Isolation, Identification and Characterization of Growth Parameters of Pseudomonas putida HSM-C2 with Coumarin-Degrading Bacteria
Abstract
:1. Introduction
2. Experimental
2.1. Sample Collection, Bacterial Isolation, and Selection
2.2. Evaluation of Growth Parameters and Coumarin Degradation Potential of HSM-C2 Strain
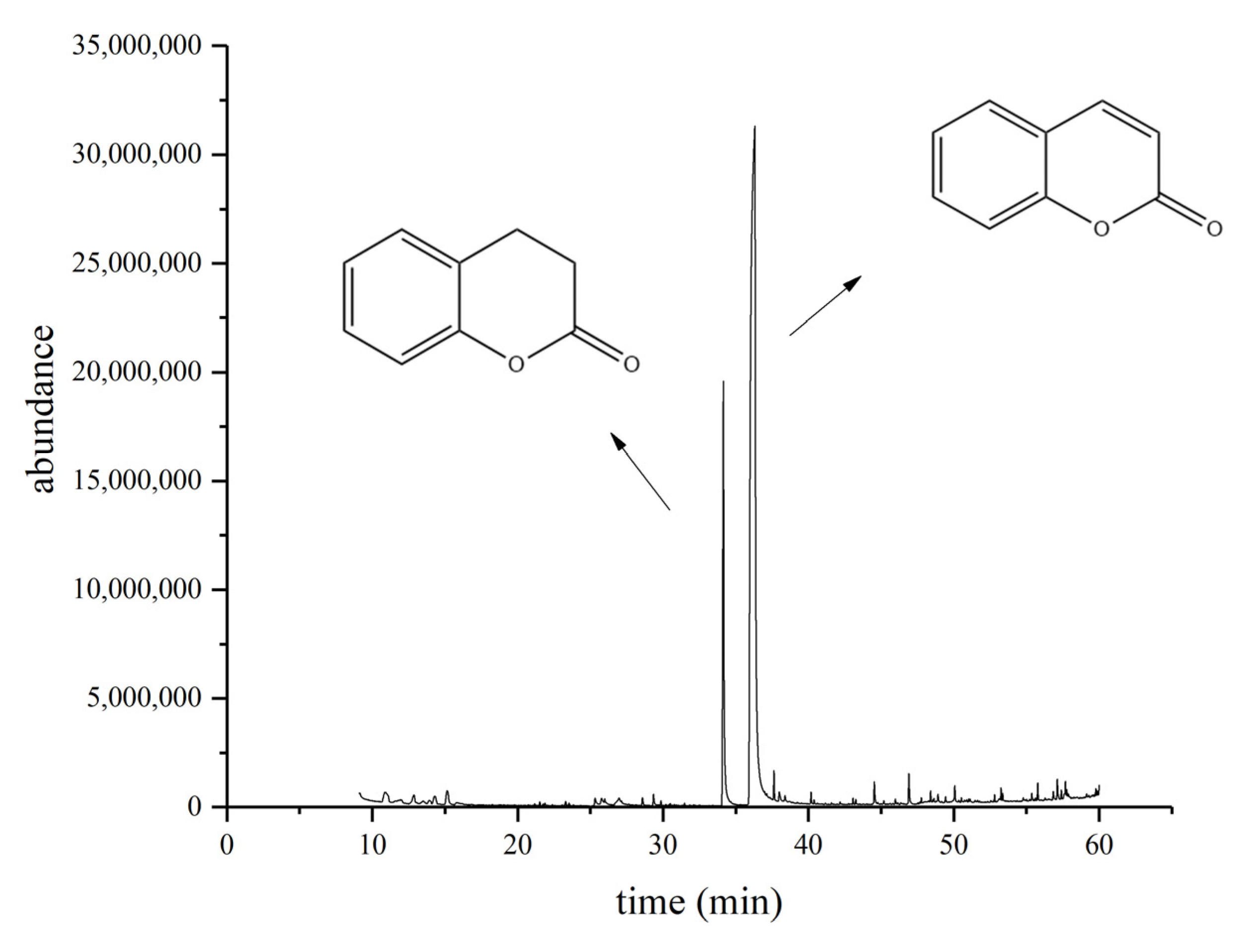
2.3. Identification of Biodegradation Products of Coumarin
2.4. Identification of HSM-C2 Strain
2.5. Whole Genome Sequencing (WGS) of Strain HSM-C2
3. Results
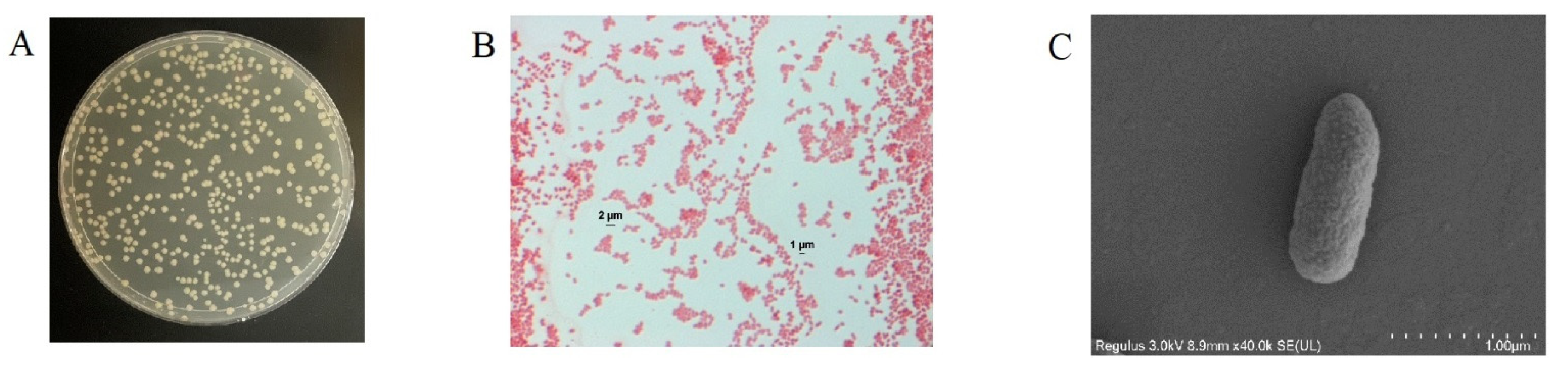
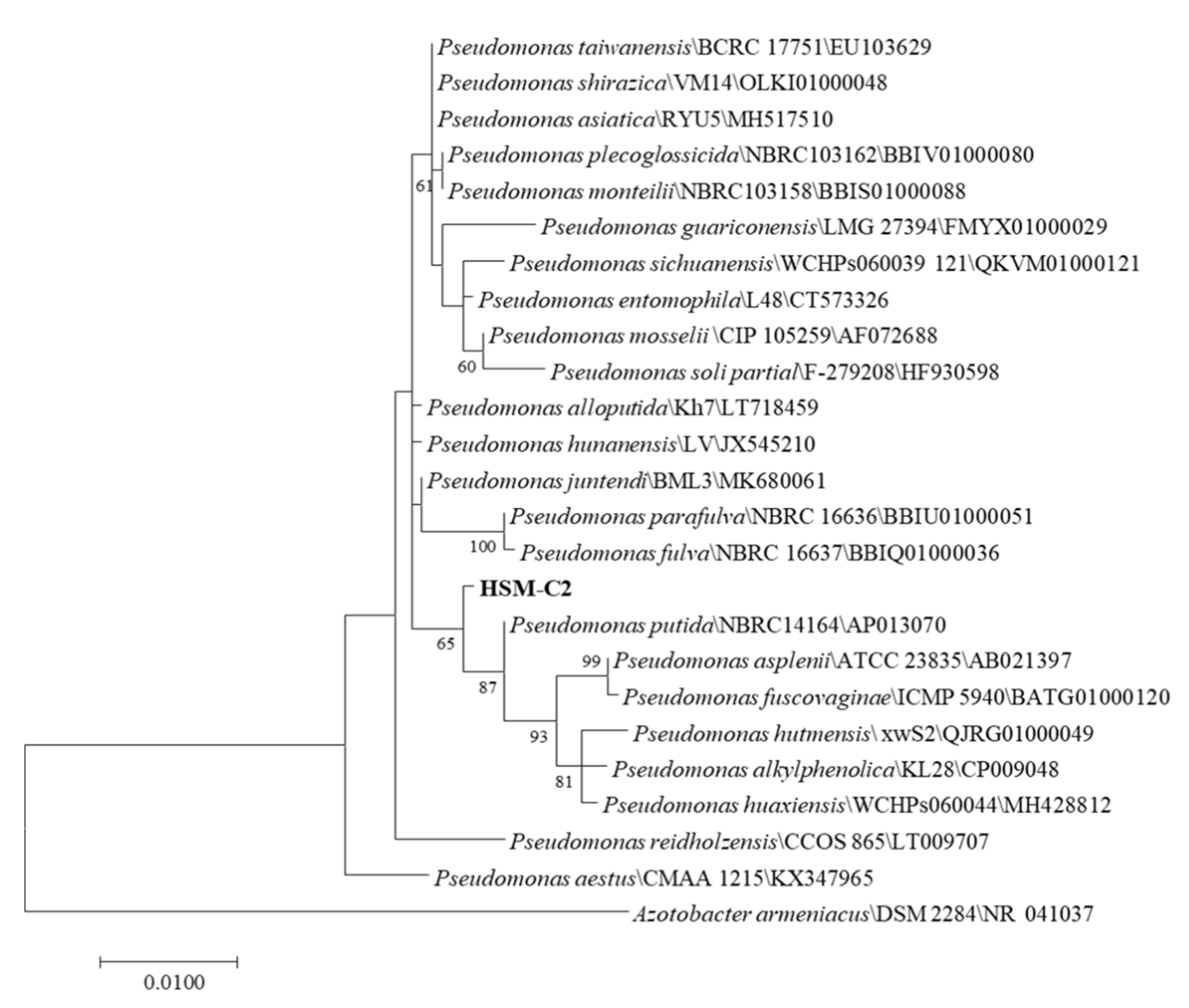
3.1. Isolation and Identification of Coumarin-Biodegrading Bacterium
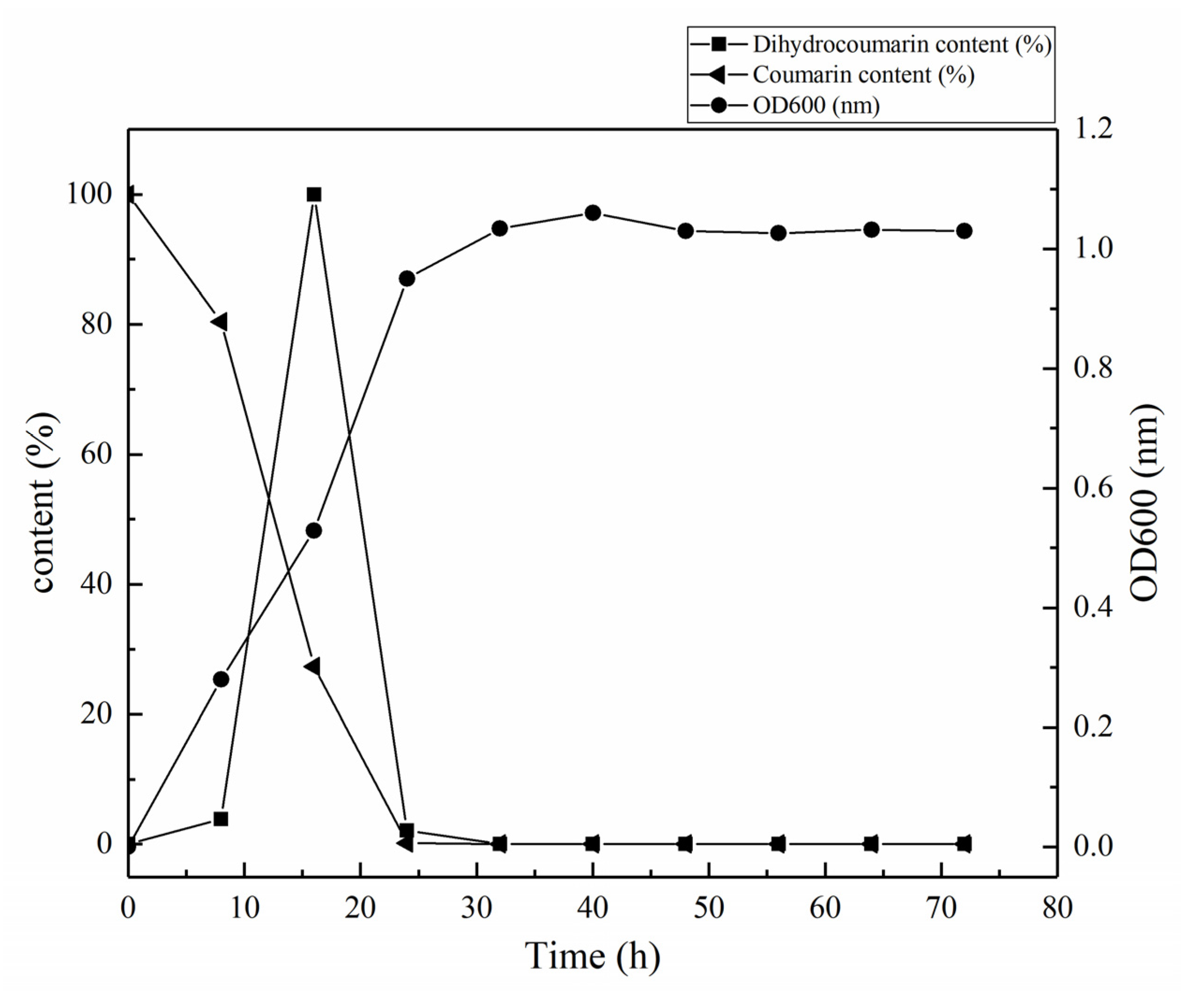
3.2. Determination of Growth Curve and Degradation Curve of HSM-C2 Strain
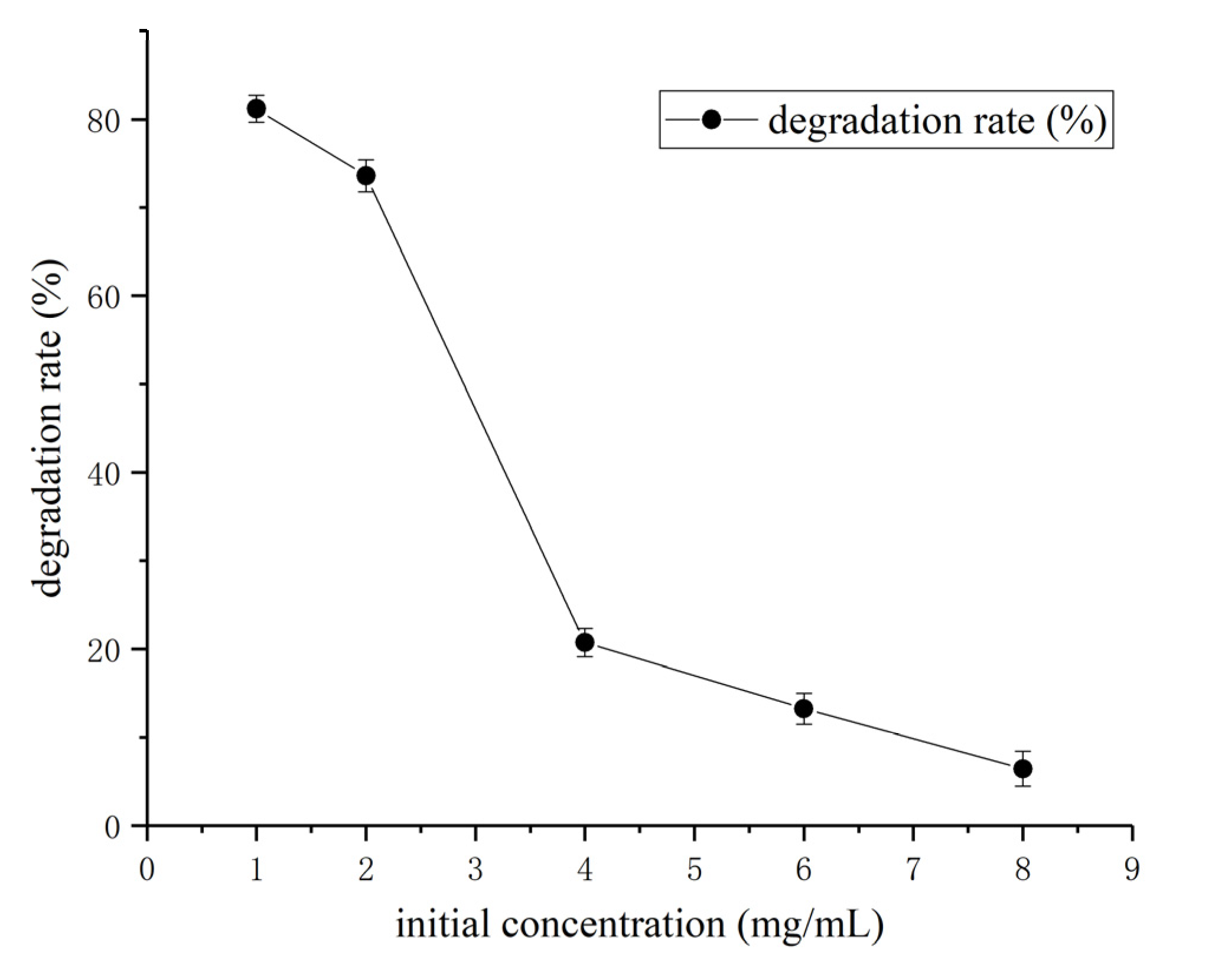
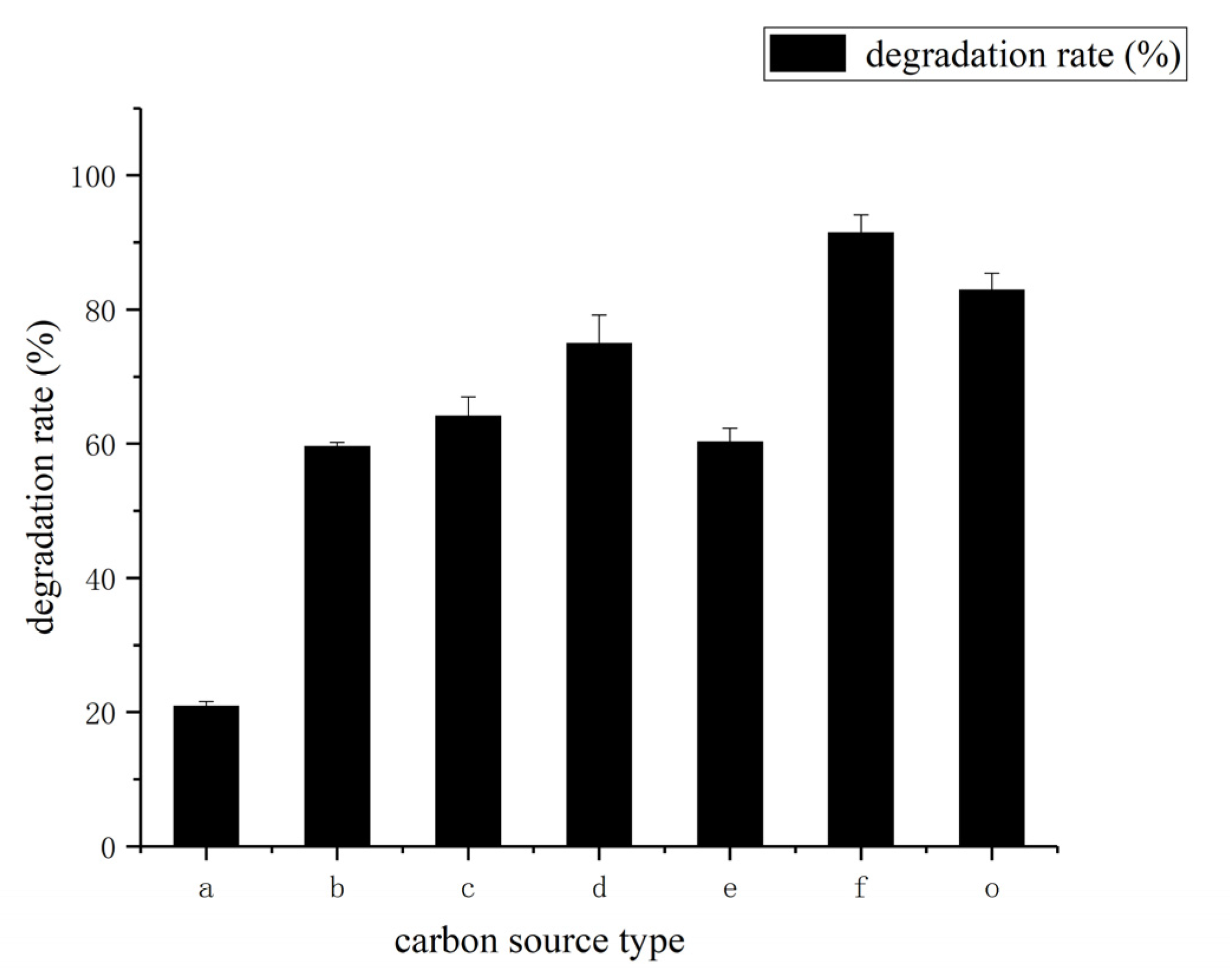
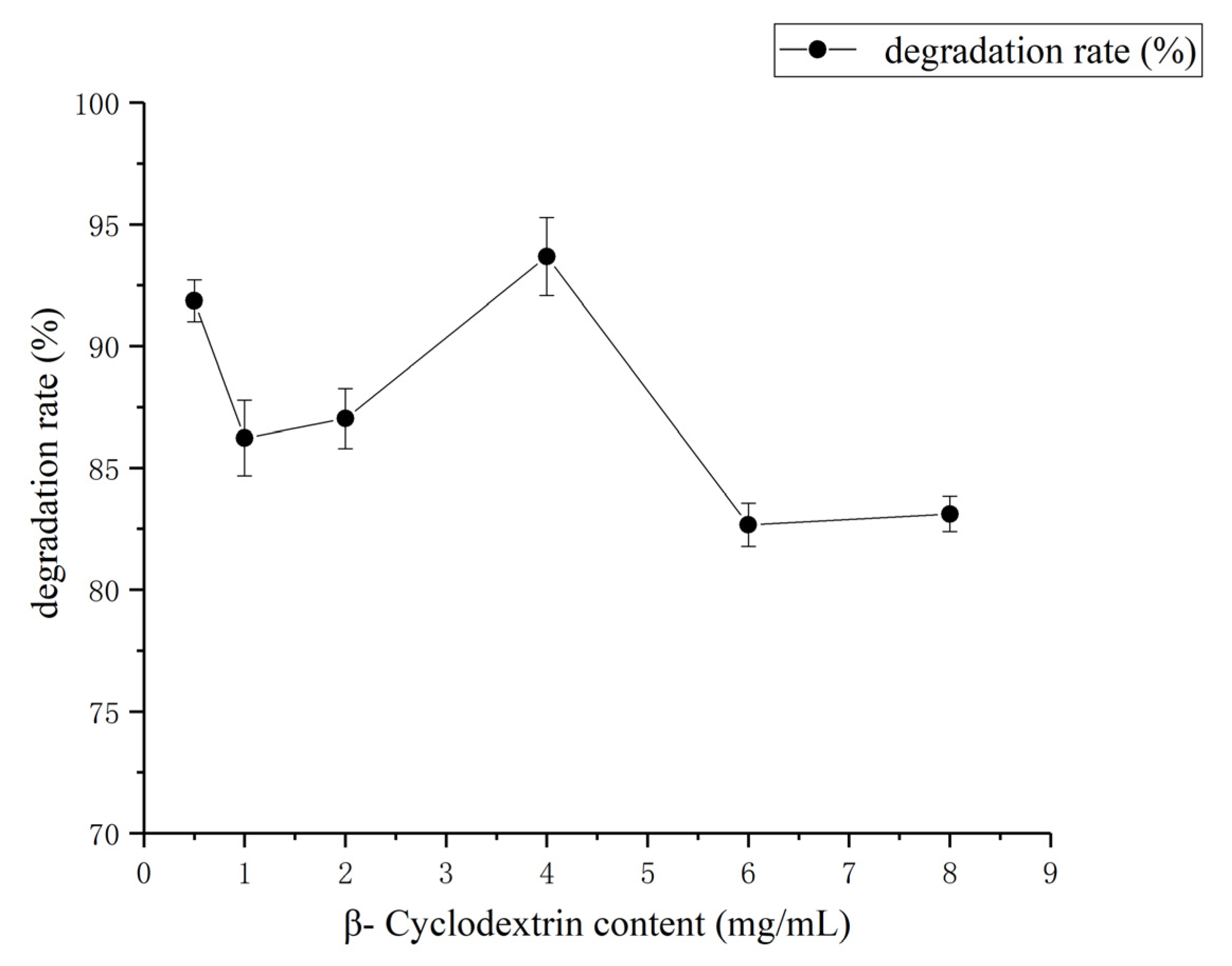
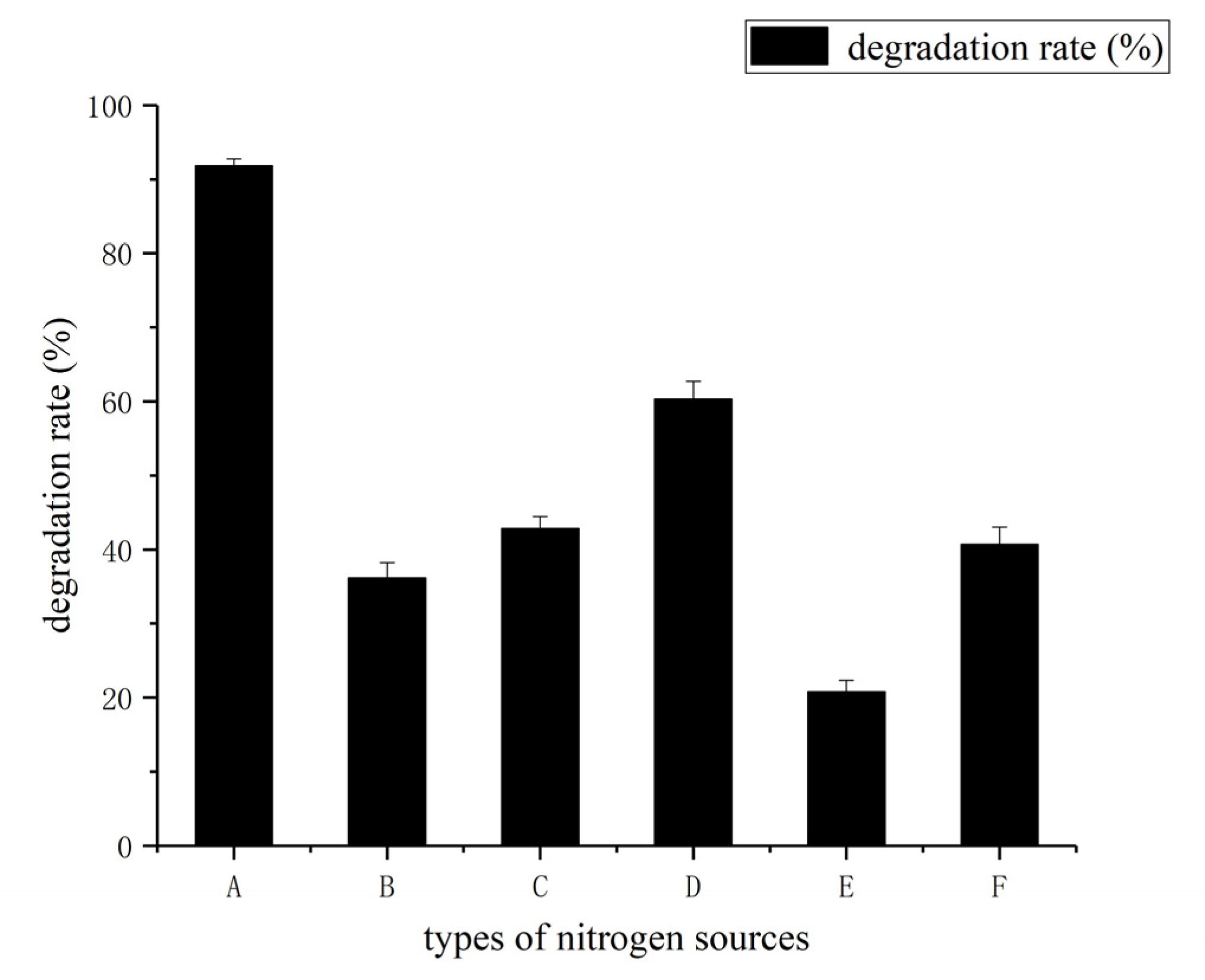
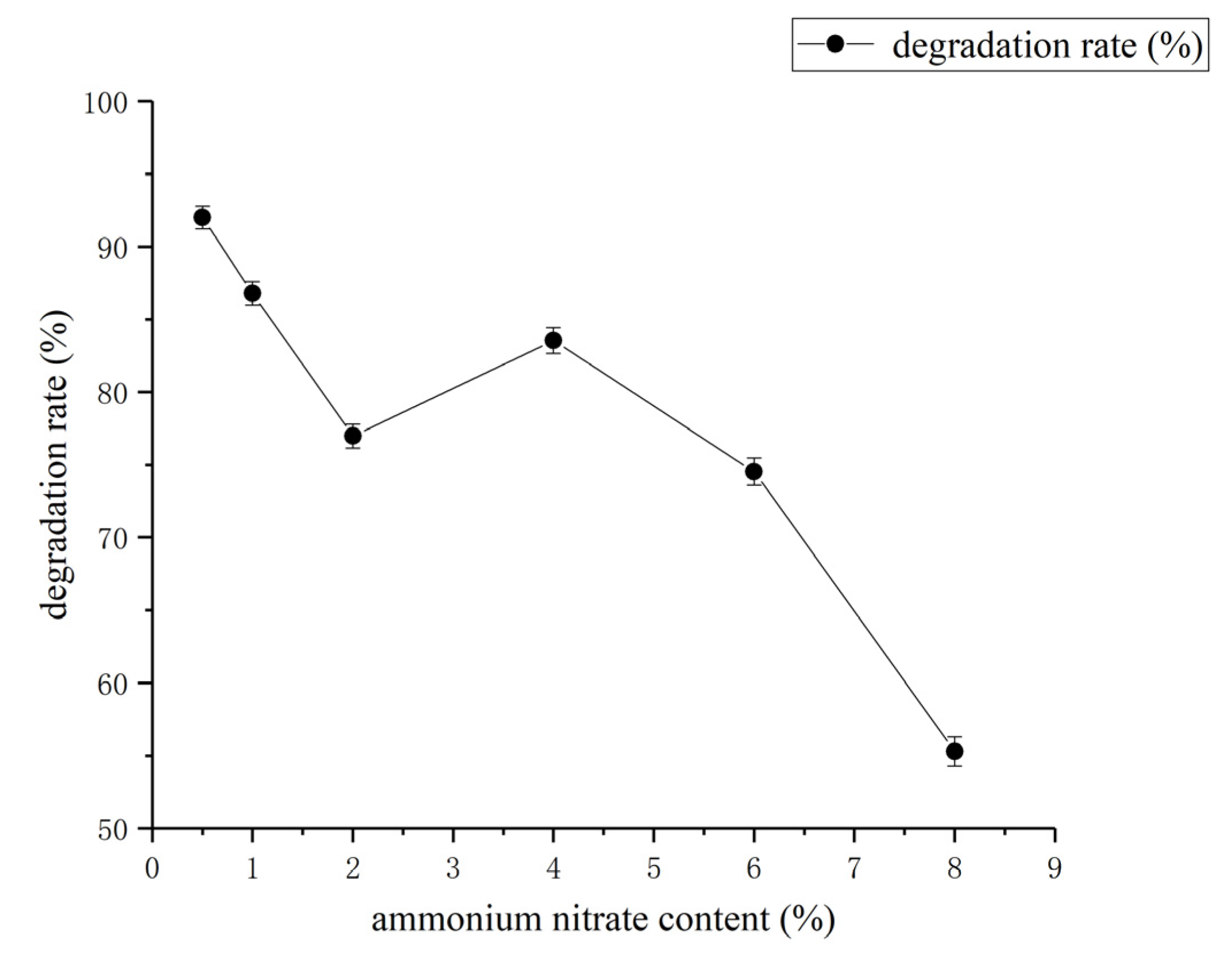
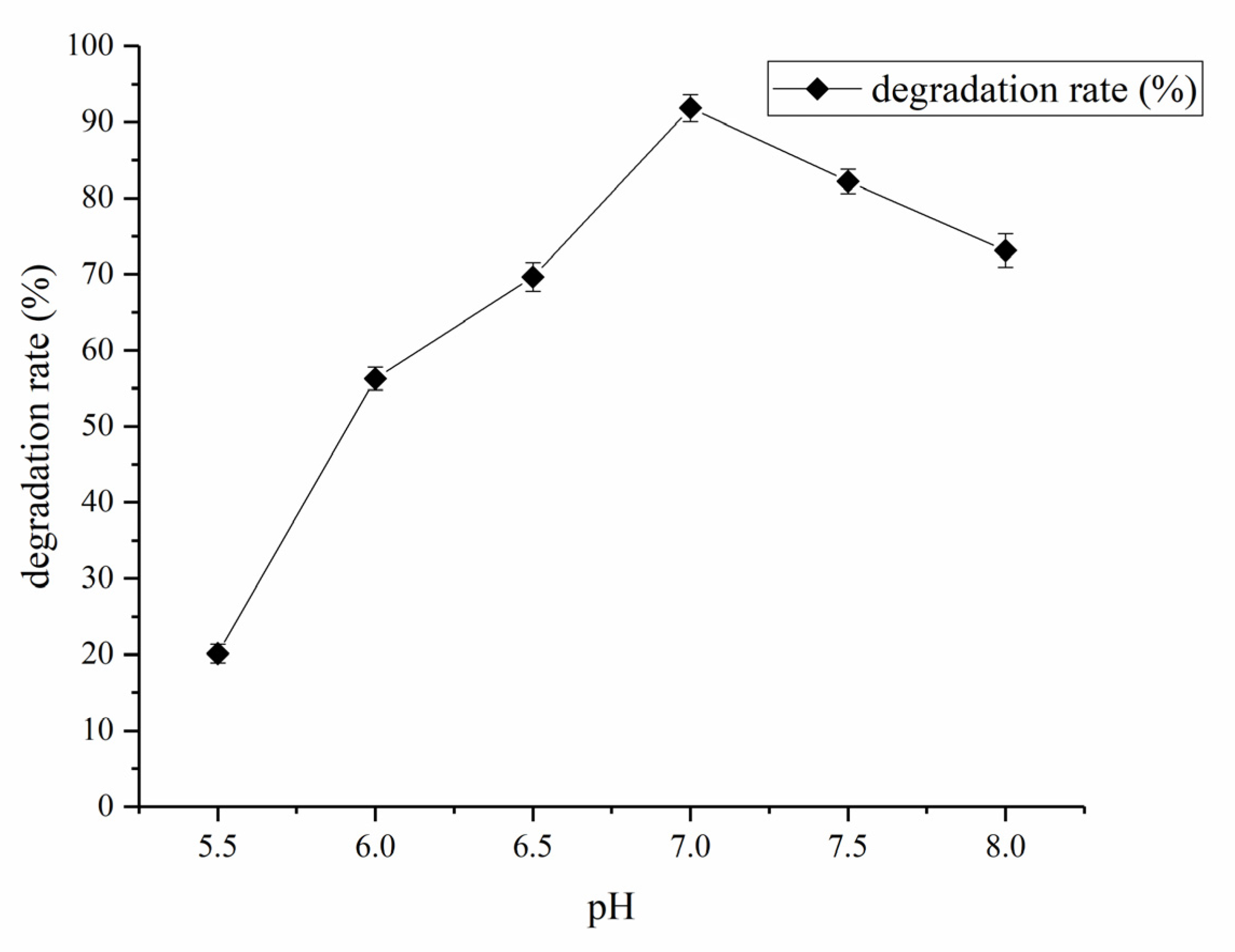
3.3. Determination of Effect of Environment Factors Biodegradation Process
