Phytochemical Analysis of Acaciaehrenbergiana (Hayne) Grown in Qatar: Identification of Active Ingredients and Their Biological Activities
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis of Acacia ehrenbergiana Grown in Qatar
2.2. Antibacterial Activity of Acacia ehrenbergiana Extracts
2.3. Antioxidant Activity of Acacia ehrenbergiana
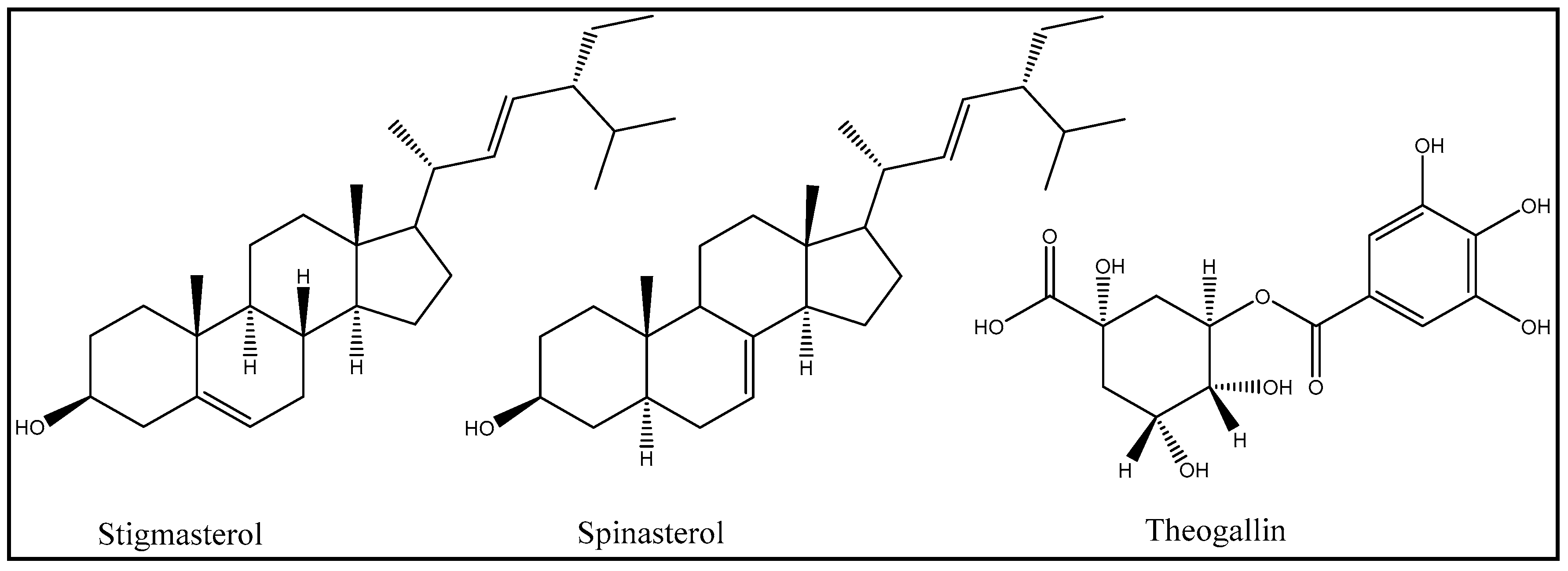
2.4. Characterization of Secondary Metabolites Present in Acetone Extracts of Acacia ehrenbergiana
2.5. Antibacterial Activity of Isolated Compounds
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Extraction of Phytochemicals and Fractionation
3.2.2. Phytochemical Analysis
3.2.3. Antibacterial Activity Tests
3.2.4. DPPH In Vitro Assay
3.2.5. Identification and Characterization of the Active Compounds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Abbas, J.A.; El-Oqlah, A.A.; Mahasneh, A.M. Herbal plants in the traditional medicine of Bahrain. Econ. Bot. 1992, 46, 158–163. [Google Scholar] [CrossRef]
- Briskin, D.P. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000, 124, 507–514. [Google Scholar] [CrossRef]
- Mahasneh, A.M. Screening of some indigenous Qatari medicinal plants for antimicrobial activity. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2002, 16, 751–753. [Google Scholar] [CrossRef]
- El-Desoukey, R.M.A. Phytochemical and Antimicrobial Activity of Acacia Ehrenbergiana hayne (Salam) as a Grazing Herb against some Animal Pathogens. Adv. Anim. Vet. Sci. 2018, 6, 246–251. [Google Scholar] [CrossRef]
- Mohammed, A.; Izeldin, A.B. Chemical Analysis of Acacia Ehrenbergiana (Salam) Tree Fruits (Seed and Pods) as Dry Season Supplement for Livestock in Arid and Semi—Arid Lands of Sudan. Anim. Rev. 2015, 2, 76–80. [Google Scholar]
- Wassel, G.M.; Abd-El-Wahab, S.M.; Aboutabl, E.A.; Ammar, N.M.; Afifi, M.S. Study of phenolic constituents and tannins isolated from Acacia nilotica L. Willd and Acacia farnesiana L. Willd growing in Egypt. Herba Hung. 1990, 29, 43–49. [Google Scholar]
- Hassan-Abdallah, A.; Merito, A.; Hassan, S.; Aboubaker, D.; Djama, M.; Asfaw, Z.; Kelbessa, E. Medicinal plants and their uses by the people in the Region of Randa, Djibouti. J. Ethnopharmacol. 2013, 148, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Maiden, J.H. The Useful Native Plants of Australia: (Including Tasmania); Turner and Henderson: Sydney, Australia, 1889. [Google Scholar]
