Behaviors of Organic Ligands and Phosphate during Biochar-Driven Nitrate Adsorption in the Presence of Low-Molecular-Weight Organic Acids
Abstract
:1. Introduction
2. Results
2.1. Variations in Eh, EC, and pH during the Period of Experiment
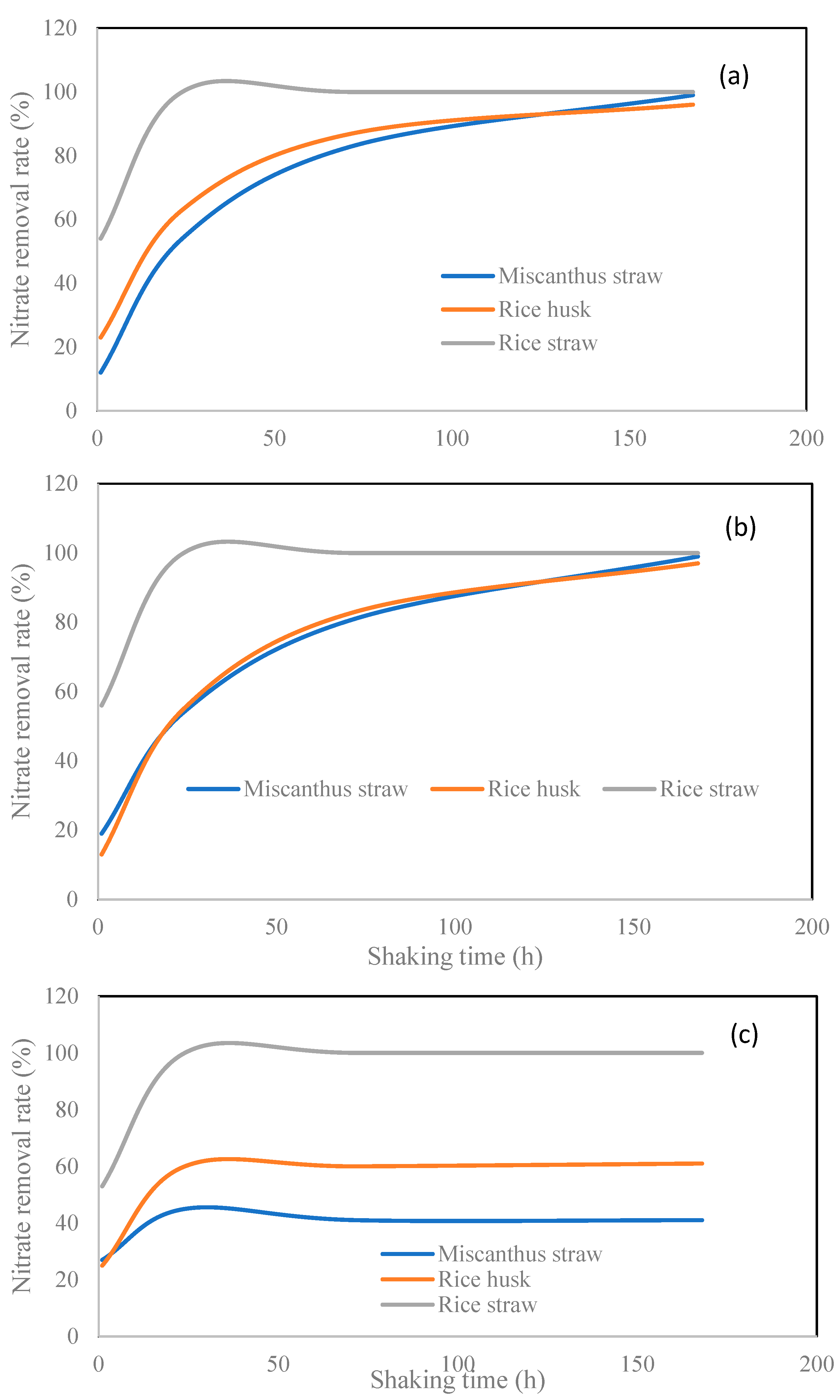
2.2. Nitrite and Nitrate
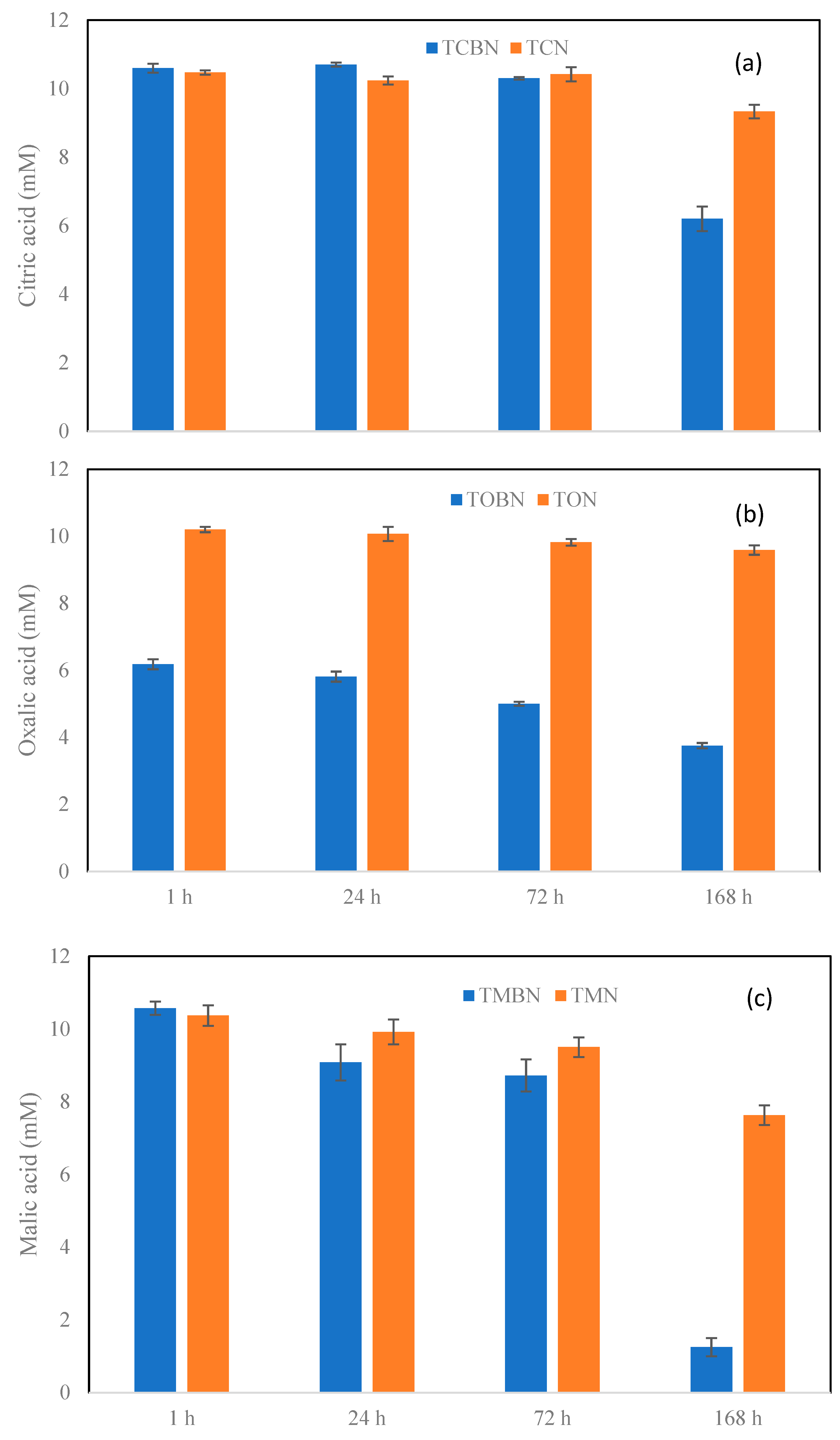
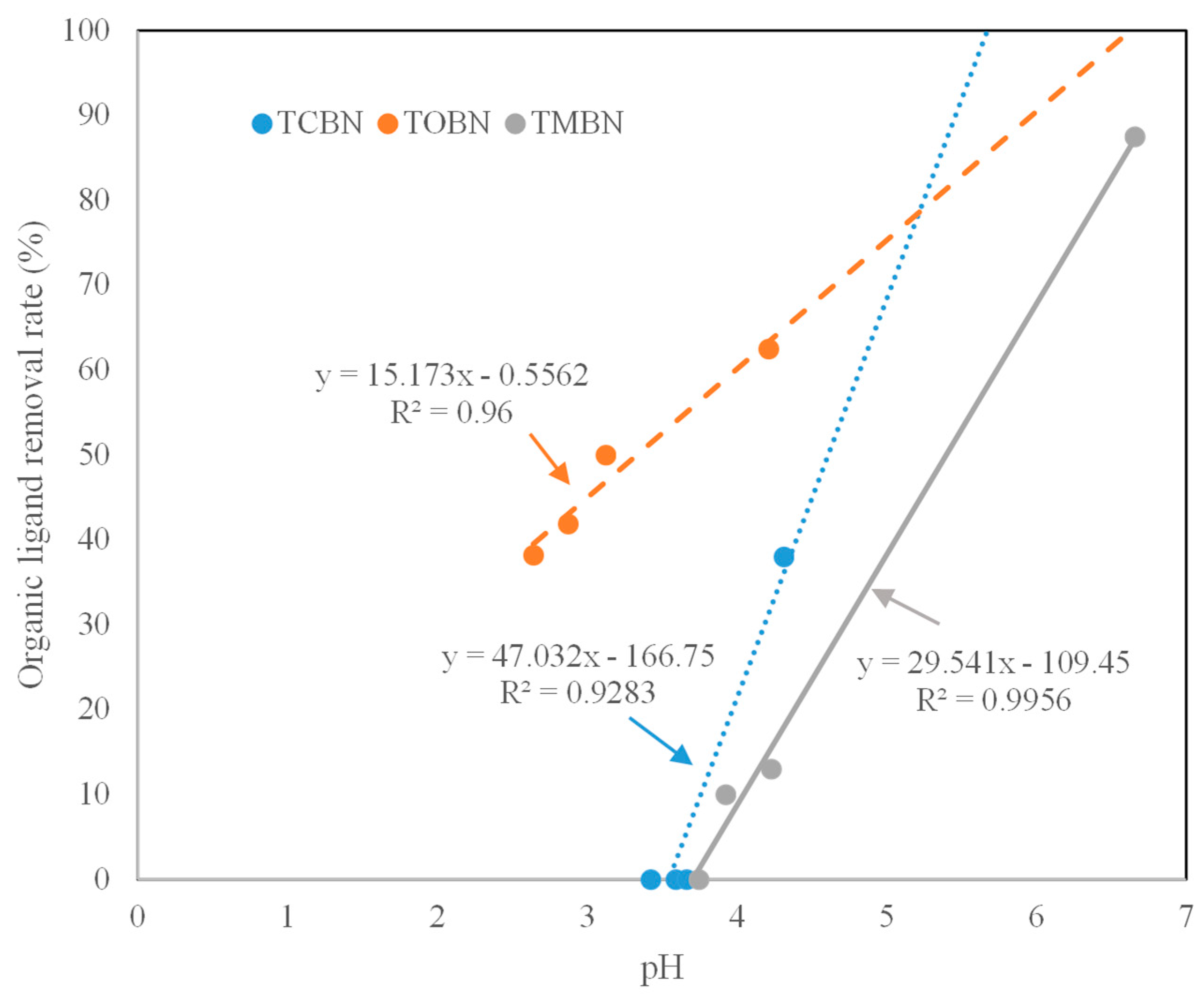
2.3. Organic Ligands
2.4. Phosphate
3. Materials and Methods
3.1. The Biochar Material Used in the Experiment
3.2. Experimental Design
3.3. Analytical Methods
3.4. Quality Assurance and Quality Control
3.5. Statistical Analysis
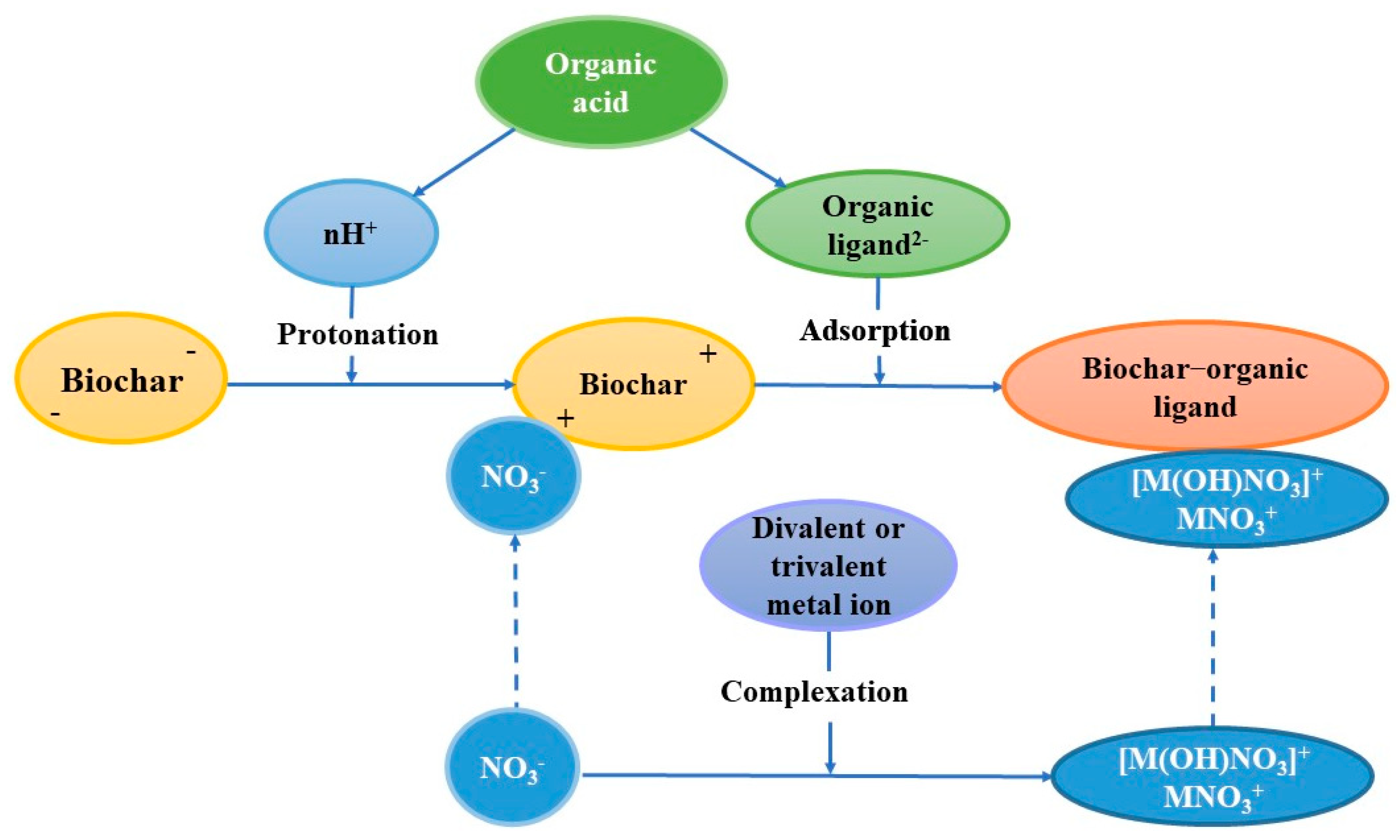
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaushal, S.S.; Groffman, P.M.; Band, L.E.; Elliott, E.M.; Shields, C.A.; Kendall, C. Tracking nonpoint source nitrogen pollution in human-impacted watersheds. Environ. Sci. Technol. 2011, 45, 8225–8232. [Google Scholar] [CrossRef]
- Wang, R.J.; Wang, Q.B.; Dong, L.S.; Zhang, J.F. Cleaner agricultural production in drinking-water source areas for the control of non-point source pollution in China. J. Environ. Manag. 2021, 285, 112096. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Alozie, N.; Heaney, N.; Lin, C. Biochar immobilizes soil-borne arsenic but not cationic metals in the presence of low-molecular-weight organic acids. Sci. Total. Environ. 2018, 630, 1188–1194. [Google Scholar] [CrossRef]
- Gautam, R.K.; Goswami, M.; Mishra, R.K.; Chaturvedi, P.; Awashthi, M.; Singh, R.; Giri, B.; Pandey, A. Biochar for remediation of agrochemicals and synthetic organic dyes from environmental samples: A review. Chemosphere 2021, 272, 129917. [Google Scholar] [CrossRef] [PubMed]
- Heaney, N.; Mamman, M.; Tahir, H.; Al-Gharib, A.; Lin, C. Effects of softwood biochar on the status of nitrogen species and elements of potential toxicity in soils. Ecotox. Environ. Safe. 2018, 166, 383–389. [Google Scholar] [CrossRef]
