Synthesis, Crystal Structure, Theoretical Calculations, Antibacterial Activity, Electrochemical Behavior, and Molecular Docking of Ni(II) and Cu(II) Complexes with Pyridoxal-Semicarbazone
Abstract
1. Introduction
2. Results and Discussion
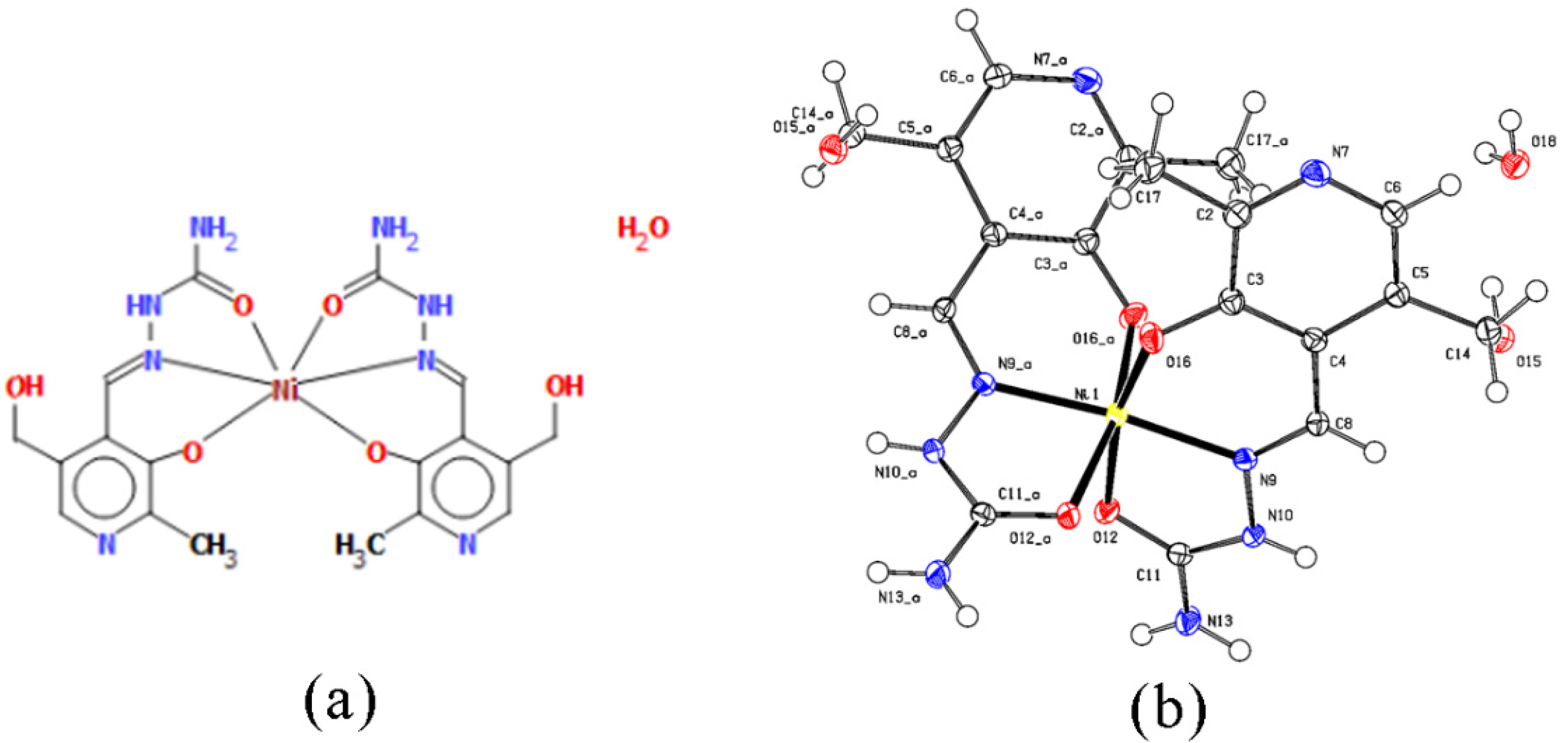
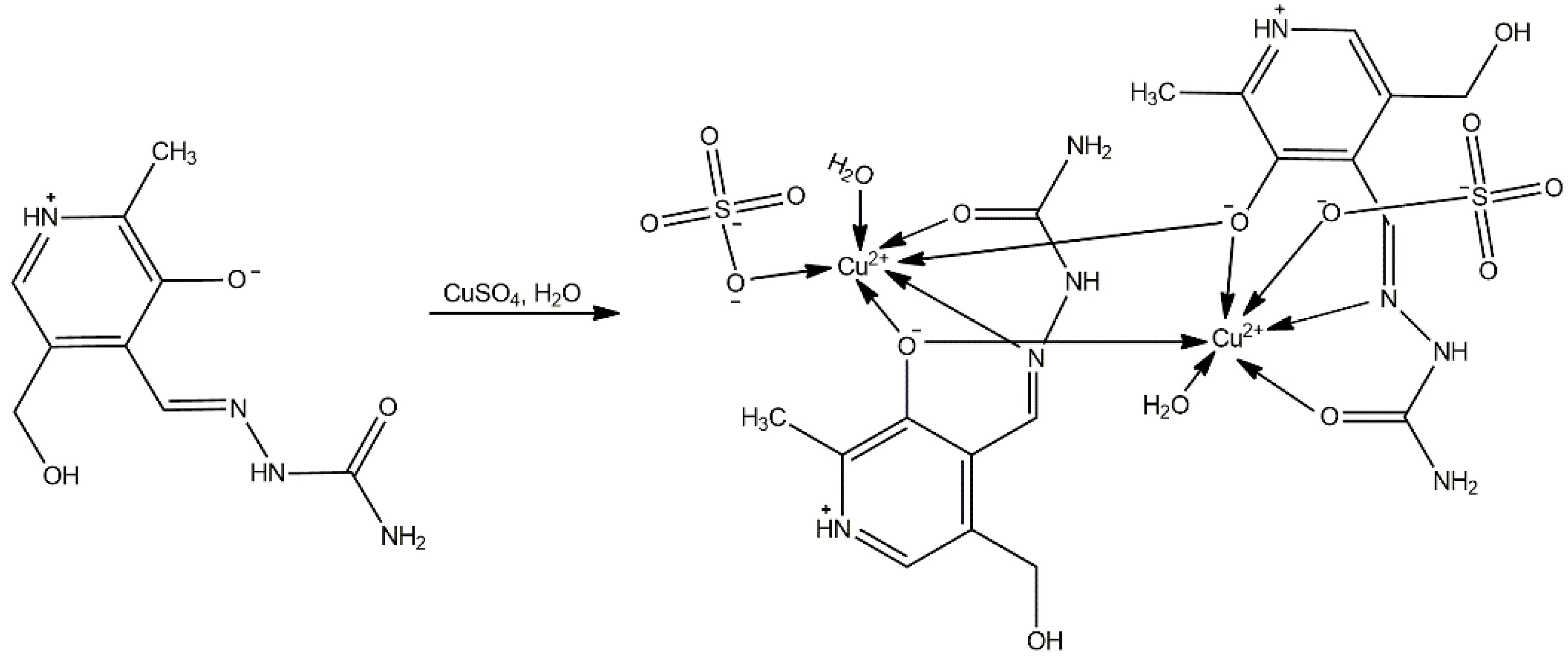
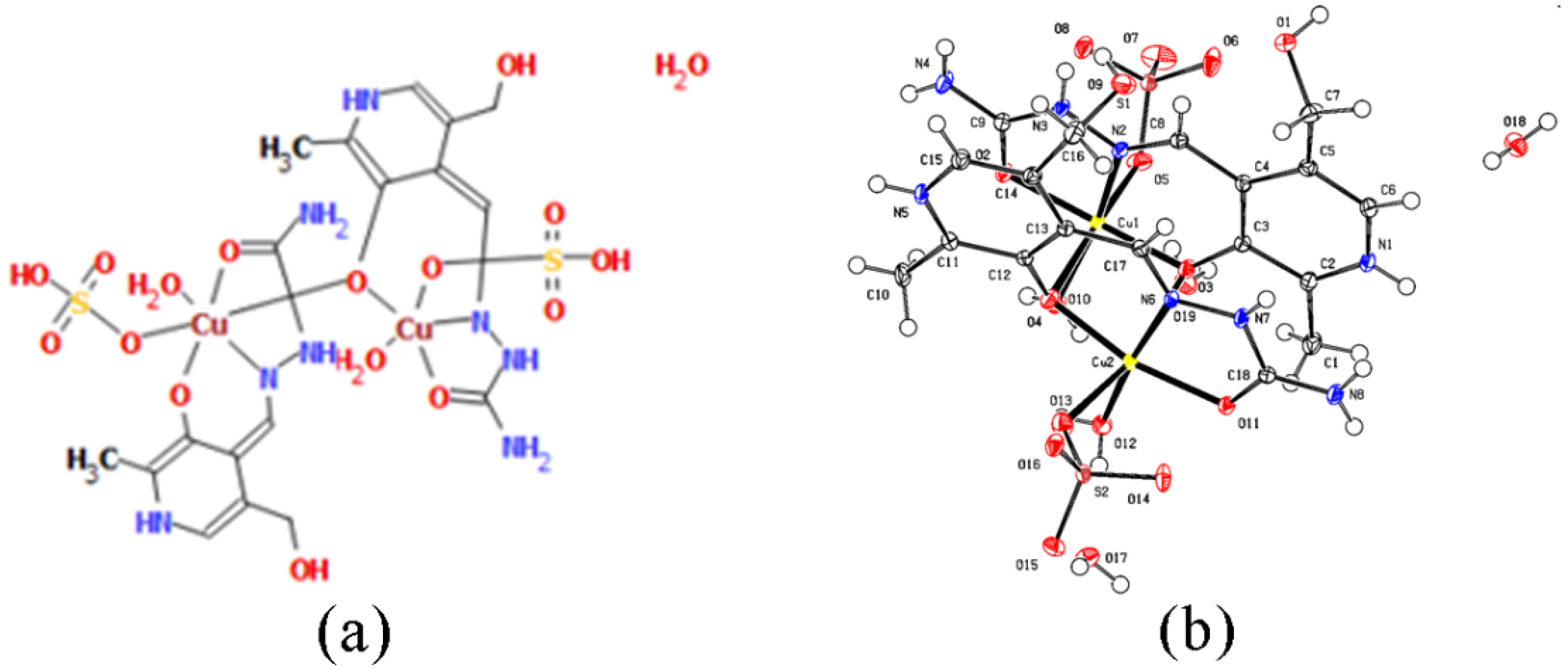
2.1. Crystal Structure
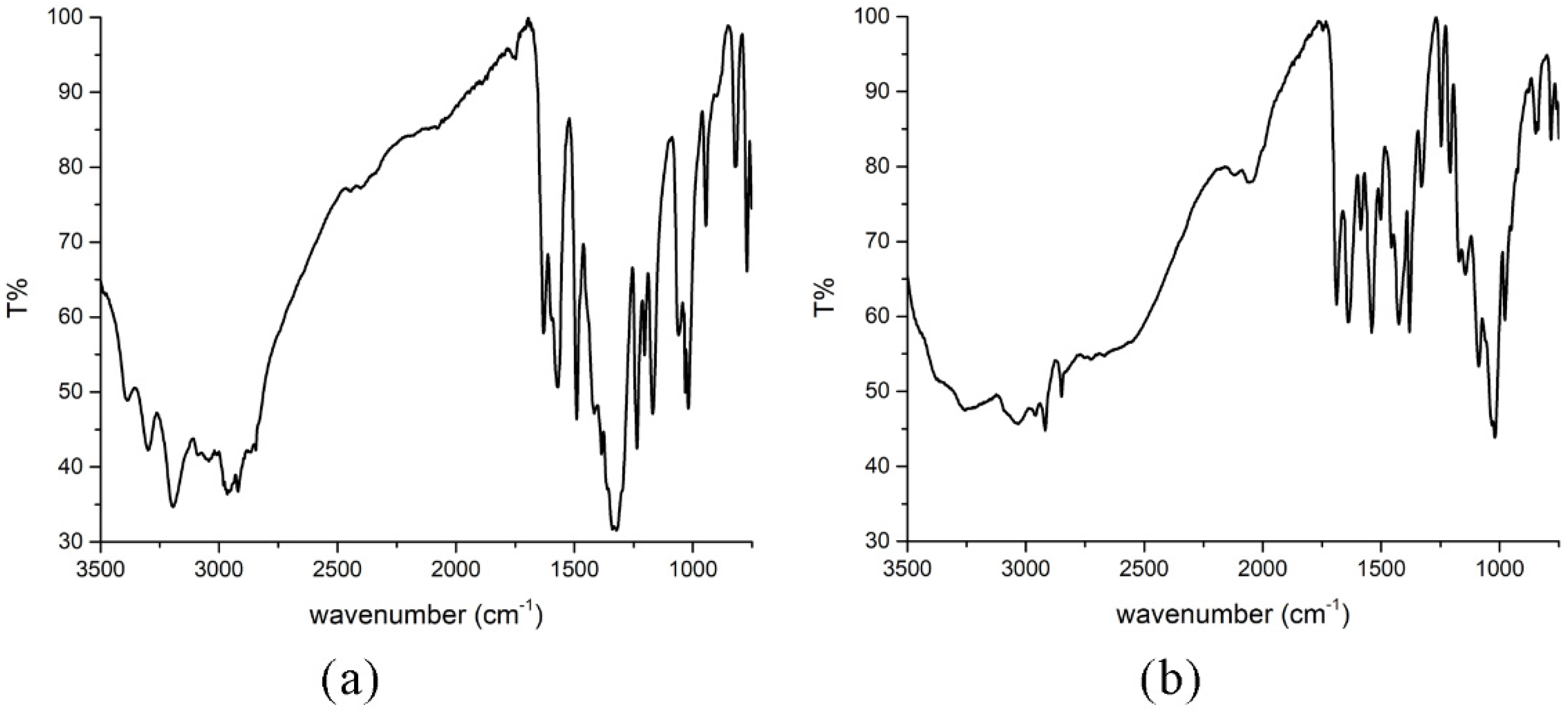
2.2. IR Spectra of Compounds 1 and 2
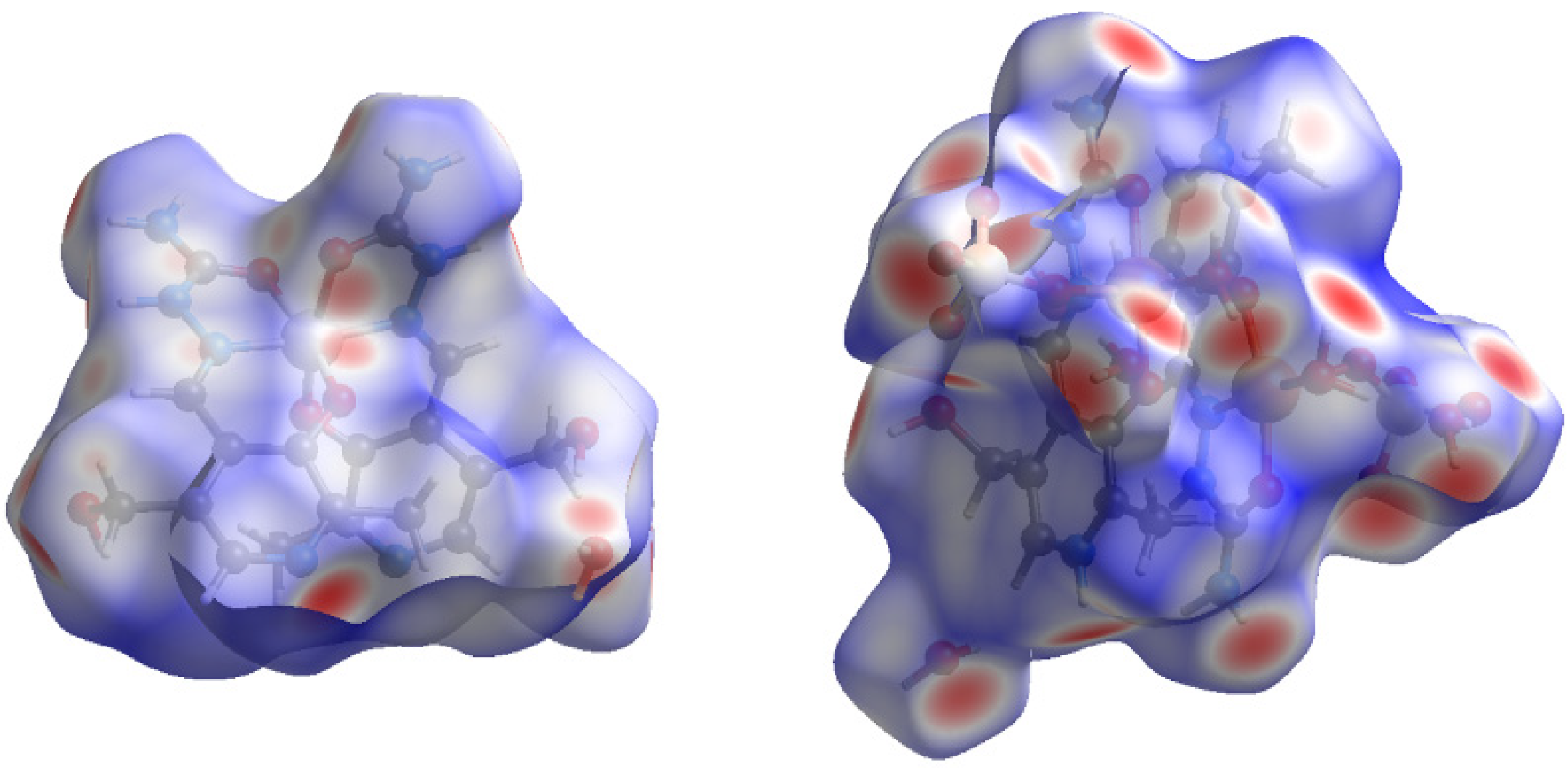
2.3. Hirshfeld Surface Analysis
2.4. Structure Optimization and NBO Analysis
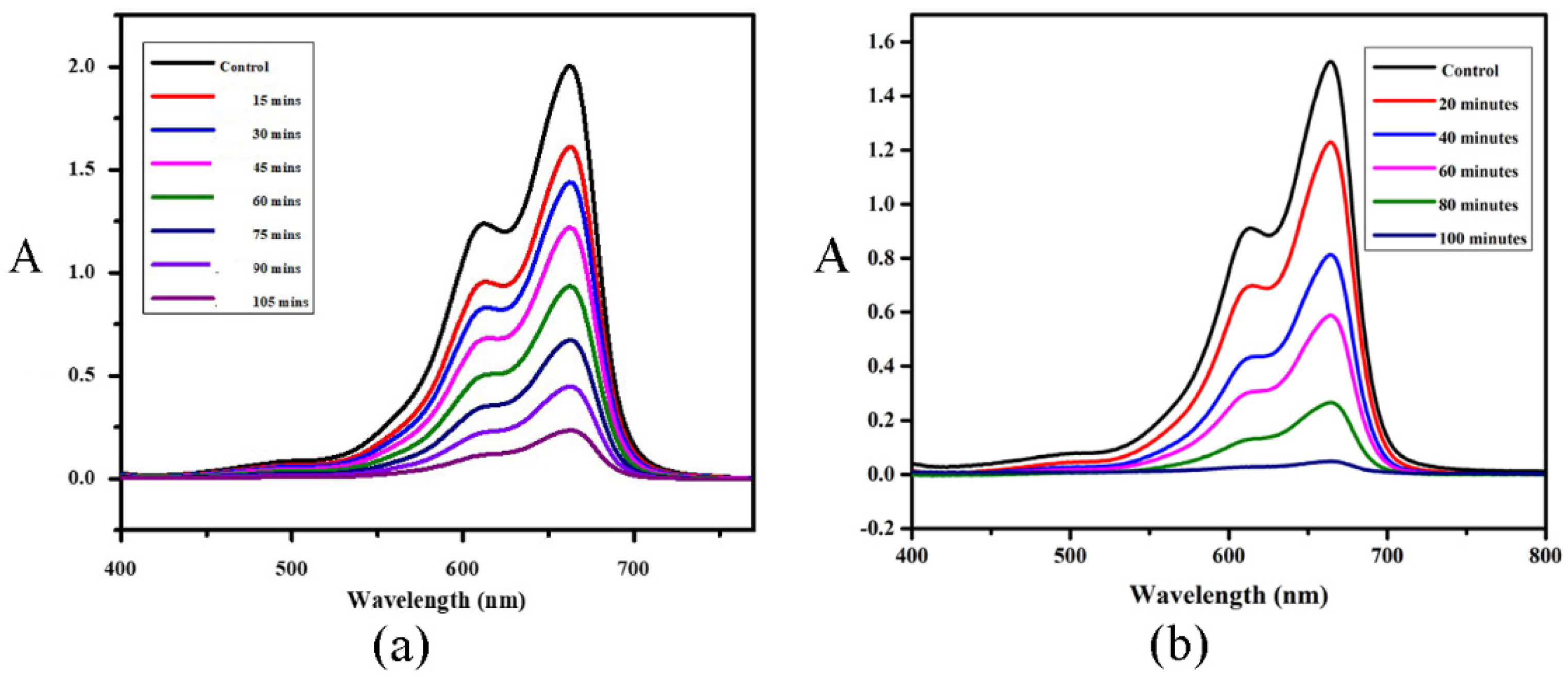
2.5. Photocatalytic Activity
2.6. Antibacterial Activity
2.7. Minimum Inhibition Concentration (MIC)
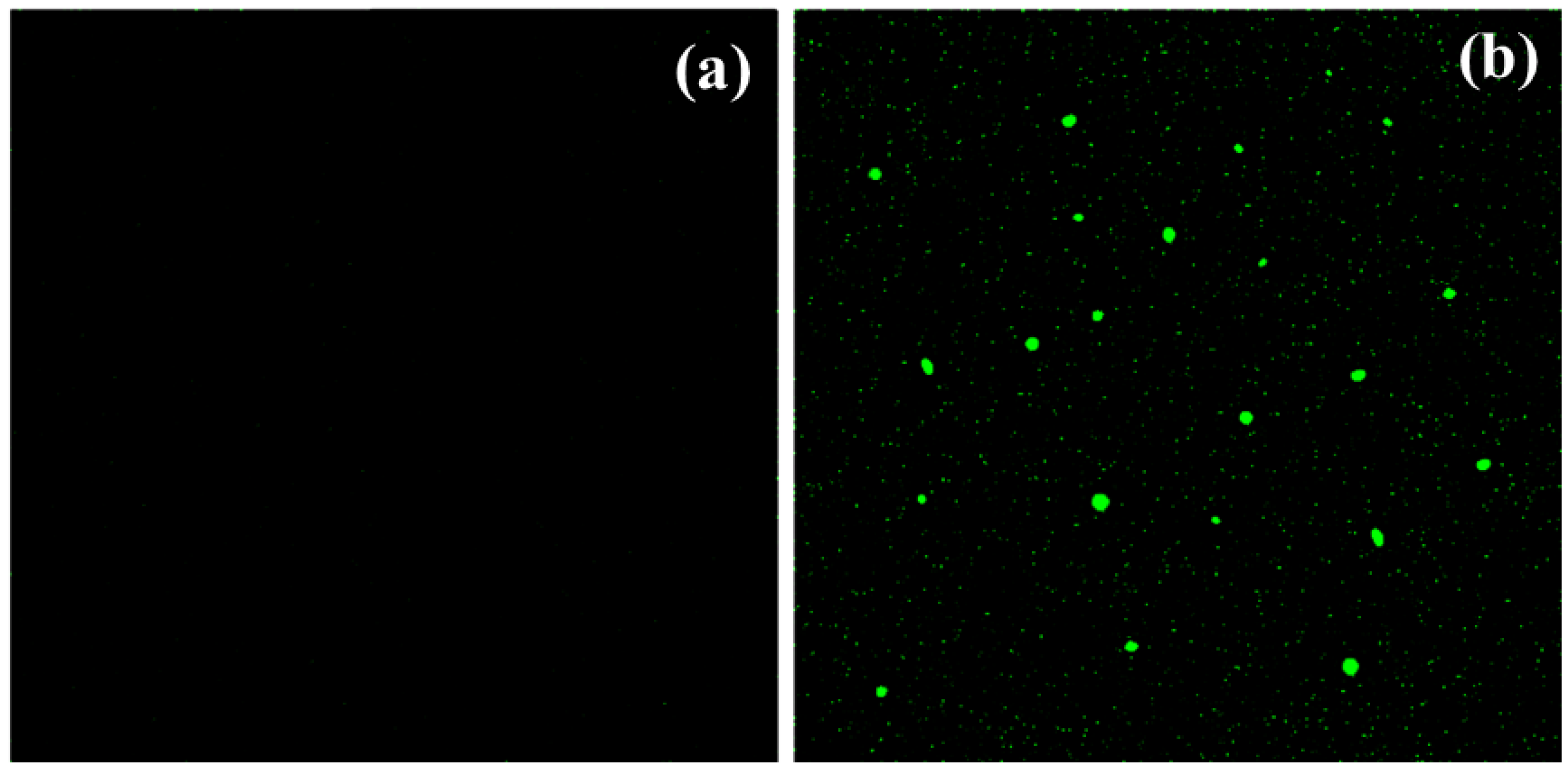
2.8. Production of Reactive Oxygen Species
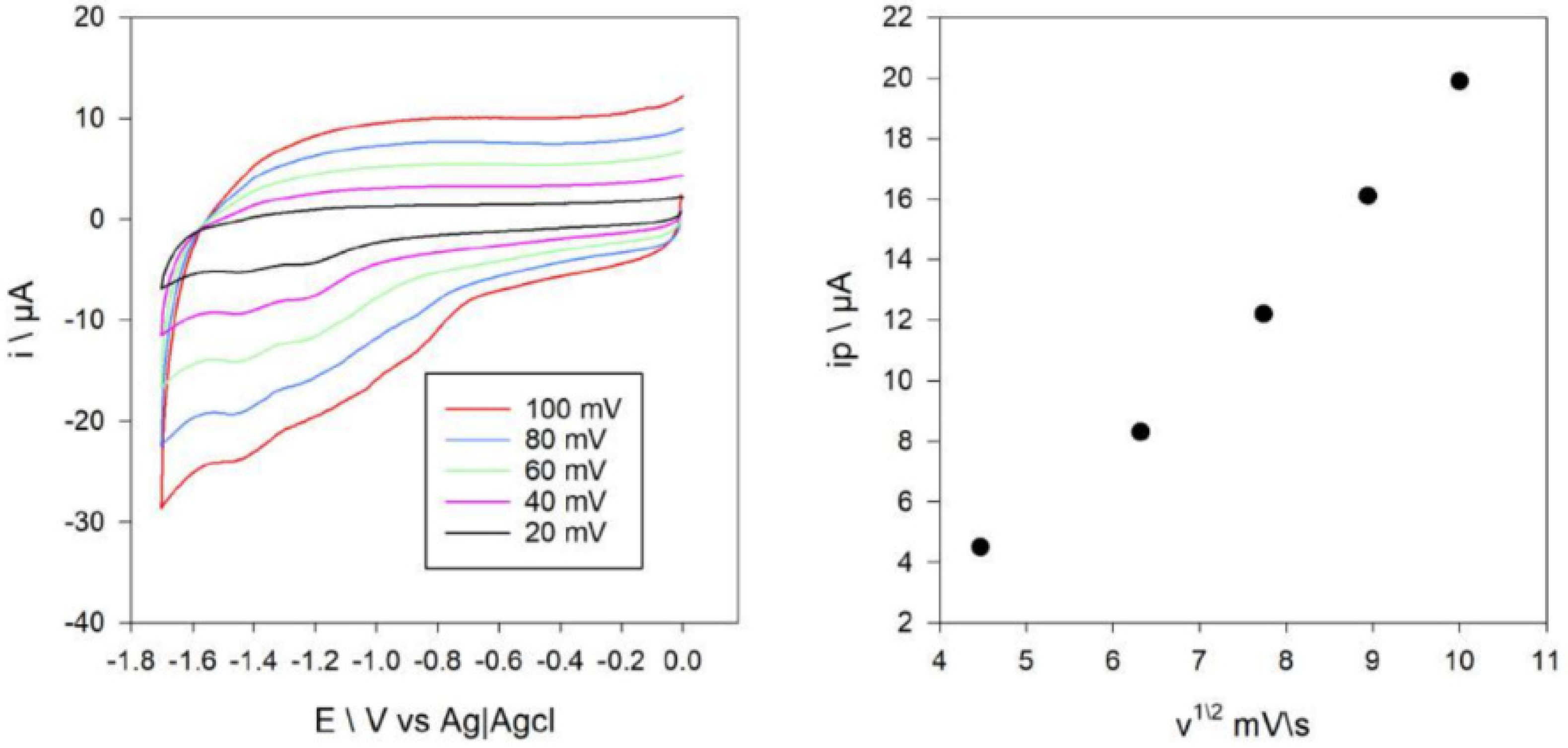
2.9. Cyclic Voltammetry of Compounds 1 and 2
2.10. Magnetic Moment Measurements and Molar Conductivity
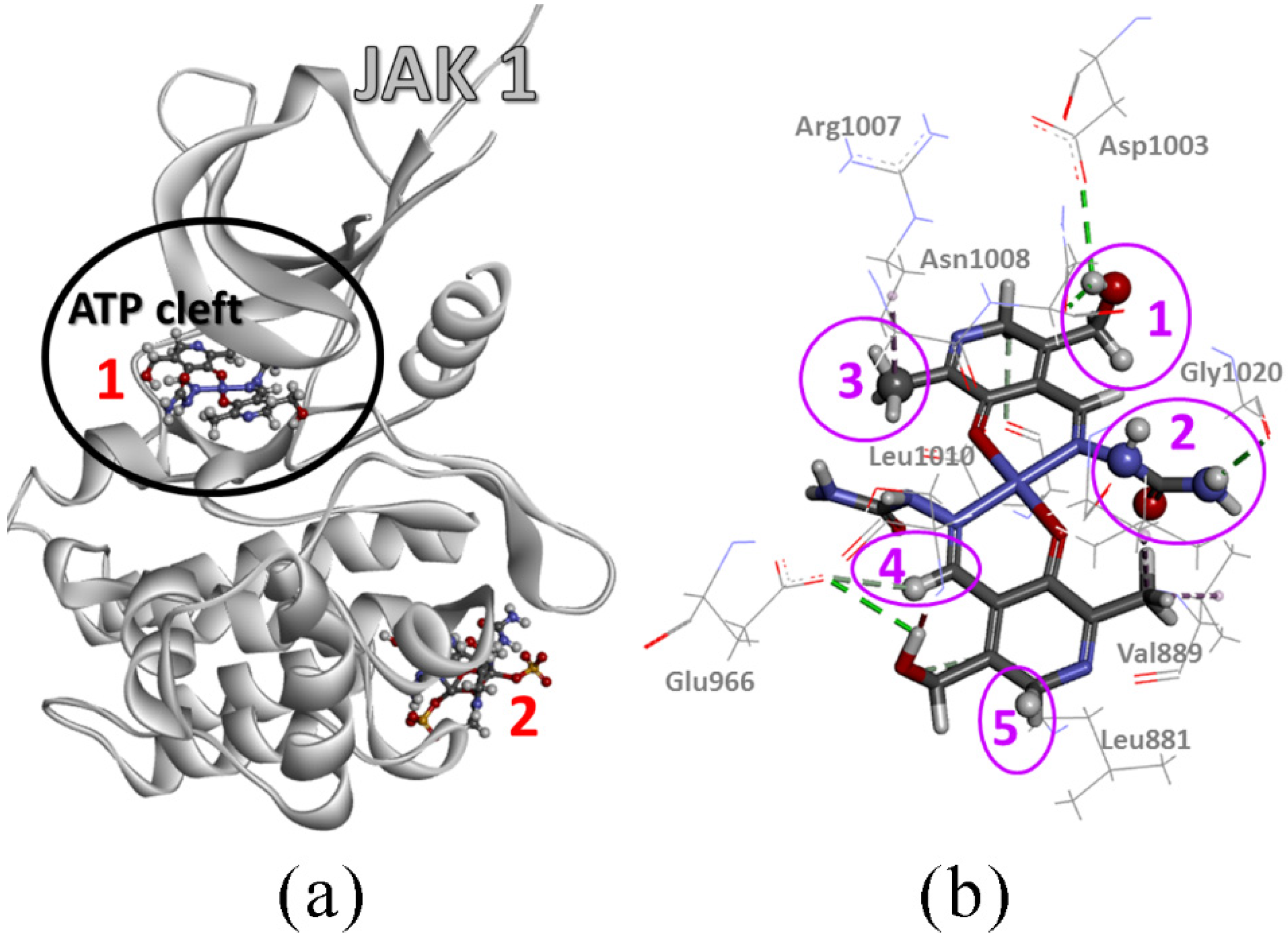
2.11. Molecular Docking
3. Materials and Methods
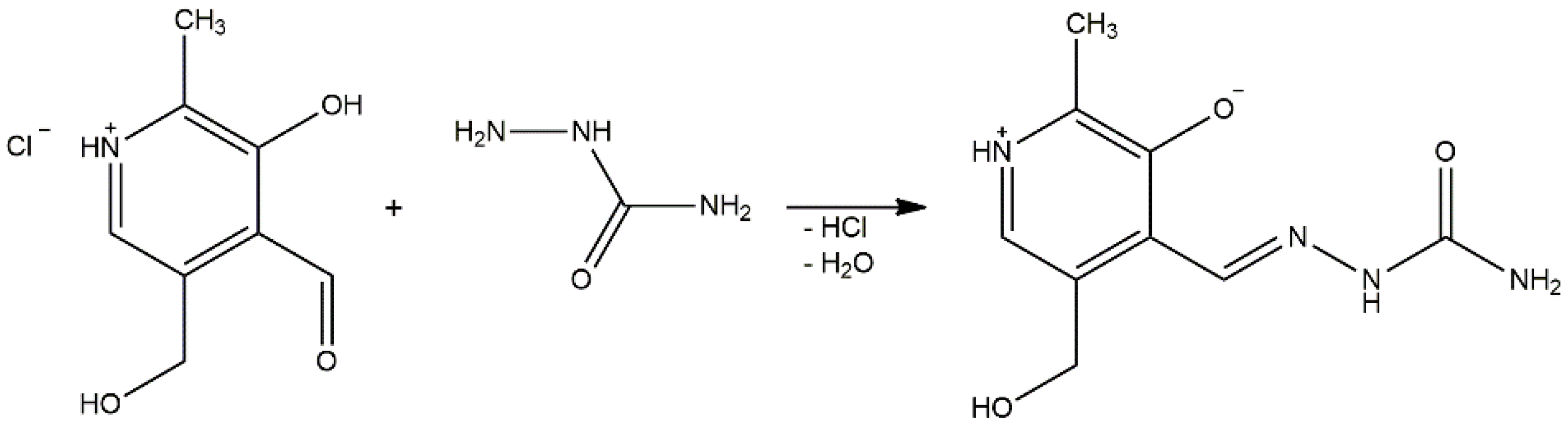
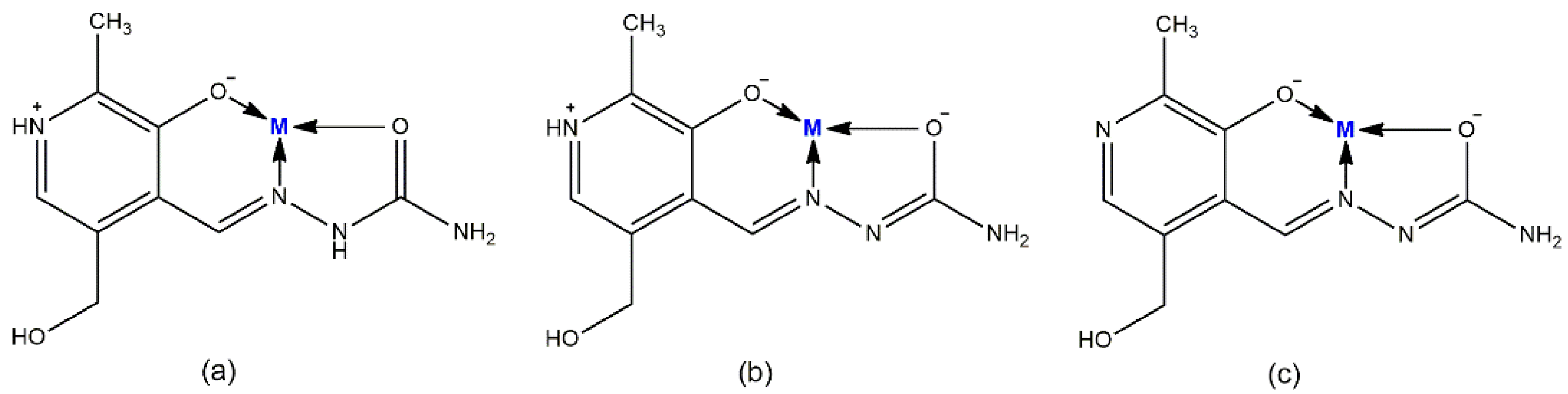
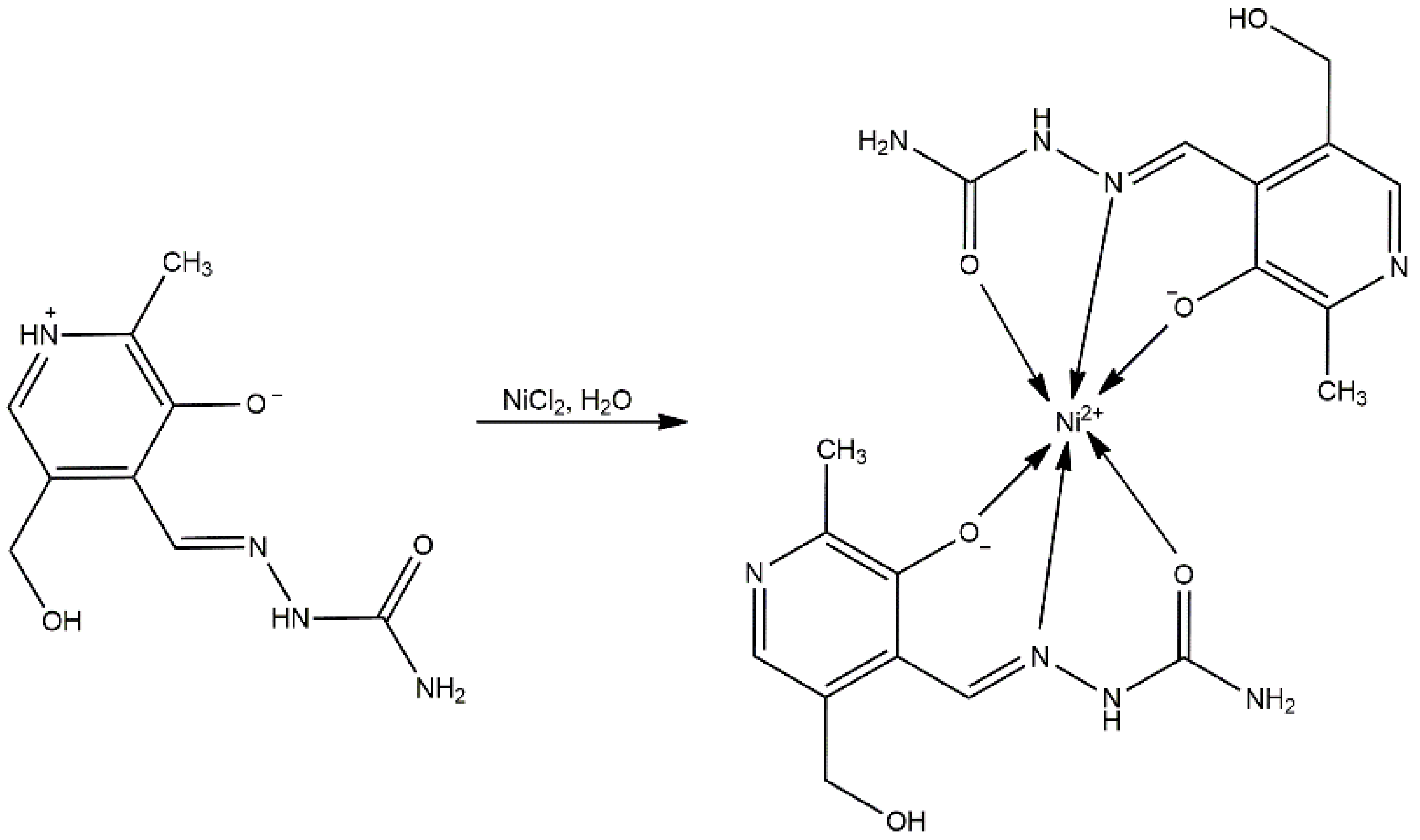
3.1. Synthesis of [Ni(PLSC-H)2]H2O
3.2. Synthesis of [Cu(PLSC)(SO4) (H2O)]2·2H2O
3.3. IR Spectroscopy, Magnetic Susceptibility, Molar Conductivity, and Elemental Analysis
3.4. X-ray Analysis
3.5. Hirshfeld Surface Analysis
3.6. Theoretical Calculations
3.7. Photocatalytic Evaluation
3.8. Antibacterial Activity of Obtained Complexes
3.9. Minimum Inhibitory Concentration (MIC)
3.10. Determination of ROS
3.11. Cyclic Voltammetry
3.12. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knežević, N.Ž.; Leovac, V.M.; Jevtović, V.S.; Grgurić-Šipka, S.; Sabo, T.J. Platinum(IV) complex with pyridoxal semicarbazone. Inorg. Chem. Commun. 2003, 6, 561–564. [Google Scholar] [CrossRef]
- Cordes, E.H.; Jencks, W.P. Semicarbazone Formation from Pyridoxal, Pyridoxal Phosphate, and Their Schiff Bases. Biochemistry 1962, 1, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, D.; Radulovic, A.; Jevtovic, V. Synthesis, characterization and structural analysis of new copper(II) complexes incorporating a pyridoxal-semicarbazone ligand. Polyhedron 2011, 30, 16–21. [Google Scholar] [CrossRef]