- von Maydell, H.-J. Trees and Shrubs of the Sahel, Their Characteristics and Uses; CABI Publishing: Preston, UK, 1986. [Google Scholar]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Al-Jeffri, J.H.; Haile, A.M. Setting up Acacia Ehrenbergiana (Salam) Plantation in the Tihama Region of Yemen Spate Irrigation; Practical Notes Series; YUMPU Publishing: Sana, Yamen, 2011. [Google Scholar]
- Rahim, S.A.A.; Almagboul, A.Z.; Mohammed, N.E.B. Antimicrobial activity of Croton zambesicus, Acacia ehrenbergiana and Fagonia cretica. Natl. J. Adv. Res. 2016, 2, 16–20. [Google Scholar]
- AbduRahim, S.A.; Mohammed, N.E.B.; Almagboul, A.Z. In Vivo antibacterial and wound healing activities of Acacia ehrenbergiana. Mediterr. J. Biosci. 2016, 1, 147–152. [Google Scholar]
- Gaara, A.H.; Nassar, M.I.; Younis, M.; Elmegeed, G.A.; Mabry, T.J.; Pare, P.W. Biologically active polyphenolic compounds from Acacia ehrenbergiana. Rev. Latinoamer. Quím. 2008, 36, 52–59. [Google Scholar]
- Mohammad, I.I.; Alsafi, M.Y.; AL-Mahdi, M.; Elsammani, T.O.; Mudawi, M.M. Evaluation of anti-inflammatory activity of Acacia ehrenbergiana (salam) leaves by in vivo and in vitro models. Indo Am. J. Pharm. Sci. 2017, 4, 26–32. [Google Scholar]
- Makeen, H.A.; Alhazmi, H.A.; Khalid, A.; Al Bratty, M.; Syame, S.M.; Abdalla, A.N.; Homeida, H.E.; Sultana, S.; Ahsan, W. Phytochemical, antimicrobial and cytotoxicity screening of ethanol extract of Acacia ehrenbergiana Hayne grown in Jazan Region of Saudi Arabia. Trop. J. Pharm. Res. 2020, 19, 313–321. [Google Scholar] [CrossRef]
- Al–Easa, H.S.; Rizk, A.M.; Abdel Bari, E.M. Chemical Constituents and Nutritive Values of Range Plants in Qatar; The Scientific and Applied Research Center; The Doha Modern Press Ltd.: Doha, Qatar, 2003. [Google Scholar]
- Fakhroo, A.; Sreerama, L. Qualitative analysis of phytochemical compounds in Ocimum basilicum grown in Qatar. Int. J. Appl. Pharm. Biol. Res. 2016, 1, 11–17. [Google Scholar]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation, and characterization of bioactive compounds from plant’s extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar]