- Qin, J.; Li, Q.; Liu, Y.; Niu, A.; Lin, C. Biochar-driven reduction of As (V) and Cr (VI): Effects of pyrolysis temperature and low-molecular-weight organic acids. Ecotox. Environ. Saf. 2020, 201, 110873. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, H.; Zhang, D.; Wu, M.; Pan, B. Limited role of biochars in nitrogen fixation through nitrate adsorption. Sci. Total. Environ. 2017, 592, 758–765. [Google Scholar] [CrossRef]
- Qin, J.; Niu, A.; Li, Q.; Liu, Y.; Lin, C. Effect of soluble calcium on enhancing nitrate retention by biochar. J. Environ. Manag. 2020, 274, 111133. [Google Scholar] [CrossRef]
- Sanford, J.R.; Larson, R.A.; Runge, T. Nitrate sorption to biochar following chemical oxidation. Sci. Total. Environ. 2019, 669, 938–947. [Google Scholar] [CrossRef]
- Heaney, N.; Ukpong, E.; Lin, C. Low-molecular-weight organic acids enable biochar to immobilize nitrate. Chemosphere 2020, 240, 124872. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Niu, A.; Liu, Y.; Lin, C. Arsenic in leafy vegetable plants grown on mine water-contaminated soils: Uptake, human health risk and remedial effects of biochar. J. Hazard. Mater. 2021, 402, 123488. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Arbestain, M.C.; Hedley, M.; Bishop, P. Predicting phosphorus bioavailability from high-ash biochars. Plant Soil. 2012, 357, 173–187. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.N.; Shao, H.B.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.; Kirkham, M.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar. 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Elad, Y.; Cytryn, E.; Harel, Y.M.; Lew, B.; Graber, E.R. The biochar effect: Plant resistance to biotic stresses. Phytopathol. Mediterr. 2011, 50, 335–349. [Google Scholar]