3.4. Sequencing and Analysis of the Whole Genome of Pseudomonas putida HSM-C2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stringlis, I.A.; Ronnie, D.J.; Pieterse, C. The Age of Coumarins in Plant–Microbe Interactions. Plant Cell Physiol. 2019, 60, 7. [Google Scholar] [CrossRef]
- Andrejak, M.; Gersberg, M.; Sgro, C.; Decocq, G.; Hamel, J.; Dominique, M.; Morin, M.; Gras, V.; Eacute, R. French pharmacovigilance survey evaluating the hepatic toxicity of coumarin. Pharmacoepidemiol. Drug Saf. 2015, 7 (Suppl. S1), S45–S50. [Google Scholar] [CrossRef]
- Smith, R.L.; Ford, R.A. Recent Progress in the Consideration in of Flavoring Ingredients Under the Food Additives Amendment III. Gras Substances. Food Technol. 1965, 19, 151–197. [Google Scholar]
- Montanaro, D.; Lavecchia, R.; Petrucci, E.; Zuorro, A. UV-assisted electrochemical degradation of coumarin on boron-doped diamond electrodes. Chem. Eng. J. 2017, 323, 512–519. [Google Scholar] [CrossRef]
- Tan, C.; Xu, Q.; Zhang, H.; Liu, Z.; Ren, S.; Li, H. Enhanced removal of coumarin by a novel O_3/SPC system: Kinetic and mechanism. Chemosphere 2019, 219, 100–108. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, C.; Xu, Q.; Liu, Y.; Yan, H. Pathway for biodegrading coumarin by a newly isolated Pseudomonas sp. USTB-Z. World J. Microbiol. Biotechnol. 2021, 37, 89. [Google Scholar] [CrossRef]
- Levy, C.C.; Frost, P. The Metabolism of Coumarin by a Microorganism. J. Biol. Chem. 1966, 241, 997–1003. [Google Scholar] [CrossRef]
- Nakayama, Y.; Nonomura, S.; Tatsumi, C. The metabolism of coumarin by a strain of pseudomonas. Agric. Biol. Chem. 1973, 37, 1423–1437. [Google Scholar] [CrossRef]
- Wenk, H.H.; Schwab, W. Biocatalytic production of dihydrocoumarin from coumarin by Saccharomyces cerevisiae. J. Agric. Food Chem. 2006, 54, 6236–6240. [Google Scholar]
- Aguirre-Pranzoni, C.; Orden, A.A.; Bisogno, F.R.; Ardanaz, C.E.; Tonn, C.E.; Kurina-Sanz, M. Coumarin metabolic routes in Aspergillus spp. Fungal Biol. 2011, 115, 245–252. [Google Scholar] [CrossRef]
- Marumoto, S.; Miyazawa, M. Microbial reduction of coumarin, psoralen, and xanthyletin by Glomerella cingulata. Tetrahedron 2011, 67, 495–500. [Google Scholar] [CrossRef]
- Aboelsauod, K. Biotransformation of coumarins by Cunninghamella elegans. Afr. J. Pharm. Pharmacol. 2016, 10, 411–418. [Google Scholar]
