Carbazole Derivatives as Potential Antimicrobial Agents
Abstract
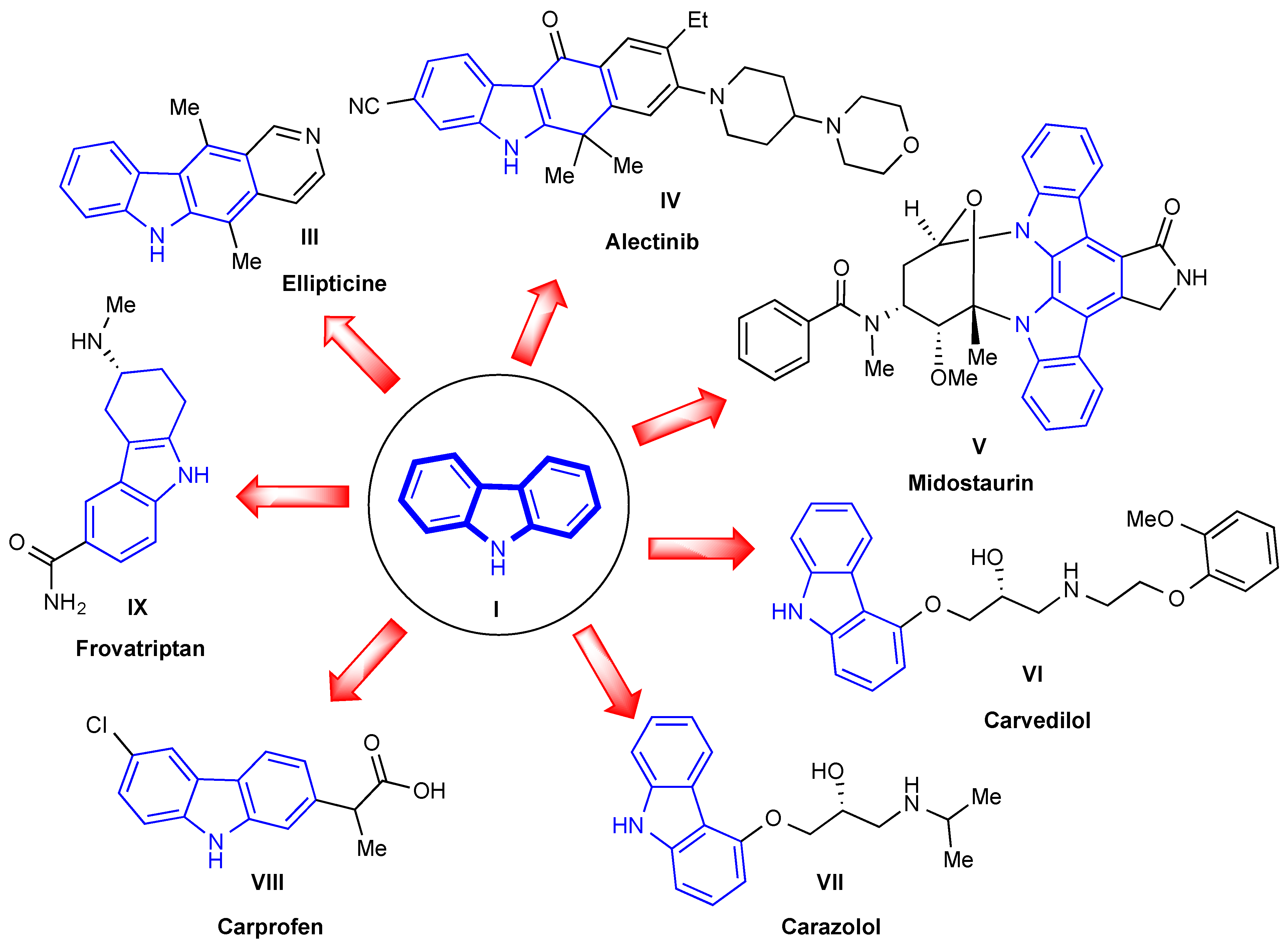
1. Introduction
