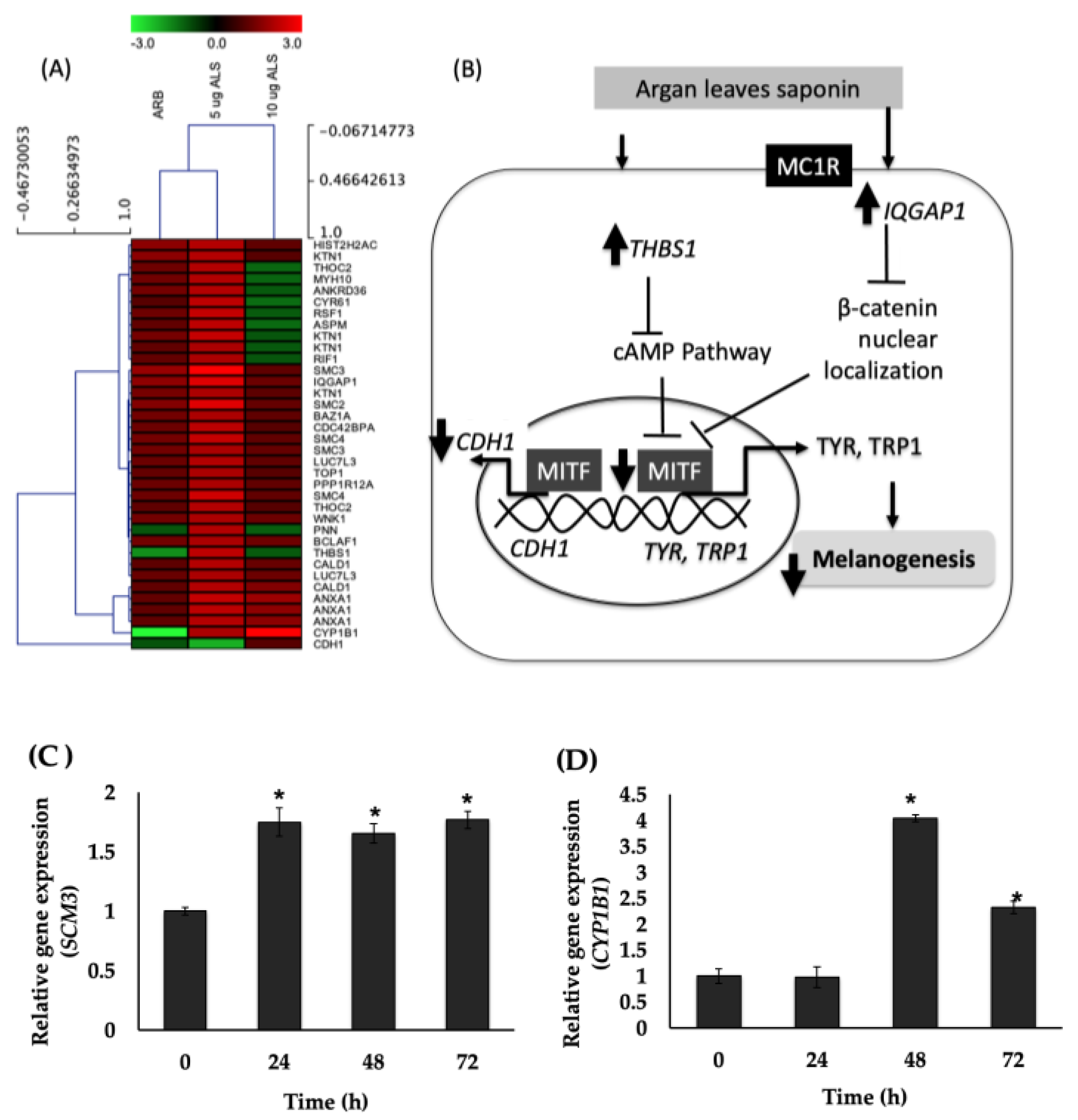
2.7. Global Gene Expression in Human Epidermal Melanocytes (HEMs) Elucidated Using DNA Microarray
3. Discussion
4. Materials and Methods
4.1. Cells and Cell Culture
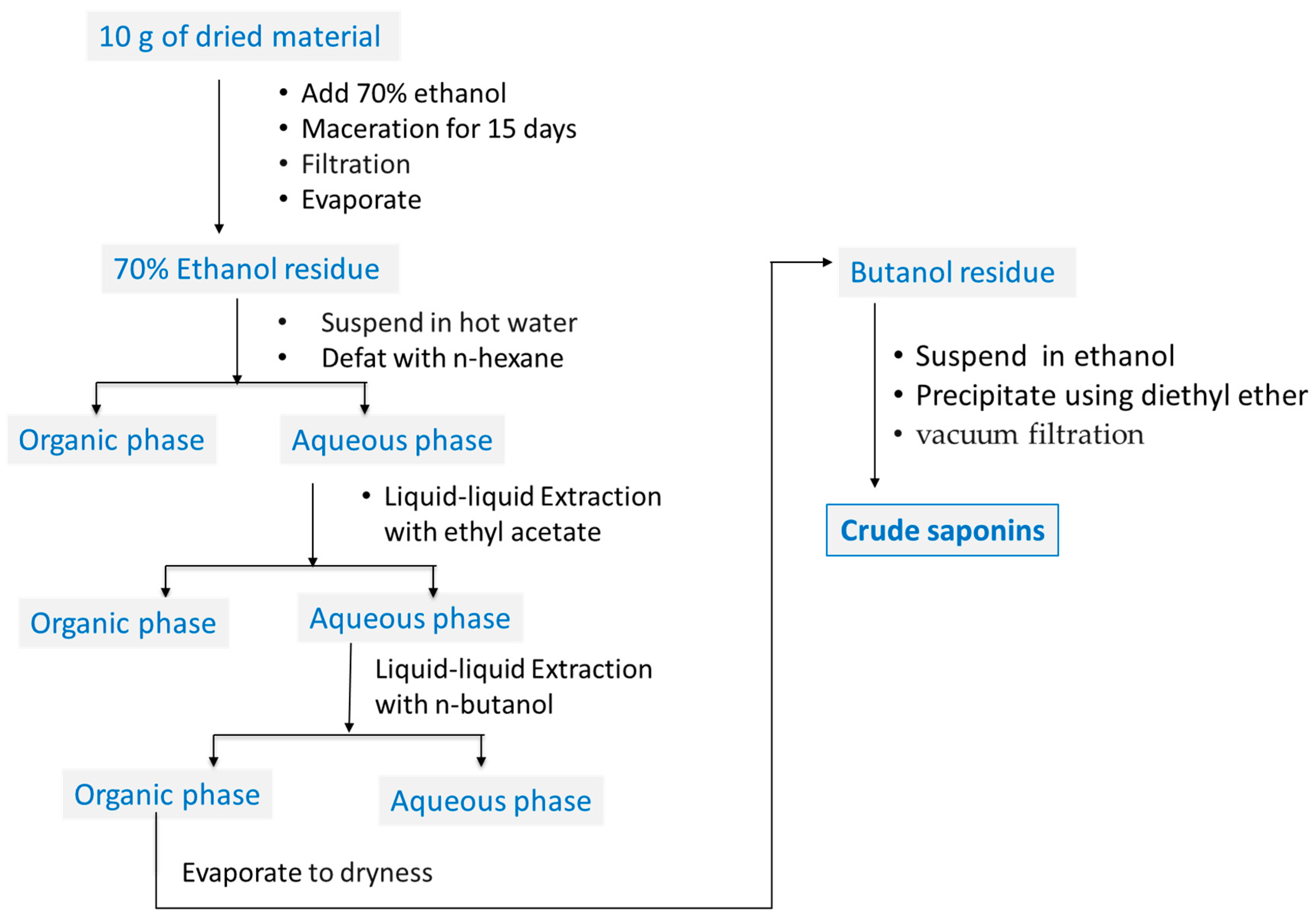
4.2. Plant Materials and Sample Preparation
4.3. Qualitative Determination of Saponins in ALS
4.4. MTT Assay
4.5. Melanin Content Determination
4.6. Western Blotting
4.7. Quantitative Real-Time PCR (qPCR) Analysis
4.8. DNA Microarray
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J. Biogenesis of pigment granules: A sensitive way to regulate melanocyte function. J. Dermatol. Sci. 2005, 37, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Jiménez-Cervantes, C.; Lozano, J.A.; Solano, F.; García-Borrón, J.C. The 5,6-dihydroxyindole-2-carboxylic acid (DHICA) oxidase activity of human tyrosinase. Biochem. J. 2001, 354, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Goding, C.R. Melanocytes: The new black. Int. J. Biochem. Cell Biol. 2007, 39, 275–279. [Google Scholar] [CrossRef]
- Rigopoulos, D.; Gregoriou, S.; Katsambas, A. Hyperpigmentation and melasma. J. Cosmetic Dermatol. 2007, 6, 195–202. [Google Scholar] [CrossRef]
- Pérez-Bernal, A.; Muñoz-Pérez, M.A.; Camacho, F. Management of facial hyperpigmentation. Am. J. Clin. Dermatol. 2000, 1, 261–268. [Google Scholar] [CrossRef]
- Kooyers, T.; Westerhof, W. Toxicology and health risks of hydroquinone in skin lightening formulations. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 777–780. [Google Scholar] [CrossRef]
- Available online: https://edition.cnn.com/2022/01/25/world/as-equals-skin-whitening-global-market-explainer-intl-cmd/index.html (accessed on 25 January 2022).
- Sofen, B.; Prado, G.; Emer, J. Melasma and post inflammatory hyperpigmentation: Management update and expert opinion. Skin Ther. Lett. 2016, 21, 1–7. [Google Scholar]
- Rendon, M.I.; Barkovic, S. Clinical evaluation of a 4% hydroquinone + 1% retinol treatment regimen for improving melasma and photodamage in Fitzpatrick skin types III-VI. J. Drugs Dermatol. 2016, 15, 1435–1441. [Google Scholar]
- Bozzo, P.; Chua-Gocheco, A.; Einarson, A. Safety of skin care products during pregnancy. Can. Fam. Physician 2011, 57, 665–667. [Google Scholar]
- Tyler, K.H. Physiological skin changes during pregnancy. Clin. Obstet. Gynecol. 2015, 58, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Darji, K.; Varade, R.; West, D.; Armbrecht, E.S.; Guo, M.A. Psychosocial impact of postinflammatory hyperpigmentation in patients with acne vulgaris. J. Clin. Aesthetic Dermatol. 2017, 10, 18–23. [Google Scholar]
