In Vitro Study of the Phytochemical Composition and Antioxidant, Immunostimulant, and Hemolytic Activities of Nigella sativa (Ranunculaceae) and Lepidium sativum Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Preparation
2.2. Chemical Reagents
2.3. Determination of Total Phenols
2.4. Determination of Condensed Tannins
2.5. Determination of Flavonoids
2.6. Determination of Total Sugars
2.7. Quantification of Mucilage
2.8. Extraction of Fixed Oils
2.9. Biological Activities
2.9.1. Antioxidant Activity: DPPH Test
2.9.2. Phyto-Hemagglutination Activity
Preparation of the Total Extract
Red Blood Cell Preparation
Hemagglutination Test
The Hemagglutination Limit
2.9.3. Hemolytic Activity
Rate of Hemolysis
2.10. Statistical Analysis
3. Results
3.1. Results of Humidity, Sugar Content, and Mucilage
3.2. Phytochemical Quantification of N. sativa and L. sativum Seeds
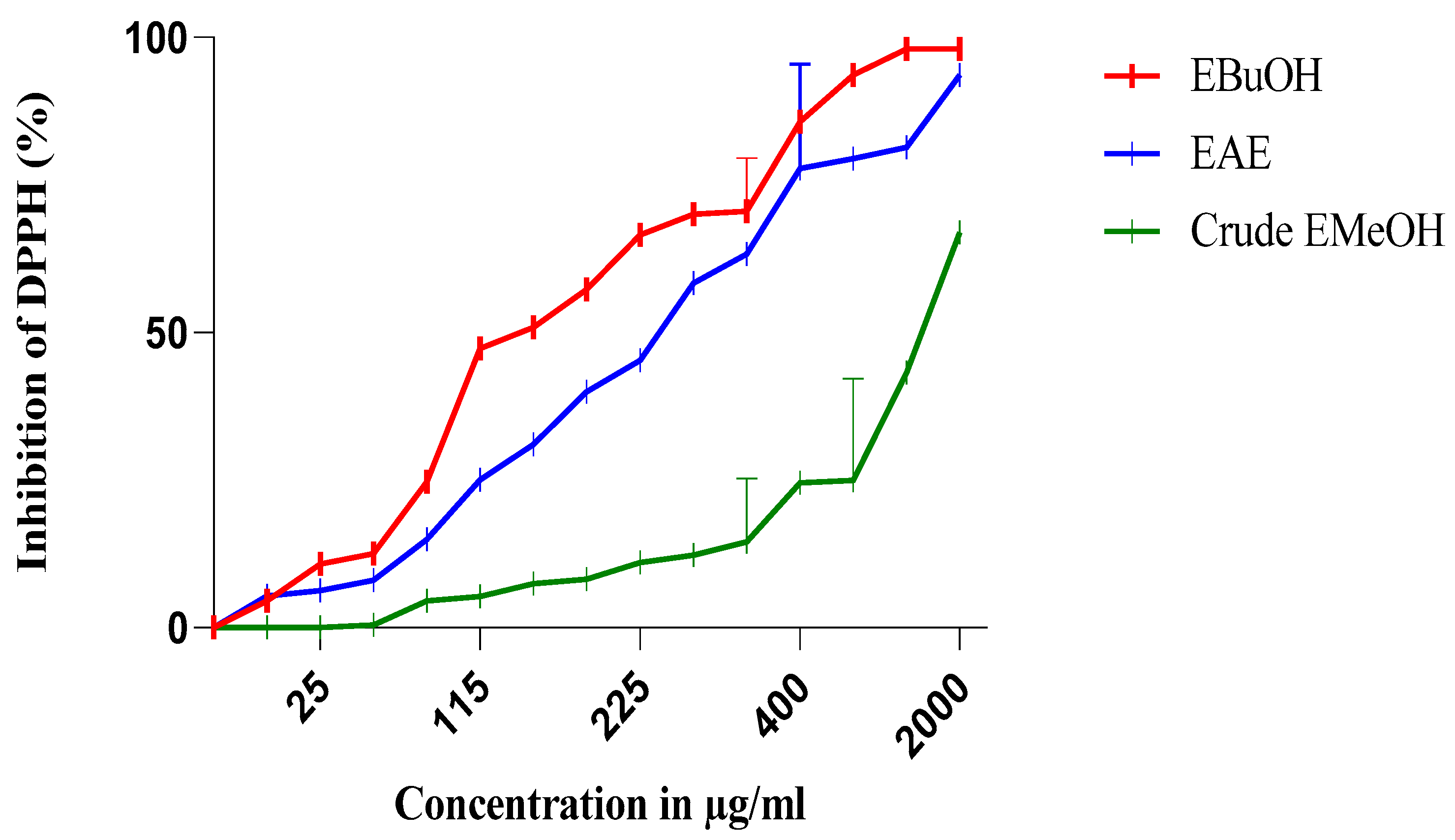
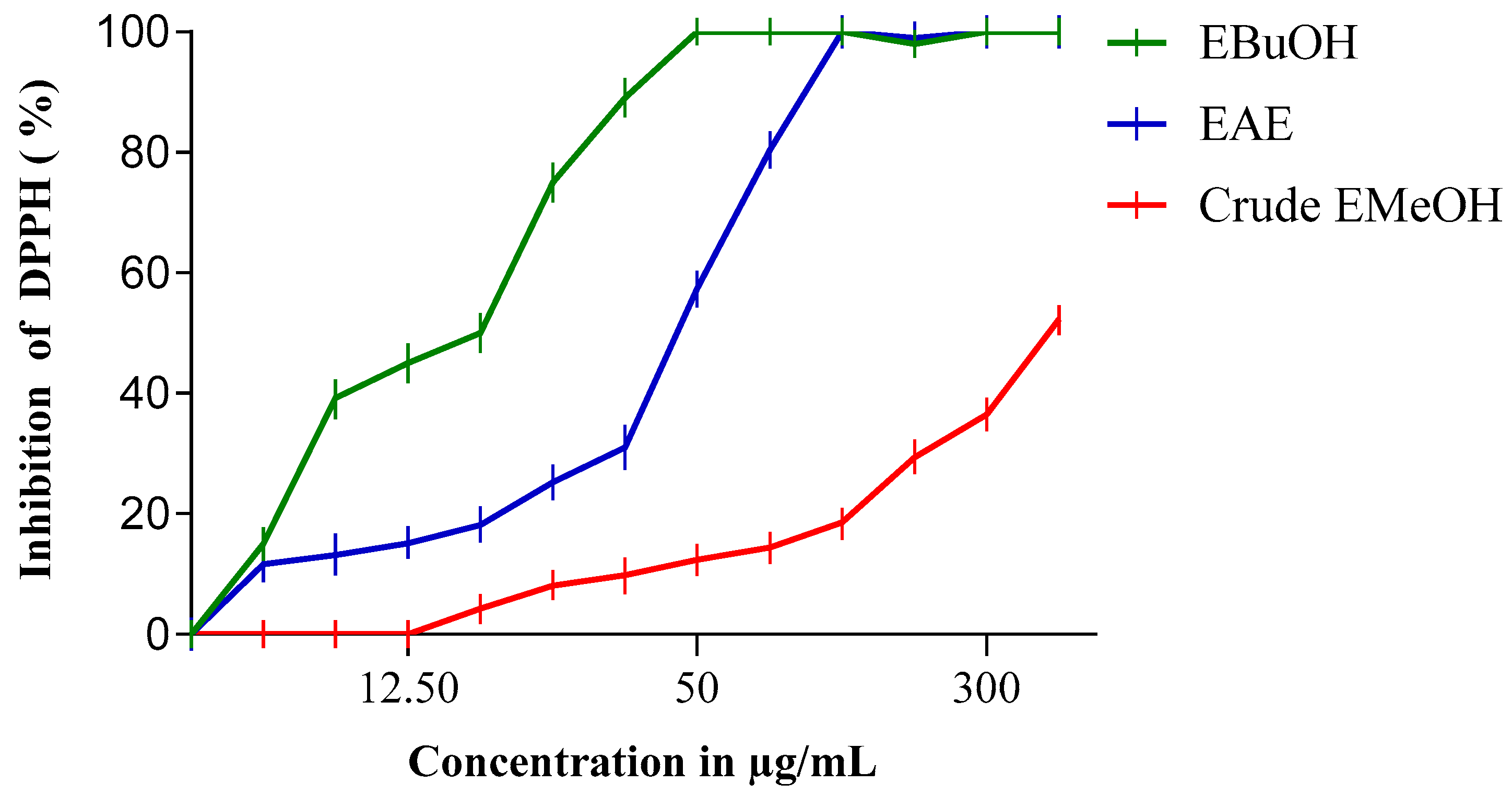
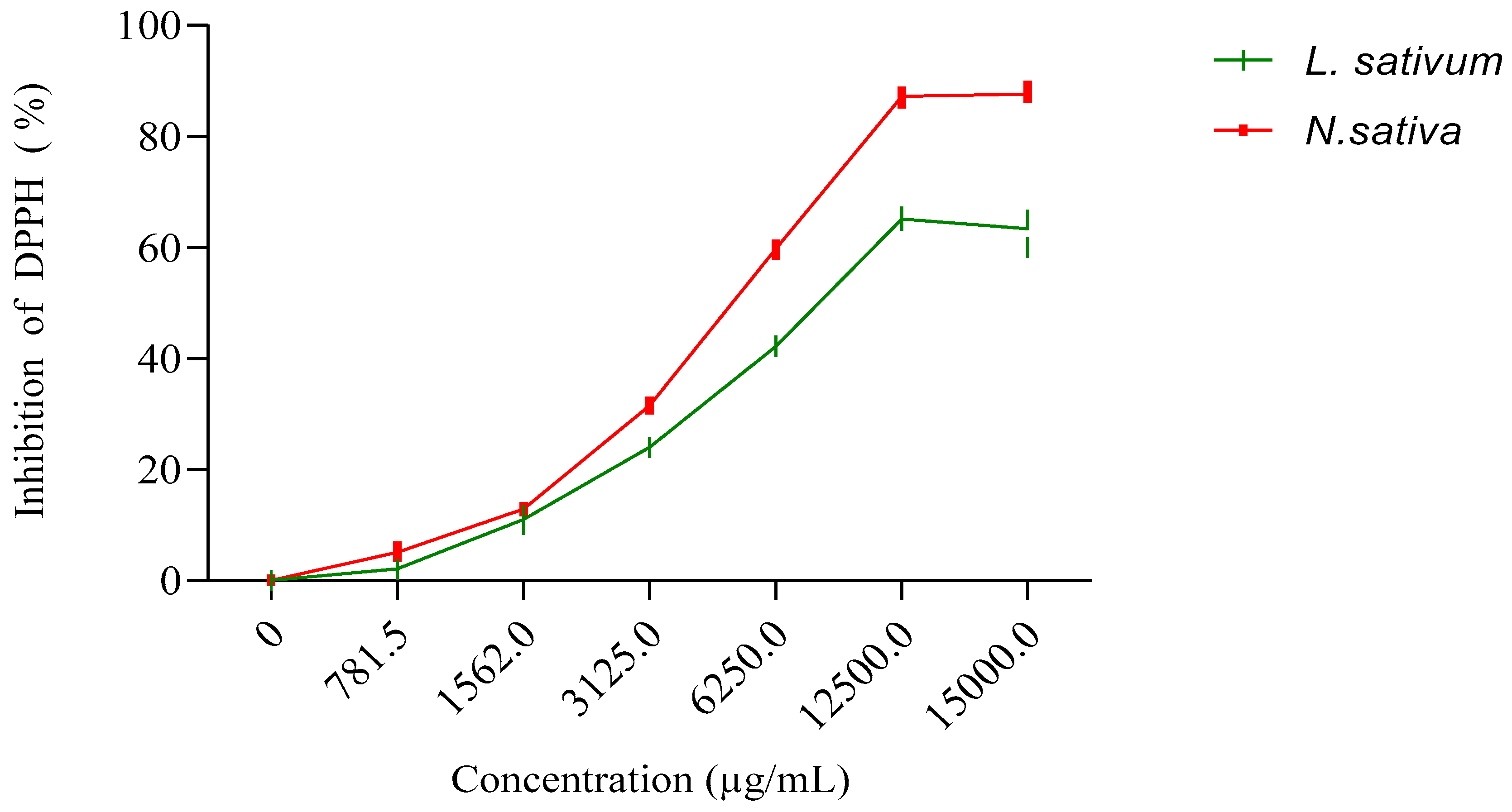
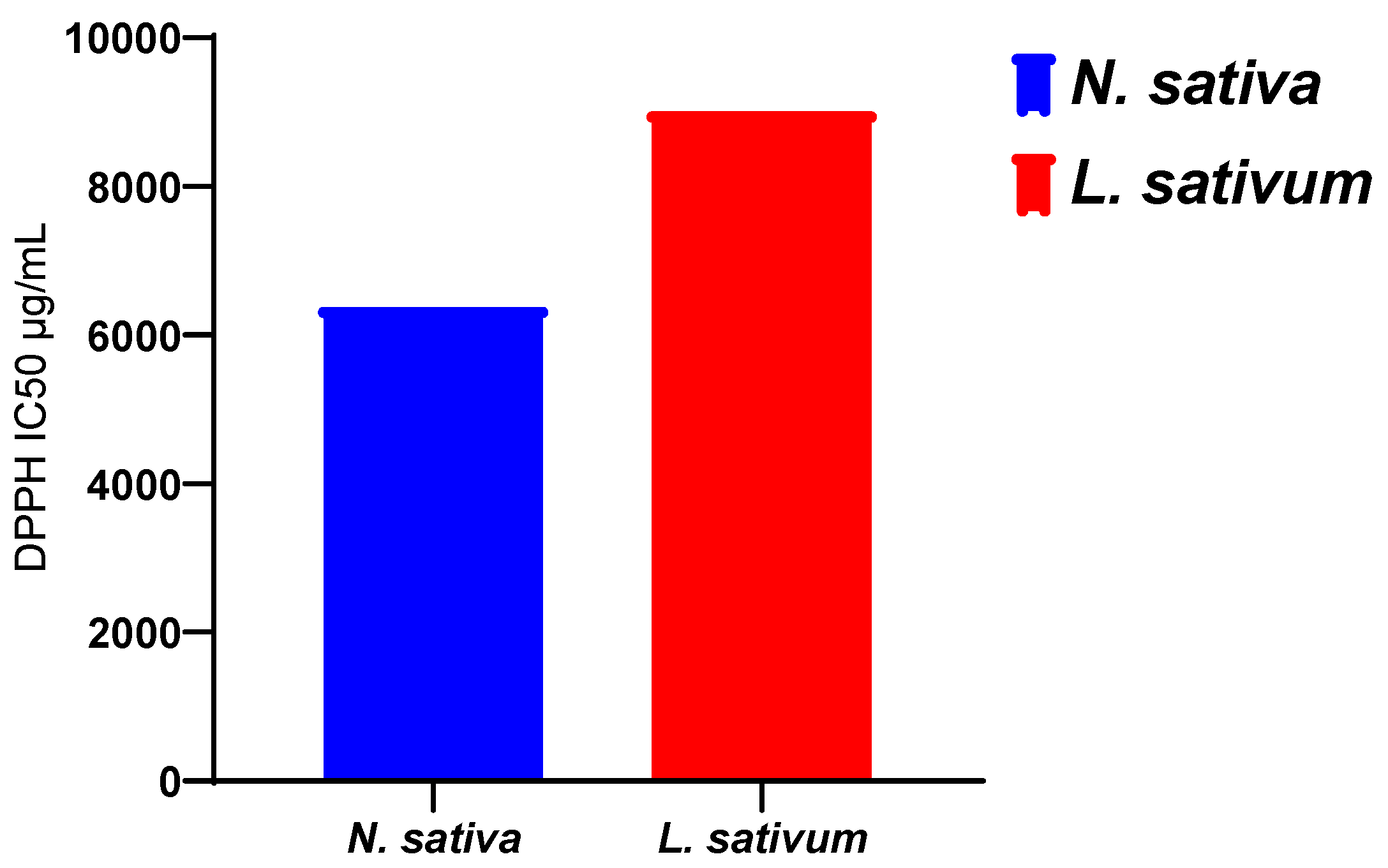
3.3. Antioxidant Activity
3.3.1. For Extracts and Fractions
3.3.2. For Vegetal Oils
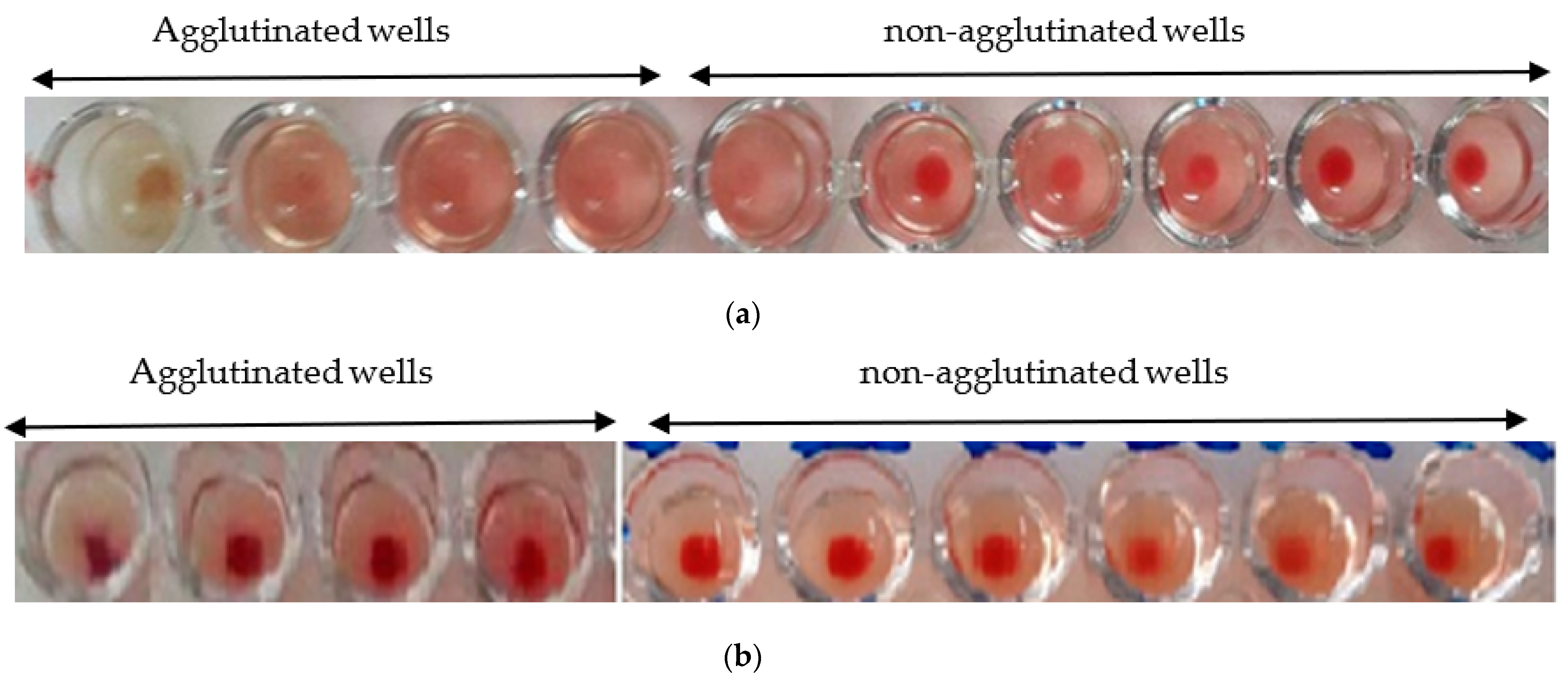
3.4. Hemagglutination Activity
3.4.1. Phyto-Hemagglutination Test
3.4.2. Hemagglutination Limit Test
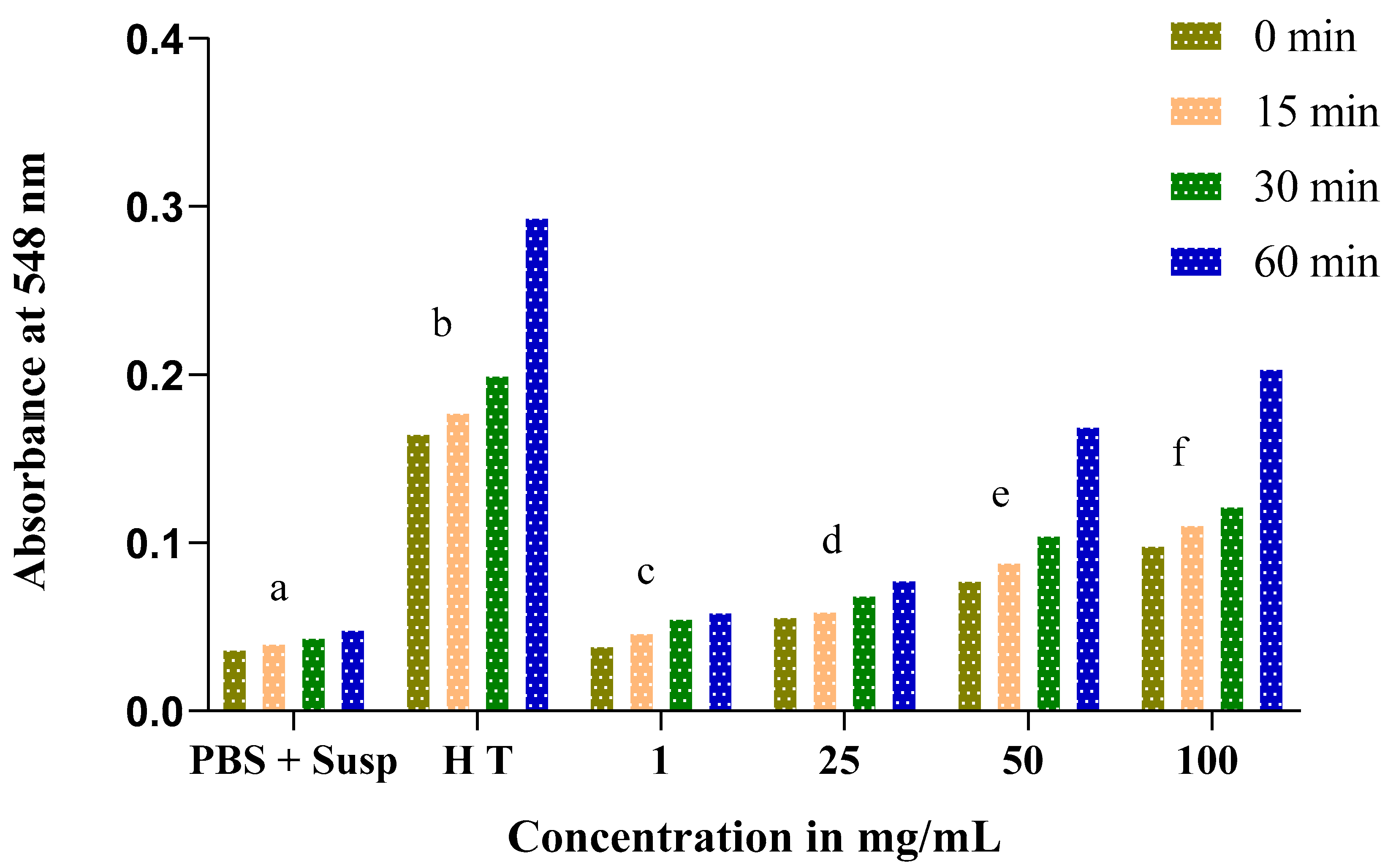
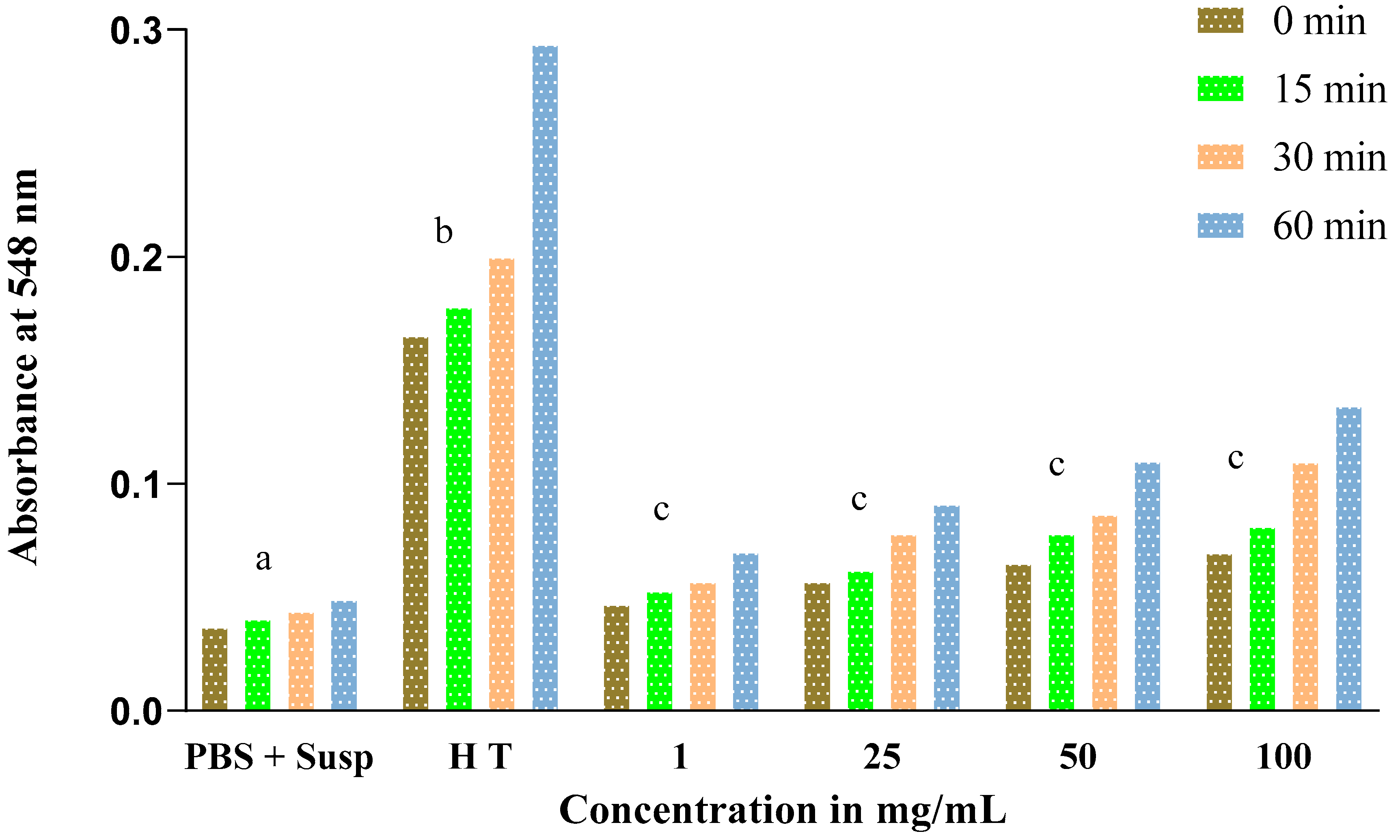
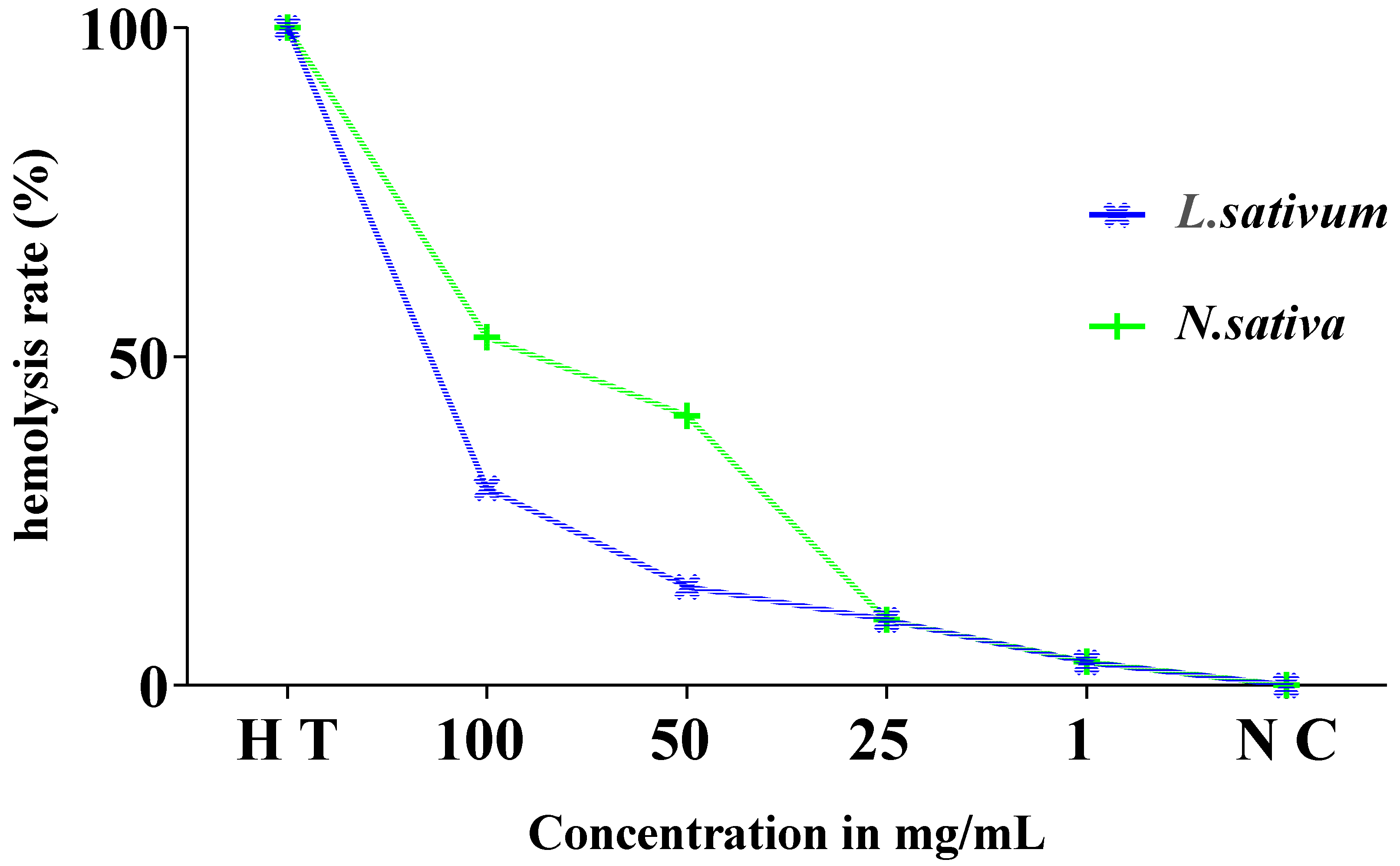
3.5. Evolution of the Hemolytic Effect of Seeds
3.6. Estimation of Hemolysis Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oulidi, J.; Lamiaceae, L. Étude ethnobotanique des plantes médicinales et aromatiques utilisées dans la ville de Fès au Maroc—Ethnobotanical survey of medicinal and aromatic plants used by the people of Fez in Morocco. Phytothérapie 2015, 14, 35–43. [Google Scholar] [CrossRef]
