A Comprehensive Literature Review on Cardioprotective Effects of Bioactive Compounds Present in Fruits of Aristotelia chilensis Stuntz (Maqui)
Abstract
1. Introduction
Maqui: Relevance and Traditional Uses
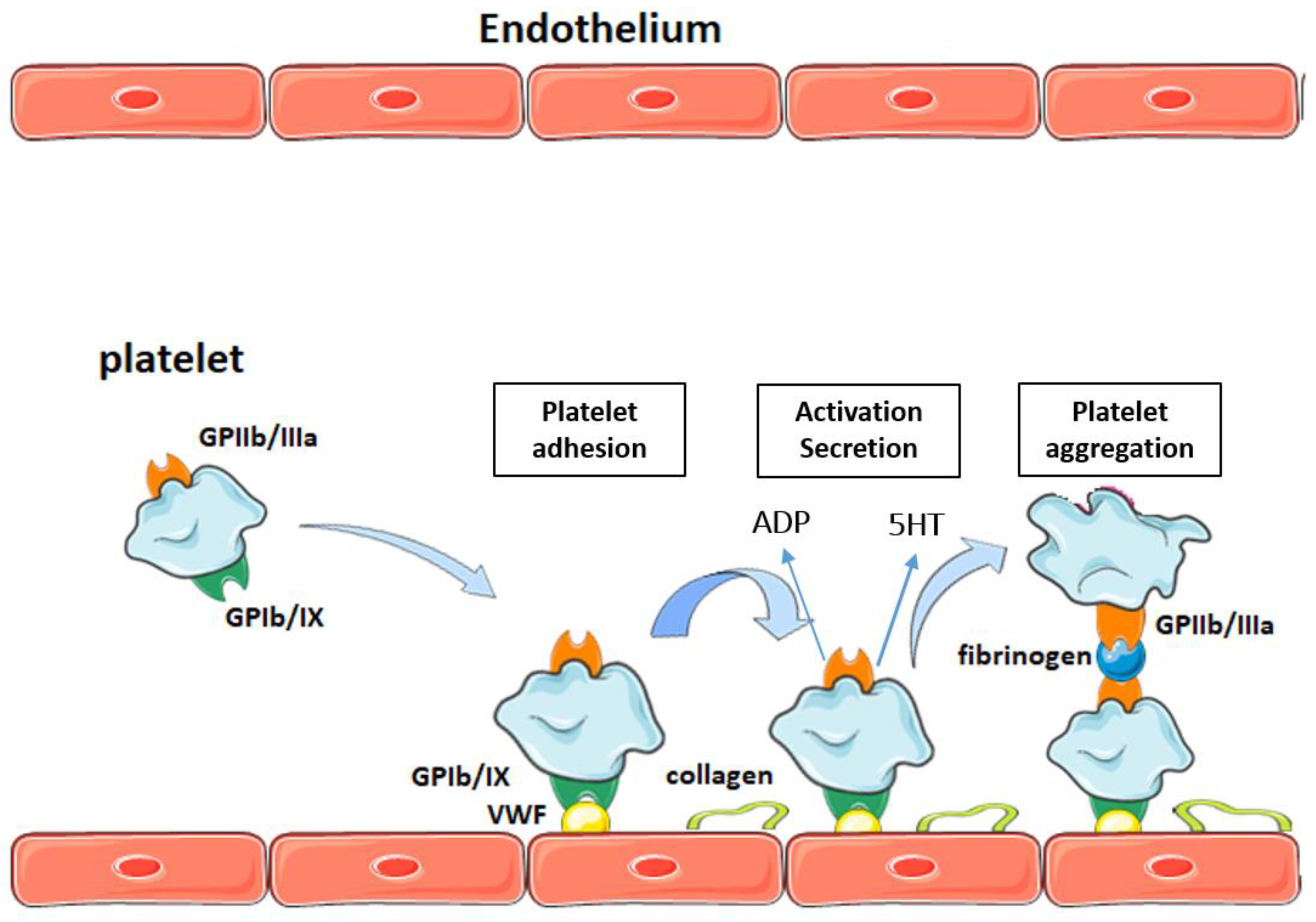
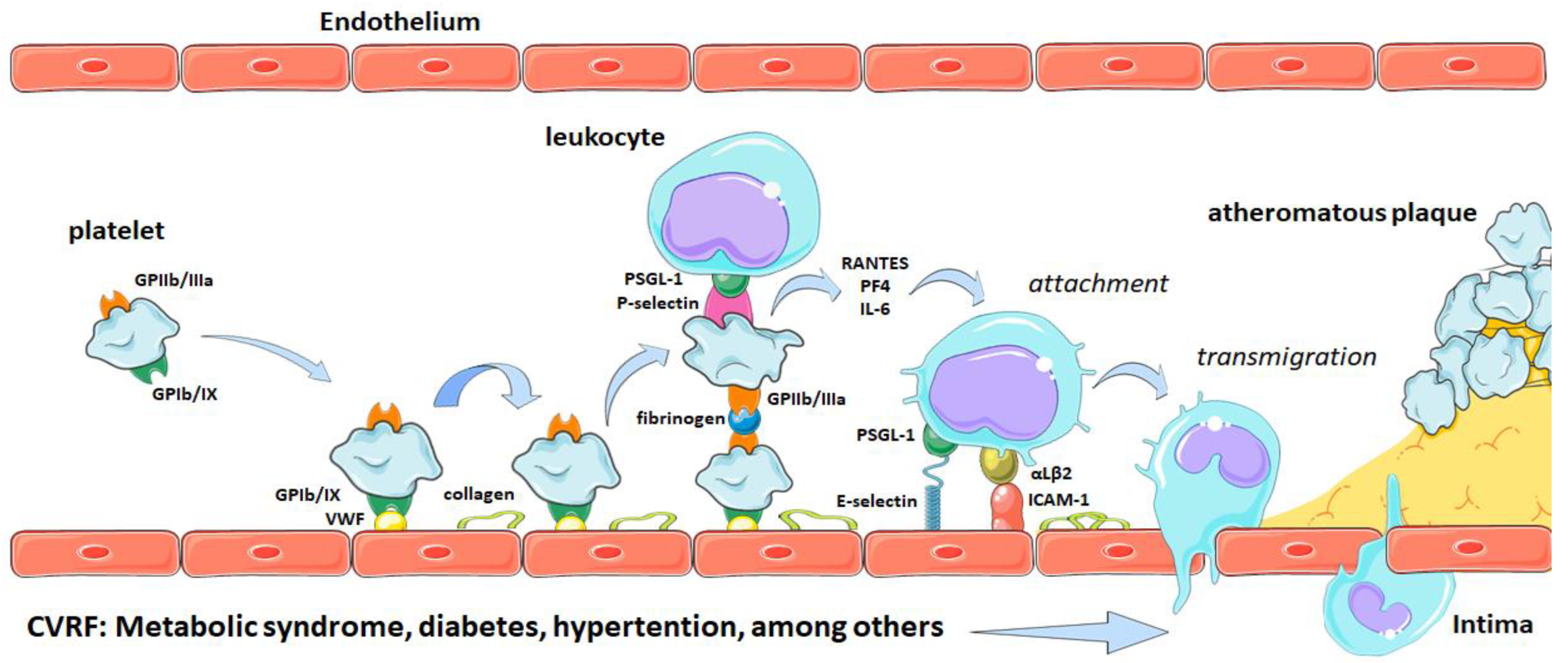
2. Platelets and Atherothrombosis
Participation of Platelets in Atherothrombosis
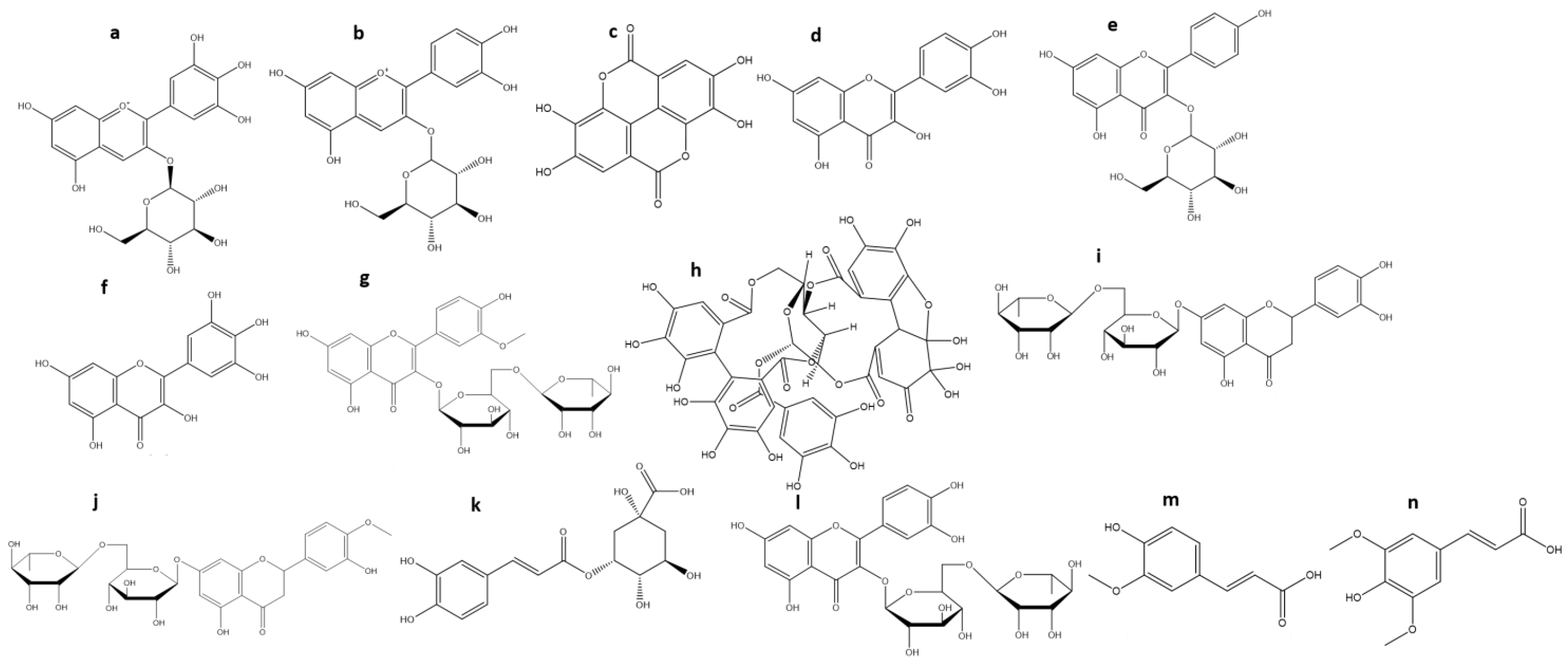
3. Chemical Characterization of Maqui
| Anthocyanins (TA) | % Average (Range) | References |
|---|---|---|
| Delphinidin-3-O-sambubioside-5-O-glucoside | 32.4 (15–49) | [21,46,52,53,61] |
| Delphinidin-3-O-glucoside (a) | 18.6 (11–28) | [21,46,52,53,61] |
| Delphinidin-3,5-O-diglucoside | 18.3 (14–24) | [46,52,53,61] |
| Delphinidin-3-O-sambubioside | 10 (6–16) | [21,46,52,53,61] |
| Cyanidin-3-O-glucoside (b) | 11 (6–16) | [46,52,61] |
| Cyanidin3,5-diglucoside | 10.8 (7–14) | [21,46,52,53] |
| Cyanidin-3-O-sambubioside-5-O-glucoside | 9 (7–11) | [52,61] |
| Cyanidin-3-O-sambubioside | 6.5 (6–7) | [21,46,52,53] |
| Cyanidin-O-glucoside-5-O-rhamnoside | 1.5 (1–2) | [21,53] |
| Phenolic Compounds (TP) | ||
| Ellagic acid (c) | 30 | [22] |
| Ellagic acid rhamnoside | 8 | [62] |
| Ellagic acid hexoside | 2.8 (2–3.5) | [22,53,62] |
| Quercetin-O-galloyl-O-hexoside | 24 | [22] |
| Quercetin-3-O-rutinoside | 10 (7–13) | [53,62] |
| Quercetin-3-O-arabinoside | 6 (5–7) | [22,53,62] |
| Quercetin-3-O-galactoside | 6 (5–7) | [22,53,62] |
| Quercetin-3-O-rhamnoside | 5.5 (4.1–6) | [53,62] |
| Quercetin-3-O-xyloside | 3.5 (2–5) | [22,53,62] |
| Quercetin-3-O-glucoside | 3 (2–4) | [22,53,62] |
| Quercetin (d) | 2 | [22] |
| Kaempferol-3-O-glucoside (e) | 18 | [53] |
| Kaempferol-3-O-galactoside | 12 | [53] |
| Kaempferol-3-O-rutinoside | 2 | [53] |
| Myricetin-3-O-glucoside | 13 (6–20) | [22,53] |
| Myricetin (f) | 8 | [22] |
| Myricetin-3-O-galactoside | 6.3 (4–10) | [22,53,62] |
| Myricetin-3-O-galoyl-O-glucoside | 4 (2–6) | [22,53,62] |
| Myricetin-3-O-galoyl-O-glucoside | 4 (2–6) | [22,53,62] |
| Isorhamnetin-3-O-rutinoside (g) | 2 | [53] |
| Granatin B (h) | 20 | [62] |
| Eriodictyol-7-O-rutinoside (i) | 11 | [62] |
| Hesperetin-7-O-rutinoside (j) | 11 | [62] |
| 5-O-caffeoylquinic acid (k) | 8.5 (5–12) | [22,53,62] |
| Rutin (l) | 6 | [22] |
| Ferulic acid (m) | 4 | [62] |
| Sinapic acid (n) | 3 | [62] |
4. Cardioprotective Role of Maqui
4.1. Antioxidant Effect
4.2. Effect on Inflammation and Endothelial Dysfunction
4.3. Effect on Diabetes and Obesity
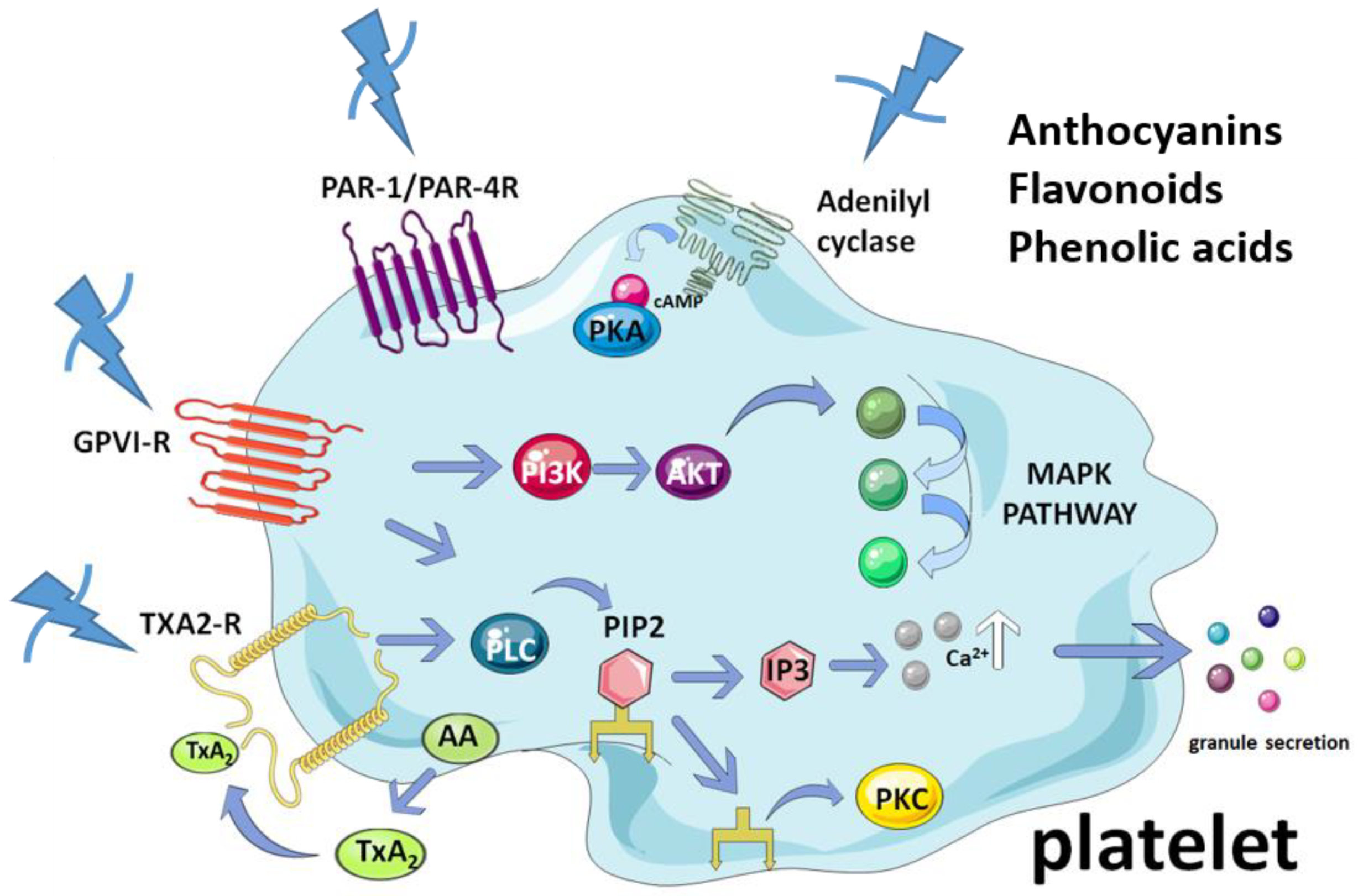
5. Antiplatelet Activity of the Compounds in Maqui
5.1. Anthocyanins
5.2. Flavonols
5.3. Flavones
5.4. Phenolic Acids
6. Limitations and Future Perspective
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Zuraini, N.Z.A.; Sekar, M.; Wu, Y.S.; Gan, S.H.; Bonam, S.R.; Rani, N.N.I.M.; Begum, M.Y.; Lum, P.T.; Subramaniyan, V.; Fuloria, N.K.; et al. Promising nutritional fruits against cardiovascular diseases: An overview of experimental evidence and understanding their mechanisms of action. Vasc. Health Risk Manag. 2021, 17, 739. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Block, G.; Humphreys, M.H.; Kopple, J.D. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003, 63, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Albala, C.; PGIGSR. Funcionalidad en los Adultos Mayores: Fragilidad y Dependencia. In Envejecimiento: Demografia, Salud e Impacto Social; Talca, U.D., Ed.; Universidad de Talca: Talca, Chile, 2016. [Google Scholar]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B., Sr.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef] [PubMed]
- Gensini, G.F.; Comeglio, M.; Colella, A. Classical risk factors and emerging elements in the risk profile for coronary artery disease. Eur. Heart J. 1998, 19, A53–A61. [Google Scholar]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Thrombosis and COVID-19: The Potential role of nutrition. Front. Nutr. 2020, 7, 177. [Google Scholar] [CrossRef]
- Palomo, I.F.; Torres, G.I.; Alarcón, M.A.; Maragaño, P.J.; Leiva, E.; Mujica, V. High prevalence of classic cardiovascular risk factors in a population of university students from south central Chile. Rev. Española Cardiol. 2006, 59, 1099–1105. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G. Coronary Atherothrombotic Disease: Progress in Antiplatelet Therapy. Rev. Española De Cardiol. 2008, 61, 501–513. [Google Scholar] [CrossRef][Green Version]
- Eduardo, F.; Andrés, T.; Lívia Mateus, R.; Mario Roberto, M., Jr.; Iván, P. Antiplatelet Effects of Bioactive Compounds Present in Tomato Pomace. Curr. Drug Targets 2021, 22, 1716–1724. [Google Scholar]
- Irfan, M.; Kwon, T.-H.; Lee, D.-H.; Hong, S.-B.; Oh, J.-W.; Kim, S.-D.; Rhee, M.H. Antiplatelet and Antithrombotic Effects of Epimedium koreanum Nakai. Evid. Based Complementary Altern. Med. 2021, 2021, 7071987. [Google Scholar] [CrossRef]
- Olas, B. Dietary supplements with antiplatelet activity: A solution for everyone? Adv. Nutr. 2018, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Meshkini, A.; Tahmasbi, M. Antiplatelet aggregation activity of walnut hull extract via suppression of reactive oxygen species generation and caspase activation. J. Acupunct. Meridian Stud. 2017, 10, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R. Elaeocarpaceae Juss. Ex DC. Flora Chile 2005, 2, 15–17. [Google Scholar]
- Misle, E.; Garrido, E.; Contardo, H.; González, W. Maqui (Aristotelia chilensis (Mol.) Stuntz) the amazing chilean tree: A review. J. Ofagricultural Sci. Technol. B 2011, 1, 473–482. [Google Scholar]
- Fredes, C.; Yousef, G.G.; Robert, P.; Grace, M.H.; Lila, M.A.; Gómez, M.; Gebauer, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef]
- Zúñiga, G.E.; Tapia, A.; Arenas, A.; Contreras, R.A.; Zúñiga-Libano, G. Phytochemistry and biological properties of Aristotelia chilensis a Chilean blackberry: A review. Phytochem. Rev. 2017, 16, 1081–1094. [Google Scholar] [CrossRef]
- Brauch, J.E.; Buchweitz, M.; Schweiggert, R.M.; Carle, R. Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) berries and juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef]
- Céspedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Mena, P.; García-Viguera, C.; Moreno, D.A. A novel beverage rich in antioxidant phenolics: Maqui berry (Aristotelia chilensis) and lemon juice. LWT-Food Sci. Technol. 2012, 47, 279–286. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; González, B.; Catenacci, G.; Doll, U. Domestication and Sustainable Production of Wild Crafted Plants with Special Reference to the Chilean Maqui Berry (Aristotelia Chilensis). Julius-Kühn-Archiv 2016, 453, 50–52. [Google Scholar]
- Salgado, P.; Prinz, K.; Finkeldey, R.; Ramírez, C.C.; Vogel, H. Genetic Variability of Aristotelia Chilensis (“Maqui”) Based on Aflp and Chloroplast Microsatellite Markers. Genetic Resources and Crop. Evolution 2017, 64, 2083–2091. [Google Scholar] [CrossRef]
- Vogel, H.; Peñailillo, P.; Doll, U.; Contreras, G.; Catenacci, G.; González, B. Maqui (Aristotelia chilensis): Morpho-phenological characterization to design high-yielding cultivation techniques. J. Appl. Res. Med. Aromat. Plants 2014, 1, 123–133. [Google Scholar] [CrossRef]
- Brauch, J.E.; Reuter, L.; Conrad, J.; Vogel, H.; Schweiggert, R.M.; Carle, R. Characterization of anthocyanins in novel Chilean maqui berry clones by HPLC–DAD–ESI/MS n and NMR-spectroscopy. J. Food Compos. Anal. 2017, 58, 16–22. [Google Scholar] [CrossRef]
- González, B.; Vogel, H.; Razmilic, I.; Wolfram, E. Polyphenol, anthocyanin and antioxidant content in different parts of maqui fruits (Aristotelia chilensis) during ripening and conservation treatments after harvest. Ind. Crops Prod. 2015, 76, 158–165. [Google Scholar] [CrossRef]
- Makinistian, F.G.; Sette, P.; Gallo, L.; Bucalá, V.; Salvatori, D. Optimized aqueous extracts of maqui (Aristotelia chilensis) suitable for powder production. J. Food Sci. Technol. 2019, 56, 3553–3560. [Google Scholar] [CrossRef]
- Rivera-Tovar, P.R.; Mariotti-Celis, M.S.; Pérez-Correa, J.R. Maqui (Aristotelia chilensis (Mol.) Stuntz) and murta (Ugni molinae Turcz): Native Chilean sources of polyphenol compounds. Mini-Rev. Org. Chem. 2019, 16, 261–276. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005, 116, 327–334. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Z.; Reheman, A.; Jin, J.W.; Li, C.; Wang, Y.; Andrews, M.C.; Chen, P.; Zhu, G.; Ling, W. Plant food delphinidin-3-glucoside significantly inhibits platelet activation and thrombosis: Novel protective roles against cardiovascular diseases. PLoS ONE 2012, 7, e37323. [Google Scholar] [CrossRef]
- Palomo, I.; Pereira, J.; Palma, J. Hematología: Fisiopatología y Diagnóstico; Universidad de Talca: Talca, Chile, 2005. [Google Scholar]
- Evstatiev, R.; Bukaty, A.; Jimenez, K.; Kulnigg-Dabsch, S.; Surman, L.; Schmid, W.; Eferl, R.; Lippert, K.; Scheiber-Mojdehkar, B.; Michael Kvasnicka, H. Iron deficiency alters megakaryopoiesis and platelet phenotype independent of thrombopoietin. Am. J. Hematol. 2014, 89, 524–529. [Google Scholar] [CrossRef]
- Jennings, L.K. Mechanisms of platelet activation: Need for new strategies to protect against platelet-mediated atherothrombosis. Thromb. Haemost. 2009, 101, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Spencer, F.A.; Becker, R.C. Platelets: Structure, function, and their fundamental contribution to hemostasis and pathologic thrombosis. In Textbook of Coronary Thrombosis and Thrombolysis; Springer: Berlin/Heidelberg, Germany, 1997; pp. 31–49. [Google Scholar]
- Robless, P.A.; Okonko, D.; Lintott, P.; Mansfield, A.O.; Mikhailidis, D.P.; Stansby, G.P. Increased platelet aggregation and activation in peripheral arterial disease. Eur. J. Vasc. Endovasc. Surg. 2003, 25, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, K.; Bhattacharyya, M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. Sci. World J. 2014, 2014, 781857. [Google Scholar] [CrossRef]
- El Haouari, M.; Rosado, J.A. Platelet signalling abnormalities in patients with type 2 diabetes mellitus: A review. Blood Cells Mol. Dis. 2008, 41, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Geisler, T.; Anders, N.; Paterok, M.; Langer, H.; Stellos, K.; Lindemann, S.; Herdeg, C.; May, A.E.; Gawaz, M. Platelet response to clopidogrel is attenuated in diabetic patients undergoing coronary stent implantation. Diabetes Care 2007, 30, 372–374. [Google Scholar] [CrossRef][Green Version]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef]
- Fuentes, Q.E.; Fuentes, Q.F.; Andrés, V.; Pello, O.M.; de Mora, J.F.; Palomo, G.I. Role of platelets as mediators that link inflammation and thrombosis in atherosclerosis. Platelets 2013, 24, 255–262. [Google Scholar] [CrossRef]
- Vilahur, G.; Badimon, L. Antiplatelet properties of natural products. Vasc. Pharmacol. 2013, 59, 67–75. [Google Scholar] [CrossRef]
- Meerson, F.Z.; Kagan, V.E.; Kozlov, Y.P.; Belkina, L.M.; Arkhipenko, Y.V. The Role of Lipid Peroxidation in Pathogenesis of Ischemic Damage and the Antioxidant Protection of the Heart; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Alarcón, M.; Fuentes, E.; Olate, N.; Navarrete, S.; Carrasco, G.; Palomo, I. Strawberry extract presents antiplatelet activity by inhibition of inflammatory mediator of atherosclerosis (sP-selectin, sCD40L, RANTES, and IL-1β) and thrombus formation. Platelets 2015, 26, 224–229. [Google Scholar] [CrossRef]
- Fuentes, E.J.; Astudillo, L.A.; Gutiérrez, M.I.; Contreras, S.O.; Bustamante, L.O.; Rubio, P.I.; Moore-Carrasco, R.; Alarcón, M.A.; Fuentes, J.A.; González, D.E. Fractions of aqueous and methanolic extracts from tomato (Solanum lycopersicum L.) present platelet antiaggregant activity. Blood Coagul. Fibrinolysis 2012, 23, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Bailón, M.T.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanins in berries of maqui [Aristotelia chilensis (Mol.) Stuntz]. Phytochem. Anal. 2006, 17, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Rodríguez-Saona, L.E.; Griffin, D.; Wrolstad, R.E. Electrospray and tandem mass spectroscopy as tools for anthocyanin characterization. J. Agric. Food Chem. 1999, 47, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Campos-Requena, V.H.J.A. Antioxidant compound extraction from maqui (Aristotelia chilensis [Mol] Stuntz) berries: Optimization by response surface methodology. Antioxidants 2017, 6, 10. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosin-Gutierrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; López-Carballo, G.; Gavara, R.; Muriel Galet, V.; Guarda, A.; Galotto, M.J. Improving polyphenolic thermal stability of Aristotelia chilensis fruit extract by encapsulation within electrospun cyclodextrin capsules. J. Food Processing Preserv. 2019, 43, e14044. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Valdez-Morales, M.; Avila, J.G.; El-Hafidi, M.; Alarcón, J.; Paredes-López, O. Phytochemical profile and the antioxidant activity of Chilean wild black-berry fruits, Aristotelia chilensis (Mol) Stuntz (Elaeocarpaceae). Food Chem. 2010, 119, 886–895. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; García-Viguera, C.; Moreno, D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Paz Robles, C.; Badilla Vidal, N.; Suarez, S.; Baggio, R. Hobartine: A tetracyclic indole alkaloid extracted from Aristotelia chilensis (maqui). Acta Crystallogr. Sect. C Struct. Chem. 2014, 70, 1075–1078. [Google Scholar] [CrossRef]
