Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials
Abstract
1. Introduction
2. Materials and Methods
3. General Characteristics of Artemisia Species
| Species | Height | Leaves | Flowers | Fruits | Occurrence |
|---|---|---|---|---|---|
| A. abrotanum | 0.7–1.5 m [56] | Gray-green leaves with numerous covering hairs on the upper side; the smooth underside of the leaves; in the lower part of the stem are doubly pinnate with ensiform sections; in the upper parts a singly pinnate, tripartite, also with ensiform shape [57,58] | Tiny yellow tubular flowers, gathered in spherical or ovoid-spherical hanging heads, panicle forms | Small oblong achenes [57,58] | Central Asia (Armenia, Iran, and Russia), Asia Minor (Turkey), Central and North Europe Europe (e.g., Albania and Croatia) [1,8] |
| A. absinthium | 0.8–1.5 m [8,9,59] | Gray-green color, densely pubescent on both sides; basal leaves with long petioles, triangular or oval blade, bi- or tripinnatisect, the lower leaves not intensely divided, and the lanceolate top leaves [8,9,59] | Capitulum inflorescences gathered in loose panicles from the axils of the leaves; light-yellow ligulate female flowers, and tubular hermaphroditic flowers [9,59] | Small achene with brown stripes [59] | Europe, West Asia, and North Africa; introduced and acclimatized in North and South America and Australia [8,9,49,50,51] |
| A. annua | 0.3–1 m [10] | Alternate arrangement [60], the tripinnatisect lower leaves from petioles, the middle leaves bipinnatisect, the upper leaves sessile with lanceolate shape [61], leaf blades can be ensiform or lanceolate, the edge of the blades serrated [8] | Flower heads in raceme-like inflorescences, small, spherical, yellow-green, only tubular flowers [8,61] | Small, long achenes [60] | Southeastern Europe, Western Asia, North and South America, Australia [8,51,60] |
| A. dracunculus | 0.5–1.5 m [2,62,63] | Alternate, sessile, the lower leaves tripartite at the apex, the middle and upper leaves lanceolate, tip of the leaf sharp and the leaf blade margins entire [2,62,63] | Yellow, tubular flowers in hanging, spherical capitula forming loose panicles [2,62,63] | Small achenes [2,62,63] | Central Asia, South Europe, Eastern Europe, North America [2] |
| A. vulgaris | 0.5–2.5 m [8,64] | Dense and alternate, primarily in the upper parts of the stem, the lower leaves with short petioles divided into segments and feathery shape, the middle and upper ones smaller and single or double pinnate, the dorsal side of the leaves with dark green color, the ventral side whitish and tomentose [65,66] | Small, almost bare, yellowish or brown-red flowers embedded in small baskets form heavily branched panicles with numerous lanceolate bracts at the top of the shoots, inflorescences with ligulate flowers and tubular flowers [65,66] | Small dark brown shiny achenes [66,67] | Europe, Asia, abundantly in North America [57,64,66,67] |
4. Phytochemical Characteristics of Artemisia Species
| Species | Sesquiterpenoid Lactones | Flavonoids | Coumarins | Phenolic Acids |
|---|---|---|---|---|
| A. abrotanum | artemisin, santonin [58] | apigenin, artemetin, casticin centaureidine, hyperoside, isoquercitrin, kaempferol, luteolin, myricetin, patuletin, rutoside, quercetin, quercetol [58] | coumarin, esculetin, herniarin, isofraxidine, scopoletin, umbelliferone [116,117] | caffeic acid, caftaric acid, p-coumaric acid, chlorogenic acid, ferulic acid, gentisic acid, isochlorogenic acid, protocatechuic acid, rosmarinic acid, sinapic acid, syryngic acid, vanillic acid [20,58,116] |
| A. absinthium | absintholide, absinthin, anabsin, anabsinthin, arabsin, artabin, artabsin, artenolide, caruifolin D, deacetyloglobicin, germacranolide, hydroxypelenolide, isoabsinthin, ketopelenolide, ketopepenolid-A, matricin, parishine B and C, β-santonin, santonin-related lactones [9,35,75,76,121,129,130] | apigenin, artemetin, Artemisia bis-isoflavonyl dirhamnoside, Artemisia isoflavonyl glucosyl diester, casticin, catechin, flavone, 5-hydroxy-3,3′,4′,6,7-pentamethoxyflavone, glycosides of quercetin, kaempferol, myristin, naringenin, quercetin, quercetin dihydrate, quercetin-3-rutinoside, 5,6,3, 5′-tetramethoxy 7,4′-hydroxyflavone, rutoside [9,34,35,84] | coumarin, herniarin [84,89] | caffeic acid, 5′-O-caffeoylquinic acid, chlorogenic acid, coumaric acid, p-coumaric acid, 1′,3′-O-dicaffeoylquinic acid, 1′,5′-O-dicaffeoylquinic acid, 3′,5′-O-dicaffeoylquinic acid, 4′,5′-O-dicaffeoylquinic acid, ferulic acid, gallic acid, rosmarinic acid, salicylic acid, syryngic acid, tannic acid, vanillic acid [35,76,84,121,122] |
| A. annua | artemisinic acid, artemisinin, artannuin B [69,70,71,72,73,74] | acacetin, apigenin, apigenin 6-C-arabinosyl-8-C-glucoside, apigenin 6-C-glucosyl-8-C-arabinoside, apigenin derivatives, artemetin, astragalin, camferol, casticin, chrysin, chrysoeriol, chrysoeriol rutinoside, chrysosplenol C, chrysosplenol D, chrysosplentin, cinaroside, cirsilineol, dihydroartemisinin, 3,5-dihydroxy-3′, 4′, 6,7-tetramethoxyflavone, 3,5-dihydroxy-6,3′, 4′-tetramethoxyflavone, 3,5-dihydroxy-6,7,4′-trimethoxyflavone, 3,5-dimethoxyquercetagentin, 3,4′-dimethyl-quercetagentin ether, ether 3-methyl-quercetin, quercetin 3-glucoside, eupatin, eupatorine, 7-O-glucoside of diosmetin, 3-O-glucoside of kaempferol, 3-O-glucoside of quercetin, 3-O-hexoside of marnsetin, isocempheride, isoquercetin, isorhamnetin, isorhamnetin derivatives, isorhamnetin 3-O-glucoside, isovitexin, jaceidin, kaempferol, kaempferol derivatives, kirsiliol, kirsimaritin, laricitrin, luteolin, luteolin derivatives, luteolin 7-O-glucoside, marnsetin glucoside, marnsetin, 8-methoxykaempferol, 3-methoxy-kaempferol glucoside, 7-methyl-luteolin ether, 3-O-methylquercetagentin, micanine, myrcetin, patulentin glucoside, quercetin, quercetin derivatives, quercetin 3-O-galactoside, quercimeritin, retina, rhamnetine, rutoside, syringetin, tamarixetine [69,74,111,119,123,124,131,132,133,134] | coumarin, esculetin, isofraxidine, cis-melilotoside, trans-melilotoside, scopoletin, scopoline, tomentin [111,118,119] | caffeic acid, 4-caffeoyl-3,5-di-succinylquinic acid, 3,5-caffeoyletherquinic acid, 3-caffeoylquinic acid, 4-caffeoylquinic acid, chlorogenic acid, coumaric acid, 3,4-di-caffeoylquinic acid, 3,5-di-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, 4,5-di-O-caffeoylquinic acid, diferulcaffeoylquinic acid, 3,4-diferuloquinic acid, 3,5-diferuloquinic acid, 4,5-diferuloquinic acid, ferulic acid, 3-feruloquinic acid, 4-feruloquinic acid, 5-feruloquinic acid, rosmarinic acid [74,111,123,124] |
| A. dracunculus | artemether, dihydroartemisinin, [77] | anangenin, apigenin, biocovertsetin, davidigenin, 5,7-dihydroxy flavone, 2′,4′-dihydroxy-4-methoxydihydrochalcone syn, 7,3′-dimethyleriodictyol, DMC-2; 4-O-methyldavidigenin, estragoniside, estroside, 7-O-β-D-glucopyranoside, hyperoside, isoquercitrin, isorhamnetin glycosides, kaempferol, kaempferol glycosides, luteolin, luteolin glycosides, 7-methylaringenine, 7-methyleriodictiol, naringenin, patuletin hexoside, patuletin malonylrhamnosylhexoside, patuletin 3-O-malonylrobinobioside, patuletin rhamnosylhexoside, 5,6,7,8,4′-pentahydroxymetoflavone, pinocembrin, quercetin, quercetin glycosides, quercetin 3-O-rutinoside, rutoside, sacuranetine, 3,5,4-trihydroxy-7,3′-dimethoxyflavone 3,5,4′-trihydroxy-7-methoxyflavone, vicenin [2,54,97,113,114,115,125,126,135,136] | arethinol, aridiodiol, artemidiol, artemidine, artemidinol, artemidynal ether, artidin, capillarin, coumarin, dacumerin, 3,4-dehydroherniarin, (+)-(S,R)-epoxyartemidine, esculetin, esculin, herniarin, 6-demethoxycapilarisine, γ,γ-dimethylallyl ether esculetin, (+)-(R)-(E)-3′-hydroxyartemidine, 8-hydroxyartemidin, 9-hydroxyartemidine, 8-hydroxycapillarin, 4-hydroxycoumarin, isocoumarin, isovalerate capillarin, (−)-(R)-20-methoxydihydro-artemidine, 7,8-methylenedioxy-6-methoxycoumarin, methylenedaphnetin, 7-methyl daphnetin ether, scoparon, scopoletin, skimming [2,54,97,102,112,113,114,115] | caffeic acid, caffeoylquinic acid, chicory acid, chlorogenic acid, p-coumaric acid, p-coumaroyl-caffeoylquinic acid, p-coumaroyl-feruloylquinic acid, 3,5-O-dicaffeoylquinic acid, 4,5-di-O-caffeoylquinic acid, ferulic acid, ferulic acid hexoside, (E) 2-hydroxy-4-methoxycinnamic acid, 5-O-caffeoylquinic acid, hydroxybenzoic acid, 2-methoxycinnamic acid, sakuranetin, syringic acid, vanillic acid [54,97,101,113,114,125,126] |
| A. vulgaris | artemisinin, 1,2,3,4-diepoxy-11(13)-eudesmen-12,8-olide, psilostachyin, psilostachyin C, vulgarin, yomogin [55,64,137,138,139,140,141] | apigenin, chrysoeriol, diosmetin, eriodictyol, eupafolin, homoeriodictyol, hyperoside, isorhamnetin, jaceosidin, kaempferol 3-glucoside, kaempferol 7-glucoside, kaempferol 3-rhamnoside, kaempferol 3-rutinoside, luteolin, luteolin 7-glucoside, quercetin, quercetin 3-galactoside, quercetin 3-glucoside, rutoside, tricine, vitexin [23,55,142,143] | esculin, esculetin, umbelliferone [55,120] | caffeic acid, 3-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 1,5-di-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, 4,5-O-di-caffeoylquinic acid, 5-feruloylquinic acid, protocatechuic acid glucoside, quinic acid [127,128] |
5. Applications in Medicine
5.1. Ethnopharmacological Uses of Artemisia Species
| Species | Traditional Activity | Traditional Medicine | References |
|---|---|---|---|
| A. abrotanum |
| Europe | [20,157] |
| A. absinthium |
| Europe | [8,9,10] |
| Hindu medicine (Unani) | [9] | |
| A. annua |
| China and India | [71,158] |
| A. dracunculus |
| Europe | [54,159] |
| Hindu traditional medicine (Ayurveda) | [54,160] | |
| Arabia | [11,12] | |
| Central Asia | [11,12] | |
| A. vulgaris |
| Europe | [55] |
| Hindu medicine (Unani) | [161,164] |
5.2. Contemporary Phytotherapy
6. Cosmetic Potential of Artemisia Species
6.1. From the History of Cosmetic Uses of Artemisia Species
6.2. CosIng Database
6.3. Potential Cosmetic Biological Activities of Artemisia ssp. Confirmed by Scientific Studies
| Direction of Activity | Species | Extract/Essential Oil | Part | Classification | Compounds | Modal/Assay | Short Description of Studies Performed | References |
|---|---|---|---|---|---|---|---|---|
| Antibacterial and antifungal activity | A. abrotanum | Ethanol | Aerial parts | nt * | nt | Cup plate method | Lethal effecton the bacteria Bacillus stearothermophilus (MIC = 250 µg/mL), Klebsiella pneumoniae (MIC = 250 µg/mL), Micrococcus luteus (MIC = 500 µg/mL), Pseudomonas cepacian (MIC = 500 µg/mL), and Salmonella typhi (MIC = 125 µg/mL), and the fungi Candida albicans (MIC = 250 µg/mL), Saccharomyces cerevisiae (MIC = 125 µg/mL), and Trichosporon beigelii (MIC = 125 µg/mL). | [232] |
| Essential oil | Aerial parts | nt | nt | In vitro/diffusion well agar method (Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus)/paper disc diffusion method (Candida albicans)/ | Inhibition of the growth of Escherichia coli (inhibition zone diameter = 16 mm), Proteus vulgaris (inhibition zone diameter = 18.89 mm), Pseudomonas aeruginosa (inhibition zone diameter = 10.33 mm), Staphylococcus aureus (inhibition zone diameter = 20 mm), and C. albicans by components of A. abrotanum essential oil and essential oil. Some activity against Aspergillus flavus Lethal effect of the essential oil of A. abrotanum herb on C. albicans (inhibition zone diameter = 20.0 mm). | [80,168,237] | ||
| Methanol | leaves | nt | nt | A microtiter plate-based protocol (microdilution) | Inhibition of the growth of the bacteria Bacillus cereus (MIC = 0.41 mg/mL), E. coli (MIC = 0.39 mg/mL), Listeria monocytogenes (MIC = 0.45 mg/mL), Micrococcus flavus (MIC = 0.57 mg/mL), P. aeruginosa (MIC = 0.47 mg/mL), and S. aureus (MIC = 0.38 mg/mL), and the fungi A. flavus (MIC = 0.39 mg/mL), Aspergillus niger (MIC = 0.78 mg/mL), Aspergillus ochraceus (MIC = 0.55 mg/mL), C. albicans (MIC = 0.86 mg/mL), Penicillium funiculosum (MIC = 0.85 mg/mL), and Penicillium ochrochloron (MIC = 0.86 mg/mL) by leaf extracts of A. abrotanum. | [20] | ||
| Ethanol | herb | nt | nt | In vitro/micromethod of diffusion in agar | Moderate inhibition of the growth of the bacteria Citrobacter freundii (inhibition zones diameter = 8.81 mm), Enterococcus faecalis (inhibition zones diameter = 6.65 mm), E. coli (inhibition zones diameter = 6.44 mm), P. aeruginosa (inhibition zones diameter = 8.52 mm), Streptococcus pyogenes (inhibition zones diameter = 5.29 mm), Streptococcus agalactiae (inhibition zones diameter = 5.19 mm), Streptococcus gordoni (inhibition zones diameter = 5.89 mm); methicillin-susceptible: S. aureus (inhibition zones diameter = 6.34 mm)and Staphylococcus epidermis (inhibition zones diameter = 6.38 mm); methicillin-resistant: S. aureus (inhibition zones diameter = 7.20 mm) and Staphylococcus haemolyticus (inhibition zones diameter = 6.85 mm); and macrolides-resistant: Propionibacterium acnes (inhibition zones diameter = 8.71 mm) strains. Decrement of C. albicans (inhibition zones diameter = 5.79 mm) and Candida tropicalis (inhibition zones diameter = 7.09 mm) colonies and A. niger (inhibition zones diameter = 13.32 mm) spore germination. Synergistic action of A. abrotanum herb ethanolic extract with erythromycin against S. aureus with efflux mechanism of MLS-resistance. | [233] | ||
| A. absinthium | Essential oil | Aerial parts | nt | nt | In vitro | Growth inhibition by the essential oil from A. absinthium and its lethal activity against Clostridium perfringens, Enterobacter aerogenes, E. coli, Klebsiella oxytoca, K. pneumoniae, L. monocytogenes, Proteus mirabilis, P. aeruginosa, S. aureus, and Staphylococcus sonnei and inhibition of growth fungi Fusarium moniliforme, Fusarium oxysporum, and Fusarium solani. The range of MIC values was from < 0.08 mg/mL for P. mirabilis and E. aerogenes isolated from stool and for P. aeruginosa and S. aureus isolated from wounds, up to 2.43 mg/mL for K. oxytoca isolated from stool. | [85,88,234] | |
| Ethanol | Herb | nt | nt | In vitro/micromethod of diffusion in agar | Lethal effect of A. absinthium extract on B. cereus (inhibition zones diameter = 20.40 mm), Bacillus subtilis (inhibition zones diameter = 14.40 mm), Haemophilus influenzae (inhibition zones diameter = 18.40 mm), P. aeruginosa (inhibition zones diameter = 7.22 mm), and S. aureus (inhibition zones diameter = 9.37 mm) and growth suppression in P. acnes (inhibition zones diameter = 7.26 mm). | [233,235] | ||
| Essential oil | Aerial parts | nt | nt | In vitro | Growth inhibition of the bacteria L. monocytogenes (inhibition zone = 20 mm) and methicillin-sensitive/resistant S. cerevisiae var. chevalieri (inhibition zone = 16 mm), S. aureus (inhibition zone = 25 mm), and the fungi Fusarium culmorum (inhibition zone = 45 mm), Fusarium graminearum (inhibition zone = 15 mm), F. oxysporum (inhibition zone = 19 mm), Rhizoctonia solani (inhibition zone = 25 mm), and Sclerotinia sp. (inhibition zone = 24 mm) by A. absinthium essential oil. | [84,87] | ||
| Aerial parts | Phenolic acids | Chlorogenic acid, 4,5-di-O-caffeoylquinic acid | In vitro | Some bactericidal activity of chlorogenic acid and efflux pump inhibition by 4,5-di-O-caffeoylquinic acid isolated from A. absinthium. | [122] | |||
| Essential oil | Aerial parts | nt | nt | In vitro | Lethal action by essential oil A. absinthium against the fungi Alternaria alternata, A. niger, Fusarium oxysporum, F. sambucinum, and F. solani and the bacteria Arthrobacter spp., Bacillus mycoides, Micrococcus lylae, and P. aeruginosa. | [236] | ||
| A. annua | Water | Leaves | nt | nt | In vitro (disk diffusion method) | Lethal activity of A. annua leaf extracts against E. coli. | [201] | |
| Essential oil | Aerial parts | Monoterpenoids | 1,8-cineole, camphor | In vitro (disk diffusion method) | Lethal activity of essential oil and 1,8-cineol, camphor, and Artemisia ketone isolated from A. annua herb against E. coli, L. monocytogenes, Salmonella enteritidis, S. typhi, and Yersinia enterocolitica. Components of essential oil penetrate through the bacterial cell membrane, causing cellular dysfunction, increasing permeability of bacterial membrane and components. Low and moderate growth inhibition of the bacteria B. cereus, E. coli, K. pneumoniae, Sarina lutea, Shigella, S. aureus, and S. enteritidis, and fungi Aspergillus fumigatus and C. albicans by essential oil and 1,8-cineol, camphor and Artemisia ketone isolated from A. annua herb. | [91,93] | ||
| Essential oil | Aerial parts | nt | nt | In vitro (disk diffusion method) | Essential oil inhibits growth of the bacteria Acinetobacter baumannii, B. subtilis, E. faecalis, E. coli, K. pneumoniae, P. aeruginosa, and S. aureus, and fungi C. albicans, Candida famata, and C. utilis, and also inhibits cell adhesion and reduces the expression of virulence factors. | [92] | ||
| A. dracunculus | Essential oil | Herb | nt | nt | In vitro (disk diffusion method) | Inhibition of the growth of B. cereus, B. subtilis, E. coli, K. pneumoniae, L. monocytogenes, M. luteus, P. aeruginosa, Salmonella sp., S. aureus, S. epidermidis, S. pyogenes, Streptococcus typhimurium, Shigella flexneri, and Shigella marcescens under the influence of the essential oil of the A. dracunculus herb. Corynebacterium diphtheriae, Proteus spp., and S. aureus colony growth inhibition after application of the essential oil. S. epidermidis showing the largest zone of inhibition (21.5 mm). | [101] | |
| Essential oil | Leaves | nt | nt | In vitro (agar well diffusion) | Essential oil of A. dracunculus leaves hampers the growth of B. cereus, Enterobacter cloacae, E. coli, L. monocytogenes, M. flavus, S. enteritidis, and S. aureus strains. P. aeruginosa, A.R P. aeruginosa, S. aureus, S. aureus MRSA (methicillin-resistant), and S. typhimurium colonies growth inhibition and bactericidal effect as well as inhibition of the growth of A. fumigatus, A. niger, A. ochraceus, A. versicolor, P. funiculosum, P. ochrochloron, Penicillium verrucosum, Trichoderma viride, and fungicidal activity under the influence of hydroethanolic extract of the Tarragon. The MIC value for these bacteria and fungi was determined using the essential oil at a concentration of 0.03 and 25 mg/mL. | [125,153,249] | ||
| Hydro-ethanol | Leaves | nt | nt | In vitro (disk diffusion method)/In vivo (mice) | Hydroethanolic extract of A. dracunculus leaves (at dose 200 mg/kg) significantly reduces the number of colony-forming units of C. albicans in the liver and kidneys of mice. Inhibition of the growth of the bacteria B. cereus, B. subtilis, E. coli, P. aeruginosa, P. vulgaris, S. aureus, and S. pyogenes, and fungi A. fumigatus, C. albicans, and Penicillium expansum under the influence of hydroethanolic herbal extract. The largest zone of growth inhibition was observed for S. pyogenes (18 mm), and the smallest for P. aeruginosa (9 mm). Inhibition of the growth of the bacteria Corynebacterium diphtheria (MIC 5.9 mg/mL), Helicobacter pylori (MIC 11.75 mg/mL), S. aureus (MIC 0.09 mg/mL), S. aureus MRSA (MIC 2.35 mg/mL), and S. epidermis (MIC 0.363 mg/mL), after the application of infusion of A. dracunculus and minimal inhibition effect in Enterococcus hirae MIC 23.5 mg/mL) and K. pneumoniae colonies (MIC 47 mg/mL). | [100,126,205] | ||
| A. vulgaris | Essential oil | Aerial parts | nt | nt | In vitro/paper disc diffusion method (Candida albicans) | Inhibitory effect of the oil fraction on the development of E. coli, K. pneumoniae, S. enteritidis, P. aeruginosa, S. enteritidis, S. aureus, and Streptococcus mutans. Inhibitory effect of the oil fraction on the development of A. niger and C. albicans (inhibition zone diameter = 12.5 mm). | [41,80,88,151,250,251,252] | |
| Antioxidant activity | A. abrotanum | Ethanol | Herb | Polyphenols | Apigenin, caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, gentisic acid, hyperoside, isoquercitrin, luteolin, rutoside, sinapic acid, quercitol, quercitrin, | In vitro | Moderate antioxidant activity (IC50 = 284.50 µg/mL) of A. abrotanum ethanolic extract in the test with DPPH (2,2-diphenyl-1-picrylhydrazyl). | [58] |
| Essential oil | Aerial parts | nt | nt | In vitro | Reducing potential and inhibition of lipid peroxidation (82.34%, 1000 µL) by the essential oil from the herb of A. abrotanum. | [237] | ||
| Methanol | Herb | Phenolic acids | Isochlorogenic acid, rosmarinic acid, quercitrin | In vitro | Reducing the potential of methanolic extract from A. abrotanum herb, in particular its components, rosmarinic acid, isochlorogenic acid, and quercitrin. | [20] | ||
| A. absinthium | Methanol | Herb | Flavonoids, phenolic acids | nt | In vitro | Antioxidant activity of flavonoids and phenolic compounds in A. absinthium. In the DPPH test, the IC50 value for radical scavenging activity was 612 μg/mL. | [238] | |
| Methanol | Herb | nt | nt | In vitro/DPPH assay, FRAP assay | Methanolic extracts from A. absinthium herb have a significant reduction potential (IC50 = 9.38 mg/mL). Herb extracts reduced iron(III) ions, the EC50 were lower than for the ascorbic acid control | [84] | ||
| Essential oil | Aerial parts | nt | nt | In vitro/DPPH assay, ABTS assay | A. absinthium essential oil has the ability to scavenge radicals in DPPH and ABTS (2,2’-azobis(3- ethylobenzotiazolino-6-sulfonian)) tests. | [88] | ||
| Methanol | Herb | nt | nt | In vivo (mice) | Reducing properties of A. absinthium extract (at dose 100 or 200 mg/kg) and the ability to capture superoxide and hydrogen peroxide anions, hydroxy and nitric oxide radicals, inhibiting oxidative stress, reducing the concentration of TBARS (thiobarbituric acid reactive substances), and increasing the concentration of superoxide and glutathione dismutases. | [217] | ||
