Hierarchically Annular Mesoporous Carbon Derived from Phenolic Resin for Efficient Removal of Antibiotics in Wastewater
Abstract
1. Introduction
2. Results and Discussion
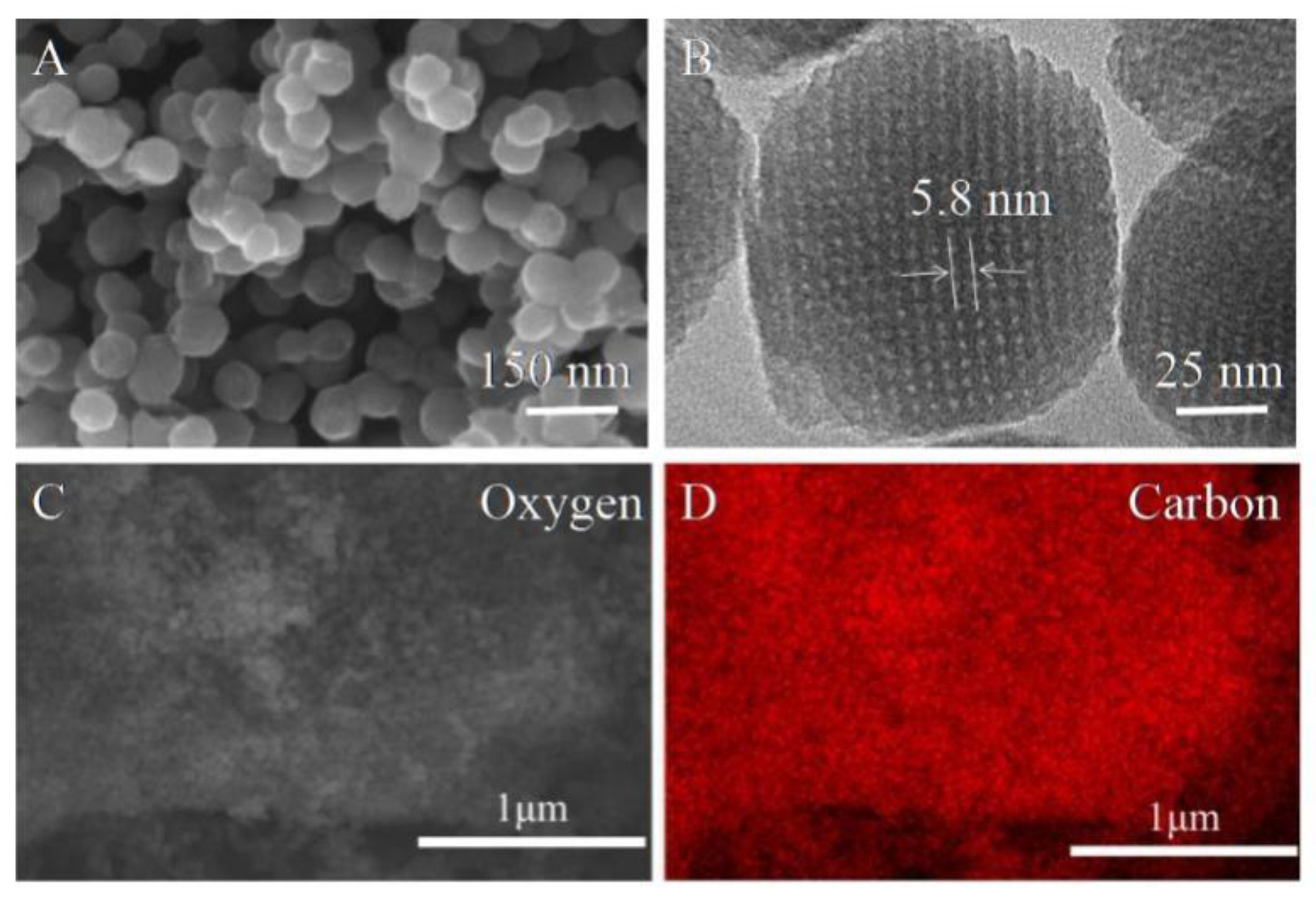
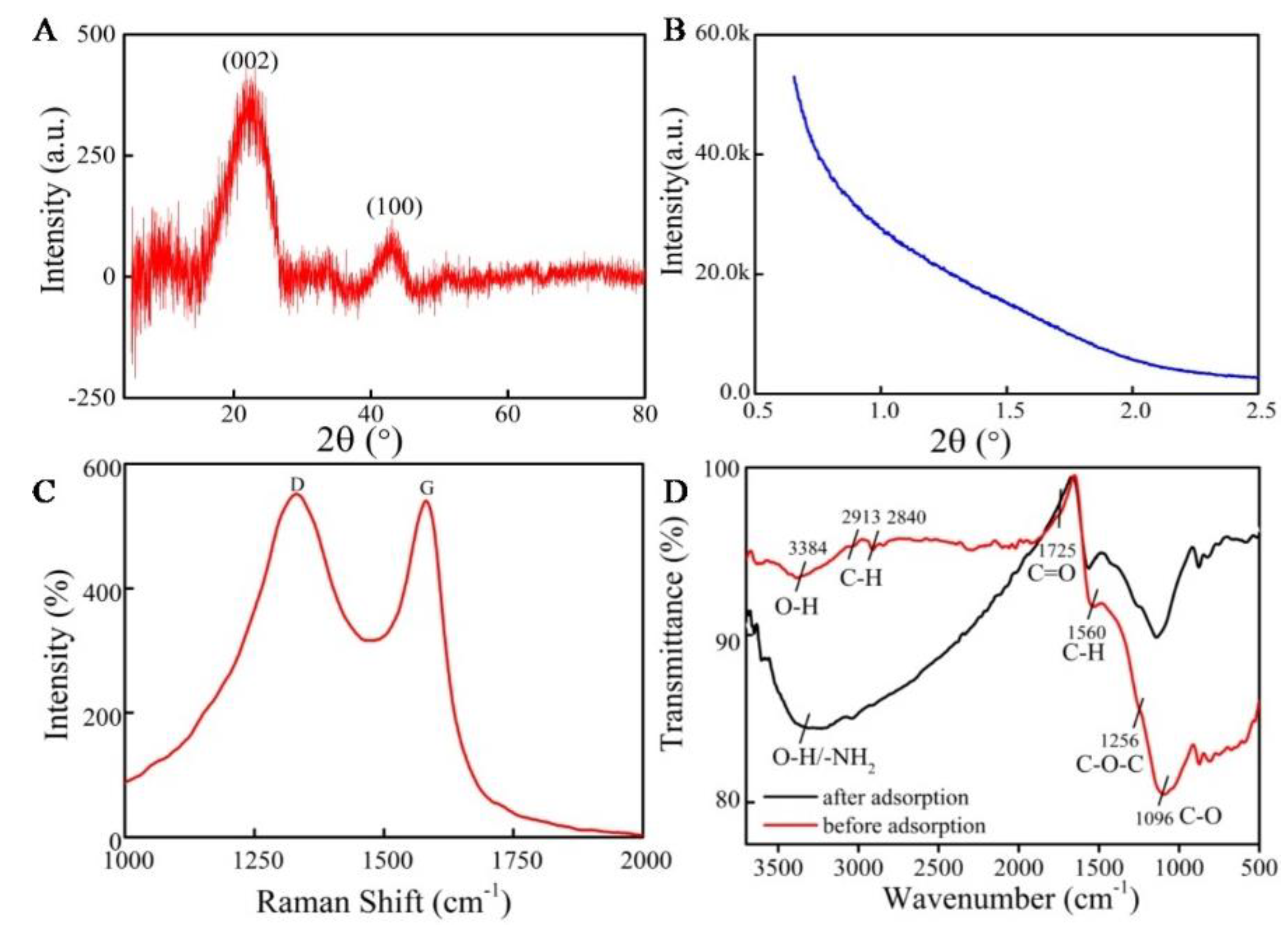
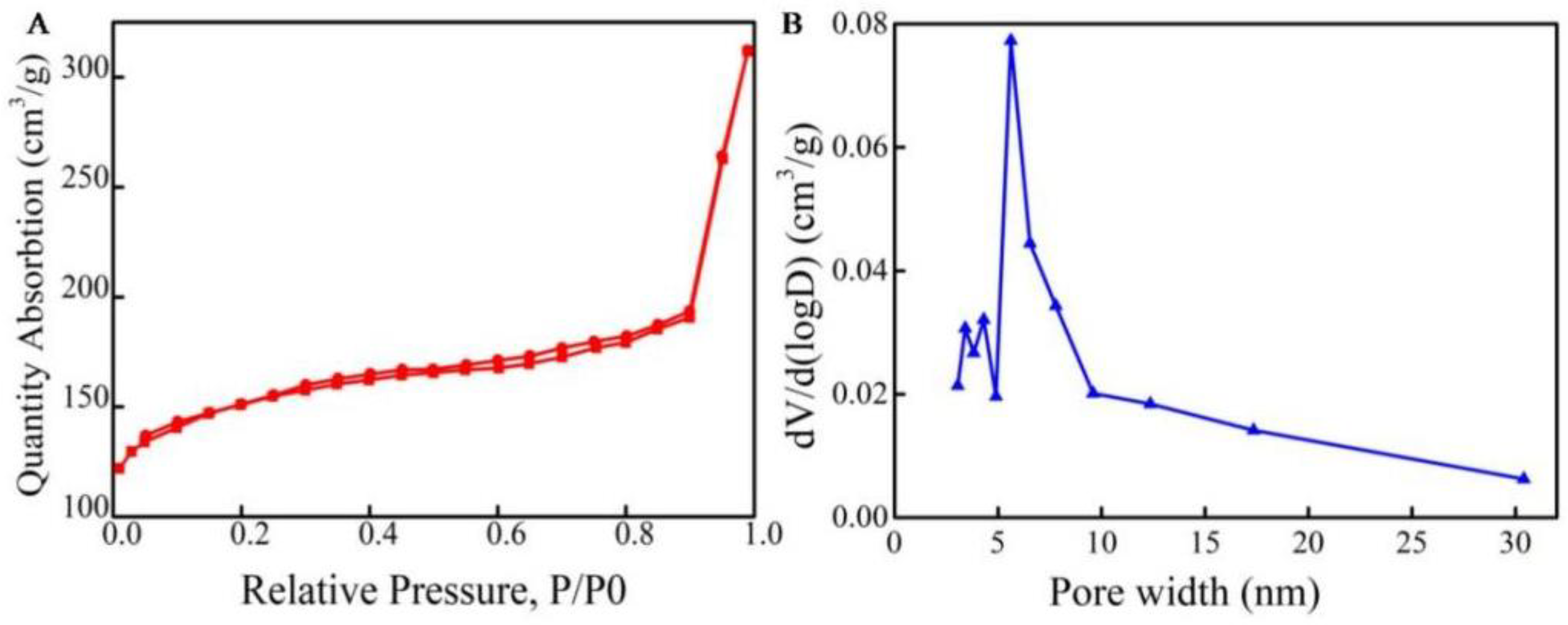
2.1. Characterization of MCNs
2.2. Adsorption of Antibiotics on MCNs
2.2.1. Adsorption Isotherm Study
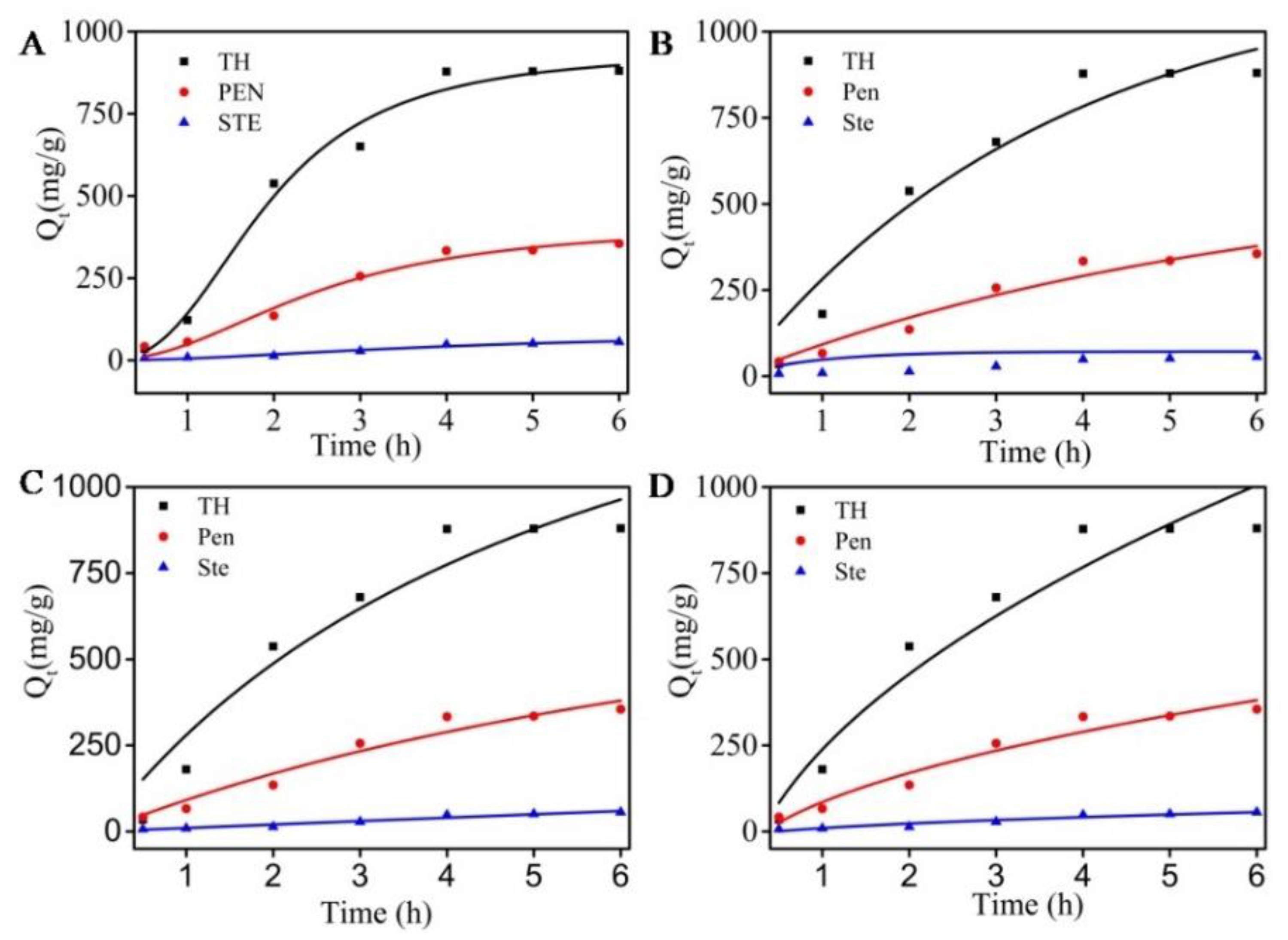
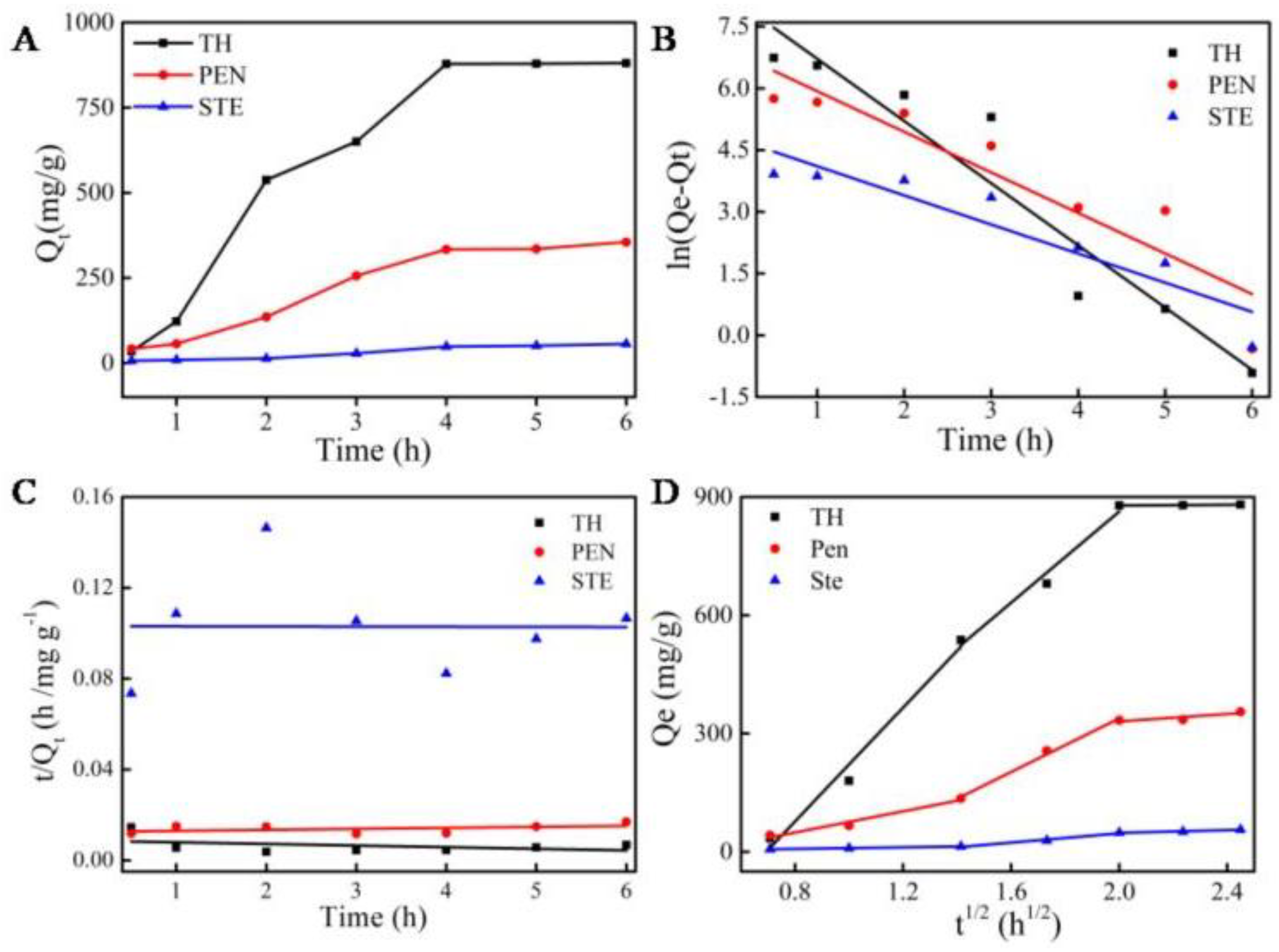
2.2.2. Adsorption Kinetics
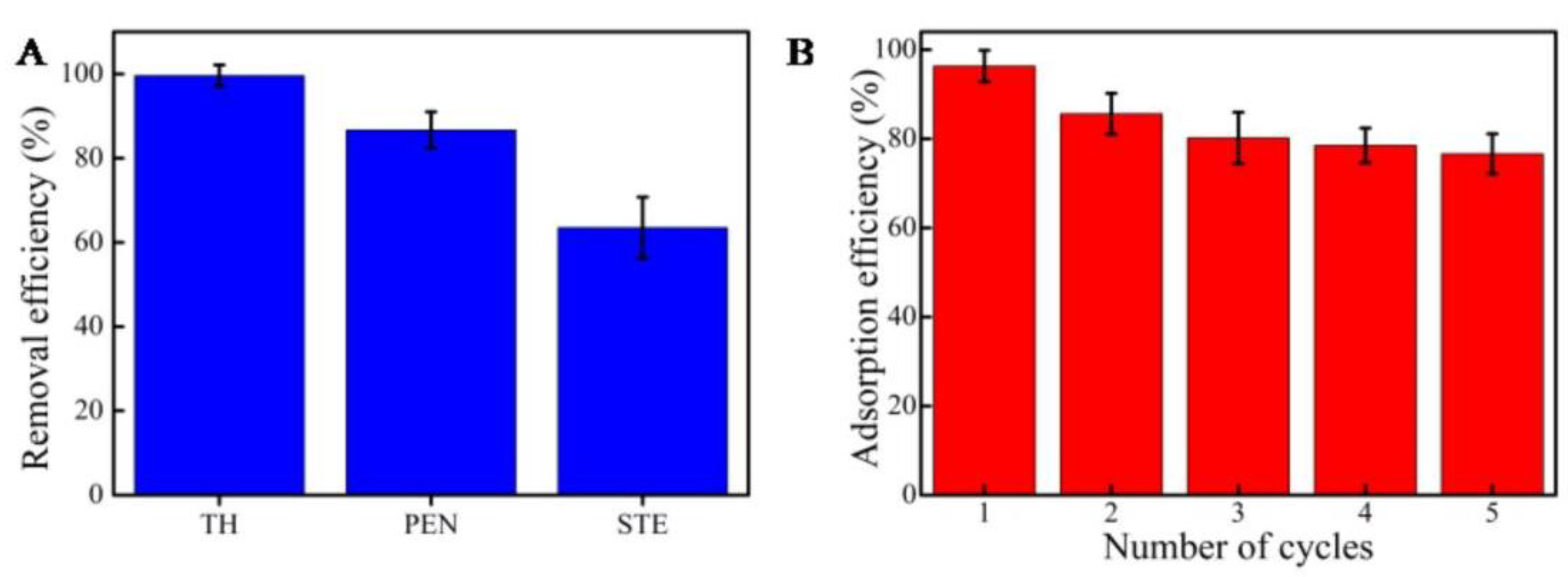
2.3. Removal Efficiency and Reuse of MCNs
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of MCNs
3.3. Characterization of MCNs
3.4. Adsorption Properties for Antibiotics Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A review of processes for removing antibiotics from breeding wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef] [PubMed]