- Muñoz, O.; Christen, P.; Cretton, S.; Backhouse, N.; Torres, V.; Correa, O.; Costa, E.; Miranda, H.; Delporte, C. Chemical study and anti-inflammatory, analgesic and antioxidant activities of the leaves of Aristotelia chilensis (Mol.) Stuntz, Elaeocarpaceae. J. Pharm. Pharmacol. 2011, 63, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.; Avello, L.; Loyola, C.; Campos, P.; Aqueveque, M.; Dungan, S.R.; Galotto, L.; Guarda, M. Microencapsulation of maqui (Aristotelia chilensis Molina Stuntz) leaf extracts to preserve and control antioxidant properties. Chil. J. Agric. Res. 2013, 73, 17–23. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Sobolev, A.P.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Di Giovanni, C.; Xiao, J.; Shirooie, S.; Sokeng, A.J.T. Antidepressive effects of a chemically characterized maqui berry extract (Aristotelia chilensis (molina) stuntz) in a mouse model of Post-stroke depression. Food Chem. Toxicol. 2019, 129, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.P.; Aradhya, S.M. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005, 93, 319–324. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Matta, F.V.; Xiong, J.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods 2020, 9, 1481. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Wang, J.; Yousef, G.; Lila, M.A.; de Mejia, E.G. Antioxidant capacity and in vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vaccinium floribundum and Aristotelia chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef]
- Gironeés-Vilaplana, A.; Valentaão Pc Moreno, D.A.; Ferreres, F.; García-Viguera, C.; Andrade, P.B. New beverages of lemon juice enriched with the exotic berries maqui, açaí, and blackthorn: Bioactive components and in vitro biological properties. J. Agric. Food Chem. 2012, 60, 6571–6580. [Google Scholar] [CrossRef]
- Fuentes, E.; Palomo, I. Antiplatelet effects of natural bioactive compounds by multiple targets: Food and drug interactions. J. Funct. Foods 2014, 6, 73–81. [Google Scholar] [CrossRef]
- Fuentes, F.; Alarcón, M.; Badimon, L.; Fuentes, M.; Klotz, K.-N.; Vilahur, G.; Kachler, S.; Padró, T.; Palomo, I.; Fuentes, E. Guanosine exerts antiplatelet and antithrombotic properties through an adenosine-related cAMP-PKA signaling. Int. J. Cardiol. 2017, 248, 294–300. [Google Scholar] [CrossRef]
- Prior, R.L.; Gu, L. Occurrence and biological significance of proanthocyanidins in the American diet. Phytochemistry 2005, 66, 2264–2280. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Rottmann, S.; Aspillaga, A.A.; Pérez, D.D.; Vasquez, L.; Martinez, A.L.; Leighton, F.J. Juice and phenolic fractions of the berry Aristotelia chilensis inhibit LDL oxidation in vitro and protect human endothelial cells against oxidative stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Céspedes-Acuña, C.L.; Xiao, J.; Wei, Z.-J.; Chen, L.; Bastias, J.M.; Avila, J.G.; Alarcon-Enos, J.; Werner-Navarrete, E.; Kubo, I. Antioxidant and anti-inflammatory effects of extracts from Maqui berry Aristotelia chilensis in human colon cancer cells. J. Berry Res. 2018, 8, 275–296. [Google Scholar] [CrossRef]
- Ortiz, T.; Argüelles-Arias, F.; Begines, B.; García-Montes, J.M.; Pereira, A.; Victoriano, M.; Vázquez-Román, V.; Pérez, J.L.; Callejon, R.M.; de Miguel, M.; et al. Native Chilean Berries Preservation and In Vitro Studies of a Polyphenol Highly Antioxidant Extract from Maqui as a Potential Agent against Inflammatory Diseases. Antioxidants 2021, 10, 843. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Villaño, D.; Moreno, D.A.; García-Viguera, C. New isotonic drinks with antioxidant and biological capacities from berries (maqui, açaí and blackthorn) and lemon juice. Int. J. Food Sci. Nutr. 2013, 64, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Martin, K. Effect of Hot Water Extracts of Maqui Berry on Human Aortic Endothelial Cells Exposed to a Hyperglycemic Environment. Curr. Dev. Nutr. 2020, 4, 435. [Google Scholar] [CrossRef]
- Fuentes, O.; Fuentes, M.; Badilla, S.; Troncoso, F. Maqui (Aristotelia chilensis) and rutin (quercetin-3-O-rutinoside) protects against the functional impairment of the endothelium-dependent vasorelaxation caused by a reduction of nitric oxide availability in diabetes. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2013, 12, 220–229. [Google Scholar]
- Fuentes, O.; Cespedes, C.; Sepulveda, R. Aristotelia chilensis, rutin and quercetin amielorates acute vascular endothelial dysfunction in rat thoracic aorta exposed to oxidative stress. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2015, 14, 11–20. [Google Scholar]
- Hidalgo, J.; Flores, C.; Hidalgo, M.; Perez, M.; Yañez, A.; Quiñones, L.; Caceres, D.; Burgos, R.J.P.M. Delphinol® standardized maqui berry extract reduces postprandial blood glucose increase in individuals with impaired glucose regulation by novel mechanism of sodium glucose cotransporter inhibition. Panminerva Med. 2014, 56, 1–7. [Google Scholar]
- Sandoval, V.; Femenias, A.; Martínez-Garza, Ú.; Sanz-Lamora, H.; Castagnini, J.M.; Quifer-Rada, P.; Lamuela-Raventós, R.M.; Marrero, P.F.; Haro, D.; Relat, J. Lyophilized maqui (Aristotelia chilensis) berry induces browning in the subcutaneous white adipose tissue and ameliorates the insulin resistance in high fat diet-induced obese mice. Antioxidants 2019, 8, 360. [Google Scholar] [CrossRef]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Schönlau, F. Nutraceutical and antioxidant effects of a delphinidin-rich maqui berry extract Delphinol®: A review. Minerva Cardioangiol 2015, 63, 1–12. [Google Scholar] [PubMed]