- Poleti, D.; Karanović, L.; Leovac, V.M.; Jevtović, V.S. Dibromo(pyridoxal semicarbazone-κ 3 N 1,O 3, O 3′ )copper(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2003, 59, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Leovac, V.M.; Jevtović, V.S.; Jovanović, L.S.; Bogdanović, G.A. Metal complexes with schiff-base ligands—Pyridoxal and semicarbazide-based derivatives. J. Serbian Chem. Soc. 2005, 70, 393–422. [Google Scholar] [CrossRef]
- Jovanović, L.S.; Jevtović, V.S.; Leovac, V.M.; Bjelica, L.J. Transition metal complexes with thiosemicarbazide-based ligands. Part 49. New complexes of iron(III) with deprotonated tridentate Schiff base—Pyridoxal derivatives. J. Serbian Chem. Soc. 2005, 70, 187–200. [Google Scholar] [CrossRef]
- Gatto, C.C.; Chagas, M.A.S.; Lima, I.J.; Mello Andrade, F.; Silva, H.D.; Abrantes, G.R.; Lacerda, E.P.S. Copper(II) complexes with pyridoxal dithiocarbazate and thiosemicarbazone ligands: Crystal structure, spectroscopic analysis and cytotoxic activity. Transit. Met. Chem. 2019, 44, 329–340. [Google Scholar] [CrossRef]
- Annaraj, B.; Neelakantan, M.A. Synthesis, crystal structure, spectral characterization and biological exploration of water soluble Cu(II) complexes of vitamin B6 derivative. Eur. J. Med. Chem. 2015, 102, 1–8. [Google Scholar] [CrossRef]
- Jevtovic, V. Syntheses, characterisation and structural analysis of new cobalt (II, III) complexes incorporating a pyridoxal-semicarbazone ligand. Int. J. Innov. Sci. Eng. Technol. 2015, 2, 591–597. [Google Scholar]
- Jevtovic, V.; Cvetkovic, D.; Vidovic, D. Synthesis, X-ray characterization and antimicrobial activity of iron(II) and cobalt(III) complexes with the schiff base derived from pyridoxal and semicarbazide or S-methylisothiosemicarbazide. J. Iran. Chem. Soc. 2011, 8, 727–733. [Google Scholar] [CrossRef]
- Manikandan, R.; Anitha, P.; Prakash, G.; Vijayan, P.; Viswanathamurthi, P. Synthesis, spectral characterization and crystal structure of Ni(II) pyridoxal thiosemicarbazone complexes and their recyclable catalytic application in the nitroaldol (Henry) reaction in ionic liquid media. Polyhedron 2014, 81, 619–627. [Google Scholar] [CrossRef]
