An Ultrastable Porous Polyhedral Oligomeric Silsesquioxane/Tetraphenylthiophene Hybrid as a High-Performance Electrode for Supercapacitors
Abstract
1. Introduction
2. Synthesis Methods
2.1. Materials
2.2. N,N′-Bis(4-bromobenzoyl)hydrazine
2.3. 2,5-Bis(4-bromophenyl)-1,3,4-oxadiazole (OXD-Br2)
2.4. 2,3,4,5-Tetrabromothiophene (Th-Br4)
2.5. 2,3,4,5-Tetraphenylthiophene (TPTh)
2.6. 2,5-Bis(4-bromophenyl)-3,4-diphenylyhiophene (TPTh-Br2)
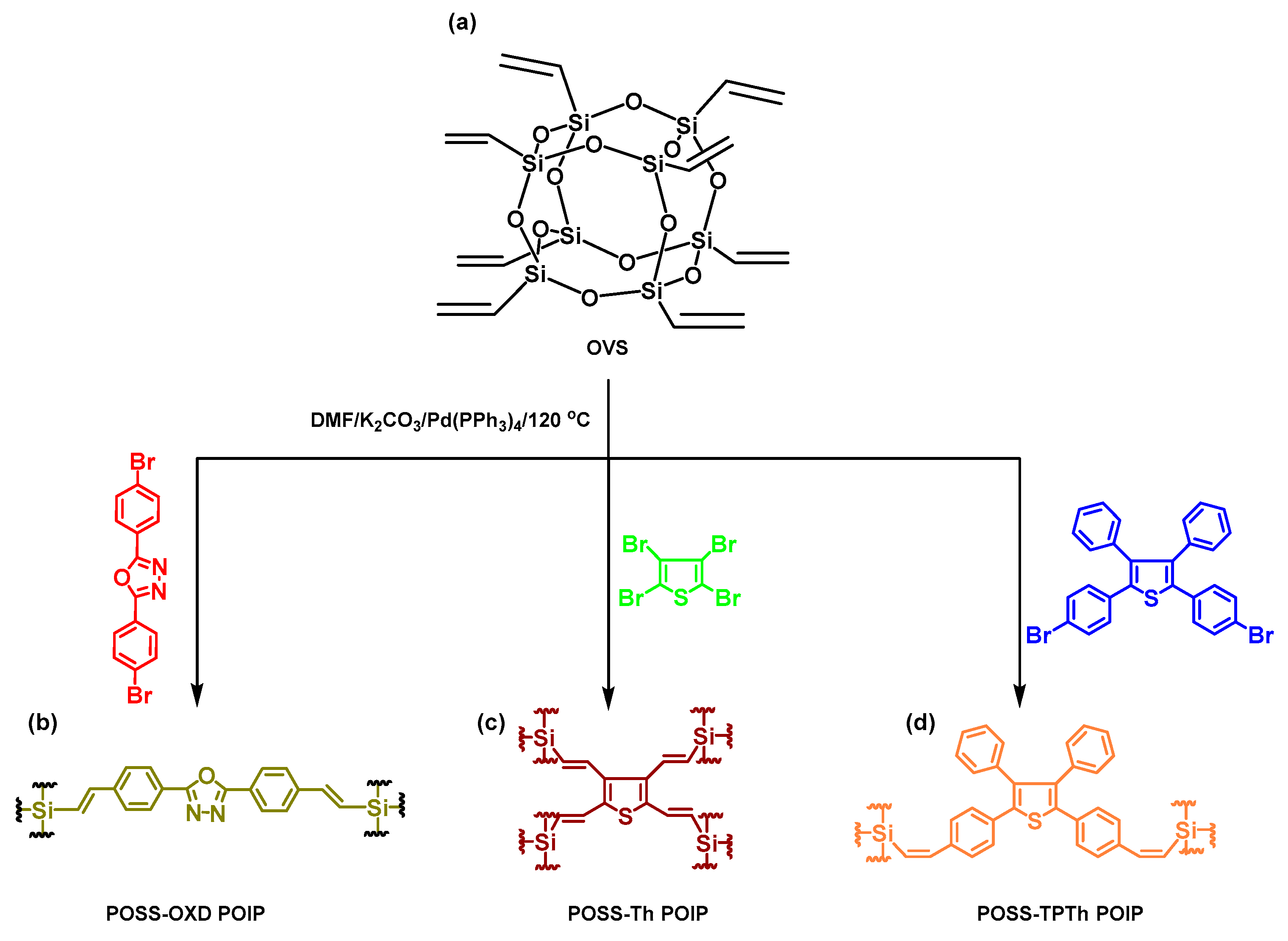
2.7. POSS-OXD, POSS-Th, and POSS-TPTh POIPs
3. Results
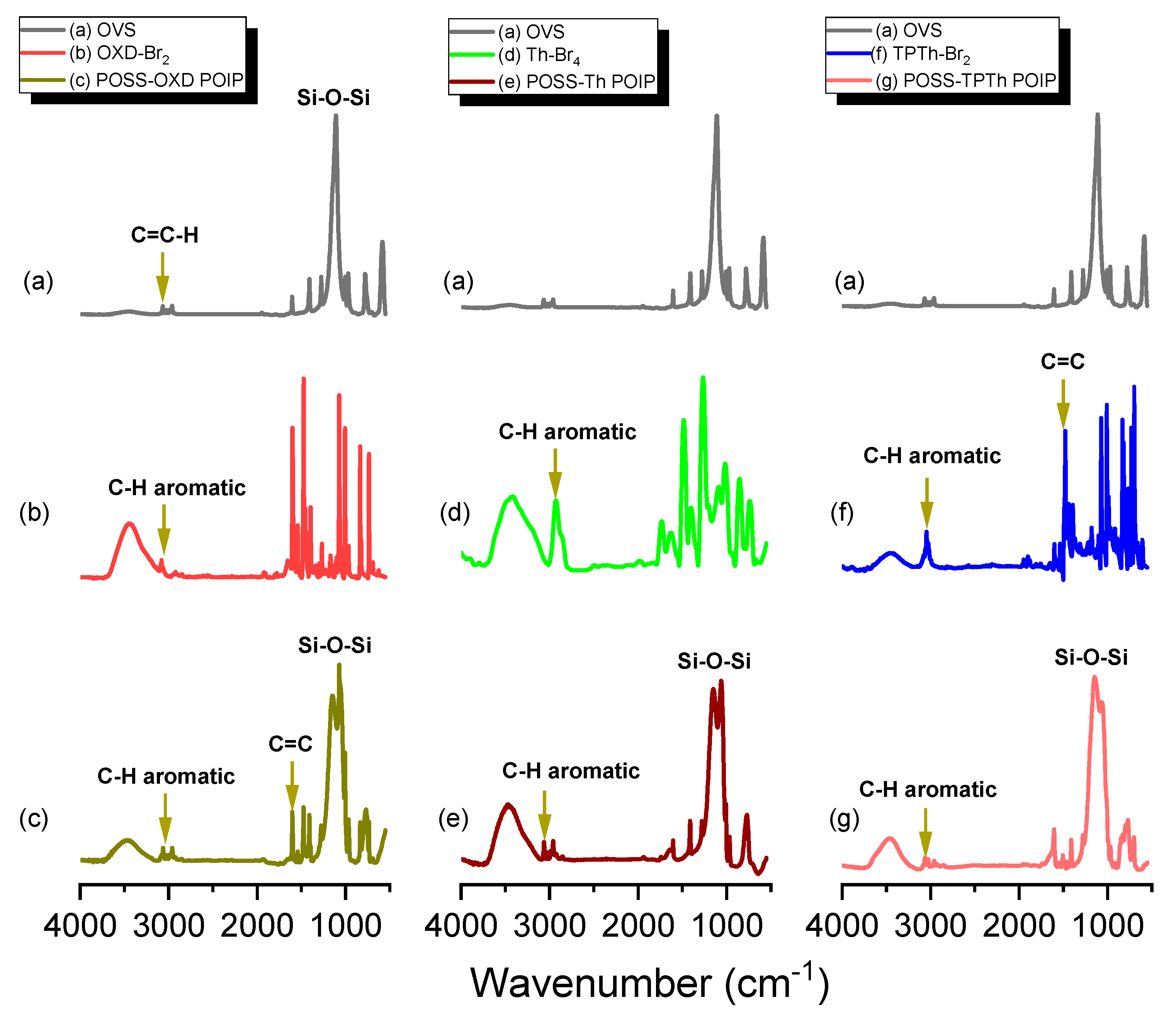
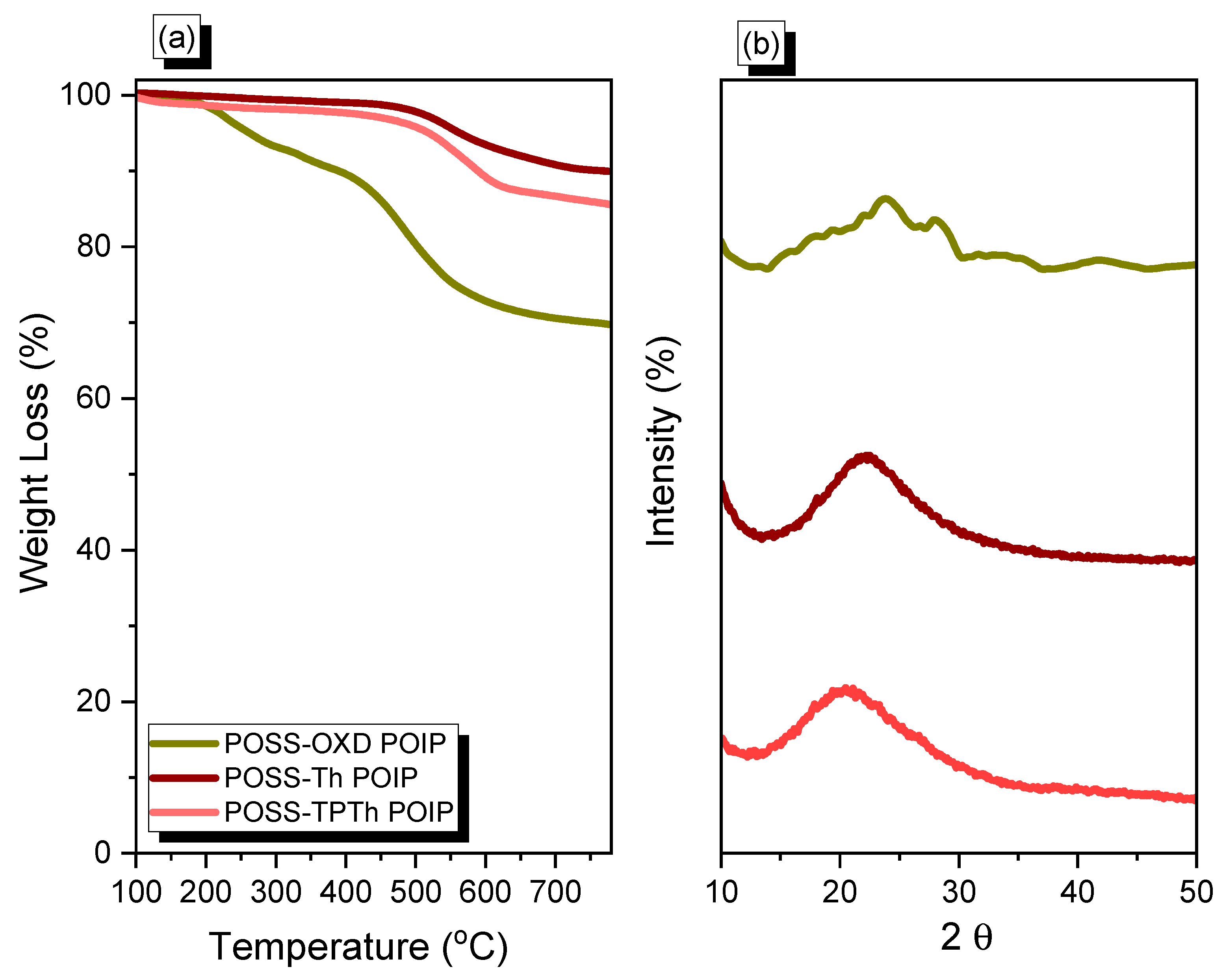
3.1. Synthesis and Character of POSS-OXD, POSS-Th and POSS-TPTh POIPs
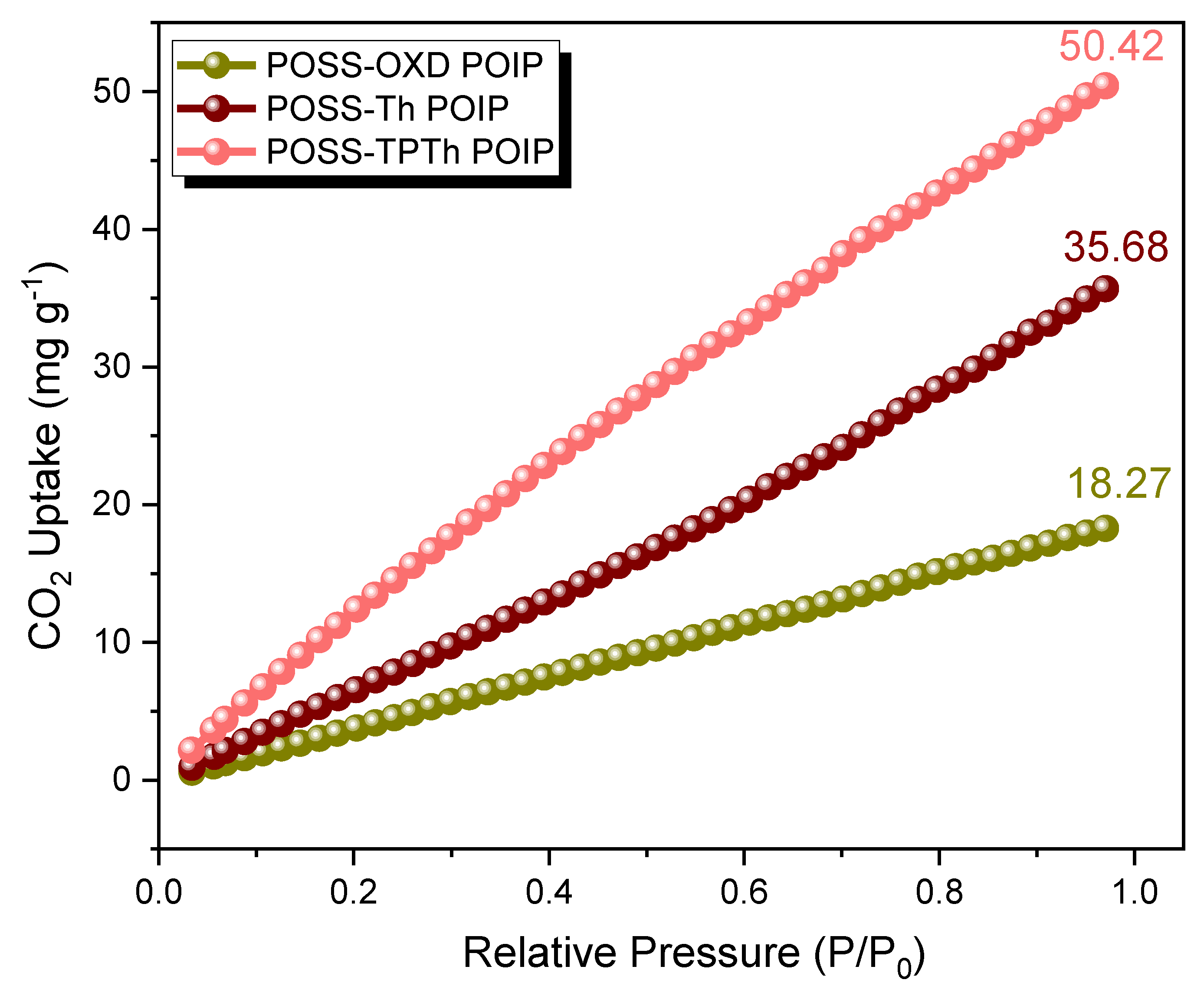
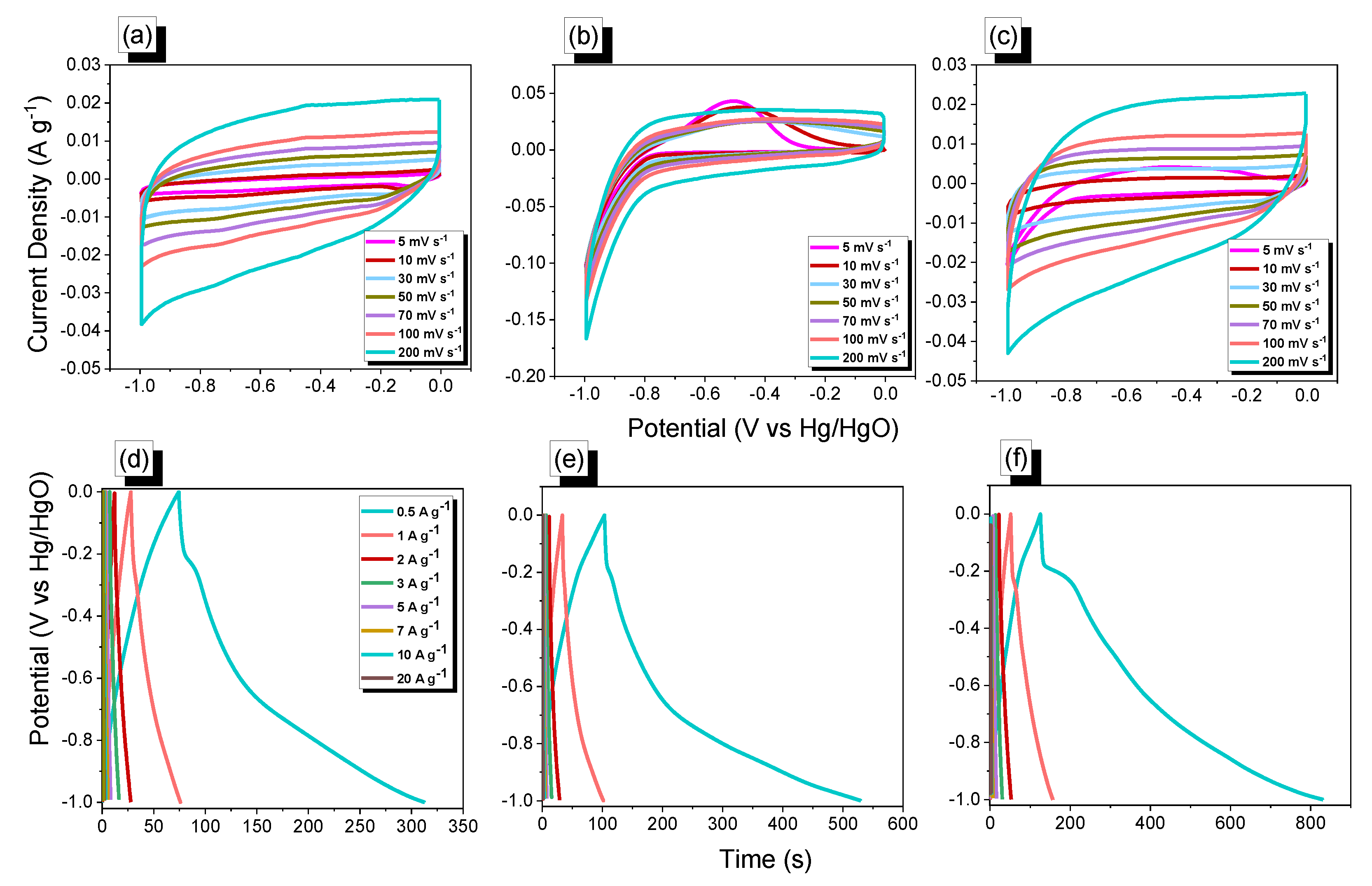
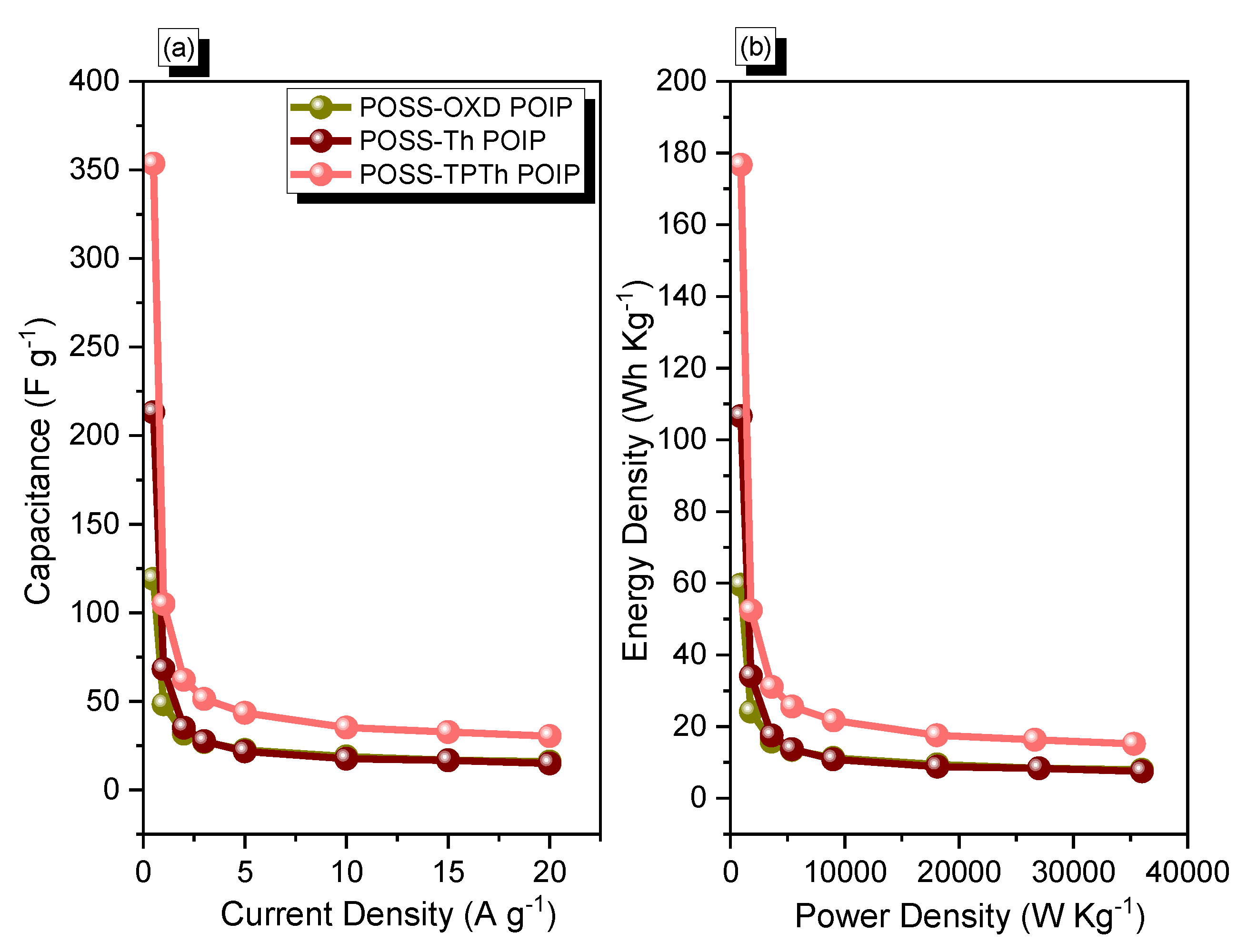
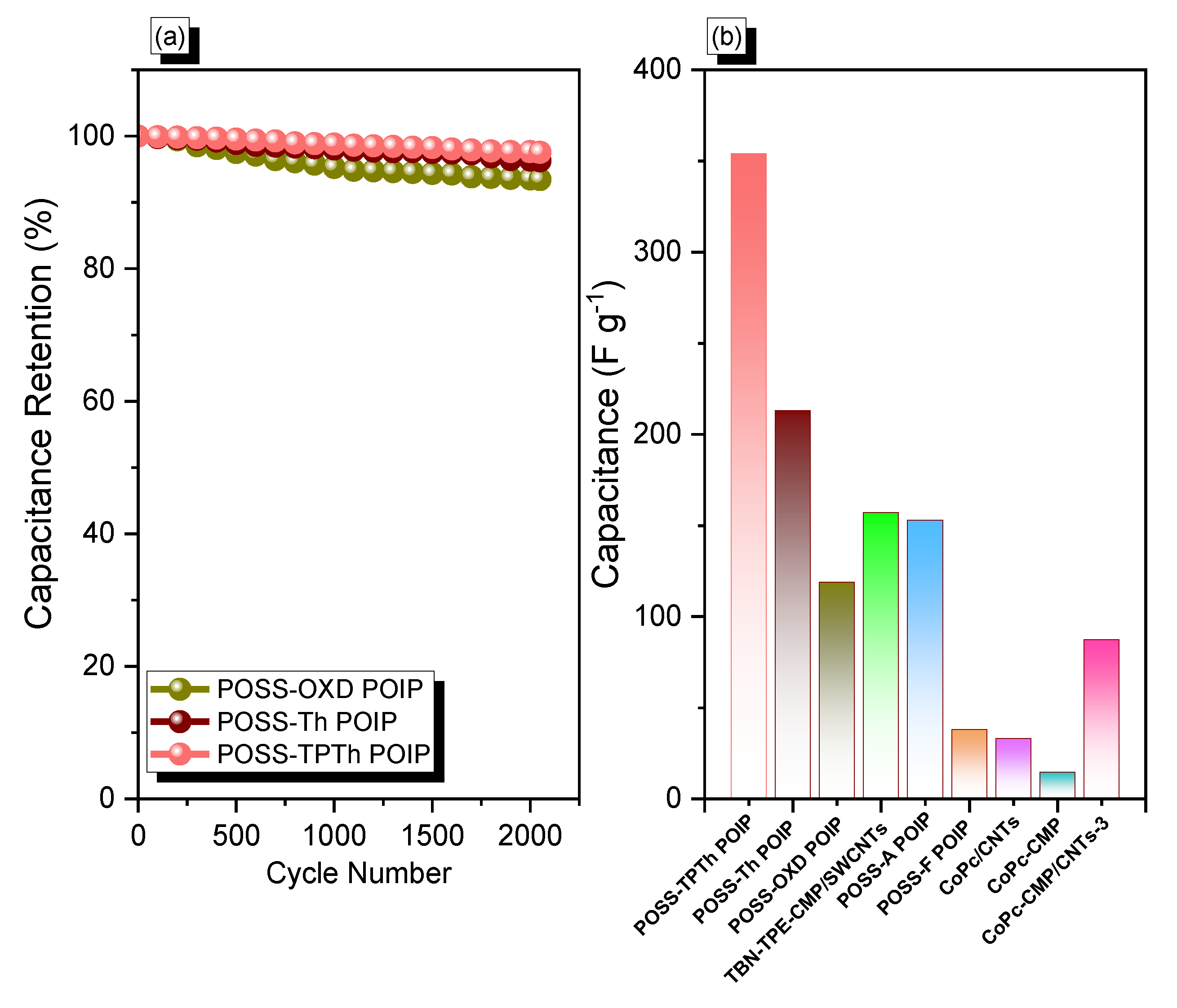
3.2. Electrochemical Performance of the POSS-OXD, POSS-Th, and POSS-TPTh POIPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wen, J.; Chen, S.; Liu, Y.; Chen, H.; Liu, B.; Yang, M.; Li, H. Triphenylimidazolium-incorporated, benzbisimidazole-linked porous organic polymers as efficient catalyst for CO2 conversion. Microporous Mesoporous Mater. 2022, 339, 111999. [Google Scholar] [CrossRef]
- Bai, W.; Wang, F.; Yan, L.; Sun, H.; Zhu, Z.; Chen, L.; Li, J.; Liang, W.; Li, A. Porous organic polymers (POPs) membrane via thiol-yne click chemistry for efficient particulate matter capture and microplastics separation. Microporous Mesoporous Mater. 2022, 329, 111509. [Google Scholar] [CrossRef]
- Chen, D.; Liu, C.; Tang, J.; Luo, L.; Yu, G. Fluorescent porous organic polymers. Polym. Chem. 2019, 10, 1168–1181. [Google Scholar] [CrossRef]
- Kiciński, W.; Dyjak, S.; Gratzke, M. Pyrolysis of Porous Organic Polymers under a Chlorine Atmosphere to Produce Heteroatom-Doped Microporous Carbons. Molecules 2021, 26, 3656. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Elsayed, M.H.; Elewa, A.M.; EL-Mahdy, A.F.M.; Yang, C.H.; Mohammed, A.A.K.; Chou, H.H.; Kuo, S.W. Pyrene-containing conjugated organic microporous polymers for photocatalytic hydrogen evolution from water. Catal. Sci. Technol. 2021, 11, 2229–2241. [Google Scholar] [CrossRef]
- Yang, Y.; Lai, Z. Ferrocene-based porous organic polymer for photodegradation of methylene blue and high iodine capture. Microporous Mesoporous Mater. 2021, 316, 110929. [Google Scholar] [CrossRef]
- Pan, X.; Ding, C.; Zhang, Z.; Ke, H.; Cheng, G. Functional porous organic polymer with high S and N for reversible iodine capture. Microporous Mesoporous Mater. 2020, 300, 110161. [Google Scholar] [CrossRef]
