An Efficient Method of Birch Ethanol Lignin Sulfation with a Sulfaic Acid-Urea Mixture
Abstract
1. Introduction
2. Results
2.1. Kinetic Study of the Process of Birch Ethanol Lignin Sulfation
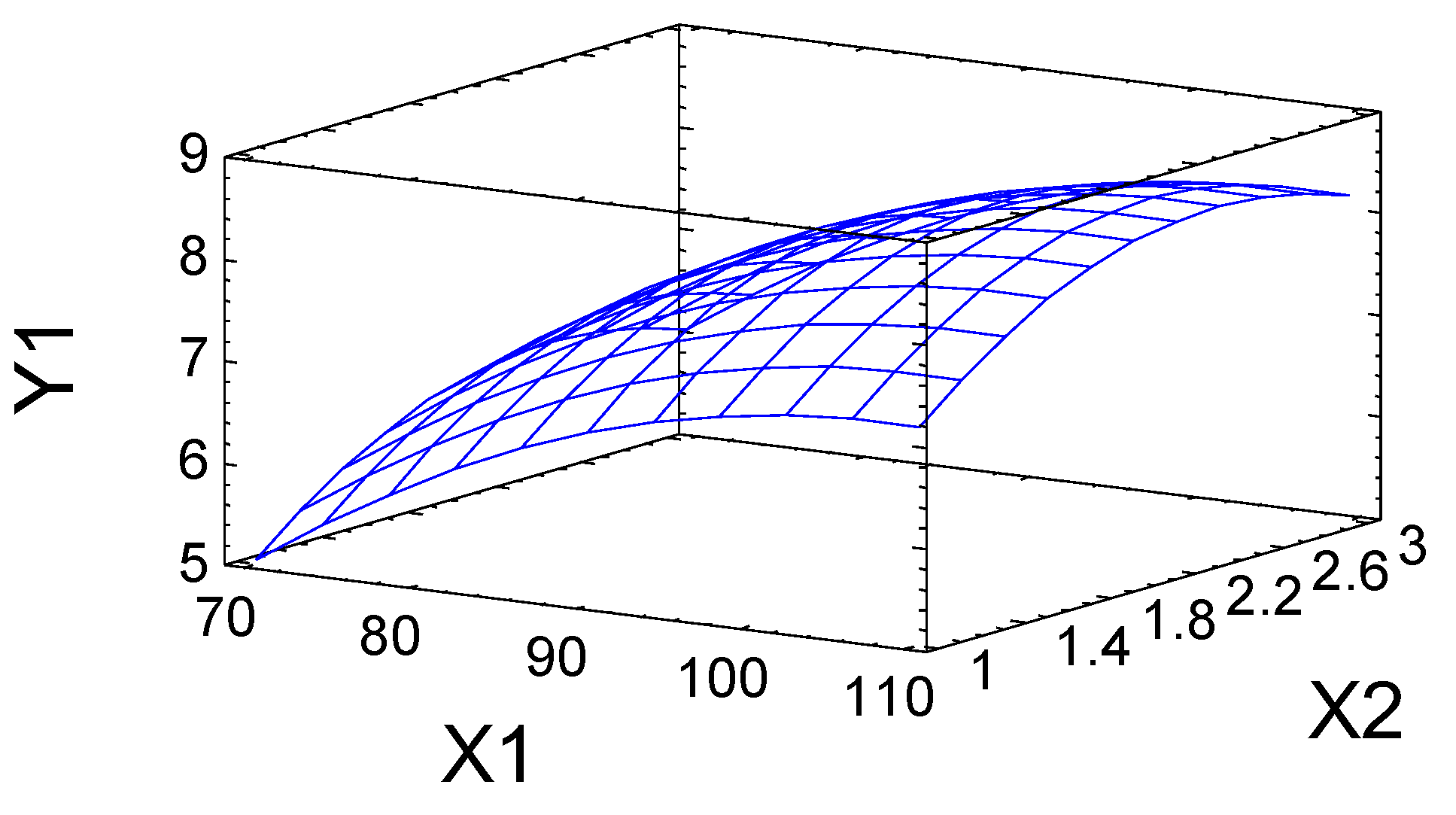
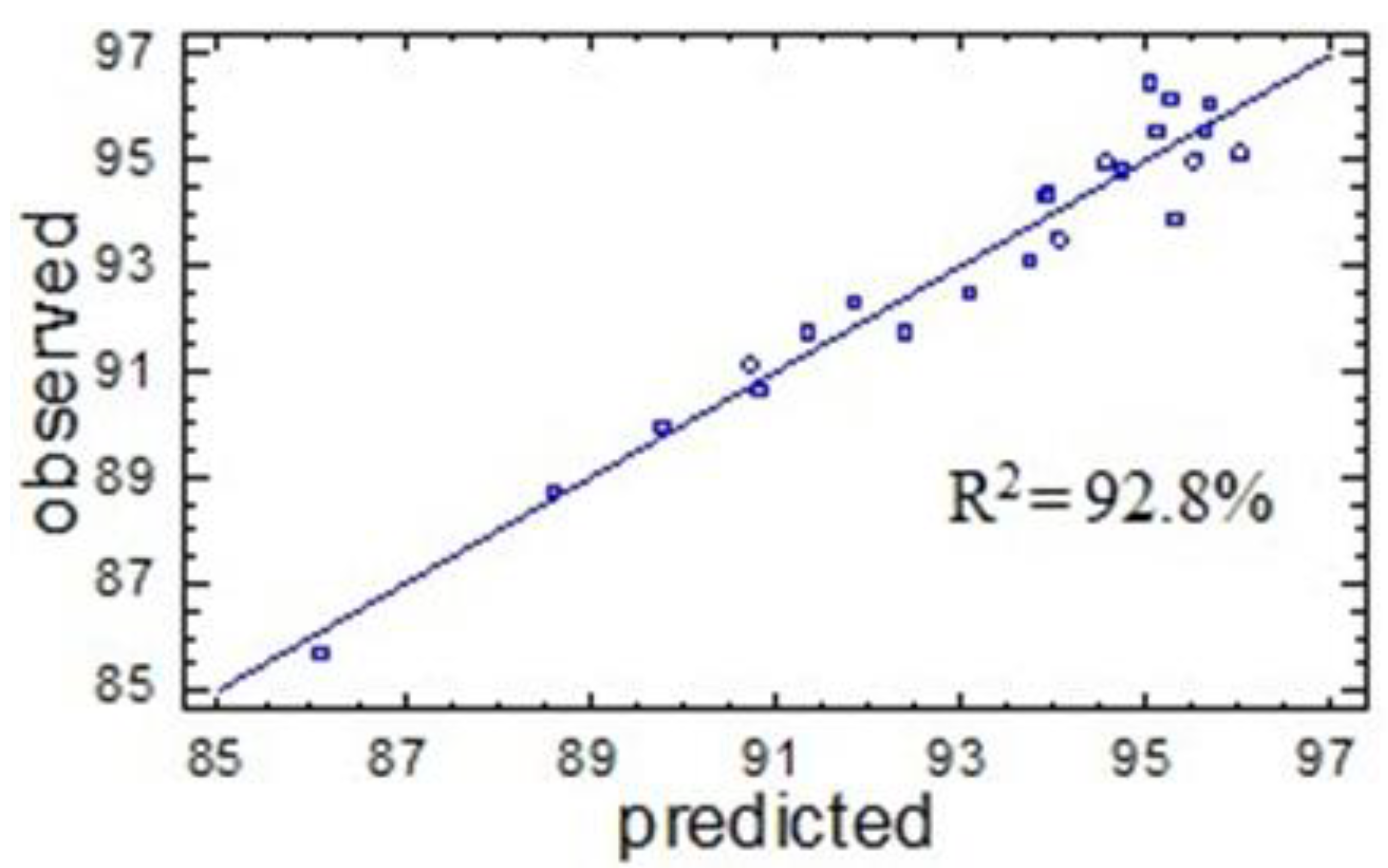
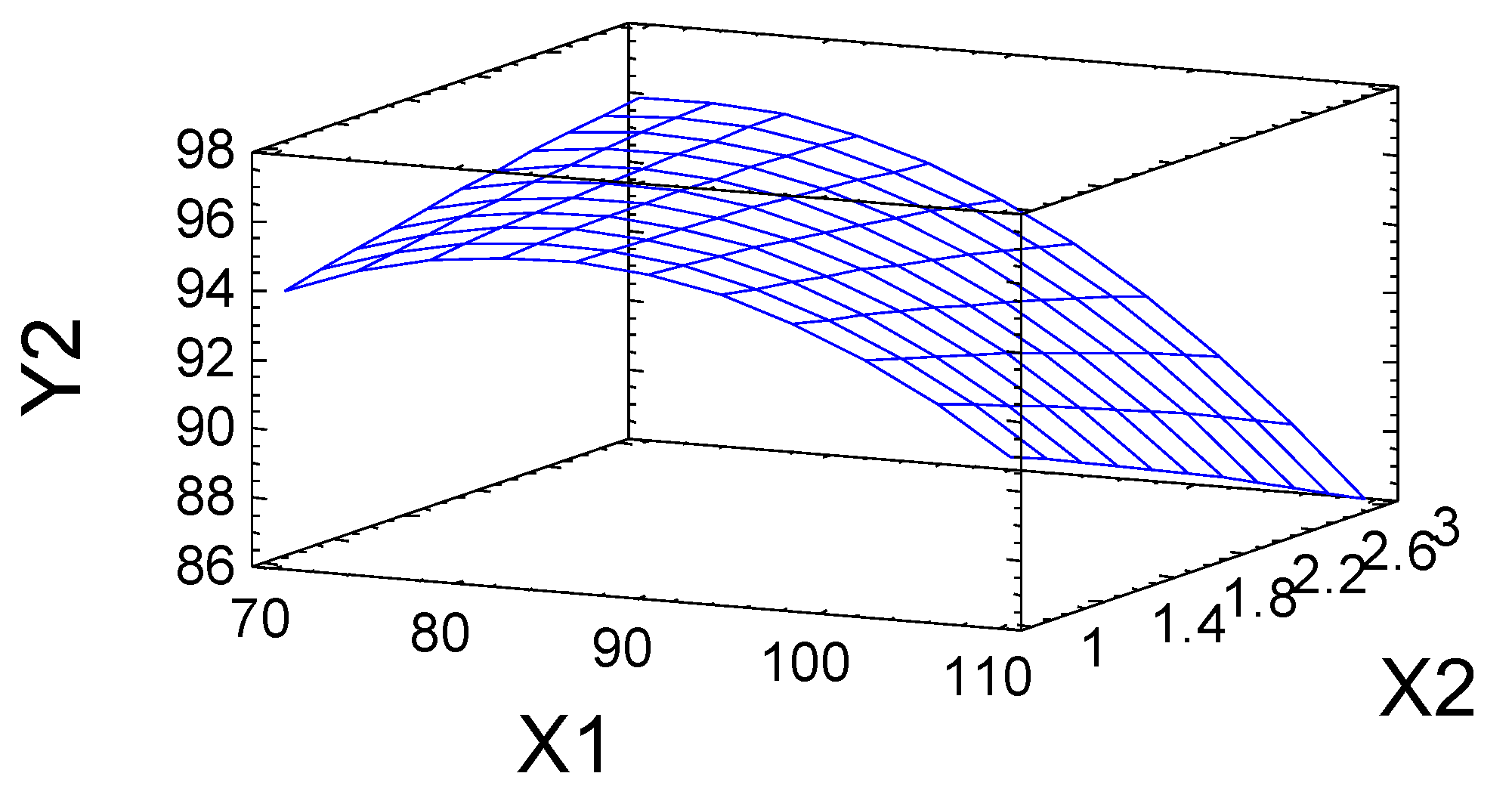
2.2. Numerical Optimization of the Process of Birch Ethanol Lignin Sulfation
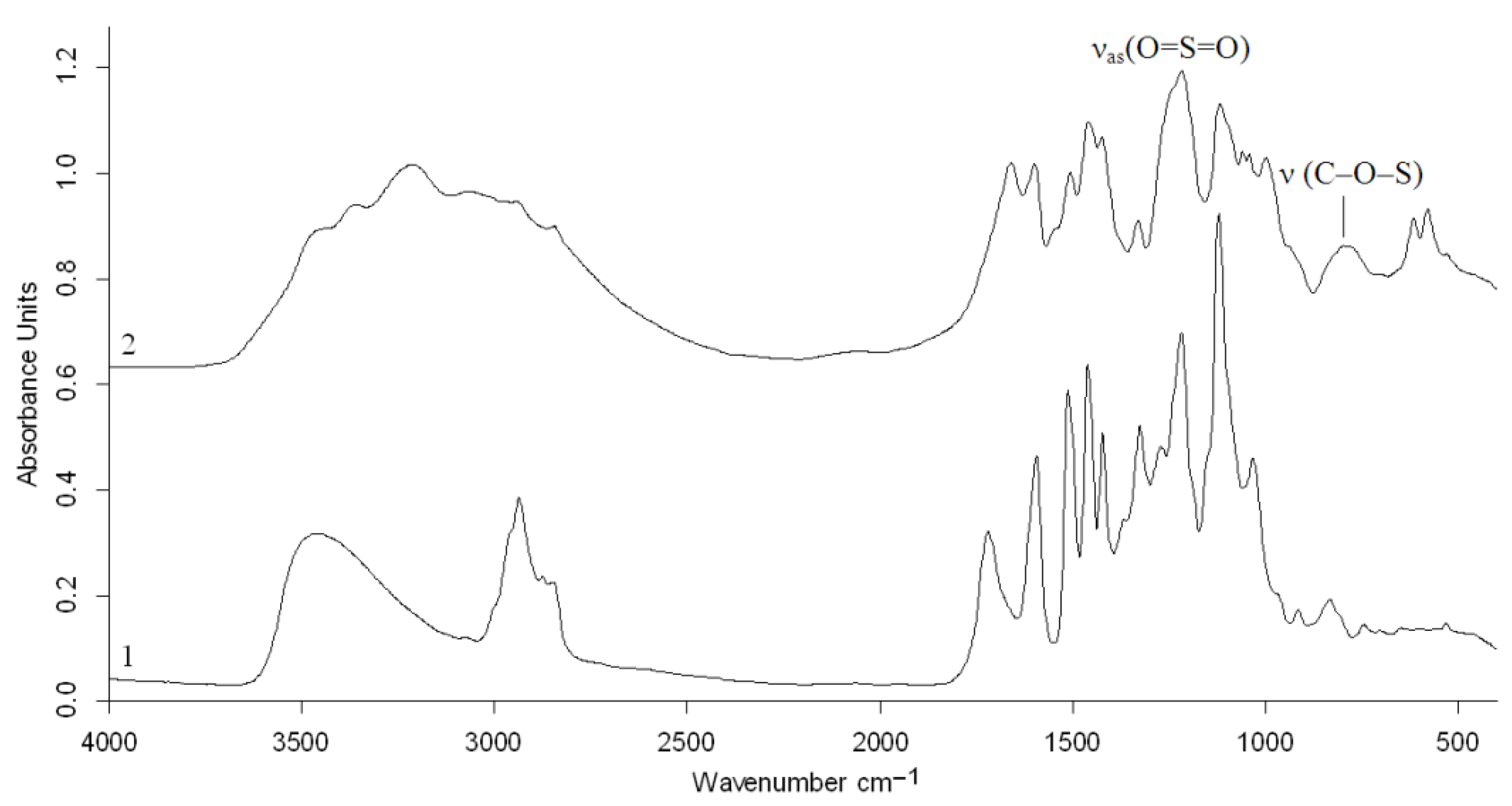
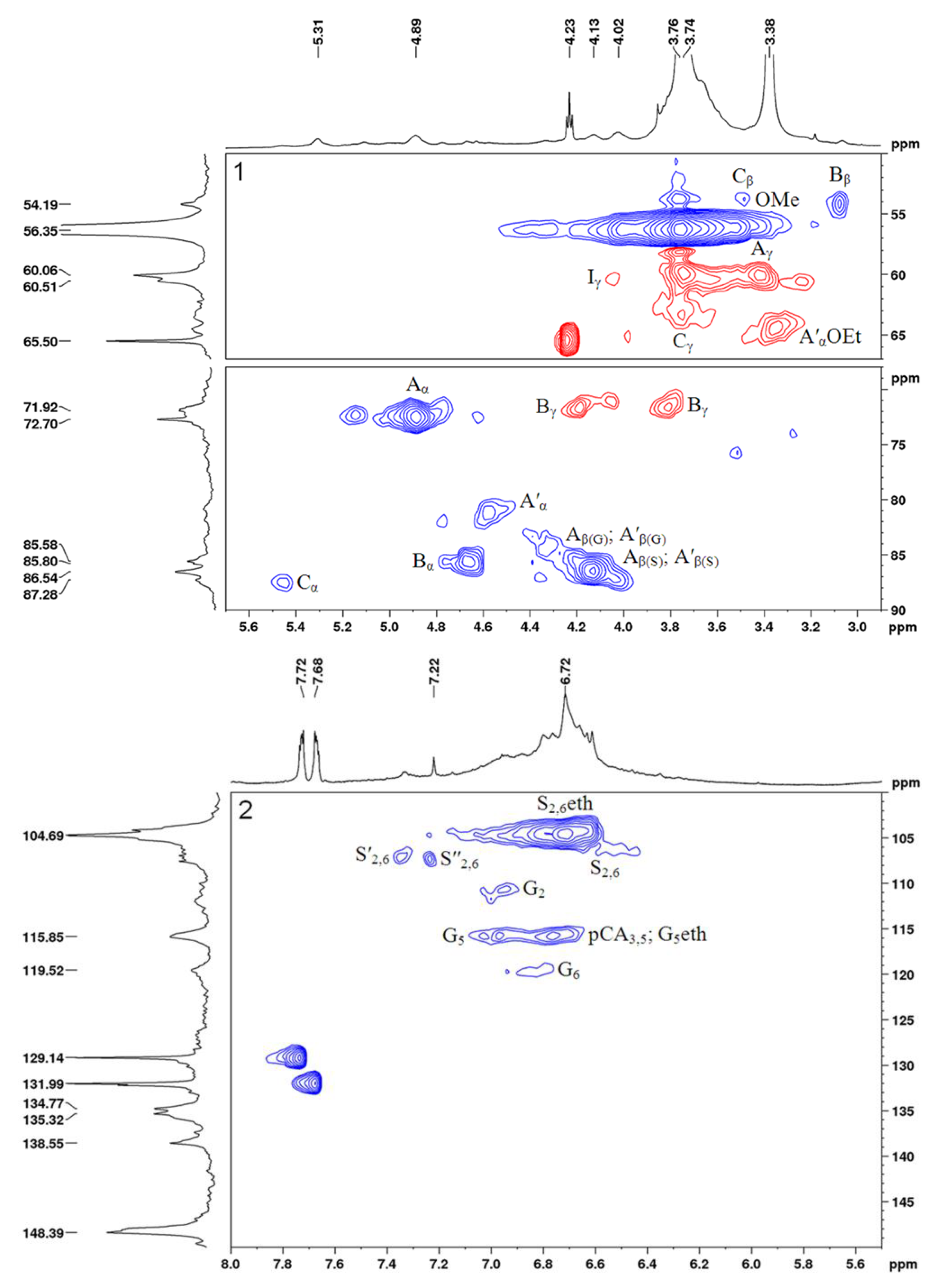
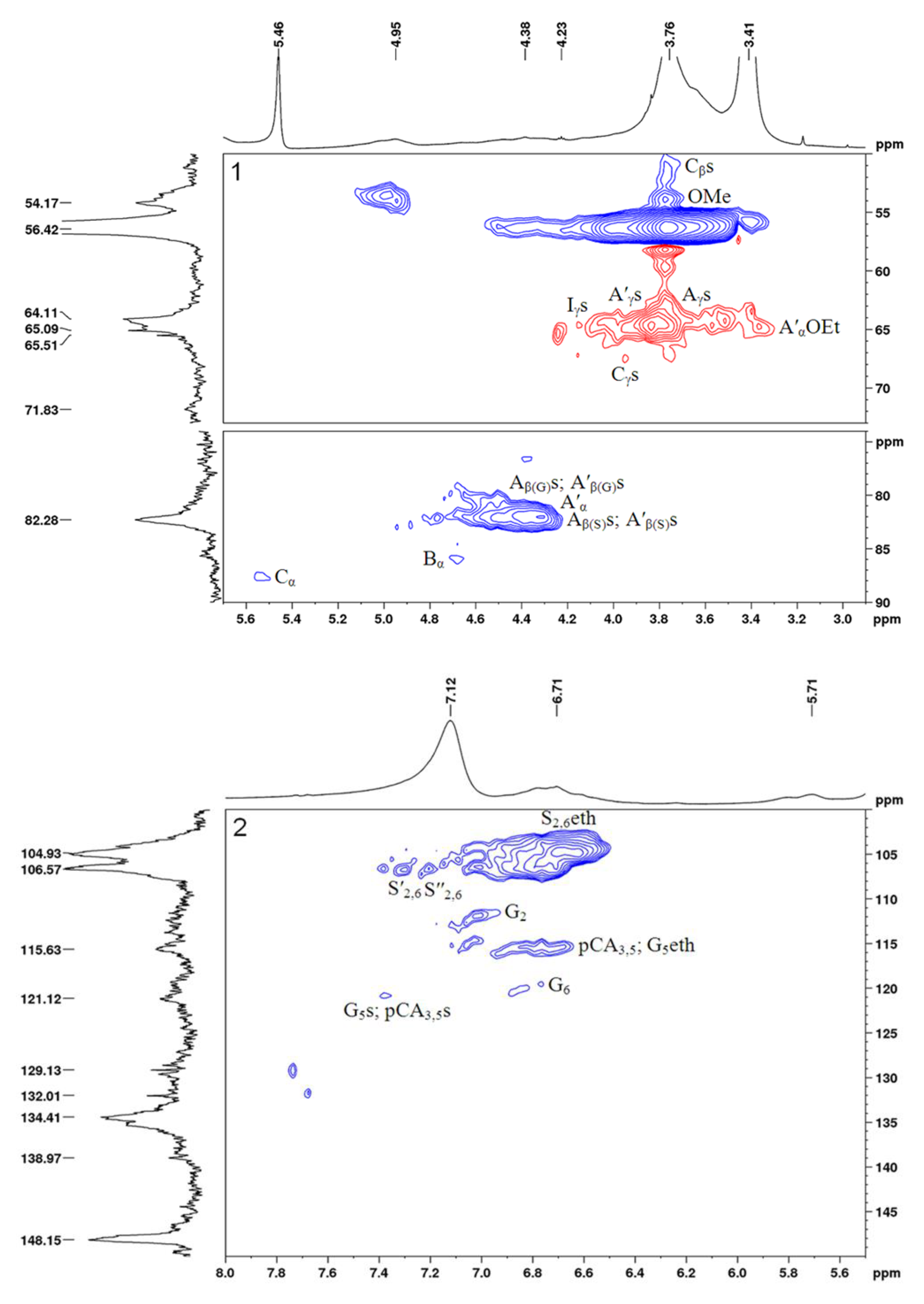
2.3. Characterization of the Sulfated Birch Ethanol Lignin
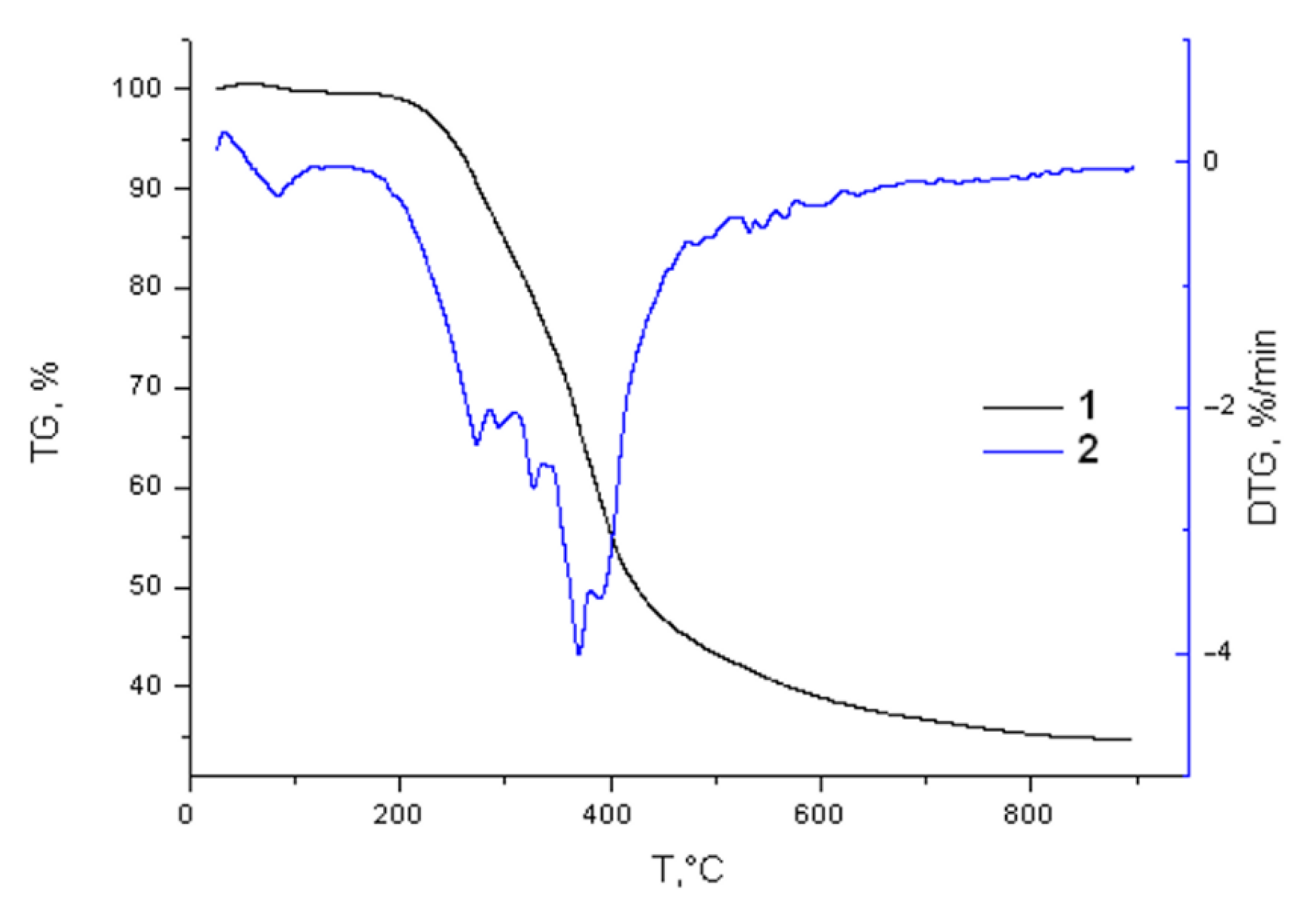
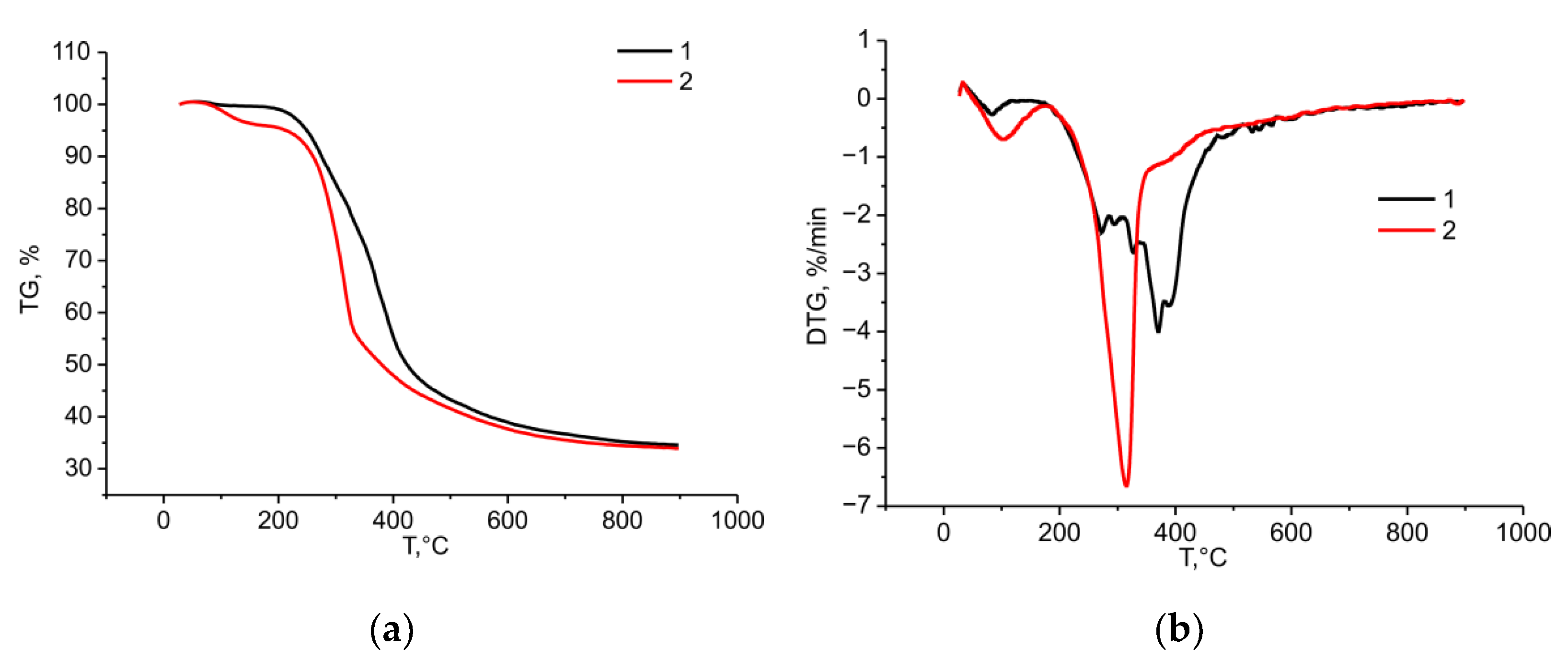
2.4. Thermochemical Properties of the Birch Ethanol Lignin
3. Materials and Methods
3.1. Materials
3.2. Ethanol Lignin Isolation
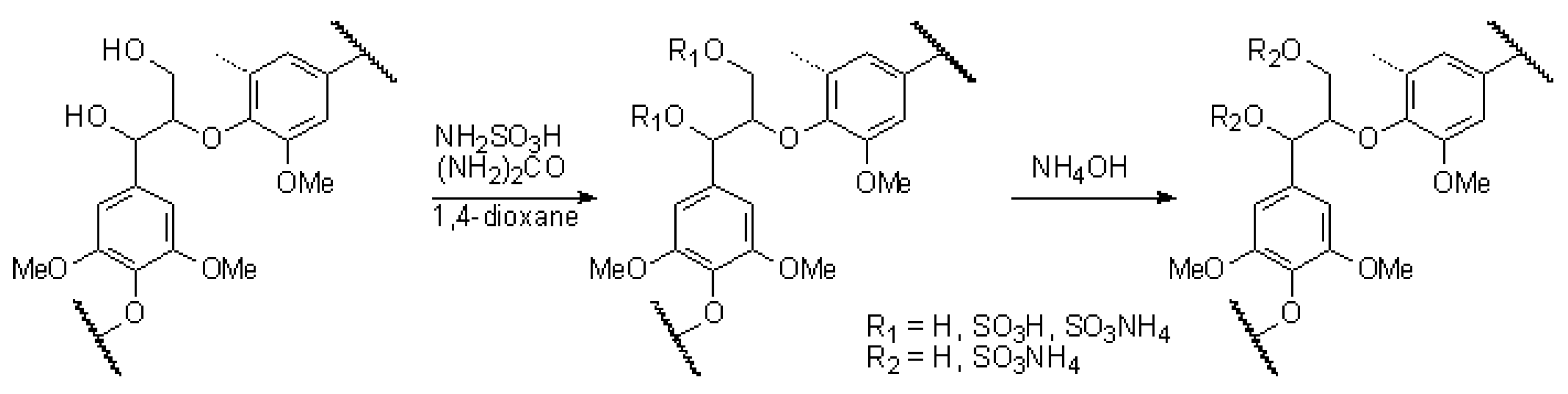
3.3. Ethanol Lignin Sulfation
3.4. Elemental Analysis
3.5. FTIR Analysis
3.6. NMR Analysis
3.7. Gel Permeation Chromatography
3.8. Thermogravimetric Analysis
3.9. Kinetic Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Faruk, O.; Sain, M. Lignin in Polymer Composites; Elsevier: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Poletto, M. Lignin: Trends and Applications; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Heitner, C.; Dimmel, D.R.; Schmidt, J.A. Lignin and Lignans: Advances in Chemistry; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Mysyakina, E.S. Lignin: Chemical structure, biodegradation, and practical application (a review). Appl. Biochem. Microbiol. 2016, 52, 573–581. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- De la Torre, M.J.; Moral, A.; Hernández, M.D.; Cabeza, E.; Tijero, A. Organosolv lignin for biofuel. Ind. Crops Prod. 2013, 45, 58–63. [Google Scholar] [CrossRef]
- Inamuddin. Green Polymer Composites Technology. Properties and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Catalytic conversion of structural carbohydrates and lignin to chemicals. Adv. Catal. 2017, 60, 59–123. [Google Scholar] [CrossRef]