| A. annua | Methanol | Leaves | Phenolic acids, flavonoids | nt | In vitro | Methanolic extracts from A. annua leaves have the highest concentration of phenolic and flavonoid compounds showing a reducing effect. | [39] | |
| Hexane, chloroform, methanol, and water | Leaves | nt | nt | In vitro | Reducing activity of A. annua leaf extracts in DPPH test. | [201] | ||
| Essential oil | Herb | Monoterpenoids | 1,8-cineol, and α-pinene | In vitro | Essential oil from A. annua herb and its components 1,8-cineol, Artemisia ketone, and α-pinene shows weak reducing activity in tests with DPPH, ABTS radical tests, and hydrogen peroxide. | [93] | ||
| A. dracunculus | Hydro-ethanol | Herb | Flavonoids, phenolic acids | nt | In vitro | Reducing properties of the hydroethanolic herbal extract related to the presence of phenolic compounds and flavonoids. Reduction in DPPH and ABTS in the presence of phenolic compounds. | [40,100,113,125] | |
| A. vulgaris | Hydro-ethanol | Herb | Flavonoids, phenolic acids | nt | In vitro | Proved by different methods, such as DPPH (IC50 value was 65.5 μg/mL), lipid peroxidation, protein glycation, xanthine oxidases, ABTS, hydroxyl, superoxide, nitric oxide, ferric reducing power activity, and inhibition of lipid peroxidation by thiobarbituric acid reactive species assays. Increasing the level of ascorbic acid and glutathione. | [41,128,243,253,254] | |
| Anti-inflammatory activity | A. absinthium | Essential oil/Methanol | Aerial parts | nt | nt | In vivo (mice) | Reduction (41%) in inflammatory edema in mice after administration of the essential oil (at dose 4 and 8 mg/kg) or methanolic extract from A. absinthium (at dose 300, 500, and 1000 mg/kg). | [86,191] |
| nt | Aerial parts | flavonoid | 5,6,3′,5′-tetramethoxy-7,4-hydroxyflavone (p7F) | In vitro, In vivo (mice) | Inhibition of the expression of nitric oxide synthase and cyclooxygenase-2, reduction in the production of prostaglandin E2, nitric oxide, and tumor necrosis factor (TNF-α), reduction in the accumulation of reactive oxygen species by 5,6,3′,5′-tetramethoxy-7,4-hydroxyflavone isolated from A. absinthium. | [239] | ||
| nt | Aerial parts | Chalcone | Cardamonin | In vitro (THP-1 (monocyte cell line of acute monocytic leukaemia) and RAW 264.7 (cell line of mouse macrophages) | Cardamonin isolated from A. absinthium inhibits the NFĸB (nuclear factor ĸB) pathway by the direct inhibition of DNA transcription factors, which leads to reduced NO release. | [255] | ||
| Methanol | Herb | nt | nt | In vivo (rats) | Reduction in paw edema in rats given carrageenan and venom of Montivipera xanthina after the application of A. absinthium extract (at dose 25 and 50 mg/kg). | [241] | ||
| A. annua | supercritical CO2 | Herb | nt | nt | In vivo | Reduction in pain and stiffness in joints and improvement in mobility after using A. annua extract (at dose 150 mg). | [242] | |
| Aqueous | Leaves | Phenolic acid | Rosmarinic acid | Use of aqueous extracts from A. annua leaves reduces secretion of proinflammatory cytokines, IL-8 and IL-6. Rosmarinic acid is largely responsible for this effect. | [119] | |||
| A. dracunculus | Ethanol, Aqueous | Herb | nt | nt | In vivo (mice) | Reduction in pain sensations and xylene-induced ear edema after the administration of the ethanolic herbal extract (at dose 50 and 100 mg/kg) to mice. Aqueous extract inhibited ROS (by 1.4%), IL-8 (by 4.0 and 4.8%), and TNF-α (by 7.8 and 5.2%). Their production imitated inflammation. | [255] | |
| A. vulgaris | Methanol | Leaves | nt | nt | In vivo (rats) | Extract (at dose 400 mg/kg) caused the normalization of serum lipid profile, an increase in paraoxonase-1 activity, and a decrease in serum malondialdehyde, nitric oxide, and TNF-α level. Proved by lipoxygenase inhibitory activity assay and “Cotton Pellet Granuloma method.” | [214,243,256] | |
| Antiallergenic activity | A. vulgaris | Aqueous | Aerial parts | nt | nt | In vivo | Decrease in skin sensitivity and eye sensitivity. | [244] |
6.4. Artemisia ssp. in Cosmetology
| Artemisia ssp. | Producer | Country of Origin | Trade Name | Cosmetic Form | The Form of Artemisia Ssp. in the Composition of the Cosmetic (INCI) | Properties of the Cosmetic According to the Producer | References |
|---|---|---|---|---|---|---|---|
| A. abrotanum | Alpha Keri | Australia | Breast Lift And Firm | Cream | A. abrotanum extract | Firming the skin of the bust | [257] |
| Dr. Hauschka | German | Sensitive care conditioner | Ampoules | A. abrotanum flower/leaf/stem extract | The treatment in sensitive ampoules for day and night is intended for sensitive skin prone to redness and dilated blood vessels | [258] | |
| Laura Mercier | Japan | Infusion De Rose Moisturizing Glow Mask | Mask | A. abrotanum extract | Hydrates and soothes skin | [259] | |
| Dermika | Poland | Neocollagen M + Phytoestrogen Anti-Wrinkle Cream | Cream | A. abrotanum extract | Regenerating, antiwrinkle effect | [260] | |
| Aveeno | USA | Fresh Essentials Daily Nourishing Moisturizer SPF 30 | Cream | A. abrotanum extract | For daily skin hydration and protection against UV radiation | [261] | |
| Christophe Robin Paris | USA | Cleansing Mask With Lemon | Mask | A. abrotanum extract | Cleans colored and thin hair | [262] | |
| RéVive | USA | Intensité Complete Anti-Aging Eye Serum | Serum | A. abrotanum extract | Antiaging decreases the appearance of lines and wrinkles and gives skin a smoother, more youthful appearance | [263] | |
| USANA Celavive® Skincare | USA | Hydrating + Lifting Sheet Mask | Mask | A. abrotanum extract | Lifts, hydrates, and rejuvenates skin’s appearance | [264] | |
| A. absinthium | Cera Skin Care | Canada | Timeless Retinol Night Mask | Mask | A. absinthium extract | Diminishes the appearance of fine lines, wrinkles, pore size, and problematic skin imperfections | [265] |
| It cosmetics | France | No. 50 Serum Collagen Veil Anti-Aging Face Primer | Serum | A. absinthium extract | Hydrating and antiaging activity | [266] | |
| Natura Siberica | Russia | Super Siberica Krasnika, Amaranth & Arginine, Care Cream | Cream | A. absinthium herb oil | Makes hair soft and manageable | [267] | |
| MAN:YO | South Korea | Zaodam Sooc Essence Toner | Toner | A. absinthium extract | Soothes essence toner to quickly treat damaged skin | [268] | |
| Mizon | South Korea | Multi-function formula all in one snail repair cream | Cream | A. absinthium extract | Intense regenerative, moisturizing effect; narrows pores; regenerates, firms, and helps to lighten discoloration | [269] | |
| Bioelements | USA | Restorative Clay | Mask | A. absinthium oil | Cleansing skin pores | [270] | |
| Kiehl’s | USA | Calendula Deep Cleansing Foaming Face Wash | Foam | A. absinthium extract | Deeply cleansing face, cleansing foam | [271] | |
| MALIN + GOETZ | USA | Resurfacing Serum | Serum | A. absinthium oil | Smoothens, clarifies, and brightens skin | [272] | |
| Neogen Dermatology | USA | Vita Lightening Serum | Serum | A. absinthium extract | Helps to reduce the appearance of discolorations for illuminating radiance and its potent antioxidant ingredients; moisturizes and revitalizes skin | [273] | |
| Pixi | USA | Rose Glow Mist | Essence | A. absinthium extract | Strengthens skin | [274] | |
| A. annua | Commonlabs | Malaysia | Vitamin E Micro Needle Spot Cream | Cream | A. annua extract | Antiacne activity | [275] |
| Kingnature | Swiss | Artemisia creme | Cream | A. annua extract | Protects and cares for the skin and has a supporting effect on skin irritations and skin problems | [276] | |
| Su:m37 | Singapore | Losec Summa Elixir Foam Cleanser | Gel | A. annua extract | Purifies and comforts the skin | [277] | |
| Dr. Oracle | South Korea | Artemisia Ultra Calming Serum | Serum | A. annua extract, A. annua leaf extract | Skin-soothing effect to irritated or sensitive skin | [278] | |
| MISSHA | South Korea | Artemisia Calming Ampoule | Essence | A. annua extract | Controls the balance of hydration and lubrication of the skin, soothes irritation and redness, controls the balance of hydration and lubrication of the skin, and soothes irritation and redness | [279] | |
| Neogen Dermatology | USA | Dermalogy Green Tea Moist PHA Gauze Peeling | Peeling | A. annua extract | Exfoliates and moisturizes skin | [273] | |
| PURE’AM | USA | Authentic Barrier Cream Balm | Cream | A. annua extract | Nourishes, repairs, and strengthens natural skin barrier | [280] | |
| A. dracunculus | ESPA | Great Britain | Age-Rebel Moisturiser | Cream | A. dracunculus oil | Moisturizes, nourishes, and smoothens skin | [281] |
| Lush | Great Britain | Dirty Shampoo | Shampoo | A. dracunculus oil | Cleanses hair | [282] | |
| Hayejin | South Korea | Blessing Of Sprout Radiance Toner | Toner | A. dracunculus leaf/stem extract | Brightens skin’s complexion, balances pH level, and moisturizes the skin | [283] | |
| Onekind | USA | Mega Multitasker All-Day Moisturizer | Cream | A. dracunculus oil | Hydrating, has antioxidant activity, and defends against daily damage | [284] | |
| A. vulgaris | Humphrey | Canada | Mugwort Anti Acne Serum | Serum | A. vulgaris extract | Treats acne, reduces inflammation on acne-prone skin, soothes and moisturizes skin | [285] |
| Vgam | Canada | Pure Artik | Gel | A. vulgaris extract | Gently removes impurities and protects skin | [286] | |
| Annayake | France | Makeup Remover Gel | Gel | A. vulgaris extract | Cleanses face and eye and removes makeup | [287] | |
| Cherry Brenchez | Great Britain | Venus Reviver Serum | Serum | A. vulgaris oil | Moisturizes skin, reduces spots and fine lines, and protects skin from sun damage | [288] | |
| Monuskin | Great Britain | Rosewood Reviving Mist | Essence | A. vulgaris oil | Refreshes and revitalizes skin | [289] | |
| R10 Labs | Great Britain | Hybrid Iq Shaving Gel-Oil | Gel | A. vulgaris oil | Softens the hair and makes it easier to shave | [290] | |
| Somethinc | Indonesia | AHA 7% BHA 1% PHA 3% Weekly Peeling Solution | Peeling | A. vulgaris extract | Helps clean clogged pores and remove dead skin cells | [291] | |
| Moraz | Israel | Body Oil Skin Saver | Oil | A. vulgaris extract | Hydrating and reduces burns, redness, itching and dryness | [292] | |
| Manuka Doctor | New Zealand | Apiclear Purifying Facial Peel | Peeling | A. vulgaris extract | Removes dead cells and stimulates cell renewal | [293] | |
| Skintific | Norway | Mugwort Anti Pores & Acne Clay Mask Pore Clarifying Wash Off Pack | Mask | A. vulgaris extract | Helps clean clogged pores, reduces skin changes, and brightens skin | [294] | |
| Natura Siberica | Russia | Anti Dandruff Shampoo | Shampoo | A. vulgaris extract | Cleanses the hair and has antidandruff properties | [267] | |
| Aprilskin | South Korea | Artemisia Essence Rice Toner | Toner | A. vulgaris extract | Calms and hydrates skin and makes skin firm | [295] | |
| I’m From | South Korea | Mugwort Spot Gel | Gel | A. vulgaris oil | Stabilizes sebum production and soothes skin | [296] | |
| Manyo Factory | South Korea | Herb Green Cleansing Oil | Cleansing oil | A. vulgaris oil | Cleanses skin | [268] | |
| Dermalogica | USA | Overnight Active Clearing Gel | Gel | A. vulgaris oil | Removes skin cells and regulates excess sebum | [297] | |
| Rms Beauty | USA | “re” Evolve Radiance Locking Hydrating Primer | Primer | A. vulgaris oil | Keeps makeup all day long | [298] |
7. Safety of Artemisia ssp. Use
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Podbielkowski, Z.; Sudnik-Wójcikowska, B. Słownik Roślin Użytkowych; Wydanie VI; Państwowe Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 2003. (In Polish) [Google Scholar]
- Aglarova, A.M.; Zilfikarov, I.N.; Severtseva, O.V. Biological characteristics and useful properties of tarragon (Artemisia dracunculus L.) (review). Pharm. Chem. J. 2008, 42, 81–86. [Google Scholar] [CrossRef]
- Majeed, I.; Rizwan, K.; Ashar, A.; Rasheed, T.; Amarowicz, R.; Kausar, H.; Zia-ul-haq, M.; Marceanu, L.G. A comprehensive review of the ethnotraditional uses and biological and pharmacological potential of the genus Mimosa. Int. J. Mol. Sci. 2021, 22, 7463. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, I.; Zubair, M.; Rizwan, K.; Rasool, N.; Jamil, M.; Khan, S.A.; Tareen, R.B.; Ahmad, V.U.; Mahmood, A.; Riaz, M.; et al. Chemical composition, antioxidant and antimicrobial potential of essential oils from different parts of Daphne mucronata Royle. Chem. Cent. J. 2018, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Rasool, N.; Afzal, S.; Riaz, M.; Rashid, U.; Rizwan, K.; Zubair, M.; Ali, S.; Shahid, M. Evaluation of antioxidant activity, cytotoxic studies and GC-MS profiling of Matthiola incana (Stock flower). Legum. Res. 2013, 36, 21–32. [Google Scholar]
- Ekiert, H.; Kubica, P.; Szopa, A. Successful cultivation and utilization of Aronia melanocarpa (Michx.) Elliott (Black Chokeberry), a species of North-American origin, in Poland and the biosynthetic potential of cells from in vitro cultures. In Medicinal Plants. Sustainable Development and Biodiversity; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Cham, Switzerland, 2021; Volume 28, pp. 69–111. [Google Scholar]
- Jafernik, K.; Ekiert, H.; Szopa, A. Schisandra chinensis and Schisandra sphenanthera—From traditional Far Eastern medicine to international utilization. In Medicinal Plants. Sustainable Development and Biodiversity; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Cham, Switzerland, 2021; Volume 28, pp. 179–227. [Google Scholar]
- The Herb Society of America. Artemesia: An Essential Guide 2014; The Herb Society of America: Kirtland, OH, USA, 2014. [Google Scholar]
- Ahamad, J.; Mir, S.R.; Amin, S. A Pharmacognostic review on Artemisia absinthium. Int. Res. J. Pharm. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- World Health Organization. WHO Monograph on Good Agricultural and Collection Practices (GACP) for Artemisia annua L.; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Sharopov, F.S.; Salimov, A.; Numonov, S.; Bakri, M.; Sangov, Z.; Habasi, M.; Akber Aisa, H.; Setzer, W.N. Chemical compositions and biological activities of essential oils-original article phytochemical study on the essential oils of tarragon (Artemisia dracunculus L.) growing in Tajikistan and its comparison with the essential oil of the species in the rest. Nat. Prod. Commun. 2020, 15, 1–7. [Google Scholar]
- Mamedov, N.; Grdner, Z.; Craker, L.E. Medicinal plants used in Central Asia for the treatment of selected skin conditions. J. Herbs Spices Med. Plants 2004, 11, 191–222. [Google Scholar] [CrossRef]
- Mohammadian, A.; Moradkhani, S.; Ataei, S.; Shayesteh, T.H.; Sedaghat, M.; Kheiripour, N.; Ranjbar, A. Antioxidative and hepatoprotective effects of hydroalcoholic extract of Artemisia absinthium L. in rat. J. HerbMed Pharmacol. 2016, 5, 29–32. [Google Scholar]
- Bora, K.S.; Sharma, A. Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J. Ethnopharmacol. 2010, 129, 403–409. [Google Scholar] [CrossRef]
- Sansar, W.; Gamrani, H. The pharmacological effect of Artemisia absinthium extract in protecting adult rats against lead neurotoxicity. J. Neurol. Sci. 2013, 333, e598. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Ebrahimzadeh, M.A.; Ansaroudi, F.; Nabavi, S.F.; Nabavi, S.M. Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. African J. Biotechnol. 2009, 8, 7170–7175. [Google Scholar]
- Shafi, G.; Hasan, T.N.; Syed, N.A.; Al-Hazzani, A.A.; Alshatwi, A.A.; Jyothi, A.; Munshi, A. Artemisia absinthium (AA): A novel potential complementary and alternative medicine for breast cancer. Mol. Biol. Rep. 2012, 39, 7373–7379. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against Gram-positive pathogenic bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef]
- McMullen, M.K.; Whitehouse, J.M.; Whitton, P.A.; Towell, A. Bitter tastants alter gastric-phase postprandial haemodynamics. J. Ethnopharmacol. 2014, 154, 719–727. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Polyphenol content and biological activities of Ruta graveolens L. and Artemisia abrotanum L. in northern Saudi Arabia. Processes 2020, 8, 531. [Google Scholar] [CrossRef]
- Navarro-Salcedo, M.H.; Delgado-Saucedo, J.I.; Siordia-Sánchez, V.H.; González-Ortiz, L.J.; Castillo-Herrera, G.A.; Puebla-Pérez, A.M. Artemisia dracunculus extracts obtained by organic solvents and supercritical CO2 produce cytotoxic and antitumor effects in mice with L5178Y lymphoma. J. Med. Food 2017, 20, 1076–1082. [Google Scholar] [CrossRef]
- Mohammadi, M.M.; Saeb, M.; Nazifi, S. Experimental hypothyroidism in adult male rats: The effects of Artemisia dracunculus aqueous extract on serum thyroid hormones, lipid profile, leptin, adiponectin, and antioxidant factors. Comp. Clin. Path. 2020, 29, 485–494. [Google Scholar] [CrossRef]
- Lee, S.J.; Chung, H.Y.; Maier, C.G.A.; Wood, A.R.; Dixon, R.A.; Mabry, T.J. Estrogenic flavonoids from Artemisia vulgaris L. J. Agric. Food Chem. 1998, 46, 3325–3329. [Google Scholar] [CrossRef]
- Shaik, A.; Kanhere, R.S.; Cuddapah, R.; Nelson, K.S.; Vara, P.R.; Sibyala, S. Antifertility activity of Artemisia vulgaris leaves on female Wistar rats. Chin. J. Nat. Med. 2014, 12, 180–185. [Google Scholar] [CrossRef]
- Zafar, M.M.; Hamdard, M.E.; Hameed, A. Screening of Artemisia absinthium for antimalarial effects on Plasmodium berghei in mice: A preliminary report. J. Ethnopharmacol. 1990, 30, 223–226. [Google Scholar]
- Ramazani, A.; Sardari, S.; Zakeri, S.; Vaziri, B. In vitro antiplasmodial and phytochemical study of five Artemisia species from Iran and in vivo activity of two species. Parasitol. Res. 2010, 107, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Siddiqui, M.M.H.; Khan, A.B. Effect of Afsanteen (Artemisia absinthium Linn.) in acute intestinal amoebiasis. Hamdard Med. 1997, 40, 24–27. [Google Scholar]
- Valdés, A.F.C.; Martínez, J.M.; Lizama, R.S.; Vermeersch, M.; Cos, P.; Maes, L. In vitro anti-microbial activity of the Cuban medicinal plants Simarouba glauca DC, Melaleuca leucadendron L and Artemisia absinthium L. Mem. Inst. Oswaldo Cruz 2008, 103, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Tariku, Y.; Hymete, A.; Hailu, A.; Rohloff, J. In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem. Biodivers. 2011, 8, 614–623. [Google Scholar] [CrossRef]
- Gonzalez-Coloma, A.; Bailen, M.; Diaz, C.E.; Fraga, B.M.; Martínez-Díaz, R.; Zuñiga, G.E.; Contreras, R.A.; Cabrera, R.; Burillo, J. Major components of Spanish cultivated Artemisia absinthium populations: Antifeedant, antiparasitic, and antioxidant effects. Ind. Crops Prod. 2012, 37, 401–407. [Google Scholar] [CrossRef]
- Bailen, M.; Julio, L.F.; Diaz, C.E.; Sanz, J.; Martinez-Diaz, R.A.; Cabrera, R.; Burillo, J.; Gonzalez-Coloma, A. Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Ind. Crops Prod. 2013, 49, 102–107. [Google Scholar] [CrossRef]
- Martínez-Díaz, R.A.; Ibáñez-Escribano, A.; Burillo, J.; de las Heras, L.; del Prado, G.; Agulló-Ortuño, M.T.; Julio, L.F.; González-Coloma, A. Trypanocidal, trichomonacidal and cytotoxic components of cultivated Artemisia absinthium Linnaeus (Asteraceae) essential oil. Mem. Inst. Oswaldo Cruz 2015, 110, 693–699. [Google Scholar] [CrossRef]
- Mendiola, J.; Bosa, M.; Perez, N.; Hernandez, H.; Torre, D. Extracts of Artemisia abrotanum and Artemisia absinthium inhibit growth of Naegleria fowleri in vitro. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 78–79. [Google Scholar] [CrossRef]
- Hernandez, H.; Mendiola, J.; Torres, D.; Garrido, N.; Perez, N. Effect of aqueous extracts of Artemisia on the in vitro culture of Plasmodium falciparum. Fitoterapia 1990, 41, 540–541. [Google Scholar]
- Wasowicz, A. Occurrence of Artemisia annua L. in Wroclaw city area (Lower Silesia, Poland). Acta Bot. Silesiaca 2004, 1, 141–146. [Google Scholar]
- Mirzaei, F.; Bafghi, A.F.; Mohaghegh, M.A.; Jaliani, H.Z.; Faridnia, R.; Kalani, H. In vitro anti-leishmanial activity of Satureja hortensis and Artemisia dracunculus extracts on Leishmania major promastigotes. J. Parasit. Dis. 2016, 40, 1571–1574. [Google Scholar] [CrossRef]
- Islamuddin, M.; Farooque, A.; Dwarakanath, B.S.; Sahal, D.; Afrin, F. Extracts of Artemisia annua leaves and seeds mediate programmed cell death in Leishmania donovani. J. Med. Microbiol. 2012, 61, 1709–1718. [Google Scholar] [CrossRef]
- Wake, G.; Court, J.; Pickering, A.; Lewis, R.; Wilkins, R.; Perry, E. CNS acetylcholine receptor activity in European medicinal plants traditionally used to improve failing memory. J. Ethnopharmacol. 2000, 69, 105–114. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Chan, K.W.; Zia-Ul-Haq, M.; Ismail, M. Chemical composition of Artemisia annua L. leaves and antioxidant potential of extracts as a function of extraction solvents. Molecules 2012, 17, 6020–6032. [Google Scholar] [CrossRef]
- Zarezade, V.; Moludi, J.; Mostafazadeh, M.; Mohammadi, M.; Veisi, A. Antioxidant and hepatoprotective effects of Artemisia dracunculus against CCl4-induced hepatotoxicity in rats. Avicenna J. Phytomedicine 2018, 8, 51–62. [Google Scholar]
- Baykan Erel, Ş.; Reznicek, G.; Şenol, S.G.; Karabay Yavaşoğulu, N.Ü.; Konyalioğlu, S. Antimicrobial and antioxidant properties of Artemisia L. species from western Anatolia. Turk J Biol 2012, 75, 75–84. [Google Scholar]
- World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 9 August 2022).
