Lichen Extracts from Cetrarioid Clade Provide Neuroprotection against Hydrogen Peroxide-Induced Oxidative Stress
Abstract
1. Introduction
2. Results
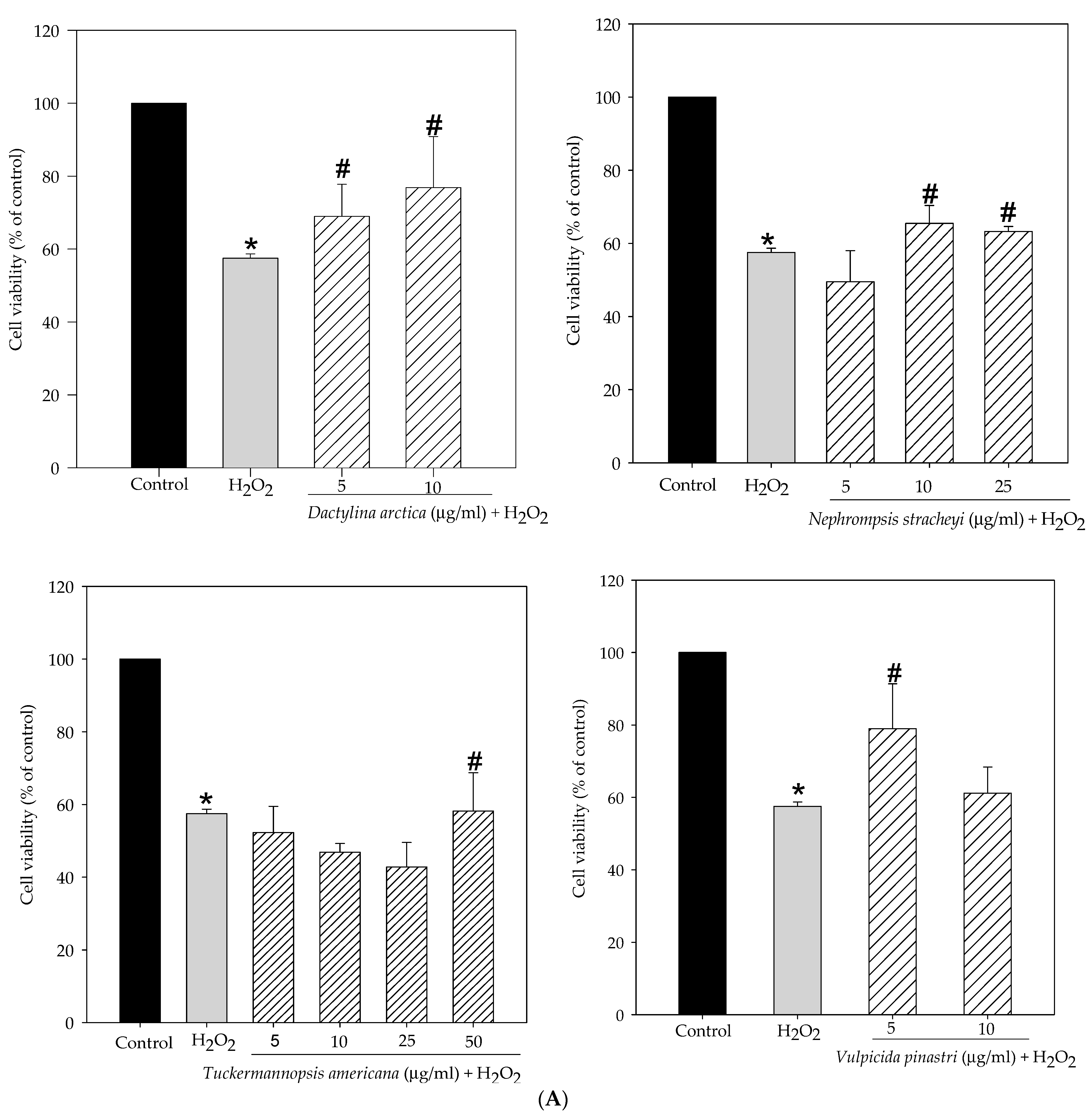
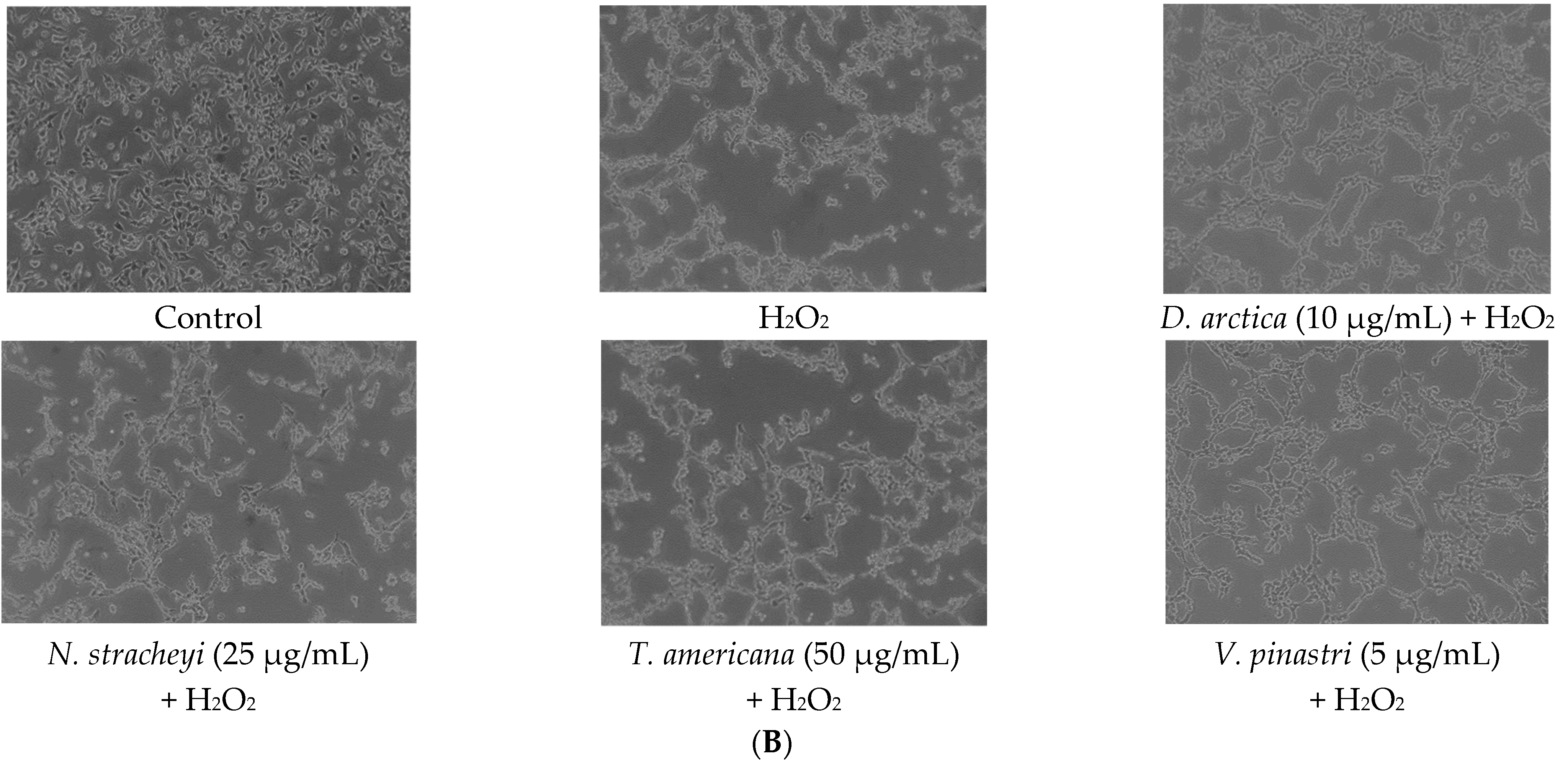
2.1. Lichen Extracts from Cetrarioid Clade Promoted Neuronal Survival after H2O2-Induced Oxidative Stress
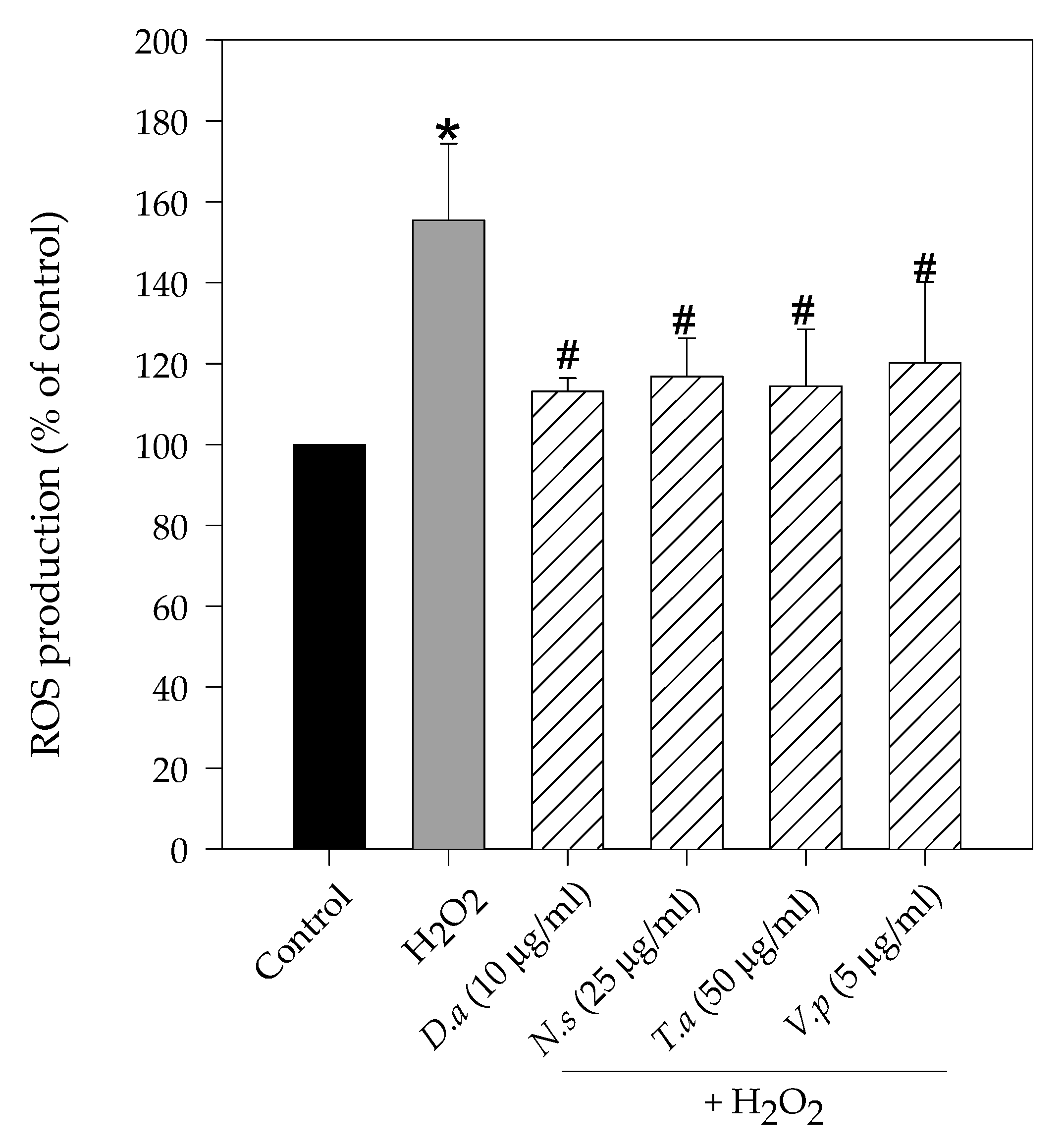
2.2. Lichen Extracts from Cetrarioid Clade Reduced ROS Production after H2O2-Induced Oxidative Stress
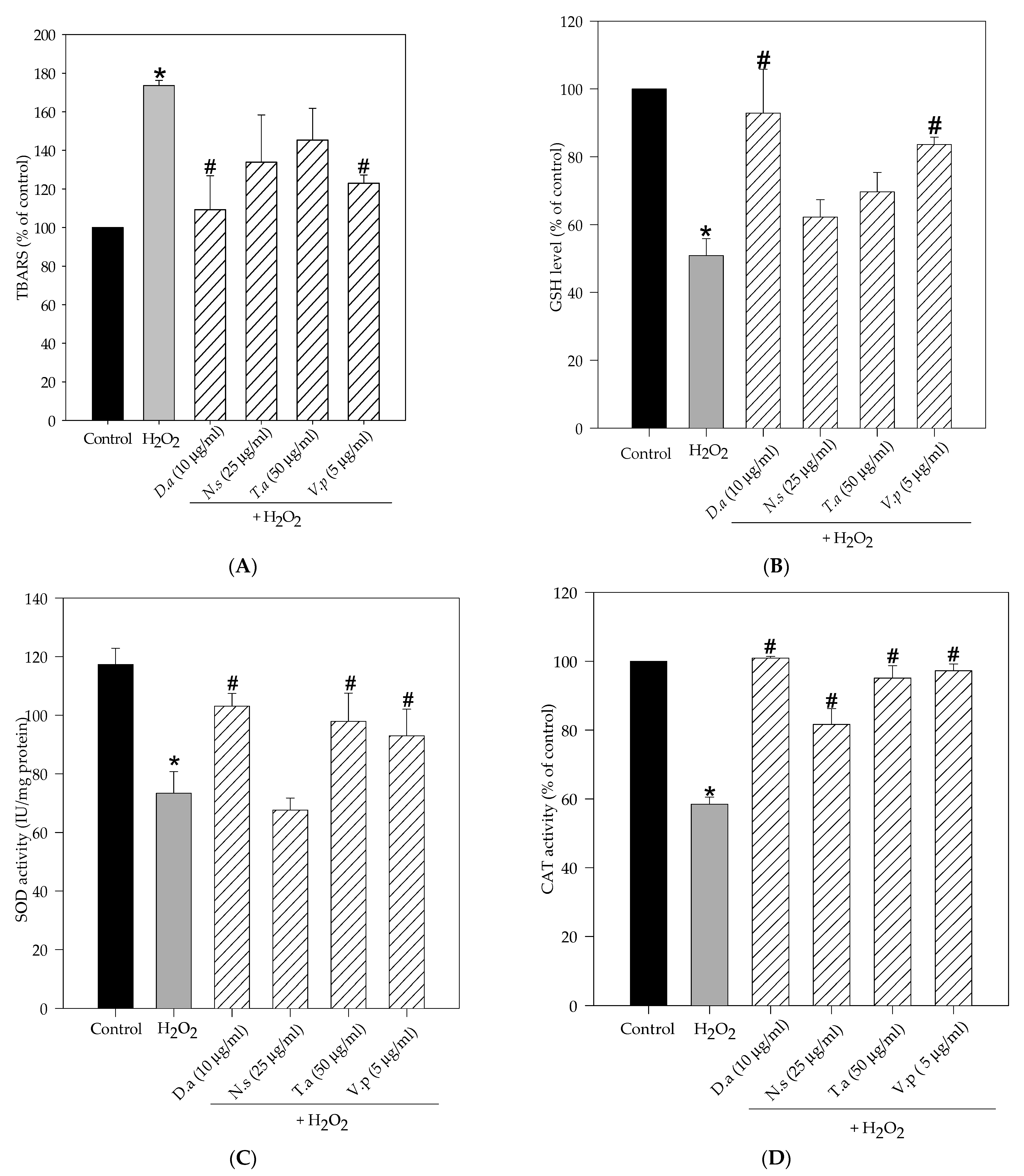
2.3. Lichen Extracts from Cetrarioid Clade Improved Oxidative Stress Markers and Antioxidant Enzyme Activity
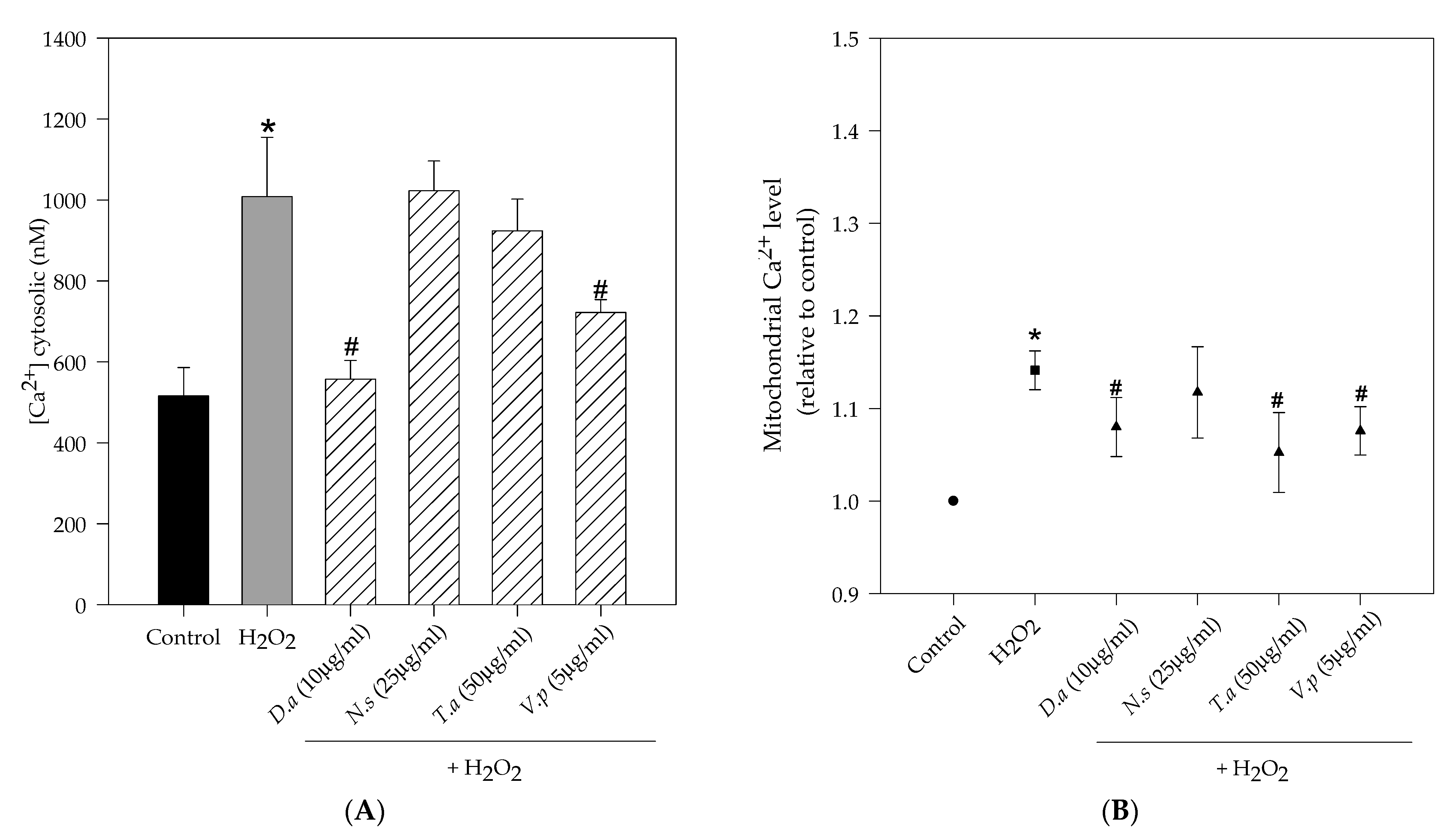
2.4. Lichen Extracts from Cetrarioid Clade Protected against H2O2-Induced Mitochondrial Dysfunction
2.5. HPLC Profile of Lichen Extracts from Cetrarioid Clade
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Lichen Collection and Preparation of Methanol Extracts
4.3. Human Neuroblastoma Cell Line (SH-SY5Y Cells)
4.4. Cell Treatments
4.5. Metabolic Activity Measurement
4.6. Intracellular ROS Production
4.7. BCA Assay
4.8. Glutathione Levels
4.9. Antioxidant Enzymatic Activity
4.9.1. SOD Enzymatic Activity
4.9.2. CAT Activity
4.10. TBARS Assay
4.11. Calcium Cytosolic Quantification
4.12. Mitochondrial Calcium Quantification
4.13. Mitochondrial Membrane Potential (MMP)
4.14. Secondary Metabolites Detection Using High Performance Liquid Chromatography
