Large-Area Ordered Palladium Nanostructures by Colloidal Lithography for Hydrogen Sensing
Abstract
:1. Introduction
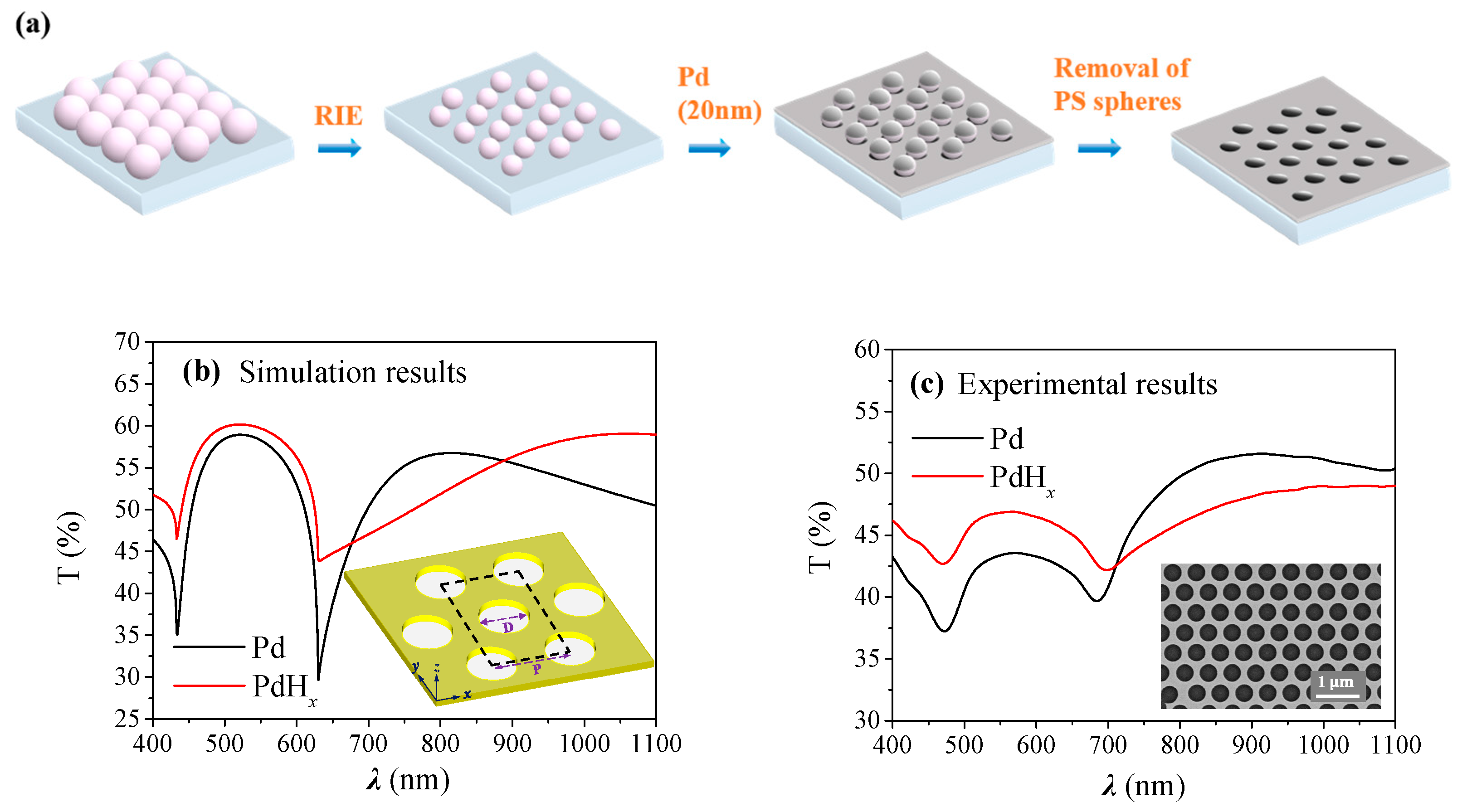
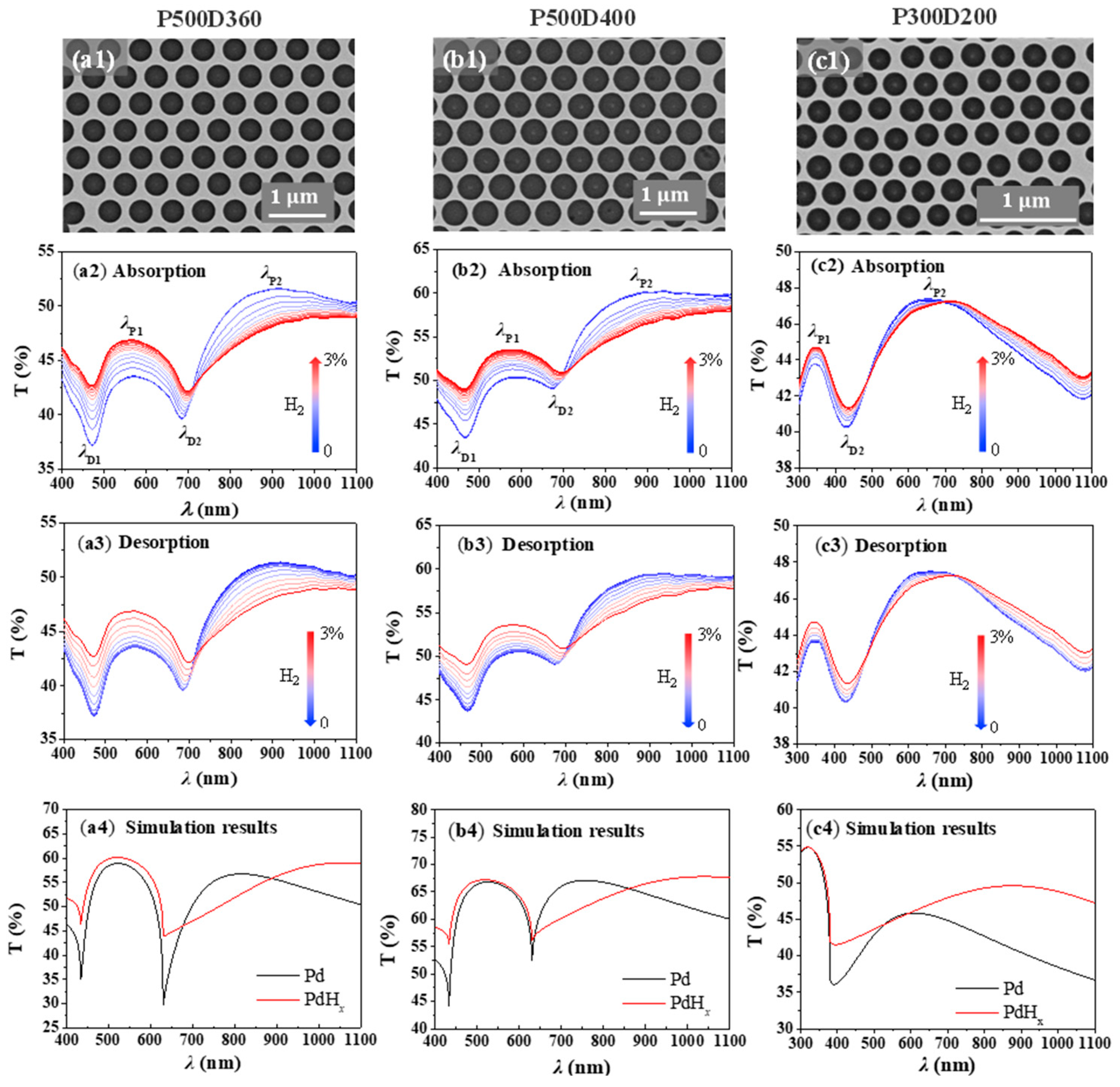
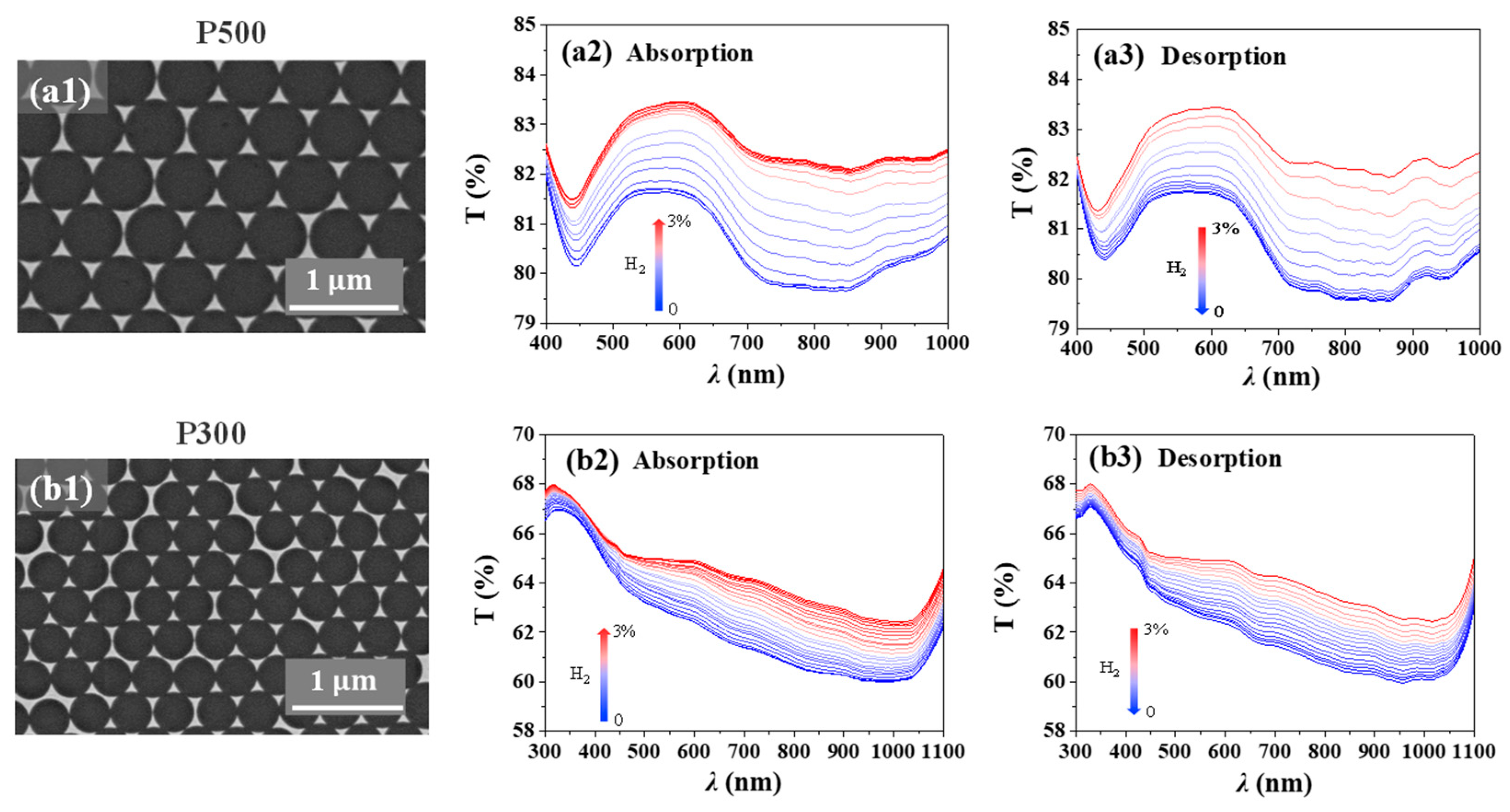
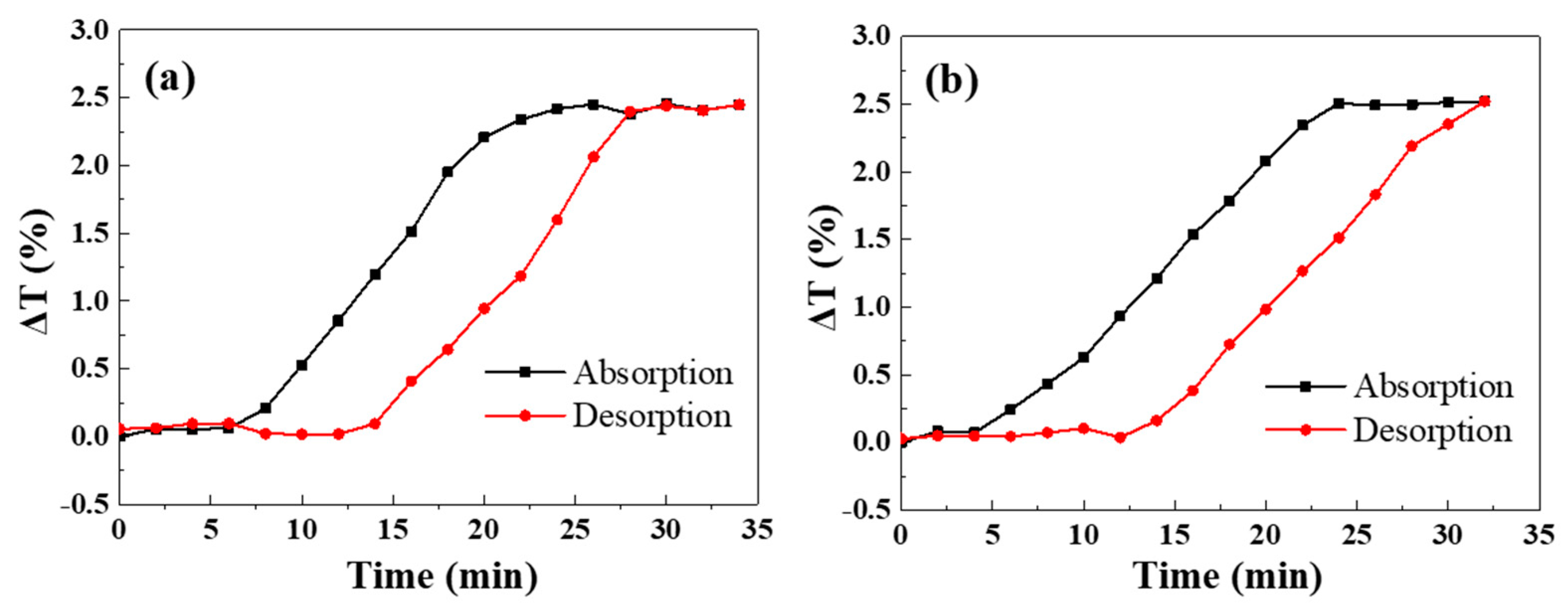
2. Results and Discussions
3. Conclusions
4. Materials and Methods
4.1. Nanohole Fabrication
4.2. Optical Characterization
4.3. Numerical Simulation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Balta, M.Ö.; Balta, M.T. Development of a sustainable hydrogen city concept and initial hydrogen city projects. Energy Policy 2022, 166, 113015. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 166, 205–213. [Google Scholar] [CrossRef]
- Butler, M.; Ginley, D. Hydrogen sensing with palladium-coated optical fibers. J. Appl. Phys. 1988, 64, 3706–3712. [Google Scholar] [CrossRef]
- Wadell, C.; Syrenova, S.; Langhammer, C. Plasmonic hydrogen sensing with nanostructured metal hydrides. ACS Nano 2014, 8, 11925–11940. [Google Scholar] [CrossRef] [PubMed]
- Buttner, W.J.; Post, M.B.; Burgess, R.; Rivkin, C. An overview of hydrogen safety sensors and requirements. Int. J. Hydrogen Energy 2011, 36, 2462–2470. [Google Scholar] [CrossRef]
- Tobiška, P.; Hugon, O.; Trouillet, A.; Gagnaire, H. An integrated optic hydrogen sensor based on SPR on palladium. Sens. Actuators B Chem. 2001, 74, 168–172. [Google Scholar] [CrossRef]
