Nanohybrid of Thymol and 2D Simonkolleite Enhances Inhibition of Bacterial Growth, Biofilm Formation, and Free Radicals
Abstract
:1. Introduction
2. Results and Discussion
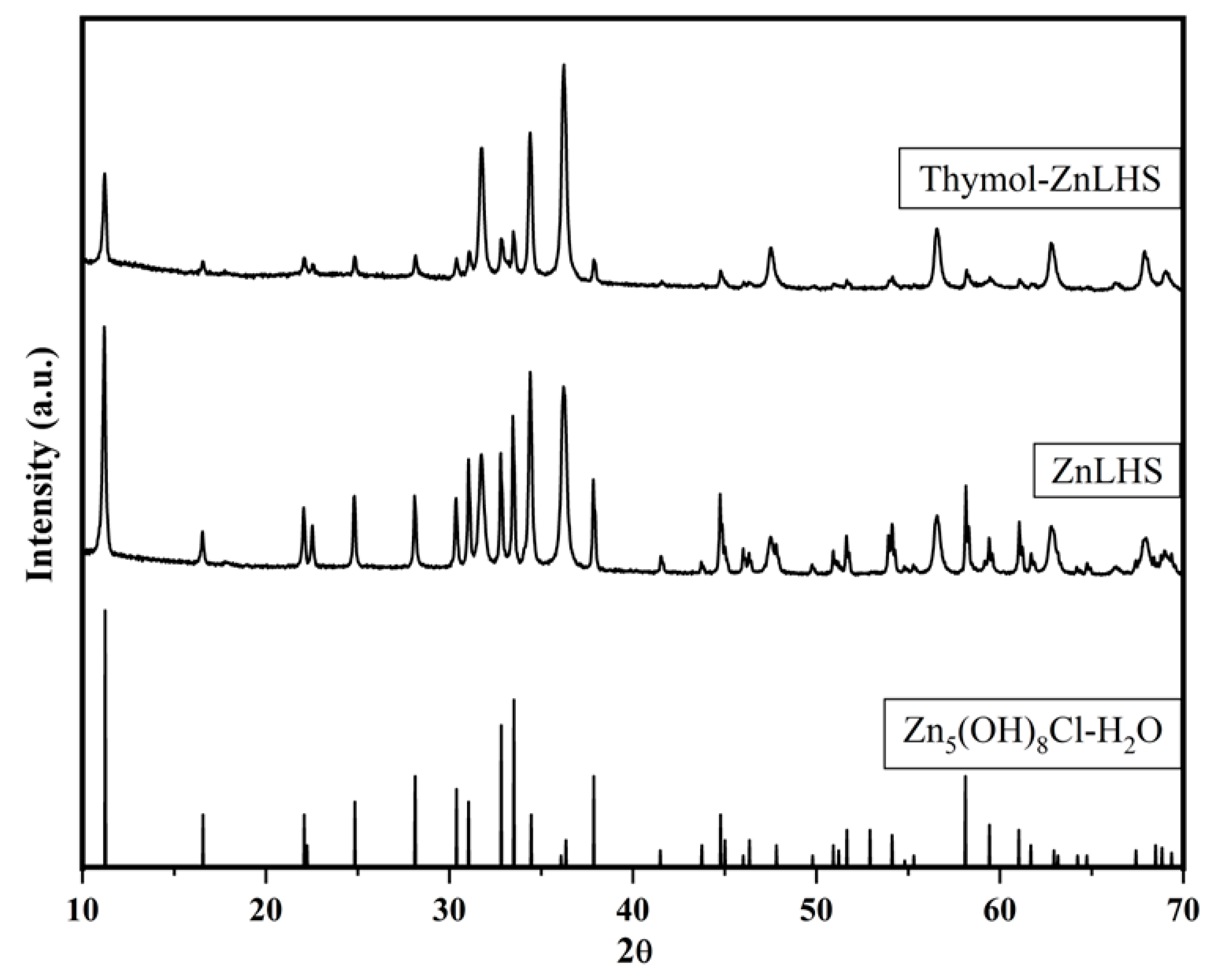
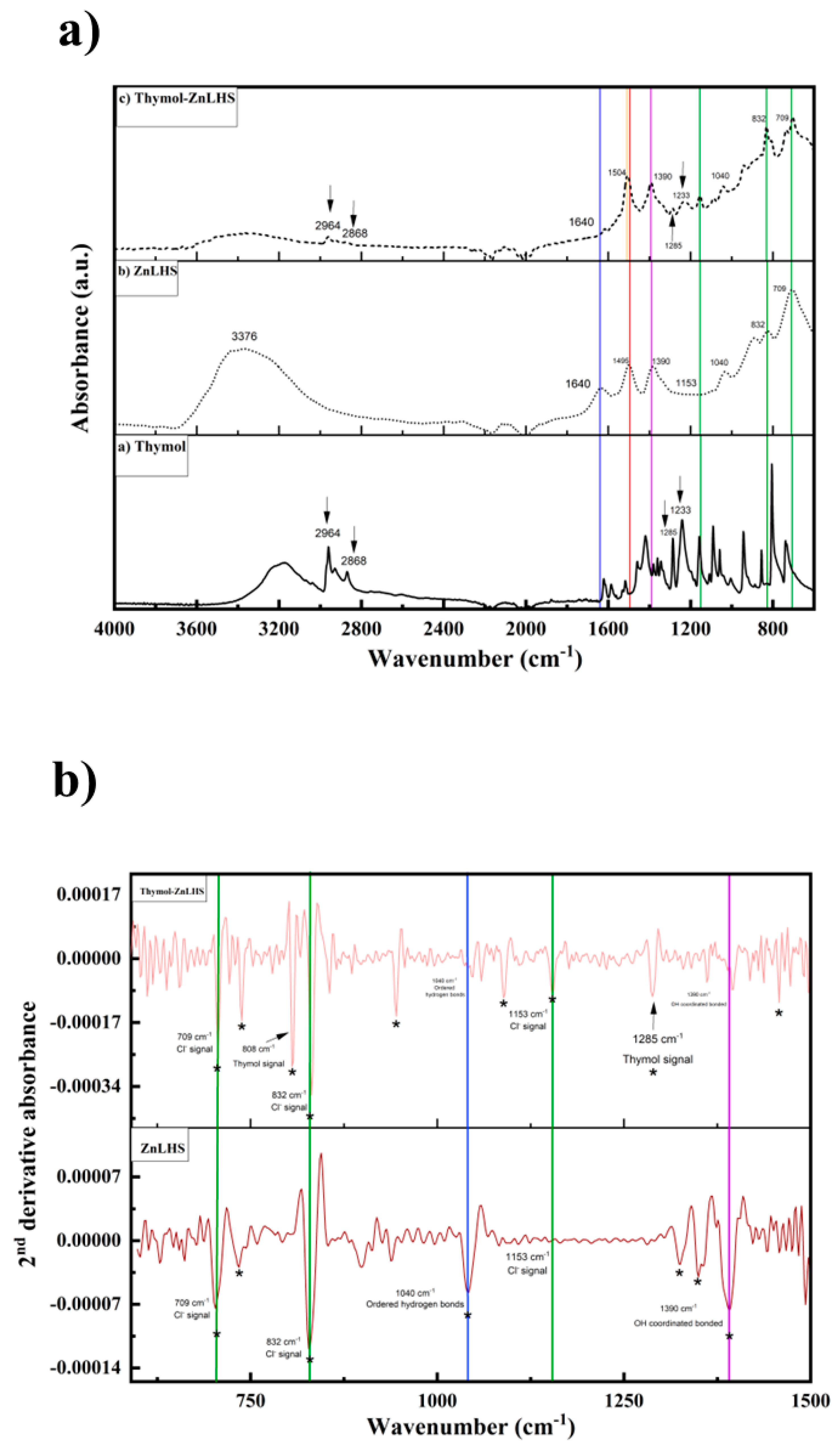
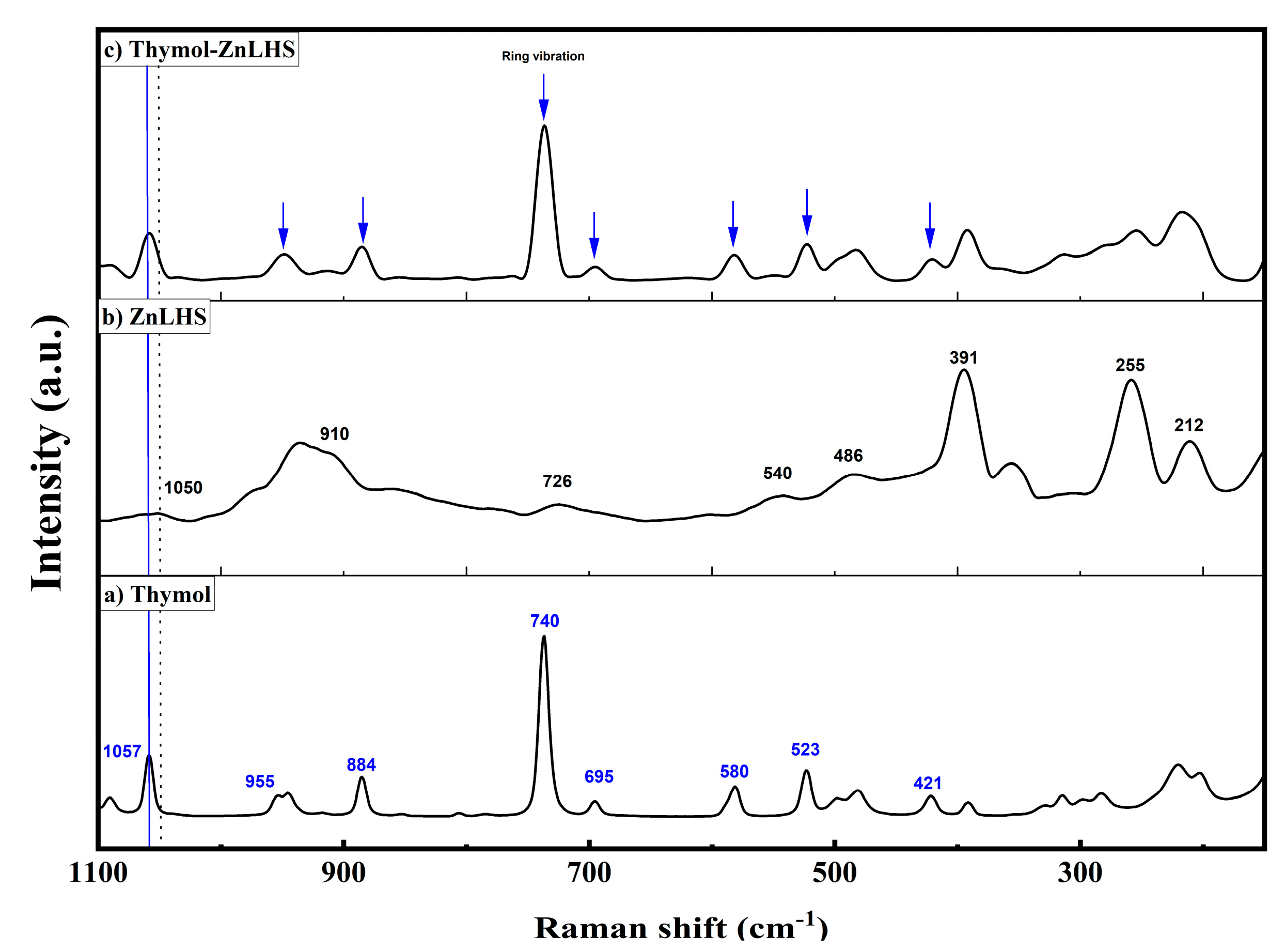
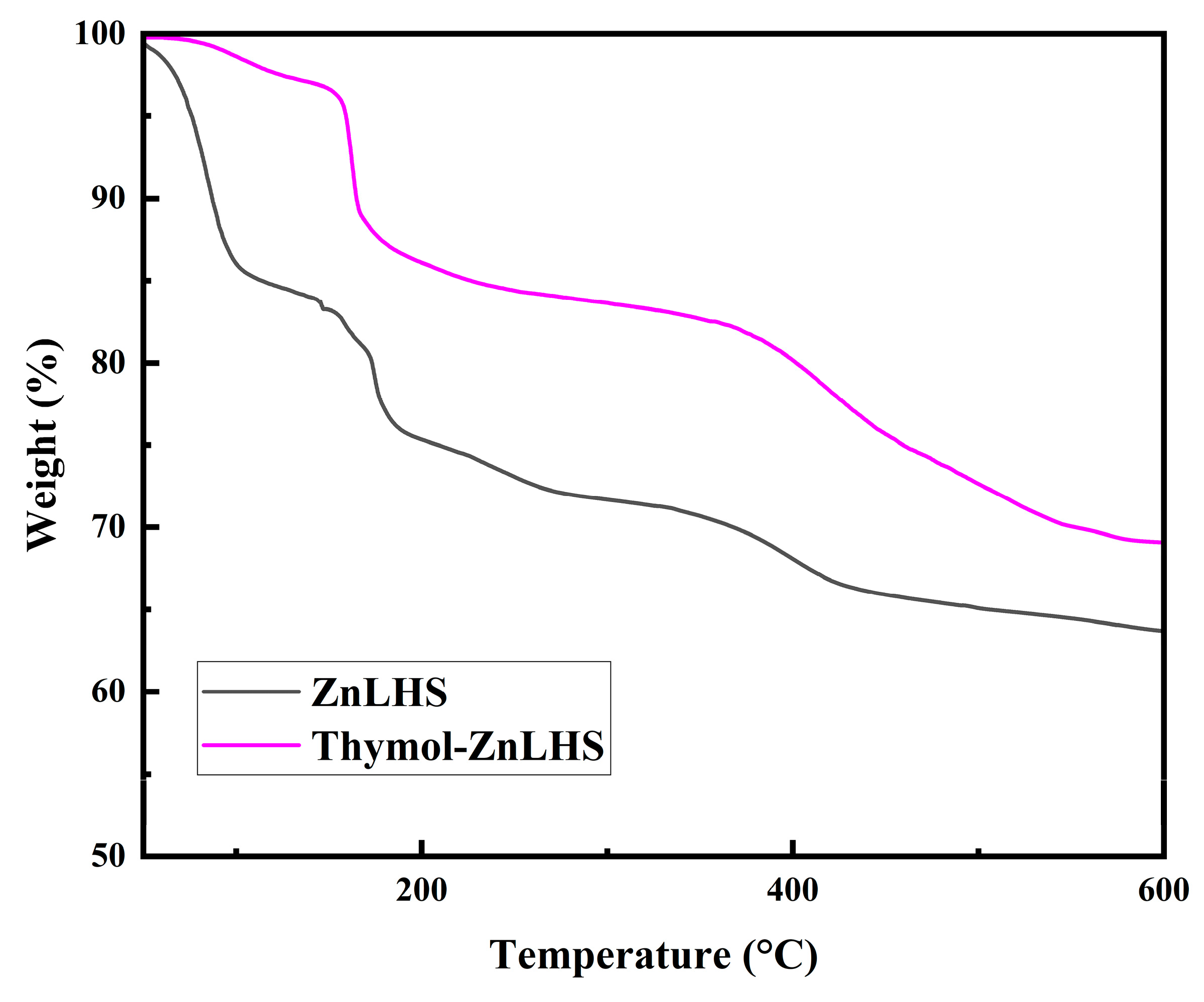
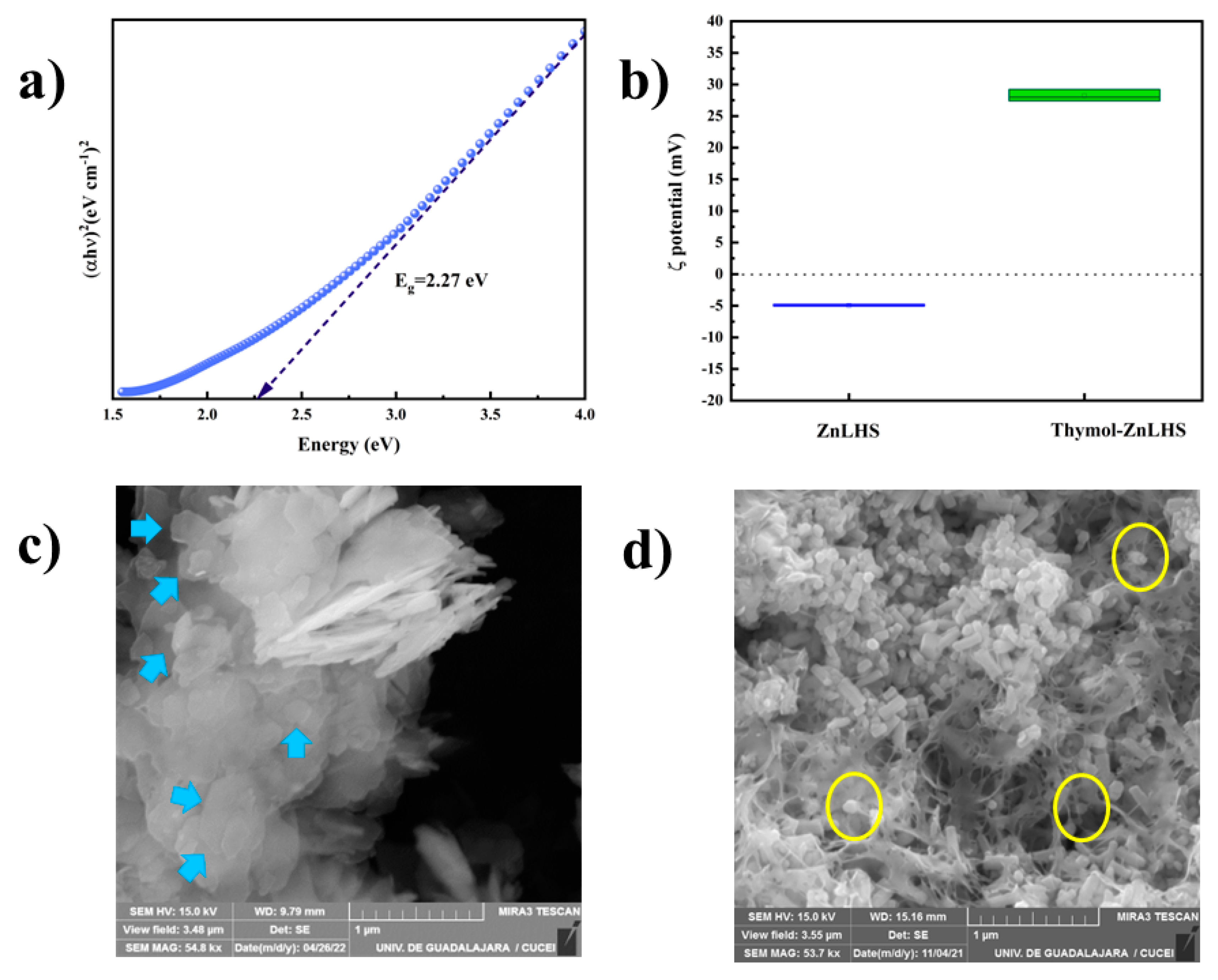
2.1. Characterization
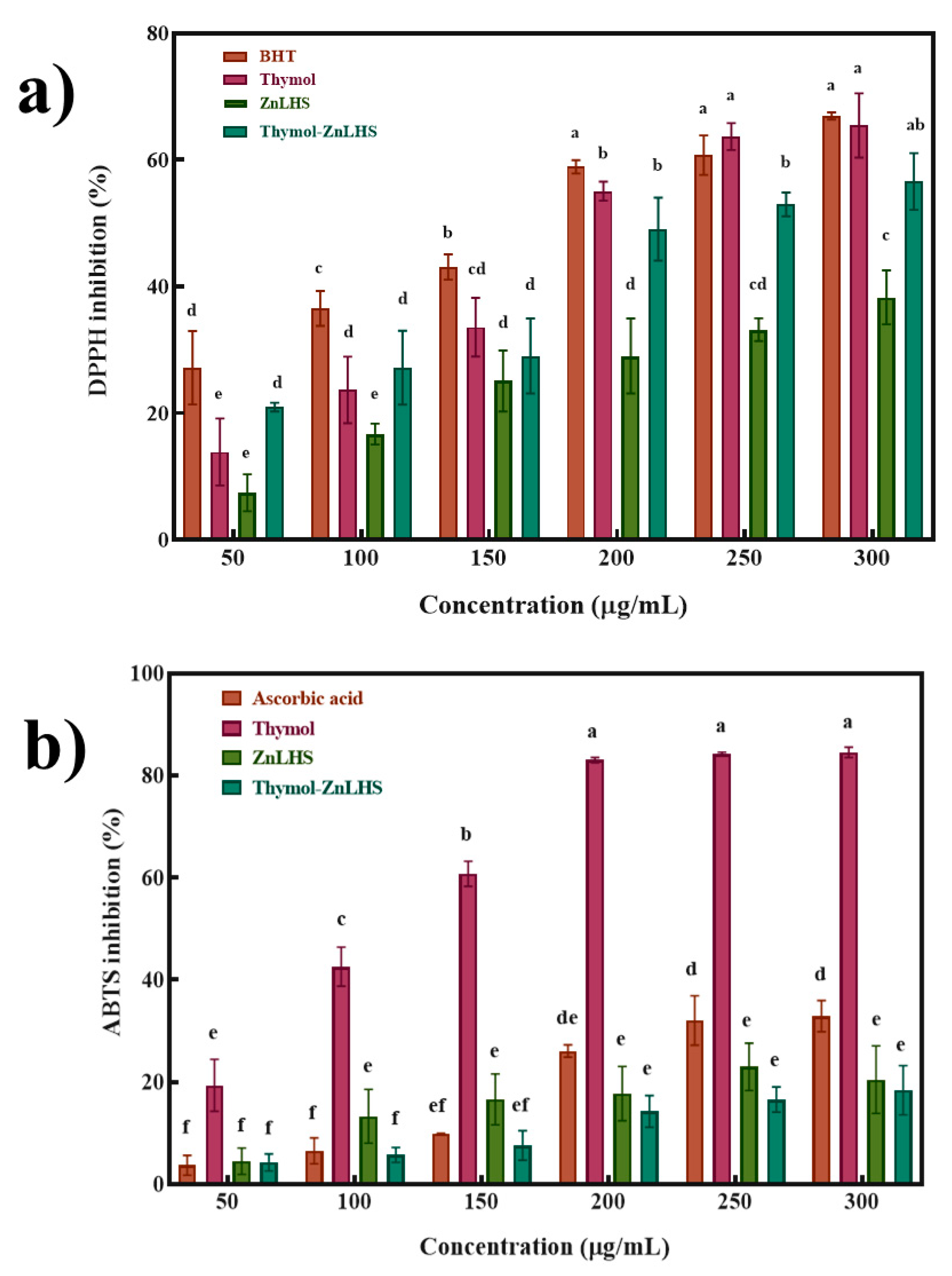
2.2. Antioxidant Activity
2.3. Antibacterial Activity
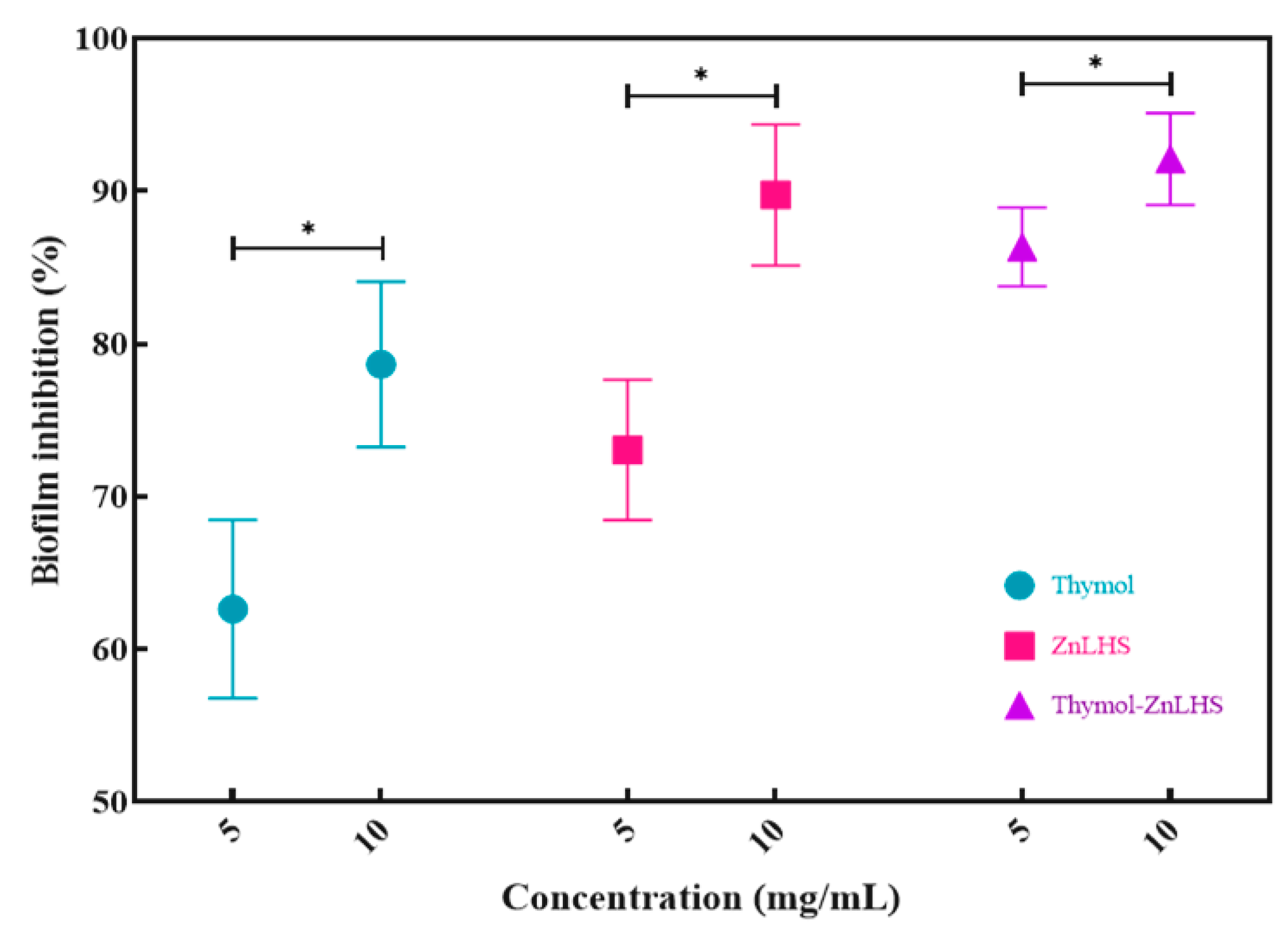
2.4. Inhibition of Biofilm Formation
3. Materials and Methods
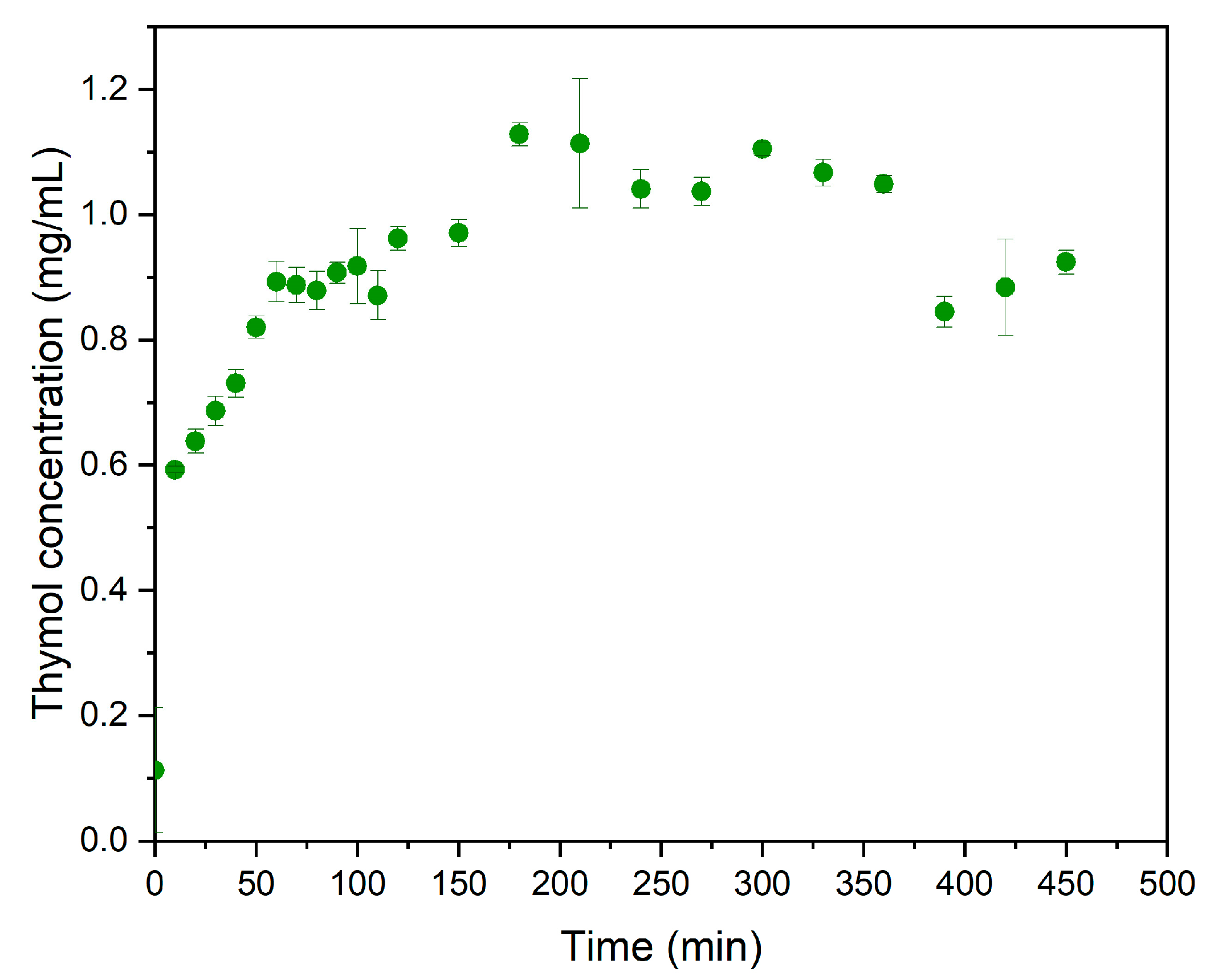
3.1. Synthesis of ZnLHS and Thymol–ZnLHS
3.2. Characterization
3.3. Antioxidant Activity
3.4. Antimicrobial Evaluation
3.5. Antibiofilm Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Flores, P.I.G.; Valenzuela, R.B.; Ruiz, L.D.; López, C.M.; Cháirez, F.E. Antibacterial activity of five terpenoid compounds: Carvacrol, limonene, linalool, α-terpinene and thymol. Trop. Subtrop. Agroecosyst. 2019, 22, 241–248. [Google Scholar]
- Rúa, J.; del Valle, P.; de Arriaga, D.; Fernández-Álvarez, L.; García-Armesto, M.R. Combination of Carvacrol and Thymol: Antimicrobial Activity Against Staphylococcus aureus and Antioxidant Activity. Foodborne Pathog. Dis. 2019, 16, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.S.; Blagojević, P.D.; Stojanović-Radić, Z.Z.; Stojanović, N.M. Antimicrobial