Proficient One-Step Heat-Up Synthesis of Manganese Sulfide Quantum Dots for Solar Cell Applications
Abstract
1. Introduction
2. Results and Discussion
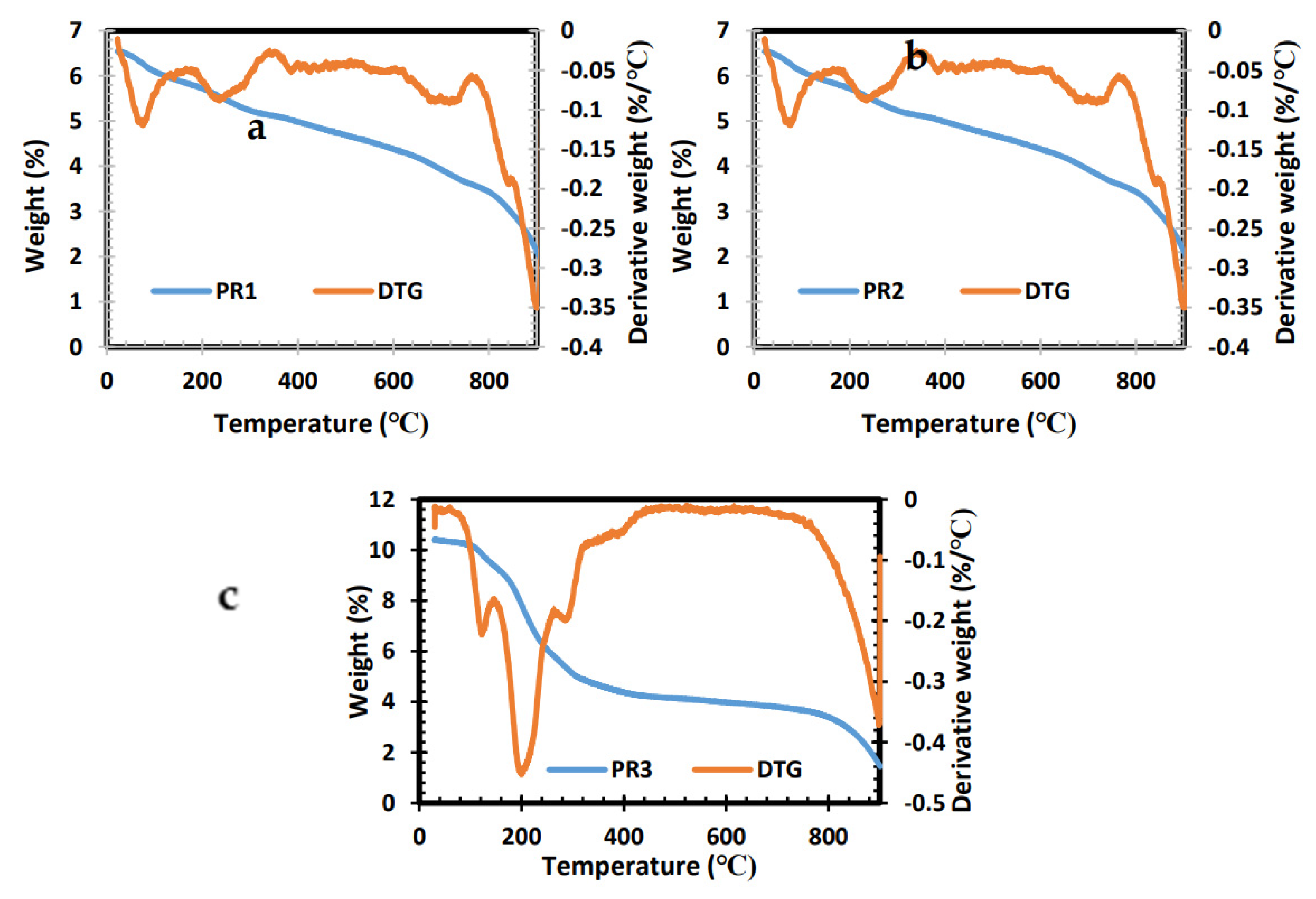
2.1. Thermogravimetric Analysis (TGA) of Precursors PR1, PR2 and PR3
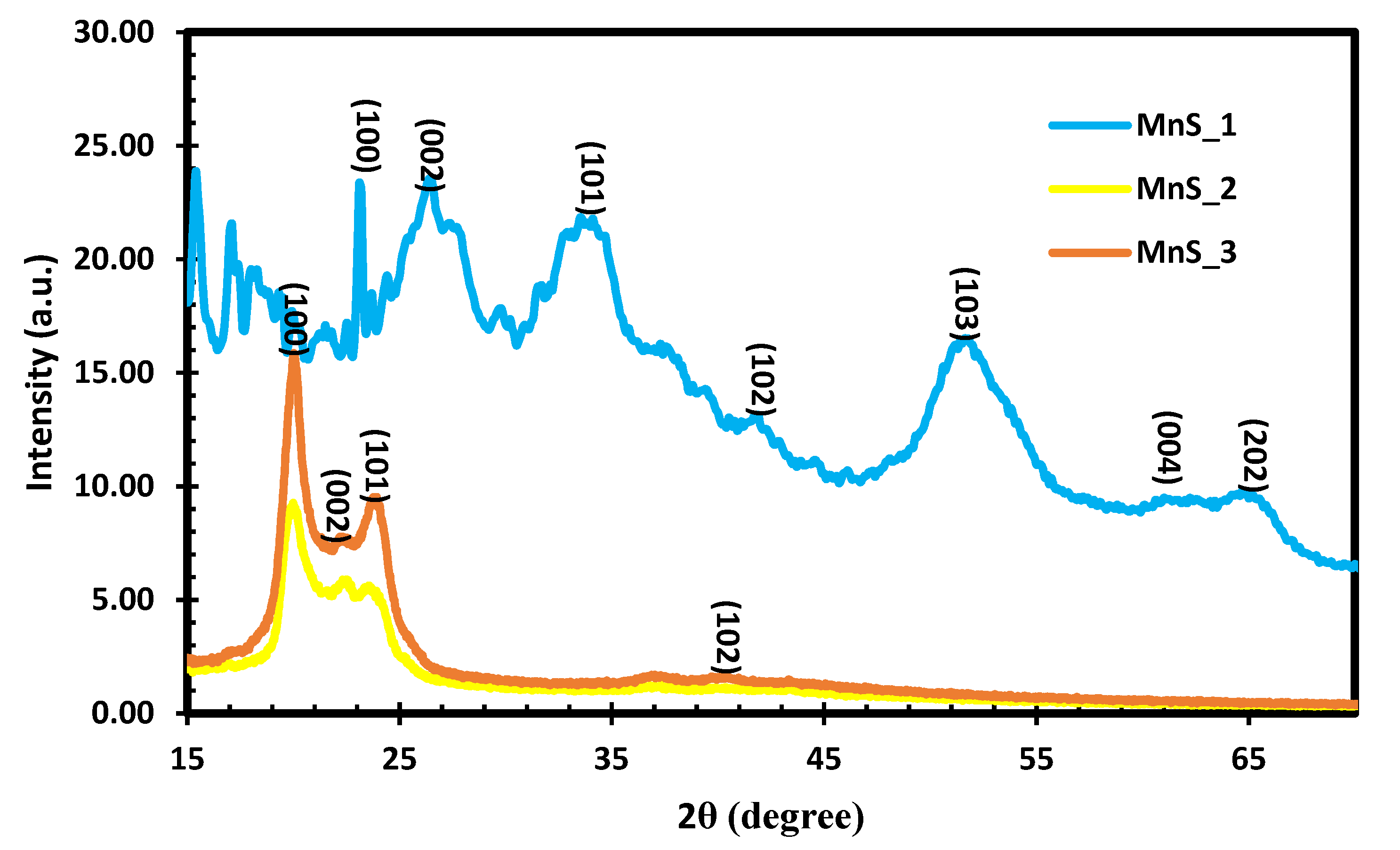
2.2. XRD
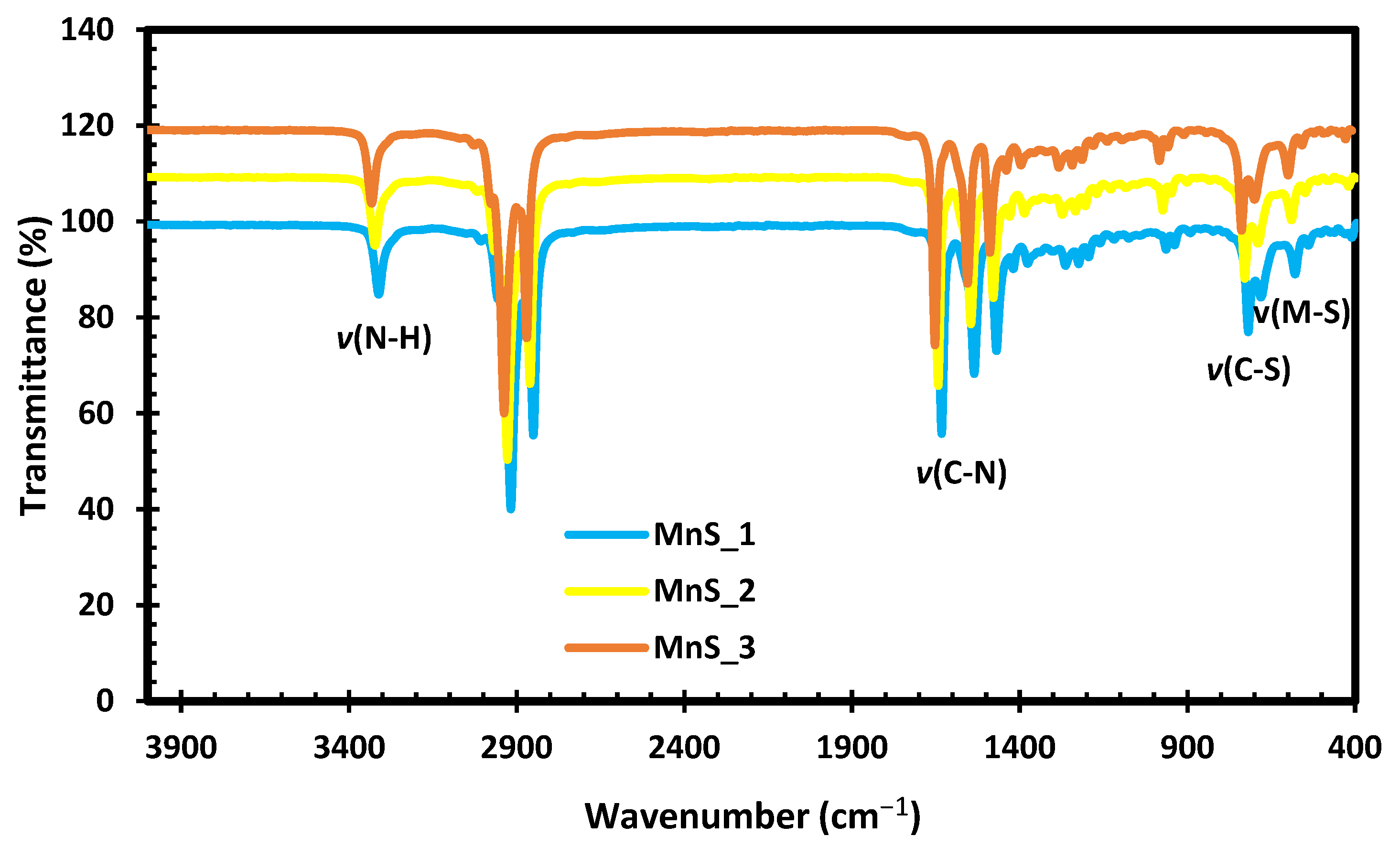
2.3. FTIR
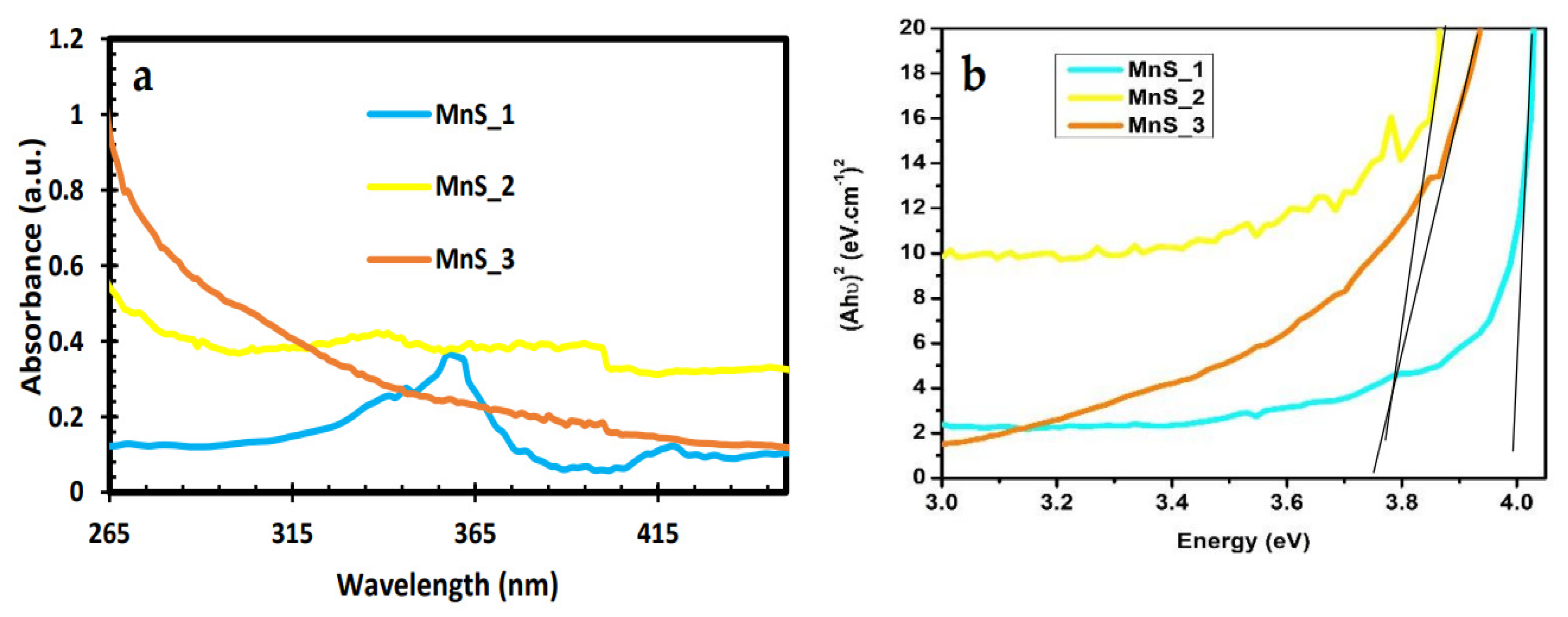
2.4. UV-Vis Spectroscopy
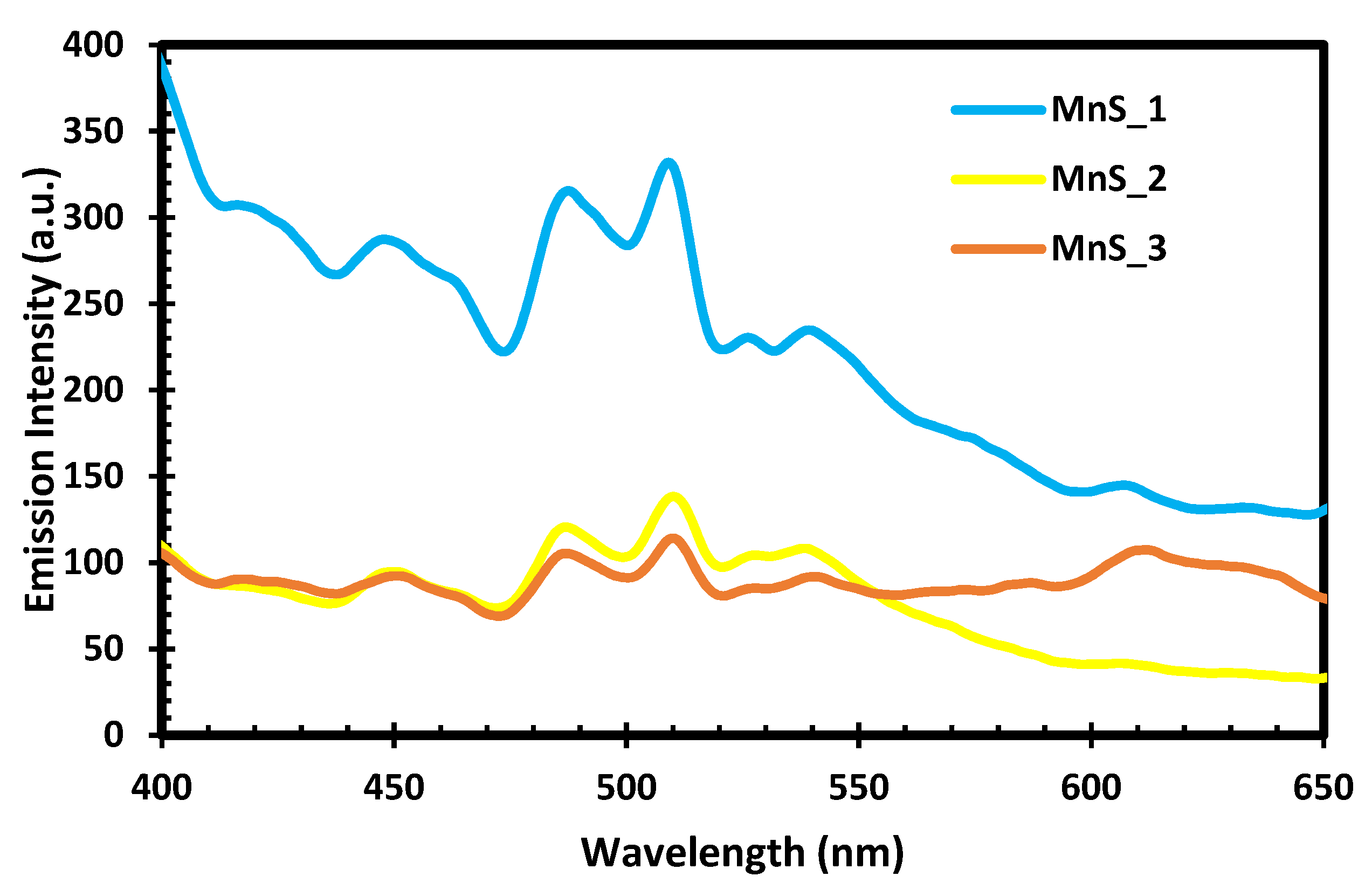
2.5. PL
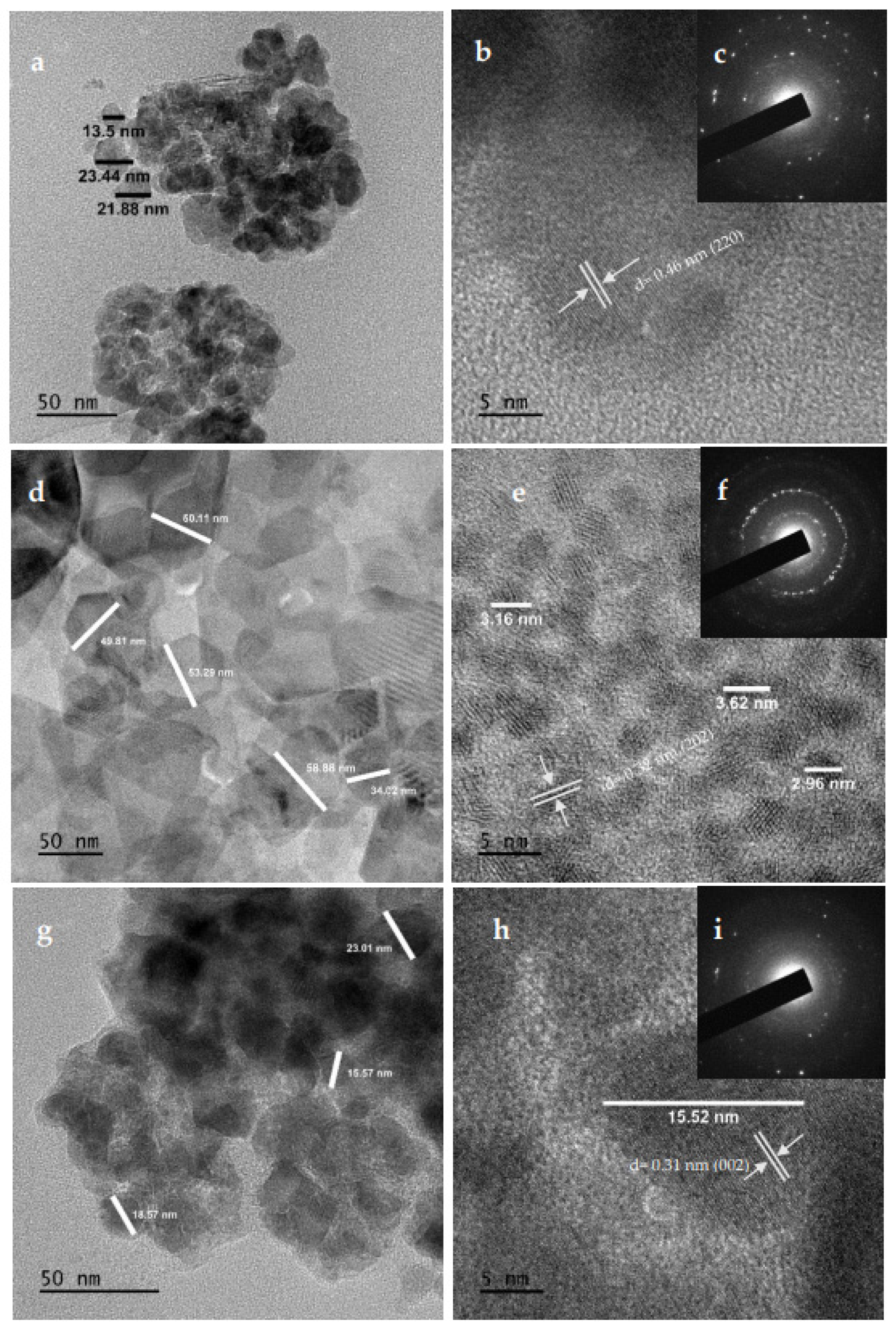
2.6. HRTEM and SAED
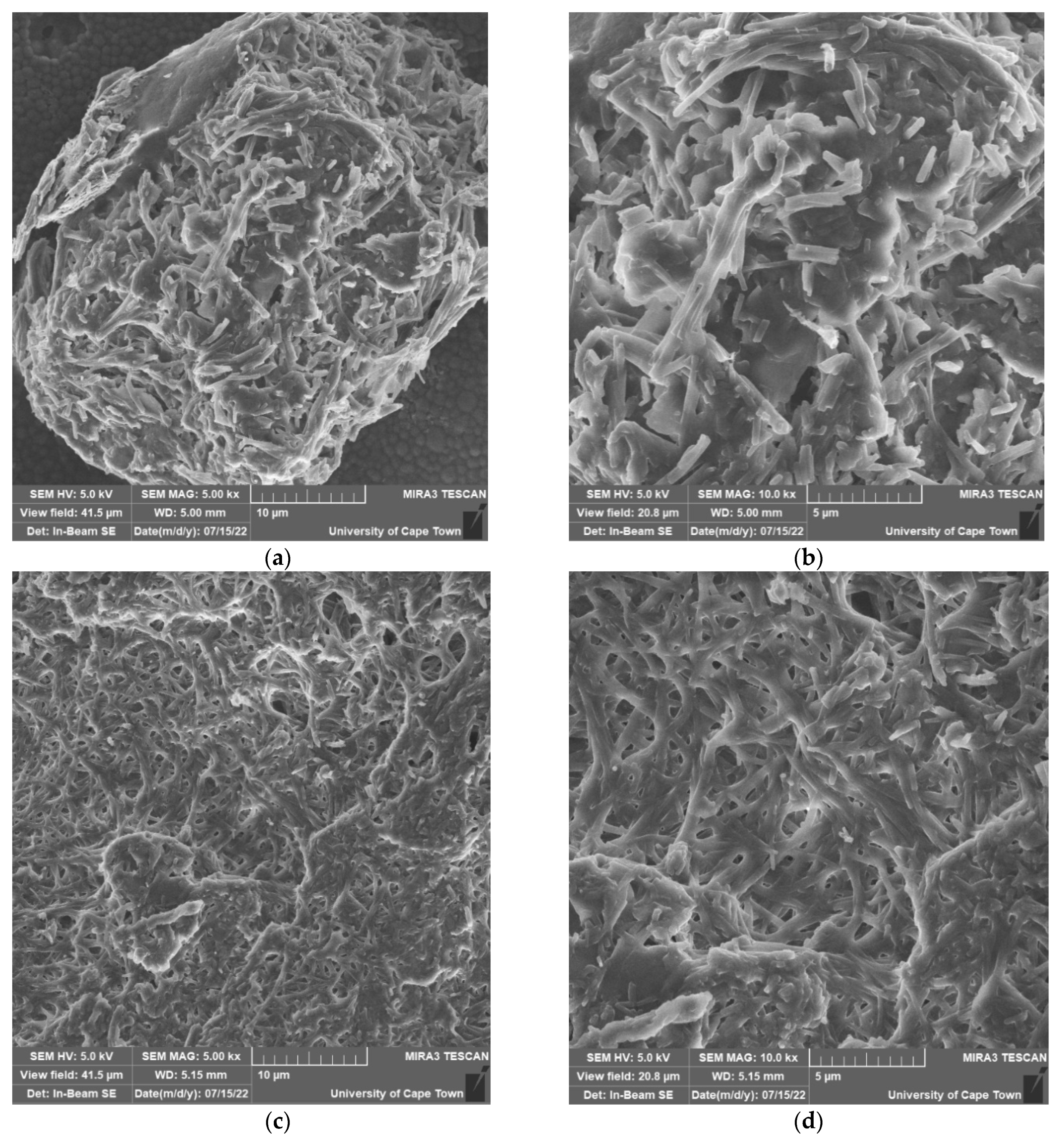
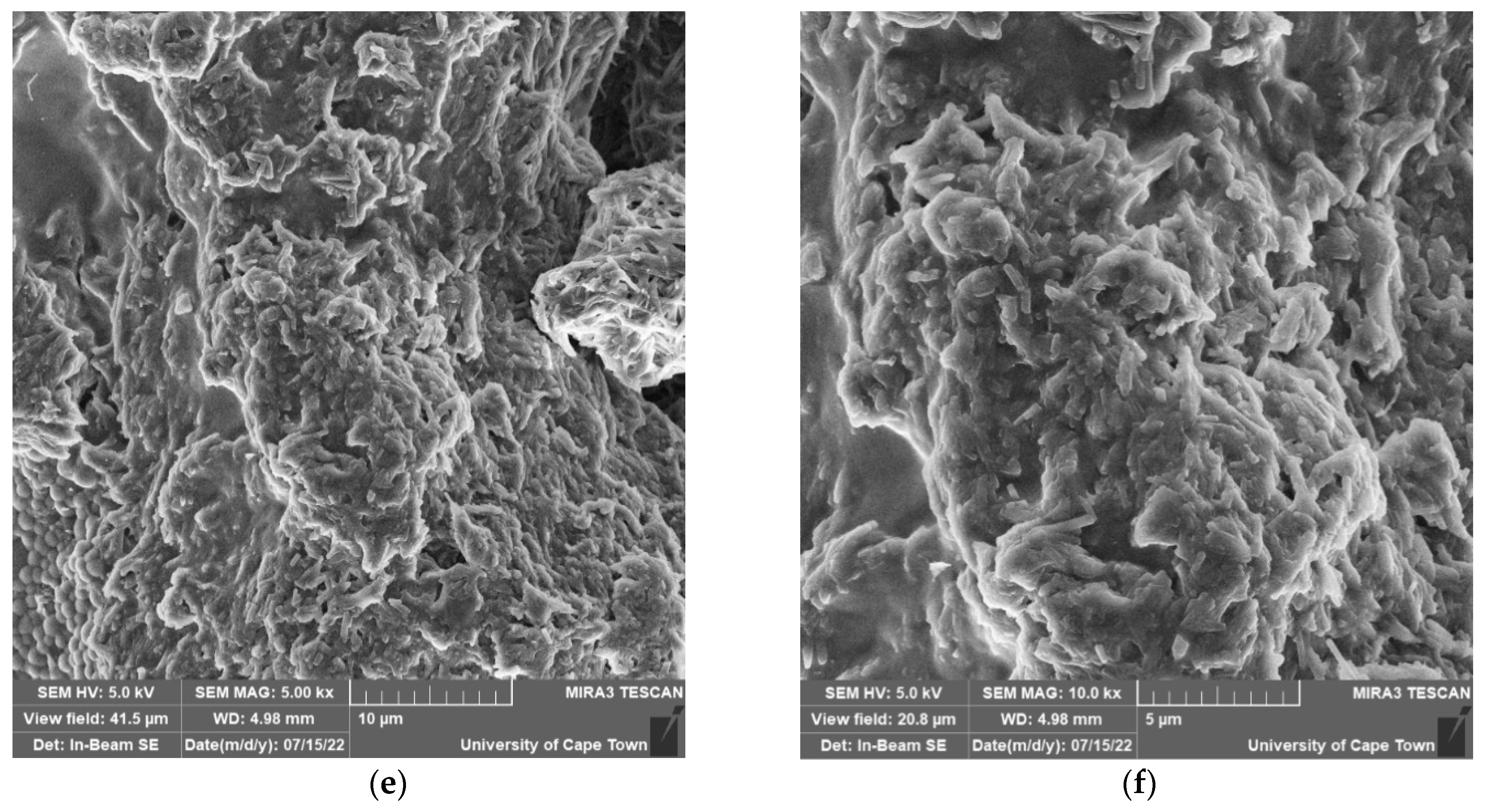
2.7. FESEM and EDS
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Dithiocarbamate Molecular Precursors
Synthesis of Ammonium N-Piperidinyldithiocarbamate
3.3. Synthesis of Bis(N-Piperl-N-p-Anisildithiocarbamato)Manganese(II) Complexes, Mn[N-Piper-N-p-Anisdtc] (PR1)
3.4. Synthesis of MnS Metals Sulfide Nanoparticles
3.5. Materials Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Agoro, M.A.; Meyer, E.L.; Mbese, J.Z.; Fuku, X.; Ahia, C.C. Aliphatic mixed ligands Sn(II) complexes as photon absorbers in quantum dots sensitized solar cell. J. Solid State Chem. 2022, 308, 122890. [Google Scholar] [CrossRef]
- Agoro, M.A.; Mbese, J.Z.; Meyer, E.L.; Onyenankeya, K. Electrochemical signature of CuS photosensitizers thermalized from alkyldithiocarbamato Cu(II) molecular precursors for quantum dots sensitized solar cells. Mater. Lett. 2021, 285, 129191. [Google Scholar] [CrossRef]
- Agoro, M.A.; Mbese, J.Z.; Meyer, E.L. Inorganic Pb(II)–P and Pb(II)–S Complexes as Photosensitizers from Primary and Secondary Amines in Dyes-Sensitized Solar Cells. ACS Omega 2021, 6, 23700–23709. [Google Scholar] [CrossRef] [PubMed]