- Akinboro, A.O.; Ezejiofor, O.I.; Olanrewaju, F.O.; Oripelaye, M.M.; Olabode, O.P.; Ayodele, O.E.; Onayemi, E.O. The impact of acne and facial post-inflammatory hyperpigmentation on quality of life and self-esteem of newly admitted Nigerian undergraduates. Clin. Cosmet. Investig. Dermatol. 2018, 11, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Villareal, M.O.; Han, J.; Yamada, P.; Shigemori, H.; Isoda, H. Hirseins inhibit melanogenesis by regulating the gene expressions of Mitf and melanogenesis enzymes. Exp. Dermatol. 2010, 19, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y. Melanin synthesis inhibiting function of β-carotene. Fragr. J. 2001, 29, 28–32. [Google Scholar]
- Villareal, M.O.; Kume, S.; Bourhim, T.; Bakhtaoui, F.Z.; Kashiwagi, K.; Han, J.; Gadhi, C.; Isoda, H. Activation of MITF by argan oil leads to the inhibition of the tyrosinase and dopachrome tautomerase expressions in B16 murine melanoma cells. Evid.-Based Complementary Altern Med. 2013, 2013, 340107. [Google Scholar] [CrossRef]
- Makbal, R.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Argania spinosa fruit shell extract-induced melanogenesis via cAMP signaling pathway activation. Int. J. Mol. Sci. 2020, 21, 2539. [Google Scholar] [CrossRef]
- Bourhim, T.; Villareal, M.O.; Couderc, F.; Hafidi, A.; Isoda, H.; Gadhi, C. Melanogenesis promoting effect, antioxidant activity, and UPLC-ESI-HRMS characterization of phenolic compounds of Argan leaves extract. Molecules 2021, 26, 371. [Google Scholar] [CrossRef]
- Bourhim, T.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Depigmenting effect of argan press-cake extract through the down-regulation of Mitf and melanogenic enzymes expression in B16 murine melanoma cells. Cytotechnology 2018, 70, 1389–1397. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Secondary metabolites from Argania spinosa (L.) Skeels. Phytochem. Rev. 2002, 1, 345–354. [Google Scholar] [CrossRef]
- Mugford, S.T.; Osbourn, A. Saponin synthesis and function. In Isoprenoid synthesis in plants and microorganisms: New Concepts Experimental Approaches; Springer: New York, NY, USA, 2012; pp. 405–424. [Google Scholar] [CrossRef]
- Goto, M.; Kuda, T.; Shikano, A.; Charrouf, Z.; Yamauchi, K.; Yokozawa, M.; Takahashi, H.; Kimura, B. Induction of superoxide anion radical-scavenging capacity in an argan press cake-suspension by fermentation using Lactobacillus plantarum Argan-L1. LWT 2019, 100, 56–61. [Google Scholar] [CrossRef]
- Akihisa, T.; Abe, M.; Manosroi, J.; Manosroi, A. Triterpenoid saponins of Sapotaceae plants and their bioactivities. Chiang Mai J. Sci. 2018, 45, 2534–2553. [Google Scholar]
- Khallouki, F.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Secondary metabolites of the argan tree (Morocco) may have disease prevention properties. African J. Biotechnol. 2005, 4, 381–388. [Google Scholar]