- Graber, E.R.; Tsechansky, L.; Mayzlish-Gati, E.; Shema, R.; Koltai, H. A humic substances product extracted from biochar reduces Arabidopsis root hair density and length under P-sufficient and P-starvation conditions. Plant Soil. 2015, 395, 21–30. [Google Scholar] [CrossRef]
- Jian, X.M.; Uchimiya, M.; Orlov, A. Particle size- and crystallinity-controlled phosphorus release from biochars. Energy Fuels. 2019, 33, 5343–5351. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Zhang, M.; Inyang, M.; Li, Y.; Alva, A.; Yang, L. Engineered carbon (biochar) prepared by direct pyrolysis of Mg-accumulated tomato tissues: Characterization and phosphate removal potential. Bioresour. Technol. 2013, 138, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A.; Morton, B.J.; Muhlack, R.; Chittleborough, D.; Kwong, C. Recovery of phosphate from calcium-containing aqueous solution resulting from biochar-induced calcium phosphate precipitation. J. Clean. Prod. 2017, 165, 27–35. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.; Ok, Y. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water. Res. 2020, 186, 116303. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Zhang, X.; Hu, J.; Jiang, H. Effects of environmental conditions on the release of phosphorus from biochar. Chemosphere 2013, 93, 2069–2075. [Google Scholar] [CrossRef]
- Nelson, N.O.; Agudelo, S.C.; Yuan, W.; Gan, J. Nitrogen and phosphorus availability in biochar-amended soils. Soil Sci. 2011, 176, 218–226. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Zhang, X.; Chen, W.; Ni, J.; Yang, L.; Wei, R. Insight into the mechanism of low molecular weight organic acids-mediated release of phosphorus and potassium from biochars. Sci. Total. Environ. 2020, 742, 140416. [Google Scholar] [CrossRef]
- Vause, D.; Heaney, N.; Lin, C. Differential release of sewage sludge biochar-borne elements by common low-molecular-weight organic acids. Ecotox. Environ. Safe 2018, 165, 219–223. [Google Scholar] [CrossRef]