- Alvarado, J.L.; Leschot, A.; Olivera-Nappa, Á.; Salgado, A.-M.; Rioseco, H.; Lyon, C.; Vigil, P. Delphinidin-Rich Maqui Berry Extract (Delphinol®) Lowers Fasting and Postprandial Glycemia and Insulinemia in Prediabetic Individuals during Oral Glucose Tolerance Tests. BioMed Res. Int. 2016, 2016, 9070537. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.A.; Čanadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Vinčić, M. Chemical composition and potential bioactivity of strawberry pomace. RSC Adv. 2015, 5, 5397–5405. [Google Scholar] [CrossRef]
- Ávila, F.; Jiménez-Aspee, F.; Cruz, N.; Gómez, C.; González, M.A.; Ravello, N. Additive effect of maqui (Aristotelia chilensis) and lemon (Citrus × limon) juice in the postprandial glycemic responses after the intake of high glycemic index meals in healthy men. NFS J. 2019, 17, 8–16. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef]
- Davinelli, S.; Bertoglio, J.C.; Zarrelli, A.; Pina, R.; Scapagnini, G. A randomized clinical trial evaluating the efficacy of an anthocyanin–maqui berry extract (Delphinol®) on oxidative stress biomarkers. J. Am. Coll. Nutr. 2015, 34, 28–33. [Google Scholar] [CrossRef]
- Bribiesca-Cruz, I.; Moreno, D.A.; García-Viguera, C.; Gallardo, J.M.; Segura-Uribe, J.J.; Pinto-Almazán, R.; Guerra-Araiza, C. Maqui Berry (Aristotelia Chilensis) Extract Improves Memory and Decreases Oxidative Stress in Male Rat Brain Exposed to Ozone. Nutr. Neurosci. 2021, 24, 477–489. [Google Scholar] [CrossRef]
- Ostertag, L.M.; O’Kennedy, N.; Horgan, G.W.; Kroon, P.A.; Duthie, G.G.; de Roos, B. In vitro anti-platelet effects of simple plant-derived phenolic compounds are only found at high, non-physiological concentrations. Mol. Nutr. Food Res. 2011, 55, 1624–1636. [Google Scholar] [CrossRef]
- Olas, B. The multifunctionality of berries toward blood platelets and the role of berry phenolics in cardiovascular disorders. Platelets 2017, 28, 540–549. [Google Scholar] [CrossRef]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganayagam, D.; Beahm, M.R.; Osman, H.E.; Krueger, C.G.; Reed, J.D.; Folts, J.D. Grape seed and grape skin extracts elicit a greater antiplatelet effect when used in combination than when used individually in dogs and humans. J. Nutr. 2002, 132, 3592–3598. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Bauer, B.A. Advising consumers about dietary supplements: Lessons from cranberry products. J. Diet. Suppl. 2009, 6, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Polagruto, J.A.; Gross, H.B.; Kamangar, F.; Kosuna, K.-i.; Sun, B.; Fujii, H.; Keen, C.L.; Hackman, R.M. Platelet reactivity in male smokers following the acute consumption of a flavanol-rich grapeseed extract. J. Med. Food 2007, 10, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Keevil, J.G.; Osman, H.E.; Reed, J.D.; Folts, J.D. Grape juice, but not orange juice or grapefruit juice, inhibits human platelet aggregation. J. Nutr. 2000, 130, 53–56. [Google Scholar] [CrossRef]
- Freedman, J.E.; Parker Iii, C.; Li, L.; Perlman, J.A.; Frei, B.; Ivanov, V.; Deak, L.R.; Iafrati, M.D.; Folts, J.D. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 2001, 103, 2792–2798. [Google Scholar] [CrossRef]
- Eccleston, C.; Baoru, Y.; Tahvonen, R.; Kallio, H.; Rimbach, G.H.; Minihane, A.M. Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans. J. Nutr. Biochem. 2002, 13, 346–354. [Google Scholar] [CrossRef]
- Fuentes, E.; Caballero, J.; Alarcón, M.; Rojas, A.; Palomo, I. Chlorogenic acid inhibits human platelet activation and thrombus formation. PLoS ONE 2014, 9, e90699. [Google Scholar] [CrossRef]
- Rodríguez, L.; Trostchansky, A.; Wood, I.; Mastrogiovanni, M.; Vogel, H.; González, B.; Maróstica Junior, M.; Fuentes, E.; Palomo, I. Antiplatelet activity and chemical analysis of leaf and fruit extracts from Aristotelia chilensis. PLoS ONE 2021, 16, e0250852. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Z.; Reheman, A.; Jin, W.; Li, C.; Zhu, G.; Wang, Y.; Freedman, J.J.; Ling, W.; Ni, H. Anthocyanins Inhibit Platelet Activation and Attenuate Thrombus Growth in Both Human and Murine Thrombosis Models. Blood 2010, 116, 3197. [Google Scholar] [CrossRef]
- Rodríguez, L.; Mendez, D.; Montecino, H.; Carrasco, B.; Arevalo, B.; Palomo, I.; Fuentes, E. Role of Phaseolus vulgaris L. in the Prevention of Cardiovascular Diseases—Cardioprotective Potential of Bioactive Compounds. Plants 2022, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, Y.; Adili, R.; McKeown, T.; Chen, P.; Zhu, G.; Li, D.; Ling, W.; Ni, H.; Yang, Y. Plant-Based Food Cyanidin-3-Glucoside Modulates Human Platelet Glycoprotein Vi Signaling and Inhibits Platelet Activation and Thrombus Formation. J. Nutr. 2017, 147, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.-H.; Deng, X.-J.; Chen, Y.-Q.; Ya, F.-L.; Zhang, X.-D.; Song, F.; Li, D.; Yang, Y. Anthocyanin cyanidin-3-glucoside attenuates platelet granule release in mice fed high-fat diets. J. Nutr. Sci. Vitaminol. 2017, 63, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, G.P.; Wolffram, S.; Lovegrove, J.A.; Gibbins, J.M. Ingestion of Quercetin Inhibits Platelet Aggregation and Essential Components of the Collagen-Stimulated Platelet Activation Pathway in Humans. J. Thromb. Haemost. 2004, 2, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, S.-H.; Ko, W.-C.; Ko, F.-N.; Teng, C.-M. Inhibition of platelet aggregation by some flavonoids. Thromb. Res. 1991, 64, 91–100. [Google Scholar] [CrossRef]
- Chung, M.-I.; Gan, K.-H.; Lin, C.-N.; Ko, F.-N.; Teng, C.-M. Antiplatelet effects and vasorelaxing action of some constituents of Formosan plants. J. Nat. Prod. 1993, 56, 929–934. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Stevens, J.M.; Cicmil, M.; Sage, T.; Jordan, P.A.; Williams, C.M.; Lovegrove, J.A.; Gibbins, J.M. Quercetin Inhibits Collagen-Stimulated Platelet Activation through Inhibition of Multiple Components of the Glycoprotein Vi Signaling Pathway. J. Thromb. Haemost. 2003, 1, 1079–1088. [Google Scholar] [CrossRef]