- Kamal, R.; Kharbach, M.; Heyden, Y.V.; Yu, H.; Bouklouze, A.; Cherrah, Y.; Alaoui, K. In vitro & in vivo anti-hyperglycemic potential of saponins cake and argan oil from Argania spinosa. Foods 2021, 10, 1078. [Google Scholar] [CrossRef]
- Silva, G.L.; Lee, I.S.; Kinghorn, A.D. Special Problems with the Extraction of Plants. In Natural Products Isolation. Methods in Biotechnology; Cannell, R.J.P., Ed.; Humana Press: Totowa, NJ, USA, 1998; Volume 4. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites; Humana Press: Totowa, NJ, USA, 2007; Volume 393, pp. 1–122. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Manzaneque, J.C.; Lane, T.F.; Ortega, M.A.; Hynes, R.O.; Lawler, J.; Iruela-Arispe, M.L. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc. Natl. Acad. Sci. USA 2001, 98, 12485–12490. [Google Scholar] [CrossRef]
- Goto, T.; Sato, A.; Adachi, S.; Iemura, S.; Natsume, T.; Shibuya, H. IQGAP1 protein regulates nuclear localization of -catenin via importin-5 protein in Wnt signaling. J. Biol. Chem. 2013, 288, 36351–36360. [Google Scholar] [CrossRef]
- Dilshat, R.; Fock, V.; Kenny, C.; Gerritsen, I.; Lasseur, R.; Travnickova, J.; Eichhoff, O.M.; Cerny, P.; Möller, K.; Sigurbjörnsdóttir, S.; et al. MITF reprograms the extracellular matrix and focal adhesion in melanoma. eLife 2021, 10, e63093. [Google Scholar] [CrossRef]
- Taylor, A.; Pawaskar, M.; Taylor, S.L.; Balkrishnan, R.; Feldman, S.R. Prevalence of pigmentary disorders and their impact on quality of life: A prospective cohort study. J. Cosmet. Dermatol. 2008, 7, 164–168. [Google Scholar] [CrossRef]
- Owolabi, J.O.; Fabiyi, O.S.; Adelakin, L.A.; Ekwerike, M.C. Effects of skin lightening cream agents—hydroquinone and kojic acid, on the skin of adult female experimental rats. Clin. Cosmet. Investig. Dermatol. 2020, 13, 283–289. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Mostafalou, S.; Baeeri, M.; Gholami, M.; Ghafour-Boroujerdi, E.; Abdollahi, M. The molecular mechanisms of liver and islets of Langerhans toxicity by benzene and its metabolite hydroquinone in vivo and in vitro. Toxicol. Mech. Methods 2015, 25, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.C.; Angra, K.; Halder, R.M. Are natural ingredients effective in the management of hyperpigmentation? A systematic review. J. Clin. Aesthet. Dermatol. 2018, 11, 28–37. [Google Scholar]
- El Aziz, M.; Ashour, A.; Melad, A. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 8, 6–12. [Google Scholar]
- Matsuda, H.; Higashino, M.; Nakai, Y.; Linuma, M.; Kubo, M.; Lang, F.A. Studies of cuticle drugs from natural sources. IV. Inhibitory effects of some Arctostaphylos plants on melanin biosynthesis. Biol. Pharm. Bull. 1996, 19, 153–156. [Google Scholar] [CrossRef][Green Version]
