Self-Assembly of Supramolecular Architectures Driven by σ-Hole Interactions: A Halogen-Bonded 2D Network Based on a Diiminedibromido Gold(III) Complex and Tribromide Building Blocks
Abstract
1. Introduction
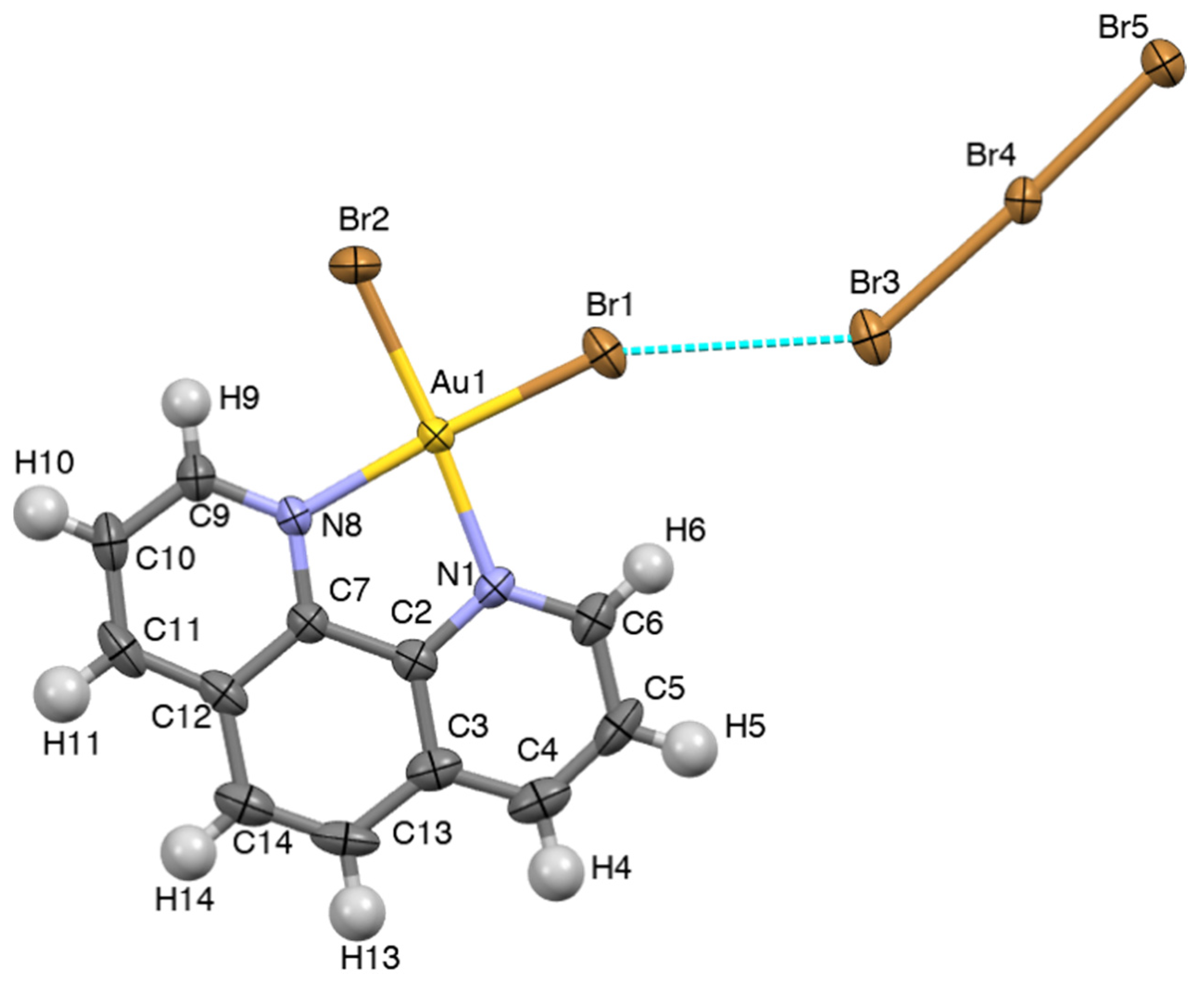
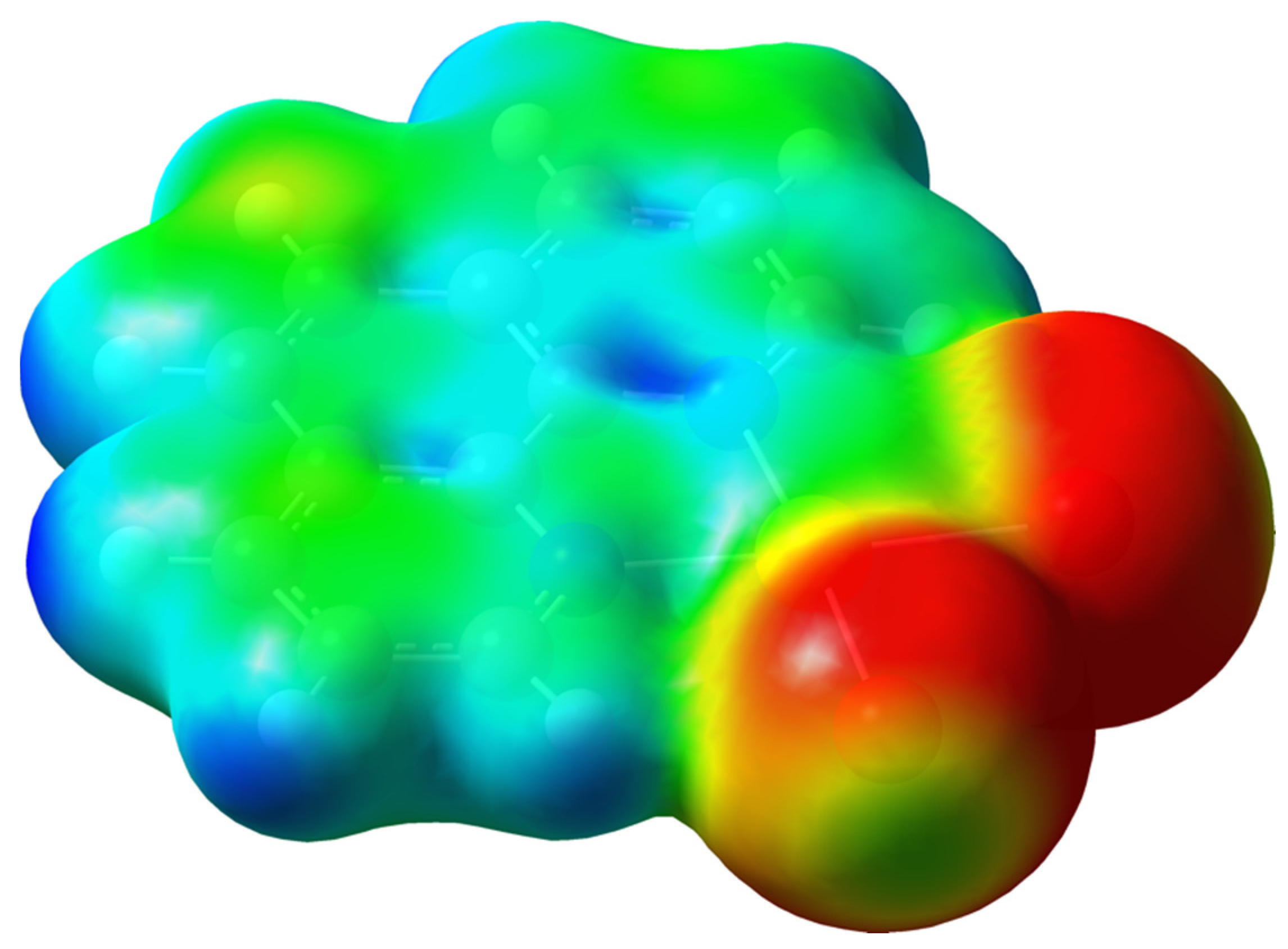
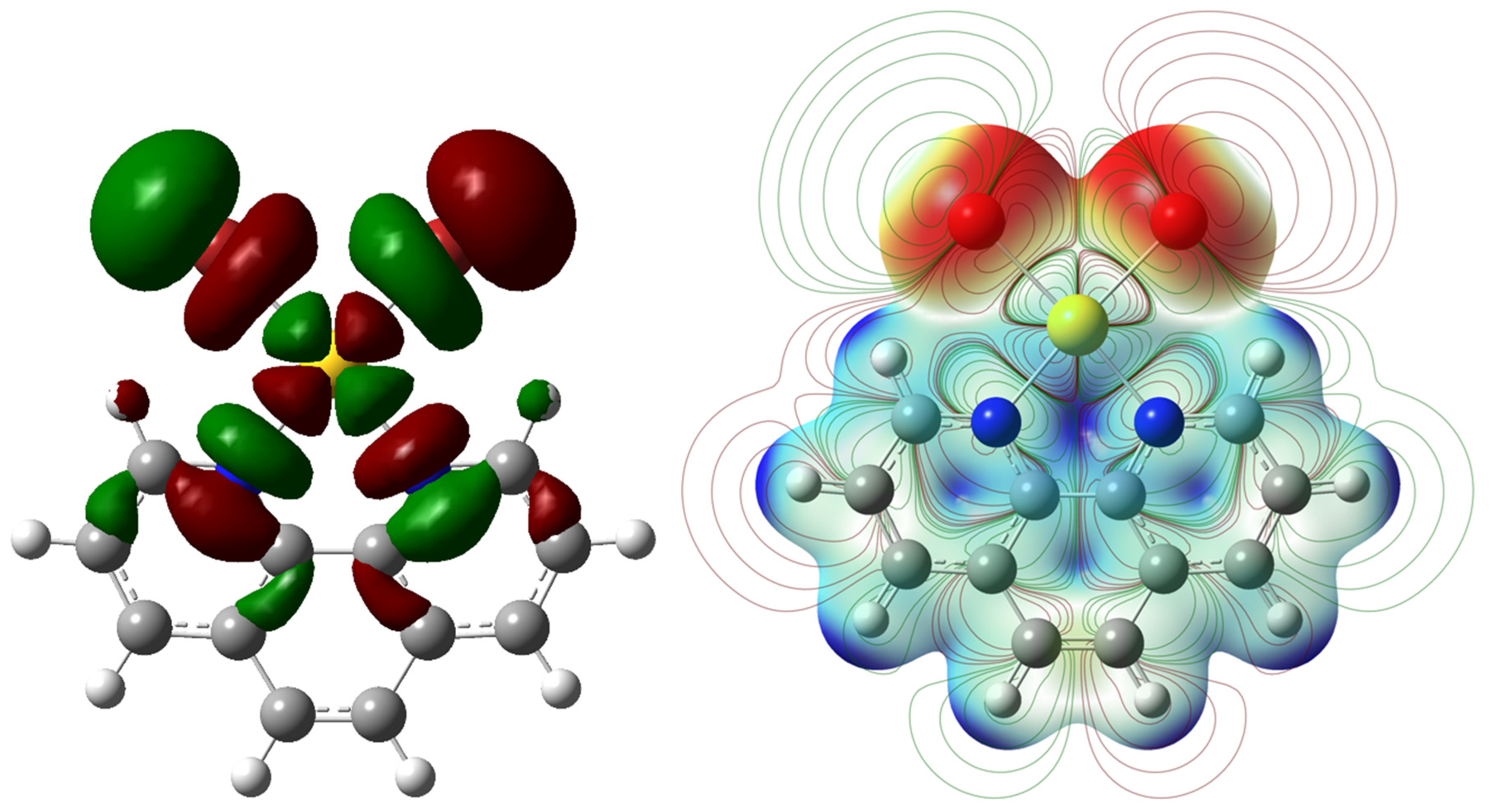
2. Results and Discussion
3. Materials and Methods
Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References and Notes
- Williams, G.T.; Haynes, C.J.E.; Fares, M.; Caltagirone, C.; Hiscock, J.R.; Gale, P.A. Advances in applied supramolecular technologies. Chem. Soc. Rev. 2021, 50, 2737–2763. [Google Scholar] [CrossRef] [PubMed]
- Gale, P. Supramolecular chemistry: From complexes to complexity. Philos. Trans. R. Soc. A 2000, 358, 431–453. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Prusty, S.; Chartrand, D.; Hanan, G.S.; Chand, D.K. Self-Assembled Molecular Squares as Supramolecular Tectons. Cryst. Growth Des. 2018, 18, 2016–2030. [Google Scholar] [CrossRef]
- Sonnenberg, K.; Mann, L.; Redeker, F.A.; Schmidt, B.; Riedel, S. Polyhalogen and Polyinterhalogen Anions from Fluorine to Iodine. Angew. Chem. Int. Ed. 2020, 59, 5464–5493. [Google Scholar] [CrossRef]
- Tebbe, K.F.; Buchem, R. The Most Iodine-Rich Polyiodide Yet: Fe3I29. Angew. Chem. Int. Ed. 1997, 36, 1345–1346. [Google Scholar] [CrossRef]
- Svensson, H.; Kloo, L.L. Synthesis, Structure, and Bonding in Polyiodide and Metal Iodide−Iodine Systems. Chem. Rev. 2003, 103, 1649–1684. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Demartin, F.; Devillanova, F.A.; Garau, A.; Isaia, F.; Lippolis, V.; Rizzato, S.; Verani, G. [Ni(L)(MeCN)]2+ complex cation as a template for the assembly of extended I3−·I5− and I5−·I7− polyiodide networks {L=2,5,8-trithia [9](2,9)-1,10-phenanthrolinophane}. Synthesis and structures of [Ni(L)(MeCN)]I8 and [Ni(L)(MeCN)]I12. Inorg. Chim. Acta 2004, 357, 3803–3809. [Google Scholar] [CrossRef]