- Punnoose, D.; Rao, S.S.; Kim, S.K.; Kim, H.J. Exploring the effect of manganese in lead sulfide quantum dot sensitized solar cell to enhance the photovoltaic performance. RSC Adv. 2015, 5, 33136–33145. [Google Scholar] [CrossRef]
- Kumar, S.; Riyajuddin, S.; Afshan, M.; Aziz, S.T.; Maruyama, T.; Ghosh, K. In-Situ Growth of Urchin Manganese Sulfide Anchored Three-Dimensional Graphene (γ-MnS@ 3DG) on Carbon Cloth as a Flexible Asymmetric Supercapacitor. J. Phys. Chem. Lett. 2021, 12, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, L.; Wu, H.B.; Xu, R.; Lou, X.W. Unusual Formation of Single-Crystal Manganese Sulfide Microboxes Co-mediated by the Cubic Crystal Structure and Shape. Angew. Chem. Int. Ed. 2012, 51, 7267–7270. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sankar, K.V.; Kundu, A.; Ma, M.; Kwon, J.Y.; Jun, S.C. Honeycomb-like interconnected network of nickel phosphide heteronanoparticles with superior electrochemical performance for supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 21829–21838. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, A.; Maghraoui-Meherzi, H. Growth of different phases and morphological features of MnS thin films by chemical bath deposition: Effect of deposition parameters and annealing. J. Solid State Chem. 2017, 247, 120–130. [Google Scholar] [CrossRef]
- Gui, Y.; Qian, L.; Qian, X. Hydrothermal synthesis of uniform rock salt (α-) MnS transformation from wurtzite (γ-) MnS. Mater. Chem. Phys. 2011, 125, 698–703. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Karuppasamy, K.; Durai, G.; Rana, A.U.H.S.; Arunachalam, P.; Sangeetha, K.; Kuppusami, P.; Kim, H.S. Recent advances in metal chalcogenides (MX; X = S, Se) nanostructures for electrochemical supercapacitor applications: A brief review. Nanomaterials 2018, 8, 256. [Google Scholar] [CrossRef]