- Saad, B.; Azaizeh, H.; Abu-hijleh, G.; Said, O. Safety of traditional Arab herbal medicine. Evid.-Based Compl. Altern. Med. 2006, 3, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Boziaris, I.S.; Nychas, G.E.; Komaitis, M. Food chemistry analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Benmakhlouf, Z.; Benserradj, O.; Kellab, R. Short communication: Identification of phytochemical constituents of Syzygium Aromaticum L. Using gas chromatography coupled with mass spectrometry and evaluation of antimicrobial activity. Biodiversitas J. Biol. Divers. 2022, 23, 2586–2593. [Google Scholar] [CrossRef]
- Ullah, R.; Alqahtani, A.S. GC-MS Analysis, Heavy Metals, Biological, and Toxicological Evaluation of Reseda muricata and Marrubium vulgare Methanol Extracts. Evidence-Based Complement. Altern. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bouslamti, M.; El Barnossi, A.; Kara, M.; Alotaibi, B.S.; Al Kamaly, O.; Assouguem, A.; Lyoussi, B.; Benjelloun, A.S. Total polyphenols content, antioxidant and antimicrobial activities of leaves of Solanum elaeagnifolium Cav. from Morocco. Molecules 2022, 27, 4322. [Google Scholar] [CrossRef] [PubMed]
- Assouguem, A.; Kara, M.; Ramzi, A.; Annemer, S.; Kowalczyk, A.; Ali, E.A.; Moharram, B.A.; Lazraq, A.; Farah, A. Evaluation of the effect of four bioactive compounds in combination with chemical product against two spider mites Tetranychus urticae and Eutetranychus orientalis (Acari: Tetranychidae). Evid.-Based Compl. Altern. Med. 2022, 2022, e2004623. [Google Scholar] [CrossRef]
- Salhi, S.; Fadli, M.; Zidane, L.; Douira, A. Etudes floristique et ethnobotanique des plantes medicinales de la ville de Kénitra (Maroc). REVESCO Rev. Estud. Coop. 2011, 104, 38–62. [Google Scholar] [CrossRef]
- El Khomsi, M.; Kara, M.; Hmamou, A.; Assouguem, A.; Al Kamaly, O.; Saleh, A.; Ercisli, S.; Fidan, H.; Hmouni, D. In vitro studies on the antimicrobial and antioxidant activities of total polyphenol content of Cynara humilis from Moulay Yacoub area (Morocco). Plants 2022, 11, 1200. [Google Scholar] [CrossRef]