- Beretz, A.; Cazenave, J.-P.; Anton, R. Inhibition of aggregation and secretion of human platelets by quercetin and other flavonoids: Structure-activity relationships. Agents Actions 1982, 12, 382–387. [Google Scholar] [CrossRef]
- Landolfi, R.; Mower, R.L.; Steiner, M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids: Structure-activity relations. Biochem. Pharmacol. 1984, 33, 1525–1530. [Google Scholar] [CrossRef]
- Choi, J.-H.; Park, S.-E.; Kim, S.-J.; Kim, S. Kaempferol inhibits thrombosis and platelet activation. Biochimie 2015, 115, 177–186. [Google Scholar] [CrossRef]
- Rolnik, A.; Żuchowski, J.; Stochmal, A.; Olas, B. Quercetin and kaempferol derivatives isolated from aerial parts of Lens culinaris Medik as modulators of blood platelet functions. Ind. Crops Prod. 2020, 152, 112536. [Google Scholar]
- Tong, Y.; Zhou, X.-M.; Wang, S.-J.; Yang, Y.; Cao, Y.-L. Analgesic activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Arch. Pharmacal. Res. 2009, 32, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.S.; da Silva, S.A.; Stapleton, J.; Fontelles, J.L.d.L.; Sousa, H.R.; Chagas, V.T.; Alsufyani, S.; Trostchansky, A.; Gibbins, J.M.; Paes, A.M.D.A. Myricetin, the main flavonoid in Syzygium cumini leaf, is a novel inhibitor of platelet thiol isomerases PDI and ERp5. Front. Pharmacol. 2020, 10, 1678. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.W.; Do, H.J.; Jeon, J.-H.; Kim, K. Quercitrin inhibits platelet activation in arterial thrombosis. Phytomedicine 2021, 80, 153363. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-R.; Hsiao, G.; Chou, P.-H.; Shen, M.-Y.; Chou, D.-S. Mechanisms involved in the antiplatelet activity of rutin, a glycoside of the flavonol quercetin, in human platelets. J. Agric. Food Chem. 2004, 52, 4414–4418. [Google Scholar] [CrossRef]
- Koleckar, V.; Brojerova, E.; Rehakova, Z.; Kubikova, K.; Cervenka, F.; Kuca, K.; Jun, D.; Hronek, M.; Opletalova, V.; Opletal, L. In vitro antiplatelet activity of flavonoids from Leuzea carthamoides. Drug Chem. Toxicol. 2008, 31, 27–35. [Google Scholar] [CrossRef]
- Jin, Y.R.; Han, X.H.; Zhang, Y.H.; Lee, J.J.; Lim, Y.; Chung, J.-H.; Yun, Y.P. Antiplatelet Activity of Hesperetin, a Bioflavonoid, Is Mainly Mediated by Inhibition of Plc-Γ2 Phosphorylation and Cyclooxygenase-1 Activity. Atherosclerosis 2007, 194, 144–152. [Google Scholar] [CrossRef]
- Sugasawa, N.; Katagi, A.; Kurobe, H.; Nakayama, T.; Nishio, C.; Takumi, H.; Higashiguchi, F.; Aihara, K.I.; Shimabukuro, M.; Sata, M.; et al. Inhibition of Atherosclerotic Plaque Development by Oral Administration of α-Glucosyl Hesperidin and Water-Dispersible Hesperetin in Apolipoprotein E Knockout Mice. J. Am. Coll. Nutr. 2019, 38, 15–22. [Google Scholar] [CrossRef]
- Hong, Q.; Ma, Z.-C.; Huang, H.; Wang, Y.-G.; Tan, H.-L.; Xiao, C.-R.; Liang, Q.D.; Zhang, H.T.; Gao, Y. Antithrombotic activities of ferulic acid via intracellular cyclic nucleotide signaling. Eur. J. Pharmacol. 2016, 777, 1–8. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, J.K.; Kim, K.M.; Lee, H.J.; Kim, S. In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. Journal of biochemical and molecular toxicology. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef]
- Fuentes, E.; Forero-Doria, O.; Carrasco, G.; Maricán, A.; Santos, L.S.; Alarcón, M.; Palomo, I. Effect of tomato industrial processing on phenolic profile and antiplatelet activity. Molecules 2013, 18, 11526–11536. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lee, J.; Lin, K.; Shen, C.; Chou, D.; Sheu, J. Antiplatelet activity of caffeic acid phenethyl ester is mediated through a cyclic GMP-dependent pathway in human platelets. Chin. J. Physiol. 2007, 50, 121. [Google Scholar] [PubMed]
- Chang, Y.; Chen, W.-F.; Lin, K.-H.; Hsieh, C.-Y.; Chou, D.-S.; Lin, L.-J.; Sheu, J.-R.; Chang, C.-C. Novel bioactivity of ellagic acid in inhibiting human platelet activation. Evid. Based Complementary Altern. Med. 2013, 2013, 595128. [Google Scholar] [CrossRef]
- Song, F.; Zhu, Y.; Shi, Z.; Tian, J.; Deng, X.; Ren, J.; Andrews, M.C.; Ni, H.; Ling, W.; Yang, Y. Plant Food Anthocyanins Inhibit Platelet Granule Secretion in Hypercholesterolaemia: Involving the Signalling Pathway of Pi3k–Akt. Thromb. Haemost. 2014, 111, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, L. Antiplatelet Effect of Aristotelia chilensis (Maqui) Extracts through In Vitro Studies; University of Talca: Talca, Chile, 2021. [Google Scholar]
- Ro, J.-Y.; Ryu, J.-H.; Park, H.-J.; Cho, H.-J. Onion (Allium cepa L.) peel extract has anti-platelet effects in rat platelets. SpringerPlus 2015, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, G.P.; Wolffram, S.; de Vos, R.; Bovy, A.; Gibbins, J.M.; Lovegrove, J.A. Ingestion of Onion Soup High in Quercetin Inhibits Platelet Aggregation and Essential Components of the Collagen-Stimulated Platelet Activation Pathway in Man: A Pilot Study. Br. J. Nutr. 2006, 96, 482–488. [Google Scholar]
- Vallance, T.M.; Ravishankar, D.; Albadawi, D.A.I.; Osborn, H.M.I.; Vaiyapuri, S. Synthetic Flavonoids as Novel Modulators of Platelet Function and Thrombosis. Int. J. Mol. Sci. 2019, 20, 3106. [Google Scholar] [CrossRef]
- Fuentes, E.; Wehinger, S.; Trostchansky, A. Regulation of Key Antiplatelet Pathways by Bioactive Compounds with Minimal Bleeding Risk. Int. J. Mol. Sci. 2021, 22, 12380. [Google Scholar] [CrossRef]
- Rodríguez, L.; Badimon, L.; Méndez, D.; Padró, T.; Vilahur, G.; Peña, E.; Carrasco, B.; Vogel, H.; Palomo, I.; Fuentes, E. Antiplatelet Activity of Isorhamnetin via Mitochondrial Regulation. Antioxidants 2021, 10, 666. [Google Scholar] [CrossRef]
- Stainer, A.R.; Sasikumar, P.; Bye, A.P.; Unsworth, A.J.; Holbrook, L.M.; Tindall, M.; Lovegrove, J.A.; Gibbins, J.M. The metabolites of the dietary flavonoid quercetin possess potent antithrombotic activity, and interact with aspirin to enhance antiplatelet effects. TH Open Companion J. Thromb. Haemost. 2019, 3, e244. [Google Scholar] [CrossRef]