Plant Metabolites: Structural Diversity and Mechanism of Action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [PubMed]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; ElNesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2020, 13, 632–641. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Paul, A.; Augustine, R.; Hasan, A.; Zahid, A.A.; Thomas, S.; Agatemor, C.; Ghosal, K. Halloysite nanotube and chitosan polymer composites: Physicochemical and drug delivery properties. J. Drug Deliv. Sci. Technol. 2022, 72, 103380. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Payal; Pandey, P. Role of Nanotechnology in Electronics: A Review of Recent Developments and Patents. Recent Patents Nanotechnol. 2022, 16, 45–66. [Google Scholar] [CrossRef]
- Ebadi, M.; Buskaran, K.; Bullo, S.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Synthesis and Cytotoxicity Study of Magnetite Nanoparticles Coated with Polyethylene Glycol and Sorafenib–Zinc/Aluminium Layered Double Hydroxide. Polymers 2020, 12, 2716. [Google Scholar] [CrossRef]
- Arizaga, G.G.C.; Satyanarayana, K.; Wypych, F. Layered hydroxide salts: Synthesis, properties and potential applications. Solid State Ion. 2007, 178, 1143–1162. [Google Scholar] [CrossRef]
- Machado, J.P.E.; de Freitas, R.A.; Wypych, F. Layered clay minerals, synthetic layered double hydroxides and hydroxide salts applied as pickering emulsifiers. Appl. Clay Sci. 2019, 169, 10–20. [Google Scholar] [CrossRef]