- Nguyen, H.M.; Nguyen, H.T.; Win, N.; Wong, C.P.; Huynh, K.L.V.; Hoang, N.N.; Do, K.M.; Nguyen, H.T.; Ho, D.H.V.; Nguyen, M.D.; et al. Antimelanogenic Activity of Ocotillol-Type Saponins from Panax vietnamensis. Chem. Biodivers. 2020, 17, e2000037. [Google Scholar] [CrossRef]
- Fujihara, K.; Takahashi, K.; Koyama, K.; Kinoshita, K. Triterpenoid saponins from Polaskia chichipe Backbg. and their inhibitory or promotional effects on the melanogenesis of B16 melanoma cells. J. Nat. Med. 2017, 71, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nakashima, S.; Tanabe, G.; Oda, Y.; Yokota, N.; Fujimoto, K.; Matsumoto, T.; Sakuma, R.; Ohta, T.; Ogawa, K.; et al. Alkaloid constituents from flower buds and leaves of sacred lotus (Nelumbo nucifera, Nymphaeaceae) with melanogenesis inhibitory activity in B16 melanoma cells. Bioorg. Med. Chem. 2013, 21, 779–787. [Google Scholar] [CrossRef]
- Kawabata, T.; Cui, M.Y.; Hasegawa, T.; Takano, F.; Ohta, T. Anti-inflammatory and anti-melanogenic steroidal saponin glycosides from Fenugreek (Trigonella foenum-graecum L.) seeds. Planta Med. 2011, 77, 705–710. [Google Scholar] [CrossRef]
- Cheli, Y.; Ohanna, M.; Ballotti, R.; Bertolotto, C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment. Cell Melanoma Res. 2010, 23, 27–40. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Borovanský, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Lin, J.; Fisher, D. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Takeda, K.; Saito, H.; Watanabe, K.; Takahashi, K.; Shibahara, S. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. 2002, 21, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, C.; Buscà, R.; Abbe, P.; Bille, K.; Aberdam, E.; Ortonne, J.P.; Ballotti, R. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: Pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol. Cell Biol. 1998, 18, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.; Magwenzi, S.; Aburima, A.; Naseem, K.M. Thrombospondin-1 induces platelet activation through CD36-dependent inhibition of the cAMP/protein kinase A signaling cascade. Blood 2010, 116, 4297–4306. [Google Scholar] [CrossRef]
- Goding, C.R. Mitf from neural crest to melanoma: Signal transduction and transcription in the melanocyte lineage. Genes Dev. 2000, 14, 1712–1728. [Google Scholar] [CrossRef]
- Orsulic, S.; Huber, O.; Aberle, H.; Arnold, S.; Kemler, R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J. Cell Sci. 1999, 112, 1237–1245. [Google Scholar] [CrossRef]
- Tarlé, R.G.; Silva de Castro, C.C.; do Nascimento, L.M.; Mira, M.T. Polymorphism of the E-cadherin gene CDH 1 is associated with susceptibility to vitiligo. Exp. Dermatol. 2015, 24, 300–302. [Google Scholar] [CrossRef]