- Mehta, A.Y.; Mohammed, B.M.; Martin, E.J.; Brophy, D.F.; Gailani, D.; Desai, U.R. Allosterism-based simultaneous, dual anticoagulant and antiplatelet action: Allosteric inhibitor targeting the glycoprotein Ibα-binding and heparin-binding site of thrombin. J. Thromb. Haemost. 2016, 14, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, A.; Tiwari, V.; Zhao, Q.; Shukla, D.; Debnath, A.K.; Desai, U.R. Viral inhibition studies on sulfated lignin, a chemically modified biopolymer and a potential mimic of heparan sulfate. Biomacromolecules 2007, 8, 1759–1763. [Google Scholar] [CrossRef]
- Raghuraman, A.; Tiwari, V.; Thakkar, J.N.; Gunnarsson, G.T.; Shukla, D.; Hindle, M.; Desai, U.R. Structural characterization of a serendipitously discovered bioactive macromolecule, lignin sulfate. Biomacromolecules 2005, 6, 2822–2832. [Google Scholar] [CrossRef] [PubMed]
- Malyar, Y.N.; Vasil’yeva, N.Y.; Kazachenko, A.S.; Skvortsova, G.P.; Korol’kova, I.V.; Kuznetsova, S.A. Sulfation of abies ethanol lignin by complexes of sulfur trioxide with 1,4-dioxane and pyridine. Russ. J. Bioorg. Chem. 2021, 47, 1368–1375. [Google Scholar] [CrossRef]
- Prinsen, P.; Narani, A.; Hartog, A.F.; Wever, R.; Rothenberg, G. Dissolving lignin in water through enzymatic sulfation with aryl sulfotransferase. ChemSusChem 2017, 10, 2267–2273. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Vasilyeva, N.Y.; Kazachenko, A.S.; Levdansky, V.A.; Kondrasenko, A.A.; Malyar, Y.N.; Skvortsova, G.P.; Lutoshkin, M.A. Optimization of the process of abies ethanol lignin sulfation by sulfamic acid–urea mixture in 1,4-dioxane medium. Wood Sci. Technol. 2020, 54, 365–381. [Google Scholar] [CrossRef]
- Gabov, K.; Gosselink, R.J.A.; Smeds, A.I.; Fardim, P. Characterization of lignin extracted from birch wood by a modified hydrotropic process. J. Agric. Food Chem. 2014, 62, 10759–10767. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, P.P.; Lange, H.; Crestini, C.; Rova, U.; Matsakas, L.; Christakopoulos, P. Characterization of organosolv birch lignins: Toward application-specific lignin production. ACS Omega 2021, 6, 4374–4385. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Chudina, A.I.; Garyntseva, N.V.; Kazachenko, A.S.; Skripnikov, A.M.; Malyar, Y.N.; Ivanov, I.P. Fractionation of birch wood biomass into valuable chemicals by the extraction and catalytic processes. Biomass Conv. Bioref. 2022, 1–15. [Google Scholar] [CrossRef]
