Biological Efficacy of Compounds from Stingless Honey and Sting Honey against Two Pathogenic Bacteria: An In Vitro and In Silico Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Pathogenic Bacteria from Milk
2.2. Identification through the Morphological, Biochemical Test, and Molecular Technique
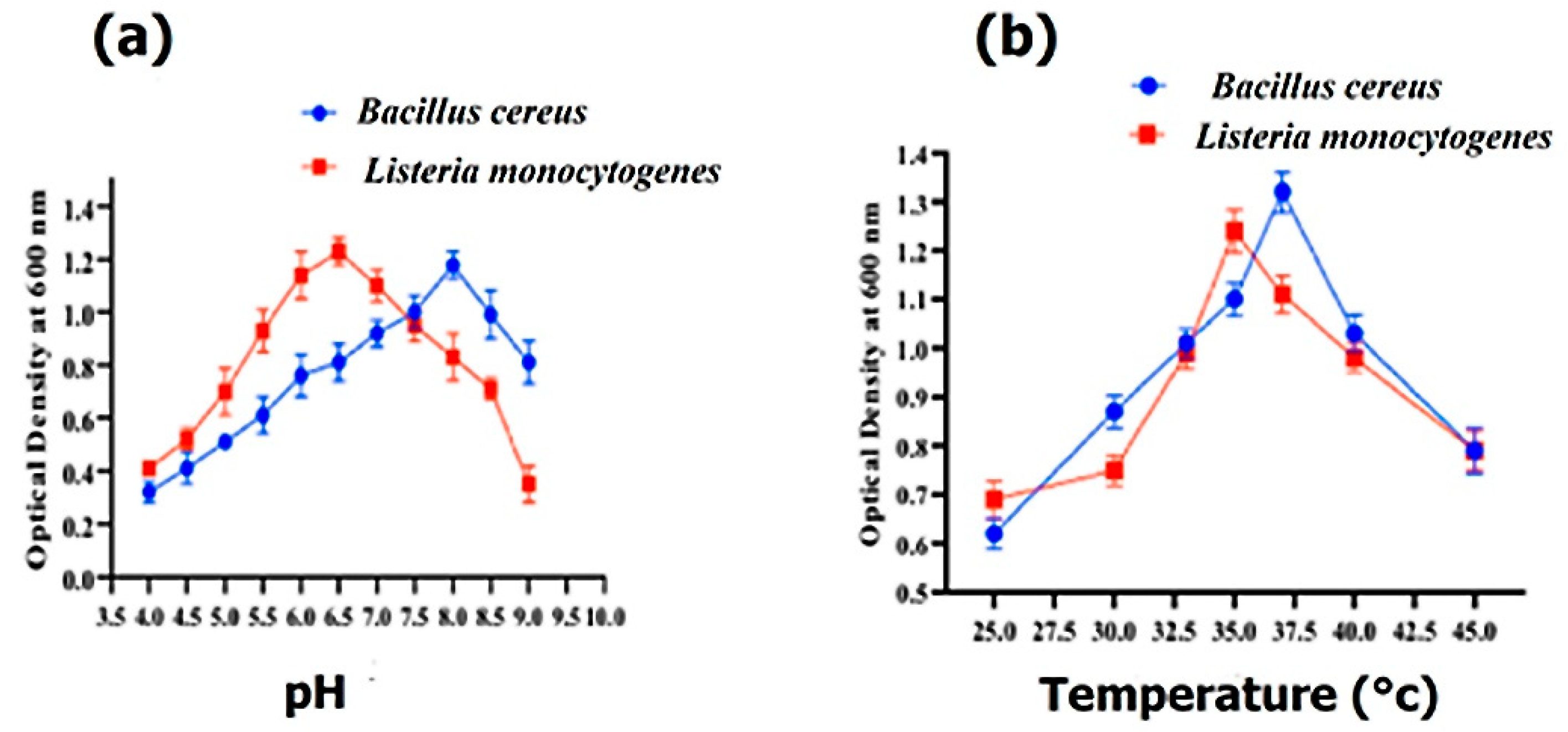
2.3. Role of pH and Temperature on Bacterial Growth
2.4. Antibiotic Sensitivity Test of Isolated Bacteria
2.5. Physicochemical Analysis of Honey
2.5.1. pH Measurement
2.5.2. Water Content
2.5.3. Hydroxymethyl Furfural (HMF)
2.5.4. Total Sugars
2.5.5. Reducing Sugars
2.5.6. Proline Content
2.6. Antimicrobial Test against the Isolated Pathogen
2.7. Assessment of Antimicrobial Activity
2.8. In Silico Experiment
2.8.1. Protein Preparation
2.8.2. Ligand Preparation
2.8.3. Molecular Docking
2.9. Molecular Dynamics Simulation
2.10. ADMET Analysis
3. Results
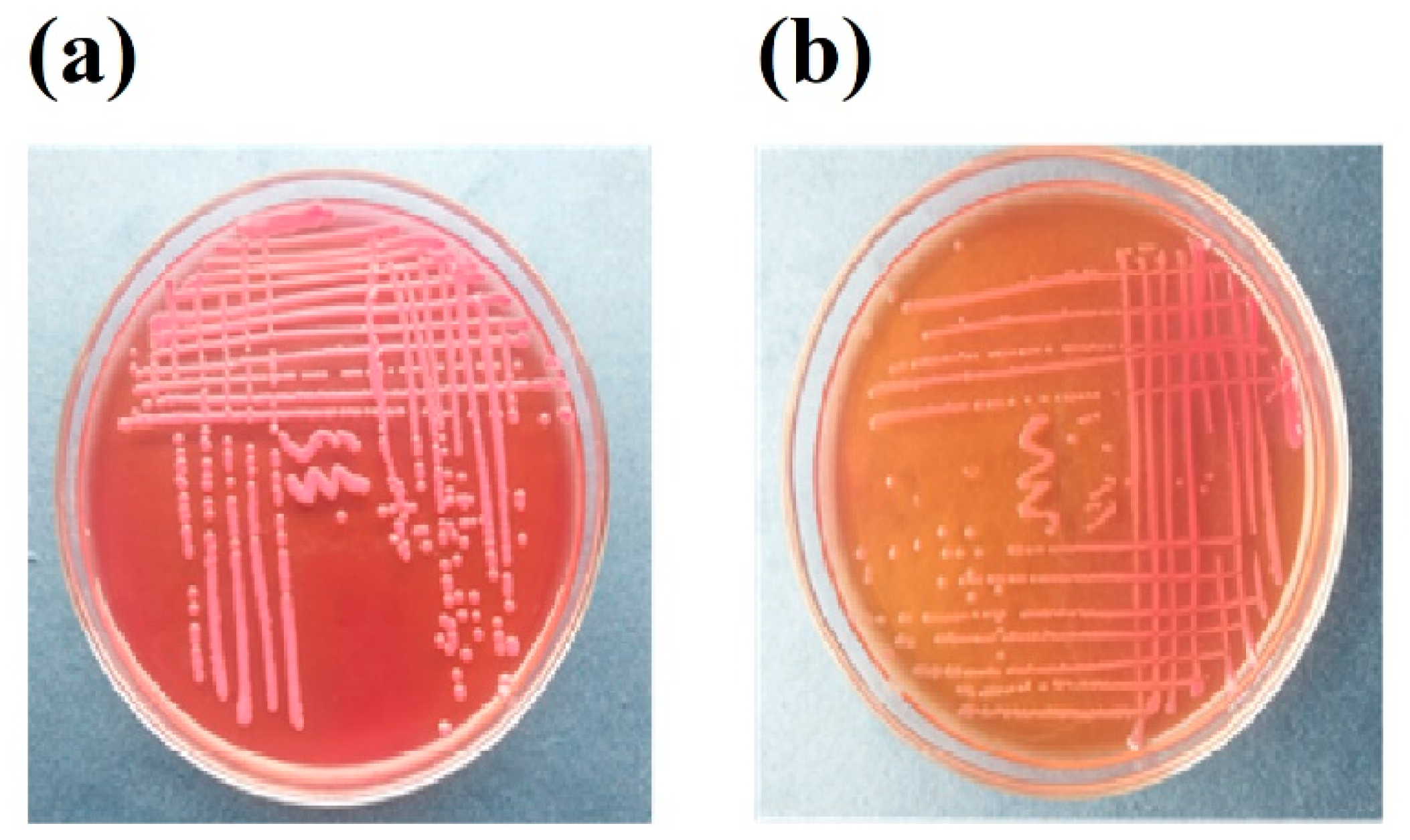
3.1. Isolation of Bacterial Strains on Selective Media
3.2. Identification of Bacterial Strains
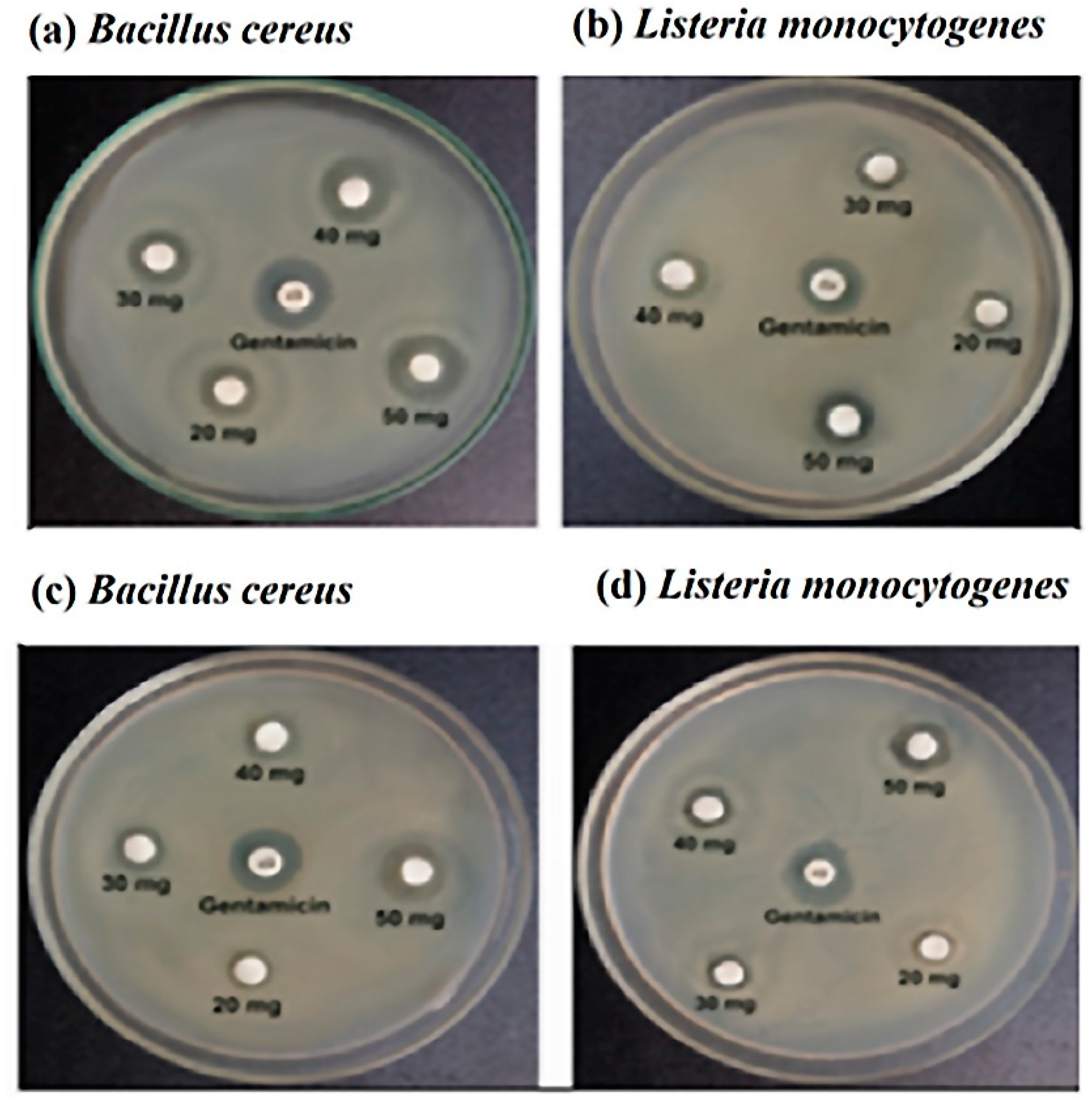
3.3. Antibiotic Sensitivity Test
3.4. Growth Characteristics
3.5. Physiochemical Characteristics of Honey Samples
3.6. Antibacterial Activity of Honey Samples against Bacillus Cereus and Listeria Monocytogenes
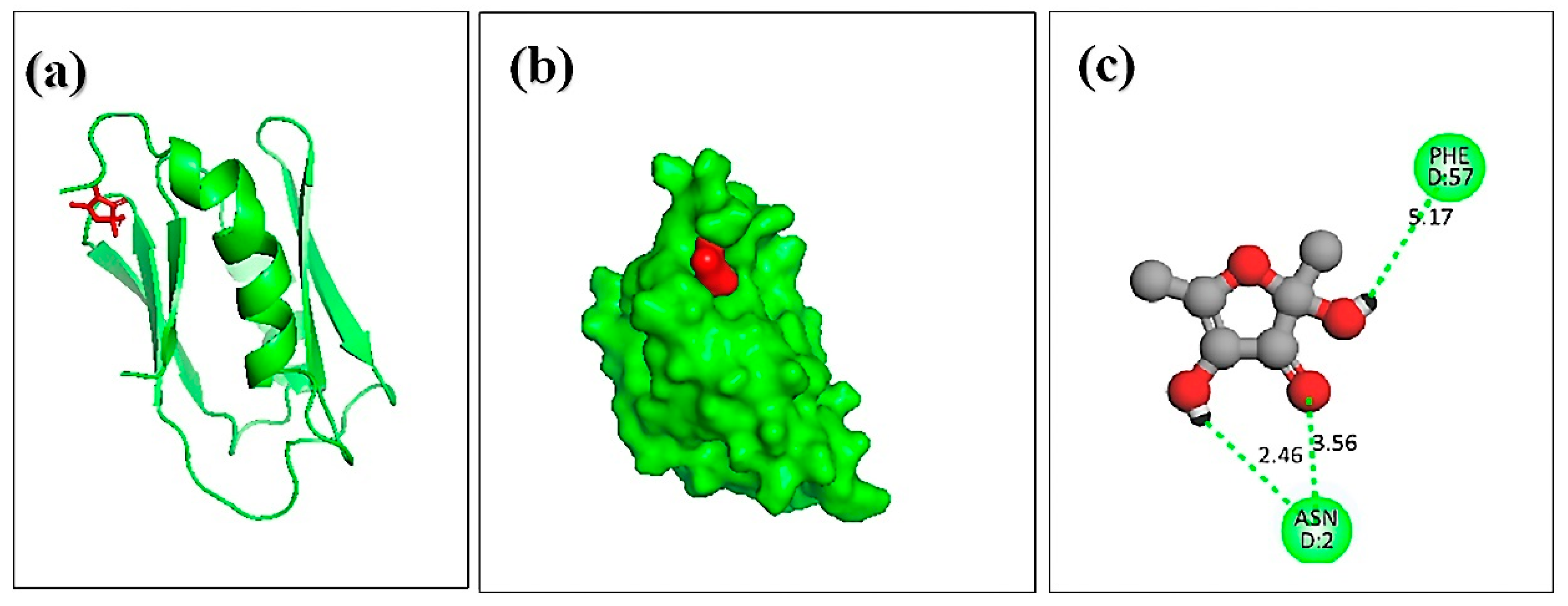