- Tahrouch, S.; Hatimi, A.; Rapior, S.; Mondolot, L.; Gargadennec, A.; Andary, C. Phenolic compounds of argan tree, Argania spinosa (endemic species of south western Morocco). TOJSAT 2011, 1, 17–23. [Google Scholar]
- Joguet, N.; Maugard, T. Characterization and quantification of phenolic compounds of Argania spinosa leaves by HPLC-PDA-ESI-MS analyses and their antioxidant activity. Chem. Nat. Compd. 2013, 48, 1069–1071. [Google Scholar] [CrossRef]
- Mercolini, L.; Protti, M.; Saracino, M.A.; Mandrone, M.; Antognoni, F.; Poli, F. Analytical profiling of bioactive phenolic compounds in argan (Argania spinosa) leaves by combined microextraction by packed sorbent (MEPS) and LC-DAD-MS/MS. Phytochem. Anal. 2015, 27, 41–49. [Google Scholar] [CrossRef]
- Hilali, M.; El Monfalouti, H.; Kartah, B.E. Study of the flavonoids and secondary metabolites of the argan tree (Argania spinosa L.). Online J. Animal Feed Res. 2020, 10, 167–171. [Google Scholar] [CrossRef]
- Guinda, A.; Rada, M.; Delgado, T.; Castellano, J.M. Pentacyclic triterpenic acids from Argania spinosa. Eur. J. Lipid Sci. Technol. 2011, 113, 231–237. [Google Scholar] [CrossRef]
- El Kabouss, A.; Charrouf, Z.; Faid, M.; Garneau, F.X.; Collin, G. Chemical composition and antimicrobial activity of the leaf essential oil of Argania spinosa L. Skeels. J. Essential Oil Res. 2002, 14, 147–149. [Google Scholar] [CrossRef]
- Tahrouch, S.; Rapior, S.; Bessiere, J.-M.; Andary, C. Les substances volatiles de Argania spinosa (Sapotaceae). Acta Bot. Gall. 1998, 145, 259–263. [Google Scholar] [CrossRef]
- Larhsini, M.; Marston, A.; Hostettmann, K. Triterpenoid saponins from the roots of Silene cucubalus. Fitoterapia 2003, 74, 237–241. [Google Scholar] [CrossRef]
- Villareal, M.O.; Chaochaiphat, T.; Bejaoui, M.; Isoda, H.; Sato, K. Tara tannin regulates pigmentation by modulating melanogenesis enzymes and melanosome transport proteins expression. Planta Med. Intl. Open 2020, 7, e34–e44. [Google Scholar] [CrossRef]
- Mossman, T. Rapid calorimetric assay for cellular growth and survival: Application to proliferation and cytotoxic assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
| Gene Symbol | Fold Change, Con vs. ALS a | Description | Biological Process |
|---|---|---|---|
| SMC3 | 3.04 | Structural maintenance of chromosomes 3 | M phase of mitotic cell cycle, cytokinesis, regulation of DNA replication, DNA repair response to DNA damage stimulus and cell cycle, mitotic spindle organization, stem cell maintenance negative regulation of DNA endoreduplication, chromosome organization, cell division, etc. |
| SMC2 | 2.46 | Structural maintenance of chromosomes 2 | Mitotic cell cycle, DNA repair, DNA recombination, cell cycle and sister chromatid cohesion, mitosis, mitotic chromosome condensation, meiotic chromosome condensation, chromosome condensation, meiotic chromosome segregation, chromosome organization, cell division, kinetochore organization |
