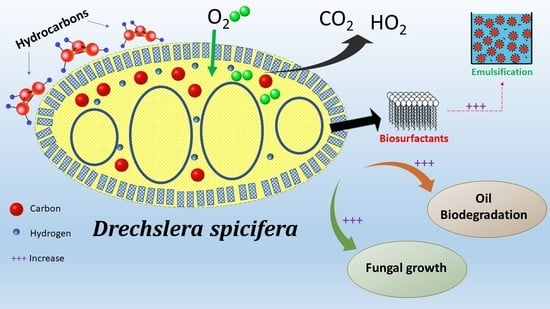
Biodegradation of Petroleum Hydrocarbons by Drechsleraspicifera Isolated from Contaminated Soil in Riyadh, Saudi Arabia
Abstract
1. Introduction
2. Results
2.1. Identification of D. spicifera Strains in Different Soil Samples
2.2. Corresponding Reactivity between D. spicifera and Different Hydrocarbons
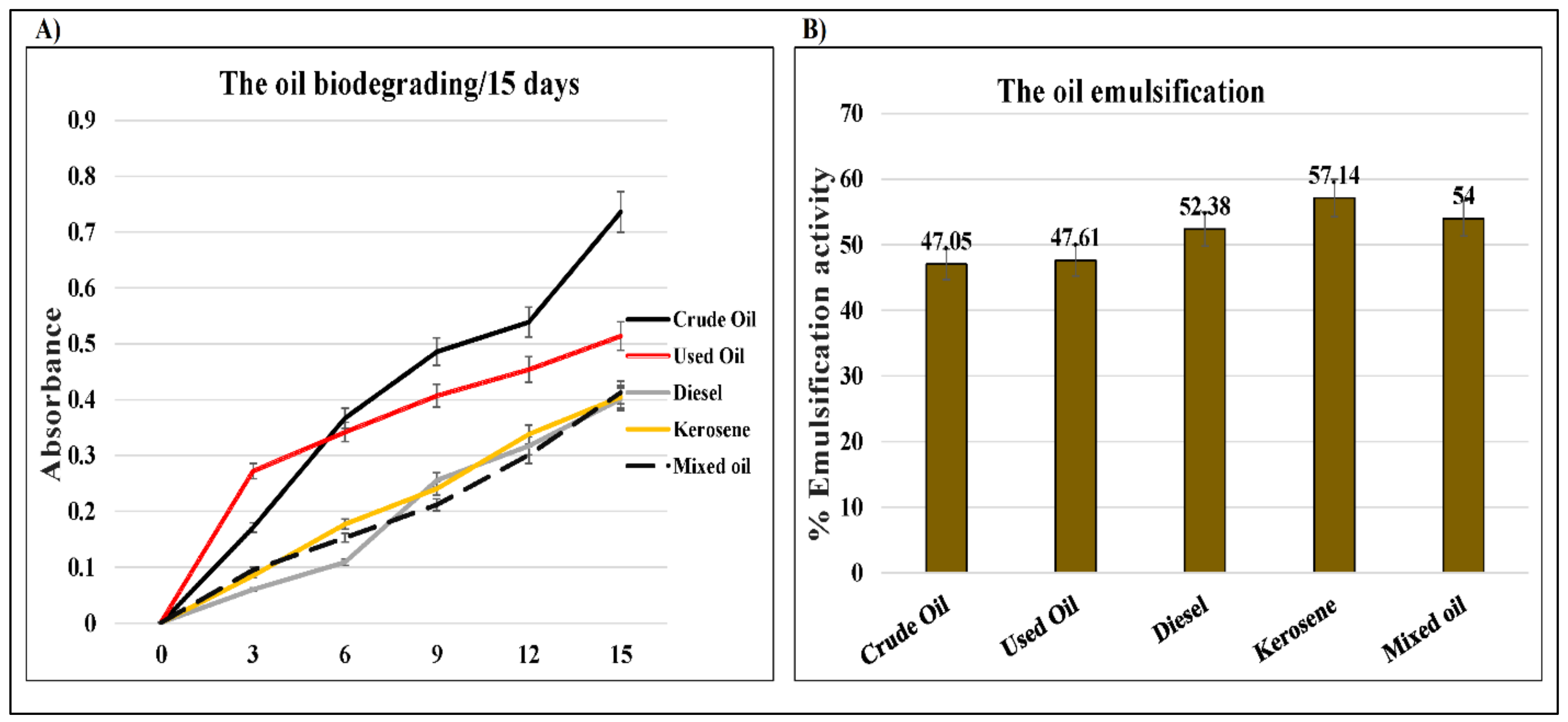
2.3. D. spicifera Biosurfactants Induced Hydrocarbons Bioremediation
2.4. Assessment of the Weight of Bio-Surfactants Recovered from D. spicifera
2.5. Bio-Surfactants of D. spicifera Improved the Growth of Tomato Seeds
3. Discussion
4. Materials and Methods
4.1. Samples Collection and Fungus Isolation
4.2. Hydrocarbons Biodegradation
4.3. Hydrocarbon Tolerance Test
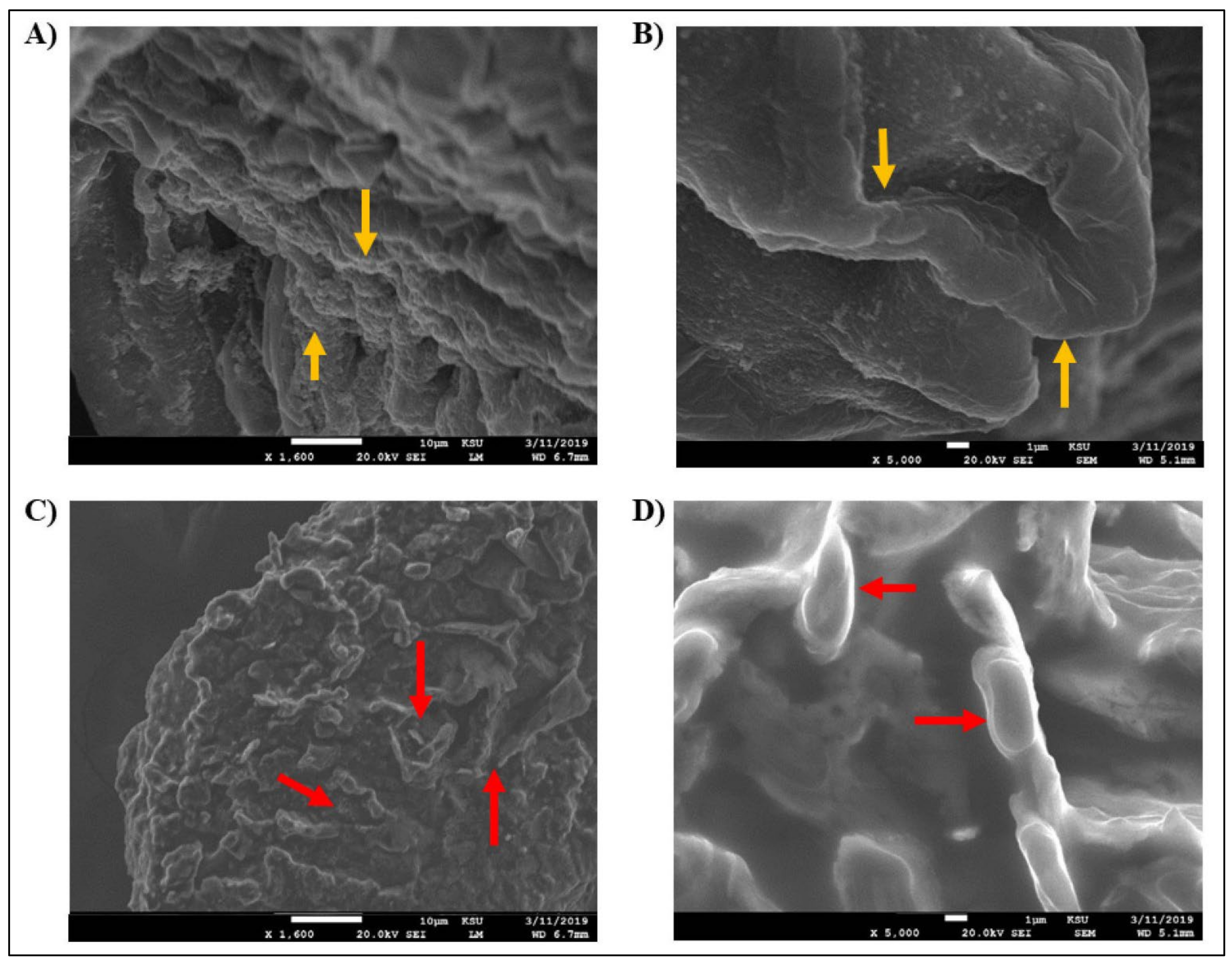
4.4. Scanning Electron Microscopy (SEM)
4.5. DCPIP Assay
4.6. Drop-Collapse Test
4.7. Emulsification Activity Measurement
4.8. Recovery of Biosurfactants
4.9. Assessment of Toxicity Using the Germination Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varjani, S.J.; Upasani, V.N. Biodegradation of petroleum hydrocarbons by oleophilic strain of Pseudomonas aeruginosa NCIM 5514. Bioresour. Technol. 2016, 222, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Meckenstock, R.U.; Boll, M.; Mouttaki, H.; Koelschbach, J.S.; Cunha Tarouco, P.; Weyrauch, P.; Dong, X.; Himmelberg, A.M. Anaerobic Degradation of Benzene and Polycyclic Aromatic Hydrocarbons. J. Mol. Microbiol. Biotechnol. 2016, 26, 92–118. [Google Scholar] [CrossRef]
- Varjani, S.J.; Rana, D.P.; Jain, A.K.; Bateja, S.; Upasani, V.N. Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int. Biodeterior. Biodegrad. 2015, 103, 116–124. [Google Scholar] [CrossRef]
- Barnes, N.M.; Khodse, V.B.; Lotlikar, N.P.; Meena, R.M.; Damare, S.R. Bioremediation potential of hydrocarbon-utilizing fungi from select marine niches of India. 3 Biotech. 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.M. Bioremediation of Oil Spill in Kingdom of Saudi Arabia by using Fungi Isolated from Polluted Soils. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 680–691. [Google Scholar] [CrossRef]
