A Review of the Phytochemistry and Pharmacology of the Fruit of Siraitia grosvenorii (Swingle): A Traditional Chinese Medicinal Food
Abstract
1. Introduction
2. Traditional Uses
3. Chemical Composition
3.1. Triterpenoids and Glycosides
3.2. Flavonoids
3.3. Miscellaneous Componds
3.4. Polysaccharides
3.5. Amino Acids & Proteins
4. Phytochemical Analysis
5. Pharmacological Activities
5.1. Antitussive, Expectorant and Anti-Asthmatic Activities
5.2. Antioxidant
5.3. Antidiabetic and Antihyperlipidemic Activies
5.4. Anti-Inflammatory
5.5. Liver Protection
5.6. Antibacterial and Antiviral Activities
5.7. Miscellaneous Activities
5.8. Summary of Pharmacologic Activities
6. Toxicological Profile: Safety and Adverse Activities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, A.M.; Zhang, Z.Y. The genus siraitia merr. in China. Guihaia 1984, 4, 27–33. [Google Scholar]
- Mahmoud, A.H.; Ehab, R.; Huang, Z.Z.; Toshiki, E.; Huang, L.; Li, L. Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chem. 2020, 303, 125400. [Google Scholar]
- Zhang, Y.L.; Zhou, G.S.; Peng, Y.; Wang, M.Y.; Li, X.B. Anti-hyperglycemic and anti-hyperlipidemic effects of a special fraction of luohanguo extract on obese t2dm rats. J. Ethnopharmacol. 2020, 247, 112273. [Google Scholar] [CrossRef]
- Nie, J.Y.; Yan, K.; Sui, L.M.; Zhang, H.T.; Liang, X.W. Mogroside v improves porcine oocyte in vitro maturation and subsequent embryonic development. Theriogenology 2020, 141, 35–40. [Google Scholar] [CrossRef]
- Chen, G.L.; Liu, C.H.; Meng, G.L.; Zhang, C.T.; Chen, F.; Tang, S.S.; Hong, H.; Zhang, C.F. Neuroprotective effect of mogrol against aβ1-42-induced memory impairment neuroinflammation and apoptosis in mice. J. Pharm. Pharmacol. 2019, 71, 869–877. [Google Scholar] [CrossRef]
- Liu, H.S.; Wang, C.C.; Qi, X.Y.; Zou, J.; Sun, Z.D. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (swingle) fruits. J. Food Sci. Technol. 2018, 55, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Li, D.P.; Zhang, H.R. Studies and uses of Chinese medicine luohanguo-A special local product of guangxi. Guihaia 2000, 20, 270–276. [Google Scholar]
- Zhang, H.; Li, X.H. Research progress on chemical compositions of fructus momordicae. Anhui Agri. Sci. 2011, 39, 4555–4556, 4559. [Google Scholar]
- S, D.D.; A, E.M.; K, A.D. Highly sweet compounds of plant origin: From ethnobotanical observations to wide utilization. J. Ethnopharmacol. 2019, 243, 112056. [Google Scholar]
- China Pharmacopoeia Commission. Chinese Pharmacopeia; China Medical Science Press: Beijing, China, 2020.
- Chu, D.H.; Yaseen, A.; Wang, L.; Chen, B.; Wang, M.K.; Hu, W.C. Two new cucurbitane glycosides from the fruits of siraitia grosvenori swingle. Chem. Pharm. Bull. 2019, 67, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.X.; Zhao, H.; Tang, Q.; Mo, C.M.; Huang, P.; Cheng, P.; Yang, P.; Yang, X.Y.; Liu, X.B.; Zheng, Y.J. Systematic identification of flavonols, flavonol glycosides, triterpene and siraitic acid glycosides from Siraitia grosvenorii using high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening strategy. J. Pharm. Biomed. Anal. 2017, 138, 240–248. [Google Scholar] [CrossRef]
- Zheng, Y.B.; Yu, J.; Liu, J.M. Determination of Total Saponin Content in the Fresh Fruit of Siraitia grosvenorii, 2007. In Proceedings of the Annual Meeting Proceedings of Sweetener Professional Board, Production and Application of Food Additives Industry Associate, London, UK, 2007. [Google Scholar]
- Li, H.B.; Zhang, M.; Wang, Y. Colorimetric determination of triterpenoid saponin in luohanguo. Food Sci. 2006, 27, 171–173. [Google Scholar]
- Lee, C.H. Intense sweetener from lo han kuo (momordica grosvenori). Experientia 1975, 31, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, T.; Arihara, S.; Nakajima, T.; Okuhira, M. Studies on the constituents of fructus momordicae i, ii, iii. Shoyakugaku Zasshi 1983, 103, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Li, D.P.; Ikeda, T.; Matsuoka, N.; Nohara, T.; Zhang, H.; Sakamoto, T. Cucurbitane glycosides from unripe fruits of lo han kuo (Siraitia grosvenorii). Chem. Pharm. Bull. 2006, 38, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- Kasai, R.; Matsumoto, K.; Nie, R.; Zhou, J.; Tanka, O. Glycosides from chinese medicinal plant, hemsleya panacis-scandens, and structure-taste relationship to cucurbitane glycosides. Chem. Pharm. Bull. 1988, 36, 234–243. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kasai, R.; Ohtani, K.; Tanaka, O. Minor cucurbitane-glycosides from fruits of siraitia grosvenori (cucurbitaceae). Chem. Pharm. Bull. 2008, 38, 2030–2032. [Google Scholar] [CrossRef]
- Kasai, R. Studies on the constituents of cucurbitaceous plants. Shoyakugaku Zasshi 2008, 128, 1369–1382. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Li, H.X.; Huang, Q.P.; Zeng, D.Y.; Lu, Y.J. Determination of mogroside v in electrolyte formula foods for special medical purpose by hplc. Food Drug 2021, 23, 258–261. [Google Scholar]