- Akman, F.; Kazachenko, A.S.; Vasilyeva, N.Y.; Malyar, Y.N. Synthesis and characterization of starch sulfates obtained by the sulfamic acid-urea complex. J. Mol. Struct. 2020, 1208, 127899. [Google Scholar] [CrossRef]
- Levdansky, A.V.; Vasilyeva, N.Y.; Kondrasenko, A.A.; Levdansky, V.A.; Malyar, Y.N.; Kazachenko, A.S.; Kuznetsov, B.N. Sulfation of arabinogalactan with sulfamic acid under homogeneous conditions in dimethylsulfoxide medium. Wood Sci. Technol. 2021, 55, 1725–1744. [Google Scholar] [CrossRef] [PubMed]
- Karger, J.; Grinberg, F.; Heitjans, P. Diffusion Fundamentals; Leipziger University: Leipzig, Germany, 2005. [Google Scholar]
- Lente, G. Deterministic Kinetics in Chemistry and Systems Biology; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Faix, O. Classification of lignin from different botanical origins by FTIR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- You, T.-T.; Mao, J.-Z.; Yuan, T.-Q.; Wen, J.-L.; Xu, F. Structural elucidation of the lignins from stems and foliage of Arundo donax Linn. J. Agric. Food Chem. 2013, 61, 5361–5370. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Structural elucidation of lignin polymers of Eucalyptus chips during organosolv pretreatment and extended delignification. J. Agric. Food Chem. 2013, 61, 11067–11075. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Sorek, H.; Mitchell, V.D.; Ibáñez, A.B.; Wemmer, D.E. Characterization of Miscanthus giganteus lignin isolated by ethanol organosolv process under reflux condition. J. Agric. Food Chem. 2012, 60, 8203–8212. [Google Scholar] [CrossRef] [PubMed]
- Lagerquist, L.; Pranovich, A.; Smeds, A.; von Schoultz, S.; Vähäsalo, L.; Rahkila, J.; Kilpeläinen, I.; Tamminend, T.; Willför, S.; Eklund, P. Structural characterization of birch lignin isolated from a pressurized hot water extraction and mild alkali pulped biorefinery process. Ind. Crops Prod. 2018, 111, 306–316. [Google Scholar] [CrossRef]
- Rencoret, J.; Marques, G.; Gutiérrez, A.; Ibarra, D.; Li, J.; Gellerstedt, G.; Santos, J.I.; Jiménez-Barbero, J.; Martínez, A.T.; del Río, J.C. Structural characterization of milled wood lifnins from different eucalypt species. Holzforschung 2008, 62, 514–526. [Google Scholar] [CrossRef]