- Manikandan, R.; Anitha, P.; Viswanathamurthi, P.; Malecki, J.G. Palladium(II) pyridoxal thiosemicarbazone complexes as efficient and recyclable catalyst for the synthesis of propargylamines by a three-component coupling reactions in ionic liquids. Polyhedron 2016, 119, 300–306. [Google Scholar] [CrossRef]
- Rufino-Felipe, E.; Valdés, H.; Morales-Morales, D. C−S Cross-Coupling Reactions Catalyzed by Well-Defined Copper and Nickel Complexes. Eur. J. Org. Chem. 2022, 2022, e202200654. [Google Scholar] [CrossRef]
- Derafa, W.; Aggoun, D.; Messasma, Z.; Houchi, S.; Bouacida, S.; Ourari, A. An unexpected single crystal structure of nickel(II) complex: Spectral, DFT, NLO, magnetic and molecular docking studies. J. Mol. Struct. 2022, 1264, 133190. [Google Scholar] [CrossRef]
- Altamimi, A.S.; Al-zahrani, S.A.; Jevtovic, V. Synthesis, Structure Analysis and Antibacterial Activity of Zn (II) and Co (III) Complexes. Am. J. Chem. 2021, 11, 43–48. [Google Scholar] [CrossRef]
- Massol, M.; Bonnet, J.J.; Laurent, J.P. Spectroscopic and x-ray structural study of the copper(II) complex with schiff base between pyridoxal and benzylamine. J. Coord. Chem. 1981, 11, 185–193. [Google Scholar] [CrossRef]
- Ferrari, M.B.; Fava, G.G.; Pelizzi, C.; Tarasconi, P.; Tosi, G. Thiosernicarbazones as Co-ordinating Agents. Part 2.* Synthesis, Spectroscopic Characterization, and X-Ray Structure of Aquachloro( pyridoxal thiosemicarbazone) manganese(II) Chloride and Aqua(pyridoxal thiosernicarbazonato)- copper(II) Chloride Monohydra. J. Chem. Soc. Dalt. Trans. 1987, 227–233. [Google Scholar] [CrossRef]
- Kocyigit, O.; Kursunlu, A.N.; Guler, E. Complexation properties and synthesis of a novel Schiff base with triphenylene nucleus. J. Hazard. Mater. 2010, 183, 334–340. [Google Scholar] [CrossRef]
- Ferrari Belicchi, M.; Fava Gasparri, G.; Leporati, E.; Pelizzi, C.; Tarasconi, P.; Tosi, G. Structure of Pyridoxa I Th iosem icar bazone Tr i hyd rate and Spectroscopic Properties of its Metal Complexes t. J. Chem. Soc. Dalt. Trans. 1986, 3, 1–6. [Google Scholar]