- Hmamou, A.; Eloutassi, N.; Alshawwa, S.Z.; Al kamaly, O.; Kara, M.; Bendaoud, A.; El-Assri, E.-M.; Tlemcani, S.; El Khomsi, M.; Lahkimi, A. Total phenolic content and antioxidant and antimicrobial activities of Papaver rhoeas L. organ extracts growing in Taounate Region, Morocco. Molecules 2022, 27, 854. [Google Scholar] [CrossRef]
- Assouguem, A.; Kara, M.; Mechchate, H.; Al-Mekhlafi, F.A.; Nasr, F.; Farah, A.; Lazraq, A. Evaluation of the Impact of Different Management Methods on Tetranychus urticae (Acari: Tetranychidae) and Their Predators in Citrus Orchards. Plants 2022, 11, 623. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of nigella sativa. Phytother. Res. Int. J. Devot. Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Ennabili, A.; Gharnit, N.; Maach, Y.; El Meskaoui, A.; Bousta, D. Exploitation des plantes medicinales et al.imentaires du bassin versant de l’oued laou (Nord-Ouest du Maroc). J Bot. Soc. Bot. Fr. 2006, 36, 71–79. [Google Scholar]
- Bnouham, M.; Mekhfi, H.; Legssyer, A.; Ziyyat, A. Ethnopharmacology forum medicinal plants used in the treatment of diabetes in Morocco. Int. J. Diabetes Metab. 2002, 10, 33–50. [Google Scholar]
- Favier, A. Le Stress Oxydant Intérêt Conceptuel et Expérimental dans la Compréhension des Mécanismes des Maladies et Potentiel Thérapeutique. L’actualité Chim. 2003, 108–115. [Google Scholar]
- Favier, A. Stress oxydant et pathologies humaines. Ann. Pharm. Fr. 2006, 64, 390–396. [Google Scholar] [CrossRef]
- Nath, K.A.; Norby, S.M. Reactive oxygen species and acute renal failure of reactive oxygen species. Am. J. Med. 2000, 109, 665–678. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Das, D.; Banerjee, R.K. Reactive oxygen species: Oxidative da and pathogenesis. Curr. Sci. 1999, 77, 658–666. [Google Scholar]