- del Río, J.C.; Rencoret, J.; Marques, G.; Li, J.; Gellerstedt, G.; Jiménez-Barbero, J.; Martínez, Á.T.; Gutiérrez, A. Structural characterization of the lignin from jute (Corchorus capsularis) fibers. J. Agric. Food Chem. 2009, 57, 10271–10281. [Google Scholar] [CrossRef]
- Kangas, H.; Liitiä, T.; Rovio, S.; Ohra-aho, T.; Heikkinen, H.; Tamminen, T.; Poppius-Levlin, K. Characterization of dissolved lignins from acetic acid Lignofibre (LGF) organosolv pulping and discussion of its delignification mechanisms. Holzforschung 2014, 69, 247–256. [Google Scholar] [CrossRef]
- Lu, F.; Karlen, S.D.; Regner, M.; Kim, H.; Ralph, S.A.; Sun, R.-C.; Kuroda, K.-i.; Augustin, M.A.; Mawson, R.; Sabarez, H.; et al. Naturally p-hydroxybenzoylated lignins in palms. BioEnergy Res. 2015, 8, 934–952. [Google Scholar] [CrossRef]
- Ragan, M.A. Phenol sulfate esters: Ultraviolet, infrared, 1H and 13C nuclear magnetic resonance spectroscopic investigation. Can. J. Chem. 1978, 56, 2681–2685. [Google Scholar] [CrossRef]
- Rencoret, J.; Marques, G.; Gutiérrez, A.; Nieto, L.; Santos, J.I.; Jiménez-Barbero, J.; Martínez, A.T.; del Río, J.C. HSQC-NMR analysis of lignin in woody (Eucalyptus globulus and Picea abies) and non-woody (Agave sisalana) ball-milled plant materials at the gel state. Holzforschung 2009, 63, 691–698. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Chesnokov, N.V.; Sudakova, I.G.; Garyntseva, N.V.; Kuznetsova, S.A.; Malyar, Y.N.; Yakovlev, V.A.; Djakovitch, L. Green catalytic processing of native and organosolv lignins. Catal. Today 2018, 309, 18–30. [Google Scholar] [CrossRef]
- Fetisova, O.Y.; Mikova, N.M.; Chesnokov, N.V. A kinetic study of the thermal degradation of fir and aspen ethanol lignins. Kinet. Catal. 2019, 60, 273–280. [Google Scholar] [CrossRef]
- Moustaqim, M.E.; Kaihal, A.E.; Marouani, M.E.; Men-La-Yakhaf, S.; Taibi, M.; Sebbahi, S.; Hajjaji, S.E.; Kifani-Sahban, F. Thermal and thermomechanical analyses of lignin. Sustain. Chem. Pharm. 2018, 9, 63–68. [Google Scholar] [CrossRef]