- Kokate, C.K.; Purohit, A.P.; Gokhale, S.B. Carbohydrate and derived Products, drugs containing glycosides, drugs containing tannins, lipid and protein alkaloids. In Text Book of Pharmacognosy, 7th ed.; Nirali Prakashan: Pune, India, 2001; pp. 133–310, 428–523. [Google Scholar]
- Vanden Berghe, D. Screening methods for antibacterial and antiviral agents from higher plants. In Methods in Plant Biochemistry; Academic Press: London, UK, 1991; pp. 47–68. [Google Scholar]
- Al-Ghanayem, A.A.; Al Sobeai, S.M.; Alhussaini, M.S.; Joseph, B.; Saadabi, A.M. Antibacterial activity of certain Saudi Arabian medicinal plants used in folk medicine against different groups of bacteria. Nusant. Biosci. 2017, 9, 392–395. [Google Scholar] [CrossRef]
- Mailafiya, M.M.; Yusuf, A.J.; Abdullahi, M.I.; Aleku, G.A.; Ibrahim, I.A.; Yahaya, M.; Abubakar, H.; Sanusi, A.; Adamu, H.W.; Alebiosu, C.O. Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J. Med. Plants Econ. Dev. 2018, 2, 1–5. [Google Scholar]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Bayer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W.; Mizaton, H.H.; Zakaria, H. The relationship between major polyphenolic acids and stigmasterol to antioxidant activity in different extracts of Myrmecodia platytyrea. S. Afr. J. Bot. 2018, 115, 94–99. [Google Scholar] [CrossRef]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar] [CrossRef]
- Kaur, N.; Chaudhary, J.; Jain, A.; Kishore, L. Stimasterol: A Comprehensive Review. Int. J. Pharm. Sci. Res. 2011, 2, 2259–2265. [Google Scholar]
- Saini, R.K.; Song, M.H.; Yu, J.W.; Shang, X.; Keum, Y.S. Phytosterol Profiling of Apiaceae Family Seeds Spices Using GC-MS. Foods 2021, 10, 2378. [Google Scholar] [CrossRef]
- Chen, H.J.; Chung, C.P.; Chiang, W.; Lin, Y.L. Anti-inflammatory effects and chemical study of a flavonoid-enriched fraction from adlay bran. Food Chem. 2011, 126, 1741–1748. [Google Scholar] [CrossRef]
- Hassan, H.S.; Sule, M.I.; Musa, A.M.; Musa, K.Y.; Abubakar, M.S.; Hassan, A.S. Anti-inflammatory activity of crude saponin extracts from five Nigerian medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 250–255. [Google Scholar] [CrossRef]
- Bredie, W.L.; Petersen, M.A. Flavour Science: Recent Advances and Trends; Elsevier: Amaterdam, The Netherlands, 2006; Volume 43. [Google Scholar]