- Aly, K.I.; Sayed, M.M.; Mohamed, M.G.; Kuo, S.W.; Younis, O. A facile synthetic route and dual function of network luminescent porous polyester and copolyester containing porphyrin moiety for metal ions sensor and dyes adsorption. Microporous Mesoporous Mater. 2020, 298, 110063. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Zhang, X.; Mansoure, T.H.; El-Mahdy, A.F.M.; Huang, C.F.; Danko, M.; Xin, Z.; Kuo, S.W. Hypercrosslinked porous organic polymers based on tetraphenylanthraquinone for CO2 uptake and high-performance supercapacitor. Polymer 2020, 205, 122857. [Google Scholar] [CrossRef]
- Du, X.H.; Jiang, Z.; Liu, Z.; Xu, C. BODIPY-linked conjugated porous polymers for dye wastewater treatment. Microporous Mesoporous Mater. 2022, 322, 111711. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Hu, H.Y.; Madhu, M.; Ejaz, M.; Sharma, S.U.; Tseng, W.L.; Samy, M.M.; Huang, C.W.; Lee, J.T.; Kuo, S.W. Construction of Ultrastable Conjugated Microporous Polymers Containing Thiophene and Fluorene for Metal Ion Sensing and Energy Storage. Micromachines 2022, 13, 1466. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chaganti, S.V.; Sharma, U.S.; Samy, M.M.; Ejaz, M.; Lee, J.T.; Zhang, K.; Kuo, S.W. Constructing Conjugated Microporous Polymers Containing the Pyrene-4,5,9,10-Tetraone Unit for Energy Storage. Acs Appl. Energy Mater. 2022, 5, 10130–10140. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chaganti, S.V.; Sharma, L.M.S.; Samy, M.M.; Sharma, S.U.; Lee, J.T.; Elsayed, M.H.; Chou, H.H.; Kuo, S.W. Ultrastable Porous Organic Polymers Containing Thianthrene and Pyrene Units as Organic Electrode Materials for Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 6442–6452. [Google Scholar] [CrossRef]
- Sachs, M.; Sprick, R.S.; Pearce, D.; Hillman, S.A.J.; Monti, A.; Guilbert, A.A.Y.; Brownbill, N.J.; Dimitrov, S.; Shi, X.; Blanc, F.; et al. Understanding structure-activity relationships in linear polymer photocatalysts for hydrogen evolution. Nat. Commun. 2018, 9, 4968. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chen, W.C.; EL-Mahdy, A.F.M.; Kuo, S.W. Porous organic/inorganic polymers based on double-decker silsesquioxane for high-performance energy storage. J. Polym. Res. 2021, 28, 219. [Google Scholar] [CrossRef]
- Sprick, R.S.; Bai, Y.; Guilbert, A.A.Y.; Zbiri, M.; Aitchison, C.M.; Wilbraham, L.; Yan, Y.; Woods, D.J.; Zwijnenburg, M.A.; Cooper, A.I. Photocatalytic hydrogen evolution from water using fluorene and dibenzothiophene sulfone-conjugated microporous and linear polymers. Chem. Mater. 2019, 2, 305–313. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, L.; Lai, G.; Wei, M.; Jiang, X.; Bai, L. Nitrogen-doped hierarchically porous carbons derived from Polybenzoxazine for enhanced Supercapacitor performance. Nanomaterials 2019, 9, 131. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Kuo, S.W. Pyrene-functionalized tetraphenylethylene polybenzoxazine for dispersing single-walled carbon nanotubes and energy storage. Compos. Sci. Technol. 2020, 199, 108360. [Google Scholar] [CrossRef]