- Barik, R.; Ingole, P.P. Challenges and prospects of metal sulfide materials for supercapacitors. Curr Opin Electrochem. 2020, 21, 327–334. [Google Scholar] [CrossRef]
- Liu, W.; Niu, H.; Yang, J.; Cheng, K.; Ye, K.; Zhu, K.; Wang, G.; Cao, D.; Yan, J. Ternary transition metal sulfides embedded in graphene nanosheets as both the anode and cathode for high-performance asymmetric supercapacitors. Chem. Mater. 2018, 30, 1055–1068. [Google Scholar] [CrossRef]
- Ulutas, C.; Guneri, E.; Kirmizigul, F.; Altindemir, G.; Gode, F.; Gumus, C. γ-MnS thin films prepared by chemical bath deposition: Effect of bath temperature on their physical properties. Mater. Chem. Phys. 2013, 138, 817–822. [Google Scholar] [CrossRef]
- Gümüş, C.; Ulutaş, C.; Ufuktepe, Y.Ü.K.S.E.L. Optical and structural properties of manganese sulfide thin films. Opt. Mater. 2007, 29, 1183–1187. [Google Scholar] [CrossRef]
- Yu, X.; Li-yun, C.; Jian-feng, H.; Jia, L.; Jie, F.; Chun-yan, Y. Influence of S/Mn molar ratio on the morphology and optical property of γ-MnS thin films prepared by microwave hydrothermal. J. Alloys Compd. 2013, 549, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Wang, B.; Yan, H.; Yoshimura, M. Low-temperature hydrothermal synthesis of pure metastable γ-manganese sulfide (MnS) crystallites. J. Cryst. Growth 2002, 243, 214–217. [Google Scholar] [CrossRef]
- Clifford, J.N.; Planells, M.; Palomares, E. Advances in high efficiency dye sensitized solar cells based on Ru(II) free sensitizers and a liquid redox electrolyte. J. Mater. Chem. 2012, 22, 24195–24201. [Google Scholar] [CrossRef]
- Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E. Light harvesting with Earth abundant d-block metals: Development of sensitizers in dye-sensitized solar cells (DSCs). Coord. Chem. Rev. 2013, 257, 3089–3106. [Google Scholar] [CrossRef]
- Agoro, M.A.; Meyer, E.L.; Mbese, J.Z.; Manu, K. Electrochemical fingerprint of CuS-hexagonal chemistry from (bis(N-1,4-Phenyl-N-(4-morpholinedithiocarbamato)copper(II) complexes) as photon absorber in quantum-dot/dye-sensitised solar cells. Catalysts 2020, 10, 300. [Google Scholar] [CrossRef]
- Agoro, M.A.; Mbese, J.Z.; Meyer, E.L. Electrochemistry of Inorganic OCT-PbS/HDA and OCT-PbS Photosensitizers Thermalized from bis(N-diisopropyl-N-octyldithiocarbamato) Pb(II) Molecular Precursors. Molecules 2020, 25, 1919. [Google Scholar] [CrossRef]
- Meyer, E.L.; Mbese, J.Z.; Agoro, M.A.; Taziwa, R. Optical and structural-chemistry of SnS nanocrystals prepared by thermal decomposition of bis(N-di-isopropyl-N-octyldithiocarbamato) tin(II) complex for promising materials in solar cell applications. Opt. Quantum Electron. 2020, 52, 90. [Google Scholar] [CrossRef]
- Ashbrook, L.N.; Elliott, C.M. Dye-sensitized solar cell studies of a donor-appended bis(2,9-dimethyl-1,10-phenanthroline) Cu(I) dye paired with a cobalt-based mediator. J. Phys. Chem. C 2013, 117, 3853–3864. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Yu, Z.T.; Zhang, J.Y.; Zou, Z.G. A copper(I) dye-sensitised TiO2-based system for efficient light harvesting and photoconversion of CO2 into hydrocarbon fuel. Dalton Trans. 2012, 41, 9594–9597. [Google Scholar] [CrossRef]
- Saha, R.; Sahana, A.; Lohar, S.; Banerjee, A.; Das, S.; Das, D. pH-controlled solid-phase enrichment of Mn(II): Confirmation of the structure of the extracted ternary Mn(II) complex by single crystal X-ray structure analysis. Desalin. Water Treat. 2014, 52, 6069–6078. [Google Scholar] [CrossRef]