- Mompon, B.; Lemaire, B.; Mengal, P.; Surbled, M. Extraction des polyphenols: Du laboratoire a la production industrielle. Colloq.-INRA 1998, 87, 31–44. [Google Scholar]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Bahorun, T.; Gressier, B.; Trotin, F.; Brunet, C.; Dine, T.; Luyckx, M.; Vasseur, J.; Cazin, M.; Cazin, J.C.; Pinkas, M. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimittelforschung 1996, 46, 1086–1089. [Google Scholar] [PubMed]
- Kara, M.; Assouguem, A.; Abdou, R.Z.; Bahhou, J. Phytochemical content and antioxidant activity of vinegar prepared from four apple varieties by different methods. Trop. J. Nat. Prod. Res. 2021, 5, 1578–1585. [Google Scholar] [CrossRef]
- Monrroy, M.; García, E.; Ríos, K.; García, J.R. Extraction and physicochemical characterization of mucilage from Opuntia cochenillifera (L.) Miller. J. Chem. 2017, 2017, 4301901. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Kroh, L.W.; Mörsel, J.-T. Radical scavenging activity of Black Cumin (Nigella sativa L.), Coriander (Coriandrum Sativum L.), and Niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Kara, M.; Assouguem, A.; Fadili, M.E.; Benmessaoud, S.; Alshawwa, S.Z.; Kamaly, O.A.; Saghrouchni, H.; Zerhouni, A.R.; Bahhou, J. Contribution to the evaluation of physicochemical properties, total phenolic content, antioxidant potential, and antimicrobial activity of vinegar commercialized in Morocco. Molecules 2022, 27, 770. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Carlini, C.R.; Udedibie, A.B. Comparative effects of processing methods on hemagglutinating and antitryptic activities of canavalia ensiformis and canavalia Braziliensis seeds. J. Agric. Food Chem. 1997, 45, 4372–4377. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, J.D. In vitro hemolysis of human erythrocytes—By plant extracts with antiplasmodial activity. J. Ethnopharmacol. 2001, 74, 239–243. [Google Scholar] [CrossRef]