- Phoon, B.L.; Ong, C.C.; Mohamed Saheed, M.S.; Show, P.-L.; Chang, J.-S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Lyu, J.; Yang, L.; Zhang, L.; Ye, B.; Wang, L. Antibiotics in soil and water in China—A systematic review and source analysis. Environ. Pollut. 2020, 266, 115147. [Google Scholar] [CrossRef]
- Li, S.; Show, P.L.; Ngo, H.H.; Ho, S.H. Algae-mediated antibiotic wastewater treatment: A critical review. Environ. Sci. Ecotechnol. 2022, 9, 100145. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Wang, J.; He, S.; Chen, C.; Wojnárovits, L.; Takács, E. Treatment of pharmaceutical wastewater by ionizing radiation: Removal of antibiotics, antimicrobial resistance genes and antimicrobial activity. J. Hazard. Mater. 2021, 415, 125724. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, M.H.; Mohammed, T.J.; Al-Najar, J.A. Advanced oxidation processes (AOPs) for treatment of antibiotics in wastewater: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012109. [Google Scholar] [CrossRef]
- Mangla, D.; Annu Sharma, A.; Ikram, S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard. Mater. 2022, 425, 127946. [Google Scholar] [CrossRef] [PubMed]
- Torkian, N.; Bahrami, A.; Hosseini-Abari, A.; Momeni, M.M.; Abdolkarimi-Mahabadi, M.; Bayat, A.; Hajipour, P.; Amini Rourani, H.; Abbasi, M.S.; Torkian, S.; et al. Synthesis and characterization of Ag-ion-exchanged zeolite/TiO2 nanocomposites for antibacterial applications and photocatalytic degradation of antibiotics. Environ. Res. 2022, 207, 112157. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, S.V.; Singh Baghel, R.; Kumar, M. Antagonistic and synergistic analysis of antibiotic adsorption on Prosopis juliflora activated carbon in multicomponent systems. Chem. Eng. J. 2020, 381, 122713. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, Z.; Cao, J.; Yu, F. Enhanced adsorption for the removal of antibiotics by carbon nanotubes/graphene oxide/sodium alginate triple-network nanocomposite hydrogels in aqueous solutions. Chemosphere 2020, 242, 125188. [Google Scholar] [CrossRef]