- Maamar, A.; Lucchesi, M.-E.; Debaets, S.; Nguyen van Long, N.; Quemener, M.; Coton, E.; Bouderbala, M.; Burgaud, G.; Matallah-Boutiba, A. Highlighting the Crude Oil Bioremediation Potential of Marine Fungi Isolated from the Port of Oran (Algeria). Diversity 2020, 12, 196. [Google Scholar] [CrossRef]
- Muhonja, C.N.; Makonde, H.; Magoma, G.; Imbuga, M. Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE 2018, 13, e0198446. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, J.; Okrasińska, A.; Kisło, K.; Aleksandrzak-Piekarczyk, T.; Szatraj, K.; Dolatabadi, S.; Muszewska, A. Carbon assimilation profiles of mucoralean fungi show their metabolic versatility. Sci. Rep. 2019, 9, 11864. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Karsani, S.A.; Alias, S.A. Fungal survival under temperature stress: A proteomic perspective. PeerJ 2020, 8, e10423. [Google Scholar] [CrossRef]
- Eliaz, N.; Ron, E.Z.; Gozin, M.; Younger, S.; Biran, D.; Tal, N. Microbial Degradation of Epoxy. Materials 2018, 11, 2123. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Characterization of biosurfactant produced during degradation of hydrocarbons using crude oil as sole source of carbon. Front. Microbiol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Sajna, K.V.; Sukumaran, R.K.; Gottumukkala, L.D.; Pandey, A. Crude oil biodegradation aided by biosurfactants from Pseudozyma sp. NII 08165 or its culture broth. Bioresour. Technol. 2015, 191, 133–139. [Google Scholar] [CrossRef]
- Wilkes, H.; Buckel, W.; Golding, B.T.; Rabus, R. Metabolism of hydrocarbons in n-alkane-utilizing anaerobic bacteria. J. Mol. Microbiol. Biotechnol. 2016, 26, 138–151. [Google Scholar] [CrossRef]
- Al-Dhabaan, F.A. Mycoremediation of crude oil contaminated soil by specific fungi isolated from Dhahran in Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 73–77. [Google Scholar] [CrossRef]
- Abdelwehab, S.A.; EL-Nagerabi, A.A.F.; Elshafie, A.E. Mycobiota Associated with Imported Seeds of Vegetable Crops in Sudan. Open Mycol. J. 2014, 8, 156–173. [Google Scholar] [CrossRef][Green Version]