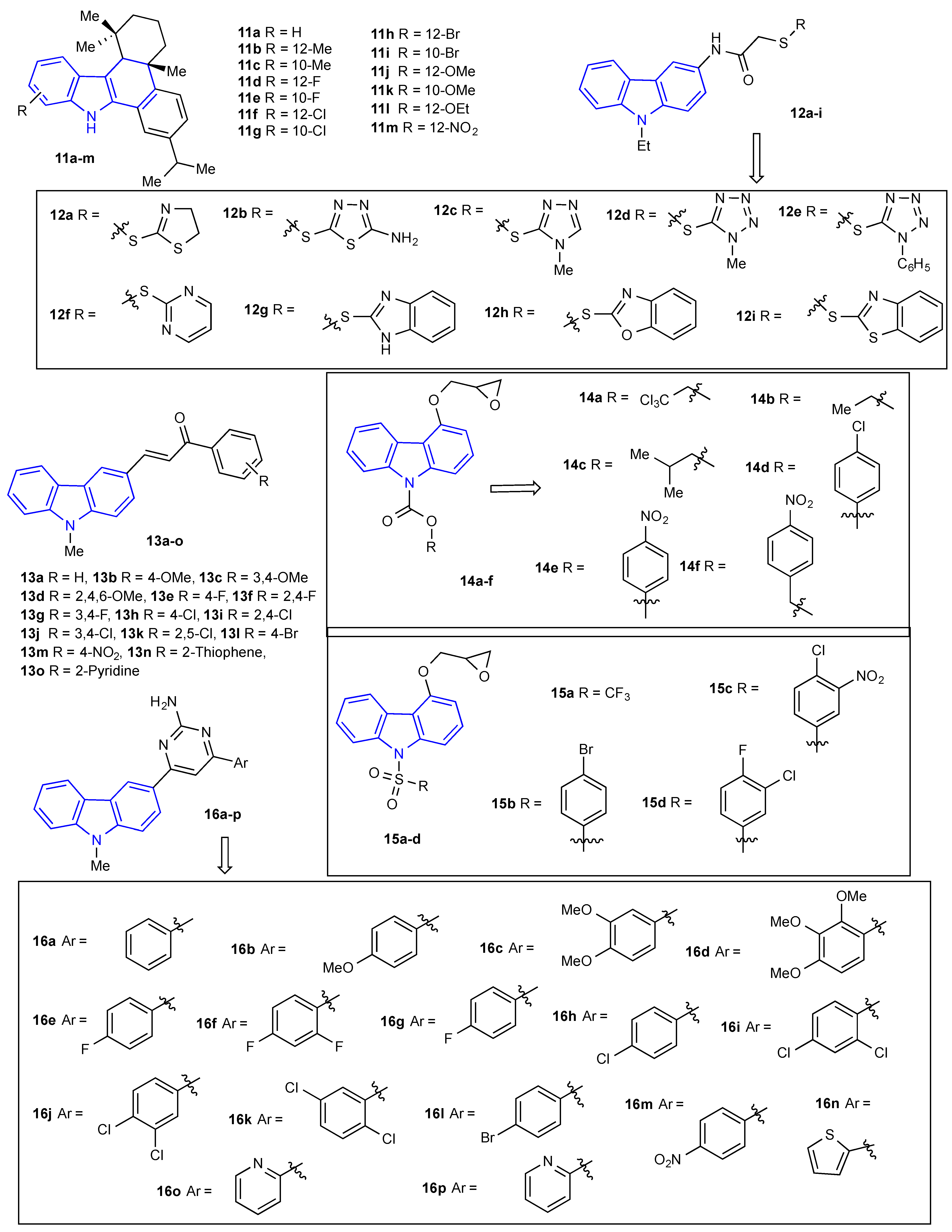
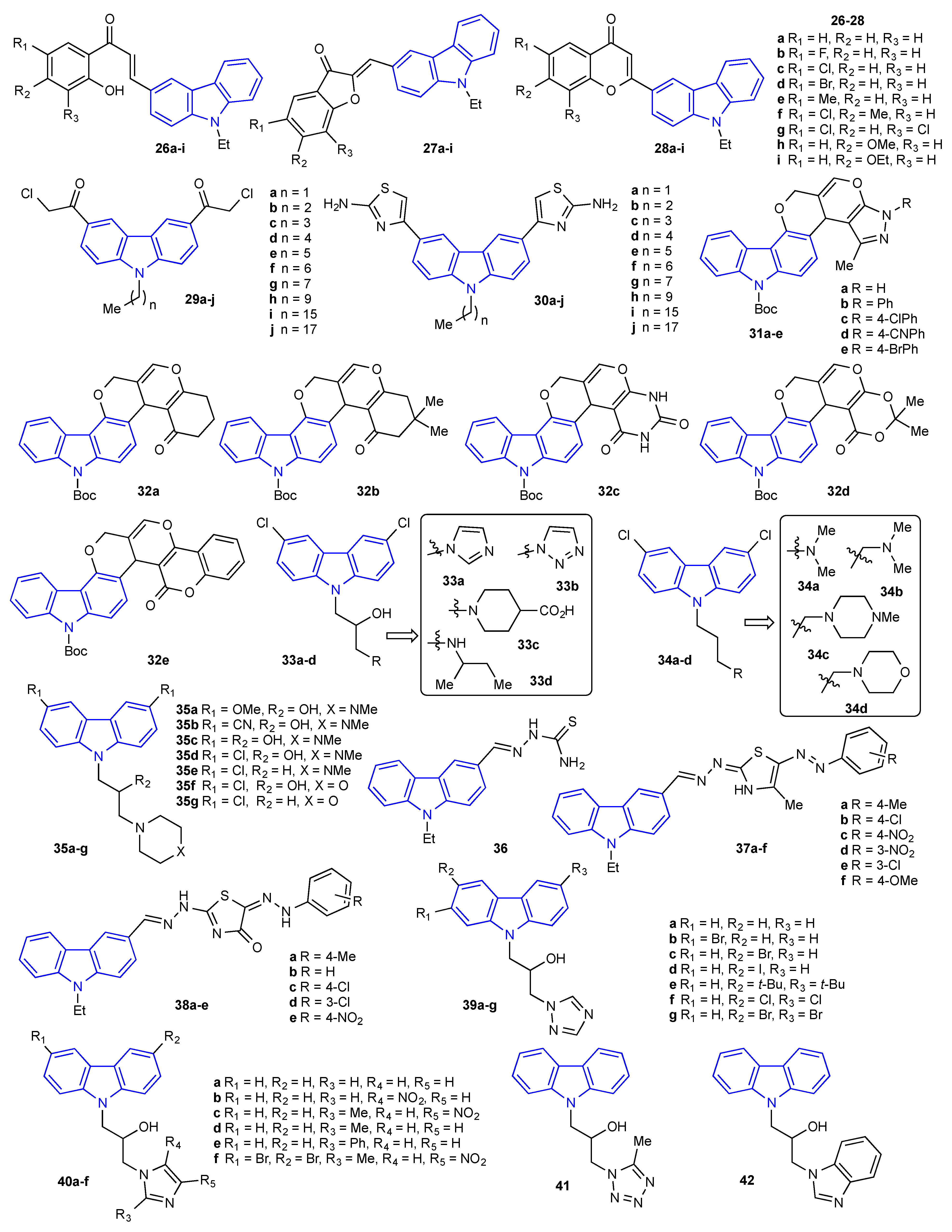
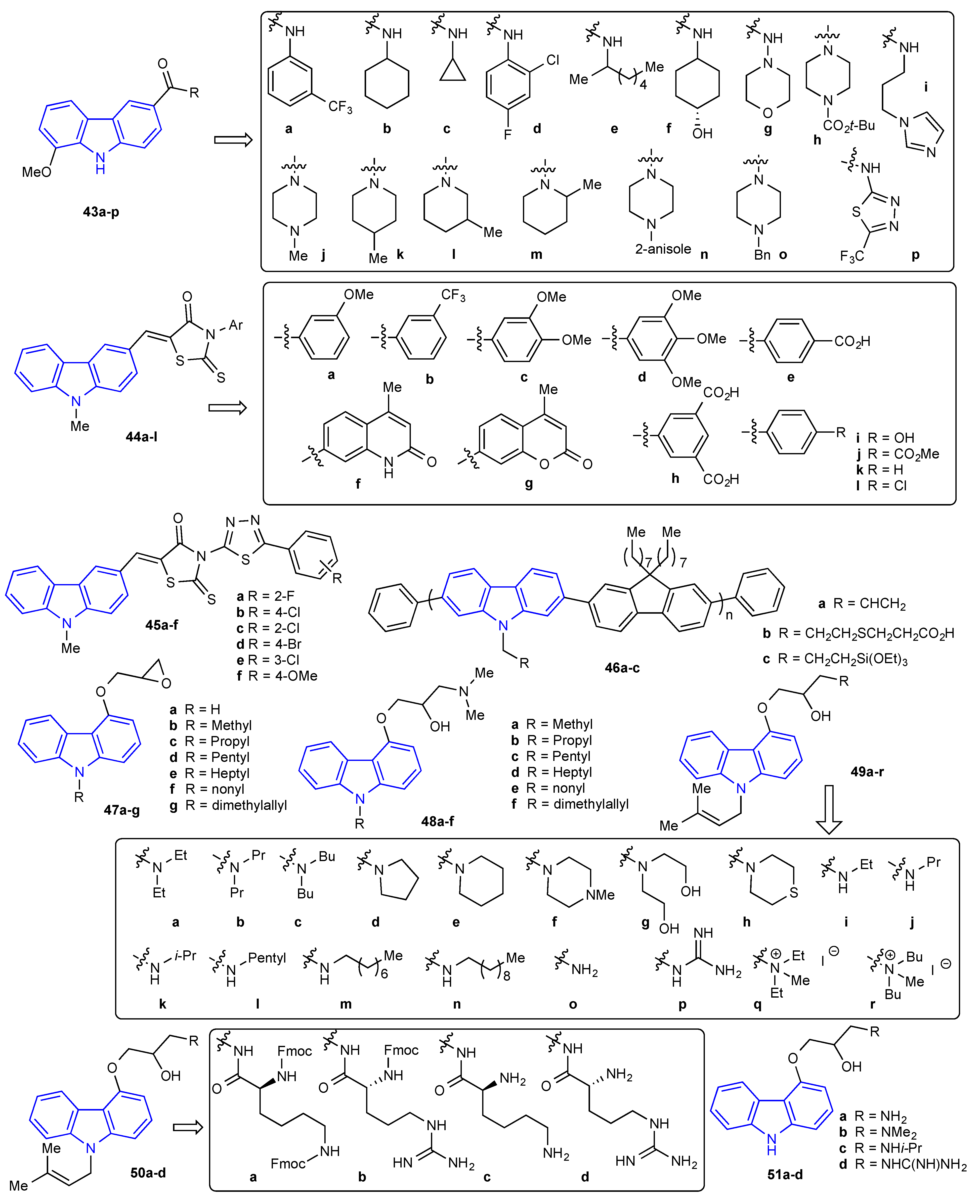