- Ekiert, H.; Knut, E.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Tomczyk, M.; Szopa, A. Artemisia abrotanum L. (southern wormwood)—history, current knowledge on the chemistry, biological activity, traditional use and possible new pharmaceutical and cosmetological applications. Molecules 2021, 26, 2503. [Google Scholar] [CrossRef]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.—importance in the history of medicine, the latest advances in phytochemistry and therapeutical, cosmetological and culinary uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua—Importance in traditional medicine and current state of knowledge on the chemistry, biological activity and possible applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef]
- Ekiert, H.; Pajor, J.; Klin, P.; Rzepiela, A.; Slesak, H.; Szopa, A. Significance of Artemisia vulgaris L. (Common Mugwort) in the history of medicine and its possible contemporary applications substantiated by phytochemical and pharmacological studies. Molecules 2020, 25, 4415. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Knut, E.; Klin, P.; Rzepiela, A.; Tomczyk, M.; Szopa, A. Artemisia dracunculus (Tarragon): A review of its traditional uses, phytochemistry and pharmacology. Front. Pharmacol. 2021, 12, 653993. [Google Scholar]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Chemical composition, traditional and professional use in medicine, application in environmental protection, position in food and cosmetics industries, and biotechnological studies of Nasturtium officinale (watercress)—A review. Fitoterapia 2018, 129, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Khare, C.P. Indian Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; Timber Press: Portland, OR, USA, 2004. [Google Scholar]
- GBIF.org. GBIF—The Global Biodiversity Information Facility. Copenhagen, Denmark. 2020. Available online: https://www.gbif.org/ (accessed on 10 December 2021).
- Watson, B.; Kennel, E. Artemesia spp. Available online: https://www.herbsociety.org (accessed on 10 August 2022).
- Eisenman, S.W.; Poulev, A.; Struwe, L.; Raskin, I.; Ribnicky, D.M. Qualitative variation of anti-diabetic compounds in different tarragon (Artemisia dracunculus L.) cytotypes. Fitoterapia 2011, 82, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Obolskiy, D.; Pischel, I.; Feistel, B.; Glotov, N.; Heinrich, M. Artemisia dracunculus L. (tarragon): A critical review of its traditional use, chemical composition, pharmacology, and safety. J. Agric. Food Chem. 2011, 59, 11367–11384. [Google Scholar] [CrossRef]
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis, 3rd ed.; Medpharm: Marburg, Germany, 2004. [Google Scholar]
- Watson, L.E.; Bates, P.L.; Evans, T.M.; Unwin, M.M.; Estes, J.R. Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera. BMC Evol. Biol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar]
- Baiceanu, E.; Vlase, L.; Baiceanu, A.; Nanes, M.; Rusu, D.; Crisan, G. New polyphenols identified in Artemisiae abrotani herba extract. Molecules 2015, 20, 11063–11075. [Google Scholar] [CrossRef]
- Prezes Urzędu Rejestracji Produktów Leczniczych Wyrobów Medycznych i Produktów Biobójczych. Farmakopea Polska XI Tom II; Polskie Towarzystwo Farmaceutyczne: Warszawa, Poland, 2017. [Google Scholar]
- Sudnik-Wójcikowska, B. Rośliny Synantropijne; Multico: Warszawa, Poland, 2011. [Google Scholar]
- Zhang, R. The Discovery and development of artemisinins and antimalarial agents. In From Artemisia annua L. to Artemisinins; Tu, Y., Ed.; Chemical Industry Press, Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128116555. [Google Scholar]
- Bakova, N.; Logvinenko, L.; Shevchuk, O. Tarragon cultivars (Artemisia dracunculus L.) of the Nikita Botanical Gardens breeding. In Proceedings of the VIII International Scientific Agriculture Symposium “AGROSYM 2017”, Jahorina, Bosnia Herzegovina, 5–8 October 2017; Volume 1, pp. 445–451. [Google Scholar]
- Koul, B.; Taak, P. The Artemisia genus: A review on traditional uses, phytochemical constituents, pharmacological properties and germplasm conservation. J. Glycom. Lipidom. 2017, 7, 142. [Google Scholar] [CrossRef]
- Weston, L.A.; Barney, J.N.; DiTommaso, A. A review of the biology and ecology of three invasive perennials in New York State: Japanese knotweed (Polygonum cuspidatum), mugwort (Artemisia vulgaris) and pale swallow-wort (Vincetoxicum rossicum). Plant Soil 2005, 277, 53–69. [Google Scholar] [CrossRef]
- Anwar, F.; Ahmad, N.; Alkharfy, K.M.; Gilani, A.H. Mugwort (Artemisia vulgaris) Oils; Preedy, V.R., Ed.; Academic Press: London, UK, 2016; ISBN 9780124166448. [Google Scholar]
- Barney, J.N.; DiTommaso, A. The biology of Canadian weeds. 118. Artemisia vulgaris L. Can. J. Plant Sci. 2003, 83, 205–215. [Google Scholar] [CrossRef]
- Gleason, H.A.; Cronquist, A. Manual of Vascular Plants of Northeastern United States and Adjacent Canada, 2nd ed.; The New York Botanical Garden: New York, NY, USA, 1991. [Google Scholar]
- Efferth, T.; Zacchino, S.; Georgiev, M.; Liu, L.; Wagner, H.; Panossian, A. Nobel Prize for artemisinin brings phytotherapy into the spotlight. Phytomedicine 2015, 22, A1–A3. [Google Scholar] [CrossRef]
- Willcox, M.; Bodeker, G.; Bourdy, G.; Dhingra, V.; Falquet, J.; Ferreira, J.F.S.; Graz, B.; Hirt, H.; Hsu, E.; De Magalhães, P.M.; et al. Traditional Medicinal Plants in Malaria; Willcox, M.L., Bodeker, G., Rasoanaivo, P., Eds.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 0415301122. [Google Scholar]
- Cala, A.C.; Ferreira, J.F.S.; Chagas, A.C.S.; Gonzalez, J.M.; Rodrigues, R.A.F.; Foglio, M.A.; Oliveira, M.C.S.; Sousa, I.M.O.; Magalhães, P.M.; Barioni, W. Anthelmintic activity of Artemisia annua L. extracts in vitro and the effect of an aqueous extract and artemisinin in sheep naturally infected with gastrointestinal nematodes. Parasitol. Res. 2014, 113, 2345–2353. [Google Scholar] [CrossRef]
- Garcia, L.C. A Review of Artemisia annua L.: Its genetics, biochemical characteristics, and anti-malarial efficacy. Int. J. Sci. Technol. 2015, 5, 38–46. [Google Scholar]
- Elfawal, M.A.; Towler, M.J.; Reich, N.G.; Golenbock, D.; Weathers, P.J.; Rich, S.M. Dried whole plant Artemisia annua as an antimalarial therapy. PLoS ONE 2012, 7, e52746. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Weathers, P.J.; Towler, M.; Hassanali, A.; Lutgen, P.; Ogwang Engeu, P. Dried-leaf Artemisia annua: A practical malaria therapeutic for developing countries? World J. Pharmacol. 2014, 3, 39–55. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Walch, S.G.; Padosch, S.A.; Kröner, L.U. Absinthe—A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 365–377. [Google Scholar] [CrossRef]
- Beigh, Y.A.; Ganai, A.M. Potential of Wormwood (Artemisia absinthium Linn.) herb for use as additive in livestock feeding: A review. Pharma Innov. J. 2017, 6, 176–187. [Google Scholar]
- Singh, P.; Bajpai, V.; Khandelwal, N.; Varshney, S.; Gaikwad, A.N.; Srivastava, M.; Singh, B.; Kumar, B. Determination of bioactive compounds of Artemisia spp. plant extracts by LC–MS/MS technique and their in-vitro anti-adipogenic activity screening. J. Pharm. Biomed. Anal. 2021, 193, 113707. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Martí, M.P. Leaf oil of Artemisia abrotanum L. grown in Cuba. J. Essent. Oil Res. 2011, 23, 119–120. [Google Scholar]
- Muangphrom, P.; Misaki, M.; Suzuki, M.; Shimomura, M.; Suzuki, H.; Seki, H.; Muranaka, T. Identification and characterization of (+)-α-bisabolol and 7-epi-silphiperfol-5-ene synthases from Artemisia abrotanum. Phytochemistry 2019, 164, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.A.; El-Gohary, A.E. Productivity of wormwood (Artemisia abrotanum) enhanced by trace elements. Bull. Natl. Res. Cent. 2020, 44, 120. [Google Scholar]
- Obistioiu, D.; Cristina, R.T.; Schmerold, I.; Chizzola, R.; Stolze, K.; Nichita, I.; Chiurciu, V. Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris. Chem. Cent. J. 2014, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Aruba, O.S.; Jasim, G.A.; Nasser, A.A. Detection of terpenes of Iraqi Artemisia abrotanum L. by GC/MS in hexane extract. Al Mustansiriyah J. Pharm. Sci. 2019, 19, 239–248. [Google Scholar] [CrossRef]
- Khodakov, G.V.; Kotikov, I.V.; Pankovetskii, V.N. Component composition of essential oil from Artemisia abrotanum and A. dracunculus. Chem. Nat. Compd. 2009, 45, 755–758. [Google Scholar] [CrossRef]
- Saunoriute, S.; Ragažinskiene, O.; Ivanauskas, L.; Marksa, M. Essential oil composition of Artemisia abrotanum L. during different vegetation stages in Lithuania. Chemija 2020, 31, 52–56. [Google Scholar] [CrossRef]
- Juteau, F.; Jerkovic, I.; Masotti, V.; Milos, M.; Mastelic, J.; Bessière, J.M.; Viano, J. Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med. 2003, 69, 158–161. [Google Scholar]
- Safayhi, H.; Sabieraj, J.; Sailer, E.; Ammon, H. An antioxidant-type inhibitor of leukotriene B4 formation. Planta Med. 1994, 60, 410–413. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, J.J.; Andres, M.F.; Ibanez-Escribano, A.; Julio, L.F.; Burillo, J.; Bolas-Fernandez, F.; Gonzalez-Coloma, A. Selective nematocidal effects of essential oils from two cultivated Artemisia absinthium populations. Z. Für Naturforsch. C 2015, 70, 275–280. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Haj Brahim, A.; et al. Chemical composition and antioxidant and antimicrobial activities of wormwood (Artemisia absinthium L.) essential oils and phenolics. J. Chem. 2015, 2015, 804658. [Google Scholar]
- Blagojević, P.; Radulović, N.; Palić, R.; Stojanović, G. Chemical composition of the essential oils of Serbian wild-growing Artemisia absinthium and Artemisia vulgaris. J. Agric. Food Chem. 2006, 54, 4780–4789. [Google Scholar]
- Ali, M.; Abbasi, B.H. Ihsan-ul-haq Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind. Crops Prod. 2013, 49, 400–406. [Google Scholar] [CrossRef]
- Hwang, D.I.; Won, K.J.; Kim, D.Y.; Yoon, S.W.; Park, J.H.; Kim, B.; Lee, H.M. Anti-adipocyte differentiation activity and chemical composition of essential oil from Artemisia annua. Nat. Prod. Commun. 2016, 11, 539–542. [Google Scholar]
- Donato, R.; Santomauro, F.; Bilia, A.R.; Flamini, G.; Sacco, C. Antibacterial activity of Tuscan Artemisia annua essential oil and its major components against some foodborne pathogens. LWT Food Sci. Technol. 2015, 64, 1251–1254. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.; Chifiriuc, M.C.; Badea, I.A.; Buleandra, M.; Lazar, V. Chemical composition and antipathogenic activity of Artemisia annua essential oil from Romania. Chem. Biodivers. 2015, 12, 1554–1564. [Google Scholar] [CrossRef]
- Radulović, N.S.; Randjelović, P.J.; Stojanović, N.M.; Blagojević, P.D.; Stojanović-Radić, Z.Z.; Ilić, I.R.; Djordjević, V.B. Toxic essential oils. Part II: Chemical, toxicological, pharmacological and microbiological profiles of Artemisia annua L. volatiles. Food Chem. Toxicol. 2013, 58, 37–49. [Google Scholar] [CrossRef]
- Kazemi, M. Essential oil of the aerial parts of Artemisia annua (Asteraceae) from Iran. J. Essent. Oil-Bear. Plants 2015, 18, 1003–1005. [Google Scholar] [CrossRef]
- Engeu, P.O.; Omujal, F.; Agwaya, M.; Kyakulaga, H.; Obua, C. Variations in antimalarial components of Artemisia annua Linn from three regions of Uganda. Afr. Health Sci. 2015, 15, 828–834. [Google Scholar]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Artemisia spp. essential oils against the disease-carrying blowfly Calliphora vomitoria. Parasites Vectors 2017, 10, 80. [Google Scholar]
- Bussmann, R.W.; Batsatsashvili, K.; Kikvidze, Z.; Khajoei Nasab, F.; Ghorbani, A.; Paniagua-Zambrana, N.Y.; Khutsishvili, M.; Maisaia, I.; Sikharulidze, S.; Tchelidze, D. Artemisia absinthium L. Artemisia annua L. Artemisia dracunculus L. Artemisia leucodes Schrenk Artemisia scoparia Waldst. and Kit. Artemisia vulgaris L. Eclipta prostrata (L.) L. Asteraceae. In Ethnobotany of the Mountain Regions of Far Eastern Europe; Batsatsashvili, K., Kikvidze, Z., Bussmann, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 131–146. [Google Scholar]
- Karimi, A.; Hadian, J.; Farzaneh, M.; Khadivi-Khub, A. Phenotypic diversity and volatile composition of Iranian Artemisia dracunculus. Ind. Crops Prod. 2015, 65, 315–323. [Google Scholar] [CrossRef]
- Ayoughi, F.; Barzegar, M.; Sahari, M.A.; Naghdibadi, H. Chemical compositions of essential oils of Artemisia dracunculus L. and endemic Matricaria chamomilla L. and an evaluation of their antioxidative effects. J. Agric. Sci. Technol. 2011, 13, 79–88. [Google Scholar]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J. Food Meas. Charact. 2017, 11, 847–863. [Google Scholar] [CrossRef]
- Abdollahnejad, F.; Kobarfard, F.; Kamalinejad, M.; Mehrgan, H.; Babaeian, M. Yield, chemical composition and antibacterial activity of Artemisia dracunculus L. essential oils obtained by two different methods. J. Essent. Oil-Bear. Plants 2016, 19, 574–581. [Google Scholar] [CrossRef]
- Osanloo, M.; Amani, A.; Sereshti, H.; Abai, M.R.; Esmaeili, F.; Sedaghat, M.M. Preparation and optimization nanoemulsion of Tarragon (Artemisia dracunculus) essential oil as effective herbal larvicide against Anopheles stephensi. Ind. Crops Prod. 2017, 109, 214–219. [Google Scholar] [CrossRef]
- Szczepanik, M.; Walczak, M.; Zawitowska, B.; Michalska-Sionkowska, M.; Szumny, A.; Wawrzeńczyk, C.; Brzezinska, M.S. Chemical composition, antimicrobial activity and insecticidal activity against the lesser mealworm Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) of Origanum vulgare L. ssp. hirtum (Link) and Artemisia dracunculus L. essential oils. J. Sci. Food Agric. 2018, 98, 767–774. [Google Scholar] [CrossRef]
- Govindaraj, S.; Ranjitha Kumari, B.D. Composition and larvicidal activity of Artemisia vulgaris L. stem essential oil against Aedes aegypti. Jordan J. Biol. Sci. 2013, 6, 11–16. [Google Scholar] [CrossRef][Green Version]
- Govindaraj, S.; Kumari, B.D.R.; Cioni, P.L.; Flamini, G. Mass propagation and essential oil analysis of Artemisia vulgaris. J. Biosci. Bioeng. 2008, 105, 176–183. [Google Scholar] [CrossRef]
- Judžentien, A.; Buzelyte, J. Chemical composition of essential oils of Artemisia vulgaris L. (mugwort) from North Lithuania. Chemija 2006, 17, 12–15. [Google Scholar]