- Milenković, D.; Avdović, E.; Dimić, D.; Sudha, S.; Ramarajan, D.; Milanović, Ž.; Trifunović, S.; Marković, Z.S. Vibrational and Hirshfeld surface analyses, quantum chemical calculations, and molecular docking studies of coumarin derivative 3-(1-m-toluidinoethylidene)-chromane-2,4-dione and its corresponding palladium(II) complex. J. Mol. Struct. 2020, 1209, 127935. [Google Scholar] [CrossRef]
- Shobana, D.; Sudha, S.; Ramarajan, D.; Ristivojević, N.; Rakić, A.; Dimić, D. Structural, spectroscopic (IR, Raman, and NMR), quantum chemical, and molecular docking analysis of (E)-2-(2,5-dimethoxybenzylidene)hydrazinecarbothioamide and its dimers. J. Mol. Struct. 2022, 1247, 131277. [Google Scholar] [CrossRef]
- Ferrari, M.B.; Fava, G.G.; Tarasconi, P. Thiosemicarbazones as co-ordinating agents. Part 3. Synthesis, spectroscopic characterization, and X-ray structure of methyl pyruvate thiosemicarbazone hemihydrate, chloro(ethyl pyruvate thiosemicarbazonato)copper(II)(green form), and chloro(pyruvic acid thiosemicarbazonato)copper(II) dihydrate (blue form). J. Chem Soc. Dalt. Trans 1989, 361–366. [Google Scholar] [CrossRef]
- Gak Simić, K.; Đorđević, I.; Lazić, A.; Radovanović, L.; Petković-Benazzouz, M.; Rogan, J.; Trišović, N.; Janjić, G. On the supramolecular outcomes of fluorination of cyclohexane-5-spirohydantoin derivatives. CrystEngComm 2021, 23, 2606–2622. [Google Scholar] [CrossRef]
- Jevtović, V.; Hamoud, H.; Al-zahrani, S.; Alenezi, K.; Latif, S.; Alanazi, T.; Abdulaziz, F.; Dimić, D. Synthesis, Crystal Structure, Quantum Chemical Analysis, Electrochemical Behavior, and Antibacterial and Photocatalytic Activity of Co Complex with. Molecules 2022, 27, 4809. [Google Scholar] [CrossRef]
- Dimić, D.S.; Marković, Z.S.; Saso, L.; Avdović, E.H.; Đorović, J.R.; Petrović, I.P.; Stanisavljević, D.D.; Stevanović, M.J.; Potočňák, I.; Samoľová, E.; et al. Synthesis and characterization of 3-(1-((3,4-dihydroxyphenethyl)amino)ethylidene)-chroman-2,4-dione as potentional anti-tumor agent. Oxid. Med. Cell. Longev. 2019, 2019, 2069250. [Google Scholar] [CrossRef]