- Poletto, M. Assessment of the thermal behavior of lignins from softwood and hardwood species. Maderas Cienc. Tecnol. 2017, 19, 63–74. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrol. 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Nakamura, T.; Kawamoto, H.; Saka, S. Condensation reactions of some lignin related compounds at relatively low pyrolysis temperature. J. Wood Chem. Technol. 2007, 27, 121–133. [Google Scholar] [CrossRef]
- Ji, X.; Guo, M.; Zhu, L.; Du, W.; Wang, H. Synthesis mechanism of an environment-friendly sodium lignosulfonate/chitosan medium-density fiberboard adhesive and response of bonding performance to synthesis mechanism. Materials 2020, 13, 5697. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Malyar, Y.N.; Kazachenko, A.S.; Vasilyeva, N.Y.; Fetisova, O.Y.; Borovkova, V.S.; Miroshnikova, A.V.; Levdansky, A.V.; Skripnikov, A.M. Sulfation of wheat straw soda lignin: Role of solvents and catalysts. Catal. Today 2022, 397–399, 397–406. [Google Scholar] [CrossRef]
- Henry, B.L.; Desai, U.R. Sulfated low molecular weight lignins, allosteric inhibitors of coagulation proteinases via the heparin binding site, significantly alter the active site of thrombin and factor xa compared to heparin. Thromb. Res. 2014, 134, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
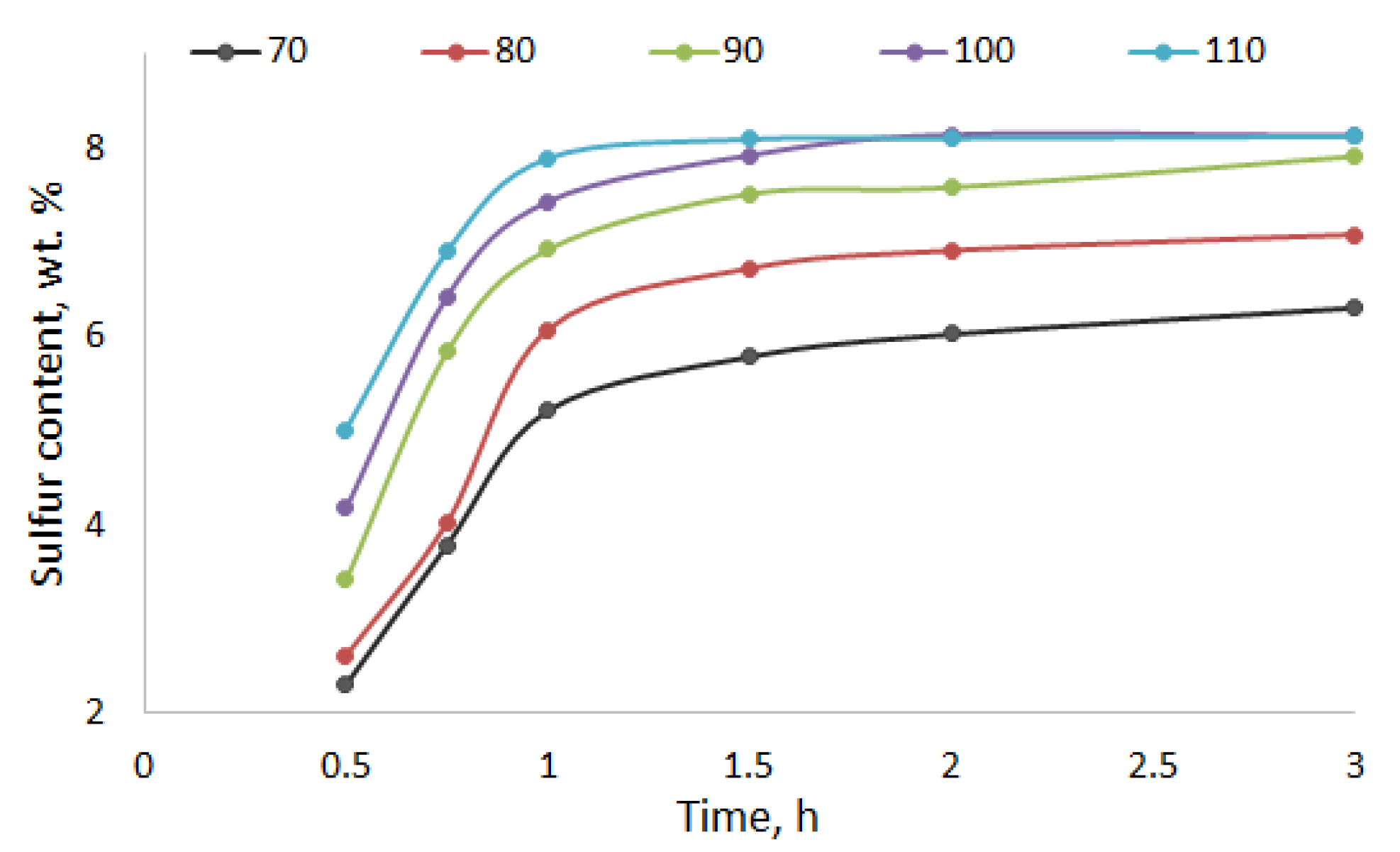


| No. | L:SC, mol/mol | Temperature, °C | Time, min | Sulfur Content, wt % | Yield, wt % |
|---|---|---|---|---|---|
| 1 | 1:3 | 70 | 30 | 2.31 ± 0.02 | * |
| 2 | 1:3 | 70 | 45 | 3.79 ± 0.02 | * |
| 3 | 1:3 | 70 | 60 | 5.22 ± 0.03 | 94.35 ± 4.04 |
| 4 | 1:3 | 70 | 90 | 5.79 ± 0.03 | 94.95 ± 3.96 |
| 5 | 1:3 | 70 | 120 | 6.03 ± 0.03 | 95.56 ± 3.93 |
| 6 | 1:3 | 70 | 180 | 6.31 ± 0.04 | 95.14 ± 3.88 |
| 7 | 1:3 | 80 | 30 | 2.62 ± 0.02 | * |
| 8 | 1:3 | 80 | 45 | 4.03 ± 0.03 | * |
| 9 | 1:3 | 80 | 60 | 6.08 ± 0.04 | 93.90 ± 3.91 |
| 10 | 1:3 | 80 | 90 | 6.73 ± 0.03 | 94.99 ± 3.83 |
| 11 | 1:3 | 80 | 120 | 6.92 ± 0.05 | 95.53 ± 3.80 |
| 12 | 1:3 | 80 | 180 | 7.09 ± 0.03 | 96.06 ± 3.77 |
| 13 | 1:3 | 90 | 30 | 3.42 ± 0.02 | * |
| 14 | 1:3 | 90 | 45 | 5.84 ± 0.04 | 95.61 ± 3.96 |
| 15 | 1:3 | 90 | 60 | 6.93 ± 0.05 | 96.17 ± 3.80 |
| 16 | 1:3 | 90 | 90 | 7.52 ± 0.05 | 96.43 ± 3.71 |
| 17 | 1:3 | 90 | 120 | 7.59 ± 0.05 | 94.82 ± 3.69 |
| 18 | 1:3 | 90 | 180 | 7.92 ± 0.05 | 94.31 ± 3.65 |
| 19 | 1:3 | 100 | 30 | 4.18 ± 0.03 | * |