- Li, D.P.; Chen, Y.Y.; Pan, Z.H. Study on variation of mogrol glycosides from fruits of Siraitia grosvenorii in different growing ages. Guihaia 2004, 24, 546–549. [Google Scholar]
- Chen, Q.B.; Yi, X.H.; Yu, L.J. Study on the variation of mogroside v and flavones glycosides in Siraitia grosvenorii fresh fruits in different growth periods. Guihaia 2005, 25, 274–277. [Google Scholar]
- Xiang, Q.; Lei, X.; Huang, L.Z. Study of metabolic conversion of mogrol glycosides in fruit of Siraitia grosvenorii. Biotechnol 2009, 19, 49–51. [Google Scholar]
- Chen, X.B.; Zhuang, J.J.; Liu, J.H.; Lei, M.; Ma, L.; Chen, J.; Shen, X.; Hu, L.H. Potential ampk activators of cucurbitane triterpenoids from Siraitia grosvenorii swingle. Bioorg. Med. Chem. 2011, 19, 5776–5781. [Google Scholar] [CrossRef] [PubMed]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Toriumi, M.; Mukainaka, T.; Banno, N. Inhibitory effects of cucurbitane glycosides and other triterpenoids from the fruits of momordica grosvenori on epstein-barr virus early antigen induced by tumor promoter 12-O-tetradecanoylphorbol-13-acetate. J. Agric. Food Chem. 2002, 50, 6710. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.Q.; Li, J.; Huang, X.S.; Huang, Y.; He, X.C.; Su, X.J. Chemical constituents of Siraitia grosvenorii (swingle) c. Jeffrey. Acta Bot. Boreali-Occident. Sin. 2008, 28, 1250–1254. [Google Scholar]
- Wang, Y.P.; Chen, J.Y. Chemical constituents of grosvenor momordica (momordica grosvenori). Chin. Tradit. Herb. Drugs 1992, 23, 61–62. [Google Scholar]
- Si, J.Y.; Chen, D.H.; Tu, G.Z. Siraitic acid F, a new nor-cucurbitacin with novel skeleton, from the roots of Siraitia grosvenorii. J. Asian Nat. Prod. Res. 2005, 7, 37–41. [Google Scholar] [CrossRef]
- Si, J.Y.; Chen, D.H.; Shen, L.G.; Tu, G.Z. Studies on chemical constituents from root of Siraitia grosvenorii. Acta Pharm. Sin. B 1999, 34, 918–920. [Google Scholar]
- Wang, X.F.; Lu, W.J.; Chen, J.Y.; Li, Y.H.; Gong, M.Y.; Lv, Y.; Wu, N.; Zheng, Q.T. Studies on the chemical constituents of root of luohanguo (siraitia grosvenori). Chin. Tradit. Herb. Drugs 1998, 293–296. [Google Scholar]
- Li, D.P.; Elaasr, M.; Ikeda, T.; Ogata, M.; Miyashita, H.; Yoshimitsu, H.; Nohara, T. Two new cucurbitane-type glycosides obtained from roots of Siraitia grosvenorii swingle. Chem. Pharm. Bull. 2009, 57, 870–872. [Google Scholar] [CrossRef]
- Huo, X.S.; Wang, J.C.; Si, J.Y. Research progress on nor-cucurbitacin triterpenes. Chin. Tradit. Herb. Drugs 2022, 53, 1558–1569. [Google Scholar]
- Wang, H.; Meng, G.L.; Zhang, C.T.; Wang, H.; Hu, M.; Long, Y.; Hong, H.; Tang, S.S. Mogrol attenuates lipopolysaccharide (lps)-induced memory impairment and neuroinflammatory responses in mice. J. Asian Nat. Prod. Res. 2020, 22, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Hayakawa, Y.; Tokuda, H.; Banno, N.; Shimizu, N.; Suzuki, T.; Kimura, Y. Cucurbitane glycosides from the fruits of Siraitia grosvenorii and their inhibitory effects on epstein-barr virus activation. J. Nat. Prod. 2007, 70, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, F.M.; Liu, X.; Wang, L.; Chen, B.; Li, L.H.; Wang, M.K. Cucurbitane glycosides from the fruit of siraitia grosvenori and their effects on glucose uptake in human HepG2 cells in vitro. Food Chem. 2017, 228, 567–573. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Yuji, M.; Hiroshi, I.; Masaki, S.; Yoshihisa, N. Triterpene glycosides of siraitia grosvenori inhibit rat intestinal maltase and suppress the rise in blood glucose level after a single oral administration of maltose in rats. J. Agric. Food Chem. 2005, 53, 2941–2946. [Google Scholar] [CrossRef]
- Cai, X.; He, L.M.; Zhou, G.A.; Li, S.H.; Liao, X.H. Mogroside IIE ameliorates cardiomyopathy by suppressing cardiomyocyte apoptosis in a type 2 diabetic model. Front. Pharmacol. 2021, 12, 650193. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.M.; Lu, F.L.; Liu, J.L.; Song, Y.F.; Li, D.P. Cucurbitane glycosides derived from mogroside iie: Structure-taste relationships, antioxidant activity, and acute toxicity. Molecules 2014, 19, 12676–12689. [Google Scholar] [CrossRef]
- Zou, C.L.; Zhang, Q.Q.; Zhang, S.H. Mogroside IIIE attenuates gestational diabetes mellitus through activating of ampk signaling pathway in mice. J. Pharmacol. Sci 2018, 138, 161–166. [Google Scholar] [CrossRef]
- Tao, L.J.; Yang, J.Y.; Cao, F.Y.; Xie, H.F.; Zhang, M.; Gong, Y.Q.; Zhang, C.F. Mogroside IIIE, a novel anti-fibrotic compound, reduces pulmonary fibrosis through toll-like receptor 4 pathways. J. Pharmacol. Exp. Ther. 2017, 361, 268–279. [Google Scholar] [CrossRef]