- Ktari, N.; Trabelsi, I.; Bardaa, S.; Triki, M.; Bkhairia, I.; Salem, R.B.S.-B.; Nasri, M.; Salah, R.B. Antioxidant and hemolytic activities, and effects in rat cutaneous wound healing of a novel polysaccharide from Fenugreek (Trigonella foenum-graecum) Seeds. Int. J. Biol. Macromol. 2017, 95, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Meziti, A.; Meziti, H.; Boudiaf, K.; Mustapha, B.; Bouriche, H. Polyphenolic profile and antioxidant activities of Nigella Sativa seed extracts in vitro and in vivo. Int. J. Biotechnol. Bioeng. 2012, 6, 109–117. [Google Scholar]
- Asdadi, A.; Harhar, H.; Gharby, S.; Bouzoubaâ, Z.; Yadini, A.E.; Moutaj, R.; Hadek, M.E.; Chebli, B.; Hassani, L.M.I. Chemical composition and antifungal activity of Nigella Sativa L. oil seed cultivated in Morocco. Int. J. Pharm. Sci. Invent. 2014, 3, 9–15. [Google Scholar]
- Al-Snafi, A.E. Chemical constituents and pharmacological effects of melilotus officinalis—A review. IOSR J. Pharm. 2020, 10, 26–36. [Google Scholar]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef] [PubMed]
- Lefahal, M.; Zaabat, N.; Ayad, R.; Makhloufi, E.H.; Djarri, L.; Benahmed, M.; Laouer, H.; Nieto, G.; Akkal, S. In vitro assessment of total phenolic and flavonoid contents, antioxidant and photoprotective activities of crude methanolic extract of aerial parts of Capnophyllum peregrinum (L.) Lange (Apiaceae) growing in Algeria. Medicines 2018, 5, 26. [Google Scholar] [CrossRef]
- Doke, S.; Guha, M. Garden Cress (Lepidium sativum L.) seed—An important medicinal source: A. Cellulose 2014, 9, 69–80. [Google Scholar]
- Dhaheri, Y.A.; Wali, A.F.; Akbar, I.; Rasool, S.; Razmpoor, M.; Jabnoun, S.; Rashid, S. Chapter 3—Nigella Sativa, a Cure for Every Disease: Phytochemistry, Biological Activities, and Clinical Trials. In Black Seeds (Nigella Sativa); Khan, A., Rehman, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 63–90. ISBN 978-0-12-824462-3. [Google Scholar]