- Achor, S.; Aravis, C.; Heaney, N.; Odion, E.; Lin, C. Response of organic acid-mobilized heavy metals in soils to biochar application. Geoderma 2020, 378, 114628. [Google Scholar] [CrossRef]
- Morales, M.M.; Comerford, N.; Guerrini, I.A.; Falc~ao, N.P.S.; Reeves, J.B. Sorption and desorption of phosphate on biochar and biocharesoil mixtures. Soil Use Manag. 2013, 29, 306–314. [Google Scholar] [CrossRef]
- Bruun, S.; Harmer, S.L.; Bekiaris, G.; Christel, W.; Zuin, L.; Hu, Y.; Jensen, L.S.; Lombi, E. The effect of different pyrolysis temperatures on the speciation and availability in soil of P in biochar produced from the solid fraction of manure. Chemosphere 2017, 169, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Matin, N.H.; Jalali, M.; Antoniadis, V.; Shaheen, S.M.; Wang, J.; Zhang, T.; Wang, H.; Rinklebe, J. Almond and walnut shell-derived biochars affect sorption-desorption, fractionation, and release of phosphorus in two different soils. Chemosphere 2020, 241, 124888. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Dabioch, V. Complex-forming organic ligands in cloud-point extraction of metal ions: A review. Talanta 2013, 110, 202–228. [Google Scholar] [CrossRef] [PubMed]
- Onireti, O.O.; Lin, C.; Qin, J. Combined effects of low-molecular-weight organic acids on mobilization of arsenic and lead from multi-contaminated soils. Chemosphere 2017, 170, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, O.Y.; Aleshina, A.R.; Tikhonov, V.V.; Lapitskiy, S.A.; Pokrovsky, O.S. Coagulation of organo-mineral colloids and formation of lowmolecular weight organic and metal complexes in boreal humic riverwater under UV-irradiation. Chemosphere 2020, 250, 126216. [Google Scholar] [CrossRef]
- Renella, G.; Landi, L.; Nannipieri, P. Degradation of low molecular weight organic acids complexed with heavy metals in soil. Geoderma 2004, 122, 311–315. [Google Scholar] [CrossRef]