- Krasucka, P.; Pan, B.; Ok, Y.S.; Mohan, D.; Sarkar, B.; Oleszczuk, P. Engineered biochar—A sustainable solution for the removal of antibiotics from water. Chem. Eng. J. 2021, 405, 126926. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Yahyaoui, S.; Hanafy, H.; Seliem, M.K.; Bonilla-Petriciolet, A.; Luiz Dotto, G.; Sellaoui, L.; Li, Q. Effective adsorption of dyes on an activated carbon prepared from carboxymethyl cellulose: Experiments, characterization and advanced modelling. Chem. Eng. J. 2021, 417, 128116. [Google Scholar] [CrossRef]
- Jawad, A.H.; Saud Abdulhameed, A.; Wilson, L.D.; Syed-Hassan, S.S.A.; Alothman, Z.A.; Rizwan Khan, M. High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption: Optimization and mechanism study. Chin. J. Chem. Eng. 2021, 32, 281–290. [Google Scholar] [CrossRef]
- Wang, B.; Xu, X.; Tang, H.; Mao, Y.; Chen, H.; Ji, F. Highly efficient adsorption of three antibiotics from aqueous solutions using glucose-based mesoporous carbon. Appl. Surf. Sci. 2020, 528, 147048. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, M.; Wang, Y.; Wei, F. Highly effective adsorption of antibiotics from water by hierarchically porous carbon: Effect of nanoporous geometry. Environ. Pollut. 2021, 274, 116591. [Google Scholar] [CrossRef]