- Arul, N.S.; Han, J.I.; Mangalaraj, D. Fabrication of highly flexible conducting electrode based on MnS nanoparticles/graphite/scotch tape for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2018, 29, 1636–1642. [Google Scholar] [CrossRef]
- Chaki, S.H.; Chauhan, S.M.; Tailor, J.P.; Deshpande, M.P. Synthesis of manganese sulfide (MnS) thin films by chemical bath deposition and their characterization. J. Mater. Res. Technol. 2017, 6, 123–128. [Google Scholar] [CrossRef]
- Ferretti, A.M.; Mondini, S.; Ponti, A. Manganese Sulfide (MnS) Nanocrystals: Synthesis, Properties, and Applications. In Advances in Colloid Science; IntechOpen: London, UK, 2017; pp. 121–123. [Google Scholar]
- Dey, S.; Kumar, V.P. The performance of highly active manganese oxide catalysts for ambient conditions carbon monoxide oxidation. Curr. Opin. Green Sustain. Chem. 2020, 3, 100012. [Google Scholar] [CrossRef]
- Kadhm, A.J.; Atwan, A.F.; Ismail, R.A. Effect of molar concentration on the structural, optical and electrical properties of the MnS thin film prepared by spray pyrolysis. J. Phys. Conf. Ser. 2021, 1795, 012032. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Ahmed, S.I.; El-Naggar, A.M.; Albassam, A.A. Effect of composition ratio on the structural and optical properties of MnS@ ZnS nanocomposites. J. Mater. Sci. Mater. Electron. 2020, 31, 14746–14755. [Google Scholar] [CrossRef]