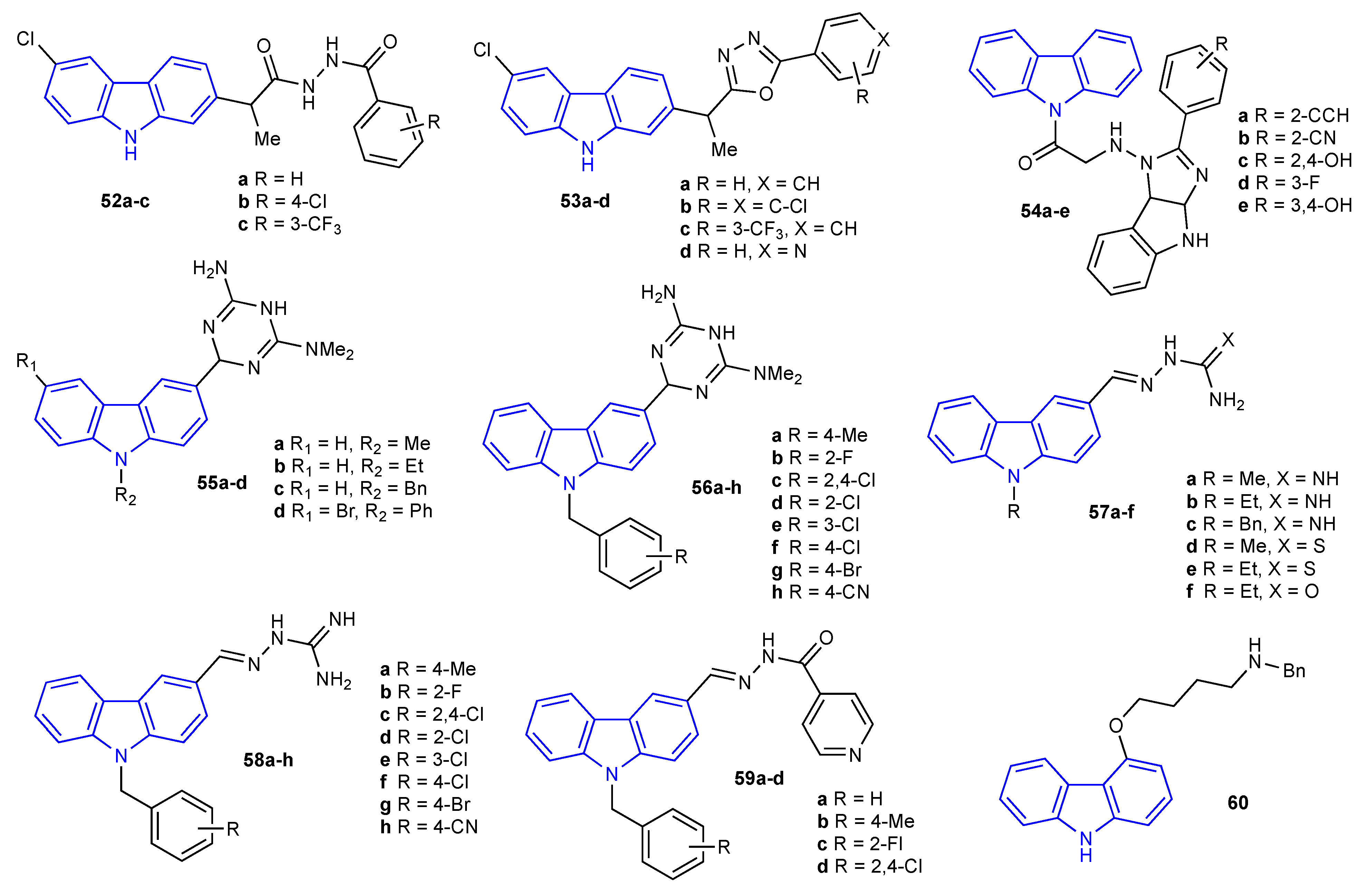
2. Antibacterial and Antifungal Activities of Carbazole Derivatives
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on antibiotic resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 15 September 2022).