- Baig, M.M.; Hassan, M.; Ali, T.; Asif, H.M.; Asghar, A.; Ullah, S.; Alsafari, I.A.; Zulfiqar, S. Green 2D simonkolleite/zinc based nanostructures for superior antimicrobial and photocatalytic applications. Mater. Chem. Phys. 2022, 287, 126292. [Google Scholar] [CrossRef]
- Velázquez-Carriles, C.A.; Carbajal-Arizaga, G.G.; Silva-Jara, J.M.; Reyes-Becerril, M.C.; Aguilar-Uscanga, B.R.; Macías-Rodríguez, M.E. Chemical and biological protection of food grade nisin through their partial intercalation in laminar hydroxide salts. J. Food Sci. Technol. 2020, 57, 3252–3258. [Google Scholar] [CrossRef] [PubMed]
- Leal, D.A.; Silva, G.M.; Tedim, J.; Wypych, F.; Marino, C.E.B. Synthesis and characterization of gordaite, osakaite and simonkolleite by different methods: Comparison, phase interconversion, and potential corrosion protection applications. J. Solid State Chem. 2020, 291, 121595. [Google Scholar] [CrossRef]
- Mohsin, S.M.N.; Hussein, M.Z.; Sarijo, S.H.; Fakurazi, S.; Arulselvan, P.; Hin, T.-Y.Y. Synthesis of (cinnamate-zinc layered hydroxide) intercalation compound for sunscreen application. Chem. Cent. J. 2013, 7, 26. [Google Scholar] [CrossRef]