- Dimpfel, W.; Kler, A.; Kriesl, E.; Lehnfeld, R. Theogallin and l-theanine as active ingredients in decaffeinated green tea extract: II. Characterization in the freely moving rat by means of quantitative field potential analysis. J. Pharm. Pharmacol. 2007, 59, 1397–1403. [Google Scholar] [CrossRef]
- Serafini, M.; Del Rio, D.; N’Dri Yao, D.; Bettuzzi, S.; Peluso, I. Health Benefits of Tea, 2nd ed.; Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
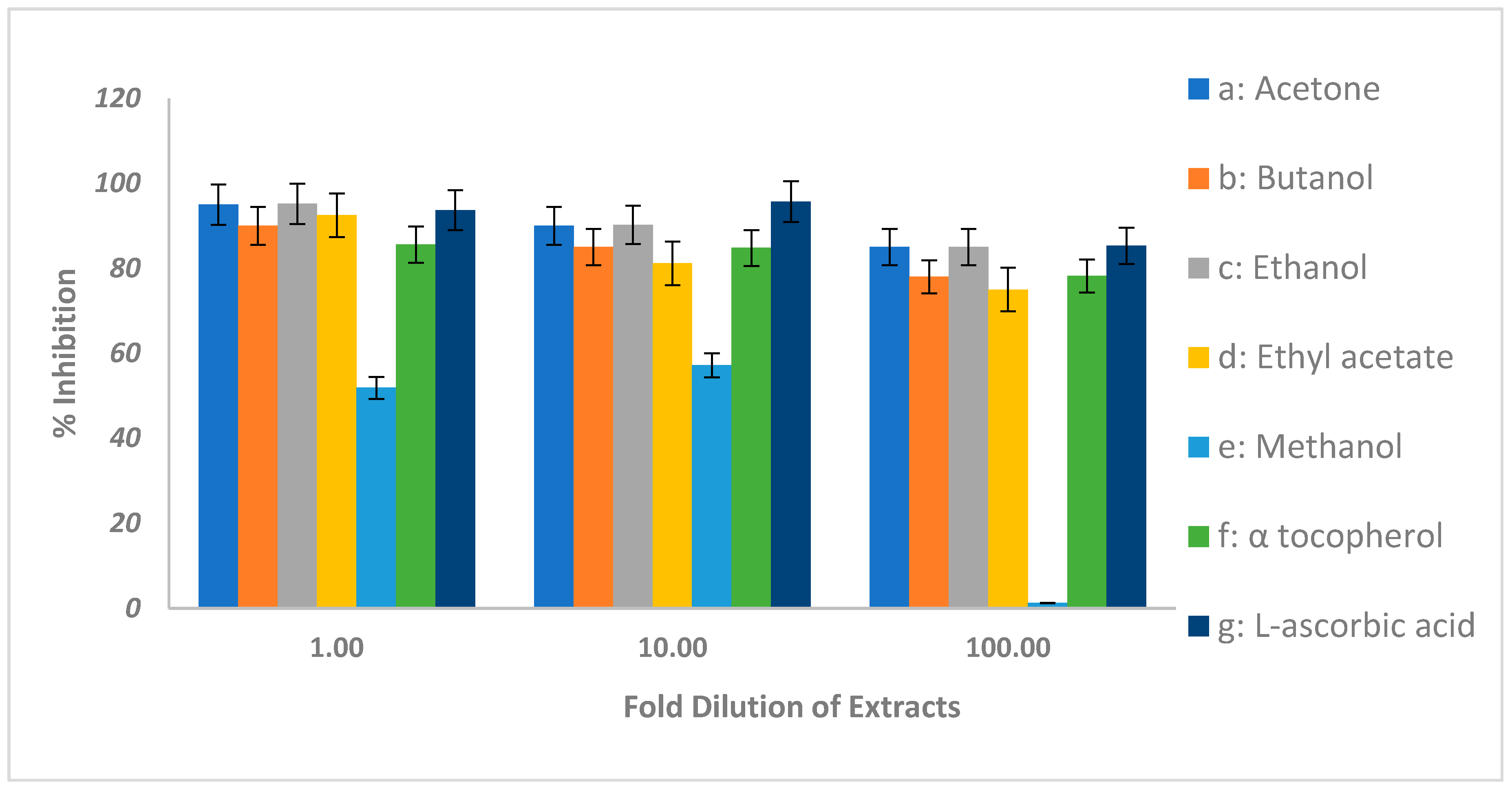
| # | Test | Procedure | Expected Positive Test Result | a 2 | b 2 | c 2 | d 2 | e 2 |
|---|---|---|---|---|---|---|---|---|
| 1 | Alkaloids | Wagner’s Test | Brown/Reddish precipitate | − | − | − | − | − |
| 2 | Glycosides | Kellar–Kiliani’s Test | Brown ring at the junction | + | + | + | + | − |
| 3 | Tannins | Braymer’s Test | Dark blue or greenish grey coloration of the solution | − | + | + | + | + |
| 4 | Flavonoids | Alkaline Reagent Test (A Drop of 10%NaOH) | Intense yellow color | + | + | + | + | + |
| 5 | Terpenoids | Salkowski Test | Reddish brown ring at the junction | + | + | + | + | + |
| 6 | Saponins | Foam Test | Stable froth produced | − | − | + | − | − |
| 7 | Phenols | FeCl3 Test | Blue-green | + | + | + | + | + |
| 8 | Anthraquinones | 1 mL benzene and 3–5 drops of 26% NH3 | Pink or violet or red coloration in the ammonical layer 3 | + | + | + | + | + |
| 9 | Phlobatannins | 2 mL extract was boiled with 2 mL of 1% hydrochloric acid HCl | Red ppt | − | − | − | − | − |
| 10 | Anthocyanins | 2 mL HCl (2M) + NH3 | Pinkish-red to bluish violet color | − | − | − | − | − |
| 11 | Proteins | Xanthoproteic Test | White precipitate | − | − | − | − | − |
| Bacterial Species Tested | Extract * | % Control at 200 µg/mL ** |
|---|---|---|
| E. coli | Acetone | 85 |
| Butanol | 0 | |
| Ethanol | 85 | |
| Ethyl acetate | 55 | |
| Methanol | 10 | |
| Bacillus sp. | Acetone | 65 |
| Butanol | 17 | |
| Ethanol | 11 | |
| Ethyl acetate | 15 | |
| Methanol | 0 | |
| Staphylococcus aureus | Acetone | 40 |
| Butanol | 65 | |
| Ethanol | 22 | |
| Ethyl acetate | 33 | |
| Methanol | 45 |
| Plant Source | Extract | Organism | Part of Plant Tested | Ref | ||
|---|---|---|---|---|---|---|
| E. coli | Bacillus | S. aureus | ||||