- Haller, H.; Riedel, S. Recent Discoveries of Polyhalogen Anions–from Bromine to Fluorine. Z. Anorg. Allg. Chem. 2014, 640, 1281–1291. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Hursthouse, M.B.; Huth, S.L.; Isaia, F.; Lippolis, V.; Mancini, A.; Ogilvie, H. Self-assembly of supramolecular architectures based on polybromide anions: Crystal structure of [H4tppz4+](Br−)2(Br42−) [tppz = tetra(2-pyridyl)pyrazine]. Inorg. Chim. Acta 2005, 8, 79–82. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Isaia, F.; Lippolis, V. Adducts of S/Se Donors with Dihalogens as a Source of Information for Categorizing the Halogen Bonding. Cryst. Growth Des. 2012, 12, 2769–2779. [Google Scholar] [CrossRef]
- Easton, M.E.; Ward, A.J.; Hudson, T.; Turner, P.; Masters, A.F.; Maschmeyer, T. The Formation of High-Order Polybromides in a Room-Temperature Ionic Liquid: From Monoanions ([Br5]− to [Br11]−) to the Isolation of [PC16H36]2[Br24] as Determined by van der Waals Bonding Radii. Chem. Eur. J. 2015, 21, 2961–2965. [Google Scholar] [CrossRef]
- Korobeynikov, N.A.; Usoltsev, A.N.; Novikov, A.S.; Abramov, P.A.; Sokolov, M.N.; Adonin, S.A. Selenium(IV) Polybromide Complexes: Structural Diversity Driven by Halogen and Chalcogen Bonding. Molecules 2022, 27, 5355. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Sonnenberg, K.; Pröhm, P.; Müller, C.; Beckers, H.; Steinhauer, S.; Lentz, D.; Riedel, S. Closing the Gap: Structural Evidence for the Missing Hexabromide Dianion [Br6]2−. Chem. Eur. J. 2018, 24, 1072–1075. [Google Scholar] [CrossRef]
- Wolff, M.; Meyer, J.; Feldmann, C. [C4MPyr]2[Br20]: Ionic-Liquid-Based Synthesis of a Three-Dimensional Polybromide Network. Angew. Chem. 2011, 50, 4970–4973. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Isaia, F.; Lippolis, V.; Mancini, A.; Pala, L.; Slawin, A.M.Z.; Woollins, J.D. First example of an infinite polybromide 2D-network. Chem. Commun. 2003, 17, 2226–2227. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Beer, P.D. Sigma-Hole Interactions in Anion Recognition. Chem 2018, 4, 731–783. [Google Scholar] [CrossRef]
- Palusiak, M. On the nature of halogen bond—The Kohn–Sham molecular orbital approach. J. Mol. Struct. THEOCHEM 2010, 945, 89–92. [Google Scholar] [CrossRef]