- Berube, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Luong, H.M.; Pham, M.T.; Guin, T.; Madhogaria, R.P.; Phan, M.-H.; Larsen, G.K.; Nguyen, T.D. Sub-second and ppm-level optical sensing of hydrogen using templated control of nano-hydride geometry and composition. Nat. Commun. 2021, 12, 2414. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tang, M.L.; Hentschel, M.; Giessen, H.; Alivisatos, A.P. Nanoantenna-enhanced gas sensing in a single tailored nanofocus. Nat. Mater. 2011, 10, 631–636. [Google Scholar] [CrossRef]
- Nishijima, Y.; Balcytis, A.; Naganuma, S.; Seniutinas, G.; Juodkazis, S. Tailoring metal and insulator contributions in plasmonic perfect absorber metasurfaces. ACS Appl. Nano Mater. 2018, 1, 3557–3564. [Google Scholar] [CrossRef]
- Ai, B.; Sun, Y.; Zhao, Y. Plasmonic Hydrogen Sensors. Small 2022, 18, 2107882. [Google Scholar] [CrossRef] [PubMed]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen gas sensors based on semiconductor oxide nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, F.A.; Darmadi, I.; Cusinato, L.; Susarrey-Arce, A.; Schreuders, H.; Bannenberg, L.J.; Fanta, A.B.D.; Kadkhodazadeh, S.; Wagner, J.B.; Antosiewicz, T.J. Metal–polymer hybrid nanomaterials for plasmonic ultrafast hydrogen detection. Nat. Mater. 2019, 18, 489–495. [Google Scholar] [CrossRef]
- Nugroho, F.A.A.; Darmadi, I.; Zhdanov, V.P.; Langhammer, C. Universal scaling and design rules of hydrogen-induced optical properties in Pd and Pd-Alloy nanoparticles. ACS Nano 2018, 12, 9903–9912. [Google Scholar] [CrossRef] [PubMed]