- Da Cunha, K.C.; Sutton, D.A.; Fothergill, A.W.; Cano, J.; Gené, J.; Madrid, H.; De Hoog, S.; Crous, P.W.; Guarro, J. Diversity of Bipolaris species in clinical samples in the United States and their antifungal susceptibility profiles. J. Clin. Microbiol. 2012, 50, 4061–4066. [Google Scholar] [CrossRef]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Hanum, S.; Fitri, L.; Lisa, O.; Samingan, S.; Alfizar, A.; Sutekad, D.; Novita, N.; Muarrif, S.; Syaukani, S. Inventory of fungi from termite nests at Gunung Leuser National Park, northern Sumatra. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 012088. [Google Scholar] [CrossRef]
- Sabatini, L.; Sisti, M.; Campana, R. Evaluation of fungal community involved in the bioderioration process of wooden artworks and canvases in Montefeltro area (Marche, Italy). Microbiol. Res. 2018, 207, 203–210. [Google Scholar] [CrossRef]
- Kottb, M.; El-Agroudy, N.; Ali, A.; Hamed, M.; Ezz El-Din, H. Biodegradation of some petroleum hydrocarbons by fungi isolated from Gulf of Suez. Catrina 2019, 18, 169–175. [Google Scholar] [CrossRef]
- Guglielminetti, M.; De Giuli Morghen, C.; Radaelli, A.; Bistoni, F.; Carruba, G.; Spera, G.; Caretta, G. Mycological and Ultrastructural studies to evaluate biodeterioration of mural paintings. Detection of fungi and mites in frescos of the Monastery of St Damian in Assisi. Int. Biodeterior. Biodegrad. 1994, 33, 269–283. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Umadhay-Briones, K.M.; Zsiros, S.M.; Morita, N.; Nodasaka, Y.; Yumoto, I.; Okuyama, H. Isolation, identification, and characterization of a novel, oil-degrading bacterium, Pseudomonas aeruginosa T1. Curr. Microbiol. 2004, 49, 108–114. [Google Scholar] [CrossRef]
- Balaji, V.; Arulazhagan, P.; Ebenezer, P. Enzymatic bioremediation of polyaromatic hydrocarbons by fungal consortia enriched from petroleum contaminated soil and oil seeds. J. Environ. Biol. 2014, 35, 521–529. [Google Scholar]