- Ni, Q.; Kirk, D.; Steven, T. Mass transport processes in the electro-oxidation of organic compounds for wastewater treatment. ECS Transactions 2012, 41, 27–33. [Google Scholar] [CrossRef]
- Russell, M.; Hall, A.J. A Hydrothermal Source of Energy and Materials at the Origin of Life. In Chemical Evolution II: From Origins of Life to Modern Society, 1st ed.; Zaikowski, L., Friedrich, J., Seidel, S.R., Eds.; American Chemical Society: Washington, DC, USA, 2009; pp. 45–62. [Google Scholar]
- Turner, B.L.; Blackwell, M.S.A. Isolating the influence of pH on the amounts and forms of soil organic phosphorus. Eur. J. Soil. Sci. 2013, 64, 249–259. [Google Scholar] [CrossRef]
| Parameter | Treatment | 1 h | 24 h | 72 h | 168 h |
|---|---|---|---|---|---|
| Eh | C1 | 481.00 ± 30.36 (a) [A] | 525.00 ± 6.30 (a) [A] | 492.03 ± 0.93 (d) [A] | 346.80 ± 0.75 (f) [B] |
| C2 | 428.57 ± 6.72 (c) [B] | 468.77 ± 3.46 (d) [A] | 414.13 ± 4.39 (f) [B] | 428.63 ± 1.60 (d) [B] | |
| C3 | 437.43 ± 2.45 (bc) [B] | 500.00 ± 1.55 (c) [A] | 445.47 ± 4.54 (e) [B] | 372.97 ± 3.22 (e) [C] | |
| TCN | 576.57 ± 0.72 (a) [A] | 513.57 ± 2.17 (b) [C] | 521.67 ± 1.78 (a) [B] | 470.17 ± 0.78 (ab) [D] | |
| TON | 578.70 ± 13.73 (a) [A] | 529.37 ± 1.65 (a) [B] | 528.70 ± 0.44 (a) [B] | 472.87 ± 1.63 (ab) [C] | |
| TMN | 539.87 ± 2.20 (bc) [A] | 529.50 ± 4.24 (a) [AB] | 523.40 ± 3.98 (a) [B] | 463.13 ± 2.24 (abc) [C] | |
| TCBN | 527.73 ± 0.81 (c) [A] | 507.03 ± 1.21 (bc) [C] | 511.30 ± 1.27 (b) [B] | 462.20 ± 0.17 (abc) [D] | |
| TOBN | 551.47 ± 1.23 (b) [A] | 503.50 ± 1.00 (c) [C] | 509.77 ± 0.07 (b) [B] | 463.50 ± 0.45 (abc) [D] | |
| TMBN | 527.10 ± 1.28 (c) [A] | 501.23 ± 0.28 (c) [B] | 508.97 ± 0.38 (b) [B] | 452.30 ± 6.43 (abc) [C] | |
| EC | C1 | 0.33 ± 0.00 (f) [B] | 0.30 ± 0.00 (cd) [C] | 0.33 ± 0.00 (cd) [B] | 0.35 ± 0.00 (c) [A] |
| C2 | 0.09 ± 0.00 (g) [A] | 0.07 ± 0.01 (d) [AB] | 0.08 ± 0.00 (d) [AB] | 0.08 ± 0.00 (c) [AB] | |