- Pickenhagen, W.; Willhalm, B. New irregular monoterpenes in Artemisia vulgaris. Helvetica 1981, 64, 1424–1430. [Google Scholar]
- Madhav, K.; Kunal, M.; Zafar, H.; Ujjwal, B.; Gaurav, N. Antioxidant analysis of essential oils and methanolic extracts of Artemisia vulgaris. Int. J. Agric. Sci. 2018, 10, 5710–5713. [Google Scholar]
- Weathers, P.J.; Towler, M.J. The flavonoids casticin and artemetin are poorly extracted and are unstable in an Artemisia annua tea infusion. Planta Med. 2012, 78, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [PubMed]
- Aydin, T.; Akincioglu, H.; Gumustas, M.; Gulcin, I.; Kazaz, C.; Cakir, A. Human monoamine oxidase (hMAO) A and hMAO B inhibitors from Artemisia dracunculus L. herniarin and skimmin: Human mononamine oxidase A and B inhibitors from A. dracunculus L. Z. Fur Naturforsch. Sect. C J. Biosci. 2020, 75, 459–466. [Google Scholar] [CrossRef]
- Mumivand, H.; Babalar, M.; Tabrizi, L.; Craker, L.E.; Shokrpour, M.; Hadian, J. Antioxidant properties and principal phenolic phytochemicals of Iranian tarragon (Artemisia dracunculus L.) accessions. Hortic. Environ. Biotechnol. 2017, 58, 414–422. [Google Scholar] [CrossRef]
- Jahani, R.; Khaledyan, D.; Jahani, A.; Jamshidi, E.; Kamalinejad, M.; Khoramjouy, M.; Faizi, M. Evaluation and comparison of the antidepressant-like activity of Artemisia dracunculus and Stachys lavandulifolia ethanolic extracts: An in vivo study. Res. Pharm. Sci. 2019, 14, 544–553. [Google Scholar]
- Güvenalp, Z.; Özbek, H.; Dursunoğlu, B.; Yuca, H.; Gözcü, S.; Çil, Y.M.; Kazaz, C.; Kara, K.; Demirezer, Ö.L. α-Amylase and α-glucosidase inhibitory activities of the herbs of Artemisia dracunculus L. and its active constituents. Med. Chem. Res. 2017, 26, 3209–3215. [Google Scholar] [CrossRef]
- Tunón, H.; Thorsell, W.; Mikiver, A.; Malander, I. Arthropod repellency, especially tick (Ixodes ricinus), exerted by extract from Artemisia abrotanum and essential oil from flowers of Dianthus caryophyllum. Fitoterapia 2006, 77, 257–261. [Google Scholar]
- Remberg, P.; Björk, L.; Hedner, T.; Sterner, O. Characteristics, clinical effect profile and tolerability of a nasal spray preparation of Artemisia abrotanum L. for allergic rhinitis. Phytomedicine 2004, 11, 36–42. [Google Scholar] [CrossRef]
- Van Der Kooy, F.; Sullivan, S.E. The complexity of medicinal plants: The traditional Artemisia annua formulation, current status and future perspectives. J. Ethnopharmacol. 2013, 150, 1–13. [Google Scholar] [CrossRef]
- Melillo De Magalhães, P.; Dupont, I.; Hendrickx, A.; Joly, A.; Raas, T.; Dessy, S.; Sergent, T.; Schneider, Y.J. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem. 2012, 134, 864–871. [Google Scholar] [CrossRef]
- Wallnofer, B.; Hofner, O.; Greger, H. Polyacetylenes from the Artemisia ‘Vulgares’ group. Phytochemistry 1989, 28, 2687–2691. [Google Scholar] [CrossRef]
- Hatziieremia, S.; Gray, A.; Ferro, V.; Paul, A.; Plevin, R. The effects of cardamonin on lipopolysaccharide- induced inflammatory protein production and MAP kinase and NFjB signalling pathways in monocytes/macrophages. Br. J. Pharmacol. 2006, 149, 188–198. [Google Scholar] [CrossRef]
- Hadi, A.; Hossein, N.; Shirin, P.; Najmeh, N.; Abolfazl, M. Anti-inflammatory and analgesic activities of Artemisia absinthium and chemical composition of its essential oil. Int. J. Pharm. Sci. Rev. Res. 2014, 38, 237–244. [Google Scholar]
- Ko, Y.S.; Lee, W.S.; Panchanathan, R.; Joo, Y.N.; Choi, Y.H.; Kim, G.S.; Jung, J.M.; Ryu, C.H.; Shin, S.C.; Kim, H.J. Polyphenols from Artemisia annua L inhibit adhesion and EMT of highly metastatic breast cancer cells MDA-MB-231. Phyther. Res. 2016, 30, 1180–1188. [Google Scholar] [CrossRef]
- Carbonara, T.; Pascale, R.; Argentieri, M.P.; Papadia, P.; Fanizzi, F.P.; Villanova, L.; Avato, P. Phytochemical analysis of a herbal tea from Artemisia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar]
- Ribeiro, A.; Barros, L.; Calhelha, R.C.; Carocho, M.; Ćirić, A.; Sokovic, M.; Dias, M.M.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. Tarragon phenolic extract as a functional ingredient for pizza dough: Comparative performance with ascorbic acid (E300). J. Funct. Foods 2016, 26, 268–278. [Google Scholar]
- Majdan, M.; Kiss, A.K.; Hałasa, R.; Granica, S.; Osińska, E.; Czerwińska, M.E. Inhibition of neutrophil functions and antibacterial effects of tarragon (Artemisia dracunculus L.) infusion—phytochemical characterization. Front. Pharmacol. 2020, 11, 947. [Google Scholar] [CrossRef]
- Carnat, A.; Heitz, A.; Fraisse, D.; Carnat, A.P.; Lamaison, J.L. Major dicaffeoylquinic acids from Artemisia vulgaris. Fitoterapia 2000, 71, 587–589. [Google Scholar]
- Melguizo-Melguizo, D.; Diaz-de-Cerio, E.; Quirantes-Piné, R.; Švarc-Gajić, J.; Segura-Carretero, A. The potential of Artemisia vulgaris leaves as a source of antioxidant phenolic compounds. J. Funct. Foods 2020, 5, 192–200. [Google Scholar] [CrossRef]
- Ahamad, J.; Naquvi, K.; Ali, M.; Mir, S. New glycoside esters from the aerial parts of Artemisia absinthium Linn. Nat. Prod. J. 2014, 3, 260–267. [Google Scholar]
- Zeng, K.W.; Liao, L.X.; Song, X.M.; Lv, H.N.; Song, F.J.; Yu, Q.; Dong, X.; Jiang, Y.; Tu, P.F. Caruifolin D from Artemisia absinthium L. inhibits neuroinflammation via reactive oxygen species-dependent c-jun N-terminal kinase and protein kinase c/NF-κB signaling pathways. Eur. J. Pharmacol. 2015, 767, 82–93. [Google Scholar] [CrossRef] [PubMed]
- De Donno, A.; Grassi, T.; Idolo, A.; Guido, M.; Papadia, P.; Caccioppola, A.; Villanova, L.; Merendino, A.; Bagordo, F.; Fanizzi, F.P. First-time comparison of the in vitro antimalarial activity of Artemisia annua herbal tea and artemisinin. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Ko, K.H.; Heo, R.W.; Yi, C.O.; Shin, H.J.; Kim, J.Y.; Park, J.H.; Nam, S.; Kim, H.; Roh, G.S. Artemisia annua leaf extract attenuates hepatic steatosis and inflammation in high-fat diet-fed mice. J. Med. Food 2016, 19, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Phadungrakwittaya, R.; Chotewuttakorn, S.; Piwtong, M.; Thamsermsang, O.; Laohapand, T.; Akarasereenont, P. Identification of apigenin and luteolin in Artemisia annua l. for the quality control. Siriraj Med. J. 2019, 71, 240–245. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.R.; Guo, D.A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef]
- Yu, Y.; Simmler, C.; Kuhn, P.; Poulev, A.; Raskin, I.; Ribnicky, D.; Floyd, Z.E.; Pauli, G.F. The designer approach helps decipher the hypoglycemic bioactive principles of Artemisia dracunculus (Russian Tarragon). J. Nat. Prod. 2019, 82, 3321–3329. [Google Scholar] [CrossRef]
- Bhutia, T.D.; Valant-vetschera, K.M. Chemodiversity of Artemisia dracunculus L. from Kyrgyzstan: Isocoumarins, coumarins, and flavonoids from aerial parts. Nat. Prod. Commun. 2008, 3, 1289–1292. [Google Scholar] [CrossRef]
- Geissmax, T.A.; Ellestad, A. Vulgarin, a sesquiterpene lactone from Artemisia vulgaris L. J. Org. Chem. 1961, 27, 1855–1859. [Google Scholar] [CrossRef]
- Natividad, G.M.; Broadley, K.J.; Kariuki, B.; Kidd, E.J.; Ford, W.R.; Simons, C. Actions of Artemisia vulgaris extracts and isolated sesquiterpene lactones against receptors mediating contraction of guinea pig ileum and trachea. J. Ethnopharmacol. 2011, 137, 808–816. [Google Scholar]
- Numonov, S.; Sharopov, F.; Salimov, A.; Sukhrobov, P.; Atolikshoeva, S.; Safarzoda, R.; Habasi, M.; Aisa, H. Assessment of artemisinin contents in selected Artemisia species from Tajikistan (Central Asia). Medicines 2019, 6, 23. [Google Scholar]
- Nganthoi, M.; Sanatombi, K. Artemisinin content and DNA profiling of Artemisias pecies of Manipur. S. Afr. J. Bot. 2019, 125, 9–15. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz, T.J.; Del Hierro, P. Two eudesmane acids from Artemisia vulgaris. Pytochemistry 1991, 30, 2403–2404. [Google Scholar] [CrossRef]
- Pires, J.M.; Mendes, F.R.; Negri, G.; Duarte-almeida, J.M.; Carlini, E.A. Antinociceptive peripheral effect of Achillea millefolium L. and Artemisia vulgaris L.: Both plants known popularly by Brand Names of analgesic drugs. Phyther. Res. 2009, 219, 212–219. [Google Scholar] [CrossRef]
- Lee, K.H.; Jung, M.Y.; Kim, S.Y. Effects of ascorbic acid on the light-induced riboflavin degradation and color changes in Milks. J. Agric. Food Chem. 1998, 46, 407–410. [Google Scholar] [CrossRef]
- Tak, I.-U.-R.; Mohiuddin, D.; Ganai, B.A.; Chishti, M.Z.; Ahmad, F.; Dar, J.S. Phytochemical studies on the extract and essential oils of Artemisia dracunculus L. (Tarragon). Afr. J. Plant Sci. 2014, 8, 72–75. [Google Scholar]
- Hassanzadeh, M.K.; Tayarani Najaran, Z.; Nasery, M.; Emami, S.A. Tarragon (Artemisia dracunculus L.) Oils; Elsevier Inc.: London, UK, 2016; ISBN 9780124166448. [Google Scholar]
- Joshi, R.; Satyal, P.; Setzer, W. Himalayan aromatic medicinal plants: A review of their ethnopharmacology, volatile phytochemistry, and biological activities. Medicines 2016, 3, 6. [Google Scholar] [CrossRef]
- European Food Safety Authority. Artemisia Vulgaris Basic Substance Application; European Food Safety Authority: Parma, Italy, 2013. [Google Scholar]
- European Food Safety Authority. Botanical Summary Report; European Food Safety Authority: Parma, Italy, 2020. [Google Scholar]
- Abtahi Froushani, S.M.; Zarei, L.; Esmaeili Gouvarchin Ghaleh, H.; Mansori Motlagh, B. Estragole and methyl-eugenol-free extract of Artemisia dracunculus possesses immunomodulatory effects. Avicenna J. Phytomedicine 2016, 6, 526–534. [Google Scholar]
- Talbi, M.; Saadali, B.; Boriky, D.; Bennani, L.; Elkouali, M.; Ainane, T. Two natural compounds—A benzofuran and a phenylpropane—from Artemisia dracunculus. J. Asian Nat. Prod. Res. 2016, 18, 724–729. [Google Scholar] [CrossRef]
- Malik, S.; de Mesquita, L.; Silva, C.; de Mesquita, J.; de Sá Rocha, E.; Bose, J.; Abiri, R.; de Maria Silva Figueiredo, P.; Costa-Júnior, L. Chemical profile and biological activities of essential oil from Artemisia vulgaris L. cultivated in Brazil. Pharmaceuticals 2019, 12, 49. [Google Scholar] [CrossRef]
- Brown, G.D. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules 2010, 15, 7603–7698. [Google Scholar] [CrossRef]
- Socaciu, M.I.; Fogarasi, M.; Semeniuc, C.A.; Socaci, S.A.; Rotar, M.A.; Mureşan, V.; Pop, O.L.; Vodnar, D.C. Formulation and characterization of antimicrobial edible films based on whey protein isolate and tarragon essential oil. Polymers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Chauhan, R.S.; Kitchlu, S.; Ram, G.; Kaul, M.K.; Tava, A. Chemical composition of capillene chemotype of Artemisia dracunculus L. from North-West Himalaya, India. Ind. Crops Prod. 2010, 31, 546–549. [Google Scholar] [CrossRef]
- Verma, M.K.; Anand, R.; Chisti, A.M.; Kitchlu, S.; Chandra, S.; Shawl, A.S.; Khajuria, R.K. Essential oil composition of Artemisia dracunculus L. (tarragon) growing in Kashmir -India. J. Essent. Oil-Bear. Plants 2010, 13, 331–335. [Google Scholar] [CrossRef]
- Suresh, J.; Ahuja, J.; Paramakrishnan, N.; Sebastian, M. Total phenolic and total flavonoids content of aerial parts of Artemisia abrotanum Linn. and A. pallens Wall. Anal. Chem. Lett. 2012, 2, 186–191. [Google Scholar] [CrossRef]
- Suresh, J.; Elango, K.; Dhanabal, S.P.; Paramakrishnan, N.; Suresh, B. A comparative pharmacognostical evaluation of two Artemisia species found in Nilgiris biosphere. Anc. Sci. Life 2007, 27, 7–13. [Google Scholar]
- Mueller, M.; Karhagomba, I.; Hirt, H. The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: Agricultural, chemical and clinical aspects. J. Ethnopharmacol. 2000, 73, 487–493. [Google Scholar] [CrossRef]
- Uhl, S.R.; Strauss, S. Handbook of Species, Seasonings and Flavorings; Technomic Publishing: Lancester, UK, 2000. [Google Scholar]
- Miller, L.; Miller, B. Ayurveda and Aromatheraphy: The Earth Essential Guide to Ancient Wisdom and Modern Healing; Motilal Banarsidass Publ: New Dehli, India, 1998. [Google Scholar]
- Khare, C.P. Indian Herbal Remedies; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Holm, L.; Doll, J.; Holm, E.; Pnacho, J.; Herberger, J. World Weeds: Natural Histories and Distribution; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Chevallier, A. The Encyclopedia of Medicinal Plants: A Practical Reference Guide to More than 500 Key Medicinal Plants and Their Uses; DK Publishing: New York, Ny, USA, 1996. [Google Scholar]
- Quisumbing, E. Medicinal Plants of the Philippines; Bureau of Printing: Manila, Philippines, 1978. [Google Scholar]
- European Medicines Agency Committee for Veterinary Medicinal Products. Artemisia abrotanum Summary Report 1999; European Medicines Agency: Amsterdam, Netherlands, 1999. [Google Scholar]
- Ożarowski, A.; Jaroniewski, W. Rośliny Lecznicze i Ich Praktyczne Zastosowanie; Panacea: Warszawa, Poland, 1987. (In Polish) [Google Scholar]
- Volak, J.; Stodola, J.; Severa, F. Rośliny Lecznicze; Państwowe Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 1987. (In Polish) [Google Scholar]
- Almahdawy, S.S.; Said, A.M.; Abbas, I.S.; Dawood, A.H. The evaluation of antimicrobial and cytotoxic activity of the essential oil extracted from the aerial parts of southernwood herb (Artemisia abrotanum L.) that recently grown in Iraq. Asian J. Pharm. Clin. Res. 2017, 10, 384–387. [Google Scholar] [CrossRef]
- Agence Nationale de Sécurité du Médicament et des Produits de Santé. Absinthium for Homoeopathic Preparations; ANSM: Saint-Denis, France, 2012. [Google Scholar]
- Lockie, A. Encyclopedia of Homeopathy; DK Publishing: New York, NY, USA, 2006; ISBN 9780756618711. [Google Scholar]
- European Medicines Agency. European Union Herbal Monograph on Artemisia absinthium L., Herba; European Medicines Agency: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Bundesinstitut für Arzneimittel und Medizinprodukte (Germany). German Commission E Monographs; Blaumenthal, M.T., Hall, R., Rister, B., Eds.; American Botanical Council: Austin, TX, USA, 1984. [Google Scholar]
- Bundesinstitut für Arzneimittel und Medizinprodukte (Germany). German Commission D Monographs; Blaumenthal, M.T., Hall, R., Rister, B., Eds.; American Botanical Council: Austin, TX, USA, 1994. [Google Scholar]
- Housselle, K. Anonymi. German Pharmacopoeia; Rudolf Ludwig Decker: Berlin, Germany, 1872; Available online: https://wiki.uibk.ac.at/noscemus/Pharmacopoea_Germanica (accessed on 4 August 2022).