- Tao, L.J.; Cao, F.Y.; Xu, G.H.; Xie, H.F.; Zhan, M.; Zhang, C.F. Mogroside IIIE attenuates LPS-induced acute lung injury in mice partly through regulation of the tlr4/MAPK/NF-κB axis via ampk activation. Phytother. Res. 2017, 31, 1097–1106. [Google Scholar] [CrossRef]
- Cao, F.Y.; Zhang, Y.F.; Li, W.G.; Shimizu, K.; Xie, H.F.; Zhang, C.F. Mogroside IVE attenuates experimental liver fibrosis in mice and inhibits HSC activation through downregulating tlr4-mediated pathways. Int. Immunopharmacol. 2018, 55, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dai, L.H.; Liu, Y.; Rong, L.; Dou, D.; Sun, Y.; Ma, L. Antiproliferative activity of triterpene glycoside nutrient from monk fruit in colorectal cancer and throat cancer. Nutrients 2016, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Yang, X. A minor, sweet cucurbitane glycoside from Siraitia grosvenorii. Nat. Prod. Commun. 2009, 4, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.F.; Lan, X.Q.; Wen, Q.W.; Liu, T.; Lu, D.; Wei, H.Y.; Al, E. Determination of mogroside V in fufang luohanguo zhike granule by HPLC-ELSD. Chin. J. Mod. Appl. Pharm. 2014, 31, 850–852. [Google Scholar]
- Mo, Q.T.; Fu, H.; Zhao, D.; Zhang, J.C.; Wang, C.; Wang, D.; Li, M. Protective effects of mogroside v on oxidative stress induced by H2O2 in skin fibroblasts. Drug Des., Dev. Ther. 2021, 15, 4901–4909. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wang, J.; Guan, X.; Yu, D.; Huangfu, M.J.; Dou, T.; Zhou, L.; Wang, L.; Liu, G.; Li, X.; et al. Mogroside V reduce ova-induced pulmonary inflammation based on lung and serum metabolomics. Phytomedicine 2021, 91, 153682. [Google Scholar] [CrossRef]
- Chen, B.; Chen, W.; Zhang, Y.M.; Wu, W. Studies on the technics of preparing effervescent tablets of Siraitia grosvenorii. Acta Med. Sin. 2015, 28, 101–103. [Google Scholar]
- Chaturvedula, V.S.; Meneni, S.R. A new cucurbitane glycoside from Siraitia grosvenorii. Nat. Prod. Commun. 2015, 10, 1521–1523. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Long, C.X.; Luo, L.; Chen, Y.Y.; Liu, P.P.; Xiao, Z.H. Effect of mogroside VI on acute liver injury induced by sepsis in mice and related mechanisms. Chin. J. Contemp. Pediatr. 2020, 22, 1233–1239. [Google Scholar]
- Niu, B.; Ke, C.Q.; Li, B.H.; Li, Y.Y.; Yi, Y.J. Cucurbitane glucosides from the crude extract of Siraitia grosvenorii with moderate effects on pgc-1α promoter activity. J. Nat. Prod. 2017, 80, 1428–1435. [Google Scholar] [CrossRef]
- Jiang, S.C.; He, L.M.; Wu, Y.; Luo, C.R. An additive for smoked Siraitia grosvenorii, their preparation and application. CN Patent 101633866, 2 November 2011. [Google Scholar]
- Yang, X.W.; Zhang, J.Y.; Qian, Z.M. Grosmomoside I, a new cucurbitane triterpenoid glycoside from fruits of momordica grosvenori. Chin. Tradit. Herb. Drugs 2005, 9–14. [Google Scholar]
- Chaturvedula, V.S.; Prakash, I. Cucurbitane glycosides from Siraitia grosvenorii. J. Carbohydr. Chem. 2011, 30, 16–26. [Google Scholar] [CrossRef]
- He, W.P.; Liu, L.J.; He, C.W.; Li, A.Y.; Li, X.M. Study on low glycemic index (ci) food and pharmacological effects of S. grosvenorii. J. Light. Ind. 2017, 27, 1. [Google Scholar]
- Ju, P.; Ding, W.; Chen, J.; Cheng, Y.; Yang, B.; Huang, L.; Zhou, Q.; Zhu, C.; Li, X.; Wang, M.; et al. The protective effects of mogroside V and its metabolite 11-oxo-mogrol of intestinal microbiota against mk801-induced neuronal damages. Psychopharmacology (Berl) 2020, 237, 1011–1026. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Huang, J.; Wu, Z.H.; Pi, F.J. Research overview of pharmacology and application development of Siraitia grosvenorii. J. Pharm. Res. 2017, 26, 164–165. [Google Scholar]
- Li, D.P.; Ikeda, T.; Nohara, T.; Liu, J.L.; Wen, Y.X.; Sakamoto, T.; Nonaka, G.I. Cucurbitane glycosides from unripe fruits of Siraitia grosvenori. Chem. Pharm. Bull. 2007, 55, 1082–1086. [Google Scholar] [CrossRef]
- Chen, W.J.; Wang, J.; Qi, X.Y.; Xie, B.J. The antioxidant activities of natural sweeteners, mogrosides, from fruits of Siraitia grosvenorii. Int. J. Food Sci. Nutr. 2007, 58, 548–556. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Murata, Y.; Sugiura, M.; Nishino, H.; Tokuda, H.; Matsumoto, K.; Kasai, R.; Yamasaki, K. Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides, from momordica grosvenori. Cancer Lett. 2003, 198, 37–42. [Google Scholar] [CrossRef]
- Prakash, I.; Chaturvedula, V.S. Additional new minor cucurbitane glycosides from Siraitia grosvenorii. Molecules 2014, 19, 3669–3680. [Google Scholar] [CrossRef]
- Si, J.Y.; Chen, D.H.; Chang, Q.; Shen, L.G. Isolation and structure determination of flavonol glycosides from the fresh fruits of siraitia grosvenori. Acta Pharm. Sin. B 1994, 29, 158–160. [Google Scholar]
- Li, C.; Lin, L.M.; Luo, M.; Ma, C.F.; Wang, Z.M. A new natural saponin from fruits of Siraitia grosvenorii. China J. Chin. Mater Med. 2011, 6, 721. [Google Scholar]