| 20 | 1:3 | 100 | 45 | 6.43 ± 0.04 | 93.50 ± 3.87 |
| 21 | 1:3 | 100 | 60 | 7.44 ± 0.05 | 93.08 ± 3.72 |
| 22 | 1:3 | 100 | 90 | 7.93 ± 0.05 | 92.48 ± 3.65 |
| 23 | 1:3 | 100 | 120 | 8.15 ± 0.05 | 91.74 ± 3.62 |
| 24 | 1:3 | 100 | 180 | 8.14 ± 0.05 | 91.14 ± 4.07 |
| 25 | 1:3 | 110 | 30 | 5.02 ± 0.03 | 92.31 ± 3.93 |
| 26 | 1:3 | 110 | 45 | 6.91 ± 0.04 | 91.74 ± 3.80 |
| 27 | 1:3 | 110 | 60 | 7.90 ± 0.05 | 90.66 ± 3.65 |
| 28 | 1:3 | 110 | 90 | 8.11 ± 0.05 | 89.93 ± 3.62 |
| 29 | 1:3 | 110 | 120 | 8.10 ± 0.05 | 88.73 ± 3.62 |
| 30 | 1:3 | 110 | 180 | 8.13 ± 0.05 | 85.71 ± 3.62 |
| Temperature | Apparent Initial Rate Constant, K × 10−4 (s−1) | Activation Energy, kJ/mol |
|---|---|---|
| 70 | 1.41 | 10.7 |
| 80 | 1.80 | |
| 90 | 2.05 | |
| 100 | 2.78 |
| Factors and Parameters | Designations in the Equations |
|---|---|
| Temperature, °C | X1 |
| Time, h | X2 |
| Sulfur content, % | Y1 |
| Product yield, % | Y2 |
| Variance Source | Output Parameters | |||
|---|---|---|---|---|
| Sulfur Content Y1 | Yield Y2 | |||
| Statistical Characteristics | ||||
| Variance Relation F | Significance Level P | Variance Relation F | Significance Level P | |
| X1 | 78.74 | 0.0000 | 200.98 | 0.0000 |
| X2 | 24.87 | 0.0001 | 10.88 | 0.0042 |
| X12 | 9.84 | 0.0060 | 59.66 | 0.0000 |
| X1X2 | 0.00 | 0.9757 | 36.11 | 0.0000 |
| X22 | 21.54 | 0.0002 | 0.26 | 0.6184 |
| R2adj | 86.1 | 92.8 | ||
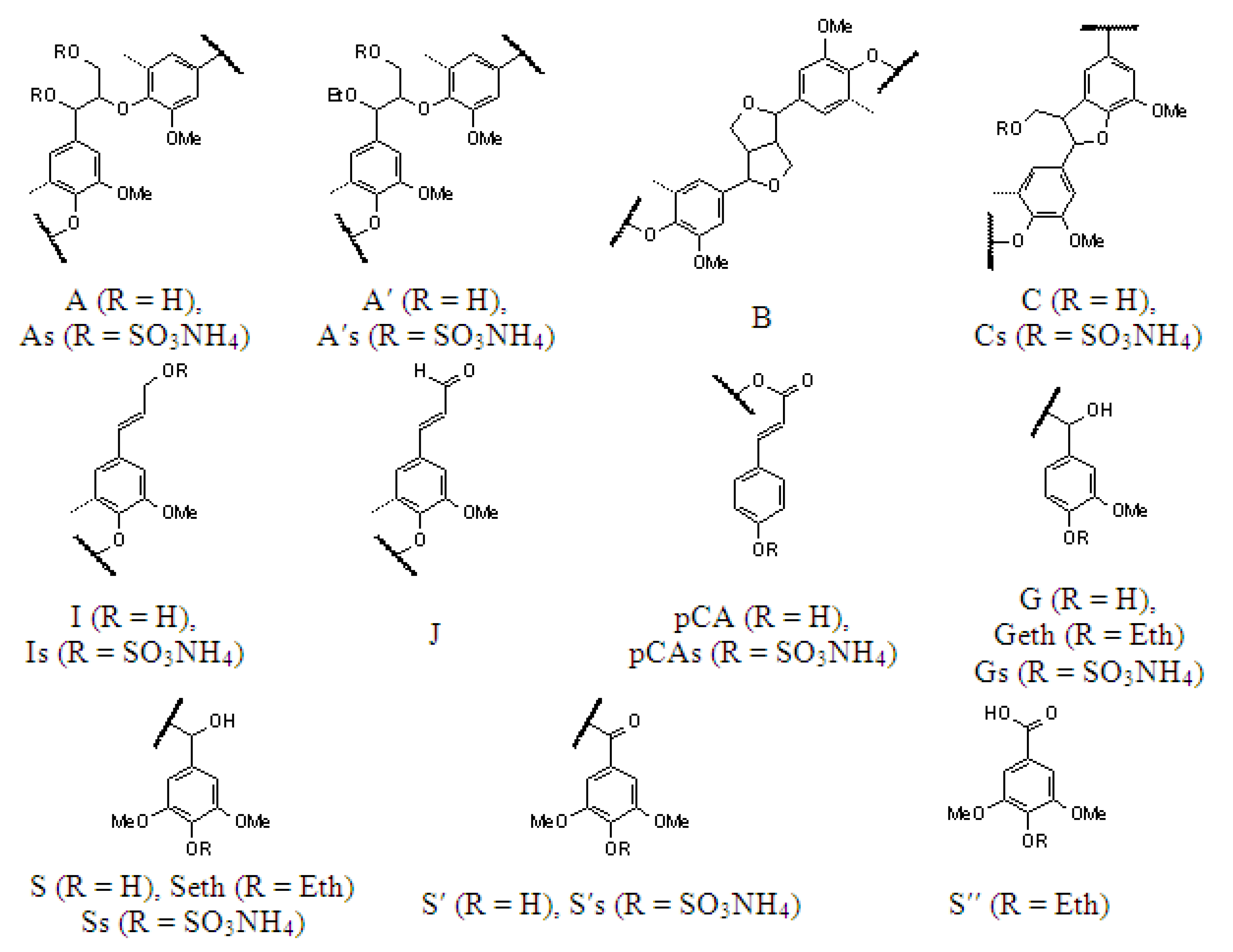
| Designation | δC/δH, ppm (Initial Lignin) | δC/δH, ppm (Sulfated Lignin) | Assignment |
|---|---|---|---|
| OMe | 56.3/3.74 | 56.3/3.76 | C-H in the methoxy groups (OMe) |
| Aγ and A′γ | 60.0/3.42−3.74 | - | Cγ-Hγ in the β-aryl ether (β-O-4′) substructures (A) and α-ethoxylated (CαOEt) β-aryl ether (β-O-4′) substructures (A′) |
| Aγs and A′γs | - | 64.6/3.51 and 3.83 | Cγ-Hγ in the γ-sulfated (γ-OSO3NH4) β-aryl ether (β-O-4′) substructures (As) and γ-sulfated (γ-OSO3NH4) α-ethoxylated (CαOEt) β-aryl ether (β-O-4′) substructures (A′s) |
| Aβ(G) and A′β(G) | 84.1/4.30 and 83.4/4.39 | - | Cβ-Hβ in the β-aryl ether (β-O-4′) substructures bonded to the G units (A) and α-ethoxylated (CαOEt) β-aryl ether (β-O-4′) substructures bonded to the G units (A′) |