- Satpute, S.K.; Bhawsar, B.D.; Dhakephalkar, P.K.; Chopade, B.A. Assessment of different screening methods for selecting biosurfactant producing marine bacteria. Indian J. Geo-Mar. Sci. 2008, 37, 243–250. [Google Scholar]
- Chandran, P.; Das, N. Role of plasmid in diesel oil degradation by yeast species isolated from petroleum hydrocarbon-contaminated soil. Environ. Technol. 2011, 33, 645–652. [Google Scholar] [CrossRef]
- Ting, A.S.Y.; Tan, C.H.C.; Aw, C.S. Hydrocarbon-degradation by isolate Pseudomonas lundensis UTAR FPE2. Malays. J. Microbiol. 2009, 5, 104–108. [Google Scholar] [CrossRef][Green Version]
- Fathepure, B.Z. Recent studies in microbial degradation of petroleum hydrocarbons in hypersaline environments. Front. Microbiol. 2014, 5, 173. [Google Scholar] [CrossRef]
- Obuekwe, C.O.; Badrudeen, A.M.; Al-Saleh, E.; Mulder, J.L. Growth and hydrocarbon degradation by three desert fungi under conditions of simultaneous temperature and salt stress. Int. Biodeterior. Biodegrad. 2005, 56, 197–205. [Google Scholar] [CrossRef]
- Obuekwe, C.O.; Al-Jadi, Z.K.; Al-Saleh, E.S. Hydrocarbon degradation in relation to cell-surface hydrophobicity among bacterial hydrocarbon degraders from petroleum-contaminated Kuwait desert environment. Int. Biodeterior. Biodegrad. 2009, 63, 273–279. [Google Scholar] [CrossRef]
- Elshafie, A.; AlKindi, A.Y.; Al-Busaidi, S.; Bakheit, C.; Albahry, S. Biodegradation of crude oil and n-alkanes by fungi isolated from Oman. Mar. Pollut. Bull. 2007, 54, 1692–1696. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Teixeira, J.A.; van der Mei, H.C.; Oliveira, R. Physicochemical and functional characterization of a biosurfactant produced by Lactococcus lactis 53. Colloids Surf. B Biointerfaces 2006, 49, 79–86. [Google Scholar] [CrossRef]
- Antoniou, E.; Fodelianakis, S.; Korkakaki, E.; Kalogerakis, N. Biosurfactant production from marine hydrocarbon-degrading consortia and pure bacterial strains using crude oil as carbon source. Front. Microbiol. 2015, 6, 274. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Ijah, U.J.J.; Manga, S.B.; Bilbis, L.S.; Umar, S. Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. Int. Biodeterior. Biodegrad. 2013, 81, 28–34. [Google Scholar] [CrossRef]
