Thermoresponsive Polyphosphoester via Polycondensation Reactions: Synthesis, Characterization, and Self-Assembly
Abstract
:1. Introduction
2. Results and Discussion
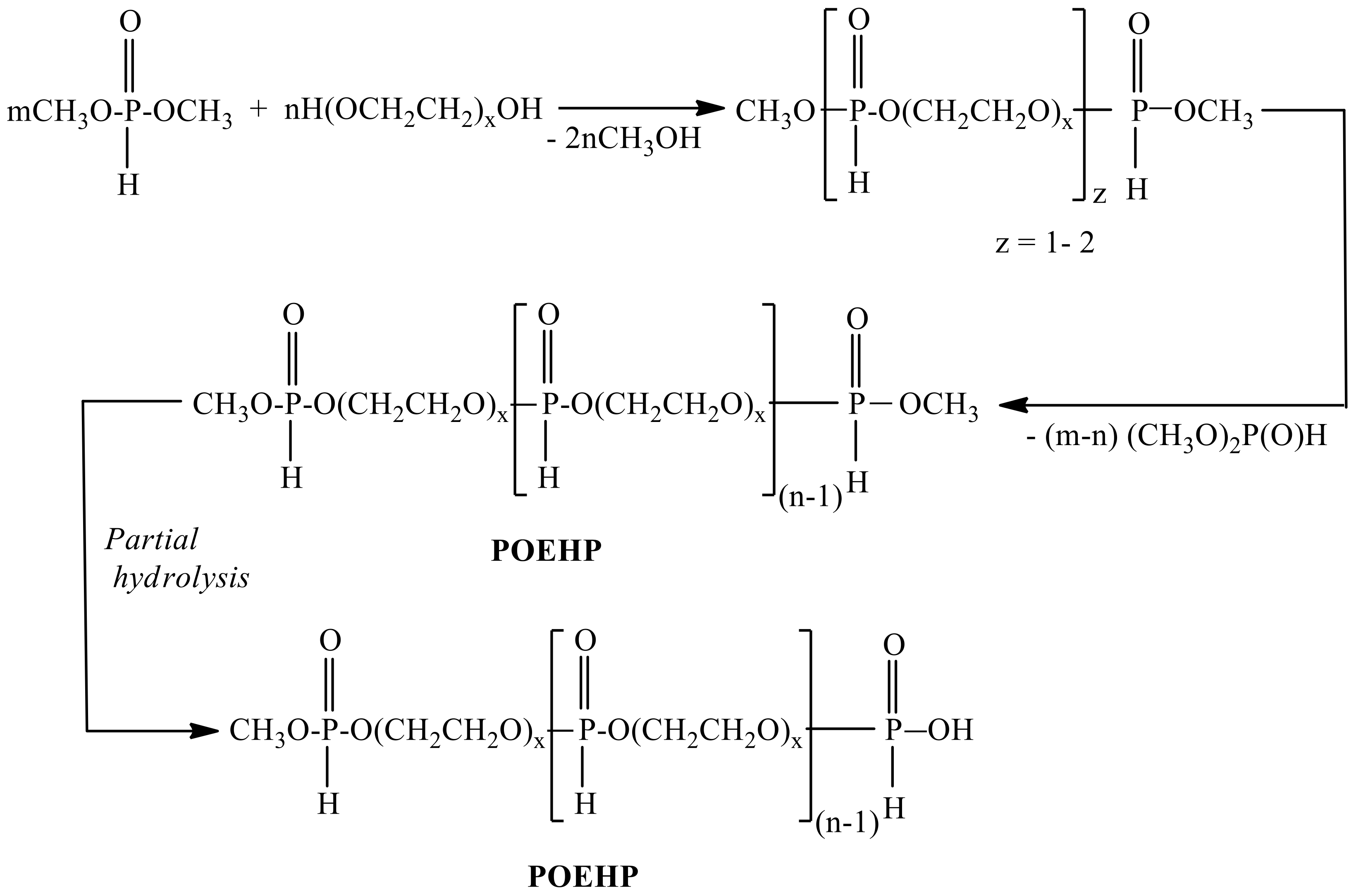
2.1. Synthesis of Poly(oxyethylene H-phosphonate)s
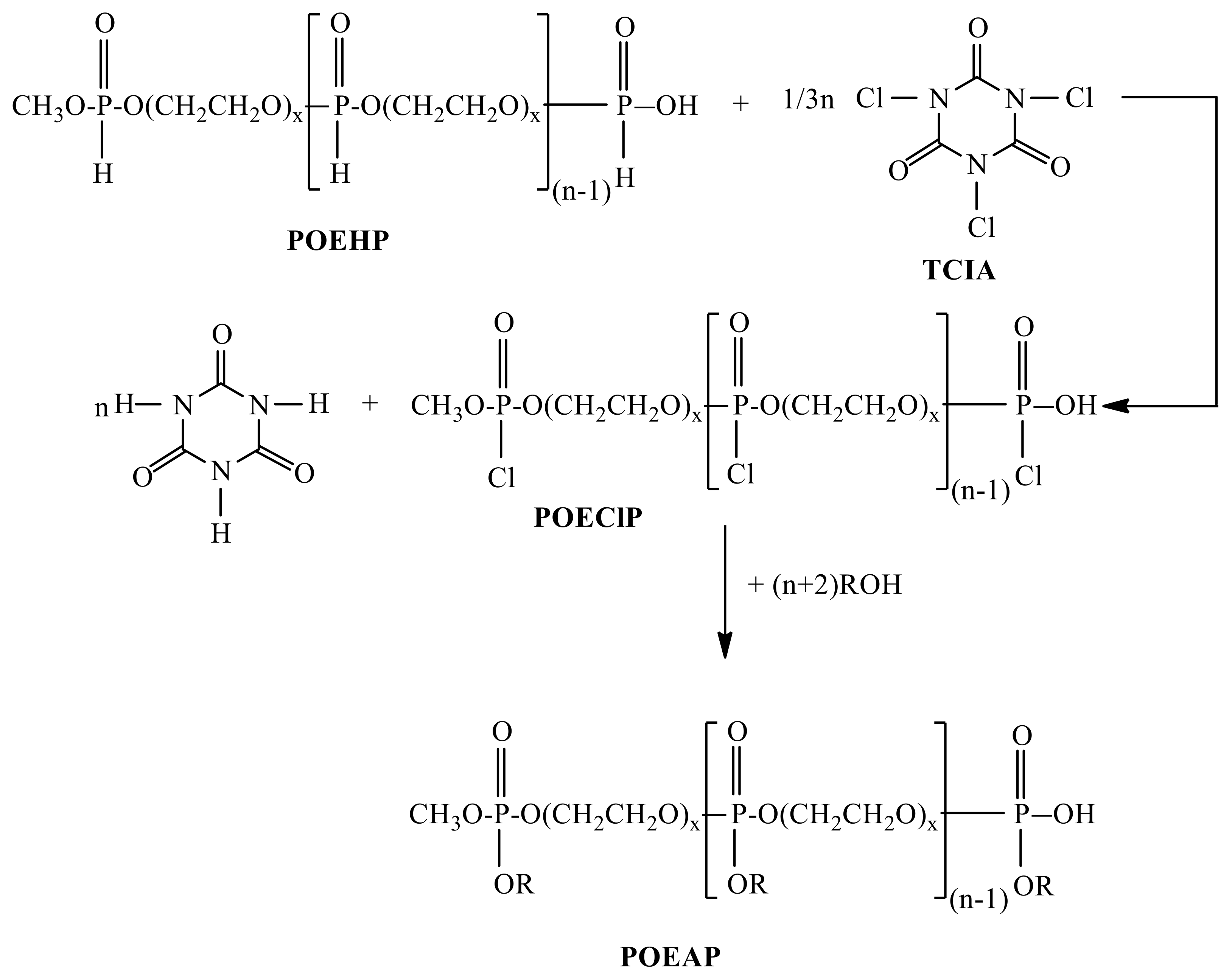
2.2. Synthesis of Poly(alkyloxyethylene phosphate)s (POEAP)
2.3. Micelle Stability
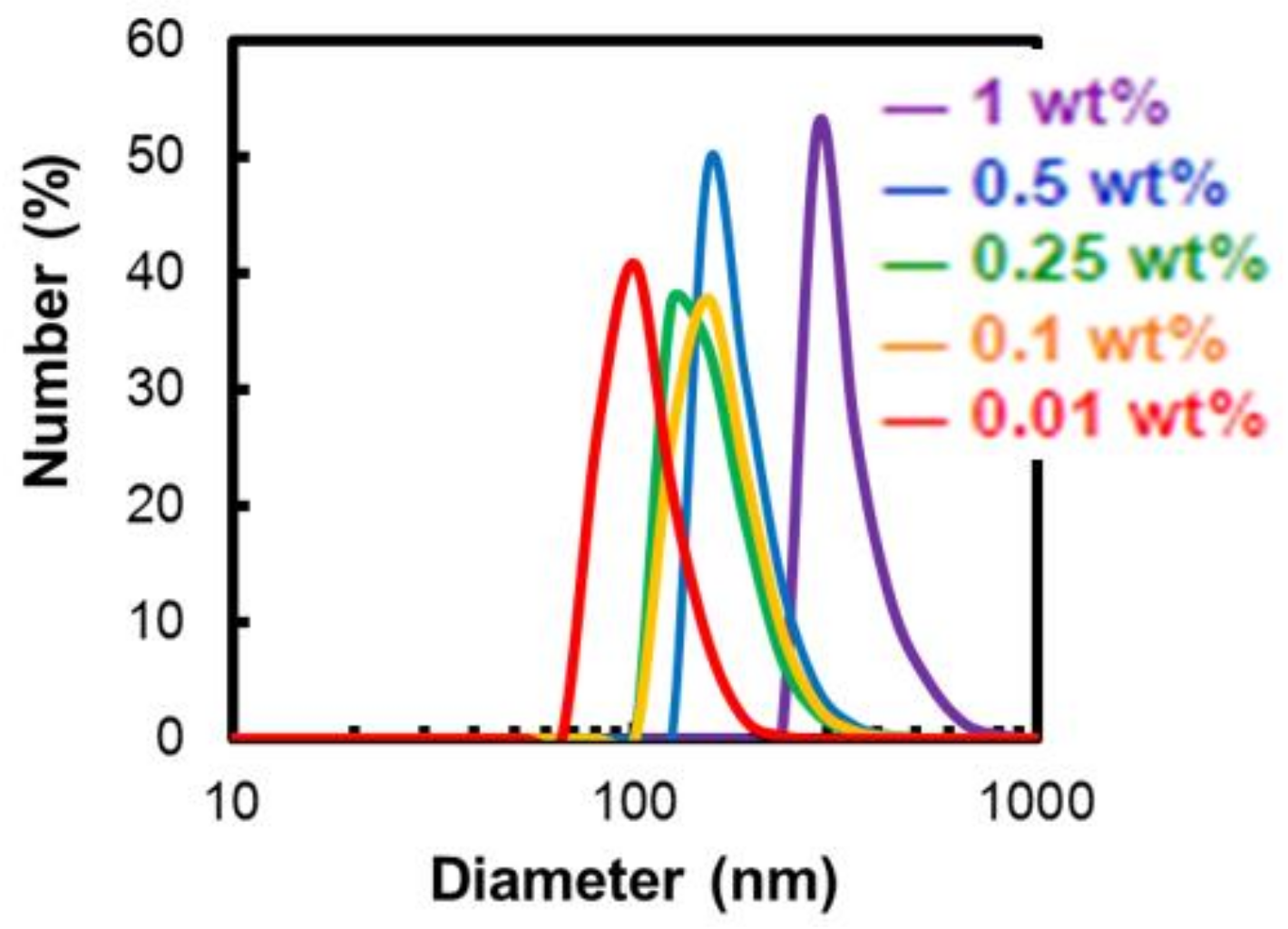
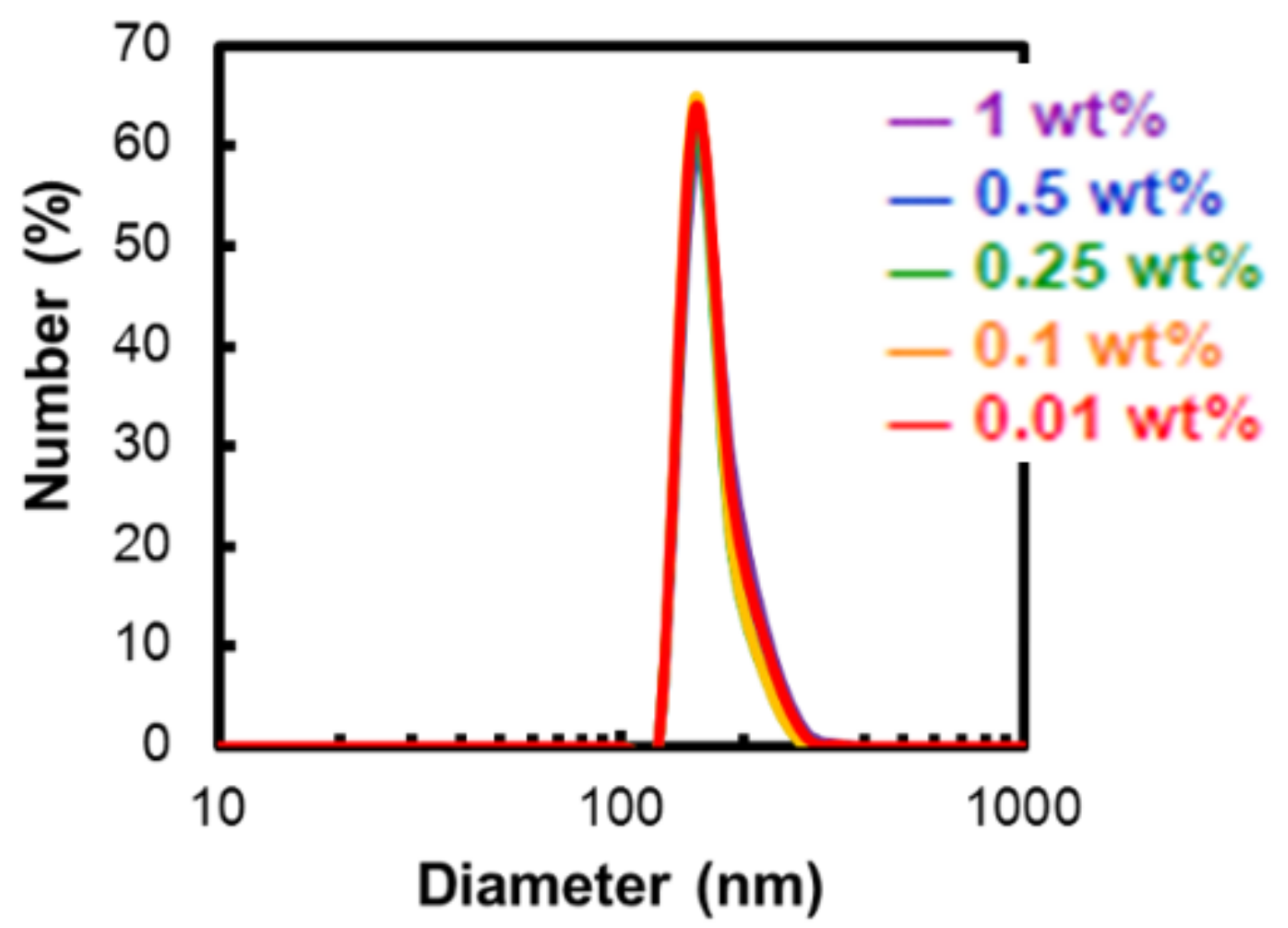
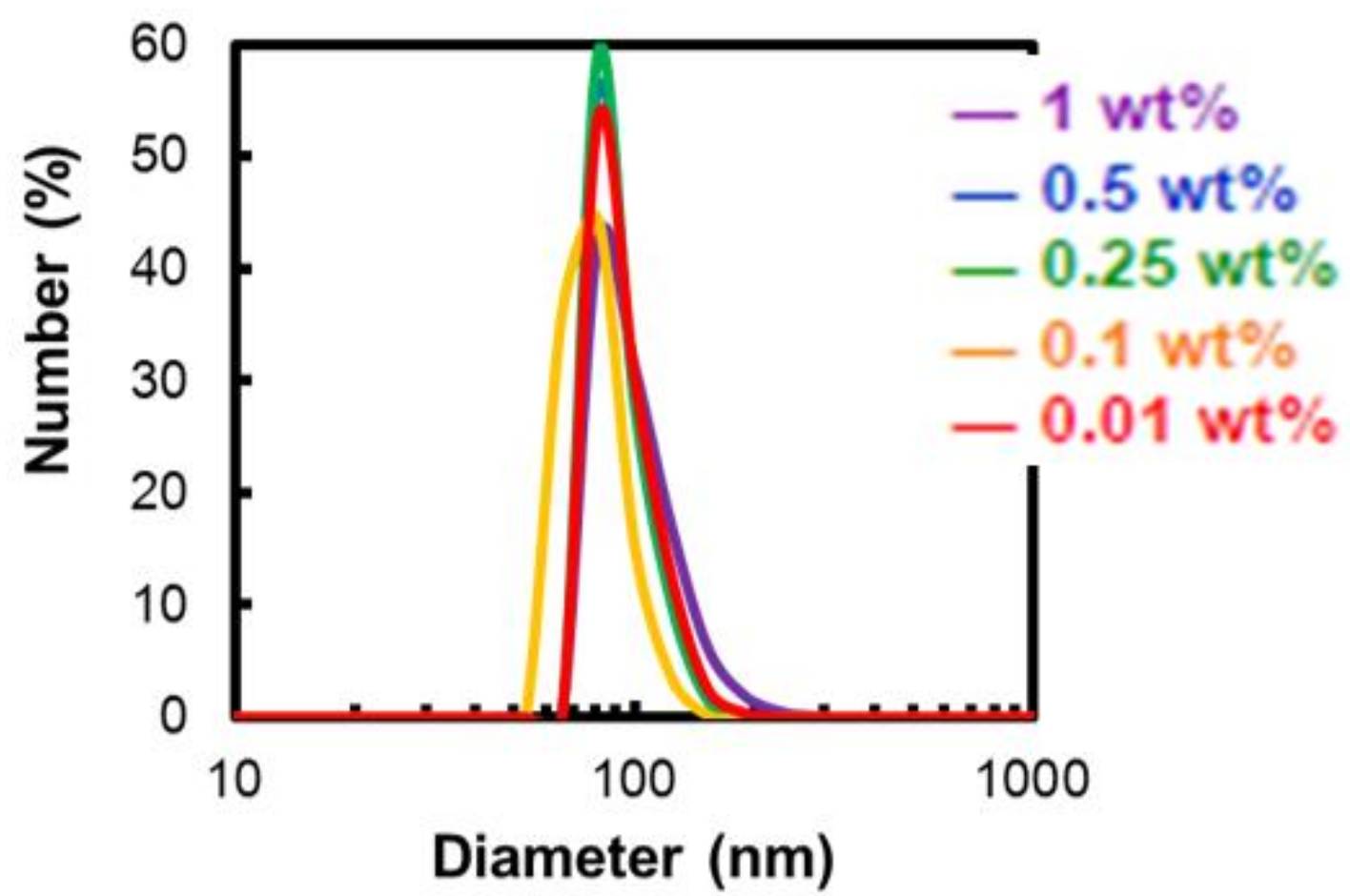
2.3.1. Dependence of Micelle Diameter on the Alkyl Chain Length and the Polymer Concentration
2.3.2. Temperature Dependence of Micelle Diameter from the Temperature and PEG’s Chain Length
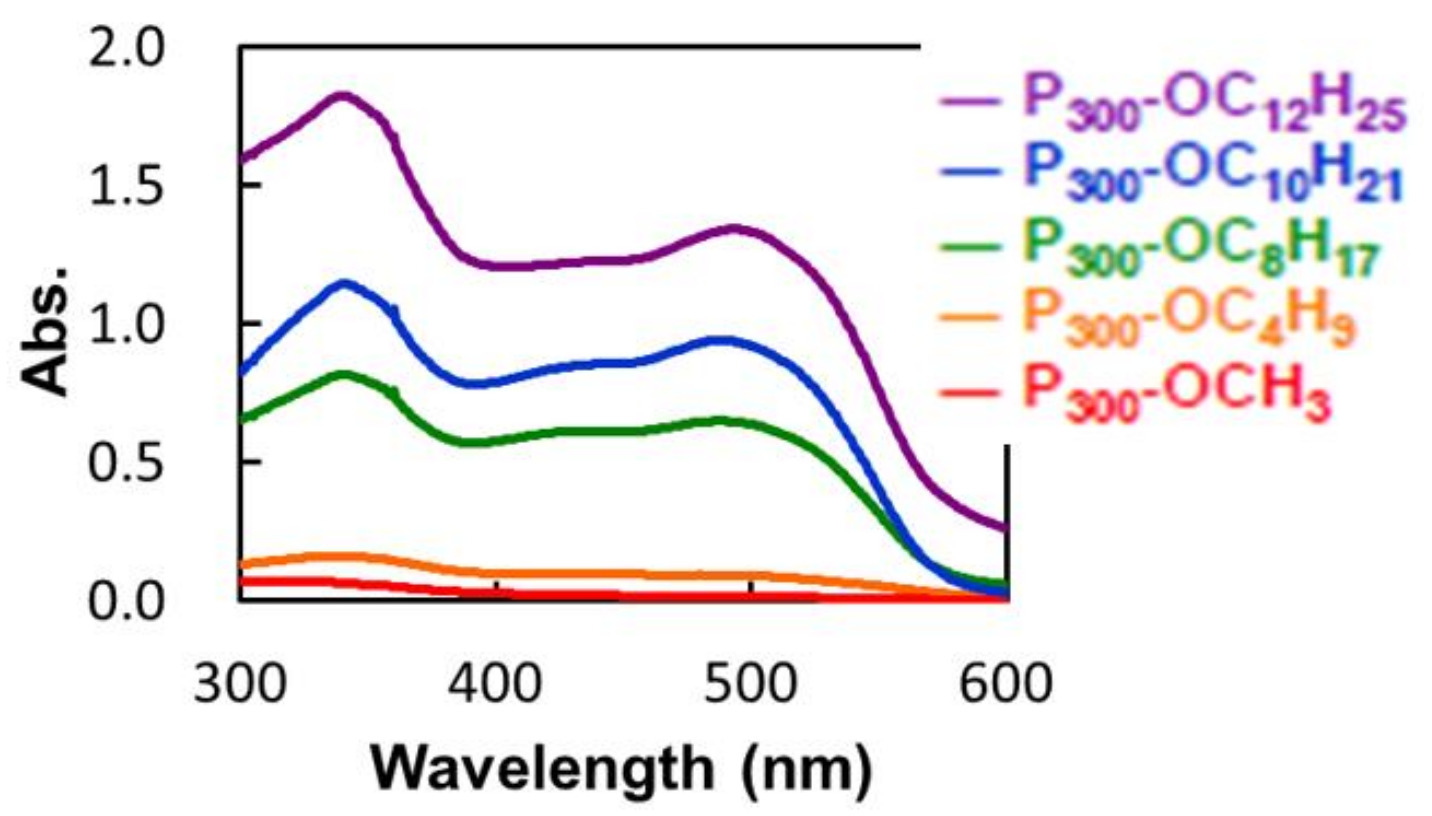
2.3.3. Solubilizing Test
3. Experimental Section
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of Poly(oxyethylene H-phosphonate)s
3.4. Synthesis of Poly(alkyloxyethylene phosphate)s
3.5. Measurement of Polymeric Micelle Size
3.6. Solubilizing Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schottler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohlr, K.; Landfester, K.; Mailander, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D. Mixed micelles in drug delivery. Nature 1992, 355, 279–280. [Google Scholar] [CrossRef]
- Xu, J.P.; Ji, J.; Chen, W.D.; Shen, J.C. Novel biomimetic polymersomes as polymer therapeutics for drug delivery. J. Control. Release 2005, 107, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Arimura, H.; Ohya, Y.; Ouchi, T. Formation of core-shell type biodegradable polymeric micelles from amphiphilic poly(aspartic acid)-block-polylactide diblock copolymer. Biomacromolecules 2005, 6, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zou, J.; Zhang, F.; Elsabahy, M.; Felder, S.; Zhu, J.; Pochan, D.J.; Wolley, K.L. Rapid and versatile construction of diverse and functional nanostructures derived from a polyphosphoester-based biomimetic block copolymer system. J. Am. Chem. Soc. 2012, 134, 18467–18474. [Google Scholar] [CrossRef]
