Antifungal Agents in Wood Protection—A Review
Abstract
1. Introduction
2. Plant-Derived Antifungal Agents
2.1. Alkaloids
2.2. Essential Oils and Their Components
2.3. Propolis Extract
2.4. Plant Extracts and Other Plant Derivatives
3. Animal-Derived Antifungal Agents
4. Synthetic Antifungal Agents
4.1. Silicon Compounds
4.2. Ionic Liquids
4.3. Nanosized Particles, Compound, and Substances
5. Complex Formulation as Antifungal Agents
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodell, B.; Winandy, J.E.; Morrell, J.J. Fungal Degradation of Wood: Emerging Data, New Insights and Changing Perceptions. Coatings 2020, 10, 1210. [Google Scholar] [CrossRef]
- Marais, B.N.; Brischke, C.; Militz, H. Wood Durability in Terrestrial and Aquatic Environments—A Review of Biotic and Abiotic Influence Factors. Wood Mater. Sci. Eng. 2020, 17, 82–105. [Google Scholar] [CrossRef]
- Tomak, E.D. Changes in Chemical Composition of Decayed Scots Pine and Beech Wood. Sci. Eng. Compos. Mater. 2014, 21, 589–595. [Google Scholar] [CrossRef]
- Daniel, G. Fungal Degradation of Wood Cell Walls. In Secondary Xylem Biology, Origins, Functions, and Applications; Academic Press: Cambridge, MA, USA, 2016; pp. 131–167. [Google Scholar] [CrossRef]
- Goodell, B. Brown-Rot Fungal Degradation of Wood: Our Evolving View. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2003; Volume 845, pp. 97–118. [Google Scholar] [CrossRef]
- Martinez, A.T.; Speranza, M.; Rui-Duenas, F.J.; Ferreira, P.; Camerero, S.; Guillen, F.; Martinez, M.J.; Gutierrez, A.; del Rio, J.C. Biodegradation of Lignocellu-Losics: Microbial, Chemical, and Enzymatic Aspects of the Fungal Attack of Lignin Lignocellulosic Materials. Int. Microbiol. 2005, 8, 195–204. [Google Scholar] [PubMed]
- Croitoru, C.; Roata, I.C. Ionic Liquids as Antifungal Agents for Wood Preservation. Molecules 2020, 25, 4289. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, D.; Kutnar, A.; Mantanis, G. Wood Modification Technologies—A Review. Ifor.-Biogeosci. For. 2017, 10, 895. [Google Scholar] [CrossRef]
- Esteves, B.M.; Pereira, H.M. Heat Treatment of Wood. BioResources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Altgen, M.; Emmerich, L.; Guigo, N.; Keplinger, T.; Kymäläinen, M.; Thybring, E.E.; Thygesen, L.G. Review of Wood Modification and Wood Functionalization Technologies. Forests 2022, 13, 1004. [Google Scholar] [CrossRef]
- Spear, M.J.; Curling, S.F.; Dimitriou, A.; Ormondroyd, G.A. Review of Functional Treatments for Modified Wood. Coatings 2021, 11, 327. [Google Scholar] [CrossRef]
- Evans, P.D.; Matsunaga, H.; Preston, A.F.; Kewish, C.M. Wood Protection for Carbon Sequestration—A Review of Existing Approaches and Future Directions. Curr. For. Rep. 2022, 8, 181–198. [Google Scholar] [CrossRef]
- Schiopu, N.; Tiruta-Barna, L. Wood Preservatives. In Toxicity of Building Materials; Woodhead Publishing: Sawston, UK, 2012; pp. 138–165. [Google Scholar] [CrossRef]
- Morais, S.; Fonseca, H.M.A.C.; Oliveira, S.M.R.; Oliveira, H.; Gupta, V.K.; Sharma, B.; de Lourdes Pereira, M. Environmental and Health Hazards of Chromated Copper Arsenate-Treated Wood: A Review. Int. J. Environ. Res. Public Health 2021, 18, 5518. [Google Scholar] [CrossRef] [PubMed]
- Teacă, C.A.; Roşu, D.; Mustaţă, F.; Rusu, T.; Roşu, L.; Roşca, I.; Varganici, C.D. Natural Bio-Based Products for Wood Coating and Protection against Degradation: A Review. BioResources 2019, 14, 4873–4901. [Google Scholar] [CrossRef]
- Pánek, M.; Reinprecht, L.; Hulla, M. Ten Essential Oils for Beech Wood Protection—Efficacy Against Wood-Destroying Fungi and Moulds, and Effect on Wood Discoloration. BioResources 2014, 9, 5588–5603. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Mi, N.; Wang, Y.; Li, G.; Wang, L.; Xie, Y. Antifungal Activity of Monoterpenes against Wood White-Rot Fungi. Int. Biodeterior. Biodegrad. 2016, 106, 157–160. [Google Scholar] [CrossRef]
- Broda, M. Natural Compounds for Wood Protection against Fungi—A Review. Molecules 2020, 25, 3538. [Google Scholar] [CrossRef]
- Singh, T.; Singh, A.P. A Review on Natural Products as Wood Protectant. Wood Sci. Technol. 2012, 46, 851–870. [Google Scholar] [CrossRef]
- Ulagesan, S.; Kim, H.J. Antibacterial and Antifungal Activities of Proteins Extracted from Seven Different Snails. Appl. Sci. 2018, 8, 1362. [Google Scholar] [CrossRef]
- Eikenes, M.; Alfredsen, G.; Christensen, B.E.; Militz, H.; Solheim, H. Comparison of Chitosans with Different Molecular Weights as Possible Wood Preservatives. J. Wood Sci. 2005, 51, 387–394. [Google Scholar] [CrossRef]
- Mai, C.; Donath, S.; Militz, H. Modification of Wood with Silicon Compounds. In Proceedings of the First European Conference on Wood Modification, Gent, Belgium, 3–4 April 2003; pp. 239–251. [Google Scholar]
- Borges, C.C.; Tonoli, G.H.D.; Cruz, T.M.; Duarte, P.J.; Junqueira, T.A. Nanoparticles-Based Wood Preservatives: The Next Generation of Wood Protection? Cerne 2018, 24, 397–407. [Google Scholar] [CrossRef]
- Cai, L.; Lim, H.; Nicholas, D.D.; Kim, Y. Bio-Based Preservative Using Methyl-β-Cyclodextrin-Essential Oil Complexes for Wood Protection. Int. J. Biol. Macromol. 2020, 147, 420–427. [Google Scholar] [CrossRef]
- Yan, L.; Zeng, F.; Chen, Z.; Chen, S.; Lei, Y. Improvement of Wood Decay Resistance by Salicylic Acid / Silica Microcapsule: Effects on the Salicylic Leaching, Microscopic Structure and Decay Resistance. Int. Biodeterior. Biodegrad. 2021, 156, 105134. [Google Scholar] [CrossRef]
- Ratajczak, I.; Woźniak, M.; Kwaśniewska-Sip, P.; Szentner, K.; Cofta, G.; Mazela, B. Chemical Characterization of Wood Treated with a Formulation Based on Propolis, Caffeine and Organosilanes. Eur. J. Wood Wood Prod. 2018, 76, 775–781. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Özgenç, Ö.; Durmaz, S.; Yildiz, Ü.C. A Comparison between Some Wood Bark Extracts: Antifungal Activity. J. For. Fac. 2017, 17, 502–508. [Google Scholar] [CrossRef]
- Bahmani, M.; Schmidt, O. Plant Essential Oils for Environment-Friendly Protection of Wood Objects against Fungi. Maderas Cienc. Tecnol. 2018, 20, 325–332. [Google Scholar] [CrossRef]
- Humar, M.; Lesar, B. Efficacy of Linseed- and Tung-Oil-Treated Wood against Wood-Decay Fungi and Water Uptake. Int. Biodeterior. Biodegrad. 2013, 85, 223–227. [Google Scholar] [CrossRef]
- Bribi, N. Pharmacological Activity of Alkaloids: A Review Intestinal Anti-Inflammatory Activity of Alkaloids in DNBS, DSS and Acetic Acid Models View Project Pharmacological Activity of Alkaloids: A Review. Asian J. Bot. 2018, 1. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Wei, X.; Ruan, W.; Vrieling, K. Current Knowledge and Perspectives of Pyrrolizidine Alkaloids in Pharmacological Applications: A Mini-Review. Molecules 2021, 26, 1970. [Google Scholar] [CrossRef]
- Soriano-Agatón, F.; Lagoutte, D.; Poupon, E.; Roblot, F.; Fournet, A.; Gantier, J.C.; Hocquemiller, R. Extraction, Hemisynthesis, and Synthesis of Canthin-6-One Analogues. Evaluation of Their Antifungal Activities. J. Nat. Prod. 2005, 68, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Gazoni, V.F.; Balogun, S.O.; Arunacham, K.; Oliveira, D.M.; Filho, V.C.; Lima, S.R.; Colodel, E.M.; Soares, I.M.; Ascêncio, S.D.; de Oliveira Martins, D.T. Assessment of Toxicity and Differential Antimicrobial Activity of Methanol Extract of Rhizome of Simaba Ferruginea A. St.-Hil. and Its Isolate Canthin-6-One. J. Ethnopharmacol. 2018, 223, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sahu, P.M.; Singh, S. Antimicrobial Activity of Pyrrolizidine Alkaloids from Heliotropium Subulatum. Fitoterapia 2002, 73, 153–155. [Google Scholar] [CrossRef]
- Kwaśniewska-Sip, P.; Cofta, G.; Mazela, B.; Ross Gobakken, L.; Przybył, A. Fungistatic Activity of Quinolizidine and Bisquinolizidine Alkaloids against A. niger. In Proceedings of the 47th IRG Annual Meeting, Lisbon, Portugal, 15–19 May 2016; The International Research Group on Wood Protection: Stockholm, Sweden, 2016; Volume 47, pp. 1–9. [Google Scholar]
- Kwaśniewska-Sip, P.; Cofta, G.; Nowak, P.B. Resistance of Fungal Growth on Scots Pine Treated with Caffeine. Int. Biodeterior. Biodegrad. 2018, 132, 178–184. [Google Scholar] [CrossRef]
- Pánek, M.; Borůvka, V.; Nábělková, J.; Šimůnková, K.; Zeidler, A.; Novák, D.; Černý, R.; Kobetičová, K. Efficacy of Caffeine Treatment for Wood Protection—Influence of Wood and Fungi Species. Polymers 2021, 13, 3758. [Google Scholar] [CrossRef]
- Šimůnková, K.; Reinprecht, L.; Nábělková, J.; Hýsek, Š.; Kindl, J.; Borůvka, V.; Lišková, T.; Šobotník, J.; Pánek, M. Caffeine—Perspective Natural Biocide for Wood Protection against Decaying Fungi and Termites. J. Clean. Prod. 2021, 304, 127110. [Google Scholar] [CrossRef]
- Kwaśniewska-Sip, P.; Bartkowiak, M.; Cofta, G.; Nowak, P.B. Resistance of Scots Pine (Pinus Sylvestris L.) after Treatment with Caffeine and Thermal Modification against Aspergillus Niger. BioResources 2019, 14, 1890–1898. [Google Scholar] [CrossRef]
- Barbero-López, A.; Ochoa-Retamero, A.; López-Gómez, Y.; Vilppo, T.; Venäläinen, M.; Lavola, A.; Julkunen-Tiitto, R.; Haapala, A. Activity of Spent Coffee Ground Cinnamates against Wood-Decaying Fungi in Vitro. BioResources 2018, 13, 6555–6564. [Google Scholar] [CrossRef]
- Lekounougou, S.; Jacquot, J.P.; Gérardin, P.; Gelhaye, E. Effects of Propiconazole on Extra-Cellular Enzymes Involved in Nutrient Mobilization during Trametes Versicolor Wood Colonization. Wood Sci. Technol. 2007, 42, 169–177. [Google Scholar] [CrossRef]