| Qatar | Acetone | + | + | + | Stem, leaves Concentrations used: 5–200 µg/mL | Present Study |
| Ethanol | + | + | + | |||
| Butanol | − | + | + | |||
| Methanol | + | − | + | |||
| Sudanese Marawi north desert | Methanol | + | + | + | Stem bark Concentration used: 200 mg/mL | [12] |
| Egypt, Aswan | Ethanol | + | + | + | Extracted compounds from aerial parts | [14] |
| Saudi Arabia | Methanol 1 | + | + | + | Fresh aerial parts, leaves | [4,8] [16,22] |
| Ethanol | + | ND | + | |||
| Isolated Compound | Organism | Results * | Literature Report * |
|---|---|---|---|
| Stigmasterol | E. coli | Inactive | Active [23] |
| Spinasterol | Inactive | N/A | |
| Theogallin | Inactive | N/A | |
| Stigmasterol | S. aureus | Inactive | Active [23] |
| Spinasterol | Inactive | N/A | |
| Theogallin | Inactive | N/A |
| Isolated Compound | Organism | Results * | Literature Report * |
|---|---|---|---|
| Stigmasterol | Saccharomyces cerevisiae | Inactive | Active [23] |
| Spinasterol | Inactive | N/A | |
| Theogallin | Inactive | N/A | |
| Stigmasterol | A. fumigatus | Inactive | Active * [23] |
| Spinasterol | Inactive | N/A | |
| Theogallin | Inactive | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thotathil, V.; Rizk, H.H.; Fakrooh, A.; Sreerama, L. Phytochemical Analysis of Acaciaehrenbergiana (Hayne) Grown in Qatar: Identification of Active Ingredients and Their Biological Activities. Molecules 2022, 27, 6400. https://doi.org/10.3390/molecules27196400
Thotathil V, Rizk HH, Fakrooh A, Sreerama L. Phytochemical Analysis of Acaciaehrenbergiana (Hayne) Grown in Qatar: Identification of Active Ingredients and Their Biological Activities. Molecules. 2022; 27(19):6400. https://doi.org/10.3390/molecules27196400
Chicago/Turabian StyleThotathil, Vandana, Hanan Hosni Rizk, Ameena Fakrooh, and Lakshmaiah Sreerama. 2022. "Phytochemical Analysis of Acaciaehrenbergiana (Hayne) Grown in Qatar: Identification of Active Ingredients and Their Biological Activities" Molecules 27, no. 19: 6400. https://doi.org/10.3390/molecules27196400
APA StyleThotathil, V., Rizk, H. H., Fakrooh, A., & Sreerama, L. (2022). Phytochemical Analysis of Acaciaehrenbergiana (Hayne) Grown in Qatar: Identification of Active Ingredients and Their Biological Activities. Molecules, 27(19), 6400. https://doi.org/10.3390/molecules27196400