- Soussan, E.; Cassel, S.; Blanzat, M.; Rico-Lattes, I. Drug delivery by soft matter: Matrix and vesicular carriers. Angew. Chem. Int. Ed. 2009, 48, 274–288. [Google Scholar] [CrossRef]
- Haag, R. Supramolecular drug-delivery systems based on polymeric core-shell architectures. Angew. Chem. Int. Ed. 2004, 43, 278–282. [Google Scholar] [CrossRef]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–897. [Google Scholar] [CrossRef]
- Savic, R.; Luo, L.; Eisenberg, A.; Maysinger, D. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science 2003, 300, 615–618. [Google Scholar] [CrossRef]
- Sun, C.Y.; Ma, Y.C.; Cao, Z.Y.; Li, D.D.; Fan, F.; Wang, J.X.; Tao, W.; Yang, X.Z. Effect of hydrophobicity of core on the anticancer efficiency of micelles as drug delivery carriers. ACS Appl. Mater. Interfaces 2014, 6, 22709–22718. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Akiyoshi, K. Design of biodegradable amphiphilic polymers: well-defined amphiphilic polyphosphates with hydrophilic graft chains via ATRP. Macromolecules 2004, 37, 7637–7642. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Akiyoshi, K. Synthesis and characterization of amphiphilic polyphosphates with hydrophilic graft chains and cholesteryl groups as nanocarriers. Biomacromolecules 2006, 7, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Wachiralarpphaithoon, C.; Akiyoshi, K. Novel thermoresponsive polymers having biodegradable phosphoester backbones. Macromolecules 2007, 40, 8136–8138. [Google Scholar] [CrossRef]