| Aβ(S) and A′β(S) | 86.5/4.12 and 84.8/4.27 | - | Cβ-Hβ in the β-aryl ether (β-O-4′) substructures bonded to the S units (A) and α-ethoxylated (CαOEt) β-aryl ether (β-O-4′) substructures boded to the S units (A′) |
| Aβ(G)s and A′β(G)s | - | 80.7/4.50 and 80.0/4.64 | Cβ-Hβ in the γ-sulfated (γ-OSO3NH4) β-aryl ether (β-O-4′) substructures bonded to the G units (As) and γ-sulfated (γ-OSO3NH4) α-ethoxylated (CαOEt) β-aryl ether (β-O-4′) substructures bonded to the G units (A′s) |
| Aβ(S)s and A′β(S)s | - | 82.1/4.35 and 82.1/4.50 | Cβ-Hβ in the γ-sulfated (γ-OSO3NH4) β-aryl ether (β-O-4′) substructures boded to the S units (As) and γ-sulfated (γ-OSO3NH4) α-ethoxylated (CαOEt) β-aryl ether (β-O-4′) substructures boded to the S units (A′s) |
| Aα | 72.5/4.88 | - | Cα-Hα in the β-aryl ether (β-O-4′) substructures (A) |
| A′αOEt | 64.4/3.33 | 64.7/3.36 | C-H of the methylene groups in the α-ethoxylated (CαOEt) β-aryl ether (β-O-4′) substructures (A′) |
| A′α | 81.2/4.56 | 81.1/4.53 | Cα-Hα in the α-ethoxylated (CαOEt) β-aryl ether (β-O-4′) substructures (A′) |
| Bβ | 54.1/3.06 | 55.1/3.06 | Cβ-Hβ in the pinoresinol (β–β′) substructures (B) |
| Bγ | 71.6/3.80 and 4.18 | - | Cγ-Hγ in the pinoresinol (β–β′) substructures (B) |
| Bα | 85.6/4.68 | 85.9/4.68 | Cα-Hα in the pinoresinol (β–β′) substructures (B) |
| Cβ | 53.7/3.48 | - | Cβ-Hβ in the phenylcoumaran (β–5′) moieties (C) |
| Cβs | - | 51.1/3.65 | Cβ-Hβ in the γ-sulfated (γ-OSO3NH4) phenyl coumaran (β–5′) moieties (Cs) |
| Cγ | 63.4/3.74 | - | Cγ-Hγ in the phenyl coumaran (β–5′) substructures (C) |
| Cγs | - | 67.5/3.94 | Cγ-Hγ in the γ-sulfated (γ-OSO3NH4) phenylcoumaran (β–5′) substructures (Cs) |
| Cα | 87.6/5.45 | 87.5/5.55 | Cα-Hα in the phenylcoumaran (β–5′) substructures (Cα) |
| Iγ | 60.4/4.03 | 59.9/4.03 | Cγ-Hγ in the cinnamyl alcohol end groups (I) |
| Iγs | - | 64.6/4.15 | Cγ-Hγ in the γ-sulfated (γ-OSO3NH4) cinnamyl alcohol end groups (Is) |
| J2,6(S) | 107.0/7.08 | 106.4/7.00 | C2,6-H2,6 in the cinnamyl aldehyde end groups (J) |
| S2,6eth | 104.5/6.70 | 104.8/6.62 | C2,6-H2,6 in the 4-etherified syringyl units (Seth) |
| S2,6 | 106.2/6.51 | - | C2,6-H2,6 in the 4-non-etherified syringyl units (S) |