- Shinu, P.; Mouslem, A.K.A.; Nair, A.B.; Venugopala, K.N.; Attimarad, M.; Singh, V.A.; Nagaraja, S.; Alotaibi, G.; Deb, P.K. Progress report: Antimicrobial drug discovery in the resistance era. Pharmaceuticals 2022, 15, 413. [Google Scholar] [CrossRef] [PubMed]
- Mitcheltree, M.J.; Pisipati, A.; Syroegin, E.A.; Silvestre, K.J.; Klepacki, D.; Mason, J.D.; Terwilliger, D.W.; Testolin, G.; Pote, A.R.; Wu, K.J.Y.; et al. A synthetic antibiotic class overcoming bacterial multidrug resistance. Nature 2021, 599, 507–512. [Google Scholar] [CrossRef]
- Johnston, S.L.; Blasi, F.; Black, P.N.; Martin, R.J.; Farrell, D.J.; Nieman, R.B.; Investigators, T. The effect of telithromycin in acute exacerbations of asthma. N. Engl. J. Med. 2006, 354, 1589–1600. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Leggett, J. Gatifloxacin, gemifloxacin, and moxifloxacin: The role of 3 newer fluoroquinolones. Clin. Infect. Dis. 2003, 37, 1210–1215. [Google Scholar] [CrossRef]
- Karpecki, P.; Depaolis, M.; Hunter, J.A.; White, E.M.; Rigel, L.; Brunner, L.S.; Usner, D.W.; Paterno, M.R.; Comstock, T.L. Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: A multicenter, prospective, randomized, doublemasked, vehicle-controlled, 5-day efficacy and safety study. Clin. Ther. 2009, 31, 514–526. [Google Scholar] [CrossRef]
- Ellis-Grosse, E.J.; Babinchak, T.; Dartois, N.; Rose, G.; Loh, E. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: Results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin. Infect. Dis. 2005, 41, S341–S353. [Google Scholar] [CrossRef]
- Ramirez, J.; Dartois, N.; Gandjini, H.; Yan, J.L.; Korth-Bradley, J.; McGovern, P.C. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenemcilastatinfor treatment of hospital-acquired pneumonia. Antimicrob. Agents Chemother. 2013, 57, 1756–1762. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Nichol, K.; Zhanel, G.G. Telavancin: Mechanisms of action, in vitro activity, and mechanisms of resistance. Clin. Infect. Dis. 2015, 61, S58–S68. [Google Scholar] [CrossRef]
- Alt, S.; Bernasconi, A.; Sosio, M.; Brunati, C.; Donadio, S.; Maffioli, S.I. Toward single-peak dalbavancin analogs through biologyand chemistry. ACS Chem. Biol. 2019, 14, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.C.; Welte, T.; File, T.M., Jr.; Strauss, R.S.; Michiels, B.; Kaul, P.; Balis, D.; Arbit, D.; Amsler, K.; Noel, G.J. A randomised, double-blind trial comparing ceftobiprole medocaril with ceftriaxone with or without linezolid for the treatment of patients with community-acquired pneumonia requiring hospitalisation. Int. J. Antimicrob. Agents 2012, 39, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Lovering, A.L.; Gretes, M.C.; Safadi, S.S.; Danel, F.; de Castro, L.; Page, M.G.P.; Strynadka, N.C.J. Structural insights into the anti-methicillin-resistant Staphylococcus aureus (MRSA) activity of ceftobiprole. J. Biol. Chem. 2012, 287, 32096–32102. [Google Scholar] [CrossRef] [PubMed]
- Graebe, C.; Glaser, C. Ueber carbazol. Justus Liebigs Ann. Chem. 1872, 163, 343–360. [Google Scholar] [CrossRef]
- Chakraborty, D.P.; Barman, B.K.; Bose, P.K. On the constitution of murrayanine, a carbazole derivative isolated from Murraya koenigii Spreng. Tetrahedron 1965, 21, 681–685. [Google Scholar] [CrossRef]
- Głuszyńska, A. Biological potential of carbazole derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. [Google Scholar] [CrossRef]
- Caruso, A.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Mauro, M.V.; Bruno, R.; Aquaro, S.; Sinicropi, M.S. Carbazole derivatives as antiviral agents: An overview. Molecules 2019, 24, 1912. [Google Scholar] [CrossRef]