- Pánek, M.; Šimůnková, K.; Novák, D.; Dvořák, O.; Schönfelder, O.; Šedivka, P.; Kobetičová, K. Caffeine and TiO2 Nanoparticles Treatment of Spruce and Beech Wood for Increasing Transparent Coating Resistance against UV-Radiation and Mould Attacks. Coatings 2020, 10, 1141. [Google Scholar] [CrossRef]
- Kobetičová, K.; Nábělková, J.; Ďurišová, K.; Šimůnková, K.; Černý, R. Antifungal Activity of Methylxanthines Based on Their Properties. BioResources 2020, 15, 8110–8120. [Google Scholar] [CrossRef]
- Kwaśniewska-Sip, P.; Woźniak, M.; Jankowski, W.; Ratajczak, I.; Cofta, G. Chemical Changes of Wood Treated with Caffeine. Materials 2021, 14, 497. [Google Scholar] [CrossRef] [PubMed]
- Kobetičová, K.; Ďurišová, K.; Nábělková, J. Caffeine Interactions with Wood Polymers. Forests 2021, 12, 533. [Google Scholar] [CrossRef]
- Moutaouafiq, S.; Farah, A.; Ez Zoubi, Y.; Ghanmi, M.; Satrani, B.; Bousta, D. Antifungal Activity of Pelargonium Graveolens Essential Oil and Its Fractions Against Wood Decay Fungi. J. Essent. Oil Bear. Plants 2019, 22, 1104–1114. [Google Scholar] [CrossRef]
- Clausen, C.A.; Woodward, B.M.; Yang, V.W. Antifungal Essential Oil Metabolites. In Proceedings of the 41st Annual Meeting of the International Research Group on Wood Protection, Biarritz, France, 9–13 May 2010; p. RG-WP 10-30531. [Google Scholar]
- Salem, M.Z.M.; Zidan, Y.E.; Mansour, M.M.A.; El Hadidi, N.M.N.; Abo Elgat, W.A.A. Antifungal Activities of Two Essential Oils Used in the Treatment of Three Commercial Woods Deteriorated by Five Common Mold Fungi. Int. Biodeterior. Biodegrad. 2016, 106, 88–96. [Google Scholar] [CrossRef]
- Yen, T.B.; Chang, S.T. Synergistic Effects of Cinnamaldehyde in Combination with Eugenol against Wood Decay Fungi. Bioresour. Technol. 2008, 99, 232–236. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, P.F.; Chang, S.T. Antifungal Activities of Essential Oils and Their Constituents from Indigenous Cinnamon (Cinnamomum Osmophloeum) Leaves against Wood Decay Fungi. Bioresour. Technol. 2005, 96, 813–818. [Google Scholar] [CrossRef]
- Yang, V.W.; Clausen, C.A. Inhibitory Effect of Essential Oils on Decay Fungi and Mold Growth on Wood. In Proceedings of the One Hundred Third Annual Meeting of the American Wood Protection Association, St. Louis, MO, USA, 6–8 May 2007; American Wood Protection Association: Hoover, AL, USA, 2007; pp. 62–70. [Google Scholar]
- Matan, N.; Matan, N. Antifungal Activities of Anise Oil, Lime Oil, and Tangerine Oil against Molds on Rubberwood (Hevea Brasiliensis). Int. Biodeterior. Biodegrad. 2008, 62, 75–78. [Google Scholar] [CrossRef]
- Chittenden, C.; Singh, T. Antifungal Activity of Essential Oils against Wood Degrading Fungi and Their Applications as Wood Preservatives. Int. Wood Prod. J. 2011, 2, 44–48. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Z.; Huang, Q.; Zhang, D. Antifungal Activity of Several Essential Oils and Major Components against Wood-Rot Fungi. Ind. Crops Prod. 2017, 108, 278–285. [Google Scholar] [CrossRef]
- Su, Y.C.; Ho, C.L.; Wang, E.I.C.; Chang, S.T. Antifungal Activities and Chemical Compositions of Essential Oils from Leaves of Four Eucalypts. Taiwan J. For. Sci. 2006, 21, 49–61. [Google Scholar] [CrossRef]
- Abo Elgat, W.A.A.; Kordy, A.M.; Böhm, M.; Černý, R.; Abdel-Megeed, A.; Salem, M.Z.M. Eucalyptus camaldulensis, Citrus aurantium, and citrus sinensis Essential Oils as Antifungal Activity against Aspergillus flavus, Aspergillus niger, Aspergillus terreus, and Fusarium culmorum. Processes 2020, 8, 1003. [Google Scholar] [CrossRef]
- De Medeiros, F.C.M.; Gouveia, F.N.; Bizzo, H.R.; Vieira, R.F.; del Menezzi, C.H.S. Fungicidal Activity of Essential Oils from Brazilian Cerrado Species against Wood Decay Fungi. Int. Biodeterior. Biodegrad. 2016, 114, 87–93. [Google Scholar] [CrossRef]
- Mohareb, A.S.O.; Badawy, M.E.I.; Abdelgaleil, S.A.M. Antifungal Activity of Essential Oils Isolated from Egyptian Plants against Wood Decay Fungi. J. Wood Sci. 2013, 59, 499–505. [Google Scholar] [CrossRef]
- Matan, N.; Woraprayote, W.; Saengkrajang, W.; Sirisombat, N.; Matan, N. Durability of Rubberwood (Hevea Brasiliensis) Treated with Peppermint Oil, Eucalyptus Oil, and Their Main Components. Int. Biodeterior. Biodegrad. 2009, 63, 621–625. [Google Scholar] [CrossRef]
- Yang, V.W.; Clausen, C.A. Antifungal Effect of Essential Oils on Southern Yellow Pine. Int. Biodeterior. Biodegrad. 2007, 59, 302–306. [Google Scholar] [CrossRef][Green Version]
- Hussain, A.; Shrivastav, A.; Jain, K. Antifungal Activity of Essential Oils against Local Wood Degrading Cellulolytic Filamentous Fungi. Adv. Biores 2013, 4, 161–167. [Google Scholar]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal Activity of the Clove Essential Oil from Syzygium Aromaticum on Candida, Aspergillus and Dermatophyte Species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef]
- Mihai, A.L.; Popa, M.E. In Vitro Activity of Natural Antimicrobial Compounds against Aspergillus Strains. Agric. Agric. Sci. Procedia 2015, 6, 585–592. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal Efficacy of Thymol, Carvacrol, Eugenol and Menthol as Alternative Agents to Control the Growth of Food-Relevant Fungi. J. Mycol. Med. 2014, 24, e51–e56. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, Z.; Cao, D.; Rong, F.; Ding, H.; Zhang, D. Antitermitic and Antifungal Activities of Eugenol and Its Congeners from the Flower Buds of Syzgium Aromaticum (Clove). Ind. Crops Prod. 2015, 77, 780–786. [Google Scholar] [CrossRef]
- Voda, K.; Boh, B.; Vrtačnik, M.; Pohleven, F. Effect of the Antifungal Activity of Oxygenated Aromatic Essential Oil Compounds on the White-Rot Trametes versicolor and the Brown-Rot Coniophora puteana. Int. Biodeterior. Biodegrad. 2003, 51, 51–59. [Google Scholar] [CrossRef]
- Zhang, J.; Du, C.; Li, Q.; Hu, A.; Peng, R.; Sun, F.; Zhang, W. Inhibition Mechanism and Antibacterial Activity of Natural Antibacterial Agent Citral on Bamboo Mould and Its Anti-Mildew Effect on Bamboo. R. Soc. Open Sci. 2021, 8, 202244. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R. Antifungal Efficacy of Some Natural Phenolic Compounds against Significant Pathogenic and Toxinogenic Filamentous Fungi. Chemosphere 2013, 93, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Kartal, S.N.; Hwang, W.J.; Imamura, Y.; Sekine, Y. Effect of Essential Oil Compounds and Plant Extracts on Decay and Termite Resistance of Wood. Holz Roh-Und Werkst. 2006, 64, 455–461. [Google Scholar] [CrossRef]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad, M.; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and Its Derivatives, a Novel Class of Antifungal Agents. Fitoterapia 2016, 112, 116–131. [Google Scholar] [CrossRef]
- Yang, D.; Wang, H.; Yuan, H.; Li, S. Quantitative Structure Activity Relationship of Cinnamaldehyde Compounds against Wood-Decaying Fungi. Molecules 2016, 21, 1563. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Abdel Rasoul, M.A.; Abdelgaleil, S.A.M. Comparative Antifungal Activities and Biochemical Effects of Monoterpenes on Plant Pathogenic Fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Ye, Z.; Chizaram, E.P.; Jiang, J.; Liu, T.; Sun, F.; Zhang, S. Mold Resistance of Bamboo after Laccase-Catalyzed Attachment of Thymol and Proposed Mechanism of Attachment. RSC Adv. 2020, 10, 7764–7770. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a Novel Antibacterial Agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Dantas Silva, R.P.; Machado, B.A.S.; de Barreto, G.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.V.; Umsza-Guez, M.A. Antioxidant, Antimicrobial, Antiparasitic, and Cytotoxic Properties of Various Brazilian Propolis Extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Giannopoulou, E.; Skalicka-Wózniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bȩben, K.; Antosiewicz, B.; et al. Characterization and Biological Evaluation of Propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the Antioxidant Activity of Propolis Samples from Different Geographical Regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef]
- Dudoit, A.; Mertz, C.; Chillet, M.; Cardinault, N.; Brat, P. Antifungal Activity of Brazilian Red Propolis Extract and Isolation of Bioactive Fractions by Thin-Layer Chromatography-Bioautography. Food Chem. 2020, 327, 127060. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent Progress of Propolis for Its Biological and Chemical Compositions and Its Botanical Origin. Evid.-Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. The Role of Seasonality on the Chemical Composition, Antioxidant Activity and Cytotoxicity of Polish Propolis in Human Erythrocytes. Braz. J. Pharmacogn. 2019, 29, 301–308. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and Functional Characterization of Italian Propolis Obtained by Different Harvesting Methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef]
- Ramanauskiene, K.; Inkeniene, A.M.; Petrikaite, V.; Briedis, V. Total Phenolic Content and Antimicrobial Activity of Different Lithuanian Propolis Solutions. Evid.-Based Complement. Altern. Med. 2013, 2013, 842985. [Google Scholar] [CrossRef]
- Kacániová, M.; Vuković, N.; Chlebo, R.; Haščík, P.; Rovná, K.; Cubon, J.; Dzugan, M.; Pasternakiewicz, A. The Antimicrobial Activity of Honey, Bee Pollen Loads and Beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. Microbiological and Calorimetric Investigations on the Antimicrobial Actions of Different Propolis Extracts: An in Vitro Approach. Thermochim. Acta 2004, 422, 115–124. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Kwaśniewska, P.; Cofta, G.; Hołderna-Kędzia, E.; Kędzia, B.; Mazela, B. The Activity of Propolis Extracts against Selected Moulds. Postępy Fitoter. 2015, 4, 205–209. [Google Scholar]