- Velazquez-Carriles, C.; Macías-Rodríguez, M.E.; Carbajal-Arizaga, G.G.; Silva-Jara, J.; Angulo, C.; Reyes-Becerril, M. Immobilizing yeast β-glucan on zinc-layered hydroxide nanoparticle improves innate immune response in fish leukocytes. Fish Shellfish Immunol. 2018, 82, 504–513. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Souto, E.B.; Durazzo, A.; Lucarini, M.; Novellino, E.; Tewari, D.; Wang, D.; Atanasov, A.G.; Santini, A. Big impact of nanoparticles: Analysis of the most cited nanopharmaceuticals and nanonutraceuticals research. Curr. Res. Biotechnol. 2020, 2, 53–63. [Google Scholar] [CrossRef]
- Ziyat, H.; Bennani, M.N.; Hajjaj, H.; Mekdad, S.; Qabaqous, O. Synthesis and characterization of crude hydrotalcite Mg–Al–CO3: Study of thymol adsorption. Res. Chem. Intermed. 2018, 44, 4163–4177. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, F.; Sánchez-Jiménez, C.; Rangel-Castañeda, I.A.; Carbajal-Arízaga, G.G.; Macías-Lamas, A.M.; Castillo-Romero, A.; Parra-Saavedra, K.J. Encapsulation of curcumin into layered double hydroxides improve their anticancer and antiparasitic activity. J. Pharm. Pharmacol. 2020, 72, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, Y.; Li, R.; Peng, S.; Ruan, R.; Li, J.; Liu, W. Fabrication of Caseinate Stabilized Thymol Nanosuspensions via the pH-Driven Method: Enhancement in Water Solubility of Thymol. Foods 2021, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, Z.; Djebbi, M.A.; Soussan, L.; Janot, J.