- Fang, Y.; Gu, D.; Zou, Y.; Wu, Z.; Li, F.; Che, R.; Deng, Y.; Tu, B.; Zhao, D. A low-concentration hydrothermal synthesis of biocompatible ordered mesoporous carbon nanospheres with tunable and uniform size. Angew. Chem. Int. Ed. 2010, 49, 7987–7991. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, L.; Deng, J.; Gong, Y.; Tang, G.; Bai, H.; Wang, Y. Annular mesoporous carbonaceous nanospheres from biomass-derived building units with enhanced biological interactions. Chem. Mater. 2019, 31, 7186–7191. [Google Scholar] [CrossRef]
- Yang, X.; Lu, P.; Yu, L.; Pan, P.; Elzatahry, A.A.; Alghamdi, A.; Luo, W.; Cheng, X.; Deng, Y. An Efficient emulsion-induced interface assembly approach for rational synthesis of mesoporous carbon spheres with versatile architectures. Adv. Funct. Mater. 2020, 30, 2002488. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 95–107. [Google Scholar] [CrossRef]
- Wen, Y.R.; Liu, Y.; Liu, D.H.; Tang, B.; Liu, C.T. Microstructural evolution of ferritic steel powder during mechanical alloying with iron oxide. Int. J. Mater. Res. 2011, 102, 160–167. [Google Scholar] [CrossRef]
- An, K.; Xu, X. Mo2C based electrocatalyst with nitrogen doped three dimensional mesoporous carbon as matrix, synthesis and HER activity study. Electrochim. Acta 2019, 293, 348–355. [Google Scholar] [CrossRef]
- Yang, J.; Dou, Y.; Yang, H.; Wang, D. A novel porous carbon derived from CO 2 for high-efficient tetracycline adsorption: Behavior and mechanism. Appl. Surf. Sci. 2021, 538, 148110. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.; Zhang, X.; Varjani, S.; Liu, Y. Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Total Environ. 2020, 720, 137662. [Google Scholar] [CrossRef]

| Name | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| kL (L/mg) | Qm, (mg/g) | R2 | n | kf (mg/g)/(mg/ L)n | R2 | |
| Tetracycline hydrochloride | 6.75 × 10−6 | 31,497 | 0.994 | 1.07 | 0.349 | 0.994 |