- Chen, Q.B.; Yang, J.X.; Chen, Z.Q.; Yi, X.H.; Wei, Z.B. Separation, purification and identification of flavonol glycoside from momordica grosvenori leafs. Guangxi Sci. 2006, 35–36. [Google Scholar]
- Li, J.; Huang, X.S.; Zhang, Y.J.; He, X.C.; Su, X.J. Chemical constituents of Siraitia grosvenorii (swingle) c. Jeffrey. China J. Chin. Mater Med. 2007, 32, 548–549. [Google Scholar]
- Li, S.; Wang, H.S.; Zhang, G.Y. The analysis of seed oil from Siraitia grosvenorii. Guangxi Med. J. 2003, 25, 850–852. [Google Scholar]
- Wang, M.Y.; Xing, S.H.; Luu, T.; Fan, M.; Li, X.B. The gastrointestinal tract metabolism and pharmacological activities of grosvenorine, a major and characteristic flavonoid in the fruits of Siraitia grosvenorii. Chem. Biodiversity 2016, 12, 1652–1664. [Google Scholar] [CrossRef]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.L.; Li, D.P. Antioxidant activity of flavonol glycosides of Siraitia grosvenorii flower. Mod. Food Sci. Technol. 2009, 25, 484–486. [Google Scholar]
- Yang, X.W.; Zhang, J.Y.; Qian, Z.M. New natural saponins form fruits of momordica grosvenorii. Chin. Tradit. Herb. Drugs 2008, 39, 810–813. [Google Scholar]
- Patel, D.K. Pharmacological activities and therapeutic potential of kaempferitrin in medicine for the treatment of human disorders: A review of medicinal importance and health benefits. Cardiovasc. Hematol. Disord. Drug Targets 2021, 21, 104–114. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Czarnomysy, R.; Buzun, K.; Bielawska, A. Anti-cancer potential of afzelin towards ags gastric cancer cells. Pharmaceuticals (Basel) 2021, 14, 973. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.; Kim, J.M.; Lee, M.K.; Seo, S.J.; Park, K.Y. Afzelin suppresses proinflammatory responses in particulate matter-exposed human keratinocytes. Int. J. Mol. Med. 2019, 43, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wen, H.; Yoo, J.G.; Ebersole, J.L.; Huang, C.B. Antibacterial compounds from Siraitia grosvenorii leaves. Nat. Prod. Res 2011, 25, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Huang, Q.Y.; Zheng, G.J.; Zeng, Z.F.; Xu, D.N.; Nong, K.L. Effects of Siraitia grosvenorii polysaccharides on immune function in immunosuppressed mice induced by cyclophosphamide. Guihaia 2019, 39, 1573–1582. [Google Scholar]
- Gong, P.; Cui, D.D.; Guo, Y.X.; Wang, M.R.; Wang, Z.N.; Huang, Z.; Yang, W.; Chen, F.; Chen, X.F. A novel polysaccharide obtained from Siraitia grosvenorii alleviates inflammatory responses in a diabetic nephropathy mouse model via the tlr4-nf-κb pathway. Food Funct. 2021, 12, 9054–9065. [Google Scholar] [CrossRef]
- Xu, W.K.; Meng, L.S. Determination of protein content of Siraitia grosvenorii. Guihaia 1986, 295–296. [Google Scholar]
- Zhou, G.S.; Wang, M.Y.; Li, Y.; Peng, Y.; Li, X.B. Rapid and sensitive analysis of 27 underivatized free amino acids, dipeptides, and tripeptides in fruits of Siraitia grosvenorii swingle using HILIC-UHPLC-QTRAP®/MS2 combined with chemometrics methods. Amino Acids 2015, 47, 1589–1603. [Google Scholar] [CrossRef]
- Luo, Z.L.; Shi, H.W.; Zhang, K.L.; Qin, X.J.; Guo, Y.H.; Ma, X.J. Liquid chromatography with tandem mass spectrometry method for the simultaneous determination of multiple sweet mogrosides in the fruits of Siraitia grosvenorii and its marketed sweeteners. J. Sep. Sci. 2016, 39, 4124–4135. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.L.; Li, D.P.; Fu, C.M.; Liu, J.L.; Huang, Y.L. Studies on chemical fingerprints of Siraitia grosvenorii fruits (luo han guo) by HPLC. J. Nat. Med. 2012, 66, 70–76. [Google Scholar] [CrossRef]
- Huang, H.H.; Wang, M.J.; Zhang, M.; Lv, S.S. Content determination of grosvenorine and kaempferol-3,7-O-α-L-dirhamnopyranoside in different parts of siraitiae fructus by UPLC. J. Hubei Univ. Chin. Med. 2020, 022, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Yang, R.J.; Deng, Y.Y.; He, X.Y.; Ye, X.C.; Liu, Y.W. Determination of total flavonoids in siraitia grosvenori swingle fruit extract and vine leaf extract. China Pharm. 2012, 15, 7–9. [Google Scholar]
- Zhou, X.X.; Song, J.S. Study on the pharmacological function of fruit and extracts from momordica grosvenorii. Chin. Ach. Tradit. Chin. Med. 2004, 22, 1723–1724. [Google Scholar]
- Sung, Y.Y.; Kim, S.H.; Yuk, H.J.; Yang, W.K.; Lee, Y.M.; Son, E.J.; Kim, D.S. Siraitia grosvenorii residual extract attenuates ovalbumin-induced lung inflammation by down-regulating IL-4, IL-5, IL-13, IL-17, and MUC5AC expression in mice. Phytomedicine 2019, 61, 152835. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, Z.H.; Jiang, Y.M.; Zhou, S.; Su, L.; Jiang, S.Y.; Liu, G.Q. Studies on the pharmaeological profile of mogroside. Chin. Tradit. Herb. Drugs 1999, 30, 914–916. [Google Scholar]