- Kellett, C.W.; Kennepohl, P.; Berlinguette, C.P. π Covalency in the halogen bond. Nat. Commun. 2020, 11, 3310. [Google Scholar] [CrossRef]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen Bonding in Supramolecular Chemistry. Angew. Chem. 2008, 47, 6114–6127. [Google Scholar] [CrossRef]
- Arca, M.; Ciancaleoni, G.; Pintus, A. Computational Methods to Study Chalcogen Bond. In Chalcogen Chemistry: Fundamentals and Applications; Lippolis, V., Santi, C., Lenardão, E.J., Braga, A.L., Eds.; RSC Publishing: London, UK, 2022; ISBN 978-1-83916-422-4. [Google Scholar]
- Oliveira, V.; Kraka, E.; Cremer, D. The intrinsic strength of the halogen bond: Electrostatic and covalent contributions described by coupled cluster theory. Phys. Chem. Chem. Phys. 2016, 18, 33031–33046. [Google Scholar] [CrossRef]
- Anderson, L.N.; Aquino, F.W.; Raeber, A.E.; Chen, X.; Wong, B.M. Halogen Bonding Interactions: Revised Benchmarks and a New Assessment of Exchange vs Dispersion. J. Chem. Theory Comput. 2018, 14, 180–190. [Google Scholar] [CrossRef]
- Dong, W.; Li, Q.; Scheiner, S. Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and Contributing Factors. Molecules 2018, 23, 1681. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. Halogen and Chalcogen Bond Energies Evaluated Using Electron Density Properties. ChemPhysChem 2020, 21, 26–31. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Hursthouse, M.B.; Huth, S.L.; Isaia, F.; Lippolis, V.; Mancini, A.; Ogilvie, H.R.; Verani, G. Reactions of pyridyl donors with halogens and interhalogens: An X-ray diffraction and FT-Raman investigation. J. Organomet. Chem. 2005, 690, 1923–1934. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Hursthouse, M.B.; Huth, S.L.; Isaia, F.; Lippolis, V.; Mancini, A. Square-pyramidal bonding of I2 molecules at the I− nodes of a polyiodide infinite pseudo-cubic 3D-network. Cryst. Eng. Comm. 2004, 6, 540–542. [Google Scholar] [CrossRef]