- Wang, C.; Tang, L.Y.; Li, Y.; Wang, J. Thermoresponsive block copolymers of poly(ethylene glycol) and polyphosphoester: Thermo-induced self-assembly, biocompatibility, and hydrolytic degradation. Biomacromolecules 2009, 10, 66–73. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Liu, X.Q.; Wang, Y.C.; Wang, J. Gold nanoparticles stabilized by thermosensitive diblock copolymers of poly(ethylene glycol) and polyphosphoester. Langmuir 2009, 25, 10298–10304. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Z.; Wang, Y.C.; Tang, L.Y.; Xia, H.; Wang, J. Synthesis and characterization of amphiphilic block copolymer of polyphosphoester and poly(L-lactic acid). J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6425–6434. [Google Scholar] [CrossRef]
- Yang, X.Z.; Sun, T.M.; Dou, S.; Wu, J.; Wang, Y.C.; Wang, J. Block copolymer of polyphosphoester and poly(L-Lactic Acid) modified surface for enhancing osteoblast adhesion, proliferation, and function. Biomacromolecules 2009, 10, 2213–2220. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Wang, Y.C.; Du, J.Z.; Wang, J. Synthesis of amphiphilic ABC 3-miktoarm star terpolymer by combination of ring-opening polymerization and “click” chemistry. Macromolecules 2008, 41, 8620–8625. [Google Scholar] [CrossRef]
- Cheng, J.; Ding, J.X.; Wang, Y.C.; Wang, J. Synthesis and characterization of star-shaped block copolymer of poly-(ɛ-caprolactone) and poly(ethyl ethylene phosphate) as drug carrier. Polymer 2008, 49, 4784–4790. [Google Scholar] [CrossRef]
- Song, W.J.; Du, J.Z.; Liu, N.J.; Dou, S.; Cheng, J.; Wang, J. Functionalized diblock copolymer of poly(ε-caprolactone) and polyphosphoester bearing hydroxyl pendant groups: Synthesis, characterization, and self-assembly. Macromolecules 2008, 41, 6935–6941. [Google Scholar] [CrossRef]