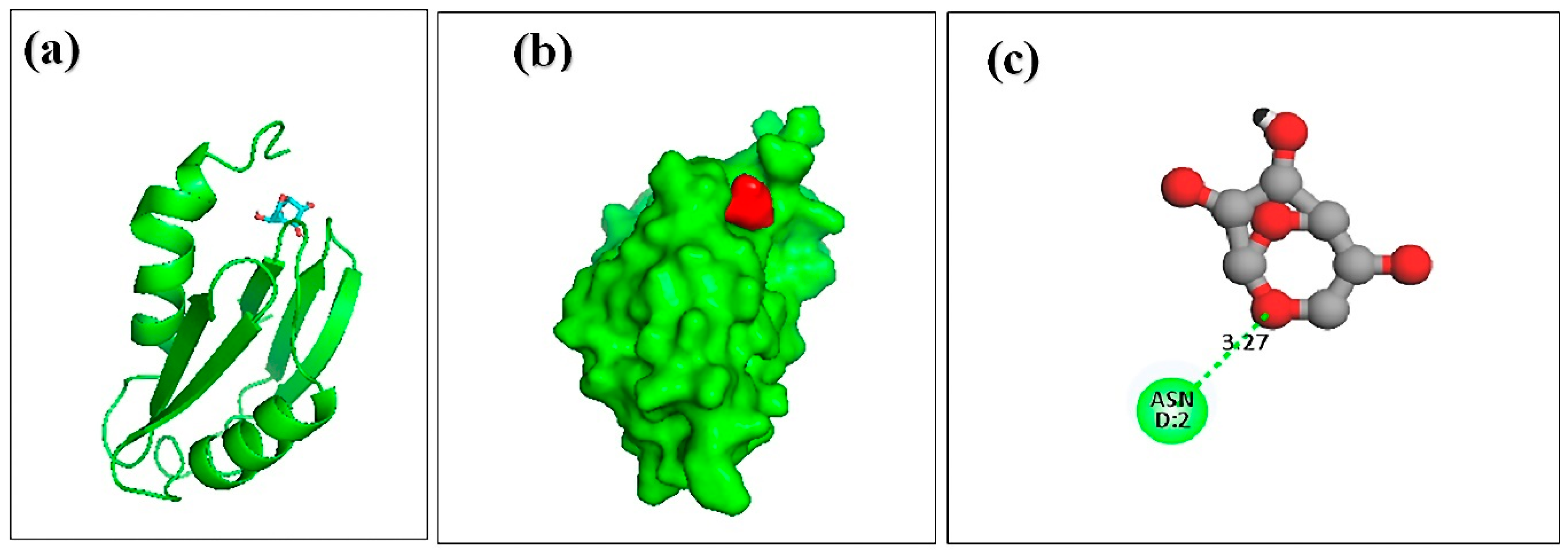
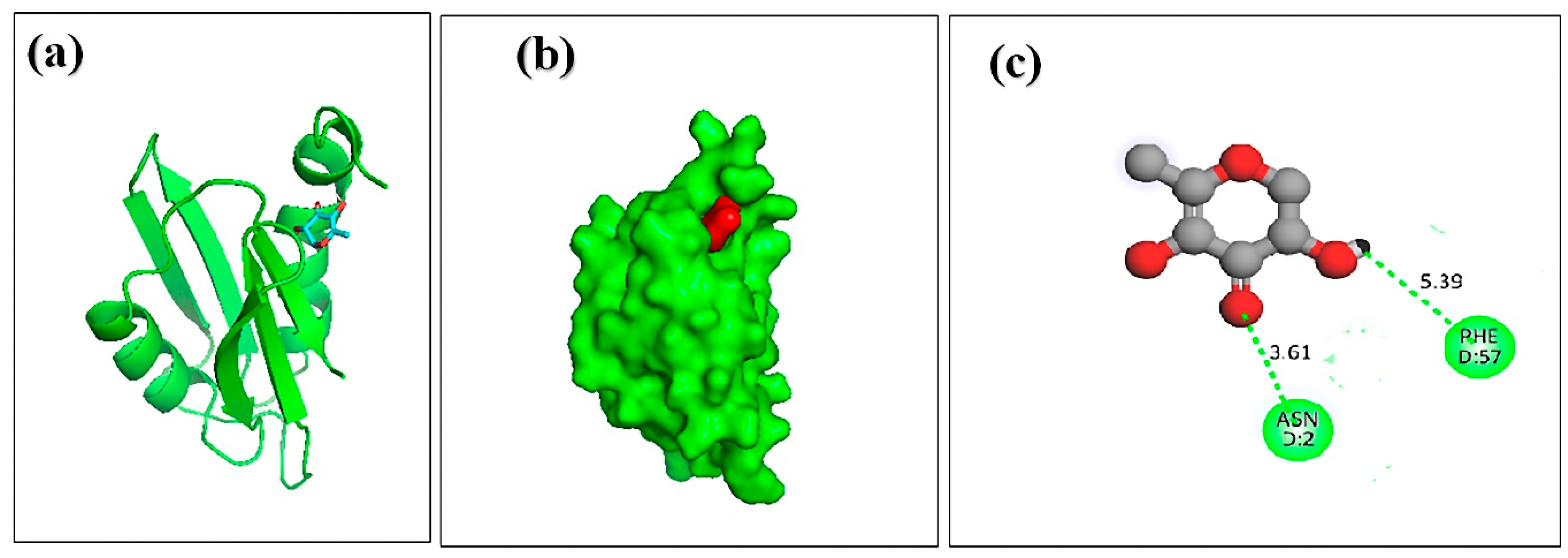
3.7. Molecular Docking of Honey Constituents against Hemolysin II (Protein) of Bacillus Cerecesus
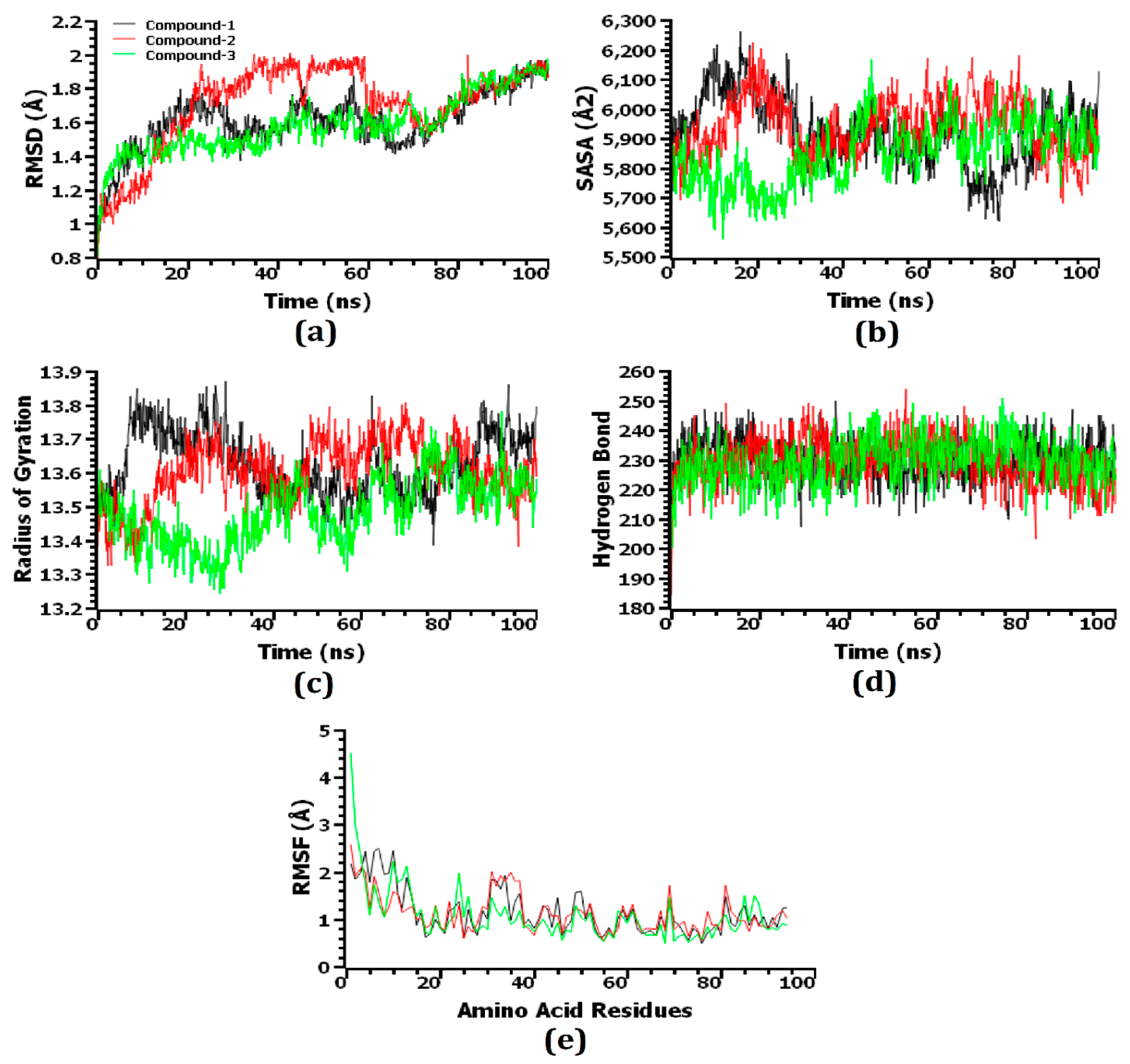
3.8. Molecular Dynamics Simulation
3.9. ADMET Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Molan, P.C. The antibacterial activity of honey: 1. The nature of the antibacterial activity. Bee World 1992, 73, 5–28. [Google Scholar] [CrossRef]
- Dixon, B. Bacteria can’t resist honey. Lancet Infect. Dis. 2003, 3, 116. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Péneau, S.; Mejean, C.; Szabo de Edelenyi, F.; Galan, P.; Hercberg, S.; Lairon, D. Profiles of organic food consumers in a large sample of French adults: Results from the Nutrinet-Sante cohort study. PLoS ONE 2013, 8, e76998. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, N.; Ugur, A.Y.S.E.L. Investigation of in vitro antimicrobial activity of honey. Riv. Biol. 2001, 94, 363–371. [Google Scholar]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Vincevica-Gaile, Z.; Klavins, M.; Rudovica, V.; Viksna, A. Geographical dissemination of trace and major elements in honey. Sustain. Today 2012, 167, 211–220. [Google Scholar]