- Blake, A.J.; Gould, R.O.; Parsons, S.; Radek, C.; Schröder, M. Self-Assembly of Polyanions at a Metal Cation Template: Syntheses and Structures of [{Ag([18]aneS6)}I7]n and [Ag([18]aneS6)]I3. Angew. Chem. Int. Ed. Engl. 1995, 34, 2374–2376. [Google Scholar] [CrossRef]
- Martínez-Camarena, Á.; Savastano, M.; Blasco, S.; Delgado-Pinar, E.; Giorgi, C.; Bianchi, A.; García-España, E.; Bazzicalupi, C. Assembly of Polyiodide Networks with Cu(II) Complexes of Pyridinol-Based Tetraaza Macrocycles. Inorg. Chem. 2022, 61, 368–383. [Google Scholar] [CrossRef]
- Schneider, D.; Schuster, O.; Schmidbaur, H. Bromination of (phosphine)gold(I) bromide complexes: Stoichiometry and structure of products. Dalton Trans. 2005, 11, 1940–1947. [Google Scholar] [CrossRef]
- Döring, C.; Jones, P.G. Two-Dimensional Networks of [AuCl4]− and [AuBr4]−Anions. Z. Anorg. Allg. Chem. 2016, 642, 930–935. [Google Scholar] [CrossRef]
- Lu, W.; Tung Chan, K.; Wu, S.-X.; Chen, Y.; Che, C.-M. Quest for an intermolecular Au(III)/Au(III) interaction between cyclometalated gold(III) cations. Chem. Sci. 2012, 3, 752–755. [Google Scholar] [CrossRef]
- Koskinen, L.; Jääskeläinen, S.; Kalenius, E.; Hirva, P.; Haukka, M. Role of C–H···Au and Aurophilic Supramolecular Interactions in Gold–Thione Complexes. Cryst. Growth Des. 2014, 14, 1989–1997. [Google Scholar] [CrossRef]
- Chernyshev, A.N.; Chernysheva, M.V.; Hirva, P.; Kukushkin, V.Y.; Haukka, M. Weak aurophilic interactions in a series of Au(III) double salts. Dalton Trans. 2015, 44, 14523–14531. [Google Scholar] [CrossRef]
- Taouss, C.; Jones, P.G. Halogenation of (phosphine chalcogenide)gold(I) halides; some unexpected products. Dalton Trans. 2011, 40, 11687–11689. [Google Scholar] [CrossRef]
- Rimmer, E.L.; Bailey, R.D.; Pennington, W.T.; Hanks, T.W. The reaction of iodine with 9-methylacridine: Formation of polyiodide salts and a charge-transfer complex. J. Chem. Soc. Perkin Trans. 2 1998, 11, 2557–2562. [Google Scholar] [CrossRef]
- Sharafie, D.; Amani, V.; Naseh, M. Synthesis, spectroscopic characterization, crystal structure determination and DFT calculations of [Au(Me2phen)Br2][AuBr2]. Chem. Pap. 2018, 72, 1427–1435. [Google Scholar] [CrossRef]