- Durairaj, P.; Malla, S.; Nadarajan, S.P.; Lee, P.G.; Jung, E.; Park, H.H.; Kim, B.G.; Yun, H. Fungal cytochrome P450 monooxygenases of Fusarium oxysporum for the synthesis of ω-hydroxy fatty acids in engineered Saccharomyces cerevisiae. Microb. Cell Factories 2015, 14, 45. [Google Scholar] [CrossRef]
- Lladó, S.; Covino, S.; Solanas, A.M.; Viñas, M.; Petruccioli, M.; D’annibale, A. Comparative assessment of bioremediation approaches to highly recalcitrant PAH degradation in a real industrial polluted soil. J. Hazard. Mater. 2013, 248–249, 407–414. [Google Scholar] [CrossRef]
- Dell’ Anno, F.; Rastelli, E.; Sansone, C.; Brunet, C.; Ianora, A.; Dell’Anno, A. Bacteria, Fungi and Microalgae for the Bioremediation of Marine Sediments Contaminated by Petroleum Hydrocarbons in the Omics Era. Microorganisms 2021, 9, 1695. [Google Scholar] [CrossRef]
- Parthipan, P.; Preetham, E.; Machuca, L.L.; Rahman, P.K.; Murugan, K.; Rajasekar, A. Biosurfactant and Degradative Enzymes Mediated Crude Oil Degradation by Bacterium Bacillus subtilis A1. Front. Microbiol. 2017, 8, 193. [Google Scholar] [CrossRef]
- Khandelwal, A.; Singh, S.B.; Sharma, A.; Nain, L.; Varghese, E.; Singh, N. Effect of surfactant on degradation of Aspergillus sp. and Trichoderma sp. mediated crude oil. Int. J. Environ. Anal. Chem. 2021, 101, 1–14. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.; Al-Othman, M.R.; Hisham, S.M.; Shehata, S.M. Evaluation of crude oil biodegradation using mixed fungal cultures. PLoS ONE 2021, 16, e0256376. [Google Scholar] [CrossRef]
- Rufino, R.D.; De Luna, J.M.; De Campos Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014, 17, 34–38. [Google Scholar] [CrossRef]
- Krawczyńska, M.; Kołwzan, B.; Rybak, J.; Gediga, K.; Shcheglova, N.S. The influence of biopreparation on seed germination and growth. Pol. J. Environ. Stud. 2012, 21, 1697–1702. [Google Scholar]