- Li, L.; Lu, F.; Xue, R.; Ma, B.L.; Li, Q.; Wu, N.; Liu, H.; Yao, W.Q.; Guo, H.; Yang, W. Ultrastabletriazine-based covalent organic framework with an interlayer hydrogen bonding for supercapacitor applications. Acs Appl. Mater. Interfaces 2019, 11, 26355–26363. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Sharma, S.U.; Liu, N.Y.; Mansoure, T.H.; Samy, M.M.; Chaganti, S.V.; Chang, Y.L.; Lee, J.Y.; Kuo, S.W. Ultrastable Covalent Triazine Organic Framework Based on Anthracene Moiety as Platform for High-Performance Carbon Dioxide Adsorption and Supercapacitors. Int. J. Mol. Sci. 2022, 23, 3174. [Google Scholar] [CrossRef]
- Elewa, A.M.; EL-Mahdy, A.F.M.; Elsayed, M.H.; Mohamed, M.G.; Kuo, S.W.; Chou, H.H. Sulfur-doped triazine-conjugated microporous polymers for achieving the robust visible-light-driven hydrogen evolution. Chem. Eng. J. 2021, 421, 129825. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, C.; Gao, X.; Shu, C.; Yan, C.; Wang, F.; Chen, Y.; Zeng, J.H.; Jiang, J.X. Structure evolution of azo-fused conjugated microporous polymers for high performance lithium-ion batteries anodes. J. Power Sources 2020, 453, 227868. [Google Scholar] [CrossRef]
- Yang, W.; Huang, B.; Li, L.; Zhang, K.; Li, Y.; Huang, J.; Tang, X.; Hu, T.; Yuan, K.; Chen, Y. Covalently Sandwiching MXene by Conjugated Microporous Polymers with Excellent Stability for Supercapacitors. Small Methods 2020, 4, 2000434. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Ahmed, M.M.M.; Du, W.T.; Kuo, S.W. Meso/microporous carbons from conjugated hyper-crosslinked polymers based on tetraphenylethene for high performance CO2 capture and supercapacitor. Molecules 2021, 26, 738. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Kuo, S.W. Directly synthesized nitrogen-and-oxygen–doped microporous carbons derived from a bio-derived polybenzoxazine exhibiting high-performance supercapacitance and CO2 uptake. Eur. Polym. J. 2020, 138, 109954. [Google Scholar] [CrossRef]
- Mei, L.; Cui, X.; Duan, Q.; Li, Y.; Lv, X.; Wang, H.G. Metal phthalocyanine-linked conjugated microporous polymer hybridized with carbon nanotubes as a high-performance flexible electrode for supercapacitors. Int. J. Hydrog. Energy 2020, 45, 22950–22958. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; EL-Mahdy, A.F.M.; Mansoure, T.H.; Wu, K.C.W.; Kuo, S.W. High-performance supercapacitor electrodes prepared from dispersions of tetrabenzonaphthalene-based conjugated microporous polymers and carbon nanotubes. Acs Appl. Mater. Interfaces 2021, 13, 51906–51916. [Google Scholar] [CrossRef]