- Sugawa, K.; Tahara, H.; Yamashita, A.; Otsuki, J.; Sagara, T.; Harumoto, T.; Yanagida, S. Refractive index susceptibility of the plasmonic palladium nanoparticle: Potential as the third plasmonic sensing material. ACS Nano 2015, 9, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Darmadi, I.; Nugroho, F.A.A.; Kadkhodazadeh, S.; Wagner, J.B.; Langhammer, C. Rationally designed pdaucu ternary alloy nanoparticles for intrinsically deactivation-resistant ultrafast plasmonic hydrogen sensing. ACS Sens. 2019, 4, 1424–1432. [Google Scholar] [CrossRef]
- Schwarz, R.; Khachaturyan, A. Thermodynamics of open two-phase systems with coherent interfaces: Application to metal–hydrogen systems. Acta Mater. 2006, 54, 313–323. [Google Scholar] [CrossRef]
- Gao, M.; Cho, M.; Han, H.J.; Jung, Y.S.; Park, I. Palladium-decorated silicon nanomesh fabricated by nanosphere lithography for high performance, room temperature hydrogen sensing. Small 2018, 14, 1703691. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, H.H. High-performance, flexible hydrogen sensors that use carbon nanotubes decorated with palladium nanoparticles. Adv. Mater. 2007, 19, 2818–2823. [Google Scholar] [CrossRef]
- Zeng, X.; Latimer, M.; Xiao, Z.; Panuganti, S.; Welp, U.; Kwok, W.; Xu, T. Hydrogen gas sensing with networks of ultrasmall palladium nanowires formed on filtration membranes. Nano Lett. 2011, 11, 262–268. [Google Scholar] [CrossRef]