-M.; Amara, A.B.H.; Balme, S. Adsorption of nisin into layered double hydroxide nanohybrids and in-vitro controlled release. Mater. Sci. Eng. C 2017, 76, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Nakhli, A.; Mbouga, M.G.N.; Bergaoui, M.; Khalfaoui, M.; Cretin, M.; Huguet, P. Non-linear analysis in estimating model parameters for thymol adsorption onto hydroxyiron-clays. J. Mol. Liq. 2017, 244, 201–210. [Google Scholar] [CrossRef]
- Jelínek, L.; Procházková, G.; Quintelas, C.; Beldíková, E.; Brányik, T. Chlorella vulgaris biomass enriched by biosorption of polyphenols. Algal Res. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Seif Zadeh, N.; Zeppa, G. Recovery and Concentration of Polyphenols from Roasted Hazelnut Skin Extract Using Macroporous Resins. Foods 2022, 11, 1969. [Google Scholar] [CrossRef]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Thymol/cyclodextrin inclusion complex nanofibrous webs: Enhanced water solubility, high thermal stability and antioxidant property of thymol. Food Res. Int. 2018, 106, 280–290. [Google Scholar] [CrossRef]
- Gonzalez, A.; Miñán, A.G.; Grillo, C.A.; Prieto, E.D.; Schilardi, P.L.; de Mele, M.A.F.L. Characterization and antimicrobial effect of a bioinspired thymol coating formed on titanium surface by one-step immersion treatment. Dent. Mater. 2020, 36, 1495–1507. [Google Scholar] [CrossRef]