- Silva-Castro, I.; Casados-Sanz, M.; Alonso-Cortés, A.L.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. Chitosan-Based Coatings to Prevent the Decay of Populus Spp. Wood Caused by Trametes versicolor. Coatings 2018, 8, 415. [Google Scholar] [CrossRef]
- Agüero, M.B.; Svetaz, L.; Baroni, V.; Lima, B.; Luna, L.; Zacchino, S.; Saavedra, P.; Wunderlin, D.; Feresin, G.E.; Tapia, A. Urban Propolis from San Juan Province (Argentina): Ethnopharmacological Uses and Antifungal Activity against Candida and Dermatophytes. Ind. Crops Prod. 2014, 57, 166–173. [Google Scholar] [CrossRef]
- Petruzzi, L.; Corbo, M.R.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods 2020, 9, 559. [Google Scholar] [CrossRef]
- Quiroga, E.N.; Sampietro, D.A.; Soberón, J.R.; Sgariglia, M.A.; Vattuone, M.A. Propolis from the Northwest of Argentina as a Source of Antifungal Principles. J. Appl. Microbiol. 2006, 101, 103–110. [Google Scholar] [CrossRef]
- Akcay, C.; Birinci, E.; Birinci, C.; Kolayli, S. Durability of Wood Treated with Propolis. BioResources 2020, 15, 1547–1562. [Google Scholar] [CrossRef]
- Budija, F.; Kričej, B.; Petrič, M. Possiblities of Use of Propolis for Wood Finishing. Wood Res. 2008, 53, 91–102. [Google Scholar]
- Casado-Sanz, M.M.; Silva-Castro, I.; Ponce-Herrero, L.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. White-Rot Fungi Control on Populus Spp. Wood by Pressure Treatments with Silver Nanoparticles, Chitosan Oligomers and Propolis. Forests 2019, 10, 885. [Google Scholar] [CrossRef]
- Woźniak, M.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Cofta, G.; Ratajczak, I. The Possibility of Propolis Extract Application in Wood Protection. Forests 2020, 11, 465. [Google Scholar] [CrossRef]
- Touzani, S.; Imtara, H.; Katekhaye, S.; Mechchate, H.; Ouassou, H.; Alqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Fearnley, H.; Fearnley, J.; et al. Determination of Phenolic Compounds in Various Propolis Samples Collected from an African and an Asian Region and Their Impact on Antioxidant and Antibacterial Activities. Molecules 2021, 26, 4589. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Mrówczyńska, L.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Nowak, P.; Ratajczak, I. Effect of the Solvent on Propolis Phenolic Profile and Its Antifungal, Antioxidant, and in Vitro Cytoprotective Activity in Human Erythrocytes under Oxidative Stress. Molecules 2020, 25, 4266. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.; Alvarez, A.; Areal, M.R.; Maldonado, L.; Marchisio, P.; Rodríguez, M.; Bedascarrasbure, E. The Effect of Different Propolis Harvest Methods on Its Lead Contents Determined by ET AAS and UV-VisS. J. Hazard. Mater. 2006, 137, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.B.; Sillero, L.; Gatto, D.A.; Labidi, J. Bioactive Molecules in Wood Extractives: Methods of Extraction and Separation, a Review. Ind. Crops Prod. 2022, 186, 115231. [Google Scholar] [CrossRef]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The Antifungal Activity of Moroccan Plants and the Mechanism of Action of Secondary Metabolites from Plants. J. Mycol. Med. 2017, 27, 303–311. [Google Scholar] [CrossRef]
- Kawamura, F.; Ramle, S.F.M.; Sulaiman, O.; Hashim, R.; Ohara, S. Antioxidant and Antifungal Activities of Extracts from 15 Selected Hardwood Species of Malaysian Timber. Eur. J. Wood Wood Prod. 2010, 69, 207–212. [Google Scholar] [CrossRef]
- Barbero-López, A. Antifungal Activity of Several Vegetable Origin Household Waste Extracts Against Wood-Decaying Fungi In Vitro. Waste Biomass Valorization 2021, 12, 1237–1241. [Google Scholar] [CrossRef]
- Awouafack, M.D.; McGaw, L.J.; Gottfried, S.; Mbouangouere, R.; Tane, P.; Spiteller, M.; Eloff, J.N. Antimicrobial Activity and Cytotoxicity of the Ethanol Extract, Fractions and Eight Compounds Isolated from Eriosema robustum (Fabaceae). BMC Complement. Altern. Med. 2013, 13, 289. [Google Scholar] [CrossRef]
- Davies, N.T.; Wu, H.F.; Altaner, C.M. The Chemistry and Bioactivity of Various Heartwood Extracts from Redwood (Sequoia sempervirens) against Two Species of Fungi. N. Zeal. J. For. Sci. 2014, 44, 17. [Google Scholar] [CrossRef]
- Vek, V.; Oven, P.; Humar, M. Phenolic Extractives of Wound-Associated Wood of Beech and Their Fungicidal Effect. Int. Biodeterior. Biodegrad. 2013, 77, 91–97. [Google Scholar] [CrossRef]
- Royer, M.; Rodrigues, A.M.S.; Herbette, G.; Beauchêne, J.; Chevalier, M.; Hérault, B.; Thibaut, B.; Stien, D. Efficacy of Bagassa guianensis Aubl. Extract against Wood Decay and Human Pathogenic Fungi. Int. Biodeterior. Biodegrad. 2012, 70, 55–59. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, C.Y.; Yen, P.L.; Yeh, T.F.; Cheng, S.S.; Chang, S.T. Antifungal Agents from Heartwood Extract of Taiwania cryptomerioides against Brown Root Rot Fungus Phellinus noxius. Wood Sci. Technol. 2017, 51, 639–651. [Google Scholar] [CrossRef]
- Mun, S.P.; Prewitt, L. Antifungal Activity of Organic Extracts from Juniperus virginiana Heartwood against Wood Decay Fungi. For. Prod. J. 2011, 61, 443–449. [Google Scholar] [CrossRef]
- Martínez-Sotres, C.; López-Albarrán, P.; Cruz-de-León, J.; García-Moreno, T.; Rutiaga-Quiñones, J.G.; Vázquez-Marrufo, G.; Tamariz-Mascarúa, J.; Herrera-Bucio, R. Medicarpin, an Antifungal Compound Identified in Hexane Extract of Dalbergia congestiflora Pittier Heartwood. Int. Biodeterior. Biodegrad. 2012, 69, 38–40. [Google Scholar] [CrossRef]
- Tascioglu, C.; Yalcin, M.; Sen, S.; Akcay, C. Antifungal Properties of Some Plant Extracts Used as Wood Preservatives. Int. Biodeterior. Biodegrad. 2013, 85, 23–28. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Zidan, Y.E.; El Hadidi, N.M.N.; Mansour, M.M.A.; Abo Elgat, W.A.A. Evaluation of Usage Three Natural Extracts Applied to Three Commercial Wood Species against Five Common Molds. Int. Biodeterior. Biodegrad. 2016, 110, 206–226. [Google Scholar] [CrossRef]
- Sen, S.; Tascioglu, C.; Tirak, K. Fixation, Leachability, and Decay Resistance of Wood Treated with Some Commercial Extracts and Wood Preservative Salts. Int. Biodeterior. Biodegrad. 2009, 63, 135–141. [Google Scholar] [CrossRef]
- Bi, Z.; Yang, F.; Lei, Y.; Morrell, J.J.; Yan, L. Identification of Antifungal Compounds in Konjac Flying Powder and Assessment against Wood Decay Fungi. Ind. Crops Prod. 2019, 140, 111650. [Google Scholar] [CrossRef]
- AdedejI, G.A.; Ogunsanwo, O.Y.; Elufioye, T.O. Quantifications of Phytochemicals and Biocide Actions of Lawsonia inermis Linn. Extracts against Wood Termites and Fungi. Int. Biodeterior. Biodegrad. 2017, 116, 155–162. [Google Scholar] [CrossRef]
- Yildiz, S.; Gürgen, A.; Can, Z.; Tabbouche, S.A.; Osman, A. Some Bioactive Properties of Acacia dealbata Extracts and Their Potential Utilization in Wood Protection. Wood 2018, 61, 81–97. [Google Scholar] [CrossRef]
- Sablík, P.; Giagli, K.; Pařil, P.; Baar, J.; Rademacher, P. Impact of Extractive Chemical Compounds from Durable Wood Species on Fungal Decay after Impregnation of Nondurable Wood Species. Eur. J. Wood Wood Prod. 2016, 74, 231–236. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Behiry, S.I.; EL-Hefny, M. Inhibition of Fusarium culmorum, Penicillium chrysogenum and Rhizoctonia solani by n-Hexane Extracts of Three Plant Species as a Wood-Treated Oil Fungicide. J. Appl. Microbiol. 2019, 126, 1683–1699. [Google Scholar] [CrossRef] [PubMed]
- Eller, F.J.; Clausen, C.A.; Green, F.; Taylor, S.L. Critical Fluid Extraction of Juniperus virginiana L. and Bioactivity of Extracts against Subterranean Termites and Wood-Rot Fungi. Ind. Crops Prod. 2010, 32, 481–485. [Google Scholar] [CrossRef]
- Mansour, M.M.A.; Salem, M.Z.M. Evaluation of Wood Treated with Some Natural Extracts and Paraloid B-72 against the Fungus Trichoderma harzianum: Wood Elemental Composition, in-Vitro and Application Evidence. Int. Biodeterior. Biodegrad. 2015, 100, 62–69. [Google Scholar] [CrossRef]
- Yildiz, Ü.C.; Kilic, C.; Gürgen, A.; Yildiz, S. Possibility of Using Lichen and Mistletoe Extracts as Potential Natural Wood Preservative. Maderas. Cienc. Tecnol. 2020, 22, 179–188. [Google Scholar] [CrossRef]
- da Silva, D.T.; Herrera, R.; Batista, B.F.; Heinzmann, B.M.; Labidi, J. Physicochemical Characterization of Leaf Extracts from Ocotea lancifolia and Its Effect against Wood-Rot Fungi. Int. Biodeterior. Biodegrad. 2017, 117, 158–170. [Google Scholar] [CrossRef]
- Behiry, S.I.; Okla, M.K.; Alamri, S.A.; EL-Hefny, M.; Salem, M.Z.M.; Alaraidh, I.A.; Ali, H.M.; Al-Ghtani, S.M.; Monroy, J.C.; Salem, A.Z.M. Antifungal and Antibacterial Activities of Musa paradisiaca L. Peel Extract: HPLC Analysis of Phenolic and Flavonoid Contents. Processes 2019, 7, 215. [Google Scholar] [CrossRef]
- Rafał, I.G.; Króliczewski, B.J.; Górniak, I.; Bartoszewski, R.; Króliczewski, Á.J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2018, 18, 241–272. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phyther. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sak, K.; Adhikary, S.; Kaur, G.; Aggarwal, D.; Kaur, J.; Kumar, M.; Parashar, N.C.; Parashar, G.; Sharma, U.; et al. Galangin: A Metabolite That Suppresses Anti-Neoplastic Activities through Modulation of Oncogenic Targets. Exp. Biol. Med. 2022, 247, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic Compounds as Nutraceuticals or Functional Food Ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yang, S.; Cheng, Y.J.; Chen, F.; Pan, S.; Fan, G. Antifungal Activity and Action Mode of Pinocembrin from Propolis against Penicillium italicum. Food Sci. Biotechnol. 2012, 21, 1533–1539. [Google Scholar] [CrossRef]