- Al-Nasrawi, H. Biodegradation of crude oil by fungi isolated from Gulf of Mexico. J. Bioremed. Biodegrad. 2012, 3, 147–152. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Whittingham, W.F. Soil microfungal changes among the profiles of disturbed conifer-hardwood forests. Ecology 1974, 55, 3–16. [Google Scholar] [CrossRef]
- Latha, R.; Kalaivani, R. Bacterial degradation of crude oil by gravimetric analysis. Adv. Appl. Sci. Res. 2012, 3, 2789–2795. [Google Scholar]
- Asemoloye, M.D.; Jonathan, S.G.; Ahmad, R. Degradation of 2, 2-Dichlorovinyl dimethyl phosphate (dichlorvos) through the rhizosphere interaction between Panicum maximum Jacq and some selected fungi. Chemosphere 2019, 221, 403–411. [Google Scholar] [CrossRef]
- Jonathan, S.G.; Oyetunji, O.J.; Olawuyi, O.J.; Asemoloye, M.D. Growth responses of Corchorus olitorius Lin. (Jute) to the application of SMC as an organic fertilizer. Acad. Arena 2012, 4, 48–56. [Google Scholar]
- Al-Hawash, A.B.; Alkooranee, J.T.; Abbood, H.A.; Zhang, J.; Sun, J.; Zhang, X.; Ma, F. Isolation and characterization of two crude oil-degrading fungi strains from Rumaila oil field, Iraq. Biotechnol. Rep. 2018, 17, 104–109. [Google Scholar] [CrossRef]
- Jakovljević, V.D.; Vrvić, M.M. The Potential Application of Selected Fungi Strains in Removal of Commercial Detergents and Biotechnology. In Application and Characterization of Surfactants; Najjar, R., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Alkhudhair, S.K.; Alharbi, R.I.; Al-Askar, A.A.; Aljowaie, R.M.; Al-Shehri, S. The Antimicrobial Activities of Silver Nanoparticles from Aqueous Extract of Grape Seeds against Pathogenic Bacteria and Fungi. Molecules 2021, 26, 6081. [Google Scholar] [CrossRef]
- Hanson, K.G.; Desai, J.D.; Desai, A.J. A rapid and simple screening technique for potential crude oil degrading microorganisms. Biotechnol. Tech. 1993, 7, 745–748. [Google Scholar] [CrossRef]
- Mariano, A.P.; Bonotto, D.M.; Angelis, D.F.; Pirôllo, M.P.S.; Contiero, J. Use of weathered diesel oil as a low-cost raw material for biosurfactant production. Braz. J. Chem. Eng. 2008, 25, 269–274. [Google Scholar] [CrossRef]