- He, X.; Wang, B.; Zhang, Q. Phenols removal from water by precursor preparation for MgAl layered double hydroxide: Isotherm, kinetic and mechanism. Mater. Chem. Phys. 2019, 221, 108–117. [Google Scholar] [CrossRef]
- Koosehgol, S.; Ebrahimian-Hosseinabadi, M.; Alizadeh, M.; Zamanian, A. Preparation and characterization of in situ chitosan/polyethylene glycol fumarate/thymol hydrogel as an effective wound dressing. Mater. Sci. Eng. C 2017, 79, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Inel, G.A.; Ungureau, E.-M.; Varley, T.S.; Hirani, M.; Holt, K.B. Solvent–surface interactions between nanodiamond and ethanol studied with in situ infrared spectroscopy. Diam. Relat. Mater. 2016, 61, 7–13. [Google Scholar] [CrossRef]
- Grdadolnik, J.; Merzel, F.; Avbelj, F. Origin of hydrophobicity and enhanced water hydrogen bond strength near purely hydrophobic solutes. Proc. Natl. Acad. Sci. USA 2016, 114, 322–327. [Google Scholar] [CrossRef]
- Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. Organic Matter and Mineral Composition of Silicate Soils: FTIR Comparison Study by Photoacoustic, Diffuse Reflectance, and Attenuated Total Reflection Modalities. Agronomy 2021, 11, 1879. [Google Scholar] [CrossRef]
- Hindawi. Study on Europium-Doped Hydroxyapatite Nanoparticles by Fourier Transform Infrared Spectroscopy and Their Antimicrobial Properties [Internet]. Available online: https://www.hindawi.com/journals/jspec/2013/284285/ (accessed on 11 September 2022).