- Nam, G.S.; Park, H.-J.; Nam, K.-S. The antithrombotic effect of caffeic acid is associated with a cAMP-dependent pathway and clot retraction in human platelets. Thromb. Res. 2020, 195, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Q.; Liu, Y.Y.; Sun, K.; Fan, J.Y.; Wang, C.S.; Han, J.Y. Inhibitory effect of caffeic acid on ADP-induced thrombus formation and platelet activation involves mitogen-activated protein kinases. Sci. Rep. 2015, 5, 13824. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kim, H.-H.; Cho, H.-J.; Bae, J.-S.; Yu, Y.-B.; Park, H.-J. Antiplatelet effects of caffeic acid due to Ca2+ mobilizationinhibition via cAMP-dependent inositol-1, 4, 5-trisphosphate receptor phosphorylation. J. Atheroscler. Thromb. 2013, 21, 18994. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.S.; Nam, K.-S.; Park, H.-J. Caffeic Acid Diminishes the Production and Release of Thrombogenic Molecules in Human Platelets. Biotechnol. Bioprocess Eng. 2018, 23, 641–648. [Google Scholar] [CrossRef]
- Kyriakidis, K.D.; Vartholomatos, E.G.; Markopoulos, G.S.; Sciences, H. Evaluation of Antiplatelet Activity of Phenolic Compounds by Flow Cytometry. Eur. J. Med. Health Sci. 2021, 3, 165–170. [Google Scholar] [CrossRef]
- Baeza, G.; Bachmair, E.-M.; Wood, S.; Mateos, R.; Bravo, L.; De Roos, B.J.F. The colonic metabolites dihydrocaffeic acid and dihydroferulic acid are more effective inhibitors of in vitro platelet activation than their phenolic precursors. Food Funct. 2017, 8, 1333–1342. [Google Scholar] [CrossRef]
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of Phenolic Compounds in the Reduction of Risk Factors of Cardiovascular Diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.J.M. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; V González de Peredo, A.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Assessment of ultrasound assisted extraction as an alternative method for the extraction of anthocyanins and total phenolic compounds from Maqui berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. [Google Scholar] [CrossRef]
- Khan, H.; Jawad, M.; Kamal, M.A.; Baldi, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Evidence and prospective of plant derived flavonoids as antiplatelet agents: Strong candidates to be drugs of future. Food Chem. Toxicol. 2018, 119, 355–367. [Google Scholar] [CrossRef]
- Marino, M.; Del Bo, C.; Martini, D.; Porrini, M.; Riso, P.J.F. A review of registered clinical trials on dietary (poly) phenols: Past efforts and possible future directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aguilar, D.M.; Grusak, M.A. Analysis, Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of Amaranthus leafy vegetables. J. Food Compos. Anal. 2017, 58, 33–39. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Mizgier, M.L.; Speisky, H.; Gotteland, M.J. Differential protective effects of quercetin, resveratrol, rutin and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem. Interact. 2012, 195, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.; Luben, R.N.; Spencer, J.P.; Schroeter, H.; Khaw, K.-T.; Kuhnle, G. Flavonoid intake in European adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L.J. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Frank, T.; Netzel, G.; Kammerer, D.R.; Carle, R.; Kler, A.; Kriesl, E.; Bitsch, I.; Bitsch, R.; Netzel, M.J. Consumption of Hibiscus sabdariffa L. aqueous extract and its impact on systemic antioxidant potential in healthy subjects. J. Sci. Food Agric. 2012, 92, 2207–2218. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]

| Plant Part(s) | Type of Extract | Dose or Concentration | Mechanism of Action or Effect of Extract and/or Pure Compound Characterization of the Extract | Characterization | Identified Compound | Reference |
|---|---|---|---|---|---|---|
| Fruit | Methanolic extract of ripe fruits of Maqui. | 100, 10, and 1 ppm/kg of rat body weight | Cardioprotection Methanolic extract of ripe fruits of Maqui has antioxidant activity and cardioprotective effect on acute ischemia/reperfusion performed in rat hearts. The extract protected from heart damage due to the incidence of reperfusion dysrhythmias and non-recovery of sinus rhythm. It also prevents harmful events in the heart of the animal by reducing lipid oxidation and reducing the concentration of substances reactive to thiobarbituric acid, and lipid peroxidation index. | No | [20] | |
| Fruit | Maqui berry extract | 150 mg standardized | Oxidative stress biomarkers Delphinol reduces levels of Ox-LDL and urinary F2-isoprostanes (8-iso-prostaglandin F2α). | No | [81] | |
| Fruit | Maqui berry powder (ground whole fruit rich in anthocyanins) | 50 and 100 mg/kg | Oxidative stress markers The administration of the aqueous extract of Maqui berry prevents the cognitive deficit caused by chronic exposure to ozone. Decreases levels of oxidative stress markers and superoxide enzymatic activity in animals exposed to ozone through four oxidative stress markers: 4HNE, MDA, Nt3, and AGEs in brain areas involved in learning and memory processes. | Yes | Ellagic acid derivatives, flavonols, and chlorogenic acid. | [82] |
| Fruit | Hydroethanolic extract of a Chilean berry Maqui. Pure compounds: rutin and quercetin | 500 µg/mL (extract) 50 µM (quercetin) and 10 µM (rutin) | Endothelial dysfunction and oxidative stress Maqui berry extracts, quercetin, and rutin protect against endothelial dysfunction induced by high glucose and pyrogallol through increased generation and bioavailability of NO. | Yes | p-Coumaric acid, rutin, gentisic acid, sinapic acid, procyanidin B; gallic acid, quercetin, myricetin, delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, delphinidin-3,5-O-diglucoside, delphinidin-3-O-sambubioside, cyanidin-3-O-sambubioside, proanthocyanidin B, proanthocyanidin blend, catechin epicatechin blend, p-coumaric acid and p-hydroxybenzoic acid blend, cyanidin catechin blend and free sugar blend. | [72] |
| Fruit | Hydroalcoholic extract of Maqui. Pure compound: rutin | 50 mg (extract) 30 mg/kg (rutin) | Vascular reactivity, hyperglycemia, and dyslipidemia Maqui reduces plasma levels of cholesterol, LDL and triglycerides. Rutin lowers blood sugar and enhances endothelium-dependent relaxation. Maqui and rutin improved the bioavailability of nitric oxide. | Yes | Gentisic acid, ferulic acid, gallic acid, p-coumaric acid, sinapic acid, 4-hydroxybenzoic acid, delphinidin, cyanidin, vanillic acid, quercetin, myricetin, mixed catechin and epicatechin, delphinidin, delphinidin-3-O-sambubioside-5-O-glucoside, delphinidin- 3,5-O-diglucoside, cyanidin-3-O-sambubioside-5-O-glucoside, cyanidin-3,5-O-diglucoside, delphinidin-3-O-sambubioside, delphinidin-3-O-glucoside, cyanidin-3-O-sambubioside, and proanthocyanidin B. | [71] |