| IQGAP1 | 2.45 | IQ motif containing GTPase activating protein 1 | Regulation of cytokine production, energy reserve metabolic process, signal transduction, small GTPase mediated signal transduction, positive regulation of Ras GTPase activity, negative regulation of dephosphorylation, positive regulation of GTPase activity, small molecule metabolic process, positive regulation of protein kinase activity, etc. |
| RSF1 | 2.37 | Remodeling and spacing factor 1 | Nucleosome assembly chromatin remodeling transcription, DNA-dependent transcription, initiation regulation of transcription, DNA-dependent chromatin modification, nucleosome positioning CENP-A containing nucleosome assembly at centromere, negative regulation of DNA binding, negative regulation of transcription, DNA-dependent positive regulation of transcription, DNA-dependent positive regulation of viral transcription |
| KTN1 | 2.32 | Kinectin 1 (kinesin receptor) | Microtubule-based movement protein transport |
| SMC4 | 2.22 | Structural maintenance of chromosomes 4 | Mitotic sister chromatid segregation, mitotic cell cycle, DNA repair, DNA recombination, chemotaxis, cell cycle, sister chromatid cohesion, mitosis, mitotic chromosome condensation, signal transduction, meiotic chromosome condensation, chromosome condensation, meiotic chromosome segregation, chromosome organization, cell division kinetochore organization |
| ANXA1 | 2.2 | Annexin A1 | Keratinocyte differentiation, neutrophil homeostasis, negative regulation of acute inflammatory response, cellular component movement and inflammatory response, cell cycle signal transduction, cell surface receptor signaling pathway, response to hormone stimulus, response to X-ray, response to organic cyclic compound, peptide cross-linking, insulin secretion, endocrine pancreas development, etc. |
| LUC7L3 | 2.18 | LUC7-like 3 (S. cerevisiae) | mRNA processing, apoptotic process, response to stress, RNA splicing |
| THBS1 | 2.15 | Thrombospondin 1 | Activation of MAPK activity, response to hypoxia, negative regulation of endothelial cell proliferation, negative regulation of endothelial cell proliferation, negative regulation of cell-matrix adhesion, negative regulation of cGMP-mediated signaling, positive regulation of transforming growth factor beta receptor signaling pathway, response to magnesium ion, response to progesterone stimulus, negative regulation of interleukin-12 production, positive regulation of transforming growth factor beta1 production, cellular response to heat, positive regulation of tumor necrosis factor biosynthetic process, positive regulation of macrophage activation, negative regulation of apoptotic process, response to calcium ion, positive regulation of protein kinase B signaling cascade, positive regulation of reactive oxygen species metabolic process, negative regulation of extrinsic apoptotic signaling pathway, etc. |