- Elango, M.; Gopalakrishnan, K.; Vairam, S.; Thamilselvan, M. Structural, optical and magnetic studies on non-aqueous synthesized CdS: Mn nanomaterials. J. Alloys Compd. 2012, 538, 48–55. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, Y.; Lin, J.; Hou, Y.; Li, D.; Wu, T. Dual emissions from MnS clusters confined in the sodalite nanocage of a chalcogenide-based semiconductor zeolite. Dalton Trans. 2017, 46, 3929–3933. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.T.; Song, E.H.; Wu, M.; Zhou, B.; Zhang, Q.Y. Exchange coupled Mn-Mn pair: An approach for super-broadband 1380 nm emission in α-MnS. Appl. Phys. Lett. 2016, 109, 191907. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, R.; Zhang, C.; Li, L.; Mei, J.; Liu, S. Solvothermal synthesis of manganese sulfides and control of their phase and morphology. J. Mater. Res. 2018, 33, 4224–4232. [Google Scholar] [CrossRef]
- Kumbhar, V.S.; Lee, Y.R.; Ra, C.S.; Tuma, D.; Min, B.K.; Shim, J.J. Modified chemical synthesis of MnS nanoclusters on nickel foam for high performance all-solid-state asymmetric supercapacitors. RSC adv. 2017, 7, 16348–16359. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Xiong, J.; Gong, X.; Ciou, J.; Lee, P.S. Encapsulation of MnS nanocrystals into N, S-Co-doped carbon as anode material for full cell sodium-ion capacitors. Nanomicro Lett. 2020, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Priya, B.A.; Sivakumar, T.; Venkateswari, P. Controlled loading of MnS_2 on porous TiO2 nanosheets for enhanced photocatalytic hydrogen evolution. J. Mater. Sci. Mater. Electron. 2022, 33, 6646–6656. [Google Scholar] [CrossRef]
- Jeyamalar, K.; Selvarajan, P. Studies of magnesium doped MnS nanomaterial synthesized by microwave-assisted solution method. J. Adv. Sci. Res. 2021, 12, 185–192. [Google Scholar] [CrossRef]
- Balpinar, N.; Göde, F. Fabrication and characterization of dye-sensitized solar cells based on murexid dye and inorganic cds: Mn Thin Films. Chalcogenide Lett 2020, 17, 429–437. [Google Scholar]