- Zhu, Y.M.; Pan, L.C.; Zhang, L.J.; Yin, Y.; Zhu, Z.Y.; Sun, H.Q.; Liu, C.Y. Chemical structure and antioxidant activity of a polysaccharide from Siraitia grosvenorii. Int. J. Biol. Macromol. 2020, 165, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Mao, J.H.; Chen, Q.J.; Ling, W.D.; Sun, Y.Q. Mogroside IIIE alleviates high glucose-induced inflammation, oxidative stress and apoptosis of podocytes by the activation of AMPK/sirt1 signaling pathway. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3821–3830. [Google Scholar] [CrossRef]
- Yao, J.W.; Yang, Y.L.; Tang, H.; Zhou, L.; Ding, X.S. Impact of momordica extracts on spon perform ance and free radical metabolism in heart muscle of mice in trainin. J. Beijing Sport Univ. 2009, 67–69. [Google Scholar]
- Boy, H.; Rutilla, A.; Santos, K.A.; Ty, A.; Yu, A.I.; Mahboob, T.; Tangpoong, J.; Nissapatorn, V. Recommended medicinal plants as source of natural products: A review. Digital Chin. Med. 2018, 1, 131–142. [Google Scholar] [CrossRef]
- Liu, H.S.; Qi, X.Y.; Yu, K.K.; Lu, A.J.; Lin, K.F.; Zhu, J.J.; Zhang, M.; Sun, Z.D. AMPK activation is involved in hypoglycemic and hypolipidemic activities of mogroside-rich extract from Siraitia grosvenorii (swingle) fruits on high-fat diet/streptozotocin-induced diabetic mice. Food Funct. 2019, 10, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Yuk, H.J.; Yang, W.K.; Kim, S.H.; Kim, D.S. Siraitia grosvenorii residual extract attenuates atopic dermatitis by regulating immune dysfunction and skin barrier abnormality. Nutrients 2020, 12, 3638. [Google Scholar] [CrossRef]
- Wang, Q.; Y, L.A.; Li, X.P. Pharmacological effects of S. grosvenorii fruit. China J. Chin. Mater Med. 1999, 24, 425–428. [Google Scholar]
- Han, B.Y.; Michael, R.; Epperly, W.; Cao, S.N.; Goff, J. Improved longevity of hematopoiesis in long-term bone marrow cultures and reduced irradiation-induced pulmonary fibrosis in toll-like receptor-4 deletion recombinant-negative mice. Vivo 2015, 28, 441–448. [Google Scholar]
- Bhattacharyya, S.; Kelley, K.; Melichian, D.S.; Tamaki, Z.; Fang, F.; Su, Y.Y.; Feng, G.; Pope, R.M.; Budinger, G.R.S.; Mutlu, G.M. Toll-like receptor 4 signaling augments transforming growth factor-β responses: A novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am. J. Pathol. 2013, 182, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Bueno, G.B.; Caso, J.R.; Madrigal, J.; Leza, J.C. Innate immune receptor toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci. Biobehav. Rev. 2016, 134–147. [Google Scholar] [CrossRef]
- Song, J.L.; Qian, B.; Pan, C.L.; Lv, F.F.; Wang, H.P.; Gao, Y.; Zhou, Y.Y. Protective activity of mogroside v against ovalbumin-induced experimental allergic asthma in kunming mice. J. Food Biochem. 2019, 43, e12973. [Google Scholar] [CrossRef]
- Carty, M.; Bowie, A.G. Evaluating the role of toll-like receptors in diseases of the central nervous system. Biochem. Pharmacol. 2011, 81, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Zhang, B.X.; Liu, J.H.; Qiao, C.Y.; Xue, N.Y.; Lv, H.M.; Li, S.Z. Mogroside V alleviates lipopolysaccharide-induced neuroinflammation via inhibition of tlr4-Myd88 and activation of akt/ampk-nrf2 signaling pathway. J. Evid.-Based Complementary Altern. Med. 2021, 2021, 5521519. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, K.; Lin, H.M.; Xia, Z.J.; Zhang, J.; Li, D.; Jin, J.F. Mogroside IIE inhibits digestive enzymes via suppression of interleukin 9/interleukin 9 receptor signalling in acute pancreatitis. Front. Pharmacol. 2020, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Song, Y.F.; Ding, Y.P.; Wang, W.; Liao, L.; Zhong, J.; Sun, P.B.; Lei, F.; Zhang, Y.O.; Xie, W.D. Effects of mogrosides on high-fat-diet-induced obesity and nonalcoholic fatty liver disease in mice. Molecules 2018, 23, 1894. [Google Scholar] [CrossRef]
- Li, L.H.; Zheng, W.F.; Wang, C.; Qi, J.M.; Li, H.B. Mogroside V protects against hepatic steatosis in mice on a high-fat diet and lo2 cells treated with free fatty acids via AMPK activation. Evid. Based Complementary Altern. Med. 2020, 2020, 7826874. [Google Scholar] [CrossRef]
- Aguero, M.B.; Gonzalez, M.; Lima, B.; Svetaz, L.; Sanchez, M.; Zacchino, S.; Feresin, G.E.; Schmeda-Hirschmann, G.; Palermo, J.; Wunderlin, D. Argentinean propolis from zuccagnia punctata cav. (Caesalpinieae) exudates: Phytochemical characterization and antifungal activity. J. Agric. Food Chem. 2010, 58, 194–201. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, T.; Li, H.Y.; Xu, D.D. Selecting active constituents of siraitiae fructus on inhibition of escherichia coli bacterial biofilms. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 51–54. [Google Scholar]