- Alam, M.T.; Parvez, N.; Sharma, P.K. FDA-approved natural polymers for fast dissolving tablets. J. Pharm. 2014, 2014, 952970. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Maham, M.; Asri-Rezaei, S.; Pournaghi, P.; Khorrami, M.-S.; Derakhshan, A. Effects of Nigella Sativa on performance, blood profiles, and antibody titer against newcastle disease in broilers. Evid. Based Compl. Altern. Med. 2021, 2021, e2070375. [Google Scholar] [CrossRef]
- Ousaaid, D.; Laaroussi, H.; Bakour, M.; El Ghouizi, A.; El Menyiy, N.; Lyoussi, B.; El Arabi, I. Effect of a combination of rosa canina fruits and apple cider vinegar against hydrogen peroxide-induced toxicity in experimental animal models. J. Food Qual. 2022, 2022, e7381378. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of antioxidant-rich propolis and bee pollen extracts against d-glucose induced type 2 diabetes in rats. Food Res. Int. 2020, 138, 109802. [Google Scholar] [CrossRef] [PubMed]
- Lafraxo, H.; Bakour, M.; Laaroussi, H.; El Ghouizi, A.; Ousaaid, D.; Aboulghazi, A.; Lyoussi, B. The synergistic beneficial effect of thyme honey and olive oil against diabetes and its complications induced by alloxan in wistar rats. Evid. Based Compl. Altern. Med. 2021, 2021, e9949056. [Google Scholar] [CrossRef] [PubMed]
- Bakour, M.; Soulo, N.; Hammas, N.; Fatemi, H.E.; Aboulghazi, A.; Taroq, A.; Abdellaoui, A.; Al-Waili, N.; Lyoussi, B. The antioxidant content and protective effect of argan oil and syzygium aromaticum essential oil in hydrogen peroxide-induced biochemical and histological changes. Int. J. Mol. Sci. 2018, 19, 610. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ousaaid, D.; Oumokhtar, B.; Lyoussi, B. Antioxidant and antibacterial effects of pollen extracts on human multidrug-resistant pathogenic bacteria. J. Food Qual. 2021, 2021, 5560182. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Ferreira-Santos, P.; Genisheva, Z.; El Ghouizi, A.; Aboulghazi, A.; Teixeira, J.A.; Lyoussi, B. Protective effect of honey and propolis against gentamicin-induced oxidative stress and hepatorenal damages. Oxid. Med. Cell. Longev. 2021, 2021, e9719906. [Google Scholar] [CrossRef]