- Akman, E. Enhanced photovoltaic performance and stability of dye-sensitized solar cells by utilizing manganese-doped ZnO photoanode with europium compact layer. J. Mol. Liq. 2020, 317, 114223. [Google Scholar] [CrossRef]
- Alavi, M.; Rahimi, R.; Maleki, Z.; Hosseini-Kharat, M. Improvement of power conversion efficiency of quantum dot-sensitized solar cells by doping of manganese into a ZnS passivation layer and cosensitization of zinc-porphyrin on a modified graphene oxide/nitrogen-doped TiO2 photoanode. ACS Omega 2020, 5, 11024–11034. [Google Scholar] [CrossRef]
- Fadaam, S.A.; Ali, H.M.; Shaban, A.H.; Ahmed, S.A. December. Improving efficiency of solar cell for MnS through annealing. AIP Conf. Proc. 2020, 2307, 020030. [Google Scholar]
- Cao, X.; Ding, R.; Zhang, Y.; Cui, Y.; Hong, K. A heterojunction film of NiS2 and MnS as an efficient counter electrode for dye-sensitized solar cells. Mater. Today Commun. 2021, 26, 102160. [Google Scholar] [CrossRef]
- Beltran-Huarac, J.; Palomino, J.; Resto, O.; Wang, J.; Jadwisienczak, W.M.; Weiner, B.R.; Morell, G. Highly-crystalline γ-MnS nanosaws. RSC Adv. 2014, 4, 38103–38110. [Google Scholar] [CrossRef]
- Zhang, N.; Yi, R.; Wang, Z.; Shi, R.; Wang, H.; Qiu, G.; Liu, X. Hydrothermal synthesis and electrochemical properties of alpha-manganese sulfide submicrocrystals as an attractive electrode material for lithium-ion batteries. Mater. Chem. Phys. 2008, 111, 13–16. [Google Scholar] [CrossRef]


| Appearance | Source | Phase | Time | T. °C | Size (nm) | Applications or Potential | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| Spherical | MnCl2·2H2O | MnS_2 | 30 min | 30–40 | Photocatalytic hydrogen | 2022 | [37] | |