- Salas, M.P.; Céliz, G.; Geronazzo, H.; Daz, M.; Resnik, S.L. Antifungal Activity of Natural and Enzymatically-Modified Flavonoids Isolated from Citrus Species. Food Chem. 2011, 124, 1411–1415. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Co, A.L.K.C.; Rideout, J.A. Antifungal Metabolites from Blumea balsamifera. Nat. Prod. Res. 2006, 19, 231–237. [Google Scholar] [CrossRef]
- Bylka, W.; Szaufer-Hajdrych, M.; Matławska, I.; Goślińska, O. Antimicrobial Activity of Isocytisoside and Extracts of Aquilegia vulgaris L. Lett. Appl. Microbiol. 2004, 39, 93–97. [Google Scholar] [CrossRef]
- Quiroga, E.N.; Sampietro, D.A.; Sgariglia, M.A.; Soberón, J.R.; Vattuone, M.A. Antimycotic Activity of 5′-Prenylisoflavanones of the Plant Geoffroea Decorticans, against Aspergillus Species. Int. J. Food Microbiol. 2009, 132, 42–46. [Google Scholar] [CrossRef]
- Da, X.; Nishiyama, Y.; Tie, D.; Hein, K.Z.; Yamamoto, O.; Morita, E. Antifungal Activity and Mechanism of Action of Ou-Gon (Scutellaria Root Extract) Components against Pathogenic Fungi. Sci. Rep. 2019, 9, 1683. [Google Scholar] [CrossRef]
- Ren, B.; Xia, B.; Li, W.; Wu, J.; Zhang, H. Two Novel Phenolic Compounds from Stenoloma chusanum and Their Antifungal Activity. Chem. Nat. Compd. 2009, 45, 182–186. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic Compounds from the Leaf Extract of Artichoke (Cynara scolymus L.) and Their Antimicrobial Activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef]
- Rashed, K.; Ćirić, A.; Glamočlija, J.; Soković, M. Antibacterial and Antifungal Activities of Methanol Extract and Phenolic Compounds from Diospyros virginiana L. Ind. Crops Prod. 2014, 59, 210–215. [Google Scholar] [CrossRef]
- Montagner, C.; De Souza, S.M.; Groposo, C.; Delle Monache, F.; Smânia, E.F.A.; Smânia, A. Antifungal Activity of Coumarins. Z. Naturforsch.-Sect. C J. Biosci. 2008, 63, 21–28. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.M.; Prietto, L.; Ribeiro, A.C.; de Souza, T.D.; Badiale-Furlong, E. Assessment of the Antifungal Activity of Spirulina Platensis Phenolic Extract against Aspergillus flavus. Ciênc. Agrotecnol. 2011, 35, 1050–1058. [Google Scholar] [CrossRef]
- Usharani, G.; Srinivasan, G.; Sivasakthi, S.; Saranraj, P. Antimicrobial Activity of Spirulina Platensis Solvent Extracts Against Pathogenic Bacteria and Fungi. Adv. Biol. Res. 2015, 9, 292–298. [Google Scholar] [CrossRef]
- Nami Kartal, S.; Terzi, E.; Kose, C.; Hofmeyr, J.; Imamura, Y. Efficacy of Tar Oil Recovered during Slow Pyrolysis of Macadamia Nut Shells. Int. Biodeterior. Biodegrad. 2011, 65, 369–373. [Google Scholar] [CrossRef]
- Singh, T.; Chittenden, C. In-Vitro Antifungal Activity of Chilli Extracts in Combination with Lactobacillus casei against Common Sapstain Fungi. Int. Biodeterior. Biodegrad. 2008, 62, 364–367. [Google Scholar] [CrossRef]
- Akçay, Ç. Determination of Decay, Larvae Resistance, Water Uptake, Color, and Hardness Properties of Wood Impregnated with Honeybee Wax. BioResources 2020, 15, 8339–8354. [Google Scholar] [CrossRef]
- Mazela, B.; Bartkowiak, M.; Ratajczak, I. Animal Protein Impact on Fungicidal Properties of Treatment Formulations. Wood Res. 2005, 52, 13–22. [Google Scholar]
- Hegedüs, N.; Marx, F. Antifungal Proteins: More than Antimicrobials? Fungal Biol. Rev. 2013, 26, 132–145. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 453. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.; Zhao, J.; Zhuang, X.; Cao, P.; Guo, X.; Liu, C.; Xiang, W. Community Composition, Antifungal Activity and Chemical Analyses of Ant-Derived Actinobacteria. Front. Microbiol. 2020, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Azeem, H.H.A.-E.; Osman, G.Y.; El-Seedi, H.R.; Fallatah, A.M.; Khalifa, S.A.M.; Gharib, M.M. Antifungal Activity of Soft Tissue Extract from the Garden Snail Helix aspersa (Gastropoda, Mollusca). Molecules 2022, 27, 3170. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.T.; Ferreira, I.C.F.R.; Barros, L.; Silva, S.; Azeredo, J.; Henriques, M. Antifungal Activity of Phenolic Compounds Identified in Flowers from North Eastern Portugal against Candida Species. Future Microbiol. 2014, 9, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Oldertrøen, K.; H-Kittikun, A.; Aam, B.B.; Larnøy, E. Resistance of Rubberwood (Hevea brasiliensis) Treated with Chitosan or Silane against Surface Molds. Eur. J. Wood Wood Prod. 2017, 75, 101–112. [Google Scholar] [CrossRef]
- Alfredsen, G.; Eikenes, M.; Militz, H.; Solheim, H. Screening of Chitosan against Wood-Deteriorating Fungi. Scand. J. For. Res. 2004, 19, 4–13. [Google Scholar] [CrossRef]
- Gorgij, R.; Tarmian, A.; Karimi, A.N. Effect of Chitosan on the Mold Resistance of Wood and Its Surface Properties. Int. J. Lignocellul. Prod. 2014, 2014, 39–49. [Google Scholar]
- El-Gamal, R.; Nikolaivits, E.; Zervakis, G.I.; Abdel-Maksoud, G.; Topakas, E.; Christakopoulos, P. The Use of Chitosan in Protecting Wooden Artifacts from Damage by Mold Fungi. Electron. J. Biotechnol. 2016, 24, 70–78. [Google Scholar] [CrossRef]
- Larnøy, E.; Dantz, S.; Eikenes, M.; Militz, H. Screening of Properties of Modified Chitosan-Treated Wood. Wood Mater. Sci. Eng. 2006, 1, 59–68. [Google Scholar] [CrossRef]
- Sivrikaya, H.; Can, A.; Yaman, B.; Palanti, S.; Morrell, J.J. Effect of Tallow Impregnation on Moisture Behavior and Decay Resistance of Various Wood Species. Wood Mater. Sci. Eng. 2020, 16, 260–268. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.M.; Grigorescu, R.M.; Iancu, L.; Constantin, M.; Stirbescu, R.M.; Gheboianu, A.I. Wood Surface Modification with Hybrid Materials Based on Multi-Walled Carbon Nanotubes. Nanomaterials 2022, 12, 1990. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.; Militz, H. Modification of Wood with Silicon Compounds. Inorganic Silicon Compounds and Sol-Gel Systems: A Review. Wood Sci. Technol. 2004, 37, 339–348. [Google Scholar] [CrossRef]
- Cui, H.; Li, Q. Nano-Titanium Dioxide Coating of Chinese Fir Treated by High-Temperature Steam to Improve the Anticorrosion and Surface Hydrophobicity. For. Prod. J. 2020, 70, 158–164. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Farahani, M.R.M.; Hale, M.D.C. The Use of Organo Alkoxysilane Coupling Agents for Wood Preservation. Holzforschung 2004, 58, 316–325. [Google Scholar] [CrossRef]
- Donath, S.; Militz, H.; Mai, C. Creating Water-Repellent Effects on Wood by Treatment with Silanes. Holzforschung 2006, 60, 40–46. [Google Scholar] [CrossRef]
- Mai, C.; Militz, H. Modification of Wood with Silicon Compounds. Treatment Systems Based on Organic Silicon Compounds—A Review. Wood Sci. Technol. 2004, 37, 453–461. [Google Scholar] [CrossRef]
- Baur, S.I.; Easteal, A.J. Improved Photoprotection of Wood by Chemical Modification with Silanes: NMR and ESR Studies. Polym. Adv. Technol. 2013, 24, 97–103. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, S.Y.; Chun, S.J.; Doh, G.H.; Paik, K.H. Effect of Silane Coupling on the Fundamental Properties of Wood Flour Reinforced Polypropylene Composites. J. Compos. Mater. 2011, 45, 1595–1605. [Google Scholar] [CrossRef]
- De Vetter, L.; Stevens, M.; Van Acker, J. Fungal Decay Resistance and Durability of Organosilicon-Treated Wood. Int. Biodeterior. Biodegrad. 2009, 63, 130–134. [Google Scholar] [CrossRef]
- Panov, D.; Terziev, N. Study on Some Alkoxysilanes Used for Hydrophobation and Protection of Wood against Decay. Int. Biodeterior. Biodegrad. 2009, 63, 456–461. [Google Scholar] [CrossRef]
- Kumar, A.; Pavla, R.; Sever, S.A.; Humar, M.; Pavlič, M.; Jan, T.; Petr, H.; Zigon, J.; Petric, M. Influence of Surface Modification of Wood with Octadecyltrichlorosilane on Its Dimensional Stability and Resistance against Coniophora puteana and Molds. Cellulose 2016, 23, 3249–3263. [Google Scholar] [CrossRef]
- Pries, M.; Wagner, R.; Kaesler, K.H.; Militz, H.; Mai, C. Effect of Short-Chain Silicones Bearing Different Functional Groups on the Resistance of Pine (Pinus sylvestris L.) and Beech (Fagus sylvatica L.) against Decay Fungi. Holzforschung 2013, 67, 447–454. [Google Scholar] [CrossRef]
- Reinprecht, L.; Grznárik, T. Biological Durability of Scots Pine (Pinus sylvestris L.) Sapwood Modified with Selected Organo-Silanes. Wood Res. 2015, 60, 687–696. [Google Scholar]
- Donath, S.; Militz, H.; Mai, C. Treatment of Wood with Aminofunctional Silanes for Protection against Wood Destroying Fungi. Holzforschung 2006, 60, 210–216. [Google Scholar] [CrossRef]
- Weigenand, O.; Humar, M.; Daniel, G.; Militz, H.; Mai, C. Decay Resistance of Wood Treated with Amino-Silicone Compounds. Holzforschung 2008, 62, 112–118. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Militz, H.; Mai, C. The Efficacy of Commercial Silicones against Blue Strain and Mould Fungi in Wood. Eur. J. Wood Wood Prod. 2009, 67, 159–167. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Pernak, J.; Zabielska-Matejuk, J.; Kropacz, A.; Foksowicz-Flaczyk, J. Ionic Liquids in Wood Preservation. Holzforschung 2004, 58, 286–291. [Google Scholar] [CrossRef]
- Pernak, J.; Zabielska-Matejuk, J.; Urbanik, E. New Quaternary Ammonium Chlorides—Wood Preservatives. Holzforschung 1998, 52, 249–254. [Google Scholar] [CrossRef]
- Zabielska-Matejuk, J. Antifungal Properties of New Quaternary Ammonium Compounds in Relation to Their Surface Activity. Wood Sci. Technol. 2005, 39, 235–243. [Google Scholar] [CrossRef]