- Wang, Y.C.; Liu, X.Q.; Sun, T.M.; Xiong, M.H.; Wang, J. Functionalized micelles from block copolymer of polyphosphoester and poly(ɛ-caprolactone) for receptor-mediated drug delivery. J. Control. Release 2008, 128, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Tang, L.Y.; Sun, T.M.; Li, C.H.; Xiong, M.H.; Wang, J. Self-assembled micelles of biodegradable triblock copolymers based on poly(ethyl ethylene phosphate) and poly(ε-caprolactone) as drug carriers. Biomacromolecules 2008, 9, 388–395. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.C.; Yan, L.F.; Wang, J. Biodegradable vesicular nanocarriers based on poly(epsilon-caprolactone)-block-poly(ethyl ethylene phosphate) for drug delivery. Polymer 2009, 50, 5048–5054. [Google Scholar] [CrossRef]
- Tang, L.Y.; Wang, Y.C.; Li, Y.; Du, J.Z.; Wang, J. Shell-detachable micelles based on disulfide-linked block copolymer as potential carrier for intracellular drug delivery. Bioconj. Chem. 2009, 20, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Li, Y.; Yang, X.Z.; Yuan, Y.Y.; Yan, L.F.; Wang, J. Tunable thermosensitivity of biodegradable polymer micelles of poly(ε-caprolactone) and polyphosphoester block copolymers. Macromolecules 2009, 42, 3026–3032. [Google Scholar] [CrossRef]
- Zhang, P.C.; Hu, L.J.; Wang, Y.C.; Wang, J.; Feng, L.Y.; Li, Y.P. Poly(ε-caprolactone)-block-poly(ethyl Ethylene Phosphate) micelles for brain-targeting drug delivery: In vitro and in vivo valuation. Pharm. Res. 2010, 27, 2657–2669. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, M.; He, J.; Ni, P. Synthesis and characterization of amphiphilic poly(ɛ-caprolactone)-b-polyphosphoester diblock copolymers bearing multifunctional pendant groups. Polymer 2012, 53, 2854–2863. [Google Scholar] [CrossRef]