- Aslan, A.; Aslan, C.; Zolbanin, N.M.; Jafari, R. Acute respiratory distress syndrome in COVID-19: Possible mechanisms and therapeutic management. Pneumonia (Nathan) 2021, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Yarza, R.; Bover, M.; Paredes, D.; López-López, F.; Jara-Casas, D.; Castelo-Loureiro, A.; Baena, J.; Mazarico, J.M.; Folgueira, M.D.; Meléndez-Carmona, M.Á.; et al. SARS-COV-2 infection in cancer patients undergoing active treatment: Analysis of clinical features and predictive factors for severe respiratory failure and death. Eur. J. Cancer 2020, 135, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Wei, M.; Wei, C. Preclinical in silico evidence indicates the pharmacological targets and mechanisms of mogroside V in patients with ovarian cancer and coronavirus disease 2019. Front. Endocrinol. (Lausanne) 2022, 13, 845404. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, D.; Simioni, C.; Neri, L.M.; Rizzo, R.; Semprini, C.M.; Occhionorelli, S.; Laface, I.; Sanz, J.M.; Schiuma, G.; Rizzo, S.; et al. Relevance of vegf and cd147 in different SARS-COV-2 positive digestive tracts characterized by thrombotic damage. FASEB J. 2021, 35, e21969. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dai, L.H.; Dou, D.Q.; Ma, L.Q.; Sun, Y.X. A natural food sweetener with anti-pancreatic cancer properties. Oncogenesis 2016, 5, e217. [Google Scholar] [CrossRef]
- Chen, J.; Jiao, D.M.; Li, Y.; Jiang, C.Y.; Chen, Q.Y. Mogroside v inhibits hyperglycemia-induced lung cancer cells metastasis through reversing emt and damaging cytoskeleton. Curr. Cancer Drug Targets 2019, 19, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Shi, G.Y.; Yang, Y.J.; Chen, W.; Zhang, L.F. Anti-aging effect of siraitia grosuenorii by enhancement of hematopoietic stem cell function. Am. J. Chin. Med. 2016, 803–815. [Google Scholar] [CrossRef]
- He, C.W.; Wen, J.; Zhu, X.Y.; He, W.P.; Lin, J.; Huang, Z.M. Safety evaluation study on the acute toxicity of fresh mogroside. Guangxi J. Light Ind. 2011, 27, 4–5. [Google Scholar]
- Jin, M.L.; Muguruma, M.; Moto, M.; Okamura, M.; Kashida, Y.; Mitsumori, K. Thirteen-week repeated dose toxicity of siraitia grosvenori extract in wistar hannover (galas) rats. Food Chem. Toxicol. 2007, 45, 1231–1237. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.H.; Huang, D.R.; Zeng, Y.; Yang, J.; Wang, S.C.; Wang, F.Y. Effects of grosvenor momordica fruit on bone marrow cell micronucleus and sperm morphology of male mice. J. Clin. Rehabil. Tissue Eng. Res. 2011, 15, 5249–5252. [Google Scholar]
- Liu, M.S.; Zhang, H.; Li, X.H.; Lan, M.B.; Qin, Y.Y. The research of mogrosides on male mice’s genotoxicity. Chin. J. Birth Health Hered. 2013, 21, 140–142. [Google Scholar]
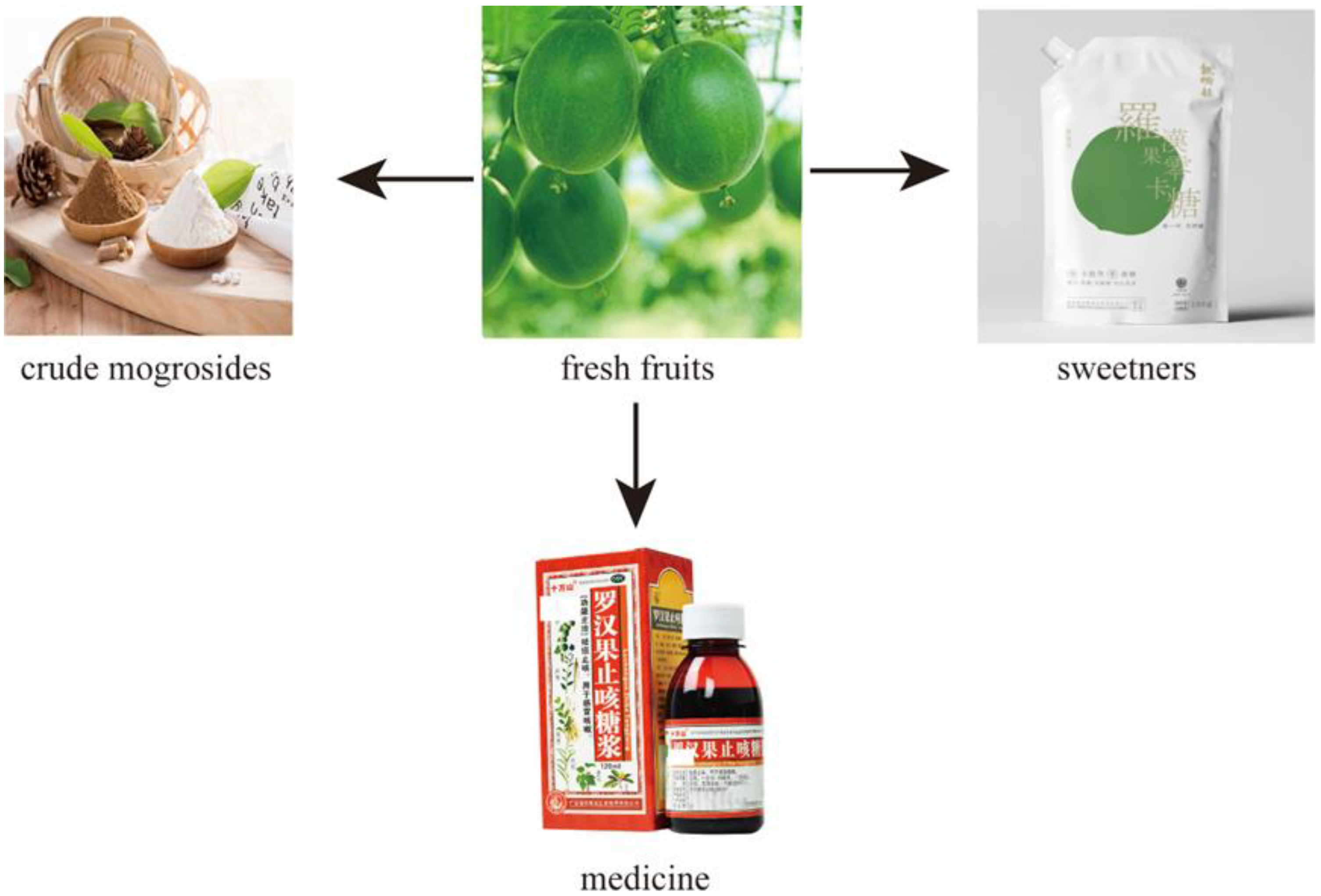
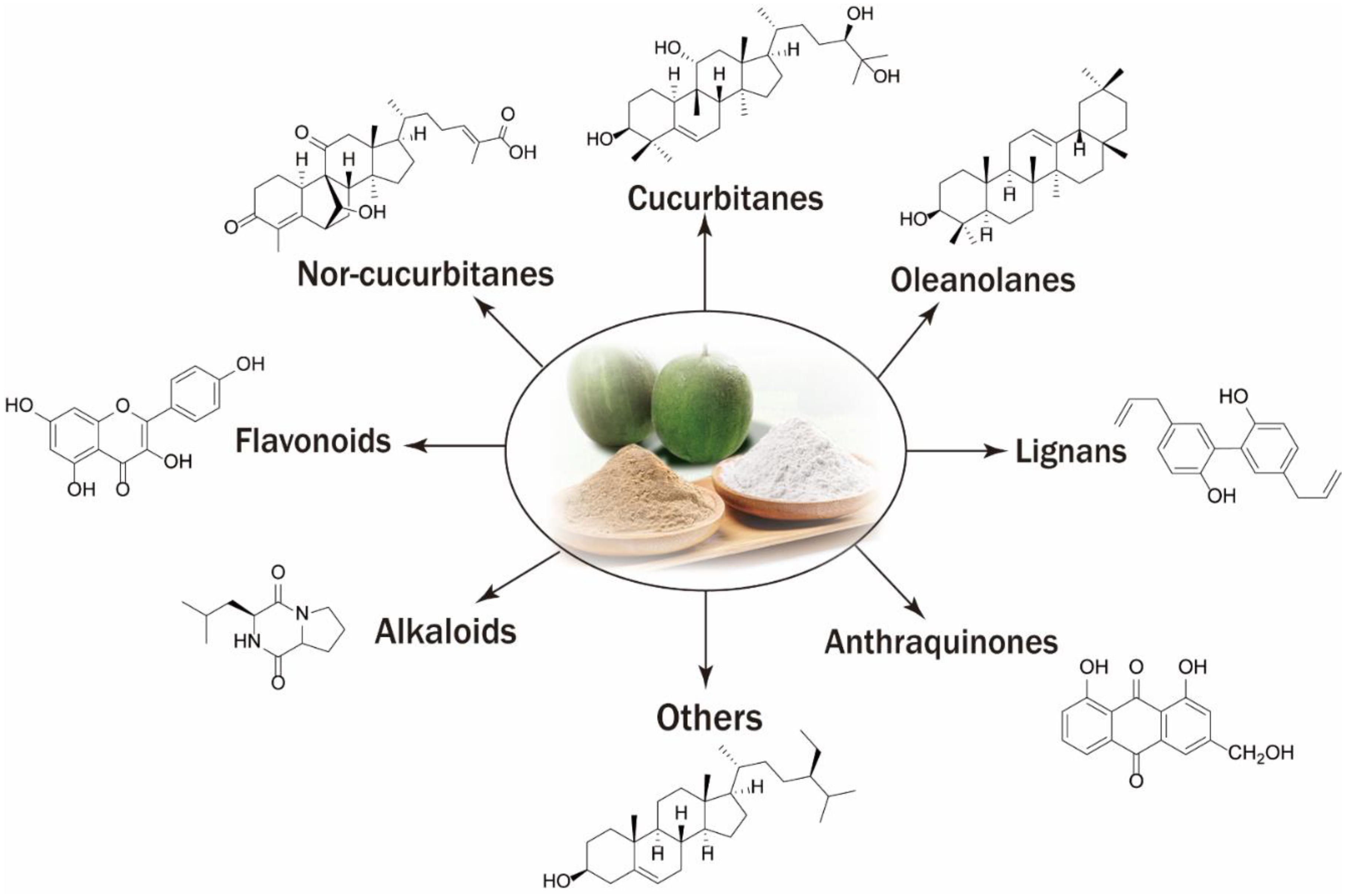
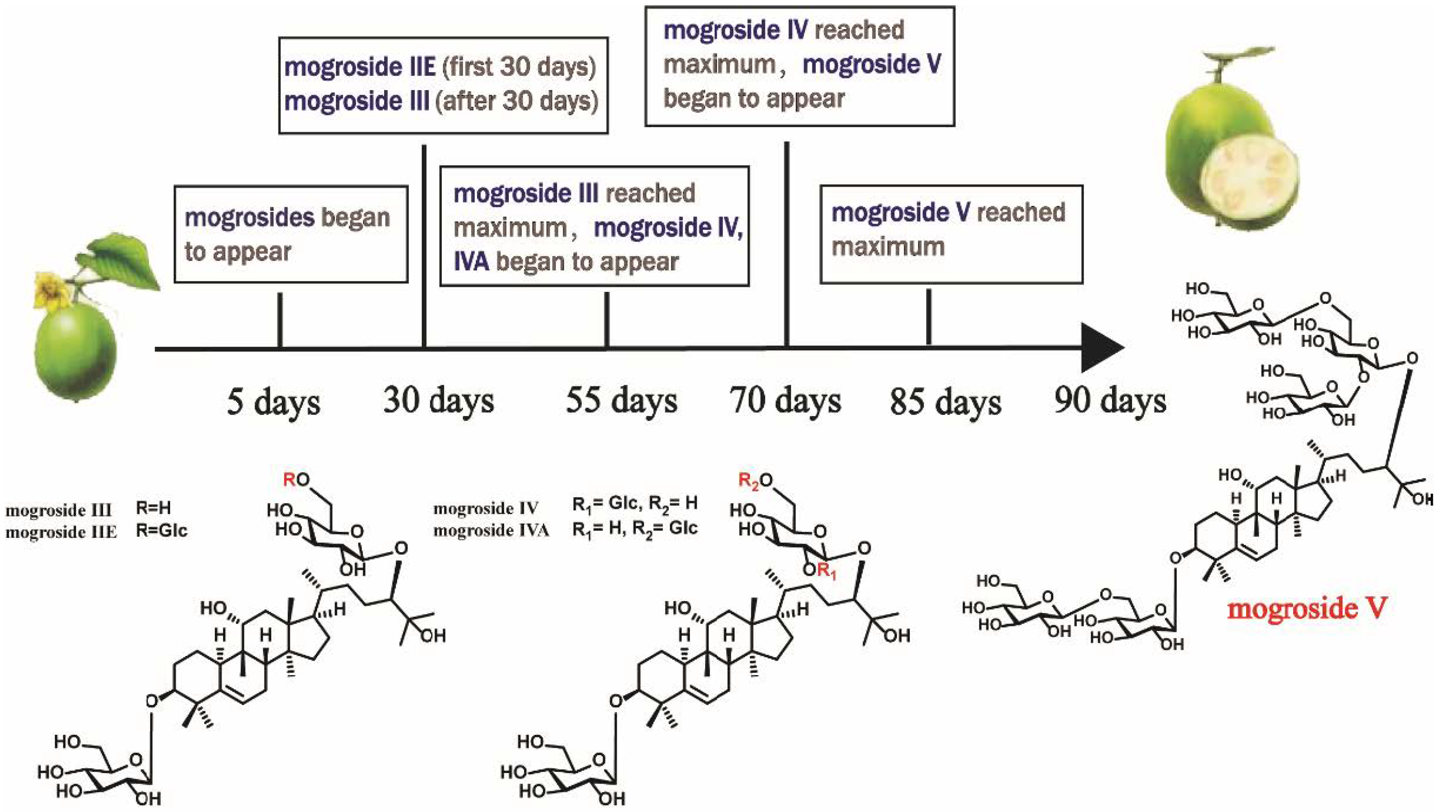
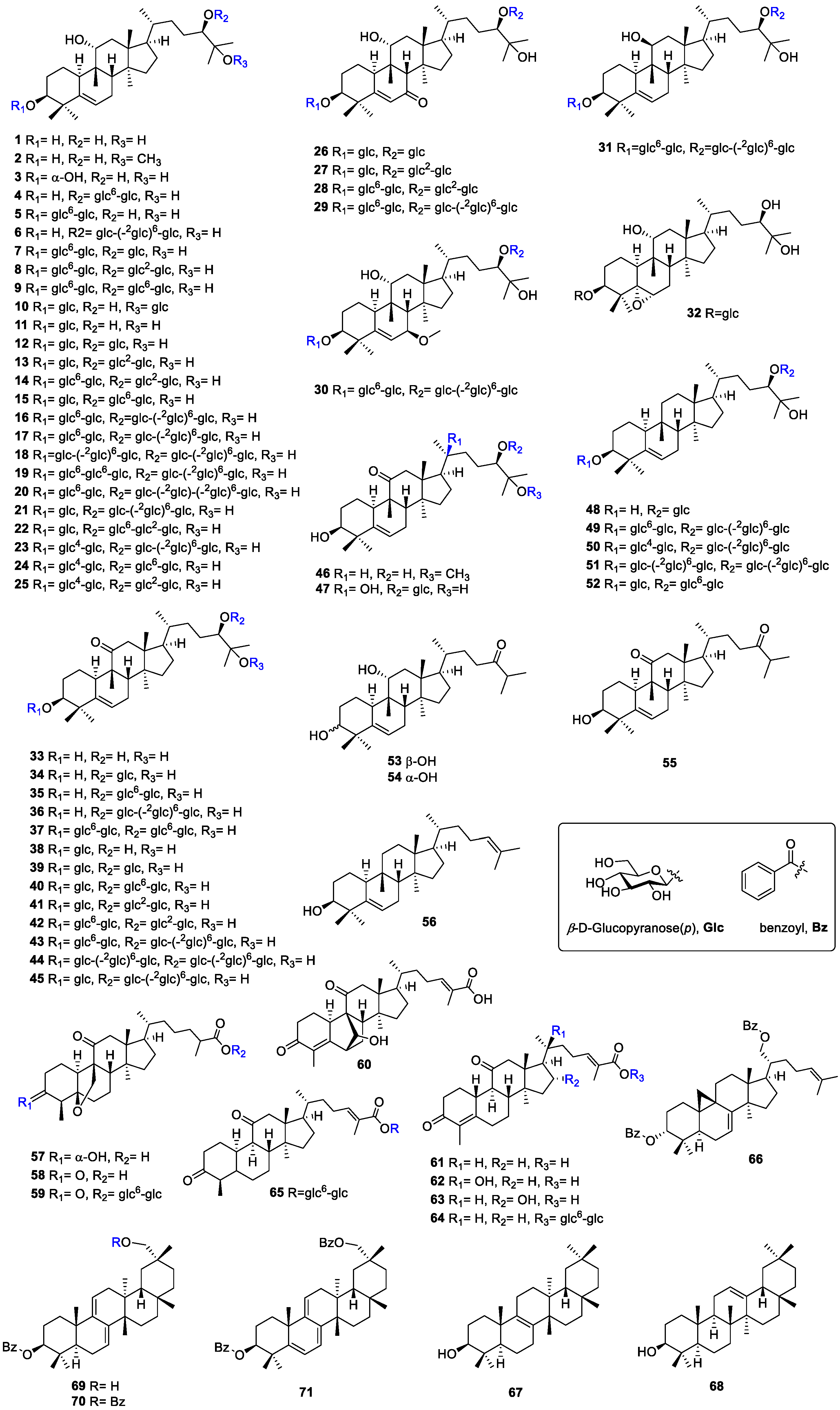
| No. | Compound Name | Pharmacological Activities | Origins | Ref. |
|---|---|---|---|---|
| 1 | mogrol | neuroprotective | fruit | [16,34] |
| 2 | 25-methoxymogrol | antidiabetic, anti-hyperlipid | fruit | [25] |
| 3 | 3α-hydroxymogrol | antidiabetic, anti-hyperlipid | fruit | [25] |
| 4 | mogroside IIA1 | antiviral | fruit | [35] |
| 5 | mogroside IIA2 | antidiabetic, antioxidant | fruit | [6,36] |
| 6 | mogroside IIIA1 | antidiabetic | fruit | [36] |
| 7 | mogroside IIIA2 | antiviral | fruit | [16,35] |
| 8 | mogroside IV | antidiabetic | fruit | [16,37] |
| 9 | mogroside IVA | - | fruit | [16,17] |
| 10 | mogroside IIB | - | fruit | [35] |
| 11 | mogroside IE1 | - | fruit | [8,16] |
| 12 | mogroside IIE | antidiabetic, antioxidant | fruit | [8,38,39] |
| 13 | mogroside IIIE | anti-inflammatory, antidiabetic, anti-fibrotic | fruit | [16,40,41,42] |
| 14 | mogroside IVE | anti-fibrotic, anti-tumor | fruit | [43,44,45] |
| 15 | mogroside III | - | fruit | [8,17,46] |
| 16 | mogroside V | anti-inflammatory, antioxidant | fruit | [8,16,47,48,49] |
| 17 | mogroside VA1 | - | fruit | [50] |
| 18 | mogroside VI | liver protective | fruit | [16,51] |
| 19 | mogroside VIA | - | fruit | [52] |
| 20 | mogroside VIB | - | fruit | [52] |
| 21 | siamenoside I | - | fruit | [8,45,49,53] |
| 22 | grosmomoside I | - | fruit | [54] |
| 23 | isomogroside V | - | fruit | [45,55] |
| 24 | isomogroside IVa | - | fruit | [36] |
| 25 | isomogroside IVe | - | fruit | [36] |
| 26 | 7-oxo-mogroside IIE | - | fruit | [56] |
| 27 | 7-oxo-mogroside IIIE | - | fruit | [52] |
| 28 | 7-oxo-mogroside IV | - | fruit | [52] |
| 29 | 7-oxo-mogroside V | - | fruit | [56] |
| 30 | 7β-methoxy-mogroside V | - | fruit | [11] |
| 31 | 11-epimogroside V | - | fruit | [8,45,49,53] |
| 32 | 5α,6α-epoxymogroside IE1 | - | fruit | [26] |
| 33 | 11-oxo-mogrol | liver protective | fruit | [57,58] |
| 34 | 11-oxo-mogroside IA1 | - | fruit | [17,58] |
| 35 | 11-oxomogroside IIA1 | - | fruit | [26] |
| 36 | 11-oxo-mogroside IIIA1 | - | fruit | [11] |
| 37 | 11-oxo-mogroside IVA | - | fruit | [45] |
| 38 | 11-oxo-mogroside IE1 | - | fruit | [16,58] |
| 39 | 11-oxo-mogroside IIE | - | fruit | [17] |