| Fruit | Maqui extract enriched with anthocyanins. Pure compound: delphinidin-3-O-sambubioside-5-O-glucoside | 125–500 mg/kg (extract) 2–10 μg/mL and 5–100 μg/mL (delphinidin-3-O-sambubioside-5-O-glucoside) | Type 2 diabetes Oral administration of anthocyanins reduces fasting blood glucose levels and glucose tolerance in hyperglycemic obese C57BL/6J mice fed a high-fat diet. Oral administration of delphinidin-3-O-sambubioside-5-O-glucoside dose-dependently lowered fasting blood glucose levels in obese C57BL/6J mice (2–10 μg/mL). It also decreases glucose production in rat liver cells (50–100 μg/mL). | Yes | Delphinidin-3-O-sambubioside-5-O-glucoside, delphinidin-3,5-O-diglucoside, delphinidin3-O-sambubioside, delphinidin-3-O-glucoside, cyanidin-3-O-sambubioside, cyanidin-3-O-glucoside, cyanidin-3-O-sambubioside-5-O-glucoside + cyanidin-3,5-O-diglucoside. | [75] |
| Fruit | Standardized extract of berries of Maqui. Pure compound: delphinidin | 20 mg/kg (extract) 50 µM (delphinidin) | Postprandial blood glucose Delphinol® lowers blood glucose and postprandial insulin. Daily oral application of Delphinol® for four months reduces fasting blood glucose levels and lowers postprandial glycemia due to sodium–glucose cotransporter inhibition in the small intestine. | No | [73] | |
| Fruit | Maqui | 250 mL containing approx. 1000 μmol GAE of polyphenols) | Postprandial blood glucose Reduction in the glycemic peak mediated by the glucose + Maqui + lemon mixture. Mixing glucose + Maqui + lemon reduces the glycemic peak of glucose (20.5 ± 8.4%) compared to glucose. This amount represents a reduction of 36.7 ± 15.0 mg/dL in postprandial blood glucose. | No | [75] | |
| Fruit | Maqui extract | 20 mg of freeze-dried Maqui/mL of filtered tap water | Obesity Maqui extract prevents diet-induced obesity and its associated comorbidities. Reduced fasting glucose. Improves insulin response and reduces weight gain, and also a differential expression of genes involved in de novo lipogenesis. | Yes | Delphinidin-3-O-sambubioside-5-O-glucoside, delphinidin-3-O-sambubioside, cyanidin-3-O-sambubioside-5-O-glucoside, cyanidin-3-O-glucoside, cyanidin-3-O- sambubioside. | [74] |
| Compounds | In Vitro | In Vivo | Reference |
|---|---|---|---|
| Anthocyanins | |||
| Delphinidin-3-O-glucoside (5–50 µg/mL) | Inhibition of platelet aggregation with collagen (10 μg/mL) and TRAP-6 (100 μM) at 0.5 μM and 50 μM in washed platelets. Inhibition of platelet aggregation with ADP (5 μM), collagen (10 μg/mL) and TRAP-6 (100 μM) at 0.5 μM and 50 μM in platelet-rich plasma Dose-dependent reduction in activated GPIIb/IIIa expression. Inhibition of platelet adhesion and aggregation in perfusion chamber assays at low and high shear rates. Decreased platelet deposition, thrombus formation, and vessel occlusion. | [94] | |
| Inhibition of platelet aggregation with ADP (5 μM), collagen (2 μg/mL), and TRAP (100 μM). Inhibition of the activation and secretion of P-selectin, CD63, CD40L, αllbβ3, and fibrinogen with ADP (200 μM), collagen (10 μg mL), thrombin (1 U/mL), and TRAP (250 μM). Mechanism: inhibition of the phosphorylation of MAPK induced by collagen (25 μg/mL). | Inhibition of collagen-induced thrombus formation (100 μg/mL), using controlled flow. Inhibition of thrombus formation induced by FeCl3 at 50 μg/mL, using intravital microscopy. | [31,95] | |
| Cyanidin-3-O-glucoside (5–50 µg/mL) | Inhibition of the activation and secretion of P-selectin, CD63, CD40L, αllbβ3, fibrinogen with collagen (10 µg/mL), thrombin (2 U/mL), and TRAP (250 µM). Inhibition of platelet aggregation with collagen (2.5 µg/mL), thrombin (0.1 U/mL), and TRAP (100 µM). Mechanism: via receiver GPVI collagen (2.5 µg/mL). Inhibition of the phosphorylation of tyrosine protein induced by collagen (2.5 µg/mL) at 5–50 µM. | Inhibition of the formation of the thrombus induced by collagen (0.5–50 µM) and FeCl3 at 5–50 µM. | [95,96] |
| Inhibition of platelet aggregation with collagen (10 μg/mL) and TRAP-6 (100 μM) at 0.5 μM-and 50 μM in washed platelets. Inhibition of platelet aggregation with ADP (5 μM), collagen (10 μg/mL) and TRAP-6 (100 μM) at 0.5 μM and 50 μM in platelet-rich plasma Dose-dependent reduction in activated GPIIb/IIIa expression. Inhibition of platelet adhesion and aggregation in perfusion chamber assays at low and high shear rates. Decreased platelet deposition, thrombus formation, and vessel occlusion. | [94] | ||
| Inhibition of platelet granules (P-selectin, CD40L, 5-HT, RANTES, and TGF-β1) with thrombin (0.5 U/mL). | Attenuated serum levels of PF4 and β-TG in mice fed high-fat diets at a dose of 1000 mg/kg. | [97] | |
| Flavonols | |||
| Quercetin- 4”-O-β-D-glucoside | Inhibition of platelet aggregation with collagen (50 μL) at 150 mg. Mechanism: inhibition of the phosphorylation of protein tyrosine kinase Syk and PLCγ2 induced by collagen (25 μg/mL) at 150 mg. | [98] | |
| Quercetin | Inhibition of platelet aggregation with AA (100 μM), ADP (20 μM), collagen (10 μg/mL) at 13 μM. It inhibits ATP release with ADP (7 μM) and epinephrine (7 μM) at 2.5 μM. Mechanism: inhibits the formation of TxA2 and PG induced by AA (100 μM) at 5 μM. | [99] | |
| Inhibition of platelet aggregation with 100 μg/mL of AA (100 μM) and collagen (10 μg/mL) at 100 μg/mL. | Relaxation in the thoracic aorta of the rat is induced by norepinephrine (3 μM) at 100 μM. | [100] | |
| Inhibition of platelet aggregation with collagen (0.5–5 μL/mL) at IC50: 2.37–8.69. Inhibition of the mobilization of Ca2+ induced by collagen (5 μL/mL) at 15 μM. Mechanism: inhibits the GPVI signaling pathways, phosphorylation of tyrosine protein, and PI3 kinase induced by collagen (25 μL/mL) at 25 μM. | [101] | ||
| Inhibition of platelet aggregation with collagen (0.5–5 μL/mL) at IC50: 2.37–8.69. Inhibition of the mobilization of Ca2+ induced by collagen (5 μL/mL) at 15 μM. Mechanism: inhibits the GPVI signaling pathways, phosphorylation of tyrosine protein, and PI3 kinase induced by collagen (25 μL/mL) at 25 μM. | [102] | ||