- Komagata, K.; Suzuki, K.I. 4 Lipid and Cell-Wall Analysis in Bacterial Systematics. Methods Microbiol. 1988, 19, 161–207. [Google Scholar]
- Minnikin, D.E.; O"Donnell, A.G.; Goodfellow, M.; Alderson, G.; Athalye, M.; Schaal, A.; Parlett, J.H. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J. Microbiol. Methods 1984, 2, 233–241. [Google Scholar] [CrossRef]
- Collins, M.D. Isoprenoid quinone analysis in classification and identification. In Chemical Methods in Bacterial Systematics; Academic Press: Cambridge, MA, USA, 1985; pp. 267–287. [Google Scholar]
- Wu, C.; Lu, X.; Qin, M.; Wang, Y.; Ruan, J. Analysis of menaquinone compound in microbial cells by HPLC. Microbiology 1989, 16, 176–178. [Google Scholar]
- Sasser, M. MIDI Technical Note 101. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids; MIDI: Newark, NJ, USA, 1990. [Google Scholar]
- Kämpfer, P.; Kroppenstedt, R.M. Numerical analysis of fatty acid patterns of coryneform bacteria and related taxa. Can. J. Microbiol. 1996, 42, 989–1005. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, S.; Khajuria, A.; Ohri, P.; Kaur, R. Biocontrol potential of chitinases produced by newly isolated Chitinophaga sp. S167. World J. Microbiol. Biotechnol. 2020, 36, 90. [Google Scholar] [CrossRef]