- Zhang, P.C.; Hu, L.J.; Yin, Q.; Zhang, Z.W.; Feng, L.; Li, Y. Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: Synthesis, preparation and in vivo evaluation. J. Control. Rel. 2012, 159, 429–434. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, J. Syntheses of amphiphilic biodegradable copolymers of poly(ethyl ethylene phosphate) and poly(3-hydroxybutyrate) for drug delivery. Sci. China B Chem. 2009, 52, 961–968. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, M.; He, J.; Ni, P. Synthesis and characterization of a new multifunctional polymeric prodrug paclitaxel–polyphosphoester–folic acid for targeted drug delivery. Polym. Chem. 2013, 4, 4515–4525. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, J.; Elsabahy, M.; Karwa, A.; Li, A.; Moore, D.A.; Dorshow, R.B.; Wooley, K.L. Poly(ethylene oxide)-block-polyphosphester-based paclitaxel conjugates as a platform for ultra-high paclitaxel-loaded multifunctional nanoparticles. Chem. Sci. 2013, 4, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, F.; Zhang, S.; Pollack, S.F.; Elsabany, M.; Fan, J.; Wooley, K.L. Poly(ethylene oxide)-block-polyphosphoester-graft-paclitaxel conjugates with acid-labile linkages as a pH-sensitive and functional nanoscopic platform for paclitaxel delivery. Adv. Healthc. Mater. 2014, 3, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Dou, S.; Du, J.Z.; Yang, X.Z.; Li, Y.P.; Wang, J. Doxorubicin conjugate of poly(ethylene glycol)-block-polyphosphoester for cancer therapy. Adv. Healthc. Mater. 2014, 3, 261–272. [Google Scholar] [CrossRef]
- Liu, X.; Ni, P.H.; He, J.L.; Zhang, M.Z. Synthesis and micellization of pH/temperature-responsive double-hydrophilic diblock copolymers polyphosphoester-block-poly[2-(dimethylamino)ethyl methacrylate] prepared via ROP and ATRP. Macromolecules 2010, 43, 4771–4781. [Google Scholar] [CrossRef]