| Penicillin | 1.88 × 10−4 | 693 | 0.945 | 1.49 | 1.121 | 0.957 |
| Streptomycin | 1.17 × 10−6 | 8483 | 0.819 | 1.17 | 0.034 | 0.823 |
| Name | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| Qe, exp (mg/g) | Qe, cal (mg/g) | k1 (h−1) | R2 | Qe (mg/g) | k2 (h−1) | R2 | |
| Tetracycline hydrochloride | 4268.2 | 3744.3 | 1.51 | 0.903 | 1480.9 | 5.83 × 10−5 | 0.013 |
| Penicillin | 886.3 | 1010.3 | 0.99 | 0.830 | 2283.1 | 1.54 × 10−5 | 0.037 |
| Streptomycin | 256.2 | 123.5 | 0.71 | 0.840 | 71,994.2 | 1.86 × 10−9 | 0.020 |
| Name | Intra-Particle Diffusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 (mg/g h−1/2) | c (mg/g) | R2 | k2 (mg/g h−1/2) | c (mg/g) | R2 | k3 (mg/g h−1/2) | c (mg/g) | R2 | |
| Tetracycline hydrochloride | 721.50 | −499.85 | 0.96 | 577.71 | −292.22 | 0.96 | 4.86 | 868.54 | 0.89 |
| Penicillin | 134.84 | −58.40 | 0.98 | 339.65 | −340.84 | 0.99 | 47.24 | 236.15 | 0.88 |