- Feder-Kubis, J.; Zabielska-Matejuk, J.; Stangierska, A.; Przybylski, P.; Jacquemin, J.; Geppert-Rybczyńska, M. Toward Designing “Sweet” Ionic Liquids Containing a Natural Terpene Moiety as Effective Wood Preservatives. ACS Sustain. Chem. Eng. 2019, 7, 15628–15639. [Google Scholar] [CrossRef]
- Schmitz, K.; Wagner, S.; Reppke, M.; Maier, C.L.; Windeisen-Holzhauser, E.; Philipp Benz, J. Preserving Cultural Heritage: Analyzing the Antifungal Potential of Ionic Liquids Tested in Paper Restoration. PLoS ONE 2019, 14, e0219650. [Google Scholar] [CrossRef]
- Zabielska-Matejuk, J.; Stangierska, A.; Kot, M. New Ammonium- and 1,2,4-Triazolium-Based Ionic Liquids for Wood Preservation. J. Wood Chem. Technol. 2015, 35, 178–192. [Google Scholar] [CrossRef]
- Stasiewicz, M.; Fojutowski, A.; Kropacz, A.; Pernak, J. 1-Alkoxymethyl-X-Dimethylaminopyridinium-Base Ionic Liquids in Wood Preservation. Holzforschung 2008, 62, 309–317. [Google Scholar] [CrossRef]
- Zabielska-Matejuk, J.; Feder-Kubis, J.; Stangierska, A.; Przybylski, P. Chiral Ionic Liquids with a (-)-Menthol Component as Wood Preservatives. Holzforschung 2017, 71, 751–757. [Google Scholar] [CrossRef]
- Ogar, A.; Tylko, G.; Turnau, K. Antifungal Properties of Silver Nanoparticles against Indoor Mould Growth. Sci. Total Environ. 2015, 521–522, 305–314. [Google Scholar] [CrossRef]
- Civardi, C.; Schwarze, F.W.M.R.; Wick, P. Micronized Copper Wood Preservatives: An Efficiency and Potential Health Risk Assessment for Copper-Based Nanoparticles. Environ. Pollut. 2015, 200, 126–132. [Google Scholar] [CrossRef]
- de Peres, M.L.; de Avila Delucis, R.; Amico, S.C.; Gatto, D.A. Zinc Oxide Nanoparticles from Microwave-Assisted Solvothermal Process: Photocatalytic Performance and Use for Wood Protection against Xylophagous Fungus. Nanomater. Nanotechnol. 2019, 9, 1847980419876201. [Google Scholar] [CrossRef]
- Akhtari, M.; Taghiyari, H.R.; Kokandeh, M.G. Effect of Some Metal Nanoparticles on the Spectroscopy Analysis of Paulownia Wood Exposed to White-Rot Fungus. Eur. J. Wood Wood Prod. 2013, 71, 283–285. [Google Scholar] [CrossRef]
- Dai, X.; Qi, Y.; Luo, H.; He, Z.; Wei, L.; Dong, X.; Ma, X.; Yang, D.Q.; Li, Y. Leachability and Anti-Mold Efficiency of Nanosilver on Poplar Wood Surface. Polymers 2022, 14, 884. [Google Scholar] [CrossRef] [PubMed]
- De Filpo, G.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing Fungal Growth in Wood by Titanium Dioxide Nanoparticles. Int. Biodeterior. Biodegrad. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- Reinprecht, L.; Vidholdová, Z. Growth Inhibition of Moulds on Wood Surfaces in Presence of Nano-Zinc Oxide and Its Combinations with Polyacrylate and Essential Oils. Wood Res. 2017, 62, 37–44. [Google Scholar]
- Lykidis, C.; Bak, M.; Mantanis, G.; Németh, R. Biological Resistance of Pine Wood Treated with Nano-Sized Zinc Oxide and Zinc Borate against Brown-Rot Fungi. Eur. J. Wood Wood Prod. 2016, 74, 909–911. [Google Scholar] [CrossRef]
- Qi, Y.; Dai, X.; Wei, L.; Luo, H.; Liu, Y.; Dong, X.; Yang, D.; Li, Y. Nano-AgCu Alloy on Wood Surface for Mold Resistance. Nanomaterials 2022, 12, 1192. [Google Scholar] [CrossRef]
- Terzi, E.; Kartal, S.N.; Yilgör, N.; Rautkari, L.; Yoshimura, T. Role of Various Nano-Particles in Prevention of Fungal Decay, Mold Growth and Termite Attack in Wood, and Their Effect on Weathering Properties and Water Repellency. Int. Biodeterior. Biodegrad. 2016, 107, 77–87. [Google Scholar] [CrossRef]
- Nair, S.; Pandey, K.K.; Giridhar, B.N.; Vijayalakshmi, G. Decay Resistance of Rubberwood (Hevea brasiliensis) Impregnated with ZnO and CuO Nanoparticles Dispersed in Propylene Glycol. Int. Biodeterior. Biodegrad. 2017, 122, 100–106. [Google Scholar] [CrossRef]
- Can, A.; Palanti, S.; Sivrikaya, H.; Hazer, B.; Stefanı, F. Physical, Biological and Chemical Characterisation of Wood Treated with Silver Nanoparticles. Cellulose 2019, 26, 5075–5084. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Wang, Y. The Preparation and Mechanism of Garlic-Templated Fluorescent Nanoparticles for Degradation of Coriolus versicolor via One-Pot. Ind. Crops Prod. 2022, 186, 115281. [Google Scholar] [CrossRef]
- Bi, Z.; Morrell, J.J.; Lei, Y.; Yan, L.; Ji, M. Eco-Friendly and Mildly Modification of Wood Cell Walls with Heat Treated Wood Extracts to Improve Wood Decay Resistance. Ind. Crops Prod. 2022, 184, 115079. [Google Scholar] [CrossRef]
- Alorbu, C.; Cai, L. Fungal Resistance and Leachability of Genipin-Crosslinked Chitosan Treated Wood. Int. Biodeterior. Biodegrad. 2022, 169, 105378. [Google Scholar] [CrossRef]
- Broda, M.; Mazela, B.; Frankowski, M. Durability of Wood Treated with AATMOS and Caffeine—Towards the Long-Term Carbon Storage. Maderas Cienc. Tecnol. 2018, 20, 455–468. [Google Scholar] [CrossRef]
- Bi, Z.; Fang, S.; Gao, Q.; Lei, Y.; Morrell, J.J.; Yan, L. Improvement of Mould Resistance of Wood with Cinnamaldehyde Chitosan Emulsion. Wood Sci. Technol. 2021, 56, 187–204. [Google Scholar] [CrossRef]
- Shiny, K.S.; Nair, S.; Mamatha, N.; Sundararaj, R. Decay Resistance of Wood Treated with Copper Oxide Nanoparticles Synthesised Using Leaf Extracts of Lantana camara L. and Nerium oleander L. Wood Mater. Sci. Eng. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Bergamonti, L.; Graiff, C.; Tegoni, M.; Predieri, G.; Elviri, L.; Palanti, S.; Paris, C.; Cappelletto, E.; di Maggio, R.; Lottici, P.P. Facile Preparation of Functionalized Poly(Amidoamine)s with Biocidal Activity on Wood Substrates. Eur. Polym. J. 2019, 116, 232–241. [Google Scholar] [CrossRef]
- Woźniak, M.; Kwaśniewska-Sip, P.; Krueger, M.; Roszyk, E.; Ratajczak, I. Chemical, Biological and Mechanical Characterization of Wood Treated with Propolis Extract and Silicon Compounds. Forests 2020, 11, 907. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Szentner, K.; Kwaśniewska, P.; Mazela, B. Propolis and Organosilanes in Wood Protection. Part I: FTIR Analysis and Biological Tests. Ann. Warsaw Univ. Life Sci.-SGGW For. Wood Technol. 2015, 91, 218–224. [Google Scholar]
- Woźniak, M.; Ratajczak, I.; Waśkiewicz, A.; Szentner, K.; Cofta, G.; Kwaśniewska-Sip, P. Investigation of the Use of Impregnating Formulation with Propolis Extract and Organosilanes in Wood Protection—Biological Analyses. Ann. Warsaw Univ. Life Sci.-SGGW. For. Wood Technol. 2016, 47, 43–47. [Google Scholar]
- Beck, G. Leachability and Decay Resistance of Wood Polyesterified with Sorbitol and Citric Acid. Forests 2020, 11, 650. [Google Scholar] [CrossRef]
- Larnøy, E.; Karaca, A.; Gobakken, L.R.; Hill, C.A.S. Polyesterification of Wood Using Sorbitol and Citric Acid under Aqueous Conditions. Int. Wood Prod. J. 2018, 9, 66–73. [Google Scholar] [CrossRef]
- Mubarok, M.; Militz, H.; Dumarçay, S.; Gérardin, P. Beech Wood Modification Based on in Situ Esterification with Sorbitol and Citric Acid. Wood Sci. Technol. 2020, 54, 479–502. [Google Scholar] [CrossRef]
- Bi, Z.; Yan, L.; Chen, Z.; Lei, Y.; Li, G. Laccase-Catalyzed Grafting of Vanillin on Wood and Its Effect on Wood Decay Resistance. Holzforschung 2022, 76, 732–743. [Google Scholar] [CrossRef]

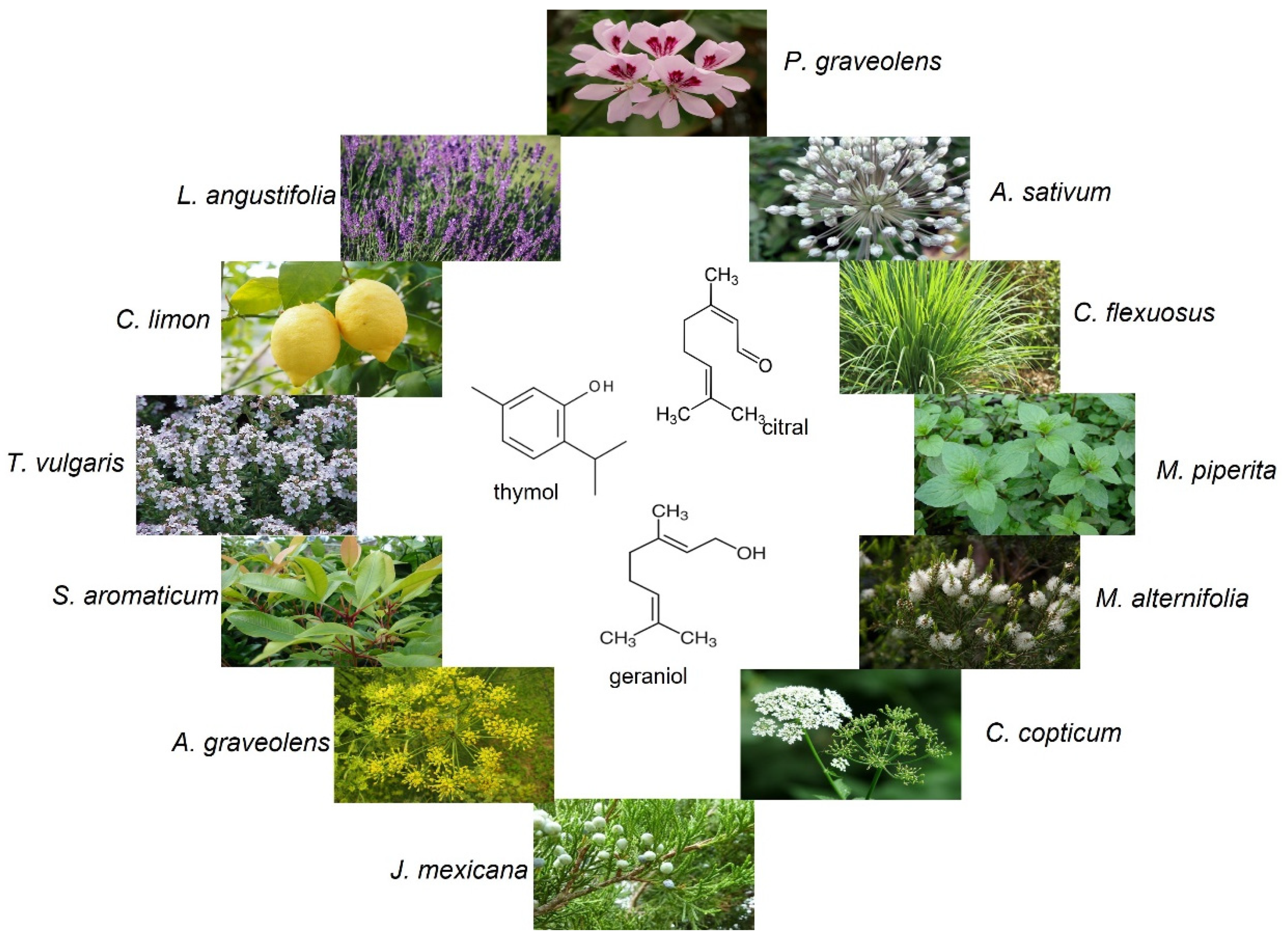
| Essential Oil | Tested Fungal Strain | Results | References |
|---|---|---|---|
| Ajowan (Carum copticum) | Wood decay fungi (G. trabeum, P. placenta, T. versicolor, C. puteana, Ch. globosum); mold and blue stain fungi (A. niger, A. flavus, P. variotii, P. chrysogenum, A. pullulans, T. viride, P. commune) | Agar diffusion plate method indicated that ajowan oil possessed a remarkable activity against mold fungi at the concentration of 0.5%. Beech and pine wood treated with ajowan oil showed resistance against decay fungi and low effect of inhibiting the growth of molds on the wood surfaces. | [30,54,63,64] |
| Bergamot (Citrus x limon) | Wood decay fungi (C. puteana, T. versicolor, Ch. globosum); mold fungi (A. niger, P. commune) | Beech and pine wood treated with bergamot oil characterized by no growth on wood surface after 10 weeks exposition to P. commune. In turn, treated wood showed no resistance against A. niger and fungi causing brown and white wood decay. | [30] |
| Birch (Betula pendula) | Wood decay fungi (C. puteana, T. versicolor); mold fungi (A. niger, P. brevicompactum) | The effective concentration of birch oil against growth of C. puteana was 3.5%, while against T. versicolor, A. niger and P. brevicompactum was 10% on treated filter paper. The mass loss of beech wood treated with 10% birch oil caused by C. puteana was 0.08%, compared to the control wood—27.01%. | [16] |
| Cedar wood (Juniperus mexicana) | Wood decay fungi (C. puteana, T. versicolor, Ch. globosum); mold fungi (A. niger, P. commune) | Beech and pine wood treated with cedar wood oil did not show resistance against both decay and mold fungi. | [30] |
| Clove (Eugenia caryophyllata, Syzygium aromaticum) | Wood decay fungi (G. trabeum, T. versicolor, C. puteana, T. hirsuta, L. sulphureus); mold fungi (A. niger. P. brevicompactum, A. flavus, A. fumigatus, P. variotii, T. viride) | Clove oil at 0.5% concentration was effective against molds, causing growth inhibition in the range from 60% (T. viride) to 84% (A. niger) tested by the agar diffusion plate method. Clove oil at a 400 µg/mL concentration totally inhibited the growth of G. trabeum and T. versicolor tested by the diffusion method. The MIC of clove oil against A. flavus was 0.64 µg/mL, while against A. fumigatus and A. niger, it was 0.32 µg/mL. The effective concentration of clove oil against the growth of C. puteana, A. niger, and P. brevicompactum was 3.5%, while against T. versicolor, it was 10% on treated filter paper. The mass loss of beech wood treated with 10% clove oil caused by C. puteana was 0.04% compared to the control wood—27.01%. | [16,57,60,64,65] |