| Inhibition of platelet aggregation with AA (150 μM) IC50: 18 μM. The increase in cAMP stimulated by PGI2 (0.5 nM) decreased at 50 μM. Mechanism: inhibition of the activity of COX-1 and lipoxygenase at 10 μM and 50 μM. | [103] | ||
| Kaempferol | Inhibition of thrombin (40 mU) and FXa (20 mU) (68 ± 1.6% and 52 ± 2.4%, respectively). Attenuated fibrin polymer formation in turbidity and phosphorylation of ERK 1/2, p38, JNK 1/2, and phosphoinositide PI3K/PKB (AKT) in cells stimulated with thrombin (0.5 U/mL). Inhibition of platelet aggregation stimulated by collagen/epinephrine (34.6%). Mechanism: inhibition of phosphorylation of ERK 1/2, p38, JNK 1/2, and PI3K/PKB. | Decreased thrombus formation in 3 animal models (collagen/epinephrine and thrombin-induced acute thromboembolism, FeCl3-induced model, and carotid arterial thrombus model). | [104] |
| Decreased collagen adhesion in resting platelets and activated platelets with thrombin at a dose of 5 μg/mL. Inhibition of platelets activated by thrombin and fibrinogen (40%). Inhibition of platelet aggregation with collagen (5 μg/mL) and AA (0.5 μmol/L) at 50 μg/kg. Thrombin-stimulated reduction of enzymatic lipid peroxidation in platelets. | [105] | ||
| Myricetin | Inhibition of platelet aggregation with collagen (5 μg/mL) and AA (0.5 μmol/L) at 50 μg/kg. Thrombin-stimulated reduction in enzymatic lipid peroxidation in platelets. | [106] | |
| Inhibition of platelet aggregation and secretion of alpha granules. with TRAP-6 (10 µM) and collagen (1 µg/mL) at 15 and 30 µM. Decreased fibrinogen binding induced by CRP (1 µg/mL) and TRAP-6 (10 µM) at 15 μM. Reduction in adhesion on collagen and thrombus formation without affecting hemostasis in vivo. Mechanism: inhibition of ERp5 and PDI. | [107] | ||
| Dose-dependent (20–30 µM) inhibition of platelet aggregation, granule secretion and activation (activation of αIIbβ3 integrin and P-selectin exposure), generation of ROS, and induced intracellular Ca2+ mobilization by CRP (0.1 µg/mL) and collagen (1 µg/mL). Mechanism: inhibition of GPVI during cell activation. | Reduction in ischemia/reperfusion-induced acute infarction in a mouse model of stroke. Blocked FeCl3-induced arterial thrombus formation in vivo and thrombus formation on collagen-coated surfaces under low shear rate. | [108] | |
| Rutin | Inhibition of platelet aggregation with collagen at 250 μM (1 μg/mL). The mobilization of Ca2+ induced by collagen (1 μg/mL) decreases to 250 μM. Mechanism: inhibition of the PLC phosphorylation and formation of TxA2, inhibits collagen-induced phosphorylation of P47 at 250 μM. | [109] | |
| Flavanones | |||
| Eriodictyol | Inhibition of platelet aggregation with collagen (2 μg/mL) and AA (0.5 mmol/L) at 50 μM. | [110] | |
| Hesperetin | Concentration-dependent inhibition of platelet aggregation induced by collagen (5 μg/mL) and AA (0.5 μmol/L) (IC50: 20.5 and at IC50: 69.2, respectively). Inhibition mobilization of cytosolic Ca2+ induced by collagen (10 μg/mL) at 20–50 μM. Inhibition of the secretion of serotonin with collagen (5 μg/mL) and AA (0.5 μmol/L) at IC50: 10.5 and at IC50: 25.2, respectively. Mechanism: inhibitionPLC-γ2 phosphorylation. Inhibition of COX-1 activity. | [111] | |
| Atherosclerosis inhibition | [112] | ||
| Phenolic acids | |||
| Ferulic acid | Inhibition of platelet aggregation induced by ADP, thrombin (0.5 U/mL), AA (2 mM), collagen (2 μg/mL), and U46619 (2 μM) at 50–200 µM. Inhibition of mobilization of cytosolic Ca2+ and TXB2 production. Increased the levels of cAMP and cGMP and phosphorylated VASP. Decreased phospho-MAPK and PDE. Mechanism: activation of cAMP and cGMP signaling. | Decreased pulmonary thrombosis and prolonged tail bleeding and coagulation time in mice without altering coagulation parameters. | [113] |
| Inhibition of platelet activation (serotonin secretion) stimulated by thrombin, collagen/epinephrine, and decreased clot retraction activity at 10 μg. Mechanism: decreased granule secretion, prolongation of the intrinsic coagulation cascade, and upregulation of αIIbβ3/FIB/AKT signaling expressions. | Decreased thrombosis in acute thromboembolism model and decreased αIIbβ3/ FIB expression and AKT phosphorylation in thrombin-stimulated platelet activation. | [114] | |
| Caffeic acid | Inhibition of platelet aggregation with ADP (8 μmol/L) and collagen (1.5 μg/mL) at 0.5 mmol/L. | [115] | |
| Inhibition of the activation and secretion of P-selectin with TRAP (25 μmol/L) at 100 μmol/L. | [83] | ||
| Inhibition of platelet aggregation with collagen (2 μg/mL) at 15–25 μM. Mechanism: inhibition of the phosphorylation of cGMP/VAS Ser /VASP Ser157 at 15–25 μM. Decreases PKC and phosphorylation of P47 at 15–25 μM. | [116] | ||
| Ellagic acid | Inhibition of platelet aggregation with collagen (1 μg/mL) at IC50: 50 μM. The mobilization of Ca2+ induced by collagen (1 μg/mL) decreases at 50 μM. Mechanism: inhibition of the PLCγ2-PKC cascade, OH* formation, MAPKs, and Akt induced by collagen (1 μg/mL) at 50 μM. | [117] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, L.; Trostchansky, A.; Vogel, H.; Wood, I.; Palomo, I.; Wehinger, S.; Fuentes, E. A Comprehensive Literature Review on Cardioprotective Effects of Bioactive Compounds Present in Fruits of Aristotelia chilensis Stuntz (Maqui). Molecules 2022, 27, 6147. https://doi.org/10.3390/molecules27196147
Rodríguez L, Trostchansky A, Vogel H, Wood I, Palomo I, Wehinger S, Fuentes E. A Comprehensive Literature Review on Cardioprotective Effects of Bioactive Compounds Present in Fruits of Aristotelia chilensis Stuntz (Maqui). Molecules. 2022; 27(19):6147. https://doi.org/10.3390/molecules27196147
Chicago/Turabian StyleRodríguez, Lyanne, Andrés Trostchansky, Hermine Vogel, Irene Wood, Iván Palomo, Sergio Wehinger, and Eduardo Fuentes. 2022. "A Comprehensive Literature Review on Cardioprotective Effects of Bioactive Compounds Present in Fruits of Aristotelia chilensis Stuntz (Maqui)" Molecules 27, no. 19: 6147. https://doi.org/10.3390/molecules27196147
APA StyleRodríguez, L., Trostchansky, A., Vogel, H., Wood, I., Palomo, I., Wehinger, S., & Fuentes, E. (2022). A Comprehensive Literature Review on Cardioprotective Effects of Bioactive Compounds Present in Fruits of Aristotelia chilensis Stuntz (Maqui). Molecules, 27(19), 6147. https://doi.org/10.3390/molecules27196147