| 40 | 11-oxo-mogroside III | - | fruit | [59] |
| 41 | 11-oxo-mogroside IIIE | - | fruit | [52] |
| 42 | 11-oxo-mogroside IV | - | fruit | [52] |
| 43 | 11-oxo-mogroside V | antioxidant, anti-tumor | fruit | [45,60,61] |
| 44 | 11-oxo-mogroside VI | antidiabetic | fruit | [36] |
| 45 | 11-oxo-siamenoside I | antidiabetic | fruit | [36] |
| 46 | 25-methoxy-11-oxomogrol | antidiabetic, anti-lipidemic | fruit | [25] |
| 47 | 20-hydroxy-11-oxomogroside I A1 | - | fruit | [17] |
| 48 | mogroside IA (mogroside, A1) | - | fruit | [16] |
| 49 | 11-deoxymogroside V | antidiabetic | fruit | [36,62] |
| 50 | 11-deoxyisomogroside V | - | fruit | [62] |
| 51 | 11-deoxymogroside VI | - | fruit | [62] |
| 52 | 11-deoxymogroside III | - | fruit | [35,59] |
| 53 | 25-dehydroxy-24-oxomogrol | antidiabetic, anti-lipidemic | fruit | [25] |
| 54 | 3-hydroxy-25-dehydroxy-24-oxomogrol | antidiabetic, anti-lipidemic | fruit | [25] |
| 55 | bryogenin | antidiabetic, anti-lipidemic | fruit | [25] |
| 56 | 10α-cucurbitadienol | - | seed oil | [26] |
| No. | Compounds Name | Pharmacological Activities | Origins | Ref. |
|---|---|---|---|---|
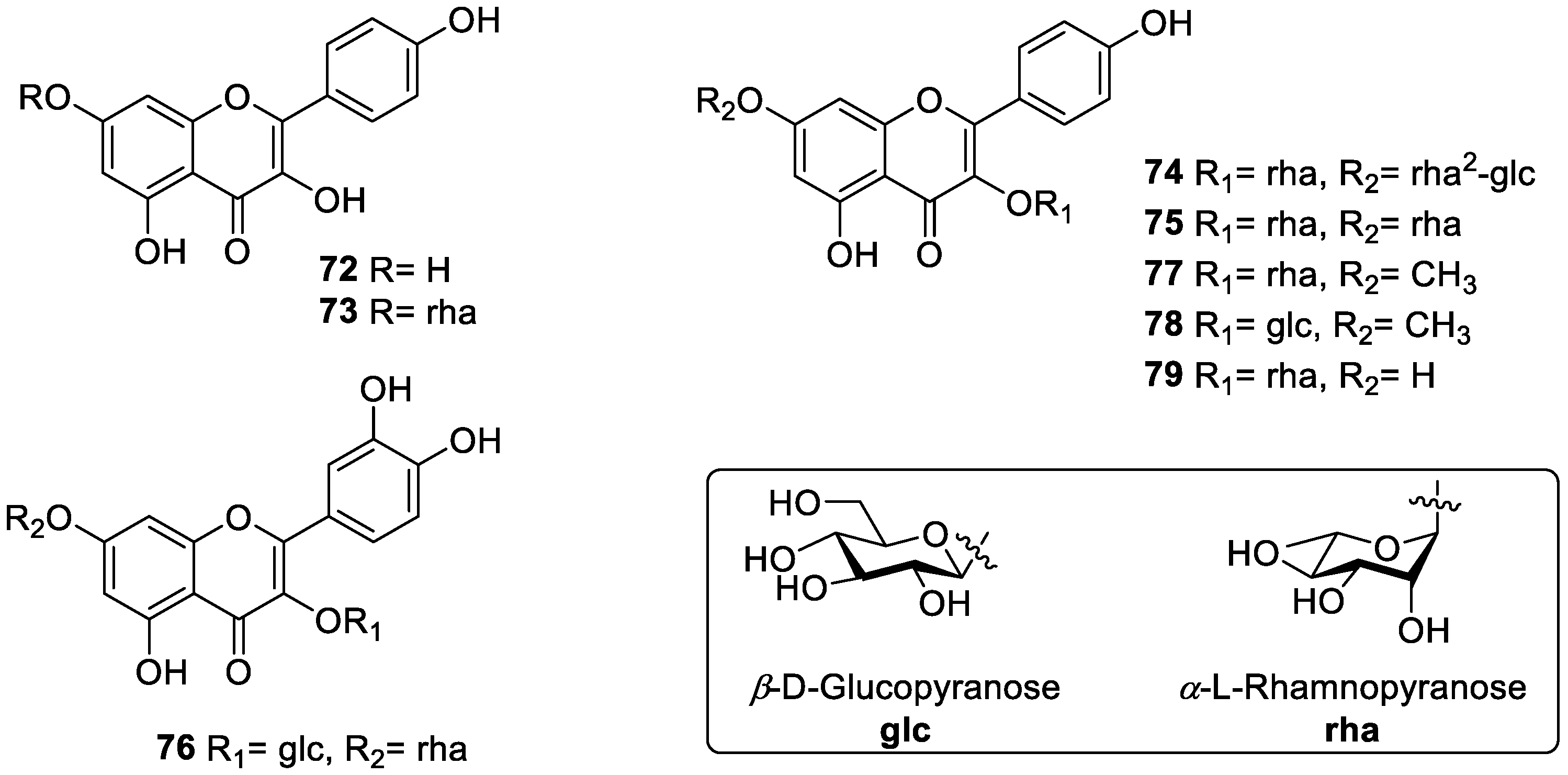
| 72 | kaempferol | antioxidant, anti-inflammatory | flower, leaf | [65,69,70] |
| 73 | kaempferol-7-O-α-L-rhamnopyranoside | antioxidant | flower, leaf, fruit | [70,71] |
| 74 | grosvenorine | antioxidant | flower, leaf, fruit | [63,70,71] |
| 75 | kaempferitrin | antioxidant, anti-inflammatory, anti-convulsant | leaf, fruit | [63,65,71,72] |
| 76 | quercetin 3-O-β-D-glucopyranosyl 7-O-α-L-rhamnopyranoside | - | leaf, fruit | [63,65,71] |
| 77 | 7-methoxy-kaempferol 3-O-α-L-rhamnopyranoside | antioxidant | flower | [70] |
| 78 | 7-methoxy-kaempferol 3-O-β-D-glucopyranoside | antioxidant | flower | [70] |
| 79 | afzelin | anti-tumor, anti-inflammatory | fruit | [68,73,74] |
| No. | Compounds Name | Pharmacological Activities | Origins | Ref. |
|---|---|---|---|---|
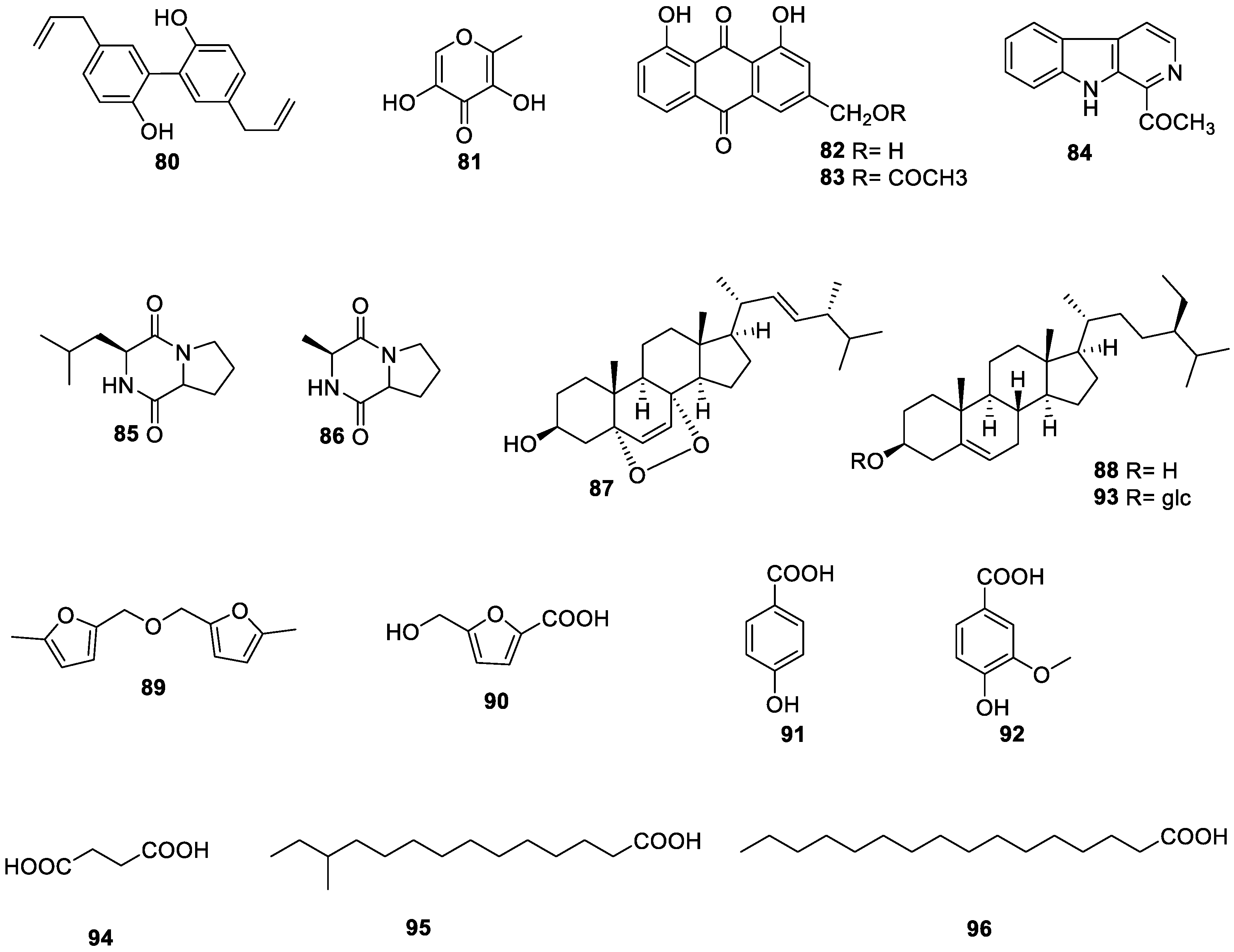
| 80 | magnolol | - | fruit | [27] |
| 81 | 5-hydroxymaltol | - | fruit | [66] |
| 82 | aloe emodin | antibacterial | leaf | [75] |
| 83 | aloe-emodin acetate | antibacterial | leaf | [75] |
| 84 | 1-acetyl-β-carboline | - | fruit | [66] |
| 85 | cyclo-(leu-pro) | - | fruit | [66] |
| 86 | cyclo-(ala-pro) | - | fruit | [66] |
| 87 | ergosterol peroxide | antibacterial | leaf | [75] |
| 88 | β-sitosterol | - | fruit | [66] |
| 89 | 5,5′-oxydimethylene-bis-(2-furfural) | - | fruit | [27] |
| 90 | 5-(hydroxymethyl)-2-furancarboxylic acid | - | fruit | [27] |
| 91 | 4-hydroxybenzoic acid | antibacterial | leaf | [75] |
| 92 | vanillic acid | - | fruit | [66] |
| 93 | daucosterol | - | leaf | [75] |
| 94 | succinic acid | - | fruit | [27] |
| 95 | 12-methyltetradecanoic acid | - | leaf | [75] |
| 96 | n-hexadecanoic acid | - | leaf | [75] |
| Activities | Detail | Extracts/Compounds | Concentration/Dose | In Vivo/In Vitro | Ref. |
|---|---|---|---|---|---|
| Antitussive, expectorant and anti-asthmatic activities | increased the secretion of phenolsulfonphthalein in mice and the excretion of sputum in rats | SGA | 4000 and 8000 mg/kg | in vivo | [84] |
| reduced Th2 cytokines (IL-4, IL-5, and IL-13) and increased the Th1cytokine IFN-γ | SGRE | 200 mg/kg | in vivo | [85] | |
| enhanced sputum secretion in mice and ciliary cell motility in the frog respiratory tract | mogrosides | 50, 100, and 200 mg/kg | in vivo | [86] | |
| Antioxidant | decrease ROS in oxide injury PC12 cells and decrease apoptotic and necrotic cells | SGP | 0.5, 1.0, 1.5, 2.0 mg/mL | in vitro | [87] |
| reduced ROS levels and MDA content, increase SOD, GSH-Px and CAT activities | mogroside V | 30, 60, and 90 µg/mL | in vitro | [47] | |
| scavenging of -OH | mogroside V | PC12 cells EC50 = 48.44 μg/mL | in vitro | [60] | |
| scavenging of O2 and H2O2, inhibited -OH induced DNA damage | 11-oxomogroside V | PC12 cells EC50 = 4.79 μg/mL, EC50 = 16.52 μg/mL, EC50 = 3.09 μg/mL | in vitro | [60] | |
| inhibited BSA glycation | MGE | 500 μg/mL | in vitro | [6] | |
| decrease the levels of inflammatory cytokines and oxidative stress-related biomarkers | mogroside IIIE | MPC-5 cells 1, 10, and 50 μM | in vitro | [88] | |
| inhibited the reduction of HB and LD and inhibited the increase of MDA, promoted the synthesis of HB and the clearance of LD | SGE | 1500 mg/kg | in vivo | [89] | |
| Hypoglycemic | increase AMPK phosphorylation | mogrol | HepG2 cell line 1, 10, and 20 μM | in vivo | [25] |
| increase AMPK phosphorylation | 3-hydroxymogrol | HepG2 cell line 1, 10, and 20 μM | in vivo | [25] | |
| increase AMPK phosphorylation | 3-hydroxy-25-dehydroxy-24-oxo-mogrol | HepG2 cell line 4 μM | in vivo | [25] | |
| downregulated mRNA levels of hepatic gluconeogenic and lipogenic genes, upregulated fat oxidation-associated genes | MGE | 300 mg/kg | in vivo | [91] | |