| C3 | 0.39 ± 0.00 (e) [B] | 0.36 ± 0.00 (cd) [C] | 0.39 ± 0.00 (cd) [B] | 0.41 ± 0.00 (c) [A] | |
| TCN | 0.75 ± 0.00 (c) [A] | 0.64 ± 0.00 (bc) [B] | 0.63 ± 0.01 (bc) [B] | 0.57 ± 0.01 (bc) [C] | |
| TON | 2.49 ± 0.01 (a) [A] | 2.14 ± 0.01 (a) [B] | 2.13 ± 0.00 (a) [B] | 2.14 ± 0.01 (a) [B] | |
| TMN | 0.61 ± 0.05 (d) [A] | 1.08 ± 0.50 (b) [A] | 1.07 ± 0.51 (b) [A] | 1.05 ± 0.52 (b) [A] | |
| TCBN | 0.76 ± 0.00 (c) [A] | 0.68 ± 0.00 (bc) [B] | 0.68 ± 0.00 (bc) [B] | 0.60 ± 0.00 (bc) [C] | |
| TOBN | 1.38 ± 0.01 (b) [A] | 0.93 ± 0.01 (b) [B] | 0.73 ± 0.01 (bc) [C] | 0.56 ± 0.01 (bc) [D] | |
| TMBN | 0.72 ± 0.01 (c) [A] | 0.64 ± 0.00 (bc) [B] | 0.64 ± 0.00 (bc) [B] | 0.61 ± 0.00 (bc) [C] | |
| pH | C1 | 8.55 ± 0.01 (a) [D] | 8.92 ± 0.05 (a) [C] | 9.40 ± 0.01 (a) [B] | 9.82 ± 0.00 (a) [A] |
| C2 | 7.03 ± 0.04 (b) [B] | 7.23 ± 0.04 (c) [A] | 6.50 ± 0.05 (c) [C] | 6.91 ± 0.01 (b) [B] | |
| C3 | 8.54 ± 0.01 (a) [D] | 8.70 ± 0.05 (b) [C] | 9.12 ± 0.08 (b) [B] | 9.77 ± 0.04 (a) [A] | |
| TCN | 2.67 ± 0.01 (e) [B] | 2.66 ± 0.00 (g) [B] | 2.62 ± 0.00 (g) [C] | 2.74 ± 0.01 (e) [A] | |
| TON | 2.19 ± 0.01 (f) [A] | 2.18 ± 0.01 (h) [A] | 2.13 ± 0.01 (h) [B] | 2.19 ± 0.01 (f) [A] | |
| TMN | 2.54 ± 0.17 (e) [A] | 2.56 ± 0.16 (g) [A] | 2.48 ± 0.17 (g) [A] | 2.54 ± 0.17 (e) [A] | |
| TCBN | 3.42 ± 0.01 (d) [D] | 3.59 ± 0.01 (e) [C] | 3.67 ± 0.01 (e) [B] | 4.31 ± 0.05 (d) [A] | |
| TOBN | 2.64 ± 0.01 (e) [D] | 2.87 ± 0.01 (f) [C] | 3.12 ± 0.03 (f) [B] | 4.21 ± 0.10 (d) [A] | |
| TMBN | 3.75 ± 0.01 (c) [C] | 3.92 ± 0.01 (d) [BC] | 4.23 ± 0.07 (d) [B] | 6.66 ± 0.26 (c) [A] |
| Parameter | Organic Acid | 1 h | 24 h | 72 h | 168 h |
|---|---|---|---|---|---|
| NO2− | C1 | n.d | n.d | n.d | n.d |
| (mg/L) | C2 | n.d | n.d | n.d | n.d |
| C3 | n.d | n.d | n.d | n.d | |
| TCN | n.d | n.d | n.d | n.d | |
| TON | n.d | n.d | n.d | n.d | |
| TMN | n.d | n.d | n.d | n.d | |