- Samy, M.M.; Mekhemer, I.M.A.; Mohamed, M.G.; Elsayed, M.H.; Lin, K.H.; Yi-Kuan Chen, Y.K.; Wu, T.L.; Chou, H.H.; Kuo, S.W. Conjugated microporous polymers incorporating Thiazolo[5,4-d]thiazole moieties for Sunlight-Driven hydrogen production from water. Chem. Eng. J. 2022, 446, 137158. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, B.; Zou, Y.; Wang, S.; Mai, W.; Cao, Y.; Wang, Y.; Chen, J.; Li, T. Green synthesized cobalt-bipyridine constructed conjugated microporous polymer: An efficient heterogeneous catalyst for cycloaddition of epoxides via CO2 fixation under ambient conditions. Microporous Mesoporous Mater. 2019, 319, 110758. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Mu, P.; Wang, X.; He, Q.; Chen, Y.; Zeng, J.; Wang, F.; Xu, Y.; Jiang, J.X. Toward high performance thiophene-containing conjugated microporous polymer anodes for lithium-ion batteries through structure design. Adv. Funct. Mater. 2018, 28, 1705432. [Google Scholar] [CrossRef]
- Zhang, C.; Qiao, Y.; Xiong, P.; Ma, W.; Bai, P.; Wang, X.; Li, Q.; Zhao, J.; Xu, Y.; Chen, Y.; et al. Conjugated microporous polymers with tunable electronic structure for high-performance potassium-ion batteries. Acs Nano. 2019, 13, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Ashraf, N.; Mao, L.; Faul, C.F.J.; Wei, Z. Conjugated microporous polymers for energy storage: Recent progress and challenges. Nano Energy 2021, 85, 105958. [Google Scholar] [CrossRef]
- Saber, A.F.; Sharma, S.U.; Lee, J.T.; El-Mahdy, A.F.M.; Kuo, S.W. Carbazole-conjugated microporous polymers from Suzuki–Miyaura coupling for supercapacitors. Polymer 2022, 254, 125070. [Google Scholar] [CrossRef]
- Lee, J.S.M.; Cooper, A.I. Advances in conjugated microporous polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef]
- Mei, L.; Cui, X.; Wei, J.; Duan, Q.; Li, Y. Metal phthalocyanine-based conjugated microporous polymer/carbon nanotube composites as flexible electrodes for supercapacitors. Dye. Pigm. 2021, 190, 109299. [Google Scholar] [CrossRef]
- Vinodh, R.; Gopi, C.V.V.M.; Kummara, V.G.R.; Atchudanc, R.; Ahamad, T.; Sambasivam, S.; Yi, M.; Obaidat, I.M.; Kim, H.J. A review on porous carbon electrode material derived from hypercrosslinked polymers for supercapacitor applications. J. Energy Stor. 2020, 32, 101831. [Google Scholar] [CrossRef]
- Seenath, J.S.; Biswal, B.P. Construction of MXene-Coupled Nitrogen-Doped Porous Carbon Hybrid from a Conjugated Microporous Polymer for High-Performance Supercapacitors. Adv. Energy Sustain. Res. 2021, 2, 2000052. [Google Scholar] [CrossRef]
- Bhanja, P.; Das, S.K.; Bhunia, K.; Pradhan, D.; Hayashi, T.; Hijikata, Y.; Irle, S.; Bhaumik, A. A new porous polymer for highly efficient capacitive energy storage. Acs Sustain. Chem. Eng. 2018, 6, 202–209. [Google Scholar] [CrossRef]