- The Scientifis Foundation for Herbal Medicinal Products. E/S/C/O/P Monographs, 2nd ed.; E/S/C/O/P: Exeter, UK, 2003. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 2005. [Google Scholar]
- Nguyen, T. Vietnamese Pharmacopoeia; Vietnamese Pharmacopoeia Commission: Hanoi, Vietnam, 2005. [Google Scholar]
- European Directorate for the Quality of Medicine & HealthCare. European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- Française Pharmacopée. Pharmacopée Française, 11th ed.; Noculak, A., Ed.; Georg Olms: Hildesheim, Germany; New York, NY, USA, 2020; Volume 37. [Google Scholar]
- Kim, S.C.; Adesogan, A.T.; Kim, J.H.; Ko, Y.D. Influence of replacing rice straw with wormwood (Artemisia montana) silage on feed intake, digestibility and ruminal fermentation characteristics of sheep. Anim. Feed Sci. Technol. 2006, 128, 1–13. [Google Scholar] [CrossRef]
- Kim, S.C.; Adesogan, A.T.; Shin, J.H. Effects of dietary addition of wormwood (Artemisia montana Pampan) silage on growth performance, carcass characteristics, and muscle fatty acid profiles of beef cattle. Anim. Feed Sci. Technol. 2012, 177, 15–22. [Google Scholar] [CrossRef]
- Shafi, N.; Khan, G.A.; Ghauri, E.G. Antiulcer effect of Artemisia absinthium L. in rats. Pak. J. Sci. Ind. Res. 2004, 47, 130–134. [Google Scholar]
- Gilani, A.U.H.; Janbaz, K.H. Preventive and curative effects of Artemisia absinthium on acetaminophen and CCl4-induced hepatotoxicity. Gen. Pharmacol. 1995, 26, 309–315. [Google Scholar] [CrossRef]
- Amat, N.; Upur, H.; Blažeković, B. In vivo hepatoprotective activity of the aqueous extract of Artemisia absinthium L. against chemically and immunologically induced liver injuries in mice. J. Ethnopharmacol. 2010, 131, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.A.; Chishti, M.Z.; Ahmad, F.; Shawl, A.S. Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet. Parasitol. 2009, 160, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Caner, A.; Döşkaya, M.; Deǧirmenci, A.; Can, H.; Baykan, Ş.; Üner, A.; Başdemir, G.; Zeybek, U.; Gürüz, Y. Comparison of the effects of Artemisia vulgaris and Artemisia absinthium growing in western Anatolia against trichinellosis (Trichinella spiralis) in rats. Exp. Parasitol. 2008, 119, 173–179. [Google Scholar] [CrossRef]
- Urban, J.; Kokoska, L.; Langrova, I.; Matejkova, J. In vitro anthelmintic effects of medicinal plants used in Czech Republic. Pharm. Biol. 2008, 46, 808–813. [Google Scholar] [CrossRef]
- Singh, O.P.; Tiwari, S.K.; Ojha, D. Pilyriasis versicolor vis-a-vis sidhma and its ayurvedic management. Sadvitra Ayurveda 1994, 46, 920. [Google Scholar]
- Shahnazi, M.; Azadmehr, A.; Hajiaghaee, R.; Mosalla, S.; Latifi, R. Effects of Artemisia absinthium L. extract on the maturation and function of dendritic cells. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e20163. [Google Scholar] [CrossRef]
- Danilets, M.G.; Bel’skii, I.P.; Gur’ev, A.M.; Belousov, M.V.; Bel’skaia, N.V.; Trofimova, E.S.; Uchasova, E.G.; Alhmedzhanov, R.R.; Ligacheva, A.A.; Iusbov, M.S.; et al. Effect of plant polysaccharides on TH1- dependent immune response: Screening investigation. Eksp. i Klin. Farmakol. 2010, 73, 19–22. [Google Scholar]
- Ahmad, F.; Khan, R.; Rasheed, S. Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scariola and Artemisia absinthium. Med. J. Islam. World Acad. Sci. 1992, 5, 309–315. [Google Scholar]
- Amirmohammadi, M.; Khajoenia, S.; Bahmani, M.; Rafieian-Kopaei, M.; Eftekhari, Z.; Qorbani, M. In vivo evaluation of antiparasitic effects of Artemisia abrotanum and Salvia officinalis extracts on Syphacia obvelata, Aspiculoris tetrapetra and Hymenolepis nana parasites. Asian Pacific J. Trop. Dis. 2014, 4, S250–S254. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Sun, H.X. Immunosuppressive effect of ethanol extract of Artemisia annua on specific antibody and cellular responses of mice against ovalbumin. Immunopharmacol. Immunotoxicol. 2009, 31, 625–630. [Google Scholar] [CrossRef]
- Noori, S.; Naderi, G.A.; Hassan, Z.M.; Habibi, Z.; Bathaie, S.Z.; Hashemi, S.M.M. Immunosuppressive activity of a molecule isolated from Artemisia annua on DTH responses compared with cyclosporin A. Int. Immunopharmacol. 2004, 4, 1301–1306. [Google Scholar] [CrossRef]
- Michaelsen, F.W.; Saeed, M.E.M.; Schwarzkopf, J.; Efferth, T. Activity of Artemisia annua and artemisinin derivatives, in prostate carcinoma. Phytomedicine 2015, 22, 1223–1231. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, Y.; Huang, S.; Jin, Y.; Liu, J.; Luo, J.; Zheng, J.; Shi, D. Influence of Artemisia annua extract derivatives on proliferation, apoptosis and metastasis of osteosarcoma cells. Pak. J. Pharm. Sci. 2005, 28, 773–779. [Google Scholar]
- Nageeb, A.; Al-Tawashi, A.; Emwas, A.-H.; Al-Talla, Z.; Al-Rifai, N. Comparison of Artemisia annua bioactivities between Traditional Medicine and Chemical Extracts. Curr. Bioact. Compd. 2014, 9, 324–332. [Google Scholar] [CrossRef]
- Willcox, M. Artemisia species: From traditional medicines to modern antimalarials—And back again. J. Altern. Complement. Med. 2009, 15, 101–109. [Google Scholar] [CrossRef]
- Mueller, M.S.; Runyambo, N.; Wagner, I.; Borrmann, S.; Dietz, K.; Heide, L. Randomized controlled trial of a traditional preparation of Artemisia annua L. (Annual Wormwood) in the treatment of malaria. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 318–321. [Google Scholar] [CrossRef]
- Zime-Diawara, H.; Ganfon, H.; Gbaguidi, F.; Yemoa, A.; Bero, J.; Jansen, O.; Evrard, B.; Moudachirou, M.; Frédérich, M.; Quetin-leclercq, J. The antimalarial action of aqueous and hydro alcoholic extracts of Artemisia annua L. cultivated in Benin: In vitro and in vivo studies. J. App. Pharm. Sci. 2015, 7, 817–823. [Google Scholar]
- Golenser, J.; Waknine, J.H.; Krugliak, M.; Hunt, N.H.; Grau, G.E. Current perspectives on the mechanism of action of artemisinins. Int. J. Parasitol. 2006, 36, 1427–1441. [Google Scholar] [CrossRef]
- Lee, A.G.; Kimura, M.; Neill, P.M.O.; Bray, P.G.; Ward, S.A.; Krishna, S. Artemisinins target the SERCA of Plasmodium falciparum. Nature 2003, 424, 957–961. [Google Scholar]
- Weathers, P.J.; Arsenault, P.R.; Covello, P.S.; McMickle, A.; Teoh, K.H.; Reed, D.W. Artemisinin production in Artemisia annua: Studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem. Rev. 2011, 10, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Safari, H.; Anani Sarab, G.; Naseri, M. Artemisia dracunculus L. modulates the immune system in a multiple sclerosis mouse model. Nutr. Neurosci. 2021, 24, 843–849. [Google Scholar] [CrossRef]
- Modaresi, M.; Zarasvand, M.A.; Madani, M. The effects of hydro-alcoholic extract of Artemisia dracunculus L. (Tarragon) on hematological parameters in mice. J. Basic Res. Med. Sci. 2018, 5, 10–14. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Fernández, A.E.; Tiano, S.; Huang, J.; Floyd, E.; Poulev, A.; Ribnicky, D.; Pasinetti, G.M. An extract of Artemisia dracunculus L. promotes psychological resilience in a mouse model of depression. Oxid. Med. Cell. Longev. 2018, 2018, 7418681. [Google Scholar] [CrossRef]
- Méndez-Del Villar, M.; Puebla-Pérez, A.M.; Sánchez-Peña, M.J.; González-Ortiz, L.J.; Martínez-Abundis, E.; González-Ortiz, M. Effect of Artemisia dracunculus administration on glycemic control, insulin sensitivity, and insulin secretion in patients with impaired glucose tolerance. J. Med. Food 2016, 19, 481–485. [Google Scholar] [CrossRef]
- Gilani, A.H.; Yaeesh, S.; Jamal, Q.; Ghayur, M.N. Hepatoprotective activity of aqueous-methanol extract of Artemisia vulgaris. Phyther. Res. 2005, 19, 170–172. [Google Scholar] [CrossRef]
- Erel, B.; Aydin, F.; Ballar, P. In vitro cytotoxic properties of six Artemisia L. species. Turkish J. Pharm. Sci. 2011, 8, 247–251. [Google Scholar]
- Jakovljević, M.R.; Grujičić, D.; Vukajlović, J.T.; Marković, A.; Milutinović, M.; Stanković, M.; Vuković, N.; Vukić, M.; Milošević-Djordjević, O. In vitro study of genotoxic and cytotoxic activities of methanol extracts of Artemisia vulgaris L. and Artemisia alba Turra. S. Afr. J. Bot. 2020, 132, 117–126. [Google Scholar] [CrossRef]
- Saleh, A.M.; Aljada, A.; Rizvi, S.A.A.; Nasr, A.; Alaskar, A.S.; Williams, J.D. In vitro cytotoxicity of Artemisia vulgaris L. essential oil is mediated by a mitochondria-dependent apoptosis in HL-60 leukemic cell line. BMC Complement. Altern. Med. 2014, 14, 226. [Google Scholar] [CrossRef]
- Khan, K.A. A preclinical antihyperlipidemic evaluation of Artemisia vulgaris root in diet induced hyperlipidemic animal model. Int. J. Pharmacol. Res. 2015, 5, 110–114. [Google Scholar]
- El-Tantawy, W.H. Biochemical effects, hypolipidemic and anti-inflammatory activities of Artemisia vulgaris extract in hypercholesterolemic rats. J. Clin. Biochem. Nutr. 2015, 57, 33–38. [Google Scholar] [PubMed]
- Tigno, X.T.; de Guzman, F.; Flora, A.M.; Theresa, V. Phytochemical analysis and hemodynamic actions of Artemisia vulgaris L. Clin. Hemorheol. Microcirc. 2000, 23, 167–175. [Google Scholar]
- Khan, A.U.; Gilani, A.H. Antispasmodic and bronchodilator activities of Artemisia vulgaris are mediated through dual blockade of muscarinic receptors and calcium influx. J. Ethnopharmacol. 2009, 126, 480–486. [Google Scholar] [CrossRef]
- Aydın, T.; Yurtvermez, B.; Şentürk, M.; Kazaz, C.; Çakır, A. Inhibitory effects of metabolites isolated from Artemisia dracunculus l. against the human carbonic anhydrase I (hCA I) and II (hCA II). Rec. Nat. Prod. 2019, 13, 216–225. [Google Scholar] [CrossRef]
- Li, Y.; Ohizumi, Y. Search for constituents with neurotrophic factor-potentiating activity from the medicinal plants of Paraguay and Thailand. Yakugaku Zasshi 2004, 124, 417–424. [Google Scholar] [CrossRef]
- de Freitas, M.V.; Rita de Cássia, M.N.; da Costa Huss, J.C.; de Souza, T.M.T.; Costa, J.O.; Firmino, C.B.; Penha-Silva, N. Influence of aqueous crude extracts of medicinal plants on the osmotic stability of human erythrocytes. Toxicol. Vitr. 2008, 22, 219–224. [Google Scholar] [CrossRef]
- Avitabile, E.; Senes, N.; D’Avino, C.; Tsamesidis, I.; Pinna, A.; Medici, S.; Pantaleo, A. The potential antimalarial efficacy of hemocompatible silver nanoparticles from Artemisia species against P. falciparum parasite. PLoS ONE 2020, 15, e0238532. [Google Scholar] [CrossRef]
- Kodippili, K.; Ratnasooriya, W.D.; Premakumara, S.; Udagama, P.V. An investigation of the antimalarial activity of Artemisia vulgaris leaf extract in a rodent malaria model. Int. J. Green Pharm. 2011, 5, 1–7. [Google Scholar]
- Bamunuarachchi, G.S.; Ratnasooriya, W.D.; Premakumara, S.; Udagama, P.V. Antimalarial properties of Artemisia vulgaris L. ethanolic leaf extract in a Plasmodium berghei murine malaria model. J. Vector Borne Dis. 2013, 50, 278–284. [Google Scholar]
- Bäumler, S. Heilpflanzen. In Praxis Heute, 2nd ed.; Urban & Fischer: München, Germany, 2007. [Google Scholar]
- Brunfels, O. Contrafayt Kreüterbuch (mit Naturgetreuen Abb. Hans Weidnitz), 2 Teile; Erste Deutsche Bearbeitung Seines Herbarum Vivae Eicones: Basel, Switzerland, 1532. [Google Scholar]
- Tabernaemontanus, J.T. Neu Vollkommen Kräuterbuch/Mit Schönen und Künstlichen Figuren/aller Gewächs der Bäumen/Stauden und Kräutern/so in Denen Teutschen und Welschen Landen/auch in Hispanien/Ost- und West-Indien/oder in der Neuen Welt; der Zeit in Offenbach am Mayn 1731. Das Erste Buch/Von Kräutern; Johann Ludwig Königs: Basel, Switzerland, 1731. [Google Scholar]
- Schröders, D.J. Apotheke/Artzney-Schatz; Verlegts Johann Hoffmann/Buch-und Kunsthändler; Gedruckt zu Jena: Nürnberg, Germany, 1685. [Google Scholar]
- Stewart, S. Cosmetics & Perfumes in the Roman World; Tempus Publishing Limited: Stroud, Gloucestershire, 2007. [Google Scholar]
- Dioscorides. De Materia Medica: Being an Herbal with Many Other Medicinal Materials Written in Greek in the First Century of the Common Era. A New Indexed Version in Modern English by Tess Anne Osbaldeston and Robert P. A. Wood; IBIDIS Press: Johannesburg, South Africa, 2000. [Google Scholar]
- Syreński, S. Zielnik Herbarzem z Języka Łacińskiego Zowią: To Iest Opisanie Własne Imion, Kształtu, Przyrodzenia, Skutkówy moc zioł Wszelakich […] Polskim Językiem Zebrany y na Ośmioro Ksiąg Rozłożony […], Cracoviae; Księgi Wtóre: Kraków, Poland, 1613. (In Polish) [Google Scholar]
- Drobnik, J.K.; Wełna, K. Cosmetic plants of the early 19th century. Rośliny kosmetyczne początku XIX wieku. Pol. J. Cosmetol. 2017, 20, 349–358. [Google Scholar]
- European Commission Cosing CosIng—Cosmetic Database. Available online: https://ec.europa.eu/growth/tools-databases/cosing/ (accessed on 4 April 2022).
- European Commission Cosing CosIng—Cosmetic Database. Available online: https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.simple (accessed on 9 August 2022).
- Suresh, J.; Vasavi Reddy, A.; Rajan, D.; Ihsanullah, M.; Nayeemmullah Khan, M. Antimicrobial activity of Artemisia abrotanum and Artemisia pallens. Int. J. Pharmacogn. Phytochem. Res. 2011, 3, 18–21. [Google Scholar]
- Hrytsyk, R.A.; Kutsyk, R.V.; Yurchyshyn, O.I.; Struk, O.A.; Kireev, I.V.; Grytsyk, A.R. The investigation of antimicrobial and antifungal activity of some Artemisia L. species. Pharmacia 2021, 68, 93–100. [Google Scholar] [CrossRef]
- Moslemi, H.R.; Hoseinzadeh, H.; Badouei, M.A.; Kafshdouzan, K.; Fard, R.M.N. Activity of Artemisia absinthium against surgical wounds infected by Staphylococcus aureus in a rat model. Indian J. Microbiol. 2012, 52, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Habibipour, R.; Rajabi, M. Antibacterial effects of Arctium lappa and Artemesia absinthium extracts in laboratory conditions. J. HerbMed Pharmacol. 2015, 4, 133–137. [Google Scholar]
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicig. J. Agric. Food Chem. 2005, 53, 9452–9458. [Google Scholar] [CrossRef]
- Al-Zubairi, A.S.; Al-Mamary, M.A.; Al-Ghasani, E. The antibacterial, antifungal, and antioxidant activities of essential oil from different aromatic plants. Glob. Adv. Res. J. Med. Med. Sci. 2017, 6, 224–233. [Google Scholar]
- Evans, T.C.; Gavrilovich, E.; Mihai, R.C.; Isbasescu, I.E.L.; Thelen, D.; Martin, J.A.; Allen, S.M.; Sa, S. Production of Organic Acid and Ammonumintrate. Patent Application Publication US 2006/0222585 A1, 5 October 2006. [Google Scholar]
- Lee, H.G.; Kim, H.; Oh, W.K.; Yu, K.A.; Choe, Y.K.; Ahn, J.S.; Kim, D.S.; Kim, S.H.; Dinarello, C.A.; Kim, K.; et al. Tetramethoxy hydroxyflavone p7F downregulates inflammatory mediators via the inhibition of nuclear factor κB. Ann. N. Y. Acad. Sci. 2004, 1030, 555–568. [Google Scholar] [CrossRef]
- Joshi, R.K. Volatile composition and antimicrobial activity of the essential oil of Artemisia absinthium growing in Western Ghats region of North West Karnataka, India. Pharm. Biol. 2013, 51, 888–892. [Google Scholar] [CrossRef]
- Nalbantsoy, A.; Erel, Ş.B.; Köksal, Ç.; Göçmen, B.; Yildiz, M.Z.; Karabay Yavaşoĝlu, N.Ü. Viper venom induced inflammation with Montivipera xanthina (Gray, 1849) and the anti-snake venom activities of Artemisia absinthium L. in rat. Toxicon 2013, 65, 34–40. [Google Scholar] [CrossRef]
- Stebbings, S.; Beattie, E.; McNmara, D.; Hunt, S. A pilot randomized, placebo-controlled clinical trial to investigate the efficacy and safety of an extract of Artemisia annua administered over 12 weeks, for managing pain, stiffness, and functional limitation associated with osteoarthritis of the hip and knee. Clin. Rheumatol. 2016, 35, 1829–1836. [Google Scholar]
- Ben Nasr, S.; Aazza, S.; Mnif, W.; Miguel, M. In-vitro antioxidant and anti-inflamatory activities of Pituranthos chloranthus and Artemisia vulgaris from Tunisia. Int. J. Appl. Pharm. Sci. Res. 2020, 11, 605–614. [Google Scholar]
- Olsen, O.T.; Frolund, L.; Heinig, J.; Jacobsen, L.; Svendsen, U.G. A double-blind, randomized study investigating the efficacy and specificity of immunotherapy with Artemisia vulgaris or Phleum pratense/Betula verrucosa. Allergol. Immunopathol. 1995, 23, 73–78. [Google Scholar]
- Aburjai, T.; Natsheh, F.M. Plants Used in Cosmetics. Phyther. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef]
- Hashemi, Z.; Ebrahimzadeh, M.A.; Khalili, M. Sun protection factor, total phenol, flavonoid contents and antioxidant activity of medicinal plants from Iran. Trop. J. Pharm. Res. 2019, 18, 1443–1448. [Google Scholar] [CrossRef]
- Varothai, S.; Bunyaratavej, S.; Leeyaphan, C.; Phaitoonwattanakij, S.; Winayanuwattikun, W. Pilot study of the efficacy and safety of nail gel containing Artemisia abrotanum extract and glycerin in the treatment of nail plate surface abnormality. Siriraj Med. J. 2021, 73, 204–208. [Google Scholar] [CrossRef]
- Park, S.H.; Cho, D.M.; Choi, B.D.; Choi, Y.J.; Choi, J.H. Antioxidative effects of skinned mugwort (Artemisia vulgaris L.) extracts on UV-irradiated hairless mouse skin. J. Korean Soc. Food Sci. Nutr. 2008, 37, 20–26. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Soleimani, N. Evaluation of the bactericidal effects of Zingiber officinale, Aloysia citrodora and Artemisia dracunculus on the survival of standard Gram-positive and Gram-negative bacterial strains. Jorjani Biomed. J. 2018, 6, 22–32. [Google Scholar] [CrossRef]
- Raj Singh, B.; Singh, V.; Karan Singh, R.; Toppo, S.; Haque, N.; Ebibeni, N. Antimicrobial effect of Artemisia vulgaris essential oil. Nat. Prod. An Indian J. 2011, 5, 5–12. [Google Scholar]
- Hiremath, S.K.; Kolume, D.G.; Muddapur, U.M. Antimicrobial activity of Artemisia vulgaris Linn. (Damanaka). Int. J. Res. Ayurveda Pharm. 2011, 2, 1674–1675. [Google Scholar]
- Singh, R.; Verma, P.; Singh, G. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. J. Intercult. Ethnopharmacol. 2012, 1, 101. [Google Scholar] [CrossRef]
- Temraz, A.; El-Tantawy, W.H. Characterization of antioxidant activity of extract from Artemisia vulgaris. Pak. J. Pharm. Sci. 2008, 21, 321–326. [Google Scholar] [PubMed]
- Oyedemi, S.; Coopoosamy, R. Preliminary studies on the antibacterial and antioxidative potentials of hydroalcoholic extract from the whole parts of Artemisia vulgaris L. Int. J. Pharmacol. 2015, 2, 561–569. [Google Scholar] [CrossRef]
- Eidi, A.; Oryan, S.; Zaringhalam, J.; Rad, M. Antinociceptive and anti-inflammatory effects of the aerial parts of Artemisia dracunculus in mice. Pharm. Biol. 2016, 54, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Afsar, S.K.; Rajesh Kumar, K.; Venu Gopal, J.; Raveesha, P. Assessment of anti-inflammatory activity of Artemisia vulgaris leaves by cotton pellet granuloma method in Wistar albino rats. J. Pharm. Res. 2013, 7, 463–467. [Google Scholar] [CrossRef]
- Alpha Keri. Available online: https://www.alphakeri.com.au (accessed on 10 December 2021).
- Dr Hauschka. Available online: https://www.drhauschka.de (accessed on 10 December 2021).
- Laura Mercier. Available online: https://www.lauramercier.com (accessed on 10 December 2021).
- Dermika. Available online: https://dermika.pl/ (accessed on 10 December 2021).
- Aveeno. Available online: https://www.aveeno.com (accessed on 10 December 2021).
- Christophe Robin Paris. Available online: https://www.christopherobin.com (accessed on 10 December 2021).
- Revive. Available online: https://reviveskincare.com/ (accessed on 10 December 2021).
- USANA Celavive Skincare. Available online: https://www.celavive.com (accessed on 10 December 2021).
- Cera Skin Care. Available online: https://caraskincare.ca/ (accessed on 10 December 2021).
- It Cosmetics. Available online: https://www.itcosmetics.com (accessed on 10 December 2021).
- Natura Siberica. Available online: http://naturasiberica.ru/ (accessed on 10 December 2021).
- MAN:YO. Available online: https://manyo.us (accessed on 10 December 2021).
- Mizon. Available online: http://www.mizon.co.kr/ (accessed on 10 December 2021).
- Bioelements. Available online: https://www.bioelements.com/ (accessed on 10 December 2021).
- Kiehl’s. Available online: https://www.kiehls.com/ (accessed on 10 December 2021).
- MALIN+GOETZ. Available online: https://www.malinandgoetz.com/ (accessed on 10 December 2021).
- Neogen Dermatology. Available online: https://www.neogenlab.us (accessed on 10 December 2021).
- Pixi. Available online: https://pixibeauty.co.uk (accessed on 10 December 2021).
- Commonlabs. Available online: https://commonlabsmalaysia.com (accessed on 10 December 2021).
- Kingnature. Available online: https://www.kingnature.ch (accessed on 10 December 2021).
- Su:m37. Available online: https://www.sum37.com.sg/ (accessed on 10 December 2021).
- Dr. Oracle. Available online: https://oraclecosmetic.com/ (accessed on 10 December 2021).
- MISSHA. Available online: https://oraclecosmetic.com/%0Ahttps://missha.com/%0A%0A (accessed on 10 December 2021).
- PURE’AM. Available online: https://www.pureambeauty.com/ (accessed on 10 December 2021).