| ANKRD36 | 2.13 | Ankyrin repeat domain 36 | None reported |
| MYH10 | 2.1 | Myosin, heavy chain 10, nonmuscle | Mitotic cytokinesis, in utero embryonic development, neuron migration, plasma membrane repair, exocytosis, substrate-dependent cell migration, cell extension, nuclear migration, signal transduction, axonogenesis, axon guidance, brain development, adult heart development, cell proliferation, regulation of cell shape, fourth ventricle development, lateral ventricle development, third ventricle development, etc. |
| CYP1B1 | 2.08 b | Cytochrome P450, family 1, subfamily B, polypeptide 1 | Angiogenesis, cellular aromatic compound metabolic process, xenobiotic metabolic process, visual perception, steroid metabolic process, estrogen metabolic process, toxin metabolic process, response to toxic substance, response to organic substance, sterol metabolic process, arachidonic acid metabolic process, epoxygenase P450 pathway, etc. |
| HIST2H2AC | 2.08 | Histone cluster 2, H2ac | Nucleosome assembly, chromatin remodeling transcription, DNA-dependent transcription, initiation regulation of transcription, DNA-dependent chromatin modification, nucleosome positioning, CENP-A containing nucleosome assembly at centromere |
| NEMF | 2.01 | Nuclear export mediator factor | Flagellum assembly, nuclear export |
| ASPM | 2.01 | asp (abnormal spindle) homolog, microcephaly associated (Drosophila) | Neuron migration, positive regulation of neuroblast proliferation, cell cycle, mitosis, spermatogenesis, brain development, forebrain neuroblast division, negative regulation of neuron differentiation, negative regulation of asymmetric cell division, oogenesis, developmental growth, cell division, maintenance of centrosome location, positive regulation of canonical Wnt receptor signaling pathway |
| CDH1 | −2.1 | Cadherin 1, type 1, E-cadherin (epithelial) | In utero embryonic development, trophectodermal cell differentiation, apoptotic process, cellular component disassembly involved in execution phase of apoptosis, cell adhesion, homophilic cell adhesion, synapse assembly, sensory perception of sound, response to toxic substance, response to organic substance, cell–cell adhesion, protein metabolic process, etc. |
| Secondary Metabolite | Compound | References |
|---|---|---|
| Polyphenols | Myricetin 3-O-rhamnoside (Myricitrin) | Tahrouch, et al. [51] Joguet and Maugard [52] Mercolini, et al. [53] Hilali, et al. [54] Bourhim, et al. [19] |