- Wang, H.G.; Cheng, Z.H.; Liao, Y.Z.; Li, J.H.; Weber, J.; Thomas, A.; Faul, C.F.J. Conjugated microporous polycarbazole networks as precursors for nitrogen enriched microporous carbons for CO2 storage and electrochemical capacitors. Chem. Mater. 2017, 29, 4885. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chang, W.C.; Kuo, S.W. Crown Ether– and Benzoxazine–Linked Porous Organic Polymers Displaying Enhanced Metal Ion and CO2 Capture through Solid State Chemical Transformation. Macromolecules 2022, 55, 7879–7892. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chen, T.C.; Kuo, S.W. Solid-state chemical transformations to enhance gas capture in benzoxazine-linked conjugated microporous polymers. Macromolecules 2021, 54, 5866–5877. [Google Scholar] [CrossRef]
- Feng, Y.; Jia, Y.; Guang, S.; Xu, H. Study on thermal enhancement mechanism of POSS-containing hybrid nanocomposites and relationship between thermal properties and their molecular structure. J. Appl. Polym. Sci. 2010, 115, 2212–2220. [Google Scholar] [CrossRef]
- Madhavan, K.; Reddy, B.S.R. Synthesis and characterization of polyurethane hybrids: Influence of the polydimethylsiloxane linear chain and silsesquioxane cubic structure on the thermal and mechanical properties of polyurethane hybrids. J. Appl. Polym. Sci. 2009, 113, 4052–4065. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H. Selective dye adsorption and metal ion detection using multifunctional silsesquioxane-based tetraphenylethene linked nanoporous polymers. J. Mater. Chem. A 2017, 5, 9156–9162. [Google Scholar] [CrossRef]
- Yang, X.; Liu, H. Ferrocene-functionalized silsesquioxane-based porous polymer for efficient removal of dyes and heavy metal ions. Chem. Eur. J. 2018, 24, 13504–13511. [Google Scholar] [CrossRef]
- Yan, J.; Choi, J.H.; Jeong, Y.G. Freestanding supercapacitor electrode applications of carbon nanofibers based on polyacrylonitrile and polyhedral oligomeric silsesquioxane. Mater. Des. 2018, 139, 72–80. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Jiang, X.; Luo, Y.; Lyu, Y. POSS-based microporous polymers: Efficient Friedel-Crafts synthesis, CO2 capture and separation properties. Micropor. Mesopor. Mater. 2017, 250, 203–209. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef]
- Shen, R.; Du, Y.; Yang, X.; Liu, H. Silsesquioxanes-based porous functional polymers for water purification. J. Mater. Sci. 2020, 55, 7518–7529. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional Silica and Carbon Nanocomposites Based on Polybenzoxazines. Macromol. Chem. Phys. 2019, 220, 1800306. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen bonding interactions in polymer/polyhedral oligomeric silsesquioxane nanomaterials. J. Polym. Res. 2022, 29, 69. [Google Scholar] [CrossRef]
- Ren, J.; Feng, J.; Wang, L.; Chen, G.; Zhou, Z.; Li, Q. High specific surface area hybrid silica aerogel containing POSS. Microporous Mesoporous Mater. 2021, 310, 110456. [Google Scholar] [CrossRef]