- ESPA. Available online: https://www.espaskincare.com (accessed on 10 December 2021).
- Lush. Available online: https://www.lush.com (accessed on 10 December 2021).
- Hayejin. Available online: https://hayejincosmetic.com (accessed on 10 December 2021).
- Onekind. Available online: https://onekind.us (accessed on 10 December 2021).
- Humphrey. Available online: https://humphreyderm.com/%0A (accessed on 10 December 2021).
- Vgam. Available online: https://vgambiome.ca (accessed on 10 December 2021).
- Annayake. Available online: https://www.annayake.com (accessed on 10 December 2021).
- Cherry Brenchez. Available online: https://brenchezbeauty.com/ (accessed on 10 December 2021).
- Monuskin. Available online: https://www.monuskin.co.uk/ (accessed on 10 December 2021).
- R10 Labs. Available online: https://www.r10labs.com (accessed on 10 December 2021).
- Somethinc. Available online: https://somethinc.com (accessed on 10 December 2021).
- Moraz. Available online: http://www.moraz.co.il (accessed on 10 December 2021).
- Manuka Doctor. Available online: https://www.manukadoctor.com (accessed on 10 December 2021).
- Skintific. Available online: https://www.skintificbeauty.com/ (accessed on 10 December 2021).
- Aprilskin. Available online: https://aprilskin.us/ (accessed on 10 December 2021).
- I’m From. Available online: https://www.coos-cosmetics.com (accessed on 10 December 2021).
- Dermalogica. Available online: http://www.dermalogica.com (accessed on 10 December 2021).
- Rms Beauty. Available online: https://www.rmsbeauty.com/ (accessed on 10 December 2021).
- Jeschke, E.; Ostermann, T.; Lüke, C.; Tabali, M.; Kröz, M.; Bockelbrink, A.; Witt, C.M.; Willich, S.N.; Matthes, H. Remedies Containing Asteraceae Extracts. Drug Saf. 2009, 32, 691–706. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Artemisia absinthium L. Extract, Basic Substance Application; European Food Safety Authority: Parma, Italy, 2014. [Google Scholar]
- Mihajilov-Krstev, T.; Jovanović, B.; Jović, J.; Ilić, B.; Miladinović, D.; Matejić, J.; Rajković, J.; Äorević, L.; Cvetković, V.; Zlatković, B. Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta Med. 2014, 80, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W. Wormwood (Artemisia absinthium L.)-A curious plant with both neurotoxic and neuroprotective properties? J. Ethnopharmacol. 2010, 131, 224–227. [Google Scholar] [CrossRef]
- Food and Drug Administration. Pollens—Weeds and Garden Plants; Food and Drug Administration: Silver Spring, MD, USA, 2011. [Google Scholar]
- Leng, X.; Ye, S.T. An investigation on in vivo allergenicity of Artemisia annua leaves and stems. Asian Pac. J. Allergy Immunol. 1987, 5, 125–128. [Google Scholar]
- Tang, R.; Sun, J.L.; Yin, J.; Li, Z. Artemisia allergy research in China. Biomed Res. Int. 2015, 2015, 179426. [Google Scholar] [CrossRef]
- European Food Safety Authority. Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. EFSA J. 2012, 10, 2663. [Google Scholar] [CrossRef]
- Food and Drug Administration. CPG Sec 525.750 Spices—Definitions FDA. Available online: https://www.fda.gov/ (accessed on 9 August 2022).
- European Commission Health & Comsumer Protection Directorate -General. Opinion of the Scientific Committee on Food on Estragole (1-Allyl-4-methoxybenzene). Int. J. Mod. Phys. Conf. Ser. 2001. [Google Scholar] [CrossRef]
- Ipsen, H.; Formgren, H.; Løswenstein, H.; Ingemann, L. Immunochemical and biological characterization of a Mugwort (Artemisia vulgaris) pollen extract. Allergy 1985, 40, 289–294. [Google Scholar] [CrossRef]
- Yong, W.L.; Soo, Y.C.; Eun, K.L.; Jung, H.S.; Park, J.W.; Hong, C.S. Cross-allergenicity of pollens from the Compositae family: Artemisia vulgaris, Dendranthema grandiflorum, and Taraxacum officinale. Ann. Allergy, Asthma Immunol. 2007, 99, 526–533. [Google Scholar]
- Ulbricht, C.E. Natural Standard, Herb and Supplement Guide, An Evidence-Based Reference; Mosby Elsevier: Maryland Heights, MO, USA, 2010. [Google Scholar]
- Wrangsjö, K.; Ros, A.M.; Wahlberg, J.E. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermat. 1990, 22, 148–154. [Google Scholar] [CrossRef]
- Haw, S.; Cho, H.-R.; Lee, M.-H. Allergic contact dermatitis associated with mugwort (Artemisia vulgaris). Contact Dermat. 2010, 62, 61–63. [Google Scholar] [CrossRef]
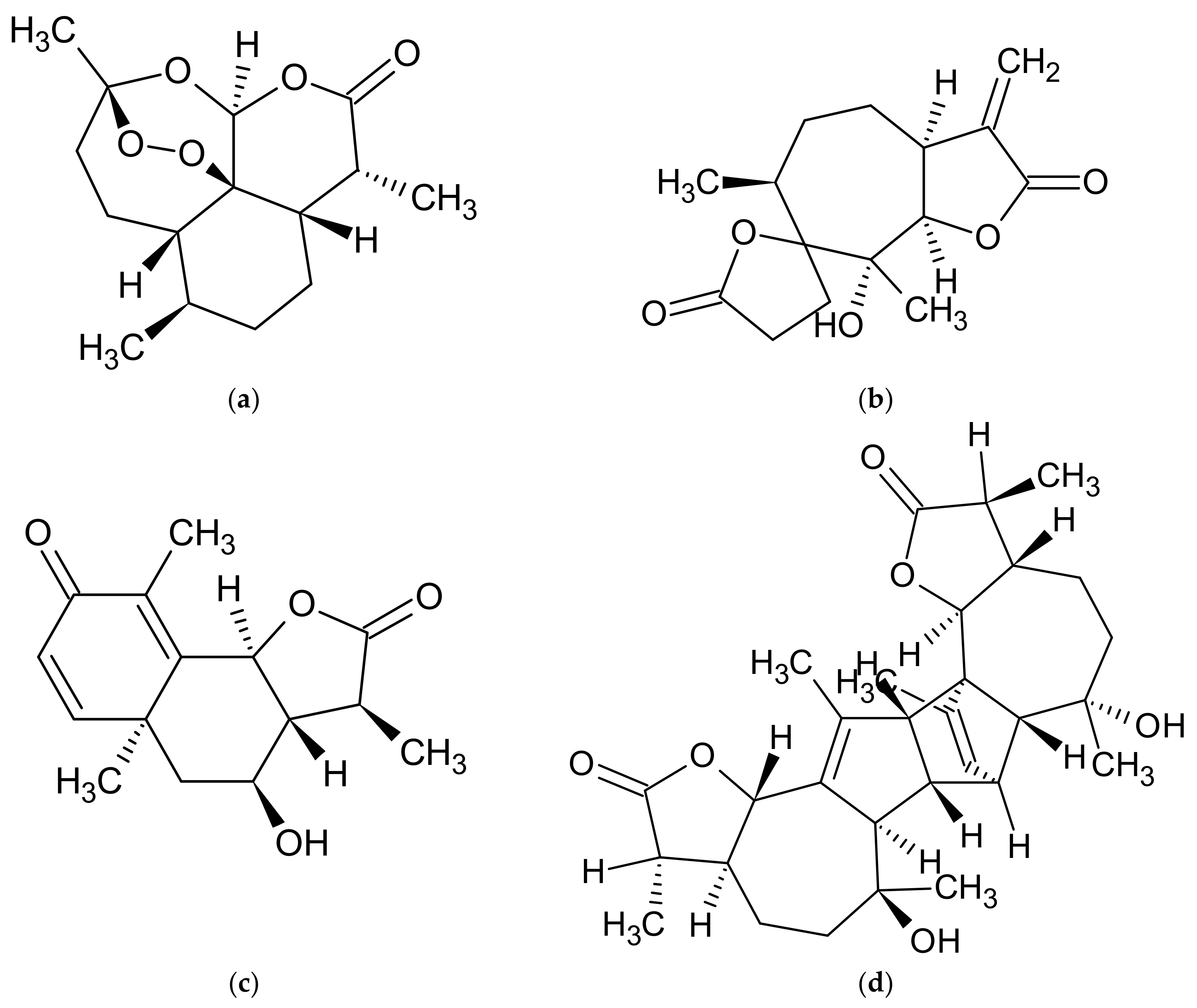
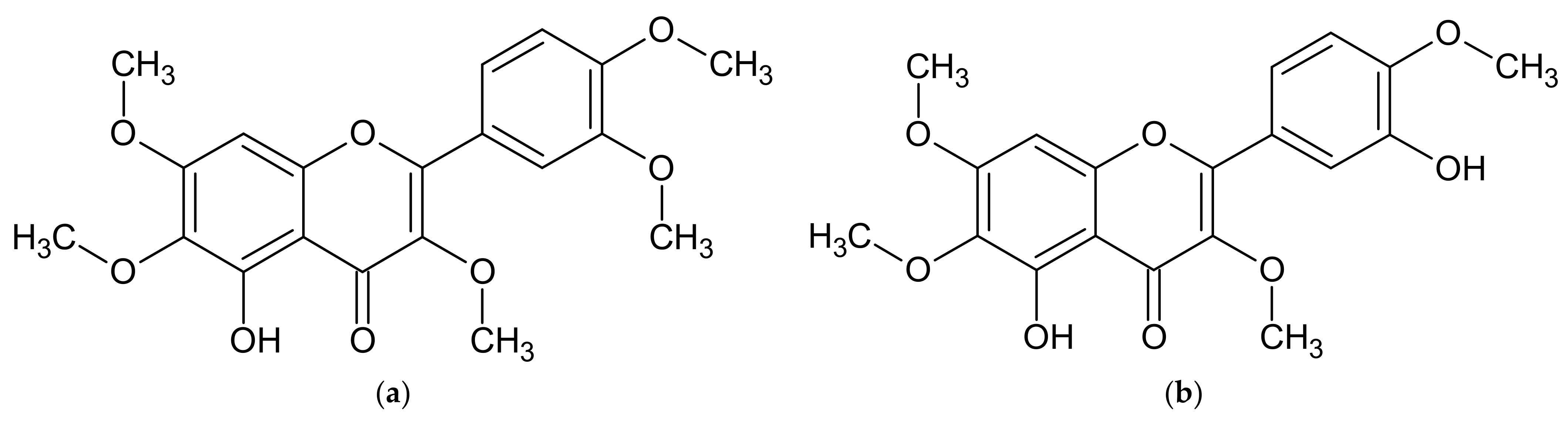
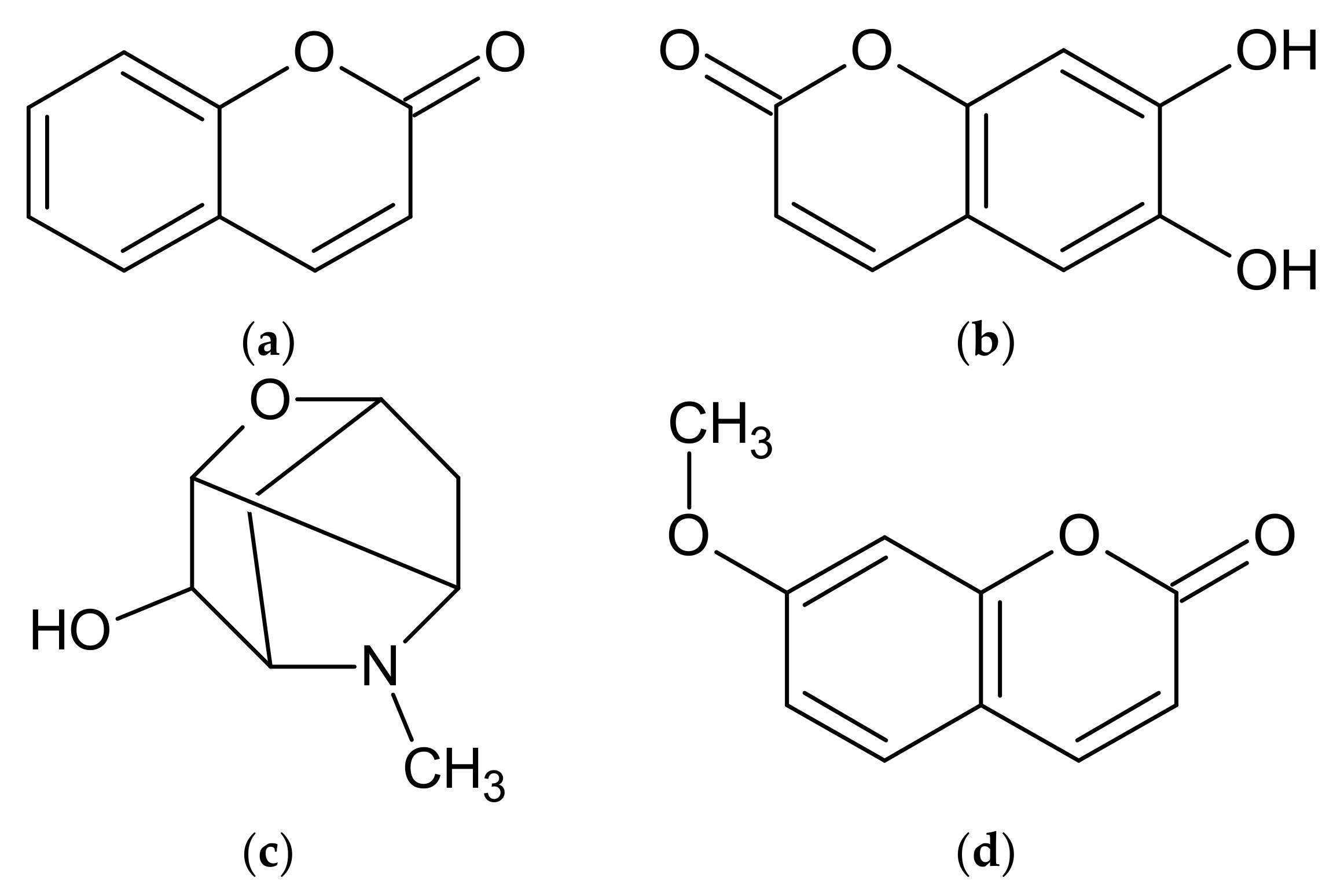
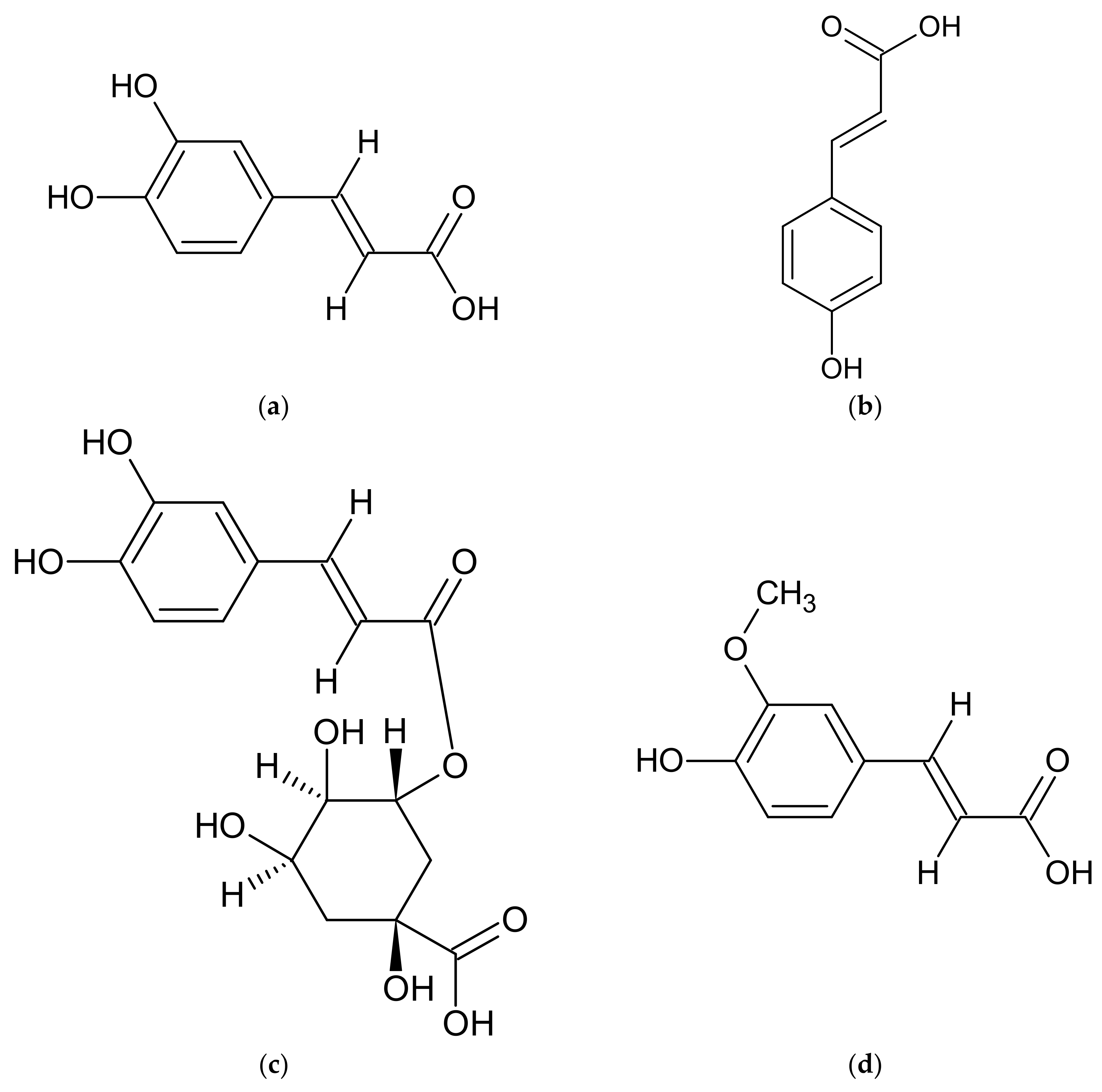
| Species | Sesquiterpenoids | Monoterpenoids | Diterpenoids | Triterpenoids | Phenylpropanoid Derivatives | Other Compounds |
|---|---|---|---|---|---|---|
| A. abrotanum | δ-amorphene, aromadendrene, artedouglasia C, artedouglasia oxide A, artedouglasia oxide B, artedouglasia oxide D, bicyclogermacrene, trans-α-bisabolen, α-bisabolol, β-bourbonene, δ-cadinene, cadinol, α-cadinol, 3-carene, caryophyllene, β-caryophyllene, caryophyllene oxide, α-copaene, davanone, davanone B cedrene, citronellol, β-copaene, α-cubebene, (E)-β-damascenone, davana ether, davanon ether, davanone B, cis-davanone, α-dehydro-ar-himachalene, γ-dehydro-ar-himachalene, β-elemene, δ-elemen, α-epi-7-epi-5-eudesmol, epi-longipinanol, 7-epi-silphiperfol-5-ene, eudesma-5-en-11-ol, α-eudesmol, β-eudesmol, γ-eudesmol acetate, farnesyl butanoate, germacrene D, germacren-D-4-ol, guaiol, α-humulene, humulene epoxide I, isospathulenol, T-muurolol, nerolidol, (E)-nerolidol, nordavanone, β-selinene, silphiperfol-4,7 (14)-diene, silphiperfol-5-ene, silphiperfol-5-en-3-ol A, silphiperfol-5-en-3-one A, silphiperfol-5-en-3-one B, silphiperfol-6-α-ol, silphiperfolen isomer, spathulenol [78,79,80,81,82,83,109] | borneol, bornyl acetate, camphene, camphor, 3 (10) -carene-2-ol, trans-carveol, cis-carvone, cis-carvyl acetate, trans-carvyl acetate, cembrene, cis-chrysanthenol, chrysanthenone, cis-chrysanthenyl acetate, trans-chrysanthenyl acetate, 1,4-cineole, 1,8-cineole, cuminyl acetate, p-cymenene, eugenol, geranyl isobutanoate, 2-hydroxy-1,8-cineole, isobornyl formate, isobornyl propionate, lavandulol, lavandulyl butanoate, lavandulyl caproate, lavandulyl isovalerate, limonene, ment-1,5-dien-7-ol, p-menth-1-en-8-ol, p-menth-2-en-1-ol, myrcene, linalool, β-myrcene, myrtanal, myrtenal, myrtenol, E-myrtenol, neryl isobutanoate, neryl propionate, β-ocimene, E-β-ocimene, Z-β-ocimene, trans-ocimene, trans-β-ocimene, trans-ocimenol, α-pinene, trans-pinocamphone, cis-piperitol, trans-piperitol, piperitone, α-phellandrene, β-phellandrene, β-pinene, 2 (10) -pinen-2-one, pinocarvone, terpenyl acetate, α-terpenyl acetate, α-terpinene, γ-terpinene, α-terpineol, 1-terpineol, 4-terpineol, cis-β-terpineol, δ-terpineol acetate, terpinolene, α-terpinolene, α-terpinyl acetate, 3-thujanol, α-thujenal, α-thujene, α-thujone, tricyclene, 4-tujanol, sabinaketone, sabinene, cis-sabinene hydrate, trans-sabinene hydrate, trans-sabinol[79,80,81,82,83,109] | lupeol, phytol isomer [80,81] | agarospirol [82] | estragol (methyl chavicol), elemicine [80,82,109] | Spiroterpenoids: methyleugenol [82,109] Jasmonates: methyl cis-jasmonate [79] Other: cis-arbusculone, trans-arbusculone, 1,4-dimethyl-4-propyl-2-one-1-(2)–cyclo-hexene, heptanal, hexanal, (E)-2-hexenal, (Z)-3-hexenol, α-(E)-ionone, isobutanoate ester of anisic acid, isopergol, cis-jasmone, (Z)-jasmone, lavender lactone, methyl p-anisate, 4-methylpent-2-enolide, nonanal, 1-octen-3-ol, 2-phenylacetaldehyde, 2,2,3-trimethyl-3-cyclopentene-1-acetaldehyde [80,81,82,83,109] |
| A. absinthium | allo-aromadendrene, ar-curcumene, α-(E)-bergamotene, bicyclogermacrene, α-bisabolene, (Z)-α-bisabolene, β-bisabolene, α-bisabolol, bisabolol oxide, bisabolol oxide B, β-bourbonene, cadinene, γ-cadinene, δ-cadinene, α-calacorene, caryophyllene, β-caryophyllene, (E)-caryophyllene, caryophyllene oxide, α-cedrene, α-copaene, γ-curcumene, cyperene, diepi-α-cedrene, curcumene, β-elemene, elemol, epi-β-santalene, β-eudesmol, (E,E)-farnesal, (Z,E)-α-farnesene, (E,E)-farnesyl acetate, (E,E)-farnesyl 3-methylbutanoate, (E)-β-farnesene, germacrene D, guaiazulene, α-gurjunene, β-gurjunene, γ-gurjunene, guaiazulene, hexahydrofarnesyl acetone, α-himachalene, α-humulene, γ-humulene, humulene oxide II, α-isocomene, β-isocomene, γ-muurolene, nerolidol, (E)-nerolidol, (E)-nerolidyl propanoate, petasitene, pethybrene, presilphiperfol-7-ene, α-santalene, β-santalene, β-selinene, silfinen-1-en, silphiperfol-6-ene, 7-α-silphiperfol-5-ene, spathulenol [33,57,84,85,86,87,88,89] | allo-ocimene, Artemisia ketone, borneol, bornyl acetate, bornyl 3-methylbutanoate, camphene, camphor, carvacrol, (Z)-carveol, carvone, chrysanthenol, (Z)-chrysanthenol, chrysanthenyl acetate, (Z)-chrysanthenyl acetate, 1,8-cineole, p-cymene, p-cymen-8-ol, (E)-epoxyocimene, (Z)-epoxyocimene, (Z)-β-epoxyocimene, (E)-6,7-epoxyocimene, (Z)-6,7-epoxyocimene, epoxyocymene, eugenol, α-fenchene, fenchone, geranial, geraniol, geranyl acetate, geranyl isovalerate, geranyl 2-methylbutanoate, geranyl 3-methylbutanoate, geranyl pentanoate, isobornyl acetate, isobornyl propanoate, iso-3-thujanol, isothujyl acetate, lavandulol, lavandulyl acetate, limonene, linalool, β-linalool, (E)-linalool oxide, (Z)-linalool oxide, linalyl acetate, linalyl butanoate, linalyl 3-methylbutanoate, linalyl propionate, lyratyl acetate, p-menth-3-en-9-ol, 3-methylbutanoate, myrcene, β-myrcene, neral, nerol, (Z)-nerolidol, neryl acetate, neryl 2-methylbutanoate, neryl 3-methylbutanoate, neryl 2-methylpropanoate, (E)-β-ocimene, (Z)-β-ocimene, phellandrene, α-phellandrene, β-phellandrene, phellandrene epoxide, pinene, α-pinene, β-pinene, 2-β-pinene, pulegone, sabinene, (E)-sabinene hydrate, (Z)-sabinene hydrate, (E)-sabinol, sabinyl acetate, (E)-sabinyl acetate, santolinatriene, α-terpinene, γ-terpinene, α-terpineol, terpinene-4-ol, terpinolene, α-terpinylacetate, α-thujene, thujol, α-thujone, β-thujone, (E)-thujone, (Z)-thujone, thujyl acetate, thujyl alcohol, thymol, tricyclene, (E)-verbenol, (Z)-verbenol [9,18,35,76,84,85,87,88,89,121] | 1-(E)-8-isopropyl-1,5-dimethyl-nona-4,8-dienyl-4-methyl-2,3-dioxa-bicyclo(2, 2, 2)oct-5-ene, iso-1-(E)-8-isopropyl-1,5-dimethyl-nona-4,8-dienyl-4-methyl-2,3-dioxa-bicyclo(2,2,2)oct-5-ene, vulgarol A, vulgarol B [9,73,80] | nd 1 | estragole, methyleugenol [89] | nd |
| A. annua | aristolon, bicyclogermacrene, β-bourbonene, β-cadinene, γ-cadinene, δ-cadinene, cis-cadin-4-en-7-ol, epi-α-cadinol, caryophyllene, β-caryophyllene, cis-β-caryophyllene, trans-β-caryophyllene, caryophyllene oxide, β-chamigrene, α-copaene, cubebin, β-cubeben, cubenol, β-elemen, γ-elemen, α-farnesan, trans-β-farnesane, germacren A, germacren B, germacren D, β-gurjunene, γ-gurjunen, humulene, α-humulene, isoledene, (–)- isolongifolen-9-one, kopaene, trans-β-kopaene, α-longipinene, γ-muurolene, nerolidol, nootkaton, β-selinene, selin-11-en-ol isomer, selin-3,11-dien-6α-ol, spathulenol [73,74,90,91,92,93,94,95] | Artemisia trien, artemisinin alcohol, artemisinin ketone, borneol, bornyl acetate, camphene, camphor, α-campholenal, cis-carveol, trans-carveol, carvone, cis-chrysanthenol, 1,8-cineole, cuminal, cis-β-O-cymene, trans-β-O-cymene, p-cymene, dehydro-1,8-cineol, dehydrosabinaketone, dehydrosabinene, eugenol, α-felandrene, ipsdienol, limonene, linalool, p-mentha-2,4 (8)-diene, myrcene, myrcenol, myrtenal, myrtenol, myrtenyl acetate, neryl acetate, α-pinene, β-pinene, β-pinene oxide, trans-pinocarveol, cis-pinocarveol acetate, pinocarvone, piperitone, sabinene, cis-sabinene hydrate, trans-sabinene hydrate, santolin alcohol, santolinatriene, α-terpineol, 4-terpineol, δ-terpineol, γ-terpinene, terpinolene, α-terpinolene, α-terpinene, thujen, α-thujone, α-thujene, verbenol, verbenone, yomogi alcohol [71,73,74,90,91,92,93,94,95,152] | vulgarone [90] | nd | nd | arteannuic acid, 2-H-1-benzopiranzone, benzyl benzoate, benzyl 3-methylbutanacetate, 1-dodekene, ethyl 2-methylbutanoate, eudesm-7(11)-en-4-ol, hexanal, 2-hexenyl 2-methylbutanoate, cis-2-hexenyl 3-methylbutanoate, isovalerate hexanoate, cis-jasmon, 2-methyl-2-butenyl 3-methylbutanoate, 3-methyl-3-butenyl 3-methylbutanoate nonanal, nonadecane, propyl 2-methylbutanoate [91,92,93,94,95] |
| A. dracunculus | acoradiene, ar-curcumen, α-bergamotene, bicyclermacren, α-bisabolol, β-bisabolen, δ-cadinene, α-epi-cadinol, caryophyllene, β-caryophyllene, E-caryophyllene, E-β-caryophyllene, caryophyllene oxide, α-cedrene, α-copaene, elemene, δ-elemene, γ-elemene farnesane, cis-trans-α-farnesene, (E)-β-farnesene, (E,E)-farnesane, E,E-α-farnesane, germacrene D, germacrene-D-4-ol, gleenol, α-himachalene, α-humulene, β-sesquiphellandrene, spathunelol, spatulenol, α-zingiberene [2,11,54,96,97,98,99,100,101,102,103] | allocimene, artemisinic ketone, borneol, bornyl acetate, camphene, camphor, 4-carene, ∆3-carene, carvacrol, trans-carveol, carvone, E-carvone oxide, 2-allo-cimene, 1,8-cineole, citronellol, citronellol acetate, citronellol formate, o-cymene, p-cymene, (E)-β-O-cymene p-mentha-1,3,8-triene, ethyl geranyl, geraniol, geranyl acetate, β-elemene, endo-isofenchene, α-fenchene, geranial, (E)-β-ionone, isobornyl acetate, isoterpinolene, limonene, D-limonene, linalool, myrcene, β-myrcene, myrtenal, nerol, neryl acetate, α-trans-ocimene, allo-ocimene, cis-β-ocimene, cis allo-ocimene, trans β-ocimene, trans-allo-ocimene, β-ocimene, β-ocimene Y, E-β-ocymene, Z-β-ocymene, neo-allo-ocymene cis allo-ocymen hydrate, phellandrene, α-phellandrene, β-phellandrene, α-pinene, β-pinene, 2-β-pinene, p-pinene, pinocarveol, pseudolimonene, sabinene, trans-sabinene acetate, cis-sabinene hydrate, β-sesquifelandrene, α-terpenyl acetate, terpineol, 4-terpineol, α-terpineol, α-terpinene, γ-terpinene, terpinolene, α-terpinolene, trans-4 thujanol, α-thujene, thymol, tricyclen [2,11,54,96,97,98,99,100,102,103,153] | phytol [99] | squalene [54] | (Z)-anethole, asarone, carpaci, dillapiole, elemycin, estragole (methylchavicol, p-allylanisole), eugenol, isoelemycin, isoeugenol methyl ether, isoeugenol methyl trans-anethole, 3-(p-methoxyphenyl)-1,2-propanediol, methyl eugenol, prestragol [11,54,80,97,98,99,100,101,102,103,115,144,149,150] | Isocoumarins: 3-(1-Z-butenyl) isocoumarin = (Z)-artemidin, 2-(1-E-butenyl)-isocoumarin = (E)-artemidin [2,11] Polyacetylenes: capillene, 1-phenyl-2,4-hexadiene, 1-phenyl-2,4-hexadiene-1-one [2,54,146,154,155] Other: acenaphthene, p-allyphenol, apiole, cinnamic acid, cinnamyl acetate, cyclohexylmorpholine, dehydro-1,8-cineole, 3-methoxycinnamaldehyde, methyl ester, methyl salicylate, myristicin, nonadecane, 1,3-oktadiene, 1-pentadecene, 5-phenyl-1,3-pentadiyne [11,102,103,146,153] |