| TCBN | n.d | n.d | n.d | n.d | |
| TOBN | n.d | n.d | n.d | n.d | |
| TMBN | n.d | n.d | n.d | n.d | |
| NO3− | C1 | 0.52 ± 0.09 (d) [B] | 1.76 ± 0.07 (c) [A] | 1.83 ± 0.15 (e) [A] | 1.83 ± 0.04 (f) [A] |
| (mg/L) | C2 | 61.92 ± 0.49 (b) [C] | 63.70 ± 0.68 (ab) [B] | 65.36 ± 0.34 (b) [A] | 65.61 ± 0.26 (a) [A] |
| C3 | 65.96 ± 0.65 (a) [A] | 64.55 ± 0.80 (a) [A] | 61.53 ± 0.31 (c) [B] | 60.92 ± 0.18 (c) [B] | |
| TCN | 60.58 ± 1.16 (b) [A] | 60.69 ± 2.18 (ab) [A] | 55.49 ± 0.30 (d) [B] | 53.26 ± 0.37 (e) [B] | |
| TON | 60.82 ± 2.67 (b) [A] | 63.17 ± 0.24 (ab) [A] | 61.00 ± 0.39 (c) [A] | 62.93 ± 0.10 (b) [A] | |
| TMN | 59.22 ± 0.27 (b) [B] | 63.01 ± 1.65 (ab) [A] | 66.42 ± 0.80 (a) [A] | 59.35 ± 1.16 (d) [B] | |
| TCBN | 28.57 ± 0.85 (c) [A] | 0.00 ± 0.00 (c) [B] | 0.00 ± 0.00 (f) [B] | 0.00 ± 0.00 (g) [B] | |
| TOBN | 29.21 ± 0.98 (c) [A] | 0.00 ± 0.00 (c) [B] | 0.00 ± 0.00 (f) [B] | 0.00 ± 0.00 (g) [B] | |
| TMBN | 27.09 ± 2.24 (c) [A] | 0.00 ± 0.00 (b) [B] | 0.00 ± 0.00 (f) [B] | 0.00 ± 0.00 (g) [B] |
| Parameter | Organic Acid | 1 h | 24 h | 72 h | 168 h |
|---|---|---|---|---|---|
| PO43− | C1 | 1.39 ± 0.15 (e) [B] | 1.66 ± 0.15 (c) [B] | 2.39 ± 0.12 (e) [A] | 2.40 ± 0.08 (e) [A] |
| (mg/L) | C2 | 0.00 ± 0.00 (e) | 0.00 ± 0.00 (b) | 0.00 ± 0.00 (f) | 0.00 ± 0.00 (f) |
| C3 | 6.45 ± 0.09 (d) [B] | 6.42 ± 0.18 (a) [B] | 7.34 ± 0.21 (d) [A] | 6.95 ± 0.32 (d) [AB] | |
| TCN | 0.00 ± 0.00 (e) | 0.00 ± 0.00 (c) | 0.00 ± 0.00 (f) | 0.00 ± 0.00 (f) | |
| TON | 0.00 ± 0.00 (e) | 0.00 ± 0.00 (c) | 0.00 ± 0.00 (f) | 0.00 ± 0.00 (f) | |
| TMN | 0.00 ± 0.00 (e) | 0.00 ± 0.00 (c) | 0.00 ± 0.00 (f) | 0.00 ± 0.00 (f) | |
| TCBN | 40.71 ± 0.70 (b) [C] | 41.97 ± 1.49 (a) [C] | 46.35 ± 0.56 (b) [B] | 51.00 ± 0.16 (b) [A] | |
| TOBN | 37.96 ± 0.94 (c) [C] | 41.84 ± 2.22 (a) [C] | 48.70 ± 0.35 (a) [B] | 53.22 ± 0.41 (a) [A] | |
| TMBN | 44.27 ± 0.89 (a) [A] | 41.17 ± 0.25 (c) [A] | 35.73 ± 2.04 (c) [B] | 24.85 ± 1.10 (c) [C] |