- Ghasemi, M.; Fahimi, Z.; Moradlou, O.; Seize, M.R. Porous gel polymer electrolyte for the solid state metal oxide supercapacitor with a wide potential window. J. Taiwan Inst. Chem. Eng. 2021, 118, 223–231. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Progress in the self-assembly of organic/inorganic polyhedral oligomeric silsesquioxane (POSS) hybrids. Soft Matter 2022, 18, 5535–5561. [Google Scholar] [CrossRef]
- Seo, S.; Chaikittisilp, W.; Koike, N.; Yokoi, T.; Okubo, T. Porous inorganic–organic hybrid polymers derived from cyclic siloxane building blocks: Effects of substituting groups on mesoporous structures. Microporous Mesoporous Mater. 2019, 278, 212–218. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Tsai, M.Y.; Wang, C.F.; Huang, C.F.; Danko, M.; Dai, L.; Chen, T.; Kuo, S.W. Multifunctional polyhedral oligomeric silsesquioxane (POSS) based hybrid porous materials for CO2 uptake and iodine adsorption. Polymers 2021, 13, 221. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Mansoure, T.H.; Takashi, Y.; Samy, M.M.; Chen, T.; Kuo, S.-W. Ultrastable porous organic/inorganic polymers based on polyhedral oligomeric silsesquioxane (POSS) hybrids exhibiting high performance for thermal property and energy storage. Microporous Mesoporous Mater. 2021, 328, 111505. [Google Scholar] [CrossRef]
- Wang, D.; Yang, W.; Feng, S.; Liu, H. Amine post-functionalized POSS-based porous polymers exhibiting simultaneously enhanced porosity and carbon dioxide adsorption properties. Rsc Adv. 2016, 6, 13749–13756. [Google Scholar] [CrossRef]
- Wang, D.; Xue, L.; Li, L.; Deng, B.; Feng, S.; Liu, H.; Zhao, X. Rational design and synthesis of hybrid porous polymers derived from polyhedral oligomeric silsesquioxanes via Heck coupling reactions. Macromol. Rapid Commun. 2013, 34, 34861–34866. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Liu, N.Y.; EL-Mandy, A.F.M.; Kuo, S.W. Ultrastable luminescent hybrid microporous polymers based on polyhedral oligomeric silsesquioxane for CO2 uptake and metal ion sensing. Microporous Mesoporous Mater. 2021, 311, 110695. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, H.; Jiang, C.; Liu, H. Silsesquioxane-based triphenylamine functionalized porous polymer for CO2, I2 capture and nitro-aromatics detection. Polymer 2020, 186, 122004. [Google Scholar] [CrossRef]
- Du, Y.; Unno, M.; Liu, H. Hybrid nanoporous materials derived from ladder- and cage-type silsesquioxanes for water treatment. ACS Appl. Nano Mater. 2020, 3, 1535–1541. [Google Scholar] [CrossRef]
- Wang, Y.; Soldatov, M.; Wang, Q.; Liu, H. Phosphazene functionalized silsesquioxane-based porous polymers for absorbing I2, CO2 and dyes. Polymer 2021, 218, 123491. [Google Scholar] [CrossRef]