- Pak, Y.; Lim, N.; Kumaresan, Y.; Lee, R.; Kim, K.; Kim, T.H.; Kim, S.M.; Kim, J.T.; Lee, H.; Ham, M.H. Palladium nanoribbon array for fast hydrogen gas sensing with ultrahigh sensitivity. Adv. Mater. 2015, 27, 6945–6952. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Duan, M.; Yu, P.; Houk, K.; Sun, J. Organocatalytic enantioselective dearomatization of thiophenes by 1, 10-conjugate addition of indole imine methides. Nat. Commun. 2021, 12, 4881. [Google Scholar] [CrossRef] [PubMed]
- Murray-Methot, M.-P.; Menegazzo, N.; Masson, J.-F. Analytical and physical optimization of nanohole-array sensors prepared by modified nanosphere lithography. Analyst 2008, 133, 1714–1721. [Google Scholar] [CrossRef]
- Im, H.; Bantz, K.C.; Lee, S.H.; Johnson, T.W.; Haynes, C.L.; Oh, S.H. Self-assembled plasmonic nanoring cavity arrays for SERS and LSPR biosensing. Adv. Mater. 2013, 25, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Wang, M.; Shen, T.; Zhou, J. Self-Assembled Large-Area Annular Cavity Arrays with Tunable Cylindrical Surface Plasmons for Sensing. ACS Nano 2015, 9, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Ai, B.; Basnet, P.; Larson, S.; Ingram, W.; Zhao, Y. Plasmonic sensor with high figure of merit based on differential polarization spectra of elliptical nanohole array. Nanoscale 2017, 9, 14710–14721. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, F.; Gao, R.; Jao, C.-Y.; Ma, C.; Li, J.; Li, X.; Guan, B.-O.; Cetin, A.E.; Chen, K. Rayleigh anomaly-enabled mode hybridization in gold nanohole arrays by scalable colloidal lithography for highly-sensitive biosensing. Nanophotonics 2022, 11, 507–517. [Google Scholar] [CrossRef]
- Chen, K.; Rajeeva, B.B.; Wu, Z.; Rukavina, M.; Dao, T.D.; Ishii, S.; Aono, M.; Nagao, T.; Zheng, Y. Moiré Nanosphere Lithography. ACS Nano 2015, 9, 6031–6040. [Google Scholar] [CrossRef]
- Avila, J.; Matelon, R.; Trabol, R.; Favre, M.; Lederman, D.; Volkmann, U.; Cabrera, A. Optical properties of Pd thin films exposed to hydrogen studied by transmittance and reflectance spectroscopy. J. Appl. Phys. 2010, 107, 023504. [Google Scholar] [CrossRef]
- Palm, K.J.; Murray, J.B.; Narayan, T.C.; Munday, J.N. Dynamic optical properties of metal hydrides. ACS Photonics 2018, 5, 4677–4686. [Google Scholar] [CrossRef]
- Maeda, E.; Matsuki, T.; Yamada, I.; Delaunay, J.-J. Hole shape effect induced optical response to permittivity change in palladium sub-wavelength hole arrays upon hydrogen exposure. J. Appl. Phys. 2012, 111, 084502. [Google Scholar] [CrossRef]
- Luong, H.M.; Pham, M.T.; Madhogaria, R.P.; Phan, M.-H.; Larsen, G.K.; Nguyen, T.D. Bilayer plasmonic nano-lattices for tunable hydrogen sensing platform. Nano Energy 2020, 71, 104558. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Ghaemi, H.; Thio, T.; Wolff, P.A. Extraordinary optical transmission through sub-wavelength hole arrays. Nature 1998, 391, 667–669. [Google Scholar] [CrossRef]