- Dimić, D.; Milenković, D.; Marković, Z.; Marković, J.D. Structural and spectral analysis of 3-metoxytyramine, an important metabolite of dopamine. J. Mol. Struct. 2017, 1134, 226–236. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kumar, A.; Basu, S.; Dutta, S. Application of Response Surface Methodology for Methylene Blue dye removal from aqueous solution using low cost adsorbent. Chem. Eng. J. 2012, 181–182, 289–299. [Google Scholar] [CrossRef]
- Garg, V.K.; Amita, M.; Kumar, R.; Gupta, R. Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: A timber industry waste. Dye. Pigment. 2004, 63, 243–250. [Google Scholar] [CrossRef]
- Pavan, F.A.; Mazzocato, A.C.; Gushikem, Y. Removal of methylene blue dye from aqueous solutions by adsorption using yellow passion fruit peel as adsorbent. Bioresour. Technol. 2008, 99, 3162–3165. [Google Scholar] [CrossRef]
- Warang, T.; Patel, N.; Santini, A.; Bazzanella, N.; Kale, A.; Miotello, A. Pulsed laser deposition of Co 3O 4 nanoparticles assembled coating: Role of substrate temperature to tailor disordered to crystalline phase and related photocatalytic activity in degradation of methylene blue. Appl. Catal. A Gen. 2012, 423–424, 21–27. [Google Scholar] [CrossRef]
- Uddin, M.T.; Islam, M.A.; Mahmud, S.; Rukanuzzaman, M. Adsorptive removal of methylene blue by tea waste. J. Hazard. Mater. 2009, 164, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Uysal, Ş.; Kurşunlu, A.N. The Synthesis and Characterization of Star Shaped Metal Complexes of Triazine Cored Schiff Bases: Their Thermal Decompositions and Magnetic Moment Values. J. Inorg. Organomet. Polym. Mater. 2011, 21, 291–296. [Google Scholar] [CrossRef]
- Mowry, S.; Ogren, P.J. Kinetics of Methylene Blue Reduction by Ascorbic Acid. J. Chem. Educ. 1999, 76, 970–974. [Google Scholar] [CrossRef]
- Pande, S.; Ghosh, S.K.; Nath, S.; Praharaj, S.; Jana, S.; Panigrahi, S.; Basu, S.; Pal, T. Reduction of methylene blue by thiocyanate: Kinetic and thermodynamic aspects. J. Colloid Interface Sci. 2006, 299, 421–427. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Ning, P. Degradation Mechanism of Methylene Blue in a Heterogeneous Fenton-like Reaction Catalyzed by Ferrocene. Ind. Eng. Chem. Res. 2014, 53, 643–649. [Google Scholar] [CrossRef]