- CSD codes: BOFSEV, EREGIT, EREGOZ, HAZMOL, JEBQUF, NUTCUD, QIRRAK, SOKCED, WOFKIM, XIZDIW, XIZDOC, XIZDUI, XIZFAQ, XIZFIY, and ZEQPES.
- A search on the CCSD reveals 253 hits of isolated tribromide anions with Br–Br mean distances of 2.54(6) Å and Br–Br–Br mean angle of 178(2)°.
- Powell, B.M.; Heal, K.M.; Torrie, B.H. The temperature dependence of the crystal structures of the solid halogens, bromine and chlorine. Mol. Phys. 1984, 53, 929–939. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond: Outline of a Comprehensive Hydrogen Bond Theory; IUCr Monographs on Crystallography, No. 23; Oxford University Press: Oxford, UK, 2009; ISBN 978-0-19-955896-4. [Google Scholar]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural-population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural Localized Molecular Orbitals. J. Chem. Phys. 1985, 83, 1736–1740. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Parthasarathy, R. The Nature of Halogen-Halogen Interactions: Are Short Halogen Contacts Due to Specific Attractive Forces or Due to Close Packing of Nonspherical Atoms? J. Am. Chem. Soc. 1989, 111, 8725–8726. [Google Scholar] [CrossRef]
- Dumitrescu, D.; Shova, S.; Man, I.C.; Caira, M.R.; Popa, M.M.; Dumitrascu, F. 5-Iodo-1-Arylpyrazoles as Potential Benchmarks for Investigating the Tuning of the Halogen Bonding. Crystals 2020, 10, 1149. [Google Scholar] [CrossRef]
- Salmasi, R.; Gholizadeh, M.; Salimi, A.; Garrison, J.C. The synthesis of 1,2-ethanediylbis(triphenylphosphonium) ditribromide as a new brominating agent in the presence of solvents and under solvent-free conditions. J. Iran. Chem. Soc. 2016, 13, 2019–2028. [Google Scholar] [CrossRef]
- Stang, S.; Lebkucher, A.; Walter, P.; Kaifer, E.; Himmel, H.-J. Redox-Active Guanidine Ligands with Pyridine and p-Benzoquinone Backbones. Eur. J. Inorg. Chem. 2012, 30, 4833–4845. [Google Scholar] [CrossRef]
- Bondarenko, M.A.; Novikov, A.S.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. The stabilization of decabromide {Br10}2− anion in the structure of Sb(V) bromide complex. J. Coord. Chem 2020, 73, 3038–3043. [Google Scholar] [CrossRef]