- Gašić, U.M.; Milojković-Opsenica, D.M.; Tešić, Ž.L. Polyphenols as possible markers of botanical origin of honey. J. AOAC Int. 2017, 100, 852–861. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Ferreira-Santos, P.; Genisheva, Z.; El Ghouizi, A.; Aboulghazi, A.; Teixeira, J.A.; Lyoussi, B. Protective effect of honey and propolis against gentamicin-induced oxidative stress and hepatorenal damages. Oxidative Med. Cell. Longev. 2021, 2021, 9719906. [Google Scholar] [CrossRef]
- Mundo, M.A.; Padilla-Zakour, O.I.; Worobo, R.W. Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int. J. Food Microbiol. 2004, 97, 1–8. [Google Scholar] [CrossRef]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. Bactericidal activity of different honeys against pathogenic bacteria. Arch. Med. Res. 2005, 36, 464–467. [Google Scholar] [CrossRef]
- Lin, S.M.; Molan, P.C.; Cursons, R.T. The in vitro susceptibility of Campylobacter spp. to the antibacterial effect of manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.; Van den Akker, J.P.; Güçlü, A.; Aslami, H.; Binnekade, J.M.; de Boer, L.; Boszhard, L.; Paulus, F.; Middelhoek, P.; te Velde, A.A.; et al. Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin. Infect. Dis. 2008, 46, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.; te Velde, A.A.; de Boer, L.; Speijer, D.; Vandenbroucke-Grauls, C.M.; Zaat, S.A. How Honey Kill Bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Robson, V.; Dodd, S.; Thomas, S. Standardized antibacterial honey (Medihoney™) with standard therapy in wound care: Randomized clinical trial. J. Adv. Nurs. 2009, 65, 565–575. [Google Scholar] [CrossRef]
- Cooper, R.A.; Jenkins, L.; Henriques, A.F.M.; Duggan, R.S.; Burton, N.F. Absence of bacterial resistance to medical-grade manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1237–1241. [Google Scholar] [CrossRef]
- Hashem, H. In silico approach of some selected honey constituents as SARS-CoV-2 main protease (COVID-19) inhibitors. chemRxiv 2020. [Google Scholar] [CrossRef]
- Yosri, N.; Abd El-Wahed, A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.; et al. Anti-viral and immunomodulatory properties of propolis: Chemical diversity, pharmacological properties, preclinical and clinical applications, and in silico potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef]
- White, J.W., Jr.; Subers, M.H.; Schepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta BBA-Spec. Sect. Enzymol. Subj. 1963, 73, 57–70. [Google Scholar] [CrossRef]
- Molan, P. Why honey is effective as a medicine: 2. The scientific explanation of its effects. Bee World 2001, 82, 22–40. [Google Scholar] [CrossRef]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Alexopoulos, A.; Plessas, S.; Karapanou, A.; Mantzourani, I.; Stavropoulou, E.; Fotou, K.; Tzora, A.; Skoufos, I.; Bezirtzoglou, E. Antibacterial activity of different honeys against pathogenic bacteria. Anaerobe 2011, 17, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Lusby, P.E.; Coombes, A.; Wilkinson, J.M. Honey: A potent agent for wound healing? J. WOCN 2002, 29, 295–300. [Google Scholar] [CrossRef]
- Wen, Q.; McClane, B.A. Detection of enterotoxigenic Clostridium perfringens type A isolates in American retail foods. Appl. Environ. Microbiol. 2004, 70, 2685–2691. [Google Scholar] [CrossRef]
- Stach, J.E.; Bathe, S.; Clapp, J.P.; Burns, R.G. PCR-SSCP comparison of 16S rDNA sequence diversity in soil DNA obtained using different isolation and purification methods. FEMS Microbiol. Ecol. 2001, 36, 139–151. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; No. BOOK; Association of Official Analytical Chemists: Rockville, MD, USA, 1990. [Google Scholar]
- Bogdanov, S.; Martin, P.; Lullmann, C. Harmonised methods of the international honey commission. Swiss Bee Res. Cent. FAM Liebefeld 2002, 5, 1–62. [Google Scholar]
- International Honey Commission. Harmonised Methods of the International Honey Commission. 2009. Available online: http://www.bee-hexagon.net/en/network.htm (accessed on 15 February 2022).