| Streptomycin | 9.77 | −0.28 | 0.98 | 59.18 | 71.29 | 0.96 | 17.01 | 14.12 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Su, M.; Fang, F.; Hong, J.; Zhang, Y.; Zhou, S.-F. Hierarchically Annular Mesoporous Carbon Derived from Phenolic Resin for Efficient Removal of Antibiotics in Wastewater. Molecules 2022, 27, 6735. https://doi.org/10.3390/molecules27196735
Lin X, Su M, Fang F, Hong J, Zhang Y, Zhou S-F. Hierarchically Annular Mesoporous Carbon Derived from Phenolic Resin for Efficient Removal of Antibiotics in Wastewater. Molecules. 2022; 27(19):6735. https://doi.org/10.3390/molecules27196735
Chicago/Turabian StyleLin, Xuexia, Mengxing Su, Feixiang Fang, Jiafu Hong, Yumeng Zhang, and Shu-Feng Zhou. 2022. "Hierarchically Annular Mesoporous Carbon Derived from Phenolic Resin for Efficient Removal of Antibiotics in Wastewater" Molecules 27, no. 19: 6735. https://doi.org/10.3390/molecules27196735
APA StyleLin, X., Su, M., Fang, F., Hong, J., Zhang, Y., & Zhou, S.-F. (2022). Hierarchically Annular Mesoporous Carbon Derived from Phenolic Resin for Efficient Removal of Antibiotics in Wastewater. Molecules, 27(19), 6735. https://doi.org/10.3390/molecules27196735