- Ahmad, A.; Syed, F.; Shah, A.; Khan, Z.; Tahir, K.; Khan, A.U.; Yuan, Q. Silver and gold nanoparticles from Sargentodoxa cuneata: Synthesis, characterization and antileishmanial activity. RSC Adv. 2015, 5, 73793–73806. [Google Scholar] [CrossRef]
- Saleh, E.A.M.; Khan, A.U.; Tahir, K.; Almehmadi, S.J.; AL-Abdulkarim, H.A.; Alqarni, S.; Muhammad, N.; Dawsari, A.M.A.; Nazir, S.; Ullah, A. Phytoassisted synthesis and characterization of palladium nanoparticles (PdNPs); with enhanced antibacterial, antioxidant and hemolytic activities. Photodiagnosis Photodyn. Ther. 2021, 36, 102542. [Google Scholar] [CrossRef]
- Shams, S.; Ahmad, W.; Memon, A.H.; Shams, S.; Wei, Y.; Yuan, Q.; Liang, H. Cu/H3BTC MOF as a potential antibacterial therapeutic agent against: Staphylococcus aureus and Escherichia coli. New J. Chem. 2020, 44, 17671–17678. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Pandey, S.K.; Mehta, J.; Bhardwaj, S.K.; Kim, K.H.; Deep, A. Bioactive nano-metal-organic frameworks as antimicrobials against Gram-positive and Gram-negative bacteria. Toxicol. Res. (Camb.) 2018, 7, 931–941. [Google Scholar] [CrossRef]
- Kizilcikli, I.; Kurt, Y.D.; Akkurt, B.; Genel, A.Y.; Birteksöz, S.; Ötük, G.; Ülküseven, B. Antimicrobial activity of a series of thiosemicarbazones and their Zn II and PdII complexes. Folia Microbiol. 2007, 52, 15–25. [Google Scholar] [CrossRef]
- Akbari, A.; Ghateazadeh, H.; Takjoo, R.; Sadeghi-Nejad, B.; Mehrvar, M.; Mague, J.T. Synthesis & crystal structures of four new biochemical active Ni(II) complexes of thiosemicarbazone and isothiosemicarbazone-based ligands: In vitro antimicrobial study. J. Mol. Struct. 2019, 1181, 287–294. [Google Scholar] [CrossRef]
- Qi, J.; Wang, X.; Liu, T.; Kandawa-Schulz, M.; Wang, Y.; Zheng, X. Synthesis, antiproliferative activity and mechanism of copper(II)-thiosemicarbazone complexes as potential anticancer and antimicrobial agents. J. Coord. Chem. 2020, 73, 1208–1221. [Google Scholar] [CrossRef]
- Dimić, D.S.; Milenković, D.A.; Avdović, E.H.; Nakarada, Đ.J.; Dimitrić Marković, J.M.; Marković, Z.S. Advanced oxidation processes of coumarins by hydroperoxyl radical: An experimental and theoretical study, and ecotoxicology assessment. Chem. Eng. J. 2021, 424, 130331. [Google Scholar] [CrossRef]