| Dill (Anethum graveolens) | Wood decay fungi (G. trabeum, P. placenta, T. versicolor); mold and blue stain fungi (A. niger, P. chrysogenum, A. pullulans, T. viride) | Southern yellow pine treated with dill oil showed resistance against tested decay, mold, and blue stain fungi. | [54,63] |
| Eucalyptus (Eucalyptus camaldulensis, E. globulus) | Wood decay fungi (C. puteana, T. versicolor, A. alternata, Ch. globosum); mold fungi (F. subglutinans, A. niger, T. viride, P. commune) | Beech and pine wood treated with eucalyptus oil showed resistance against Ch. globosum and P. commune, and no resistance against C. puteana, T. versicolor, and A. niger. The wood (P. sylvestris, P. rigida, and F. sylvatica) treated with eucalyptus oil showed better resistance against Ch. globosum and A. niger than against T. viride. | [30,51] |
| Geranium (Pelargonium graveolens) | Wood decay fungi (C. puteana, C. versicolor, P. placenta, G. trabeum, T. hirsuta, L. sulphureus, T. versicolor, Ch. globosum); mold and blue stain fungi (A. niger, P. chrysogenum, A. pullulans, T. viride, P. commune) | Beech and pine wood treated with geranium oil showed resistance against decay fungi—C. puteana, T. versicolor, Ch. globosum (except of treated beech against C. puteana), and P. commune. Southern yellow pine treated with 100% geranium oil showed a resistance against decay fungi (G. trabeum, P. placenta and T. versicolor) with no weight loss in the wood specimens. Pine wood treated with geranium oil exhibited resistance against mold fungi (P. chrysogenum, A. niger and T. viride). | [30,49,54,57,63] |
| Lavender (Lavandula angustifolia) | Wood decay fungi (C. puteana, T. versicolor, Ch. globosum); mold fungi (A. niger, P. brevicompactum, P. commune) | The effective concentration of lavender oil against the growth of C. puteana was 10%, while against T. versicolor and A. niger, it was 100% on the treated filter paper. Beech and pine wood treated with lavender oil showed resistance against decay fungi (C. puteana, T. versicolor, Ch. globosum) and molds (A. niger, P. commune). The mass loss of beech wood treated with 10% lavender oil caused by C. puteana was 8.02% compared to the control wood—27.01%. | [16,30] |
| Lemongrass (Cymbopogon flexuosus, C. winterianus) | Wood decay fungi (G. trabeum, P. placenta, T. versicolor, C. puteana, Ch. globosum); mold and blue stain fungi (A. niger, P. chrysogenum, A. pullulans, T. viride, P. commune) | Beech and pine wood treated with lemongrass oil showed resistance against decay fungi (C. puteana, T. versicolor, Ch. globosum) and molds (A. niger, P. commune). Pine wood treated with 10% and 100% lemongrass oil showed resistance against decay fungi—G. trabeum, P. placenta, and T. versicolor. In turn, the inhibitory effect on the surface of the wood specimens treated with lemongrass oil against molds (A. niger, T. viride and P. chrysogenum) was low. | [30,54,63] |
| Neem (Azadirachta indica) | Mold fungi (A. niger, A. flavus, P. variotii, T. viride) | Neem oil at a 0.5% concentration possessed a remarkable antifungal activity against all of the tested fungi, which was evaluated for its ability to inhibit weight loss by soil wood block tests. | [64] |
| Oregano (Origanum vulgare) | Wood decay fungi (T. hirsuta, L. sulphureus, C. puteana, T. versicolor); mold fungi (A. niger, P. brevicompactum) | The effective concentration of oregano oil against growth of T. versicolor, A. niger and P. brevicompactum was 10%, while against C. puteana—1%, on treated filter paper. Oregano oil at 400 µg/mL concentration showed 100% inhibition of T. hirsuta and L. sulphureus growth. The mass loss of beech wood treated with 10% oregano oil caused by C. puteana was 0.02%, compared to control wood—27.01%. | [16,57] |
| Peppermint (Mentha x piperita) | Wood decay fungi (T. versicolor, C. puteana, Ch. globosum) and mold fungi (A. niger, P. chrysogenum, P. commune) | Beech and pine wood treated with peppermint oil showed resistance against P. commune and moderate activity against A. niger. Treated beech exhibited activity against C. puteana and no resistance against T. versicolor, while impregnated pine wood showed resistance against T. versicolor and moderate resistance against C. puteana. | [30,62] |
| Pinus (Pinus rigida—wood) | Wood decay fungi (A. alternata, Ch. globosum); mold fungi (F. subglutinans, A. niger, T. viride) | The wood (P. sylvestris, P. rigida and F. sylvatica) treated with Pinus oil showed resistance against all tested fungi. | [51] |
| Rosemary (Rosmarinus officinalis) | Wood decay fungi (G. trabeum, P. placenta, T. versicolor); mold and blue stain fungi (A. niger, A. flavus, A. ochraceus, P. chrysogenum, A. pullulans, T. viride) | The inhibitory effect on the surface of the wood specimens treated with rosemary oil against molds (A. niger, T. viride and P. chrysogenum) was low. Pine wood treated with 10% and 100% rosemary oil showed resistance against decay fungi—G. trabeum, P. placenta, and T. versicolor, with no weight loss of the wood samples. | [54,63,66] |
| Sage (Salvia officinalis) | Wood decay fungi (C. puteana, T. versicolor); mold fungi (A. niger, P. brevicompactum) | The effective concentration of sage oil against the growth of decay fungi was 100%, while against molds, sage oil was non-active on the treated filter paper. The mass loss of beech wood treated with 10% sage oil caused by C. puteana was 17.80% compared to the control wood—27.01%. | [16] |
| Savory (Satureja hortensis) | Wood decay fungi (C. puteana, T. versicolor); mold fungi (A. niger, P. brevicompactum) | The effective concentration of savory oil against the growth of C. puteana was 3.5%, while against T. versicolor and molds, it was 10% on the treated filter paper. The mass loss of beech wood treated with 10% savory oil caused by C. puteana was 0.07% compared to the control wood—27.01%. | [16] |
| Sweet flag (Acorus calamus) | Wood decay fungi (C. puteana, T. versicolor); mold fungi (A. niger, P. brevicompactum) | The effective concentration of sweet flag oil against the growth of C. puteana and P. brevicompactum was 3.5%, while against T. versicolor and A. niger, it was 10% on the treated filter paper. The mass loss of beech wood treated with 10% sweet flag oil caused by C. puteana was 0.04% compared to the control wood—27.01%. | [16] |
| Tea tree (Melaleuca alternifolia) | Wood decay fungi (G. trabeum, P. placenta, T. versicolor, C. puteana, Ch. globosum); mold and blue stain fungi (A. niger, P. chrysogenum, A. pullulans, T. viride, P. brevicompactum, P. commune) | The inhibitory effect on the surface of wood specimens treated with tea tree oil against molds (A. niger, T. viride, and P. chrysogenum) was low. The effective concentration of tea tree oil against the growth of decay (C. puteana and T. versicolor) and mold fungi (A. niger and P. brevicompactum) was 100%. The mass loss of beech wood treated with 10% tea tree oil caused by C. puteana was 8.52% compared to the control wood—27.01%. | [16,30,54,63] |
| Thyme (Thymus vulgaris) | Wood decay fungi (T. hirsuta, L. sulphureus, G. trabeum, P. placenta, T. versicolor, C. puteana, Ch. globosum); mold fungi (A. niger, P. chrysogenum, A. pullulans, T. viride, P. brevicompactum, P. commune) | The effective concentration of thyme oil against the growth of C. puteana and P. brevicompactum was 3.5%, while against T. versicolor and A. niger it was 10%. The mass loss of beech wood treated with 10% thyme oil caused by C. puteana was 0.06% compared to the control wood—27.01%. Pine wood treated with 10% and 100% thyme oil showed resistance against decay fungi—G. trabeum, P. placenta, and T. versicolor, with no weight loss of the wood samples. | [16,30,54,57,63] |
| Chemical Compounds | Tested Fungal Strain | Results | References |
|---|---|---|---|
| α-pinene | Wood decay fungi (T. hirsuta, S. commune, P. sanguineus); mold fungi (A. niger, A. flavus, A. ochraceus) | The diameter of the inhibition zone of A. flavus was in the range from 0.85 cm (2.5 µL concentration) to 3.61 cm (20 µL concentration). Pinene at a 50 µg/mL concentration did not show activity against wood decay fungi in the fluid medium test. | [17,66] |
| Borneol | Wood decay fungi (T. hirsuta, S. commune, P. sanguineus) | Borneol at 50 µg/mL concentration did not show activity against wood decay fungi in the fluid medium test. | [17] |
| Carvacrol | Wood decay fungi (T. versicolor, C. puteana, T. hirsuta, S. commune, P. sanguineus, L. sulphureus, A. alternata); mold fungi (A. flavus, A. fumigatus, A. niger, A. ochraceus, P. citrinum, P. chrysogenum, F. oxysporum) | IC50 values of carvacrol against S. commune and P. sanguineus were 53.6 and 71.7 µg/mL, respectively. In turn, IC50 values of carvacrol against T. hirsuta and L. sulphureus were 33.6 and 17.2 μg/mL, respectively. The MIC of carvacrol against T. versicolor and C. puteana was 1.25 and 0.625 mmol/L, respectively. Carvacrol showed activity against Aspergillus species with MIC vales from 50 (A. niger) to 100 µg/mL (A. fumigatus, A. flavus, A. ochraceus). | [17,57,67,69,71] |