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Gottfredsen, R.H.; Larsen, U.G.; Enghild, J.J.; Petersen, S.V. Hydrogen peroxide induce modifications of human extracellular superoxide dismutase that results in enzyme inhibition. Redox Biol. 2013, 1, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Zhao, Z. Iron and oxidizing species in oxidative stress and Alzheimer’s disease. Aging Med. 2019, 2, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. Bcl-2 family proteins as targets for anticancer drug design. Oncogene 2000, 19, 6627–6631. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Neuroprotective activity and cytotoxic potential of two Parmeliaceae lichens: Identification of active compounds. Phytomedicine 2015, 22, 847–855. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; González-Burgos, E.; De Vita, S.; Divakar, P.K.; Bifulco, G.; Gómez-Serranillos, M.P. Phytochemical Characterization and Pharmacological Properties of Lichen Extracts from Cetrarioid Clade by Multivariate Analysis and Molecular Docking. Evid.-Based Complement. Altern. Med. 2022, 2022, 5218248. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M.K. Mechanisms of Cell Death in Oxidative Stress. Antioxid. Redox Signal. 2006, 9, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Ofoedu, C.E.; You, L.; Osuji, C.M.; Iwouno, J.O.; Kabuo, N.O.; Ojukwu, M.; Agunwah, I.M.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Hydrogen Peroxide Effects on Natural-Sourced Polysacchrides: Free Radical Formation/Production, Degradation Process, and Reaction Mechanism-A Critical Synopsis. Foods 2021, 10, 699. [Google Scholar] [CrossRef]