- Georgieva, R.; Tsevi, R.; Kossev, K.; Kusheva, R.; Balgjiska, M.; Petrova, R.; Tenchova, V.; Gitsov, I.; Troev, K. Immobilization of aminothiols on poly(oxyalkylene phosphates). Formation of poly(oxyethylene phosphates)/cysteamine complexes and their radioprotective efficiency. J. Med. Chem. 2002, 45, 5797–5801. [Google Scholar] [CrossRef]
- Troev, K.; Mitova, V.; Ivanov, I. On the design of polymeric 5′-O-ester prodrugs of 3′-azido-2′,3′-dideoxythymidine (AZT). Tetrah. Lett. 2010, 51, 6123–6125. [Google Scholar] [CrossRef]
- Bogomilova, A.; Hohn, M.; Gunther, M.; Troev, K.; Wagner, E.; Schreiner, L. A polyphosphoester conjugate of melphalan as antitumoral agent. Eur. J. Pharm. Sci. 2013, 50, 410–419. [Google Scholar] [CrossRef]
- Koseva, N.; Tsacheva, I.; Mitova, V.; Vodenicharova, E.; Jessica, M.; Mason, K.; Troev, K. Polymer complex of WR 2721. Synthesis and radioprotective efficiency. Eur. J. Pharm. Sci. 2014, 65, 9–14. [Google Scholar] [CrossRef]
- Troev, K.; Tsacheva, I.; Koseva, N.; Georgieva, R.; Gitsov, I. Immobilization of aminothiols on poly(oxyethylene H-phosphonate)s and poly(oxyethylene phosphate)s—An approach to polymeric protective agents for radiotherapy of cancer. J. Polym. Sci. A Polym. Chem. 2007, 45, 1349–1363. [Google Scholar] [CrossRef]
- Troev, K.D. Polyphosphoesters: Chemistry and Application; Elsevier: New York, NY, USA; Oxford, UK; Amsterdam, The Netherlands; Tokyo, Japan, 2012. [Google Scholar]
- Troev, K.; Naruoka, A.; Terada, H.; Kikuchi, A.; Makino, K. New efficient method of oxidation of poly(alkylene H-phosphonate)s: A promising route to novel co-polyphosphoesters. Macromolecules 2012, 45, 5698–5703. [Google Scholar] [CrossRef]
- Pretula, J.; Penczek, S. Poly(ethy1ene glycol) ionomers with phosphate diester linkages. Makromol. Chem. Rapid Commun. 1988, 9, 731–737. [Google Scholar] [CrossRef]