- Arzami, A.N.; Ho, T.M.; Mikkonen, K.S. Valorization of cereal by-product hemicelluloses: Fractionation and purity considerations. Food Res. Int. 2022, 151, 110818. [Google Scholar] [CrossRef]
- Ben Jalloul, A.; Chaar, H.; Tounsi, M.S.; Abderrabba, M. Variations in phenolic composition and antioxidant activities of Scabiosa Maritima (Scabiosa Atropurpurea Sub. Maritima L.) crude extracts and fractions according to growth stage and plant part. S. Afr. J. Bot. 2022, 146, 703–714. [Google Scholar] [CrossRef]
- Solaiman, M.M. Agglutination effect of selected medicinal plant leaf crude extracts on ABO blood group. Am. J. Plant Biol. 2021, 6, 11–18. [Google Scholar] [CrossRef]
- El Abdali, Y.; Allali, A.; Agour, M.; Lahkimi, A.; Eloutassi, N.; Bouia, A. Phytochemical screening, and in vitro antiradical and immunostimulant potential of Linum usitatissimum L. Pharmacologyonline 2021, 3, 602–614. [Google Scholar]
- Senthilganesh, J.; Ravichandran, S.; Durairajan, R.; BalaSubramaniyan, S.; Krishnasamy, L.; Veerappan, A.; Paramasivam, N. Metal sulfide nanoparticles based phytolectin scaffolds inhibit vulvovaginal candidiasis causing Candida Albicans. J. Clust. Sci. 2021, 33, 1361–1372. [Google Scholar] [CrossRef]
- Cavada, B.S.; Osterne, V.J.S.; Oliveira, M.V.; Pinto-Junior, V.R.; Silva, M.T.L.; Bari, A.U.; Lima, L.D.; Lossio, C.F.; Nascimento, K.S. Reviewing mimosoideae lectins: A group of under explored legume lectins. Int. J. Biol. Macromol. 2020, 154, 159–165. [Google Scholar] [CrossRef] [PubMed]

| Humidity % | Sugar Content (mg/g) | Mucilage (mg/g) | |
|---|---|---|---|
| N. sativa seeds | 8.40 ± 0.03 | 58.17 ± 0.42 | 8.2 |
| L. sativum seeds | 9.54 ± 0.18 | 67.86 ± 0.87 | 240 |
| TPC mg GAE/g | TFC mg QE/g | CT mg/g | |
|---|---|---|---|
| N. sativa seeds | 10.48 ± 1.33 | 0.258 ± 0.058 | 7.2 ± 0.025 |
| L. sativum seeds | 10.35 ± 0.73 | 3.09 ± 0.048 | 1.4 ± 0.22 |