- Bay, M.V.; Nam, P.C.; Quang, D.T.; Mechler, A.; Hien, N.K.; Hoa, N.T.; Vo, Q.V. Theoretical Study on the Antioxidant Activity of Natural Depsidones. ACS Omega 2020, 5, 7895–7902. [Google Scholar] [CrossRef]
- Hoa, N.T.; Van Bay, M.; Mechler, A.; Vo, Q.V. Is Usnic Acid a Promising Radical Scavenger? ACS Omega 2020, 5, 17715–17720. [Google Scholar] [CrossRef]
- Le Roux, A.; Kuzmanovski, I.; Habrant, D.; Meunier, S.; Bischoff, P.; Nadal, B.; Thetiot-Laurent, S.A.L.; Le Gall, T.; Wagner, A.; Novič, M. Design and Synthesis of New Antioxidants Predicted by the Model Developed on a Set of Pulvinic Acid Derivatives. J. Chem. Inf. Model. 2011, 51, 3050–3059. [Google Scholar] [CrossRef]
- Angelova, P.R.; Esteras, N.; Abramov, A.Y. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med. Res. Rev. 2021, 41, 770–784. [Google Scholar] [CrossRef]
- Lin, M.C.; Liu, C.C.; Lin, Y.C.; Liao, C.S. Resveratrol Protects against Cerebral Ischemic Injury via Restraining Lipid Peroxidation, Transition Elements, and Toxic Metal Levels, but Enhancing Anti-Oxidant Activity. Antioxidants 2021, 10, 1515. [Google Scholar] [CrossRef]
- Yoshida, Y.; Umeno, A.; Shichiri, M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J. Clin. Biochem. Nutr. 2013, 52, 9–16. [Google Scholar] [CrossRef]