- Martin-Moreno, L.; Garcia-Vidal, F.; Lezec, H.; Pellerin, K.; Thio, T.; Pendry, J.; Ebbesen, T. Theory of extraordinary optical transmission through subwavelength hole arrays. Phys. Rev. Lett. 2001, 86, 1114. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Wang, X.; Wang, T.; Liu, H.; Yang, H.; Gong, Y.; Gao, J. Structurally tunable plasmonic absorption bands in a self-assembled nano-hole array. Nanoscale 2018, 10, 19117–19124. [Google Scholar] [CrossRef]
- Larson, S.; Carlson, D.; Ai, B.; Zhao, Y. The extraordinary optical transmission and sensing properties of Ag/Ti composite nanohole arrays. Phys. Chem. Chem. Phys. 2019, 21, 3771–3780. [Google Scholar] [CrossRef]
- Strohfeldt, N.; Tittl, A.; Giessen, H. Long-term stability of capped and buffered palladium-nickel thin films and nanostructures for plasmonic hydrogen sensing applications. Opt. Mater. Express 2013, 3, 194–204. [Google Scholar] [CrossRef]
- Maeda, E.; Mikuriya, S.; Endo, K.; Yamada, I.; Suda, A.; Delaunay, J.-J. Optical hydrogen detection with periodic subwavelength palladium hole arrays. Appl. Phys. Lett. 2009, 95, 133504. [Google Scholar] [CrossRef]
- Sil, D.; Gilroy, K.D.; Niaux, A.; Boulesbaa, A.; Neretina, S.; Borguet, E. Seeing is believing: Hot electron based gold nanoplasmonic optical hydrogen sensor. ACS Nano 2014, 8, 7755–7762. [Google Scholar] [CrossRef] [PubMed]