- Płaza, G.A.; Zjawiony, I.; Banat, I.M. Use of different methods for detection of thermophilic biosurfactant-producing bacteria from hydrocarbon-contaminated and bioremediated soils. J. Pet. Sci. Eng. 2006, 50, 71–77. [Google Scholar] [CrossRef]
- Karthik, L.; Kumar, G.; Rao, K.V.B. Comparison of methods and screening of biosurfactant producing marine actinobacteria isolated from Nicobar marine sediment. IIOAB J. 2010, 1, 34–38. [Google Scholar]
- Morikawa, M.; Daido, H.; Takao, T.; Murata, S.; Shimonishi, Y.; Imanaka, T. A new lipopeptide biosurfactant produced by Arthrobacter sp. strain MIS38. J. Bacteriol. 1993, 175, 6459–6466. [Google Scholar] [CrossRef]
- Adebajo, S.; Akintokun, P.; Ojo, A.; Akintokun, A.; Badmos, O. Recovery of Biosurfactant Using Different Extraction Solvent by Rhizospheric Bacteria Isolated from Rice-husk and Poultry Waste Biochar Amended Soil. Egypt. J. Basic Appl. Sci. 2020, 7, 252–266. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Kim, S.; Park, I.; Lee, Y.; Chung, S.; Choi, Y. Characterization of new biosurfactant produced by Klebsiella sp. Y6-1 isolated from waste soybean oil. Bioresour. Technol. 2008, 99, 2288–2292. [Google Scholar] [CrossRef]
- Shah, M.U.; Sivapragasam, M.; Moniruzzaman, M.; Yusup, S.B. A comparison of recovery methods of rhamnolipids produced by Pseudomonas aeruginosa. Procedia Eng. 2016, 148, 494–500. [Google Scholar] [CrossRef]
- Sobrinho, H.B.S.; Luna, J.M.; Rufino, R.D.; Porto, A.L.; Sarubbo, L.A. Assessment of toxicity of a biosurfactant from Candida sphaerica UCP 0995 cultivated with industrial residues in a bioreactor. Electron. J. Biotechnol. 2013, 16, 4. [Google Scholar] [CrossRef]
- Pele, M.A.; Ribeaux, D.R.; Vieira, E.R.; Souza, A.F.; Luna, M.A.C.; Rodríguez, D.M.; Andrade, R.F.S.; Alviano, D.S.; Alviano, C.S.; Barreto-Bergter, E.; et al. Conversion of renewable substrates for biosurfactant production by Rhizopus arrhizus UCP 1607 and enhancing the removal of diesel oil from marine soil. Electron. J. Biotechnol. 2019, 38, 40–48. [Google Scholar] [CrossRef]
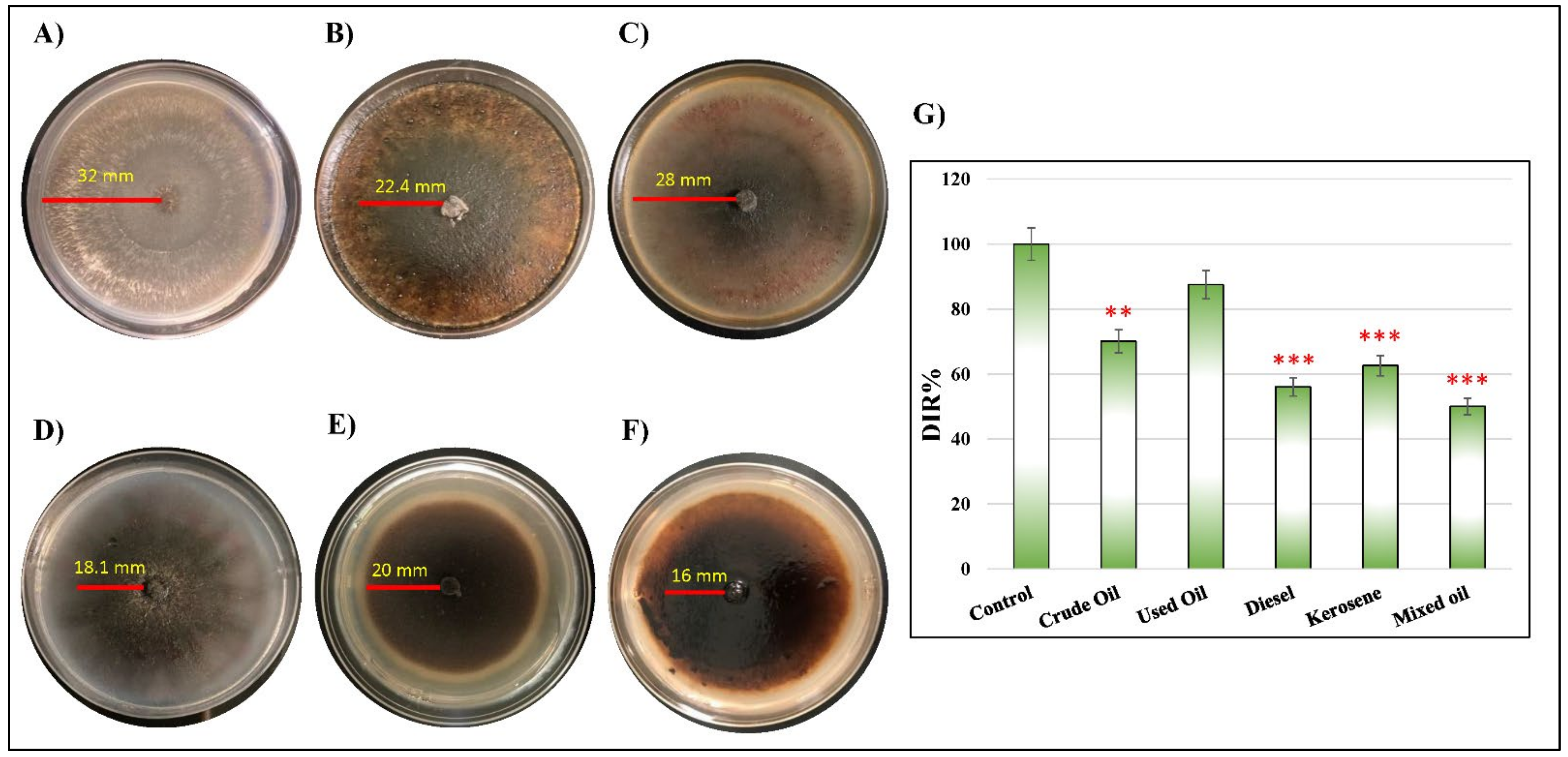