- Caruso, A.; Iacopetta, D.; Puoci, F.; Rita Cappello, A.; Saturnino, C.; Stefania Sinicropi, M. Carbazole derivatives: A promising scenario for breast cancer treatment. Mini Rev. Med. Chem. 2016, 16, 630–643. [Google Scholar] [CrossRef]
- Knölker, H.-J.; Reddy, K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002, 102, 4303–4428. [Google Scholar] [CrossRef]
- Garbett, N.C.; Graves, D.E. Extending nature’s leads: The anticancer agent ellipticine. Curr. Med. Chem. Anticancer Agents 2004, 4, 149–172. [Google Scholar] [CrossRef]
- Ruiz-Ceja, K.A.; Chirino, Y.I. Current FDA-approved treatments for non-small cell lung cancer and potential biomarkers for its detection. Biomed. Pharmacother. 2017, 90, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Manley, P.W.; Larson, R.A.; Capdeville, R. Midostaurin: Its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv. 2018, 2, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Jang, M.; Zhang, T.; Akhtari, M.; Alachkar, H. Midostaurin reduces regulatory T cells markers in acute myeloid leukemia. Sci. Rep. 2018, 8, 17544. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.D. Carvedilol and the Food and Drug Administration (FDA) approval process: The FDA paradigm and reflections on hypothesis testing. Control. Clin. Trials 1999, 20, 16–39. [Google Scholar] [CrossRef]
- Méjean, A.; Guillaume, J.-L.; Strosberg, A.D. Carazolol: A potent, selective β3-adrenoceptor agonist. Eur. J. Pharmacol. Mol. Pharmacol. 1995, 291, 359–366. [Google Scholar] [CrossRef]
- Papich, M.G. An update on nonsteroidal anti-inflammatory drugs (NSAIDs) in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1243–1266. [Google Scholar] [CrossRef]
- Balbisi, E. Frovatriptan succinate, a 5-HT1B/1D receptor agonist for migraine. Int. J. Clin. Pract. 2004, 58, 695–705. [Google Scholar] [CrossRef]
- Ramsewak, R.S.; Nair, M.G.; Strasburg, G.M.; DeWitt, D.L.; Nitiss, J.L. Biologically active carbazole alkaloids from Murraya koenigii. J. Agric. Food Chem. 1999, 47, 444–447. [Google Scholar] [CrossRef]
- Danish, I.A.; Prasad, K.J.R. A one pot synthesis and evaluation of 13-oxo-quino[3,4-b]carbazol-N-oxides as antimicrobial agents. Acta Pharm. 2003, 53, 287–294. [Google Scholar]
- Sangeetha, V.; Rajendra Prasad, K.J. Synthesis of isoxazolo and pyrazolino annelated carbazoles from 2-(4′-methoxy)benzylidene-1-oxo-1,2,3,4-tetrahydrocarbazoles. Asian J. Chem. 2004, 16, 1165–1170. [Google Scholar]
- Vandana, T.; Rajendra Prasad, K.J. Synthesis of 3-phenyl-4-oxopyrano[2,3-a]carbazoles (indoloisoflavones). Indian J. Chem. Sect. B 2005, 44B, 1101–1104. [Google Scholar] [CrossRef]
- Mukhlesur Rahman, M.; Gray, A.I. A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity. Phytochemistry 2005, 66, 1601–1606. [Google Scholar] [CrossRef]
- Surendiran, T.; Balasubramanian, S.; Sivaraj, D. Synthesis and characterization of novel isoxazolyl and pyrazolyl 2, 3, 4, 9 tetrahydro-1H-carbazoles and their antimicrobial studies. OCAIJ 2008, 4, 427–433. [Google Scholar]
- Rajakumar, P.; Sekar, K.; Shanmugaiah, V.; Mathivanan, N. Synthesis of novel carbazole based macrocyclic amides as potential antimicrobial agents. Eur. J. Med. Chem. 2009, 44, 3040–3045. [Google Scholar] [CrossRef]
- Gu, W.; Wang, S. Synthesis and antimicrobial activities of novel 1H-dibenzo[a,c]carbazoles from dehydroabietic acid. Eur. J. Med. Chem. 2010, 45, 4692–4696. [Google Scholar] [CrossRef]