- Nombré, I.; Schweitzer, P.; Boussim, J.I.; Rasolodimby, J.M. Impacts of storage conditions on physicochemical characteristics of honey samples from Burkina Faso. Afr. J. Food Sci. 2010, 4, 458–463. [Google Scholar]
- Oddo, L.P.; Piazza, M.G.; Pulcini, P. Invertase activity in honey. Apidologie 1999, 30, 57–65. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Millogo, J.; Romito, M.; Nacoulma, O.G. Physiochemical analyses of Burkina Fasan honey. Acta Vet. Brno 2005, 74, 147–152. [Google Scholar] [CrossRef]
- Ough, C.S. Rapid determination of proline in grapes and wines. J. Food Sci. 1969, 34, 228–230. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, K.C.; Truck, M. Antibiotic susceptibility testing by a standardized single disk method. Amer. Clin. Path 1966, 45, 493–496. [Google Scholar]
- Taormina, R.J. Organizational socialization: The missing link between employee needs and organizational culture. J. Manag. Psychol. 2009, 24, 650–676. [Google Scholar] [CrossRef]
- Akmar, S.L.; Ansari, M.; Berahim, Z.; Shahidan, W.N.S. Phytochemical compound and non-cytotoxicity effect of sting bee and stingless bee honey against normal human gingival cell lines. Bangladesh J. Med. Sci. 2022, 21, 158–164. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, D.K.; Lee, J.; Kim, Y.I.; Seo, J.M.; Kim, Y.G.; Jeong, J.H.; Kim, M.; Kim, J.I.; Kim, P.; et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Crawford, B.; Kasmidi, M.; Korompis, F.; Pollnac, R.B. Factors influencing progress in establishing community-based marine protected areas in Indonesia. Coast. Manag. 2006, 34, 39–64. [Google Scholar] [CrossRef]
- Nath, A.; Kumer, A.; Zaben, F.; Khan, M.W. Investigating the binding affinity, molecular dynamics, and ADMET properties of 2, 3-dihydrobenzofuran derivatives as an inhibitor of fungi, bacteria, and virus protein. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A tool to obtain structural guidance in biocatalytic investigations. In Protein Engineering; Humana Press: New York, NY, USA, 2018; pp. 43–67. [Google Scholar]
- Krieger, E.; Vriend, G.; Spronk, C. YASARA–yet another scientific artificial reality application. YASARA Org. 2013, 993, 51–78. [Google Scholar]
- Wang, W.R.; Wolf, R.J.; Caldwell, J.W.; Kollman, P.A. Case. DA J. Comput. Chem. 2014, 25, 92. [Google Scholar]
- Mahmud, S.; Biswas, S.; Paul, G.K.; Mita, M.A.; Promi, M.M.; Afrose, S.; Hasan, M.R.; Zaman, S.; Uddin, M.S.; Dhama, K.; et al. Plant-based phytochemical screening by targeting main protease of SARS-CoV-2 to design effective potent inhibitors. Biology 2021, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Harrach, M.F.; Drossel, B. Structure and dynamics of TIP3P, TIP4P, and TIP5P water near smooth and atomistic walls of different hydroaffinity. J. Chem. Phys. 2014, 140, 174501. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.K.; Mahmud, S.; Aldahish, A.A.; Afroze, M.; Biswas, S.; Gupta, S.B.; Razu, M.H.; Zaman, S.; Uddin, M.S.; Nahari, M.H.; et al. Computational screening and biochemical analysis of Pistacia integerrima and Pandanus odorifer plants to find effective inhibitors against Receptor-Binding domain (RBD) of the spike protein of SARS-Cov-2. Arab. J. Chem. 2022, 15, 103600. [Google Scholar] [CrossRef] [PubMed]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Krieger, E.; Nielsen, J.E.; Spronk, C.A.; Vriend, G. Fast empirical pKa prediction by Ewald summation. J. Mol. Graph. Model. 2006, 25, 481–486. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. New ways to boost molecular dynamics simulations. J. Comput. Chem. 2015, 36, 996–1007. [Google Scholar] [CrossRef]
- Afrose, S.; Hasan, R.; Sharmin, M.; Shimu, S. Antiviral peptides against the main protease of SARS-CoV-2: A molecular docking and dynamics study. Arab. J. Chem 2021, 14, 103315. [Google Scholar]
- Mahfuz, A.M.U.B.; Khan, M.A.; Biswas, S.; Afrose, S.; Mahmud, S.; Bahadur, N.M.; Ahmed, F. In search of novel inhibitors of anti-cancer drug target fibroblast growth factor receptors: Insights from virtual screening, molecular docking, and molecular dynamics. Arab. J. Chem. 2022, 15, 103882. [Google Scholar] [CrossRef]
- Mahmud, S.; Hasan, M.R.; Biswas, S.; Paul, G.K.; Afrose, S.; Mita, M.A.; Sultana Shimu, M.S.; Promi, M.M.; Hani, U.; Rahamathulla, M.; et al. Screening of Potent Phytochemical Inhibitors Against SARS-CoV-2 Main Protease: An Integrative Computational Approach. Front. Bioinform. 2021, 1, 717141. [Google Scholar] [CrossRef]
- Mahmud, S.; Mita, M.A.; Biswas, S.; Paul, G.K.; Promi, M.M.; Afrose, S.; Hasan, R.; Shimu, S.S.; Zaman, S.; Uddin, S.; et al. Molecular docking and dynamics study to explore phytochemical ligand molecules against the main protease of SARS-CoV-2 from extensive phytochemical datasets. Expert Rev. Clin. Pharmacol. 2021, 14, 1305–1315. [Google Scholar] [CrossRef]