- Milenković, D.A.; Dimić, D.S.; Avdović, E.H.; Amić, A.D.; Dimitrić Marković, J.M.; Marković, Z.S. Advanced oxidation process of coumarins by hydroxyl radical: Towards the new mechanism leading to less toxic products. Chem. Eng. J. 2020, 395, 124971. [Google Scholar] [CrossRef]
- Lončar, A.; Negrojević, L.; Dimitrić-Marković, J.; Dimić, D. The reactivity of neurotransmitters and their metabolites towards various nitrogen-centered radicals: Experimental, theoretical, and biotoxicity evaluation. Comput. Biol. Chem. 2021, 95, 107573. [Google Scholar] [CrossRef]
- Khan, A.U.; Nazir, S.; El-Keblawy, A.; Tahir, K.; Abdel-Hafez, S.H.; AL-Abdulkarim, H.A.; Jevtovic, V.; Ibrahim, M.M.; Al-Shehri, H.S.; Hegab, K.H. Uncaria rhynchophylla mediated Ag/NiO nanocomposites: A new insight for the evaluation of cytotoxicity, antibacterial and photocatalytic applications. Photodiagnosis Photodyn. Ther. 2022, 37, 102681. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef]
- Mabbott, G.A. An introduction to cyclic voltammetry. J. Chem. Educ. 1983, 60, 697–702. [Google Scholar] [CrossRef]
- Su, Q.; Banks, E.; Bebernitz, G.; Bell, K.; Borenstein, C.F.; Chen, H.; Chuaqui, C.E.; Deng, N.; Ferguson, A.D.; Kawatkar, S.; et al. Discovery of (2 r)- n-[3-[2-[(3-methoxy-1-methyl-pyrazol-4-yl)amino]pyrimidin-4-yl]-1 h-indol-7-yl]-2-(4-methylpiperazin-1-yl)propenamide (azd4205) as a potent and selective janus kinase 1 inhibitor. J. Med. Chem. 2020, 63, 4517–4527. [Google Scholar] [CrossRef]
- Williams, N.K.; Bamert, R.S.; Patel, O.; Wang, C.; Walden, P.M.; Wilks, A.F.; Fantino, E.; Rossjohn, J.; Lucet, I.S. Dissecting Specificity in the Janus Kinases: The Structures of JAK-Specific Inhibitors Complexed to the JAK1 and JAK2 Protein Tyrosine Kinase Domains. J. Mol. Biol. 2009, 387, 219–232. [Google Scholar] [CrossRef]
- Jevtovic, V. Cu, Fe, Ni and V Complexes with Pyridoxal Semicarbazones, Synthesis, Physical and Chemical Properties, Structural Analyses and Biological Activities; Lambert Academic Publishing: Chinsau, Republic of Moldova, 2010. [Google Scholar]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17 2017. Available online: https://wiki.crystalexplorer.net/how-to-cite (accessed on 3 September 2022).