- Wang, D.; Yang, W.; Feng, S.; Liu, H. Constructing hybrid porous polymers from cubic octavinyl silsequioxane and planar halogenated benzene. Polym. Chem. 2014, 5, 3634–3642. [Google Scholar] [CrossRef]
- Sajjad, M.; Tao, R.; Kang, K.; Luo, S.; Qiu, L. Phosphine-based porous organic polymer/rGO aerogel composites for high-performance asymmetric supercapacitor. Acs Appl. Energy Mater. 2021, 4, 828–838. [Google Scholar] [CrossRef]
- Xu, J.; He, Y.; Bi, S.; Wang, M.; Yang, P.; Wu, D.; Wang, J.; Zhang, F. An olefin-linked covalent organic framework as a flexible thin-film electrode for a high-performance micro-supercapacitor. Angew. Chem. Int. Ed. 2019, 131, 12193–12197. [Google Scholar] [CrossRef]
- He, X.; Xie, X.; Wang, J.; Ma, X.; Xie, Y.; Gu, J.; Xiao, N.; Qiu, J. From fluorine molecules to ultrathin carbon nanonets with an enhanced charge transfer capability for supercapacitors. Nanoscale 2019, 11, 6610–6619. [Google Scholar] [CrossRef]
- Khattak, A.M.; Ghazi, Z.A.; Liang, B.; Khan, N.A.; Iqbal, A.; Li, L.; Tang, Z. A redox active 2D covalent organic framework with pyridine moieties capable of faradaic energy storage. J. Mater. Chem. A 2016, 4, 16312–16317. [Google Scholar] [CrossRef]
- Xu, L.; Shi, R.; Li, H.; Han, C.; Wu, M.; Wong, C.P.; Kang, F.; Li, B. Pseudocapacitive anthraquinone modified with reduced graphene oxide for flexible symmetric all solid-state supercapacitors. Carbon 2018, 127, 459–468. [Google Scholar] [CrossRef]
- Khattak, A.M.; Sin, H.; Ghazi, Z.A.; He, X.; Liang, B.; Khan, N.A.; Alanagh, H.R.; Iqbal, A.; Li, L.; Tang, Z. Controllable fabrication of redox-active conjugated microporous polymers on reduced graphene oxide for high performance faradaic energy storage. J. Mater. Chem. 2018, 6, 18827–18832. [Google Scholar] [CrossRef]
- Kusuma, H.D.; Rochmadi; Prasetyo, I.; Ariyanto, T. Mesoporous Manganese Oxide/Lignin-Derived Carbon for High Performance of Supercapacitor Electrodes. Molecules 2021, 26, 7104. [Google Scholar] [CrossRef] [PubMed]
- DeBlase, C.R.; Silberstein, K.E.; Truong, T.T.; Abruña, H.D.; Dichtel, W.R. β-Ketoenamine-Linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 2013, 135, 16821–16824. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Yang, Y.; Hu, Z.; An, Y.; Zhang, Q.; Yang, X.; Wang, X.; Wu, H. Redox-active organic molecules functionalized nitrogen-doped porous carbon derived from metal-organic framework as electrode materials for supercapacitor. Electrochim. Acta 2017, 223, 74–84. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Zhang, T.; Song, X.; Chen, W.; Chen, L. 2D redox-active covalent organic frameworks for supercapacitors: Design, synthesis, and challenges. Small 2021, 17, 2005073. [Google Scholar] [CrossRef]