| Spherical | MnCl2·2H2O | MnS | 30 min | 400 | 9.19–14.85 | Antibacterial activity | 2021 | [38] |
| Flower-like | Mn(CH3COO)24·H2O | γ-MnS Mn3O4 | 2 h | 220 | 400–600 | Photocatalytic activity | 2018 | [34] |
| Nanoparticles | Mn(CH3COO)2·4H2O | MnS | 3 h | 800 | Sodium-ion capacitors | 2020 | [36] | |
| Heterostructures | Mn(CH3COO)2·4H2O | γ-MnS α-MnS MnS | 2 h | 300 | 15–20 | Optoelectronics | 2020 | [29] |
| Nanoparticles | Mn(NO3)2·4H2O | γ-MnS | 24 h | 90 | 15 | Supercapacitors | 2018 | [25] |
| Nanopores | Mn(NO3)2·4H2O | MnS | 24 h | 25 | Optoelectronics | 2017 | [32] | |
| Spherical | C4H6MnO4·4H2O | γ-MnS | 2 h | 40 | 21–45 | Photoconductors | 2017 | [26] |
| Nanoparticles | Mn(NO3)2 | γ-MnS | 3 h | 85 | 20–30 | Supercapacitors | 2017 | [35] |
| Spherical | MnCl₂·4H₂O | γ-MnS | 2 h | 260 | 2–15 | QDSC | 2022 | PS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agoro, M.A.; Meyer, E.L. Proficient One-Step Heat-Up Synthesis of Manganese Sulfide Quantum Dots for Solar Cell Applications. Molecules 2022, 27, 6678. https://doi.org/10.3390/molecules27196678
Agoro MA, Meyer EL. Proficient One-Step Heat-Up Synthesis of Manganese Sulfide Quantum Dots for Solar Cell Applications. Molecules. 2022; 27(19):6678. https://doi.org/10.3390/molecules27196678
Chicago/Turabian StyleAgoro, Mojeed A., and Edson L. Meyer. 2022. "Proficient One-Step Heat-Up Synthesis of Manganese Sulfide Quantum Dots for Solar Cell Applications" Molecules 27, no. 19: 6678. https://doi.org/10.3390/molecules27196678
APA StyleAgoro, M. A., & Meyer, E. L. (2022). Proficient One-Step Heat-Up Synthesis of Manganese Sulfide Quantum Dots for Solar Cell Applications. Molecules, 27(19), 6678. https://doi.org/10.3390/molecules27196678