| Nigella sativa | Lepidium sativum | |||||
|---|---|---|---|---|---|---|
| Crude Extract EMetOH | Fraction | Crude Extract EMetOH | Fraction | |||
| EButOH | EAE | EButOH | EAE | |||
| DPPH IC50 (µg/mL) | 1331.53 | 48.7 | 50.65 | 380 | 15.7 | 52.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouattar, H.; Zouirech, O.; Kara, M.; Assouguem, A.; Almutairi, S.M.; Al-Hemaid, F.M.; Rasheed, R.A.; Ullah, R.; Abbasi, A.M.; Aouane, M.; et al. In Vitro Study of the Phytochemical Composition and Antioxidant, Immunostimulant, and Hemolytic Activities of Nigella sativa (Ranunculaceae) and Lepidium sativum Seeds. Molecules 2022, 27, 5946. https://doi.org/10.3390/molecules27185946
Ouattar H, Zouirech O, Kara M, Assouguem A, Almutairi SM, Al-Hemaid FM, Rasheed RA, Ullah R, Abbasi AM, Aouane M, et al. In Vitro Study of the Phytochemical Composition and Antioxidant, Immunostimulant, and Hemolytic Activities of Nigella sativa (Ranunculaceae) and Lepidium sativum Seeds. Molecules. 2022; 27(18):5946. https://doi.org/10.3390/molecules27185946
Chicago/Turabian StyleOuattar, Hafssa, Otmane Zouirech, Mohammed Kara, Amine Assouguem, Saeedah Musaed Almutairi, Fahad M. Al-Hemaid, Rabab Ahmed Rasheed, Riaz Ullah, Arshad Mehmood Abbasi, Mahjoub Aouane, and et al. 2022. "In Vitro Study of the Phytochemical Composition and Antioxidant, Immunostimulant, and Hemolytic Activities of Nigella sativa (Ranunculaceae) and Lepidium sativum Seeds" Molecules 27, no. 18: 5946. https://doi.org/10.3390/molecules27185946
APA StyleOuattar, H., Zouirech, O., Kara, M., Assouguem, A., Almutairi, S. M., Al-Hemaid, F. M., Rasheed, R. A., Ullah, R., Abbasi, A. M., Aouane, M., & Mikou, K. (2022). In Vitro Study of the Phytochemical Composition and Antioxidant, Immunostimulant, and Hemolytic Activities of Nigella sativa (Ranunculaceae) and Lepidium sativum Seeds. Molecules, 27(18), 5946. https://doi.org/10.3390/molecules27185946