- Saitou, N. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731. [Google Scholar] [CrossRef]
- Patel, R.K.; Mukesh, J.; Liu, Z. NGS QC Toolkit: A Toolkit for Quality Control of Next Generation Sequencing Data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Schubert, M.; Lindgreen, S.; Orlando, L. AdapterRemoval v2: Rapid adapter trimming, identification, and read merging. BMC Res. Notes 2016, 9, 88. [Google Scholar] [CrossRef]
- Chin, C.S.; Peluso, P.; Sedlazeck, F.J.; Nattestad, M.; Concepcion, G.T.; Clum, A.; Dunn, C.; O’Malley, R.; Figueroa-Balderas, R.; Morales-Cruz, A. Phased diploid genome assembly with single-molecule real-time sequencing. Nat. Methods 2016, 13, 1050–1054. [Google Scholar] [CrossRef]
- Koren, S.; Brian, P.; Walenz, K.; Berlin, J.; Jason, R.; Miller, N.H.; Bergman, C. Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef]
- John, B.; Alexandre, L.; Mark, B. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar]
- Buchfink, B.; Chao, H.; Daniel, H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Mulet, M.; Sánchez, D.; Lalucat, J.; Lee, K.; Garcia-Valdes, E. Pseudomonas alkylphenolica sp. nov. a bacterial species able to form special aerial structures when grown on p-cresol. Int. J. Syst. Evol. Microbiol. 2015, 65, 4013–4018. [Google Scholar] [CrossRef]
- Mulet, M.; Gomila, M.; Scotta, C.; Sánchez, D.; Lalucat, J.; García-Valdés, E. Concordance between whole-cell matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry and multilocus sequence analysis approaches in species discrimination within the genus Pseudomonas. Syst. Appl. Microbiol. 2012, 35, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Krikštaponis, A.; Meškys, R. Biodegradation of 7-Hydroxycoumarin in Pseudomonas mandelii 7HK4 via ipso-Hydroxylation of 3-(2,4-Dihydroxyphenyl)-propionic Acid. Molecules 2018, 23, 2613. [Google Scholar] [CrossRef]
- Chen, Q.; Hui, T.; Xue, L.; Zhang, B.; Cui, Z. The Regulation of para-Nitrophenol Degradation in Pseudomonas putida DLL-E4. PLoS ONE 2016, 11, e0155485. [Google Scholar] [CrossRef]