| Cinnamaldehyde | Wood decay fungi (L. betulina, L. sulphureus, C. versicolor, P. coccineus, T. abietinum, O. lowei, A. taxa, F. pinicola, P. schweinitzii, T. hirsute, T. palustris, T. versicolor, G. trabeum); mold fungus (A. niger) | IC50 value of cinnamaldehyde against L. betulina was 0.65 mM and against L. sulphureus was 0.23 mM. Cinnamaldehyde at 100 ppm concentration showed high activity against wood rot fungi including C. puteana, T. versicolor, P. coccineus, A. taxa, and P. schweinitzii. Cinnamaldehyde below a 100 µg/mL concentration totally inhibited the growth of T. hirsute and L. sulphureus. The MIC of cinnamaldehyde against T. versicolor and C. puteana was 1.25 and 0.625 mmol/L, respectively. | [52,53,57,72,73,74] |
| Citral | Wood decay fungi (T. hirsuta, S. commune, P. sanguineus, L. sulphureus); molds (T. viride, A. niger, P. citrinum) | The MIC of citral against P. citrinum, T. viride, and A. niger resulting in the inhibition of fungal growth on the bamboo wood surfaced was 0.180, 0.265, and 0.226 mg/mL, respectively. IC50 values of citral against T. hirsuta, S. commune, and P. sanguineus were 184.5, 207.5, and 178.5 µg/mL, respectively. | [17,57,70] |
| Citronellol | Wood decay fungi (P. placenta, G. trabeum, T. hirsuta, S. commune, P. sanguineus, L. sulphureus); mold fungi (A. niger, P. chrysogenum, T. viride) | IC50 values of citronellol against T. hirsuta and L. sulphureus were 80.5 and 61.6 µg/mL, respectively. IC50 value of monoterpene against decay wood fungi was in a range from 190.2 µg/mL (P. sanguineus) to 219.5 µg/mL (T. hirsuta). | [17,50,57] |
| Eugenol | Wood decay fungi (T. versicolor, C. puteana, T. hirsuta, S. commune, P. sanguineus, L. betulina, L. sulphureus, C. versicolor, A. alternata); mold fungi (A. niger, A. flavus, A. ochraceus, A. fumigatus, P. citrinum, P. chrysogenum, F. oxysporum) | IC50 values of eugenol against T. hirsuta, S. commune, and P. sanguineus were 85.1, 122.2, and 137.7 µg/mL, respectively. Eugenol at the concentration of 100 µg/mL showed high activity against L. betulina and L. sulphureus. IC50 value of eugenol against L. betulina was 0.37 mM and against L. sulphureus was 0.25 mM. The MIC value of eugenol against T. versicolor and C. puteana was 5.0 and 1.25 mmol/L, respectively. | [17,52,53,57,66,67,68,69,71] |
| Geraniol | Wood decay fungi (P. placenta, G. trabeum, T. hirsuta, S. commune, P. sanguineus, L. sulphureus); mold fungi (A. niger, P. chrysogenum, T. viride) | IC50 values of geraniol against T. hirsuta, S. commune, and P. sanguineus were 189.3, 224.4, and 367.6 µg/mL, respectively. IC50 values of geraniol against A. niger was 128.7 mg/L. | [17,50,57,75] |
| Menthol | Wood decay fungi (T. hirsuta, S. commune, P. sanguineus, T. versicolor, A. alternata); mold fungi (A. niger, P. chrysogenum, A. fumigatus, A. flavus, P. citrinum, F. oxysporum, A. ochraceus) | Menthol did not show activity against decay wood fungi—T. hirsuta, S. commune, and P. sanguineus. The MIC of menthol against A. niger and T. versicolor was 250 µL/mL, while against P. chrysogenum was 150 µL/mL. The MIC value of menthol against A. flavus and A. ochraceus was 100 µg/mL, 150 µg/mL against A. fumigatus, and 450 µg/mL against A. alternata. | [17,62,67] |
| Nerol | Wood decay fungi (T. hirsuta, S. commune, P. sanguineus); mold fungi (A. flavus) | Nerol in a concentration higher than 7.5 μL totally inhibited the growth of the A. flavus strain and the diameter of inhibition zone was in the range from 2.68 cm (2.5 µL concentration) to 6.09 cm (20 µL concentration). IC50 values of nerol against T. hirsuta, S. commune, and P. sanguineus were 166.3, 337.6, and 242.7 µg/mL, respectively. | [17,75] |
| Thymol | Wood decay fungi (P. placenta, G. trabeum, T. versicolor, C. puteana, A. alternata); mold fungi (A. niger, P. chrysogenum, T. viride, A. flavus, A. fumigatus, P. citrinum, F. oxysporum) | IC50 values of thymol against S. commune and P. sanguineus were 67.1 and 116.8 µg/mL, respectively. The MIC value of thymol against T. versicolor and C. puteana was 1.25 and 0.313 mmol/L, respectively. IC50 of thymol against T. hirsuta and L. sulphureus was 45.8 and 23.2 µg/mL, respectively. In turn, IC50 values of thymol against A. niger was 23.80 mg/L. | [50,57,67,69,71,75,76] |
| Vanillin | Wood decay fungi (T. versicolor, C. puteana); mold fungi (A. flavus, A. fumigatus) | The MIC value of vanillin against T. versicolor and C. puteana was 10 mmol/L. Inhibitory effect of vanillin on A. flavus and A. fumigatus at a concentration 1000 µg/mL was 46.34 and 100%, respectively. | [69,71] |
| Plant Source | Tested Fungal Strain | Results | References |
|---|---|---|---|
| Eriosema robustum | A. fumigatus | The MIC of crude ethanolic extract against A. fumigatus was 0.63 mg/mL, while the MIC for the eight compounds identified in this extract was in a range from 65 (6-prenylpinocembrin) to 250 µg/mL (orostachyscerebroside A). | [105] |
| Sequoia sempervirens | G. trabeum, T. versicolor | Among the extracts prepared using various solvents (water, ethanol, acetone, ethyl acetate, and dichloromethane), the acetone-soluble extract and the ethyl acetate-soluble fraction of the ethanol extract caused the greatest reduction in the growth of both tested fungi. | [106] |
| Fagus sylvatica L. | G. trabeum, T. versicolor | The paper disc screening test of the methanolic extracts indicated that both wound-wood as well as to healthy sapwood possessed fungicidal potential against the tested fungi. In turn, the extracts of the reaction zones did not exhibit a corresponding inhibitory effect toward the examined fungi. | [107] |
| Bagassa guianensis Aubl (heartwood) | P. sanguineus | The ethyl acetate extract showed activity against the wood decay fungus and exhibited a lower MIC value (2 µg/mL) than the six components isolated from this extract including 6-O-methyl-moracin N (8 µg/mL), moracin N (4 µg/mL), and oxyresveratrol (32 µg/mL). | [108] |
| Taiwania cryptomerioides Hayata | P. noxius | The hexane-soluble fraction demonstrated a significant inhibition of the growth of wood brown rot fungus among the four examined fractions (hexane, ethyl acetate, butanol, and water) using the agar dilution method. Moreover, constituents of the hexane-soluble fraction (ferruginol, T-cadinol, α-cadinol, and T-muurolol) exhibited excellent antifungal activities against P. noxius with IC50 values equal 16.9, 25.8, 33.8, and 50.6 µg/mL, respectively. | [109] |
| Juniperus virginiana (heartwood) | T. versicolor, G. trabeum | Among the four extracts obtained using solvents with different polarity (hexane, chloroform, ethyl acetate, methanol), the hexane and chloroform soluble fraction of the extract showed a high inhibitory effect on the growth of the tested wood decay fungi. The component of extracts (mainly cedrol and thujopsene) exhibited an inhibitory effect against the tested fungi. | [110] |
| Dalbergia congestiflora Pittier (heartwood) | T. versicolor | The hexane extract completely inhibited the growth of the tested wood decay fungus, and the main component of this extract was the isoflavonoid—medicarpin. | [111] |
| Plant Source | Tested Fungal Strain | Wood Species | Results | References |
|---|---|---|---|---|
| Lawsonia inermis | G. lucidum, S. rolfsii | Vitex doniana, Triplochiton scleroxylon | Wood samples treated with extracts, both from the bark and leaves exhibited lower values of weight loss caused by the action of tested fungi in comparison to the untreated wood samples. | [116] |
| Acacia dealbata | C. puteana | Scots pine (P. sylvestris L.) | The wood samples were impregnated with extracts (methanolic and aqueous) from the bark, sapwood, and heartwood of A. dealbata at 3 and 5% concentration. The wood treated with 5% methanolic extracts from bark showed the lowest mass loss (7.45%), while the highest weight loss (23.5%) was determined for wood treated with 3% aqueous extract from sapwood. | [117] |
| Robinia pseudoacacia | T. versicolor | European beech (F. sylvatica L.) | The treated wood was characterized by higher resistance (mass loss of 12.7%) against the tested fungus compared to the untreated wood samples (mass loss of 43.6%). | [118] |
| Eucalyptus camaldulensis (aerial parts) | Fusarium culmorum, Rhizoctonia solani, P. chrysogenum | Melia azedarach | The wood treated with the n-hexane extract showed growth inhibition of the tested fungi. The main components of the extract were β-fenchol, eucalyptol, and subinene. | [119] |
| Vitex agnus-castus (leaves) | F. culmorum, R. solani, P. chrysogenum | Melia azedarach | The impregnation of wood with the n-hexane extract showed a higher resistance against the tested fungi compared to the unprotected wood. The main ingredients of the extract were eucalyptol, β-caryophyllene, and β-sitosterol. | [119] |
| Juniperus virginiana L. | P. placenta, G. trabeum | Southern pine | The wood treated with cedar extract obtained by ethanolic extraction and by liquid carbon dioxide extraction showed a higher resistance against the tested fungi compared to the control wood samples. | [120] |
| Cupressus sempervirens | T. harzianum | Acacia saligna | The treated wood with the methanolic extract of C. sempervirens wood showed an inhibition zone against the growth of T. harzianum around the treated wood at the concentrations of 5, 10, and 20%. | [121] |
| Usnea filipendula | C. puteana | Scots pine (P. sylvestris L.) | The wood samples treated with the methanolic and aqueous extract of lichen were characterized by lower weight loss than the control wood samples. | [122] |