- Leong, P.K.; Ko, P.M. Induction of the Glutathione Antioxidant Response/Glutathione Redox Cycling by Nutraceuticals: Mechanism of Protection against Oxidant-induced Cell Death. J. Nutraceuticals Food Sci. 2016, 1, 1. [Google Scholar]
- Salminen, L.E.; Paul, R.H. Oxidative stress and genetic markers of suboptimal antioxidant defense in the aging brain: A theoretical review. Rev. Neurosci. 2014, 25, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of lichen metabolite fumarprotocetraric acid via intracellular redox modulation. Toxicol. Appl. Pharmacol. 2017, 316, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Protective effects of lichen metabolites evernic and usnic acids against redox impairment-mediated cytotoxicity in central nervous system-like cells. Food Chem. Toxicol. 2017, 105, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Sieteiglesias, V.; González-Burgos, E.; Bermejo-Bescós, P.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichens of Parmelioid Clade as Promising Multitarget Neuroprotective Agents. Chem. Res. Toxicol. 2019, 32, 1165–1177. [Google Scholar] [CrossRef]
- Lenaz, G. The mitochondrial production of reactive oxygen species: Mechanisms and implications in human pathology. IUBMB Life 2001, 52, 159–164. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef]
- Goga, M.; Elečko, J.; Marcinčinová, M.; Ručová, D.; Bačkorová, M.; Backor, M. Lichen Metabolites: An Overview of Some Secondary Metabolites and Their Biological Potential. In Co-Evolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–36. [Google Scholar]
- Calcott, M.J.; Ackerley, D.F.; Knight, A.; Keyzers, R.A.; Owen, J.G. Secondary metabolism in the lichen symbiosis. Chem. Soc. Rev. 2018, 47, 1730–1760. [Google Scholar] [CrossRef]
- Lauterwein, M.; Oethinger, M.; Belsner, K.; Peters, T.; Marre, R. In vitro activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid, and (−)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob. Agents Chemother. 1995, 39, 2541–2543. [Google Scholar] [CrossRef] [PubMed]
- Candan, M.; Yilmaz, M.; Tay, T.; Erdem, M.; Türk, A.O. Antimicrobial activity of extracts of the lichen Parmelia sulcata and its salazinic acid constituent. Z. Nat. C J. Biosci. 2007, 62, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Bačkorová, M.; Bačkor, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. Vitr. 2011, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Koparal, A.T. Anti-angiogenic and antiproliferative properties of the lichen substances (−)-usnic acid and vulpinic acid. Z. Nat. C J. Biosci. 2015, 70, 159–164. [Google Scholar] [CrossRef]
- Varol, M.; Türk, A.; Candan, M.; Tay, T.; Koparal, A.T. Photoprotective Activity of Vulpinic and Gyrophoric Acids toward Ultraviolet B-Induced Damage in Human Keratinocytes. Phytother. Res. 2016, 30, 9–15. [Google Scholar] [CrossRef]