- Cabral, L.; Yu, R.-Q.; Crane, S.; Giovanella, P.; Barkay, T. Methylmercury degradation by Pseudomonas putida V1. Ecotoxicol. Environ. Saf. 2016, 130, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kurzbaum, E.; Raizner, Y.; Cohen, O.; Suckeveriene, R.Y.; Kulikov, A.; Hakimi, B.; Kruh, L.I.; Armon, R.; Farber, Y.; Menashe, O. Encapsulated Pseudomonas putida for phenol biodegradation: Use of a structural membrane for construction of a well-organized confined particle. Water Res. 2017, 121, 37–45. [Google Scholar] [CrossRef]
- Yang, L.; Cai, T.; Ding, D.; Cai, T.; Jiang, C.; Li, H.; Yang, Q.; Chen, L. Biodegradation of 2-hydroxyl-1,4 naphthoquinone (lawsone) by Pseudomonas taiwanensis LH-3 isolated from activated sludge. Sci. Rep. 2017, 7, 6795. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, H. The Metabolism of Coumarin by a Pseudomonas Sp.; McGill University: Montreal, QC, Canada, 1962. [Google Scholar]
- Kosuge, T.; Conn, E.E. The metabolism of aromatic compounds in higher plants. V. Purification and properties of dihydrocoumarin hydrolase of Melilotus alba. J. Biol. Chem. 1962, 237, 1653–1656. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Wang, M.; Mao, D.; Rasool, A.; Jia, C.; Yang, P.; Han, L.; Yan, M. Isolation, Identification and Characterization of Growth Parameters of Pseudomonas putida HSM-C2 with Coumarin-Degrading Bacteria. Molecules 2022, 27, 6007. https://doi.org/10.3390/molecules27186007
Huang S, Wang M, Mao D, Rasool A, Jia C, Yang P, Han L, Yan M. Isolation, Identification and Characterization of Growth Parameters of Pseudomonas putida HSM-C2 with Coumarin-Degrading Bacteria. Molecules. 2022; 27(18):6007. https://doi.org/10.3390/molecules27186007
Chicago/Turabian StyleHuang, Shen, Menghuan Wang, Duobin Mao, Aamir Rasool, Chunxiao Jia, Pengfei Yang, Li Han, and Meiling Yan. 2022. "Isolation, Identification and Characterization of Growth Parameters of Pseudomonas putida HSM-C2 with Coumarin-Degrading Bacteria" Molecules 27, no. 18: 6007. https://doi.org/10.3390/molecules27186007
APA StyleHuang, S., Wang, M., Mao, D., Rasool, A., Jia, C., Yang, P., Han, L., & Yan, M. (2022). Isolation, Identification and Characterization of Growth Parameters of Pseudomonas putida HSM-C2 with Coumarin-Degrading Bacteria. Molecules, 27(18), 6007. https://doi.org/10.3390/molecules27186007