| Viscum album | C. puteana | Scots pine (P. sylvestris L.) | The wood impregnated with methanolic and aqueous extracts from the leaves of mistletoe showed lower mass loss values compared to the mass loss of the untreated wood. | [122] |
| Ocotea lancifolia | T. versicolor, G. trabeum | Downy birch (Betula pubescens) | Wood veneers impregnated with ethanolic extract from the leaves of the native Brazilian tree and ethyl acetate phenolic-rich fraction of this extract at a concentration of 4% showed higher resistance to wood-destroying fungi compared to the untreated wood veneers. | [123] |
| Musa paradisiaca L. | F. culmorum, R. solani | Melia azedarach | The wood treated with the 3% methanolic extract of M. paradisiaca peels showed activity against F. culmorum and R. solani. The mycelial growth inhibition percentages reached 68.88% for F. culmorum and 94.07% for R. solani. | [124] |
| Chemical Compound | Plant Source | Tested Fungal Strain | Results | References |
|---|---|---|---|---|
| Icthyothereol acetate | Blumea balsamifera | A. niger | The compound possessed moderate activity against A. niger with an activity index of 0.4 at a mass of 30 µg determined by the agar cup method. | [132] |
| 4′-methoxy-5,7-dihydroxyflavone 6-C-glucoside | Aquilegia vulgaris | A. niger | The antimicrobial activity of isocytisoside (4′-methoxy-5,7-dihydroxyflavone 6-C-glucoside) tested by the method of series dilutions against A. niger was 62·5 µg/mL. | [133] |
| (3R)-5,7,2′,3′-tetrahydroxy-4′-methoxy-5′-prenylisoflavanone | Geoffroea decorticans | A. flavus, A. parasiticus, A. nomius | The percentage of hyphal radial growth inhibition produced by the compound was 31.2% for A. flavus, 40.3% for A. parasiticus, and 60.8% for A. nomius. | [134] |
| (3R)-7-2′-3′-trihydroxy-4′-methoxy-5′-prenylisoflavanone | Geoffroea decorticans | A. flavus, A. parasiticus, A. nomius | The percentage of hyphal radial growth inhibition caused by the compound was 28.9% for A. flavus, 35.8% for A. parasiticus, and 57.2% for A. nomius. | [134] |
| Baicalein | Scutellaria baicalensis Georgi (root) | A. fumigatus | Baicalein showed antifungal activity toward A. fumigatus and the minimal inhibitory concentration (MIC50) was 0.23 mM. | [135] |
| Wogonin | Scutellaria baicalensis Georgi (root) | A. fumigatus | Wogonin exhibited antifungal activity toward A. fumigatus and the minimal inhibitory concentrations (MIC50) was 0.23 mM. | [135] |
| Phenolic compounds | Stenoloma chusanum (L.) Ching | A. niger | The minimum inhibitory concentration (MIC) of 4-O-β-d-(6-O-gentisoylglucopyranosyl) vanillic acid, vanillic, syringic, and gentisic acids against A. niger determined by the test tube dilution method on dextrose agar was 100 µg/mL. | [136] |
| Phenolic compounds | Cynara scolymus L. | A. niger | The MIC of chlorogenic acid, cynarin, 3,5-di-O-caffeoylquinic acid, and luteolin 7-rutinoside against A. niger was 100 µg/mL, the MIC of 4,5-di-O-caffeoylquinic acid, apigenin 7-rutinoside, and apigenin-7-O-β-d-glucopyranoside was 200 µg/mL, and the MIC of cynaroside was 50 µg/mL. | [137] |
| Phenolic compounds | Diospyros virginiana (fruits) | A. fumigatus, A. versicolor, A. ochraceus, A. niger, P. funiculosum | The phenolic compounds isolated from the D. virginana extract including m-gallate, gallic acid, luteolin, quercetin, myricetin, myricetin 3-O-α-rhamnoside, myricetin 3-O-β-glucoside, and myricetin 3-O-β-glucuronide demonstrated significant activity against molds. | [138] |
| Preparation | Tested Fungal Strain | Wood Species | Results | References |
|---|---|---|---|---|
| Caffeine—silicon compounds (AATMOS) | C. puteana | Scots pine (P. sylvestris L.) | Mass loss of the unleached treated wood exposure to C. puteana was 1.6% compared to the control wood—42.3%. After the leaching procedure (EN 84) mass loss of the treated wood was 2.2% and the weight loss of unprotected wood was 46.9%. | [199] |
| Caffeine—propolis—silicon compounds (MTMOS and OTEOS) | C. puteana | Scots pine (P. sylvestris L.) | Mass loss of the unleached treated wood was 7.18% compared to the unprotected wood—50.5%, while the weight loss of wood after leaching (EN 84) was 1.61% for the treated wood and 49.7% for the untreated wood. | [26] |
| Chitosan—cinnamaldehyde | A. niger | Poplar (Populus tomentosa Carr.) | Wood treated with different molar ratios of cinnamaldehyde and chitosan showed different activity against A. niger, which caused the inhibition of fungus growth from 16.7% (0.5:1.0 ratio) to 95.8% (3.0:1.0 ratio). | [200] |
| Chitosan—propolis—nanoAg | T. versicolor | Poplar (Populus spp.) | Mass losses of the treated wood were in the range from 0% (after 5 days of fungal exposure) to 39.94% (after 30 days of fungal exposure). | [90] |
| Genipin—chitosan | G. trabeum, P. placenta, T. versicolor, I. lacteus | Southern pine and poplar | Mass loss of the treated wood caused by all fungal species decreased with an increasing concentration of the formulation regardless of the leaching procedure (AWPA E11). However, all treated wood samples recorded significantly lower weight loss compared to the unprotected samples. | [198] |
| Methyl-β-cyclodextrin—eugenol | P. placenta, G. trabeum | Southern pine wood | Mass loss of the wood treated with eugenol and 50% solution of MβCD caused by P. placenta was 4.84%, and by G. trabeum—6.12%. | [24] |
| Methyl-β-cyclodextrin—cinnamaldehyde | P. placenta, G. trabeum | Southern pine wood | Mass loss of the wood treated with cinnamaldehyde and 50% solution of MβCD caused by P. placenta was 5.50%, and by G. trabeum—6.86%. | [24] |
| Methyl-β-cyclodextrin—carvacrol | P. placenta, G. trabeum | Southern pine wood | Mass loss of the wood treated with carvacrol and 50% solution of MβCD caused by P. placenta was 8.40%, and by G. trabeum—7.94%. | [24] |
| Methyl-β-cyclodextrin—thymol | P. placenta, G. trabeum | Southern pine wood | Mass loss of the wood treated with thymol and 50% solution of MβCD caused by P. placenta was 7.75%, and by G. trabeum—6.75%. | [24] |
| Nano-CuO—extract of Lantana camara | T. hirsuta, P. placenta | Rubberwood (H. brasiliensis) | Mass loss of the wood treated with the formulation caused by T. hirsuta was 15.01% compared to the untreated wood—28.28%, and the weight loss caused by P. placenta was 23.12% for the treated and 51.59% for untreated wood. | [201] |
| Nano-CuO—extract of Nerium oleander | T. hirsuta, P. placenta | Rubberwood (H. brasiliensis) | Mass loss of the wood treated with the formulation caused by T. hirsuta was 24.75%, compared to the untreated wood—28.28%, and the weight loss caused by P. placenta was 13.19% for the treated and 51.59% for the untreated wood. | [201] |
| Nano-ZnO—essential oils (clove, oregano, thyme oils) | Ch. globosum, A. niger, P. brevicompactum, A. alternata | Lime tree (T. cordata), maple (A. pseudoplatanus L.) | Clove and oregano oils mixed with ZnO significantly improved the resistance of treated wood against a mixture of tested fungi, mainly in the first days of the test. | [190] |
| Linear poly(amidoamine)s—nanoAg | C. puteana, T. versicolor | Scots pine (P. sylvestris L.), beech (F. sylvatica L.) | The wood treated with the formulation after exposure to the tested fungi showed a weight loss well below 5%. | [202] |
| Propolis extract—silicon compounds | C. puteana | Scots pine (P. sylvestris L.) | Wood treated with propolis and VTMOS/MTMOS after exposure to C. puteana showed a mass loss of 3.7%, wood treated with propolis and MTMOS/MPTMOS before and after leaching (EN 84) were characterized by a weight loss equal to 3.8 and 3.0%, respectively. Pine wood impregnated with propolis and VTMOS/TEOS showed a mass loss of 3.3% (before leaching) and 3.5% (after leaching—EN 84), while wood impregnated with propolis and MPTMOS/TEOS were characterized by a mass loss equal to 2.9 and 3.2% (EN 84). | [203,204,205] |
| Salicylic acid—silica microcapsules | T. versicolor, G. trabeum | Poplar (P. nigra L.) | Mass losses of the untreated wood attacked by white rot fungi and brown rot fungi were 42.50% and 62.82%, respectively, while weight losses of the leached treated wood were 14.42% and 15.87% respectively. | [25] |
| Sorbitol—citric acid | P. placenta, T. versicolor, C. puteana | Scots pine (P. sylvestris L.), European beech (F. sylvatica) | Treatment of wood with sorbitol and citric acid caused an increase in the decay resistance against brown rot and white rot fungi. | [206,207,208] |
| Thymol—laccase | A. niger | Bamboo (P. pubescens) | Impregnation of wood with thymol with laccase improved the resistance to mold compared to the unprotected wood. Moreover, wood treated with thymol with laccase exhibited a higher resistance to mold than wood treated with thymol alone, even after the leaching procedure. | [76] |
| Vanillin—laccase | T. versicolor, G. trabeum | Poplar (P. nigra L.) | The weight loss of wood exposed to white and brown rot fungi decreased from 46 and 13% to 9 and 4% for the treated wood, respectively. | [209] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, M. Antifungal Agents in Wood Protection—A Review. Molecules 2022, 27, 6392. https://doi.org/10.3390/molecules27196392
Woźniak M. Antifungal Agents in Wood Protection—A Review. Molecules. 2022; 27(19):6392. https://doi.org/10.3390/molecules27196392
Chicago/Turabian StyleWoźniak, Magdalena. 2022. "Antifungal Agents in Wood Protection—A Review" Molecules 27, no. 19: 6392. https://doi.org/10.3390/molecules27196392
APA StyleWoźniak, M. (2022). Antifungal Agents in Wood Protection—A Review. Molecules, 27(19), 6392. https://doi.org/10.3390/molecules27196392