| A. vulgaris | aromadendrene, α-trans-bergamotene, bicyclogermacrene, β-bisabolene, α-bisabololene, β-burbonen, α-cadinol, α-calacorene, caryophylla-4(14),8(15)-diene-5-α-ol, caryophyllene, trans-caryophyllene, caryophyllene oxide, α-cedrene, β-chamigrene, α-copaen, cubebene, davanone, α-elemene, β-elemene, β-eudesmol, farnesene, farnesyl acetate, germacrene D, germacrene D-4-ol, α-humulene, humulene epoxide II, humulene oxide, α-isocomene, lanceol acetate, ledol, β-longipinene, modhephene, epi-α-muurolol, (E)-nerolidol, petasitene, presilphiperfol-7-ene, trans-salvene, salvial-4(14)-en-1-one, epi-β-santalene, silphin-1-ene, 7-α-silphiperfol-5-ene, silphiperfol-5-en-3-ol (Z)-β-farnesene, silphiperfol-4,7(14)-diene, spathulenol, valeranone [55,65,80,88,104,105,106,107,108] | Artemisia alcohol, Artemisia ketone, artemisyl acetate, borneol, bornyl acetate, camphene, camphor, trans-carveol, carvone, cis-chrysanthenol, chrysanthenyl acetate, 1,8-cineol, cuminol, cymene, p-cymene-8-ol, dehydrosabinaketone, α-fenchen, isoborneol, isobornyl acetate, iso-3-thujanol, limonene, linalool, menthol, methyleugenol, p-mentha-1,4-dien-7-ol, β-myrcene, (E)-β-ocymen, (Z)-β-ocymen, α-pinene, β-pinene, trans-pinocarveol, piperitone, sabinaketone, sabinene, cis-sabinene hydrate, santolina triene, α-terpinene, γ-terpinene, α-terpineol, 4-terpineol, terpinolene, 3-thujanol, α-thujene, α-thujone, β-thujone, cis-thujone, thymol, trans-verbenol, verbenyl acetate [63,65,73,88,104,105,106,107,108,147,148] | phytol, γ-terpineol [106,108,151] | nd | nd | nd |
| Direction of Activity | Species | Extract/Essential Oil | Part | Classification | Compounds | Model/Assay | Short Description of Performed Studies | References |
|---|---|---|---|---|---|---|---|---|
| Antitumor activity | A. abrotanum | Essential oil | Aerial part | Monoterpenoids | Borneol, cymene, camphor, terpineol, 1,8-cineole, and aromadendrene | In vitro | Decrease in the survival of neoplastic cells of the RD (rhabdomyosarcoma). The viability of RD cells after the application of the essential oil at concentrations of 25, 50, and 100 μg/mL was 29.679%, 20.833%, and 20.256%, respectively. | [168] |
| Methanolic extract | Leaves | Phenolic acids | Chlorogenic and isochlorogenic acids | In vitro | Methanolic extract of A. abrotanum leaves in serial concentrations of 50, 100, 200, 300, and 400 µg/mL and its components (including chlorogenic acid and isochlorogenic acid) inhibits the proliferation of cells of the Jurkat line (T-lymphoblastic leukemia line, IC50 = 82.64 µg/mL), MCF-7 line (breast adenocarcinoma line, IC50 = 71.04 µg/mL), HeLa line (cervical adenocarcinoma line, IC50 = 49.97 µg/mL), and HT-29 line (colorectal adenocarcinoma line, IC50 = 54.75 µg/mL). | [20] | ||
| A. dracunculus | hexane, ethyl acetate, acetone, ethanol, acetonitrile and supercritical carbon dioxide (scCO2) | Leaves | Polyphenols, alkamides | nt * | In vitro (mouse lymphoma L5178YD cells) | Inhibition of the proliferation of mouse lymphoma cells (L5178YD) due to the presence of polyphenols and alkamides in leaf extracts. In the control group the tumor cell count was 17.969 × 106, the acetonitrile extract from A. dracunculus leaves reduced the cell count to 0.1 × 106. | [21] | |
| Alleviating allergy symptoms | A. abrotanum | Essential oil and isolated flavonoids | Aerial part | Monoterpenoids, flavonoids | 1,8-Cineole, davanone, linalool, centaureidine dimethylether, casticin and quercetin | In vivo | Relief of symptoms of allergic rhinitis with possible concomitant allergic conjunctivitis, symptoms of bronchial obstruction, and symptoms of exercise-induced asthma by using a nasal spray with a mixture of essential oils and flavonoids present in A. abrotanum. | [117] |
| Digestion-stimulating activity | A. absinthium | Ethanol | Herb | nt | nt | In vivo | Change in postprandial hemodynamics in the gastric digestive phase with increased hyperemia, probably due to the effects of bitter compounds contained in the herb of the plant. | [19] |
| Appetite-stimulating activity | A. absinthium | nt | Aerial part | nt | nt | In vivo | Enrichment of sheep fodder with silage containing A. absinthium increases the amount of fodder consumed, improves digestion, induces nitrogen retention, and has a positive effect on the development of microorganisms involved in nitrogen assimilation. | [180] |
| nt | Aerial part | nt | nt | In vivo | Improvement in nutrient supply and digestion, faster growth, improvement in carcass quality, and the amount of fatty acids among Hanwoo steers. | [181] | ||
| Antiulcer activity | A. absinthium | carbon tetrachloride, chloroform, methanol, ethanol, hexane | Aerial part and root | nt | nt | In vivo (rats) | Decrease in gastric juice volume, reduction in gastric acid and pepsin secretion, and decrease in the digestion rate. | [182] |
| Hepatoprotective activity | A. absinthium | Hydro-methanol | Herb | nt | nt | In vivo (rats) | A. absinthium extracts (in dose 500 mg/kg) inhibit liver microsomal enzymes (20%) that are responsible for the metabolism of xenobiotics. | [183] |
| Methanol | Herb | nt | nt | In vivo (rats) | Methanolic extracts from the herb of the plant (in dose 50 mg/kg) protect liver cells by reducing ALAT (alanine aminotransferase) and ASPAT (aspartate aminotransferase) levels and by reducing oxidative damage. | [13] | ||
| Aqueous | Herb | nt | nt | In vivo (mice) | Protection of the liver due to the immunomodulatory and/or antioxidant properties of A. absinthium (in dose 500, 100, or 200 mg/kg body weight/day). | [184] | ||
| A. dracunculus | Hydro-ethanol | Herb | nt | nt | In vivo (rats) | The extract (at dose 50, 100, or 200 mg/kg) decreased the levels of ALAT, ASPAT, alkaline phosphatase, and total bilirubin and increased total protein levels. | [40] | |
| A. vulgaris | Hydro-ethanol | Aerial part | nt | nt | In vivo (mice) | Prophylactic protective effect limiting inflammation, cellular edema, apoptotic cell count, and hyperemia of the hepatic parenchyma of hydro-ethanolic extract (at dose 600 mg/kg). | [209] | |
| Antispasmolytic activity | A. vulgaris | Chloroform and methanol | Herb | Sesquiterpenoids | Yomogin and 1,2,3,4-diepoxy-11(13)-eudesmen-12,8-olide | In vivo (guinea pigs) | Antagonism toward H1 histamine receptors. | [138,142] |
| Anthelmintic activity | A. absinthium | Aqueous and an ethanolic | Aerial part | nt | nt | In vivo (sheep) | Extracts from A. absinthium (in dose 2 g/kg body weight) cause paralysis and/or death of Haemonchus contortus nematodes and reduce (80.49%) the number of the parasite’s eggs in the host’s feces. | [185] |
| Essential oil | Aerial part | nt | nt | In vivo (mice) | Lethal effect on Trichinella spiralis larvae. | [86,186] | ||
| Ethanolic | Herb | nt | nt | In vivo (rabbits) | Lethal effect of A. absinthium ethanolic extract on Ascaris suum eggs and Trichostrongylus colubriformis larvae. | [187] | ||
| Ethanolic extract | Aerial part | nt | nt | In vivo (sheep), in vitro (parasite motility inhibition test) | Lethal effect on H. contortus tested in vivo; reduction in its mobility in vitro. | [188] | ||
| A. vulgaris | Methanol | Herb | nt | nt | In vivo (rats) | Extract (at dose 300 mg/kg) inhibited activity against T. spiralis by 75.6% and 63.5% in the tongue, 53.4% and 37.7% in the diaphragm, 67.8% and 46.2% in the quadriceps, and 66.7% and 60.5% in the biceps–triceps muscles of rats. | [186] | |
| Antiprotozoal activity | A. absinthium | Aqueous and ethanolic extracts | Aerial part | nt | nt | In vitro (mice) | Lethal effect of aqueous and ethanolic extracts from A. absinthium on Plasmodium berghei (in dose 74 mg/kg). | [25] |
| Hydro-ethanolic | Herb | nt | nt | In vitro (chloroquine-resistant (K1) and chloroquine-sensitive (CY27) strains of Plasmodium berghei) | Lethal effect of the hydro-ethanolic extract P. berghei. IC50 = 0.46 μg/mL for the K1 strain and IC50 = 0.195 μg/mL for the CY27. | [26] | ||
| nt | Herb powdered | nt | nt | In vivo (human) | Lethal effect of capsuled powdered herb of A. absinthium in dose 500 mg on Entamoeba histolytica. | [30] | ||
| Essential oil | Aerial part | nt | nt | In vitro | Lethal activity against the promastigotes and amastigotes forms of the protozoa Leishmania aethiopica and Leishmania donovani. MIC for both microorganisms in the promastigote form was 0.1565 μL/mL. | [32] | ||
| Ethanol | Aerial part | Flavonoids, sesquiterpenoid lactone | Artemetin, casticin, hydroxypelenolide | In vitro | Lethal activity in vitro against Leishmania infantum and Trypanosoma cruzi | [33,34] | ||
| Essential oil | Aerial part | Sesquiterpenoids | (E)-Caryophyllene and 3,6-dihydrochamazulene | In vitro | Lethal effect of the essential oil on T. cruzi and on Trichomonas vaginalis. The compounds likely to be responsible for this activity are (E)-caryophyllene and 3,6-dihydrochamazulene. | [35] | ||
| Aqueous and ethanolic | Aerial part | Sesquiterpenoids lactones | Artemisinin, dihydroartemisinin | In vitro | Inhibition (100%) of Naegleria fowleri growth by sesquiterpenoid lactones from A. absinthium. | [36] | ||
| Aqueous | Aerial part | nt | nt | In vitro | Inhibition (88.9%) of A. absinthium aqueous extract against Plasmodium falciparum. | [37] | ||
| A. annua | Methanol, ethanol, aqueous | Herb | Sesquiterpene lactone | Artemisinin | In vivo/In vitro | Lethal activity against Artemisia castellani of artemisinin and methanolic, ethanolic, and aqueous extracts from A. annua herb. | [27] | |
| n-Hexane, ethanol, and water | Leaves and seeds | nt | nt | In vitro | Compounds present in A. annua seed and leaf extracts have lethal activity against L. donovani. | [29] | ||
| A. dracunculus | Hydro-ethanol | Herb | nt | nt | In vitro | The extract (at dose (100–1000 μg/mL) inhibited the development of the promastigote form of Leishmania major. The recorded MIC values of the extract after 24 h, 48 h and 72 h were: 962.03, 688.36 and 585.51 μg/mL. | [28] | |
| Immunostimulating activity | A. absinthium | Ethanolic | Herb | nt | nt | In vivo (mice) | Induction of dendritic cell maturation by increasing the level of CD40 surface expression and by induction of cytokines. It was found that at 100 μg/mL extract the proliferation of T-lymphocytes was reduced by 78.2% relative to the control. | [189] |
| nt | Herb | Polysaccharides | nt | In vivo (mice) | Induction of TH1 immune response and stimulation of nitric oxide production by macrophages. | [190] | ||
| Immunosuppressive activity | A. annua | Ethanol | Herb | nt | nt | In vitro/In vivo | Inhibition of lymphocyte proliferation and reduction in IgG, IgG1, and IgG2b antibody levels after the administration of A. annua whole-plant extract (at dose 0.25, 0.5, and 1. 0 mg). | [91] |
| nt | Herb | Sesquiterpene lactone | Artemisinin | In vivo (mice) | Artemisinin obtained from A. annua inhibits late-type hypersensitivity response and has a suppressive effect on calmodulin responsible for activation of T lymphocytes. | [198] | ||
| A. dracunculus | Aqueous | Herb | nt | nt | In vivo (mice) | The extract (at dose 100 mg/kg) reduced IL-17 (interleukin 17) and IFN-γ (interferon gamma) production and intensification of the phagocytosis process carried out by macrophages. | [149] | |
| Aqueous | Herb | nt | nt | In vivo (mice) | Lowering of IL-17 and IL-23 (interleukin-23) levels and reduction in the infiltration of leukocytes into brain cells. | [204] | ||
| Hydro-ethanol | Leaves | nt | nt | In vivo (mice) | Increased neutrophil levels and decreased lymphocyte levels after intraperitoneal administration of the hydroethanolic extract from the leaves (at dose 200 mg/kg). | [205] | ||
| Cytotoxic activity | A. absinthium | Methanol | Leaves | nt | nt | In vitro | Inhibition of proliferation of breast cancer cells of MDA-MB-231 (50% at 20 g/mL) and MCF-7 lines (50%, at 25 g/mL). | [17] |
| Essential oil | Aerial part | Sesquiterpenoids | (E)-Caryophyllene, germacrene D | In vitro | The essential oil, in particular (E)-caryophyllene and/or germacrene D, is toxic to tumor lines A548, NCI-H292, HCT116, MCF-7, and SK-MEL-5. | [18] | ||
| A. annua | Ethyl acetate | Aerial part | Polyphenols | Caffeic acid, syringic aldehyde, dicaffeoylquinic acid isomer, quercetin 3-O-galactoside, dicaffeoylquinic acid isomer, mearnsetin 3-O-hexoside isomer, kaempferol 3-O-glucoside, quercetin 3-O-glucoside, ferulic acid, caffeoylferuloylquinic acid isomer, isorhamnetin 3-O-glucoside, diosmetin 7-O-glucoside, luteolin 7-O-glucoside, diferuloylquinic acid, quercetin, dicaffeoylferuloylquinic acid isomer, 3-O-methylquercetagetin, luteolin, 8-methoxykaempferol, 3,5-dimethoxyquercetagetin, caffeoyldiferuloyl quinic acid, kaempferol, 3,5-dihydroxy-6,7,4′-trimethoxyflavone, and 3,5-dihydroxy-6,7,3′,4′-tetramethoxyflavone | In vitro | Polyphenols present in A. annua inhibit adhesion of cancer cells to endothelial cells and inhibit epithelial–mesenchymal transition. | [123] | |
| nt | Herb | Sesquiterpenoid lactone | Artemisinin | In vivo | Regression of prostate cancer in a patient treated (at dose 5 mg/day) with capsules containing a concentrate with A. annua and bicalutamide. | [199] | ||
| Methanol | Leaves | nt | nt | In vitro | Methanolic extract from A. annua leaves collected in Egypt showed significant cytotoxic activity against MCF-7 human breast adenocarcinoma cell line, human lung cancer cell line, and Chinese hamster ovary (CHO) cell line. | [201] | ||
| A. vulgaris | Methanol | Aerial part | nt | nt | In vitro | Inhibition of tumor cell growth in cancer cell lines: MCF-7 (IC50 = 190 ng/mL), HeLa (IC50 = 284 ng/mL), A7R5 (IC50 = 382 ng/mL), 293T (IC50 = 317 ng/mL), and SW-480 (IC50 = 778 ng/mL). | [210,211,212] | |
| Analgesic activity | A. absinthium | Methanolic | Herb | nt | nt | In vivo (mice) | Reduction in temperature-induced pain in mice at doses of 300 mg/kg, 500 mg/kg or 1000 mg/kg. | [191] |
| Essential oil/Aqueous | Aerial part | nt | nt | In vivo (mice) | Reduction in episodes in the writhing test and delay in pain response in the hot plate test in mice after the administration of A. absinthium essential oil (at doses of 2, 4, or 8 mg/kg) or aqueous extract (50, 100, or 200 mg/kg). | [122] | ||
| A. annua | Essential oil | Herb | Monoterpenoids | Camphor, 1,8-cineol, and α-pinene | In vivo (mice) | Administration of essential oil (at dose 400 mg/kg) from A. annua herb, camphor, 1,8-cineol, and α-pinene in mice reduces (57%) writhing episodes caused by acetic acid. | [93] | |