- Karrouchi, K.; Brandán, S.A.; Sert, Y.; Karbane, M.E.; Radi, S.; Ferbinteanu, M.; Garcia, Y.; Ansar, M. Synthesis, structural, molecular docking and spectroscopic studies of (E)-N’-(4-methoxybenzylidene)-5-methyl-1H-pyrazole-3-carbohydrazide. J. Mol. Struct. 2021, 1225, 129072. [Google Scholar] [CrossRef]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- Ben Issa, T.; Ghalla, H.; Marzougui, S.; Benhamada, L. Crystal structure and theoretical studies on quinoline phosphate. J. Mol. Struct. 2017, 1150, 127–134. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitale. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Behjatmanesh-Ardakani, R. NBO–NEDA, NPA, and QTAIM studies on the interactions between aza-, diaza-, and triaza-12-crown-4 (An-12-crown-4, n = 1, 2, 3) and Li+, Na+, and K+ ions. Comput. Theor. Chem. 2015, 1051, 62–71. [Google Scholar] [CrossRef]
- Kavitha, G.; Dhandapani, A.; Gunasekaran, B.; Suresh, M. Synthesis, crystal structure, Hirshfeld surface, DFT calculations, Z-scan and nonlinear optical studies of novel flourinated hexahydropyrimidine. J. Mol. Struct. 2021, 1228, 129484. [Google Scholar] [CrossRef]
- Hadacek, F.; Greger, H. Testing of antifungal natural products: Methodologies, comparability of results and assay choice. Phytochem. Anal. 2000, 11, 137–147. [Google Scholar] [CrossRef]
- Khan, A.U.; Arooj, A.; Tahir, K.; Ibrahim, M.M.; Jevtovic, V.; AL-Abdulkarim, H.A.; Saleh, E.A.M.; Al-Shehri, H.S.; Amin, M.A.; Li, B. Facile fabrication of novel Ag2S-ZnO/GO nanocomposite with its enhanced photocatalytic and biological applications. J. Mol. Struct. 2022, 1251, 131991. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, Q.U.; Tahir, K.; Ullah, S.; Arooj, A.; Li, B.; Rehman, K.U.; Nazir, S.; Khan, M.U.; Ullah, I. A Tagetes minuta based eco-benign synthesis of multifunctional Au/MgO nanocomposite with enhanced photocatalytic, antibacterial and DPPH scavenging activities. Mater. Sci. Eng. C 2021, 126, 112146. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Tahir, K.; Irshad, R.; Khan, Q.U.; Khan, S.; Khan, I.U.; Nawaz, A.; Rehman, F. Photo-assisted inactivation of highly drug resistant bacteria and DPPH scavenging activities of zinc oxide graphted Pd-MCM-41 synthesized by new hydrothermal method. Photodiagnosis Photodyn. Ther. 2021, 33, 102162. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- BIOVIA. Dassault Systèmes, Discovery Studio 2016; Dassault Systèmes: San Diego, CA, USA, 2016. [Google Scholar]
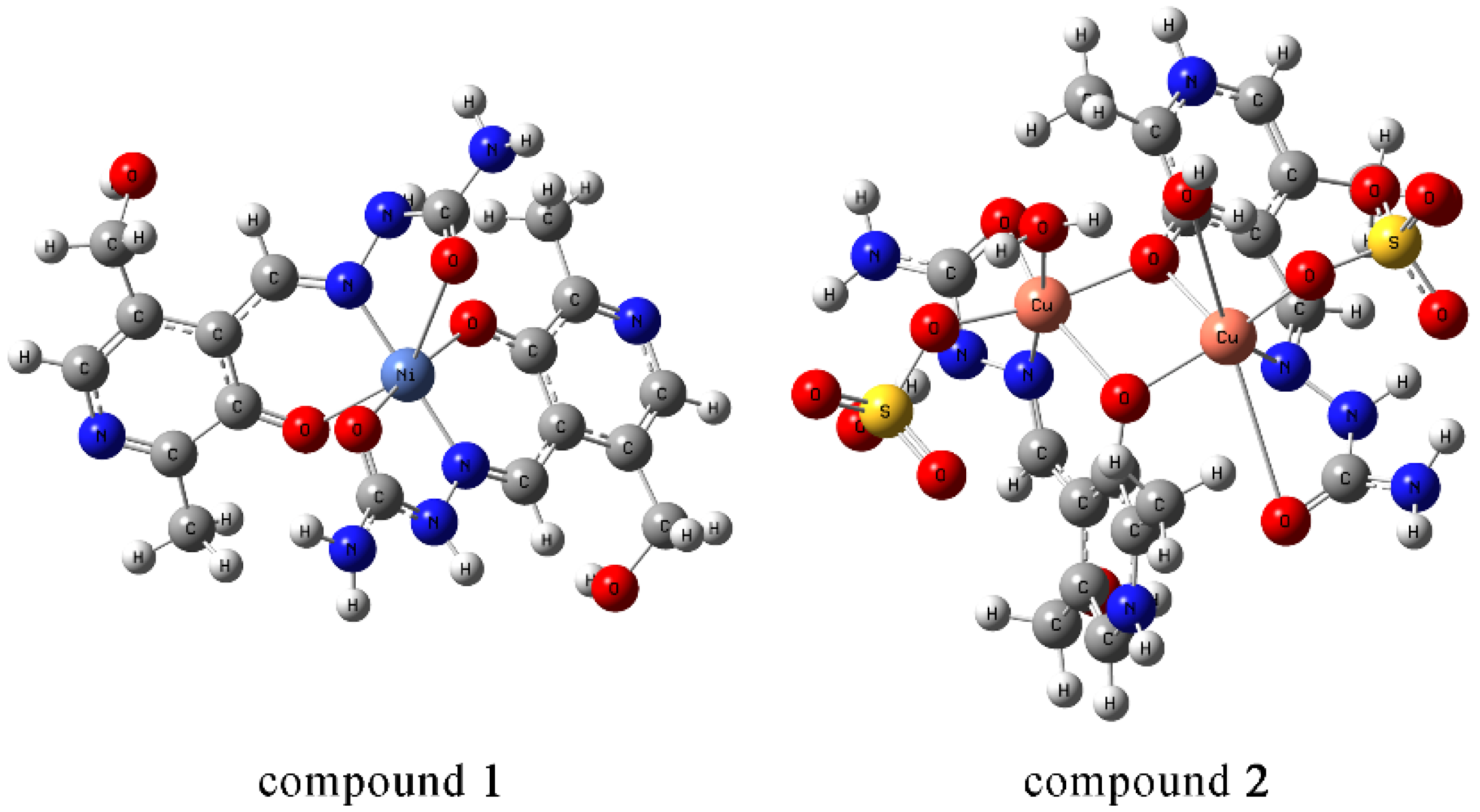
| Empirical Formula | C18H26N8NiO8 | C18H36N8O19S2Cu2 |
|---|---|---|
| Formula weight | 541.16 | 859.77 |
| Temperature | 123 K | 123 K |
| Wavelength | 1.54184 | 0.71073 |
| Crystal System | I 2/a | P-1 |
| Space group | monoclinic | triclinic |
| Volume | 2215.48 (6) | 1492.58 (3) |
| Unit cell dimension | a = 12.4040 (2) | a = 9.2533 (1) |
| b = 13.5306 (2) | b = 9.5788 (1) | |
| c = 13.5689 (2) | c = 17.0995 (2) | |
| α = 90 | α = 83.858 (1) | |
| β = 103.383 (1) | β = 82.899 (1) | |
| γ = 90 | γ = 85.791 (1) | |
| Z | 4 | 2 |
| Microorganisms | Concentration (mg mL−1) | Zone of Inhibition (mm) | |||
|---|---|---|---|---|---|
| Compound 1 | Compound 2 | Positive Control | Negative Control | ||
| E. coli | 0.25 | 10 ± 0.1 | 9 ± 0.2 | 15 ± 0.3 | Zero inhibition |
| 0.5 | 14 ± 0.2 | 13 ± 0.2 | |||
| 1 | 17 ± 0.3 | 18 ± 0.3 | |||
| 2 | 21 ± 0.3 | 24 ± 0.4 | |||
| S. aureus | 0.25 | 12 ± 0.2 | 11 ± 0.1 | 16 ± 0.2 | Zero inhibition |
| 0.5 | 16 ± 0.2 | 15 ± 0.2 | |||
| 1 | 21 ± 0.3 | 20 ± 0.3 | |||
| 2 | 24 ± 0.4 | 26 ± 0.3 | |||
| Compound | Bacterium | Concentration (μg mL−1) | ||||
|---|---|---|---|---|---|---|
| 60 | 50 | 40 | 30 | 20 | ||
| Compound 1 | S. aureus | − | − | − | − | + |
| E. coli | − | − | − | + | + | |
| Compound 2 | S. aureus | − | − | − | − | − |
| E. coli | − | − | − | − | + | |
| Chloramphenicol | S. aureus | − | − | − | − | − |
| E. coli | − | − | − | − | − | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jevtovic, V.; Alshammari, N.; Latif, S.; Alsukaibi, A.K.D.; Humaidi, J.; Alanazi, T.Y.A.; Abdulaziz, F.; Matalka, S.I.; Pantelić, N.Đ.; Marković, M.; et al. Synthesis, Crystal Structure, Theoretical Calculations, Antibacterial Activity, Electrochemical Behavior, and Molecular Docking of Ni(II) and Cu(II) Complexes with Pyridoxal-Semicarbazone. Molecules 2022, 27, 6322. https://doi.org/10.3390/molecules27196322
Jevtovic V, Alshammari N, Latif S, Alsukaibi AKD, Humaidi J, Alanazi TYA, Abdulaziz F, Matalka SI, Pantelić NĐ, Marković M, et al. Synthesis, Crystal Structure, Theoretical Calculations, Antibacterial Activity, Electrochemical Behavior, and Molecular Docking of Ni(II) and Cu(II) Complexes with Pyridoxal-Semicarbazone. Molecules. 2022; 27(19):6322. https://doi.org/10.3390/molecules27196322
Chicago/Turabian StyleJevtovic, Violeta, Njood Alshammari, Salman Latif, Abdulmohsen Khalaf Dhahi Alsukaibi, Jamal Humaidi, Tahani Y. A. Alanazi, Fahad Abdulaziz, Samah I. Matalka, Nebojša Đ. Pantelić, Milica Marković, and et al. 2022. "Synthesis, Crystal Structure, Theoretical Calculations, Antibacterial Activity, Electrochemical Behavior, and Molecular Docking of Ni(II) and Cu(II) Complexes with Pyridoxal-Semicarbazone" Molecules 27, no. 19: 6322. https://doi.org/10.3390/molecules27196322
APA StyleJevtovic, V., Alshammari, N., Latif, S., Alsukaibi, A. K. D., Humaidi, J., Alanazi, T. Y. A., Abdulaziz, F., Matalka, S. I., Pantelić, N. Đ., Marković, M., Rakić, A., & Dimić, D. (2022). Synthesis, Crystal Structure, Theoretical Calculations, Antibacterial Activity, Electrochemical Behavior, and Molecular Docking of Ni(II) and Cu(II) Complexes with Pyridoxal-Semicarbazone. Molecules, 27(19), 6322. https://doi.org/10.3390/molecules27196322