| Immunology and anti-inflammatory | increased filaggrin expression and reduced DfE induced phosphorylation of ERK, JNK and p38 | NHGR | 200 or 400 mg/kg | in vivo | [92] |
| enhanced monocyte phagocytosis in hydrocortisone injured | SGA | 2500 and 5000 mg/kg | in vivo | [93] | |
| decreased expression of IL-1b, IL-6, and TNF-a | mogroside IIIE | GDM model 20.0 mg/kg | in vivo | [40] | |
| inhibited LPS-induced inflammatory | mogroside IIIE | RAW264.7 cells 10 μM | in vitro | [40] | |
| inhibited LPS-induced inflammatory | mogroside IIIE | RAW264.7 cells 1, 10 or 50 μM | in vitro | [42] | |
| reduced the OVA-induced activation of NF-κB | mogroside V | 2, 5, and 10 mg/kg | in vivo | [97] | |
| inhibited LPS-induced inflammatory | mogroside V | BV-2 cells 6.25, 12.5 and 25 μM | in vitro | [99] | |
| inhibited the IL-9/IL-9R/calcium overload/cathepsin B activation/trypsinogen activation pathway | mogroside IIE | AR42J cells 5, 10 and 20 μM | in vitro | [100] | |
| Liver protection | inhibited reactive oxygen species production and upregulated sequestosome-1 (SQSTM1, p62) expression | mogroside V | 200, 400, and 800 mg/kg | in vivo | [101] |
| activated AMPK ameliorates HFD-induced hepatic steatosis | mogroside V | LO2 cells 15, 30, 60, and 120 μM | in vitro | [102] | |
| Antibacterial and anti-viral | inhibitory effects against gram-positive bacteria | grosvenorine | MIC less than 70 μg/mL | in vitro | [68] |
| inhibitory effects against gram-positive bacteria | kaempferitrin | MIC less than 70 μg/mL | in vitro | [68] | |
| inhibitory effects against gram-positive bacteria and MSSA | kaempferol | MIC less than 70 μg/mL | in vitro | [68] | |
| inhibitory effects against MSSA and MRSA | afzelin | MIC less than 70 μg/mL | in vitro | [68] | |
| inhibitory effects against Streptococcus mutans, Actinobacillus actinobacillus, Clostridium sclerotiorum, and Candida albicans | β-amyrin | MIC = 48.80, >100, 48.40, and >100 μg/mL | in vitro | [75] | |
| inhibitory effects against Streptococcus mutans, Actinobacillus actinobacillus, Clostridium sclerotiorum, and Candida albicans | ergosterol peroxide | MIC = 4.88, 48.80, 48.80, and 12.20 μg/mL | in vitro | [75] | |
| inhibitory effects against Streptococcus mutans, Actinobacillus actinobacillus, Clostridium sclerotiorum, and Candida albicans | aloe-emodin | MIC = 1.22, 6.10, 12.20, and 6.10 μg/mL | in vitro | [75] | |
| inhibitory effects against Streptococcus mutans, Actinobacillus actinobacillus, Clostridium sclerotiorum, and Candida albicans | aloe-emodin acetate | MIC = 6.10, 12.20, >100, and 6.10 μg/mL | in vitro | [75] | |
| inhibitory effects against Streptococcus mutans, Actinobacillus actinobacillus, Clostridium sclerotiorum, and Candida albicans | 4-hydroxybenzoic acid | MIC = 12.20, >100, 12.20, and 12.20 μg/mL | in vitro | [75] | |
| inhibitory effects against Escherichia coli bacterial biofilms | different ethanol-eluting parts of S. grosvenorii | MIC = 55.58, 78.32, and 87.62% | in vitro | [103] | |
| regulation of VEGF | mogroside V | - | in vitro | [107] | |
| Miscellaneous activities | inhibited expression of the STAT3 pathway | mogroside V | PANC-1 cells 10, 100, and 250 μM | in vivo and in vitro | [109] |
| inhibited expression levels of Rho A, Rac1, Cdc42 and p-PAK1 | mogroside V | A549 and H1299 cells 0–50 μM | in vitro | [110] | |
| inhibited expression of IL1β, IL-6, NF-κB p65, TNF-a induced by Aβ1–42 | mogrol | 20, 40, 80 mg/kg | in vivo | [5] | |
| decrease the intercellular levels of ROS | extract of S. grosvenorii | 200 mg/kg | in vivo | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Jian, Y.; Wang, H.; Huang, H.; Gong, L.; Liu, G.; Yang, Y.; Wang, W. A Review of the Phytochemistry and Pharmacology of the Fruit of Siraitia grosvenorii (Swingle): A Traditional Chinese Medicinal Food. Molecules 2022, 27, 6618. https://doi.org/10.3390/molecules27196618
Wu J, Jian Y, Wang H, Huang H, Gong L, Liu G, Yang Y, Wang W. A Review of the Phytochemistry and Pharmacology of the Fruit of Siraitia grosvenorii (Swingle): A Traditional Chinese Medicinal Food. Molecules. 2022; 27(19):6618. https://doi.org/10.3390/molecules27196618
Chicago/Turabian StyleWu, Juanjiang, Yuqing Jian, Huizhen Wang, Huaxue Huang, Liming Gong, Genggui Liu, Yupei Yang, and Wei Wang. 2022. "A Review of the Phytochemistry and Pharmacology of the Fruit of Siraitia grosvenorii (Swingle): A Traditional Chinese Medicinal Food" Molecules 27, no. 19: 6618. https://doi.org/10.3390/molecules27196618
APA StyleWu, J., Jian, Y., Wang, H., Huang, H., Gong, L., Liu, G., Yang, Y., & Wang, W. (2022). A Review of the Phytochemistry and Pharmacology of the Fruit of Siraitia grosvenorii (Swingle): A Traditional Chinese Medicinal Food. Molecules, 27(19), 6618. https://doi.org/10.3390/molecules27196618