- Khamlich, S.; Bello, A.; Fabiane, M.; Ngom, B.D.; Manyala, N. Hydrothermal synthesis of simonkolleite microplatelets on nickel foam-graphene for electrochemical supercapacitors. J. Solid State Electrochem. 2013, 17, 2879–2886. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Ussia, M.; Mendichi, R.; Zampino, D.; Puglisi, C.; Carroccio, S.C. Halloysite nanotubes and thymol as photo protectors of biobased polyamide 11. Polym. Degrad. Stab. 2018, 152, 43–51. [Google Scholar] [CrossRef]
- Bin Hussein, M.Z.; Sarijo, S.H.; Yahaya, A.H.; Zainal, Z. Synthesis of 4-chlorophenoxyacetate-zinc-aluminium-layered double hydroxide nanocomposite: Physico-chemical and controlled release properties. J. Nanosci. Nanotechnol. 2007, 7, 2852–2862. [Google Scholar] [CrossRef]
- Rajput, M.K.; Konwar, M.; Sarma, D. Preparation of a novel environmentally friendly hydrophobic deep eutectic solvent ChCl-THY and its application in removal of hexavalent chromium from aqueous solution. Water Environ. Res. 2021, 93, 2250–2260. [Google Scholar] [CrossRef]
- Franco-Tabares, S.; Stenport, V.F.; Hjalmarsson, L.; Tam, P.L.; Johansson, C.B. Chemical Bonding to Novel Translucent Zirconias: A Mechanical and Molecular Investigation. J. Adhes. Dent. 2019, 21, 107–116. [Google Scholar]
- Çakır, M.A.; Icyer, N.C.; Tornuk, F. Optimization of production parameters for fabrication of thymol-loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 151, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Dembek, M.; Bocian, S.; Buszewski, B. Solvent Influence on Zeta Potential of Stationary Phase—Mobile Phase Interface. Molecules 2022, 27, 968. [Google Scholar] [CrossRef] [PubMed]
- Ribes, S.; Ruiz-Rico, M.; Pérez-Esteve, É.; Fuentes, A.; Talens, P.; Martínez-Máñez, R.; Barat, J.M. Eugenol and thymol immobilised on mesoporous silica-based material as an innovative antifungal system: Application in strawberry jam. Food Control 2017, 81, 181–188. [Google Scholar] [CrossRef]
- Mattos, B.D.; Tardy, B.L.; Pezhman, M.; Kämäräinen, T.; Linder, M.; Schreiner, W.H.; Magalhães, W.L.E.; Rojas, O.J. Controlled biocide release from hierarchically-structured biogenic silica: Surface chemistry to tune release rate and responsiveness. Sci. Rep. 2018, 8, 5555. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Y.; Luo, J.; Zhong, Z.; Borgna, A.; Guo, Z.; O’Hare, D. Synthesis of nano-sized spherical Mg3Al–CO3 layered double hydroxide as a high-temperature CO2 adsorbent. RSC Adv. 2012, 3, 3414–3420. [Google Scholar] [CrossRef]
- Pavón, E.; Martín-Rodríguez, R.; Perdigón, A.; Alba, M. New Trends in Nanoclay-Modified Sensors. Inorganics 2021, 9, 43. [Google Scholar] [CrossRef]
- Deng, L.; Taxipalati, M.; Que, F.; Zhang, H. Physical characterization and antioxidant activity of thymol solubilized Tween 80 micelles. Sci. Rep. 2016, 6, 38160. [Google Scholar] [CrossRef]
- Gayani, B.; Dilhari, A.; Wijesinghe, G.K.; Kumarage, S.; Abayaweera, G.; Samarakoon, S.R.; Perera, I.C.; Kottegoda, N.; Weerasekera, M.M. Effect of natural curcuminoids-intercalated layered double hydroxide nanohybrid against Staphylococcus aureus, Pseudomonas aeruginosa, and Enterococcus faecalis: A bactericidal, antibiofilm, and mechanistic study. Microbiologyopen 2019, 8, e00723. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Li, H.; Hossen, A.; Sameen, D.E.; Dai, J.; Qin, W.; Lee, K. Synthesis and properties of core-shell thymol-loaded zein/shellac nanoparticles by coaxial electrospray as edible coatings. Mater. Des. 2021, 212, 110214. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.-P.; Pei, R.-S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Mu, B.; Yan, P.; Jing, Y.; Hui, A.; Wang, A. A comparative study on surface/interface mechanism and antibacterial properties of different hybrid materials prepared with essential oils active ingredients and palygorskite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126455. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Bellissimo, A.; Pinto, L.; Petrella, A.; De Vietro, N.; Iannaccone, G.; Baruzzi, F.; De Giglio, E. A green approach to develop zeolite-thymol antimicrobial composites: Analytical characterization and antimicrobial activity evaluation. Heliyon 2022, 8, e09551. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mijakovic, I. Antibacterial Effect of Silver Nanoparticles Is Stronger If the Production Host and the Targeted Pathogen Are Closely Related. Biomedicines 2022, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Köse, H.; Yapar, N. The comparison of various disinfectants’ efficacy on Staphylococcus aureus and Pseudomonas aeruginosa biofilm layers. Turk. J. Med. Sci. 2017, 47, 1287–1294. [Google Scholar] [CrossRef]