- Kaplancikli, Z.A. Synthesis of some novel carbazole derivatives and evaluation of their antimicrobial activity. Marmara Pharm. J. 2011, 15, 105–109. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Adsul, L.K.; Lonikar, S.V.; Chavan, H.V.; Shringare, S.N.; Patil, S.A.; Jalde, S.S.; Koti, B.A.; Dhole, N.A.; Gacche, R.N.; et al. Synthesis of novel carbazole chalcones as radical scavenger, antimicrobial and cancer chemopreventive agents. J. Enzym. Inhib. Med. Chem. 2013, 28, 593–600. [Google Scholar] [CrossRef]
- Lakshmi Reddy, S.V.; Naresh, K.; Raju, C.N. New sulfonamide and carbamate derivatives of 4-(oxiran-2-ylmethoxy)-9H-carbazole: Synthesis, characterization, antimicrobial and antioxidant activities. Der. Pharm. Lett. 2013, 5, 221–231. [Google Scholar]
- Adsul, L.K.; Bandgar, B.P.; Chavan, H.V.; Jalde, S.S.; Dhakane, V.D.; Shirfule, A.L. Synthesis and biological evaluation of novel series of aminopyrimidine derivatives as urease inhibitors and antimicrobial agents. J. Enzym. Inhib. Med. Chem. 2013, 28, 1316–1323. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, N.; Pathak, D. Synthesis, characterization and biological evaluation of some newer carbazole derivatives. J. Serb. Chem. Soc. 2014, 79, 125–132. [Google Scholar] [CrossRef]
- Chakraborty, B.; Chakraborty, S.; Saha, C. Antibacterial Activity of murrayaquinone A and 6-methoxy-3,7-dimethyl-2,3-dihydro-1H-carbazole-1,4(9H)-dione. Int. J. Microbiol. 2014, 2014, 540208. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Qiao, C.; Wang, S.; Hao, Y.; Miao, T.T. Synthesis and biological evaluation of novel N-substituted 1H-dibenzo[a,c]carbazole derivatives of dehydroabietic acid as potential antimicrobial agents. Bioorganic Med. Chem. Lett. 2014, 24, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hao, Y.; Zhang, G.; Wang, S.-F.; Miao, T.-T.; Zhang, K.-P. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorganic Med. Chem. Lett. 2015, 25, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, G.; Sadiq, Z.; Hamid, A.; Fatima, A.; Ijaz, Z. Conventional-microwave mediated synthesis and in vitro antimicrobial activity of novel carbazole-efflux pump inhibitor hybrid antibacterials. J. Chem. 2017, 2017, 7243279. [Google Scholar] [CrossRef]
- Sathiya, M.; Guhanathan, S. Electrophilic and free radical bromination of biologically active bromo derivative of [b] carbazole using NBS/H2SO4. World J. Pharm. Res. 2018, 7, 291–305. [Google Scholar]
- Ochung, A.A.; Manguro, L.A.O.; Owuor, P.O.; Jondiko, I.O.; Nyunja, R.A.; Akala, H.; Mwinzi, P.; Opiyo, S.A. Bioactive carbazole alkaloids from Alysicarpus ovalifolius (Schumach). J. Korean Soc. Appl. Biol. Chem. 2015, 58, 839–846. [Google Scholar] [CrossRef]
- Ashok, D.; Ravi, S.; Ganesh, A.; Lakshmi, B.V.; Adam, S.; Murthy, S.D.S. Microwave-assisted synthesis and biological evaluation of carbazole-based chalcones, aurones and flavones. Med. Chem. Res. 2016, 25, 909–922. [Google Scholar] [CrossRef]
- Addla, D.; Wen, S.-Q.; Gao, W.-W.; Maddili, S.K.; Zhang, L.; Zhou, C.-H. Design, synthesis, and biological evaluation of novel carbazole aminothiazoles as potential dna-targeting antimicrobial agents. Med. Chem. Commun. 2016, 7, 1988–1994. [Google Scholar] [CrossRef]
- Reddy, P.N.; Padmaja, P.; Reddy, B.R.; Rambabu, G.; Kumar, M.P. synthesis, molecular docking, antiproliferative, and antimicrobial activity of novel pyrano[3,2-c]carbazole derivatives. Med. Chem. Res. 2016, 25, 2093–2103. [Google Scholar] [CrossRef]