- Pretula, J.; Kaluzynski, K.; Szymanski, R.; Penczek, S. Preparation of Poly(alkylene H-phosphonate)s and Their Derivatives by Polycondensation of Diphenyl H-Phosphonate with Diols and Subsequent Transformations. Macromolecules 1997, 30, 8172–8176. [Google Scholar] [CrossRef]
- Pretula, J.; Penczek, S. High-molecular-weight poly(alky1ene phosphonate)s by condensation of dialkylphosphonates with diols. Makromol. Chem. 1990, 191, 671–680. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J. High-molecular-weight poly(alkylene phosphates) and preparation of amphiphilic polymers thereof. Macromolecules 1993, 26, 2228–2233. [Google Scholar] [CrossRef]

| Entry | Yield (%) | Mn (×103) 1 | Mw (×103) 1 | Mw/Mn (PDI) 1 | DP |
|---|---|---|---|---|---|
| P200-H | 84.3 | 3.6 | 6.6 | 1.83 | 14.8 |
| P300-H | 86.8 | 6.4 | 8.5 | 1.32 | 18.6 |
| P400-H | 84.2 | 11.5 | 15.5 | 1.34 | 25.8 |
| P600-H | 80.1 | 12.4 | 17.4 | 1.40 | 19.2 |
| Pa-b | Reagent | Reaction Time (h) |
|---|---|---|
| P400-OCH3 | methanol | 3 |
| P400-OC4H9 | 1-butanol | 5 |
| P400-OC8H17 | 1-octanol | 15 |
| P400-OC10H21 | 1-decanol | 20 |
| P400-OC12H25 | 1-dodecanol | 24 |
| Entry Pa-b | Degree of Alkylation 1 (%) | Micelle Diameter 2 (nm) |
|---|---|---|
| P200-OC12H25 | 95 | 102.7 ± 25.0 |
| P300-OC12H25 | 93 | 93.6 ± 17.5 |
| P400-OC12H25 | 93 | 93.8 ± 18.2 |
| P600-OC12H25 | 90 | 2.5 ± 0.4 |
| Entry Pa-b | Degree of Alkylation 1 (%) | Micelle Diameter 2 (nm) |
|---|---|---|
| P300-OC8H17 | 92 | 145.3 ± 34.0 |
| P300-OC10H21 | 92 | 123.7 ± 28.4 |
| P300-OC12H25 | 93 | 93.6 ± 17.5 |
| Entry Pa-b | Diameter 1 (nm) | ||||
|---|---|---|---|---|---|
| 1.0 wt% | 0.5 wt% | 0.25 wt% | 0.1 wt% | 0.01 wt% | |
| P400-OC8H17 | 340.1 ± 81.8 | 183.2 ± 44.2 | 158.0 ± 41.9 | 165.7 ± 42.6 | 106.0 ± 23.9 |
| P400-OC10H21 | 173.2 ± 30.9 | 170.2 ± 29.8 | 165.6 ± 23.3 | 165.2 ± 23.0 | 170.4 ± 29.1 |
| P400-OC12H25 | 101.9 ± 26.3 | 93.8 ± 18.2 | 92.2 ± 16.4 | 80.3 ± 14.7 | 94.6 ± 18.3 |