| A. vulgaris | Hydro-ethanol | Aerial part | Flavonoids, phenolic acids | Rutoside, hydroxybenzoic acid derivatives, and caffeic acid and its derivatives. | In vivo (mice) | Mild peripheral antinociceptive effect of extract (at dose 100 and 250 mg/kg). | [142] | |
| Inhibiting the activity of carbonic anhydrase I and II | A. dracunculus | Dichloromethane | Herb | Phenylpropanoid derivatives, sterols, coumarin | trans-Anethole, stigmasterol, herniarin, (2E,4E)-N-isobutylundeca-2,4-diene-8,10-diynamide, (2E,4E)-1-(piperidin-1-yl)undeca-2,4-diene-8,10-diyn-1-one and 1-(4’-methoxyphenyl)-1,2,3-trihydroxypropane | In vitro | Compounds present in herbal extracts reduce the activity of carbonic anhydrase I (hCA I) and II (hCA II) (IC50 = 0.02 μg/mL for hCA I, and IC50 = 0.31 μg/mL for hCA II). | [216] |
| Neuroprotective activity | A. absinthium | Methanol | Aerial part | nt | nt | In vivo (rats) | Methanolic extract (at dose 100 and 200 mg/kg) from A. absinthium, because of its antioxidant potential, reduces brain damage, inhibits lipid peroxidation, and restores the activity of enzymes involved in reducing oxidative stress. | [14] |
| Aqueous | Herb | nt | nt | In vivo (rats) | Protective effect of A. absinthium aqueous extract (at dose 200 mg/L) on glial cells and the dopaminergic system when exposed to lead. | [15] | ||
| Herb | Sesquiterpenoid dimer | Caruifolin D | In vitro (BV2 microglial cells) | Caruifolin D in Absinthii herba inhibits the production of proinflammatory microglia mediators and reactive oxygen species and also inhibits protein C kinase and stress-activated kinases. | [130] | |||
| Antidepressant activity | A. absinthium | Methanol | Aerial part | nt | nt | In vivo (mice) | Shortening of the period of mouse immobility in the forced swim test (at dose 1000 mg/kg) and in the tail suspension test (at dose 500 mg/kg). | [16] |
| A. dracunculus | Ethanol | Herb | nt | nt | In vivo (mice) | Increased resistance to stressful situations and reduction in stress-related levels of inflammatory cytokines. | [206] | |
| Ethanol | Herb | Phenolic acids, flavonoids | Chlorogenic acid, caffeic acid or luteolin and quercetin | In vivo (mice) | Phenolic compounds and flavonoids contained in the A. dracunculus herb extract (at dose dose of 200 mg/kg) reduce the immobility response time in mice in the writhing test and in the forced swim test. | [114] | ||
| Ethanol | Herb | Coumarins | Herniarin, skimmin c | In vitro | Mild inhibition of hMAO-A (human monoamine oxidase A) and hMAO-B (human monoamine oxidase B) by extracts of A. dracunculus and compounds. Herniarin and skimmin c showed the inhibitory effects against hMAO A (IC50 = 51.76 and 73.47 μM, respectively) and hMAO B (IC50 = 0.84 and 1.63 mM, respectively). | [112] | ||
| Procognitive activity | A. absinthium | Ethanol | Aerial part | nt | nt | In vitro (human cortical brain cells) | Extract in concentration 29 mg/mL had affinity for human muscarinic (99.8%) and nicotinic receptors (99.8%) responsible for cognitive functions. | [38] |
| Neurotrophic activity | A. absinthium | Methanol, ethanol and aqueous | Aerial part | nt | nt | In vitro (PC12D cells (cell line of rat pheochromocytoma tumor) | Methanolic, ethanolic, and aqueous extracts from A. absinthium induce the nerve growth factor, which stimulates development of neurites. | [217] |
| Nephroprotective activity | A. annua | Essential oil | Aerial part | nt | nt | In vivo (rats) | Administration of A. annua essential oil to rats exposed to carbon tetrachloride prevents kidney damage. | [93] |
| Stabilizing cell membrane activity | A. absinthium | Hydroalcoholic | Aerial part | nt | nt | In vitro | Hydroalcoholic extract from A. absinthium prevents hemolysis of erythrocytes. | [218] |
| Auxiliary action in obesity treatment | A. annua | Essential oil | Aerial part | nt | nt | In vitro | Reduction in fat droplet accumulation and inhibition of PPARγ (peroxisome proliferator- activated receptor gamma), C/EBPα (CCAAT/enhancer-binding protein), SREBP-1c (Sterol regulatory element-binding protein 1), FAS, and ACC (Acetyl-CoA carboxylase) protein expression under the influence of A. annua essential oil. | [202] |
| Hydro-ethanol | Leaves | nt | nt | In vivo (mice) | Reduction in insulin resistance, liver steatosis, and fibrosis. Lowering the levels of SREBP-1c, ChREBP (carbohydrate-responsive element-binding protein), and COX-2 (cyclooxygenase-2). Inhibition of TGF-β1 and connective tissue growth factor. | [203] | ||
| Hypoglycemic activity | A. dracunculus | Ethanol | Herb | nt | nt | In vivo | Encapsulated ethanolic extract of A. dracunculus (at dose 1000 mg for 90 days) decrease in glycated hemoglobin (5.8% in the control group, 5.6% in the test group), area under the curve for insulin (56.136 to 27.426 pmol/L in the control group, 44.472 to 23.370 pmol/L in the test group), total insulin secretion (0.45 to 0.23 in the control group, 0.35 to 0.18 in the test group), and systolic blood pressure (120 mm Hg in the control group, 113 mmHg in the test group), and increase in HDL-C. | [207] |
| Hypolipemic activity | A. vulgaris | Aqueous | Root | nt | nt | In vivo (rat) | Normalized serum lipid profile, a significant increase in paraoxonase-1 activity, and decrease in serum malondialdehyde, nitric oxide, and tumor necrosis factor-α levels and in hydroxymethylglutaryl-CoA reductase activity. Lowering total cholesterol, triglycerides, LDL (low-density lipoprotein), and VLDL (very low density lipoprotein), and increasing HDL (high density lipoprotein) and atherogenicity indicator (aqueous extract of A. vulgaris roots). | [213,214] |
| Antihypertensive activity | A. vulgaris | Aqueous and chloroform | Aerial part | nt | nt | In vivo (rats) | A 10% solution of the aqueous extract inhibiting the hypertensive effect of noradrenaline. | [215] |
| Bronchodilatory activity | A. vulgaris | Methanol | Aerial part | Alkaloids, coumarins, flavonoids, saponins, sterols, tannins, and terpenoids | nt | In vivo (rabbit jejunum and guinea pig trachea) | Anticholinergic and Ca2+ antagonist mechanisms. Histamine H1 antagonism in the ileum and trachea. | [138,208] |
| Normalizing the profile of thyroid hormones | A. dracunculus | Aqueous | Herb | nt | nt | In vivo (rats) | Extract (at dose 300 mg/kg) caused increase in thyroxine and triiodothyronine levels, decrease in thyrotropin levels, increase in total antioxidant capacity, increase in glutathione, and decrease in malondialdehyde levels. | [22] |
| Estrogenic activity | A. vulgaris | Ethyl acetate | Aerial part | Flavonoids | Eriodictyol and apigenin | In vivo (rats) | Antagonism toward the estrogen receptor and activation of gene transcription. Induction of gene transcription by eriodictyol and apigenin. Anti-implantation activity and estrogenic activity on female Wistar rats. | [23,24] |
| Insect repellent activity | A. abrotanum | Toluene extract | Herb | Monoterpenoids, coumarins, phenolic acids | Camphor, coumarin and thujyl alcohol, chlorogenic acid and caffeic acid | In vivo | Toluene extract from the herb A. abrotanum and the individual components of the extract showed an insect repellent effect against Ixodes ricinus and Aedes aegypti. After 4 and 8 h from the time of applying the ethanolic suspension of the toluene extract from the herb A. abrotanum, the recorded repellency rates were, respectively, 69.1% and 56.8% against Ixodes ricinus, and 100% and 86.7% against Aedes aegypti. | [116] |
| A. dracunculus | Essential oil | Herb | nt | nt | In vitro | Inhibition of Calliphora vomitoria egg laying on fresh beef, on which the essential oil of A. dracunculus herb (at dose 0.05 μL/cm2) was applied. | [96] | |
| Essential oil | Herb | nt | nt | In vitro | Larvacidal effect against Anopheles stephensi under the influence of nanoemulsion of A. dracunculus essential oil (consisting of 0.35% tarragon oil, 10% of Tween 20 and deionized water). | [102] | ||
| Anti-animal parasites activity | A. abrotanum | Reduction in the number of eggs of Hymenolepis nana (dwarf tapeworm), Syphacia obvelata, and Aspiculuris tetraptera (rodent pinworms) in the feces of mice after administration of ethanolic extract from A. abrotanum leaves. | [192] | |||||
| A. annua | Water, 0.1% sodium bicarbonate solution, dichloromethane, and methanol | Leaves | Sesquiterpenoid lactones | Artemisinin | In vivo | Extracts from A. annua leaves inhibit the growth of larvae and the hatching of eggs of Haemnochus contortus (parasite of sheep and goats). | [118] | |
| Antiplasmodial activity | A. abrotanum | Ethanol/water (1/1) | Leaves | nt | nt | In vitro/Hemolysis assay | Notable antiprotozoal activity against P. falciparum under the influence of A. abrotanum-AgNPs in concentration ranging from 0.6 to 7.5 µg/mL. The inhibition dependent on concentration was 50%, 90%, and 99%. | [219] |
| Antimalarial activity | A. annua | Methanol | Herb | nt | nt | In vivo | Improvement in malaria symptoms after treating patients with infusion of A. annua herb. Inactivation of protozoan calcium pump. | [193] |
| Hydro-ethanol and aqueous | Leaves | nt | nt | In vivo | Lethal activity of hydroethanolic and aqueous extracts from A. annua leaves (at dose 20 mg/kg) against P. falciparum and P. berghei. | [194] | ||
| nt | Herb | Sesquiterpenoid lactones | Artemisinin | In vitro | Interference of artemisinin with protein metabolism and mitochondrial activity of Plasmodium spp. protozoa. | [195] | ||
| nt | Leaves | Sesquiterpenoid lactones | Artemisinin | In vitro | Synergism of action of artemisinin and other compounds present in A. annua leaves against P. falciparum. | [131] | ||
| A. vulgaris | Ethanol | Leaves | nt | nt | In vitro | Activity against Plasmodium yoelii and P. berghei. The extract at doses of 500, 750, and 1000 mg/kg significantly inhibited parasitemia by 79.3%, 79.6%, and 87.3%, respectively. | [220,221] |
| Species | INCI Name | Description | Functions |
|---|---|---|---|
| A. abrotanum | Artemisia abrotanum extract | Extract of the whole plant of the Southernwood, A. abrotanum | Skin protecting |
| Artemisia abrotanum leaf/stem extract | Extract of the flowers, leaves, and stems of the Southernwood, A. abrotanum | Moisturizing Skin conditioning | |
| A. absinthium | Artemisia absinthium extract | Extract of the whole herb of the Wormwort, A. absinthium | Skin conditioning |
| Artemisia absinthium herb extract | Extract obtained from the flowering herb of the Wormwort, A. absinthium | Perfuming | |
| Artemisia absinthium herb oil | “Wormwood Oil”, essential oil obtained from the flowering herb of the Wormwort, A. absinthium. It contains thujyl alcohol, thujyl acetate, thujone, phellandrene, cadinene, and a blue oil | Perfuming | |
| Artemisia absinthium oil | Volatile oil obtained from the whole plant of the Wormwort, A. absinthium | Antimicrobial | |
| Artemisia absinthium/Chamaecyparis obtusa wood extract | Extract of the whole plant, A. absinthium, and the wood of C. obtusa | Antimicrobial Hair conditioning Skin conditioning—emollient | |
| A. annua | Artemisia annua (leaf/stem)/Ficus carica fruit/Ginkgo biloba leaf extract | Extract of the leaves and stems of A. annua, the fruit of F. carica, and the leaves of G. biloba | Skin conditioning |
| A. annua callus extract | Extract of the callus of A. annua grown in culture | Antimicrobial Antioxidant Hair conditioning Skin conditioning Skin protecting | |
| Artemisia annua extract | Extract of the whole herb, A. annua | Fragrance | |
| Artemisia annua flower/leaf/stem extract | Extract of the flowers, leaves, and stems of A. annua | Skin conditioning—miscellaneous | |
| Artemisia annua herb oil | Essential oil obtained from the whole herbs of the plant A. annua | Perfuming | |
| Artemisia annua leaf extract | Extract obtained from the leaves of the plant A. annua | Antiseborrheic Antimicrobial Perfuming Skin conditioning | |
| Artemisia annua leaf/stem extract | Extract of the leaves and stems of A. annua | Skin conditioning | |
| Artemisia annua meristem cell extract | Extract of the cultured meristem cells of A. annua | Antioxidant | |
| Artemisia annua oil | Volatile oil obtained from the whole plant, A. annua | Antioxidant Humectant Skin conditioning Skin conditioning—emollient | |
| Artemisia annua seed extract | Extract of the seeds of A. annua | Antioxidant | |
| Artemisia annua/Citrus junos fruit/Pinus densiflora leaf extract | Extract of the whole plant A. annua, the fruit of C. junos, and the leaves of P. densiflora | Skin protecting | |
| A. dracunculus | Artemisia dracunculus flower | Flower of A. dracunculus | Skin conditioning |
| Artemisia dracunculus herb extract | Extract obtained from the whole herb of the Tarragon, A. dracunculus | Perfuming | |
| Artemisia dracunculus leaf/stem extract | Extract of the leaves and stems of the Tarragon, A. dracunculus | Fragrance | |
| Artemisia dracunculus oil | Essential oil obtained from the whole herbs of the Tarragon, A. dracunculus | Perfuming Skin conditioning | |
| Artemisia dracunculus root extract | Extract of the roots of the Tarragon, A. dracunculus | Skin conditioning | |
| Artemisia dracunculus seed/Anthemis nobilis seed/Hypericum androsaemum seed extract | Extract of the seeds of the Tarragon, A. dracunculus, A. nobilis, and H. androsaemum | Skin conditioning | |
| A. vulgaris | Artemisia vulgaris extract | Extract of the whole plant of the Common Mugwort, A. vulgaris | Skin conditioning |
| Artemisia vulgaris herb extract | Extract obtained from the whole herb of the Common Mugwort, A. vulgaris | Perfuming | |
| Artemisia vulgaris leaf extract | Extract of the leaves of A. vulgaris | Antioxidant Skin conditioning—emollient Skin protecting | |
| Artemisia vulgaris oil | Volatile oil obtained from the whole herb of the Common Mugwort, A. vulgaris | Perfuming Skin conditioning |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials. Molecules 2022, 27, 6427. https://doi.org/10.3390/molecules27196427
Ekiert H, Klimek-Szczykutowicz M, Rzepiela A, Klin P, Szopa A. Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials. Molecules. 2022; 27(19):6427. https://doi.org/10.3390/molecules27196427
Chicago/Turabian StyleEkiert, Halina, Marta Klimek-Szczykutowicz, Agnieszka Rzepiela, Paweł Klin, and Agnieszka Szopa. 2022. "Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials" Molecules 27, no. 19: 6427. https://doi.org/10.3390/molecules27196427
APA StyleEkiert, H., Klimek-Szczykutowicz, M., Rzepiela, A., Klin, P., & Szopa, A. (2022). Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials. Molecules, 27(19), 6427. https://doi.org/10.3390/molecules27196427