| Concentration | Non-Treated (cm/day) | Crude Oil (cm/day) | Used Oil (cm/day) | Diesel (cm/day) | Kerosene (cm/day) | Mixed Oil (cm/day) |
|---|---|---|---|---|---|---|
| 1% | 3.20 | 2.24 | 2.80 | 1.81 | 2.00 | 1.60 |
| 5% | 3.20 | 1.15 | 1.26 | 1.09 | 1.05 | 0.91 |
| 10% | 3.20 | 0.55 | 0.78 | 0.70 | 0.62 | 0.53 |
| DIR (%) | 100 | 70.01 | 87.50 | 56.06 | 62.58 | 50 |
| Incubation Period (Days) | 0 | 3 | 6 | 9 | 12 | 15 |
|---|---|---|---|---|---|---|
| Control | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Crude oil | 0.000 | 0.171 | 0.367 | 0.486 | 0.539 | 0.736 |
| Used oil | 0.002 | 0.272 | 0.342 | 0.407 | 0.454 | 0.514 |
| Diesel | 0.001 | 0.060 | 0.109 | 0.256 | 0.317 | 0.401 |
| Kerosene | 0.001 | 0.085 | 0.177 | 0.241 | 0.338 | 0.405 |
| Mixed oil | 0.001 | 0.095 | 0.153 | 0.212 | 0.301 | 0.413 |
| Hydrocarbon Source | ||||||
|---|---|---|---|---|---|---|
| −C | +C | Crude Oil | Used Oil | Diesel | Kerosene | Mixed Oil |
| − | +++ | + | ++ | + | + | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zahrani, R.M.; Al-Otibi, F.; Marraiki, N.; Alharbi, R.I.; Aldehaish, H.A. Biodegradation of Petroleum Hydrocarbons by Drechsleraspicifera Isolated from Contaminated Soil in Riyadh, Saudi Arabia. Molecules 2022, 27, 6450. https://doi.org/10.3390/molecules27196450
Al-Zahrani RM, Al-Otibi F, Marraiki N, Alharbi RI, Aldehaish HA. Biodegradation of Petroleum Hydrocarbons by Drechsleraspicifera Isolated from Contaminated Soil in Riyadh, Saudi Arabia. Molecules. 2022; 27(19):6450. https://doi.org/10.3390/molecules27196450
Chicago/Turabian StyleAl-Zahrani, Rasha M., Fatimah Al-Otibi, Najat Marraiki, Raedah I. Alharbi, and Horiah A. Aldehaish. 2022. "Biodegradation of Petroleum Hydrocarbons by Drechsleraspicifera Isolated from Contaminated Soil in Riyadh, Saudi Arabia" Molecules 27, no. 19: 6450. https://doi.org/10.3390/molecules27196450
APA StyleAl-Zahrani, R. M., Al-Otibi, F., Marraiki, N., Alharbi, R. I., & Aldehaish, H. A. (2022). Biodegradation of Petroleum Hydrocarbons by Drechsleraspicifera Isolated from Contaminated Soil in Riyadh, Saudi Arabia. Molecules, 27(19), 6450. https://doi.org/10.3390/molecules27196450