- Cetin Cakmak, K.; Gulçin, I. Anticholinergic and antioxidant activities of usnic acid-an activity-structure insight. Toxicol. Rep. 2019, 6, 1273–1280. [Google Scholar] [CrossRef]
- Manojlovic, N.T.; Vasiljevic, P.J.; Maskovic, P.Z.; Juskovic, M.; Bogdanovic-Dusanovic, G. Chemical Composition, Antioxidant, and Antimicrobial Activities of Lichen Umbilicaria cylindrica (L.) Delise (Umbilicariaceae). Evid.-Based Complement. Alternat. Med. 2012, 2012, 452431. [Google Scholar] [CrossRef]
- Mohammadi, M.; Bagheri, L.; Badreldin, A.; Fatehi, P.; Pakzad, L.; Suntres, Z.; van Wijnen, A.J. Biological Effects of Gyrophoric Acid and Other Lichen Derived Metabolites, on Cell Proliferation, Apoptosis and Cell Signaling pathways. Chem. Biol. Interact. 2022, 351, 109768. [Google Scholar] [CrossRef]
- Martinčič, R.; Kuzmanovski, I.; Wagner, A.; Novič, M. Development of models for prediction of the antioxidant activity of derivatives of natural compounds. Anal. Chim. Acta 2015, 868, 23–35. [Google Scholar] [CrossRef]
- Ahmadi, S.; Ghanbari, H.; Lotfi, S.; Azimi, N. Predictive QSAR modeling for the antioxidant activity of natural compounds derivatives based on Monte Carlo method. Mol. Divers. 2021, 25, 87–97. [Google Scholar] [CrossRef]
- Lohézic-Le Dévéhat, F.; Tomasi, S.; Elix, J.A.; Bernard, A.; Rouaud, I.; Uriac, P.; Boustie, J. Stictic acid derivatives from the lichen Usnea articulata and their antioxidant activities. J. Nat. Prod. 2007, 70, 1218–1220. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Resende, R.; Ferreiro, E.; Pereira, C.; Resende de Oliveira, C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1–42: Involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience 2008, 155, 725–737. [Google Scholar] [CrossRef]
- Takahashi, A.; Camacho, P.; Lechleiter, J.D.; Herman, B. Measurement of intracellular calcium. Physiol. Rev. 1999, 79, 1089–1125. [Google Scholar] [CrossRef]
- Correia, S.C.; Santos, R.X.; Cardoso, S.M.; Santos, M.S.; Oliveira, C.R.; Moreira, P.I. Cyanide preconditioning protects brain endothelial and NT2 neuron-like cells against glucotoxicity: Role of mitochondrial reactive oxygen species and HIF-1α. Neurobiol. Dis. 2012, 45, 206–218. [Google Scholar] [CrossRef] [PubMed]
- de Paz, G.A.; Raggio, J.; Gómez-Serranillos, M.P.; Palomino, O.M.; González-Burgos, E.; Carretero, M.E.; Crespo, A. HPLC isolation of antioxidant constituents from Xanthoparmelia spp. J. Pharm. Biomed. Anal. 2010, 53, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, I.; Kinoshita, Y.; Yamamoto, Y.; Huneck, S.; Yamada, Y. Analysis of secondary metabolites from lichen by high performance liquid chromatography with a photodiode array detector. Phytochem. Anal. 1994, 5, 197–205. [Google Scholar] [CrossRef]



| Compounds | Retention Time (tR, min) | UV Detection (λ max, nm) |
|---|---|---|
| Alectoronic acid (ALE) | 29.1 ± 0.001 | 214, 254, 316 |
| Gyrophoric acid (GYR) | 25.9 ± 0.003 | 212, 270, 304 |