| S′2,6 | 107.1/7.35 | 106.7/7.30 | C2,6-H2,6 in the oxidized (Cα=O) syringyl units (S′) |
| S″2,6 | 107.2/7.23 | 106.5/7.20 | C2,6-H2,6 in the oxidized (CαOOH) syringyl units (S″) |
| pCA3,5 | 115.8/6.79 | 115.5/6.79 | C3,5-H3,5 in the p-coumarates (pCA) |
| pCA3,5s | - | 120.8/7.32 | C3,5-H3,5 in the 4-sulfated (4-OSO3NH4) p-coumarates (pCAs) |
| G2 | 110.6/6.94 | 111.9/7.00 | C2-H2 in the 4-etherified guaiacyl units (Geth) |
| G5 | 115.7/6.97 | - | C5-H5 in the 4-non-etherified guaiacyl units (G) |
| G5s | - | 120.8/7.38 | C5-H5 in the 4-sulfated (4-OSO3NH4) guaiacyl units (Gs) |
| G5eth | 115.7/6.76 | 115.4/6.76 | C5-H5 in the 4-etherified guaiacyl units (Geth) |
| G6 | 119.4/6.79 | 119.5/6.76 | C6-H6 in the 4-etherified guaiacyl units (Geth) |
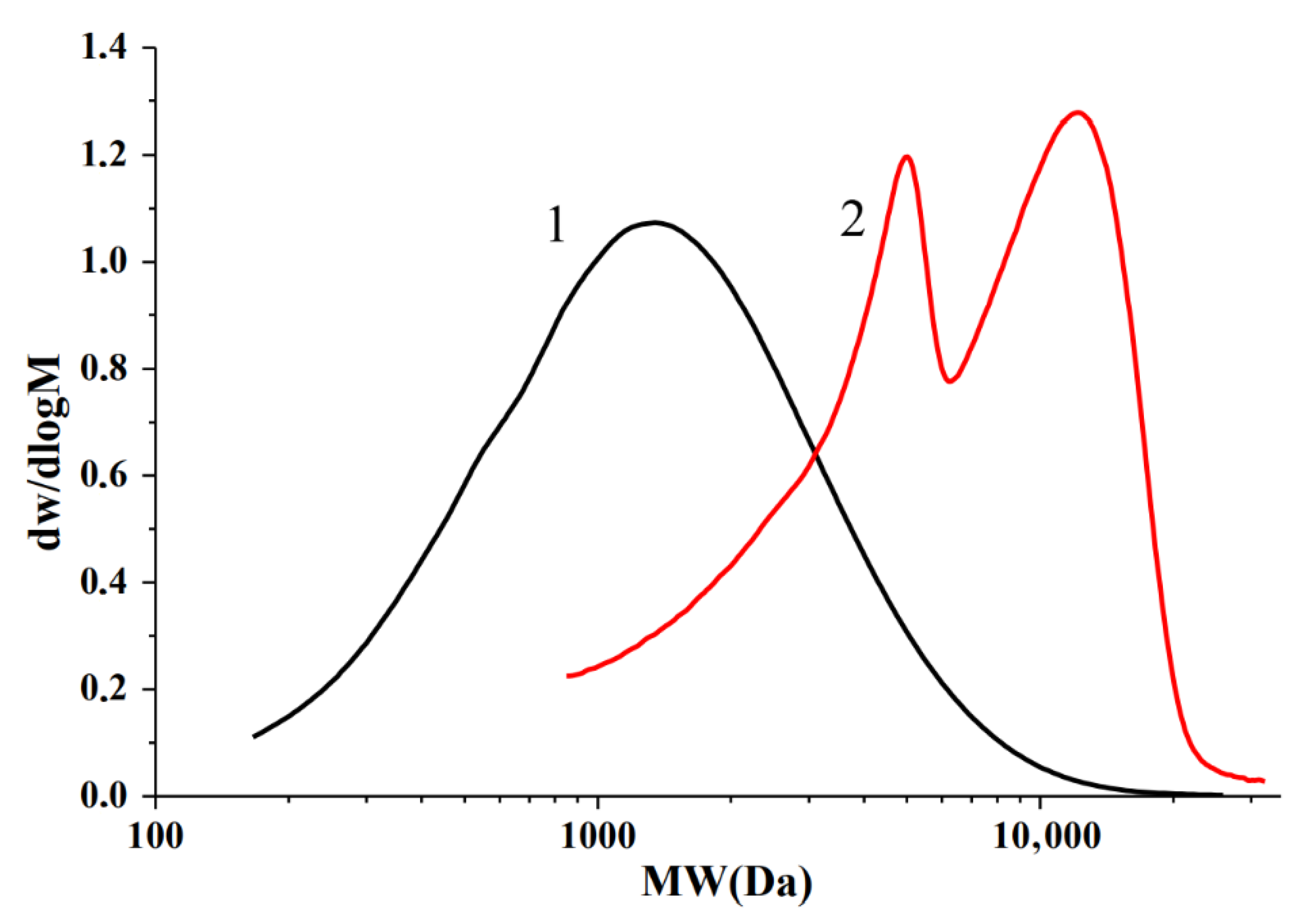
| Sample | Mn (Da) | Mw (Da) | PD |
|---|---|---|---|
| Birch ethanol lignin | 902 | 1828 | 2.02 |
| Sulfated birch ethanol lignin | 4199 | 7599 | 1.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levdansky, A.V.; Vasilyeva, N.Y.; Malyar, Y.N.; Kondrasenko, A.A.; Fetisova, O.Y.; Kazachenko, A.S.; Levdansky, V.A.; Kuznetsov, B.N. An Efficient Method of Birch Ethanol Lignin Sulfation with a Sulfaic Acid-Urea Mixture. Molecules 2022, 27, 6356. https://doi.org/10.3390/molecules27196356
Levdansky AV, Vasilyeva NY, Malyar YN, Kondrasenko AA, Fetisova OY, Kazachenko AS, Levdansky VA, Kuznetsov BN. An Efficient Method of Birch Ethanol Lignin Sulfation with a Sulfaic Acid-Urea Mixture. Molecules. 2022; 27(19):6356. https://doi.org/10.3390/molecules27196356
Chicago/Turabian StyleLevdansky, Alexander V., Natalya Yu. Vasilyeva, Yuriy N. Malyar, Alexander A. Kondrasenko, Olga Yu. Fetisova, Aleksandr S. Kazachenko, Vladimir A. Levdansky, and Boris N. Kuznetsov. 2022. "An Efficient Method of Birch Ethanol Lignin Sulfation with a Sulfaic Acid-Urea Mixture" Molecules 27, no. 19: 6356. https://doi.org/10.3390/molecules27196356
APA StyleLevdansky, A. V., Vasilyeva, N. Y., Malyar, Y. N., Kondrasenko, A. A., Fetisova, O. Y., Kazachenko, A. S., Levdansky, V. A., & Kuznetsov, B. N. (2022). An Efficient Method of Birch Ethanol Lignin Sulfation with a Sulfaic Acid-Urea Mixture. Molecules, 27(19), 6356. https://doi.org/10.3390/molecules27196356