- Luo, B.C.; Chen, Y.; Zhang, Y.B.; Huo, J.Q. Nitrogen-rich anthraquinone–triazine conjugated microporous polymer networks as high-performance supercapacitor. New J. Chem. 2021, 45, 17278–17286. [Google Scholar] [CrossRef]
- He, Y.; Cheng, Z.H.; Zuo, H.Y.; Yan, C.N.; Liao, Y.Z. Green Synthesis of Pyridyl Conjugated Microporous Polymers as Precursors for Porous Carbon Microspheres for Efficient Electrochemical Energy Storage. ChemElectroChem 2019, 7, 959–966. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, C.W.; Lee, S.M.; Kim, H.J.; Ko, Y.; Choi, J.; Son, S.U. Nanoparticulate Conjugated Microporous Polymer with Post-Modified Benzils for Enhanced Pseudocapacitor Performance. Chem. Eur. J. 2020, 26, 12343–12348. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.-F.; Lai, W.-Y.; Huang, W. Porous Organic Polymers as Promising Electrode Materials for Energy Storage Devices. Adv. Mater. Technol. 2020, 5, 2000154. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Santos, C.S.; Garcia, J.R.; Vidotti, M.; Marchesi, L.F.; Pessoa, C.A. IR drop studies of poly(aniline)-based modified electrodes. J. Electroanal. Chem. 2020, 878, 114662. [Google Scholar] [CrossRef]



| Sample | Td10 (°C) | Char Yield (wt.%) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Capacity at 0.5 A g−1 (F g−1) |
|---|---|---|---|---|---|---|
| POSS-OXD POIP | 386 | 69 | 94 | 0.02 | 2.34 | 119 |
| POSS-Th POIP | 786 | 90 | 605 | 0.23 | 1.98 | 213 |
| POSS-TPTh POIP | 586 | 86 | 682 | 0.23 | 1.87 | 354 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejaz, M.; Mohamed, M.G.; Sharma, S.U.; Lee, J.-T.; Huang, C.-F.; Chen, T.; Kuo, S.-W. An Ultrastable Porous Polyhedral Oligomeric Silsesquioxane/Tetraphenylthiophene Hybrid as a High-Performance Electrode for Supercapacitors. Molecules 2022, 27, 6238. https://doi.org/10.3390/molecules27196238
Ejaz M, Mohamed MG, Sharma SU, Lee J-T, Huang C-F, Chen T, Kuo S-W. An Ultrastable Porous Polyhedral Oligomeric Silsesquioxane/Tetraphenylthiophene Hybrid as a High-Performance Electrode for Supercapacitors. Molecules. 2022; 27(19):6238. https://doi.org/10.3390/molecules27196238
Chicago/Turabian StyleEjaz, Mohsin, Mohamed Gamal Mohamed, Santosh U. Sharma, Jyh-Tsung Lee, Chih-Feng Huang, Tao Chen, and Shiao-Wei Kuo. 2022. "An Ultrastable Porous Polyhedral Oligomeric Silsesquioxane/Tetraphenylthiophene Hybrid as a High-Performance Electrode for Supercapacitors" Molecules 27, no. 19: 6238. https://doi.org/10.3390/molecules27196238
APA StyleEjaz, M., Mohamed, M. G., Sharma, S. U., Lee, J.-T., Huang, C.-F., Chen, T., & Kuo, S.-W. (2022). An Ultrastable Porous Polyhedral Oligomeric Silsesquioxane/Tetraphenylthiophene Hybrid as a High-Performance Electrode for Supercapacitors. Molecules, 27(19), 6238. https://doi.org/10.3390/molecules27196238