- Adonin, S.A.; Bondarenko, M.A.; Novikov, A.S.; Abramov, P.A.; Plyusnin, P.E.; Sokolov, M.N.; Fedin, V.P. Halogen bonding-assisted assembly of bromoantimonate(V) and polybromide-bromoantimonate-based frameworks. CrystEngComm 2019, 21, 850–856. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Robertson, K.N.; Bakshi, P.K.; Cameron, T.S.; Knop, O. Polyhalide Anions in Crystals. The Br82− anion in diquinuclidinium octabromide, the crystal structures of Me4PBr3 and quinuclidinium tribromide, and Ab initio calculations on polybromide anions. Z. Anorg. Allg. Chem. 1997, 623, 104–114. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT: Integrating space group determination and structure solution. Acta Cryst. 2014, A70, C1437. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Pintus, A.; Aragoni, M.C.; Bellec, N.; Devillanova, F.A.; Lorcy, D.; Isaia, F.; Lippolis, V.; Randall, R.A.M.; Slawin, A.M.Z.; Woollins, J.D.; et al. Structure-Property Relationships in PtII Diimine-Dithiolate Nonlinear Optical Chromophores Based on Arylethylene-1,2-Dithiolate and 2-Thioxothiazoline-4,5-Dithiolate. Eur. J. Inorg. Chem. 2012, 22, 3577–3594. [Google Scholar] [CrossRef]
- Pintus, A.; Aragoni, M.C.; Isaia, F.; Lippolis, V.; Lorcy, D.; Slawin, A.M.Z.; Woollins, J.D.; Arca, M. On the Role of Chalcogen Donor Atoms in Diimine-Dichalcogenolate PtII SONLO Chromophores: Is It Worth Replacing Sulfur with Selenium? Eur. J. Inorg. Chem. 2015, 31, 5163–5170. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Binda, M.; Caltagirone, C.; Lippolis, V.; Natali, D.; Podda, E.; Sampietro, M.; Pintus, A. Platinum Diimine-Dithiolate Complexes as a New Class of Photoconducting Compounds for Pristine Photodetectors: Case Study on [Pt(Bipy)(Naph-Edt)] (Bipy = 2,2′-Bipyridine; Naph-Edt2− = 2-Naphthylethylene-1,2-Dithiolate). Dalton Trans. 2021, 50, 7527–7531. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Ross, R.B.; Powers, J.M.; Atashroo, T.; Ermler, W.C.; LaJohn, L.A.; Christiansen, P.A. Ab Initio Relativistic Effective Potentials with Spin–Orbit Operators. IV. Cs through Rn. J. Chem. Phys. 1998, 93, 6654–6670. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Dennington, R.D.; Keith, T.A.; Millam, J.M. GaussView, 6.0. 16; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Skripnikov, L.V. Chemissian, Version 4.53, Visualization Computer Program. 2017. Available online: https://www.chemissian.com (accessed on 7 September 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragoni, M.C.; Cherchi, M.F.; Lippolis, V.; Pintus, A.; Podda, E.; Slawin, A.M.Z.; Woollins, J.D.; Arca, M. Self-Assembly of Supramolecular Architectures Driven by σ-Hole Interactions: A Halogen-Bonded 2D Network Based on a Diiminedibromido Gold(III) Complex and Tribromide Building Blocks. Molecules 2022, 27, 6289. https://doi.org/10.3390/molecules27196289
Aragoni MC, Cherchi MF, Lippolis V, Pintus A, Podda E, Slawin AMZ, Woollins JD, Arca M. Self-Assembly of Supramolecular Architectures Driven by σ-Hole Interactions: A Halogen-Bonded 2D Network Based on a Diiminedibromido Gold(III) Complex and Tribromide Building Blocks. Molecules. 2022; 27(19):6289. https://doi.org/10.3390/molecules27196289
Chicago/Turabian StyleAragoni, M. Carla, M. Francesca Cherchi, Vito Lippolis, Anna Pintus, Enrico Podda, Alexandra M. Z. Slawin, J. Derek Woollins, and Massimiliano Arca. 2022. "Self-Assembly of Supramolecular Architectures Driven by σ-Hole Interactions: A Halogen-Bonded 2D Network Based on a Diiminedibromido Gold(III) Complex and Tribromide Building Blocks" Molecules 27, no. 19: 6289. https://doi.org/10.3390/molecules27196289
APA StyleAragoni, M. C., Cherchi, M. F., Lippolis, V., Pintus, A., Podda, E., Slawin, A. M. Z., Woollins, J. D., & Arca, M. (2022). Self-Assembly of Supramolecular Architectures Driven by σ-Hole Interactions: A Halogen-Bonded 2D Network Based on a Diiminedibromido Gold(III) Complex and Tribromide Building Blocks. Molecules, 27(19), 6289. https://doi.org/10.3390/molecules27196289