- Sreelatha, S.; Kumar, N.; Yin, T.S.; Rajani, S. Evaluating the Antibacterial Activity and Mode of Action of Thymol-Loaded Chitosan Nanoparticles Against Plant Bacterial Pathogen Xanthomonas campestris pv. campestris. Front. Microbiol. 2022, 12, 792737. [Google Scholar] [CrossRef]
- Soumya, E.A.; Ibnsouda, K.S.; Hassan, L.; Ghizlane, Z.; Hind, M.; Adnane, R. Carvacrol and thymol components inhibiting Pseudomonas aeruginosa adherence and biofilm formation. Afr. J. Microbiol. Res. 2011, 5, 3229–3232. [Google Scholar]
- Nabipour, H.; Sadr, M.H.; Thomas, N. Synthesis, controlled release and antibacterial studies of nalidixic acid–zinc hydroxide nitrate nanocomposites. New J. Chem. 2015, 40, 238–244. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Li, P.; Li, Y.; Wang, H. Comparative study of antioxidant activity of grape (Vitis vinifera) seed powder assessed by different methods. J. Food Drug Anal. 2008, 16, 12. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
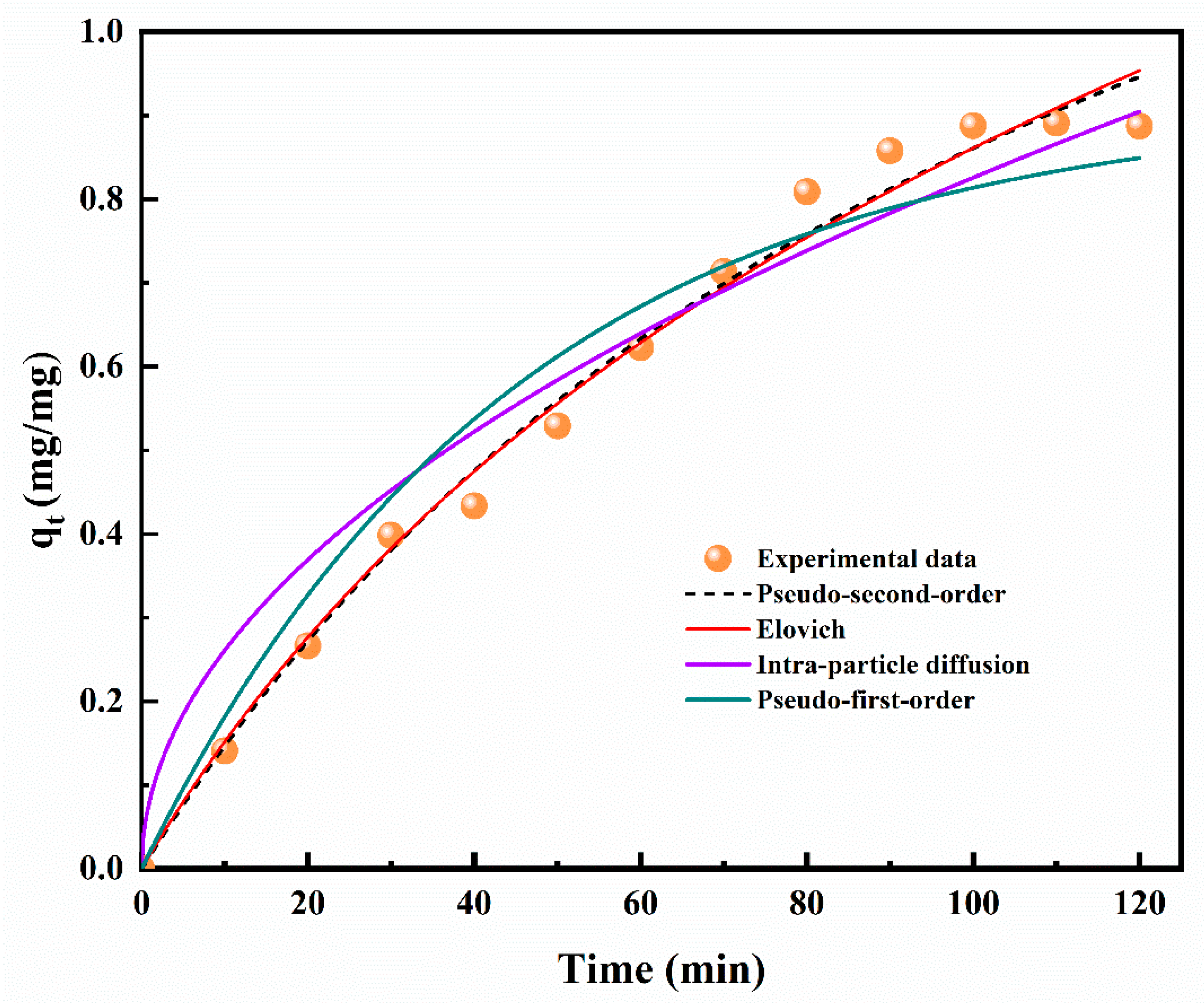
| Kinetic Model | Statistical Validation | |||||
|---|---|---|---|---|---|---|
| R2 | RMSE | ARE% | χ2 | AIC | BIC | |
| Lagergren’s pseudo-first order | 0.954 | 0.060 | 15.727 | 0.092 | −64.464 | −65.436 |
| Ho and McKay’s pseudo-second order | 0.989 | 0.031 | 4.654 | 0.017 | −81.752 | −82.724 |
| Elovich | 0.986 | 0.033 | 4.431 | 0.019 | −80.157 | −81.129 |
| Intra-particle diffusion | 0.950 | 0.065 | 15.636 | 0.131 | −62.407 | −63.379 |
| Concentration (mg/mL) | Escherichia coli (mm) | Staphylococcus aureus (mm) | ||||
|---|---|---|---|---|---|---|
| Thymol | ZnHSL | Thymol–ZnLHS | Thymol | ZnHSL | Thymol–ZnLHS | |
| 1 | 10.3 ± 0.1 f | 10.3 ± 0.2 f | 11.0 ± 0.5 e | 10.3 ± 0.6 F | 10.5 ± 0.2 F | 16.2 ± 2.3 D |
| 3 | 10.6 ± 0.5 f | 10.6 ± 0.5 f | 10.9 ± 0.2 e | 11.8 ± 0.4 F | 10.5 ± 0.3 F | 19.5 ± 0.5 C |
| 5 | 21.2 ± 0.1 c | 10.4 ± 0.5 f | 11.7 ± 0.6 e | 13.0 ± 1.0 E | 10.4 ± 0.4 F | 20.7 ± 0.6 B |
| 7 | 22.4 ± 0.4 b | 10.2 ± 0.3 f | 15.5 ± 0.5 d | 19.3 ± 1.5 C | 10.2 ± 0.3 F | 22.7 ± 0.8 A |
| 10 | 23.9 ± 0.3 a | 10.7 ± 0.3 e | 21.1 ± 0.1 c | 21.2 ± 0.7 B | 10.7 ± 0.6 E | 24.0 ± 1.0 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez-Carriles, C.; Macías-Rodríguez, M.E.; Ramírez-Alvarado, O.; Corona-González, R.I.; Macías-Lamas, A.; García-Vera, I.; Cavazos-Garduño, A.; Villagrán, Z.; Silva-Jara, J.M. Nanohybrid of Thymol and 2D Simonkolleite Enhances Inhibition of Bacterial Growth, Biofilm Formation, and Free Radicals. Molecules 2022, 27, 6161. https://doi.org/10.3390/molecules27196161
Velázquez-Carriles C, Macías-Rodríguez ME, Ramírez-Alvarado O, Corona-González RI, Macías-Lamas A, García-Vera I, Cavazos-Garduño A, Villagrán Z, Silva-Jara JM. Nanohybrid of Thymol and 2D Simonkolleite Enhances Inhibition of Bacterial Growth, Biofilm Formation, and Free Radicals. Molecules. 2022; 27(19):6161. https://doi.org/10.3390/molecules27196161
Chicago/Turabian StyleVelázquez-Carriles, Carlos, María Esther Macías-Rodríguez, Omar Ramírez-Alvarado, Rosa Isela Corona-González, Adriana Macías-Lamas, Ismael García-Vera, Adriana Cavazos-Garduño, Zuamí Villagrán, and Jorge Manuel Silva-Jara. 2022. "Nanohybrid of Thymol and 2D Simonkolleite Enhances Inhibition of Bacterial Growth, Biofilm Formation, and Free Radicals" Molecules 27, no. 19: 6161. https://doi.org/10.3390/molecules27196161
APA StyleVelázquez-Carriles, C., Macías-Rodríguez, M. E., Ramírez-Alvarado, O., Corona-González, R. I., Macías-Lamas, A., García-Vera, I., Cavazos-Garduño, A., Villagrán, Z., & Silva-Jara, J. M. (2022). Nanohybrid of Thymol and 2D Simonkolleite Enhances Inhibition of Bacterial Growth, Biofilm Formation, and Free Radicals. Molecules, 27(19), 6161. https://doi.org/10.3390/molecules27196161