| Hydrogen Sensors Type | Sensitivity | Response Time | Recovery Time | Ref. | |
|---|---|---|---|---|---|
| Transmission | Wavelength | ||||
| Pd nano-disk | ~6.5% at 3% H2 | 30 nm at 3% H2 | <10 s | <30 s | [40] |
| Pd hole arrays | ~7% at 2% H2 | 200 nm at 2% H2 | - | - | [41] |
| Pd hole arrays | ~7% at 2% H2 | 200 nm at 2% H2 | - | - | [34] |
| Au nano-disk | ~1.8% at 10% H2 | ~3 nm at 10% H2 | 0.3–1 s | - | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Zhang, Z.; Ma, J.; Ma, C.; Guan, B.-O.; Chen, K. Large-Area Ordered Palladium Nanostructures by Colloidal Lithography for Hydrogen Sensing. Molecules 2022, 27, 6100. https://doi.org/10.3390/molecules27186100
Xu F, Zhang Z, Ma J, Ma C, Guan B-O, Chen K. Large-Area Ordered Palladium Nanostructures by Colloidal Lithography for Hydrogen Sensing. Molecules. 2022; 27(18):6100. https://doi.org/10.3390/molecules27186100
Chicago/Turabian StyleXu, Feng, Zhiliang Zhang, Jun Ma, Churong Ma, Bai-Ou Guan, and Kai Chen. 2022. "Large-Area Ordered Palladium Nanostructures by Colloidal Lithography for Hydrogen Sensing" Molecules 27, no. 18: 6100. https://doi.org/10.3390/molecules27186100
APA StyleXu, F., Zhang, Z., Ma, J., Ma, C., Guan, B.-O., & Chen, K. (2022). Large-Area Ordered Palladium Nanostructures by Colloidal Lithography for Hydrogen Sensing. Molecules, 27(18), 6100. https://doi.org/10.3390/molecules27186100