| Myricetin-3-O-galactoside | Tahrouch, et al. [51] Mercolini, et al. [53] Hilali, et al. [54] Bourhim, et al. [19] | |
| Quercetin-3-O-galactoside (Hyperoside) | Tahrouch, et al. [51] Joguet and Maugard [52] Mercolini, et al. [53] Hilali, et al. [54] | |
| Quercetin-3-O-rhamnoside (Quercitrin) | Tahrouch, et al. [51] Joguet and Maugard [52] Mercolini, et al. [53] Hilali, et al. [54] Bourhim, et al. [19] | |
| Quercetin-3-O-rutinoside (Rutin) | Mercolini, et al. [53] Bourhim, et al. [19] | |
| Quercetin-3-O-Glucuronide | Bourhim, et al. [19] | |
| Quercetin-7-O-rhamnoside | Bourhim, et al. [19] | |
| Quercetin-O-pentoside | Joguet and Maugard [52] Mercolini, et al. [53] | |
| Myricetin-3-O-glucoside | Mercolini, et al. [53] | |
| Myricetin-3-O-hexose | Joguet and Maugard [52] | |
| Myricetin-O-pentoside | Joguet and Maugard [52] Mercolini, et al. [53] | |
| Catechin | Mercolini, et al. [53] Bourhim, et al. [19] | |
| Epicatechin | Mercolini, et al. [53] Bourhim, et al. [19] | |
| Myricetin | Mercolini, et al. [53] Bourhim, et al. [19] | |
| Quercetin. | Mercolini, et al. [53] Bourhim, et al. [19] | |
| Gallic acid | Bourhim, et al. [19] | |
| (+)−Gallocatechin | Bourhim, et al. [19] | |
| (−)−Epigallocatechin | Bourhim, et al. [19] | |
| Triterpenoids | Ursolic acid | Guinda, et al. [55] |
| Oleanolic acid | Guinda, et al. [55] | |
| Volatile compounds | 1,10-di-epi-cubenol (major component of essential oil) | El Kabouss, et al. [56] |
| 14-methylidene-2,6, 10-trimethylhexadecene (major component of essential oil) | Tahrouch, et al. [57] | |
| Amino acid derivative | Melatonin | Mercolini, et al. [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villareal, M.O.; Chaochaiphat, T.; Makbal, R.; Gadhi, C.; Isoda, H. Molecular Analysis of the Melanogenesis Inhibitory Effect of Saponins-Rich Fraction of Argania spinosa Leaves Extract. Molecules 2022, 27, 6762. https://doi.org/10.3390/molecules27196762
Villareal MO, Chaochaiphat T, Makbal R, Gadhi C, Isoda H. Molecular Analysis of the Melanogenesis Inhibitory Effect of Saponins-Rich Fraction of Argania spinosa Leaves Extract. Molecules. 2022; 27(19):6762. https://doi.org/10.3390/molecules27196762
Chicago/Turabian StyleVillareal, Myra O., Thanyanan Chaochaiphat, Rachida Makbal, Chemseddoha Gadhi, and Hiroko Isoda. 2022. "Molecular Analysis of the Melanogenesis Inhibitory Effect of Saponins-Rich Fraction of Argania spinosa Leaves Extract" Molecules 27, no. 19: 6762. https://doi.org/10.3390/molecules27196762
APA StyleVillareal, M. O., Chaochaiphat, T., Makbal, R., Gadhi, C., & Isoda, H. (2022). Molecular Analysis of the Melanogenesis Inhibitory Effect of Saponins-Rich Fraction of Argania spinosa Leaves Extract. Molecules, 27(19), 6762. https://doi.org/10.3390/molecules27196762