| Entry Pa-b | Diameter 1 (nm) | |||
|---|---|---|---|---|
| 25 °C | 30 °C | 35 °C | 40 °C | |
| P200-OC12H25 | 118.3 ± 23.9 | 104.7 ± 23.1 | 100.7 ± 21.6 | 95.0 ± 18.5 |
| P300-OC12H25 | 120.0 ± 30.4 | 124.4 ± 29.1 | 123.5 ± 28.5 | 122.8 ± 26.8 |
| P400-OC12H25 | 1.3 ± 0.1 | 116.4 ± 22.0 | 73.6 ± 16.7 | 64.9 ± 13.7 |
| P600-OC12H25 | 2.5 ± 0.4 | 1.4 ± 0.2 | 29.1 ± 7.5 | 18.8 ± 5.4 |
| Entry Pa-b | Abs. (500 nm) | Solubilizing Capacity (%) |
|---|---|---|
| P300-OC12H25 | 1.332 | 0.21 |
| P300-OC10H21 | 0.920 | 0.18 |
| P300-OC8H17 | 0.637 | 0.11 |
| P300-OC4H9 | 0.091 | - |
| P300-OCH3 | 0.015 | - |
| Only water | 0.004 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamakita, Y.; Takeuchi, I.; Makino, K.; Terada, H.; Kikuchi, A.; Troev, K. Thermoresponsive Polyphosphoester via Polycondensation Reactions: Synthesis, Characterization, and Self-Assembly. Molecules 2022, 27, 6006. https://doi.org/10.3390/molecules27186006
Yamakita Y, Takeuchi I, Makino K, Terada H, Kikuchi A, Troev K. Thermoresponsive Polyphosphoester via Polycondensation Reactions: Synthesis, Characterization, and Self-Assembly. Molecules. 2022; 27(18):6006. https://doi.org/10.3390/molecules27186006
Chicago/Turabian StyleYamakita, Yoshihiro, Issei Takeuchi, Kimiko Makino, Hiroshi Terada, Akihiko Kikuchi, and Kolio Troev. 2022. "Thermoresponsive Polyphosphoester via Polycondensation Reactions: Synthesis, Characterization, and Self-Assembly" Molecules 27, no. 18: 6006. https://doi.org/10.3390/molecules27186006
APA StyleYamakita, Y., Takeuchi, I., Makino, K., Terada, H., Kikuchi, A., & Troev, K. (2022). Thermoresponsive Polyphosphoester via Polycondensation Reactions: Synthesis, Characterization, and Self-Assembly. Molecules, 27(18), 6006. https://doi.org/10.3390/molecules27186006