- Mahmud, S.; Rafi, M.; Paul, G.K.; Promi, M.M.; Shimu, M.; Sultana, S.; Biswas, S.; Emran, T.B.; Dhama, K.; Alyami, S.A.; et al. Designing a multi-epitope vaccine candidate to combat MERS-CoV by employing an immunoinformatics approach. Sci. Rep. 2021, 11, 15431. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Paul, G.K.; Biswas, S.; Afrose, S.; Mita, M.A.; Hasan, M.R.; Shimu, M.S.; Hossain, A.; Promi, M.M.; Ema, F.K.; et al. Prospective role of peptide-based antiviral therapy against the main protease of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 628585. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Paul, G.K.; Afroze, M.; Islam, S.; Gupt, S.B.; Razu, M.H.; Biswas, S.; Zaman, S.; Uddin, M.S.; Khan, M.; et al. Efficacy of phytochemicals derived from Avicennia officinalis for the management of COVID-19: A combined in silico and biochemical study. Molecules 2021, 26, 2210. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Mahmud, S.; Mita, M.A.; Afrose, S.; Hasan, M.R.; Shimu, M.S.; Saleh, M.A.; Mostafa-Hedeab, G.; Alqarni, M.; Obaidullah, A.J.; et al. Molecular Docking and Dynamics Studies to Explore Effective Inhibitory Peptides Against the Spike Receptor Binding Domain of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 791642. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness, and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Lannigan, R. Mechanism of honey bacteriostatic action against MRSA and VRE involves hydroxyl radicals generated from honey’s hydrogen peroxide. Front. Microbiol. 2012, 3, 36. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Deans, S.G. Bioactivity of selected plant essential oils against Listeria monocytogenes. J. Appl. Microbiol. 1997, 82, 759–762. [Google Scholar] [CrossRef]
- Laallam, H.; Boughediri, L.; Bissati, S.; Menasria, T.; Mouzaoui, M.S.; Hadjadj, S.; Hammoudi, R.; Chenchouni, H. Modeling the synergistic antibacterial effects of honey characteristics of different botanical origins from the Sahara Desert of Algeria. Front. Microbiol. 2015, 6, 1239. [Google Scholar] [CrossRef]
- Khan, S.N. Macrophomina phaseolina as causal agent for charcoal rot of sunflower. Mycopath 2007, 5, 111–118. [Google Scholar]
- Allen, J.D.; Martin, G.P.; Marriott, C.; Hassan, I.; Williamson, I. Drug transport across a novel mucin secreting cell model: Comparison with the Caco-2 cell system. J. Pharm. Pharmacol. 1991, 43, 63P. [Google Scholar]
- Zaika, L.L. Spices and herbs: Their antimicrobial activity and its determination. J. Food Saf. 1988, 9, 97–118. [Google Scholar] [CrossRef]
- Rudenko, N.; Nagel, A.; Zamyatina, A.; Karatovskaya, A.; Salyamov, V.; Andreeva-Kovalevskaya, Z.; Siunov, A.; Kolesnikov, A.; Shepelyakovskaya, A.; Boziev, K.; et al. A monoclonal antibody against the C-terminal domain of Bacillus cereus hemolysin II inhibits HlyII cytolytic activity. Toxins 2020, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.; Siunov, A.; Zamyatina, A.; Melnik, B.; Nagel, A.; Karatovskaya, A.; Borisova, M.; Shepelyakovskaya, A.; Andreeva-Kovalevskaya, Z.; Kolesnikov, A.; et al. The C-terminal domain of Bacillus cereus hemolysin II oligomerizes by itself in the presence of cell membranes to form ion channels. Int. J. Biol. Macromol. 2022, 200, 416–427. [Google Scholar] [CrossRef]
- Tran, S.L.; Guillemet, E.; Ngo-Camus, M.; Clybouw, C.; Puhar, A.; Moris, A.; Gohar, M.; Lereclus, D.; Ramarao, N. Haemolysin II is a Bacillus cereus virulence factor that induces apoptosis of macrophages. Cell. Microbiol. 2011, 13, 92–108. [Google Scholar] [CrossRef]
- Ojatula, A.O. Developmental botanic: A case study on chromatographic determination of phytocompounds in the roots of anthocleista nobilis (G. Don.) as pro-fertility. J. Infertil. Reprod. Biol. 2021, 9, 7–21. [Google Scholar]
- Kuś, P.M.; Czabaj, S.; Jerković, I. Comparison of Volatile Profiles of Meads and Related Unifloral Honeys: Traceability Markers. Molecules 2022, 27, 4558. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. Review of the medicinal effects of tualang honey and a comparison with manuka honey. Malays. J. Med. Sci. MJMS 2013, 20, 6. [Google Scholar]
- Nik Man, N.M.K.; Hassan, R.; Ang, C.Y.; Abdullah, A.D.; Mohd Radzi, M.A.R.; Sulaiman, S.A. Antileukemic effect of tualang honey on acute and chronic leukemia cell lines. BioMed Res. Int. 2015, 2015, 307094. [Google Scholar] [CrossRef]