| Parameter | Biochar |
|---|---|
| pH | 9.94 |
| EC (dS/m) | 8.05 |
| BET surface area (m2/g) | 37.85 |
| TOC (g/kg) | 420 |
| Soluble Ca (g/kg) | 0.31 |
| Soluble Mg (g/kg) | 0.06 |
| Soluble Na (g/kg) | 0.69 |
| Total N (g/kg) | 7.9 |
| Total P (mg/kg) | 2.35 |
| Available P (mg/kg) | 59.23 |
| Available K (g/kg) | 9.65 |
| Treatment | Organic Acid | Biochar (g) | NO3− (mM) | Solution (mL) |
|---|---|---|---|---|
| C1 | No | 1.0 | 0.0 | 100 mL |
| C2 | No | 0.0 | 1.0 | 100 mL |
| C3 | No | 1.0 | 1.0 | 100 mL |
| TCN | Citric | 0 | 1.0 | 100 mL |
| TON | Oxalic | 0 | 1.0 | 100 mL |
| TMN | Malic | 0 | 1.0 | 100 mL |
| TCBN | Citric | 1.0 | 1.0 | 100 mL |
| TOBN | Oxalic | 1.0 | 1.0 | 100 mL |
| TMBN | Malic | 1.0 | 1.0 | 100 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, W.; Li, Y.; Ying, J.; Lin, C.; Qin, J. Behaviors of Organic Ligands and Phosphate during Biochar-Driven Nitrate Adsorption in the Presence of Low-Molecular-Weight Organic Acids. Molecules 2022, 27, 5811. https://doi.org/10.3390/molecules27185811
Xiong W, Li Y, Ying J, Lin C, Qin J. Behaviors of Organic Ligands and Phosphate during Biochar-Driven Nitrate Adsorption in the Presence of Low-Molecular-Weight Organic Acids. Molecules. 2022; 27(18):5811. https://doi.org/10.3390/molecules27185811
Chicago/Turabian StyleXiong, Wenming, Yongjun Li, Jidong Ying, Chuxia Lin, and Junhao Qin. 2022. "Behaviors of Organic Ligands and Phosphate during Biochar-Driven Nitrate Adsorption in the Presence of Low-Molecular-Weight Organic Acids" Molecules 27, no. 18: 5811. https://doi.org/10.3390/molecules27185811
APA StyleXiong, W., Li, Y., Ying, J., Lin, C., & Qin, J. (2022). Behaviors of Organic Ligands and Phosphate during Biochar-Driven Nitrate Adsorption in the Presence of Low-Molecular-Weight Organic Acids. Molecules, 27(18), 5811. https://doi.org/10.3390/molecules27185811