- Clausen, J.D.; Kjellerup, L.; Cohrt, K.O.; Hansen, J.B.; Dalby-Brown, W.; Winther, A.-M.L. Elucidation of antimicrobial activity and mechanism of action by n-substituted carbazole derivatives. Bioorganic Med. Chem. Lett. 2017, 27, 4564–4570. [Google Scholar] [CrossRef]
- Jasass, R.S.; Alshehrei, F.; Farghaly, T.A. Microwave-assisted synthesis of antimicrobial agents containing carbazole and thiazole moieties. J. Heterocycl. Chem. 2018, 55, 2099–2106. [Google Scholar] [CrossRef]
- Zhang, Y.; Tangadanchu, V.K.R.; Cheng, Y.; Yang, R.-G.; Lin, J.-M.; Zhou, C.-H. Potential antimicrobial isopropanol-conjugated carbazole azoles as dual targeting inhibitors of Enterococcus Faecalis. ACS Med. Chem. Lett. 2018, 9, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.S.; Chandrasekaran, B.; Palkar, M.B.; Kanhed, A.M.; Kajee, A.; Mlisana, K.P.; Singh, P.; Ghai, M.; Cleopus Mahlalela, M.; Karpoormath, R. Synthesis and biological evaluation of novel carbazole hybrids as promising antimicrobial agents. Chem. Biodivers. 2020, 17, e1900550. [Google Scholar] [CrossRef] [PubMed]
- Meriç, Ç.; Kanbur, S.; Baycan, F. Side chain functional carbazole-fluorene electroactive polymers: Optical, electrochemical properties, antimicrobial activity and thin film morphologies. J. Appl. Polym. Sci. 2021, 138, 50325. [Google Scholar] [CrossRef]
- Lin, S.; Liu, J.; Li, H.; Liu, Y.; Chen, Y.; Luo, J.; Liu, S. Development of highly potent carbazole amphiphiles as membrane-targeting antimicrobials for treating gram-positive bacterial infections. J. Med. Chem. 2020, 63, 9284–9299. [Google Scholar] [CrossRef]
- Bordei Telehoiu, A.T.; Nuță, D.C.; Căproiu, M.T.; Dumitrascu, F.; Zarafu, I.; Ioniță, P.; Bădiceanu, C.D.; Avram, S.; Chifiriuc, M.C.; Bleotu, C.; et al. Design, synthesis and in vitro characterization of novel antimicrobial agents based on 6-chloro-9h-carbazol derivatives and 1,3,4-oxadiazole scaffolds. Molecules 2020, 25, 266. [Google Scholar] [CrossRef]
- Hegden, P.R.; Emmanuel, B.D.; Beevi, J.; Dharan, S.S. In Silico Design, synthesis and biological evaluation of novel carbazole derivatives. J. Pharm. Chem. Res. 2021, 13, 8–18. [Google Scholar]
- Xue, Y.-J.; Li, M.-Y.; Jin, X.-J.; Zheng, C.-J.; Piao, H.-R. Design, synthesis and evaluation of carbazole derivatives as potential antimicrobial agents. J. Enzym. Inhib. Med. Chem. 2021, 36, 296–307. [Google Scholar] [CrossRef]
- Zawadzka, K.; Felczak, A.; Głowacka, I.E.; Piotrowska, D.G.; Lisowska, K. Evaluation of the antimicrobial potential and toxicity of a newly synthesised 4-(4-(benzylamino)butoxy)-9h-carbazole. Int. J. Mol. Sci. 2021, 22, 12796. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, S.A.; Patil, S.A.; Ble-González, E.A.; Isbel, S.R.; Hampton, S.M.; Bugarin, A. Carbazole Derivatives as Potential Antimicrobial Agents. Molecules 2022, 27, 6575. https://doi.org/10.3390/molecules27196575
Patil SA, Patil SA, Ble-González EA, Isbel SR, Hampton SM, Bugarin A. Carbazole Derivatives as Potential Antimicrobial Agents. Molecules. 2022; 27(19):6575. https://doi.org/10.3390/molecules27196575
Chicago/Turabian StylePatil, Siddappa A., Shivaputra A. Patil, Ever A. Ble-González, Stephen R. Isbel, Sydney M. Hampton, and Alejandro Bugarin. 2022. "Carbazole Derivatives as Potential Antimicrobial Agents" Molecules 27, no. 19: 6575. https://doi.org/10.3390/molecules27196575
APA StylePatil, S. A., Patil, S. A., Ble-González, E. A., Isbel, S. R., Hampton, S. M., & Bugarin, A. (2022). Carbazole Derivatives as Potential Antimicrobial Agents. Molecules, 27(19), 6575. https://doi.org/10.3390/molecules27196575