- Nurul, S.M.S.; Gan, S.H.; Halim, A.S.; Shah, N.S.M.; Sukari, H.A. Analysis of volatile compounds of Malaysian Tualang (Koompassia excelsa) honey using gas chromatography mass spectrometry. Afr. J. Tradit. Complementary Altern. Med. 2013, 10, 180–188. [Google Scholar] [CrossRef]
- Xu, S.; Butkevich, A.N.; Yamada, R.; Zhou, Y.; Debnath, B.; Duncan, R.; Zandi, E.; Petasis, N.A.; Neamati, N. Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proc. Natl. Acad. Sci. USA 2012, 109, 16348–16353. [Google Scholar] [CrossRef] [PubMed]
| Isolate A | Isolate B | |
|---|---|---|
| Gram Staining | + | + |
| Motility test | + | + |
| Indole test | - | - |
| Urease hydrolysis test | + | - |
| TSI | + | - |
| Methyl red test | - | + |
| Citrate test | + | - |
| Catalase test | + | + |
| Eosin methylene blue (EMB) agar test | - | + |
| Mannitol agar test | - | - |
| Starch hydrolysis test | + | - |
| Antibiotics | Bacillus Cereus | Listeria Monocytogenes | ||
|---|---|---|---|---|
| Zone of Inhibition (mm) | Resistance Pattern | Zone of Inhibition (mm) | Resistance Pattern | |
| Penicillin G | 7 ± 0.52 | R | 8 ± 0.34 | R |
| Ampicillin | 7.5 ± 0.48 | R | 7 ± 0.65 | R |
| Amoxicillin, | 6 ± 0.67 | R | 17 ± 0.76 | S |
| Ciprofloxacin | 16 ± 0.43 | S | 8 ± 0.71 | R |
| Chloramphenicol | 16 ± 0.56 | S | 9 ± 0.81 | R |
| Erythromycin | 18 ± 0.72 | S | 8 ± 0.29 | R |
| Gentamycin | 18 ± 0.59 | S | 6 ± 0.34 | R |
| Tetracycline | 19 ± 0.39 | S | 13 ± 0.32 | I |
| Parameters | Stingless Honey | Sting Honey |
|---|---|---|
| Color | Blackish | Light Black |
| pH | 4.35 ± 0.52 | 4.56 ± 0.62 |
| Water content (%) | 18 ± 0.76 | 16 ± 0.67 |
| Hydroxy methyl furfural (mg/kg) | 123.05 ± 1.54 | 94.76 ± 2.01 |
| Total sugars (%) | 87.34 ± 1.34 | 81.09 ± 2.09 |
| Reducing sugars (%) | 77.84 ± 1.32 | 67.10 ± 1.82 |
| Proline (mg/kg) | 833.02 ± 2.89 | 783.76 ± 2.91 |
| Bacteria | Dose (mg/Disc) | Stingless Honey | Sting Honey |
|---|---|---|---|
| Zone of Inhibition (mm) | Zone of Inhibition (mm) | ||
| Bacillus cereus | 20 | 10 ± 0.67 | 7.5 ± 0.60 |
| 30 | 11 ± 0.76 | 9 ± 0.39 | |
| 40 | 13 ± 0.54 | 9.5 ± 0.40 | |
| 50 | 14 ± 0.89 | 12 ± 0.52 | |
| Gentamycin (10 µg) | 15 ± 0.91 | 16 ± 0.74 | |
| Listeria monocytogenes | 20 | 7.5 ± 0.29 | 7 ± 0.41 |
| 30 | 8.5 ± 0.76 | 8 ± 0.42 | |
| 40 | 10 ± 0.89 | 9.5 ± 0.48 | |
| 50 | 12 ± 0.54 | 11 ± 0.51 | |
| Gentamycin (10 µg) | 13 ± 0.49 | 14 ± 0.21 |
| SI NO | Compound | Docking Score | Hydrogen Bond | |
|---|---|---|---|---|
| Residues | Distance (A°) | |||
| 1. | 2,4-dihydroxy-2,5-dimethyl 3(2H)-furan-3-one (furan) | –5.4 | PHE-57 ASN-2 ASN-2 | 5.17 3.56 2.46 |
| 2. | beta.-D-glucopyranose,1,6-anhydro | –5.2 | PHE-57 ASN-2 | 5.39 3.61 |
| 3. | 4H-pyran-4-one,2,3-dihydro 3,5-dihydroxy-6-methyl | –4.8 | ASN-2 | 3.27 |
| Parameters | 2,4-Dihydroxy-2,5-Dimethyl 3(2H)-Furan-3-One (Furan) | Beta.-D-Glucopyranose,1,6-Anhydro | 4H-Pyran-4-One,2,3-Dihydro 3,5-Dihydroxy-6-Methyl |
|---|---|---|---|
| Molecular weight | 144.13 g/mol | 162.14 g/mol | 144.13 g/mol |
| Num. H-bond acceptors | 4 | 5 | 4 |
| Num. H-bond donors | 2 | 3 | 2 |
| TPSA (S) | 66.76 Ų | 79.15 Ų | 66.76 Ų |
| BBB permeability | No | No | |
| Human intestinal absorption | High | High | High |
| P-glycoprotein substrate | No | No | No |
| Lipinski rule of five | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.; Pramanik, M.J.; Biswas, S.; Moniruzzaman, M.; Biswas, J.; Akhtar-E-Ekram, M.; Zaman, S.; Uddin, M.S.; Saleh, M.A.; Hassan, S. Biological Efficacy of Compounds from Stingless Honey and Sting Honey against Two Pathogenic Bacteria: An In Vitro and In Silico Study. Molecules 2022, 27, 6536. https://doi.org/10.3390/molecules27196536
Islam S, Pramanik MJ, Biswas S, Moniruzzaman M, Biswas J, Akhtar-E-Ekram M, Zaman S, Uddin MS, Saleh MA, Hassan S. Biological Efficacy of Compounds from Stingless Honey and Sting Honey against Two Pathogenic Bacteria: An In Vitro and In Silico Study. Molecules. 2022; 27(19):6536. https://doi.org/10.3390/molecules27196536
Chicago/Turabian StyleIslam, Shirmin, Mohammad Joy Pramanik, Suvro Biswas, Mohammad Moniruzzaman, Jui Biswas, Mohammad Akhtar-E-Ekram, Shahriar Zaman, Mohammad Salah Uddin, Mohammad Abu Saleh, and Sabry Hassan. 2022. "Biological Efficacy of Compounds from Stingless Honey and Sting Honey against Two Pathogenic Bacteria: An In Vitro and In Silico Study" Molecules 27, no. 19: 6536. https://doi.org/10.3390/molecules27196536
APA StyleIslam, S., Pramanik, M. J., Biswas, S., Moniruzzaman, M., Biswas, J., Akhtar-E-Ekram, M., Zaman, S., Uddin, M. S., Saleh, M. A., & Hassan, S. (2022). Biological Efficacy of Compounds from Stingless Honey and Sting Honey against Two Pathogenic Bacteria: An In Vitro and In Silico Study. Molecules, 27(19), 6536. https://doi.org/10.3390/molecules27196536