| Lecanoric acid (LEC) | 19.4 ± 0.0312 | 212, 270, 304 |
| Methyl orsellinate (Me-ORS) | 13.9 ± 0.002 | 210, 262, 298 |
| Orsellinic acid (ORS) | 9.8 ± 0.011 | 210, 262, 298 |
| Pinastric acid (PIN) | 26.9 ± 0.047 | <210, 246, 392 |
| Usnic acid (USN) | 32.3 ± 0.046 | 226/234, 282 |
| Vulpinic acid (VUL) | 25.1 ± 0.034 | <210, 234, 282, 354 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen Extracts from Cetrarioid Clade Provide Neuroprotection against Hydrogen Peroxide-Induced Oxidative Stress. Molecules 2022, 27, 6520. https://doi.org/10.3390/molecules27196520
Ureña-Vacas I, González-Burgos E, Divakar PK, Gómez-Serranillos MP. Lichen Extracts from Cetrarioid Clade Provide Neuroprotection against Hydrogen Peroxide-Induced Oxidative Stress. Molecules. 2022; 27(19):6520. https://doi.org/10.3390/molecules27196520
Chicago/Turabian StyleUreña-Vacas, Isabel, Elena González-Burgos, Pradeep Kumar Divakar, and María Pilar Gómez-Serranillos. 2022. "Lichen Extracts from Cetrarioid Clade Provide Neuroprotection against Hydrogen Peroxide-Induced Oxidative Stress" Molecules 27, no. 19: 6520. https://doi.org/10.3390/molecules27196520
APA StyleUreña-Vacas, I., González-Burgos, E., Divakar, P. K., & Gómez-Serranillos, M. P. (2022). Lichen Extracts from Cetrarioid Clade Provide Neuroprotection against Hydrogen Peroxide-Induced Oxidative Stress. Molecules, 27(19), 6520. https://doi.org/10.3390/molecules27196520






