Simple Extraction of Cannabinoids from Female Inflorescences of Hemp (Cannabis sativa L.)
Abstract
:1. Introduction
2. Results
2.1. Cannabinoids Content in Inflorescences
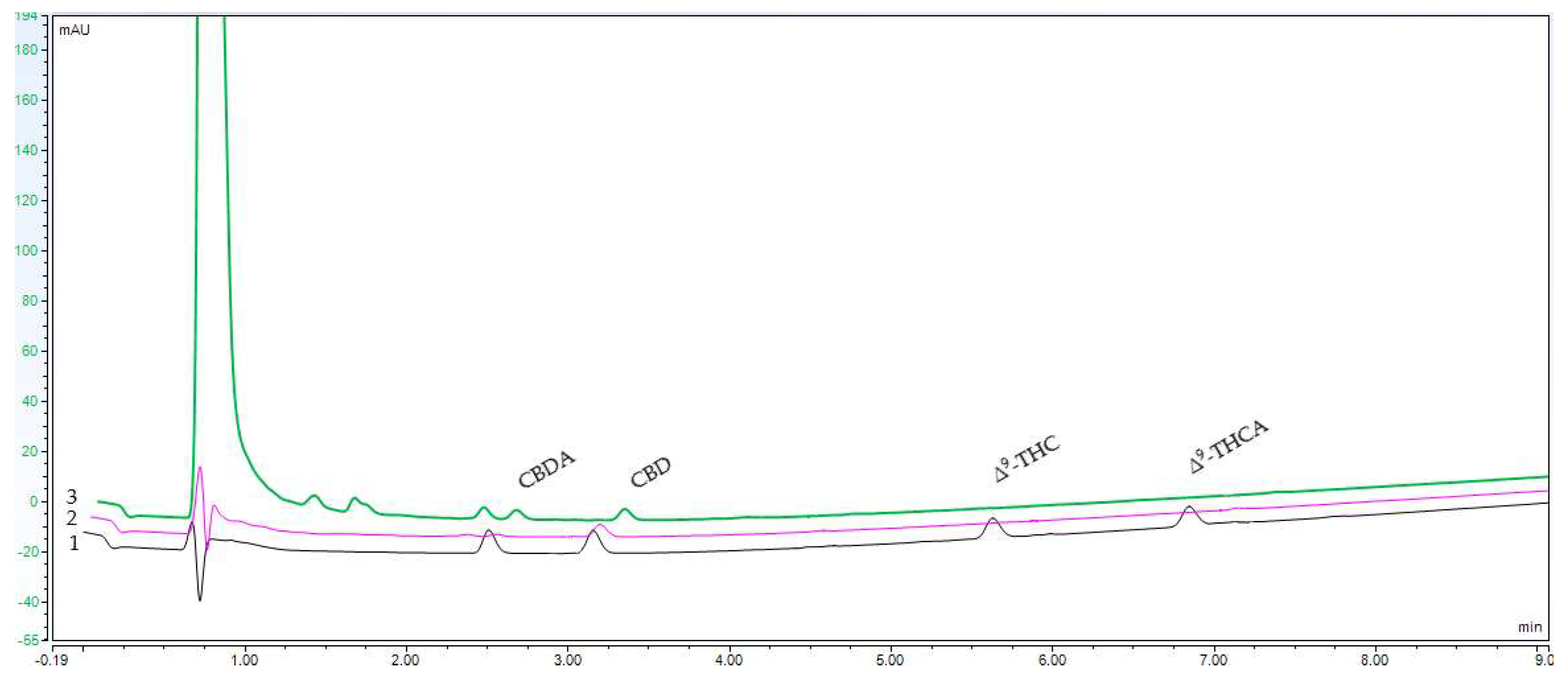
2.2. Extraction Methods
2.2.1. Water Extraction
2.2.2. Ethanol Extraction
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Sampling
4.3. Cannabinoids Analysis in Inflorescences
4.4. Extraction
4.5. High-Performance Liquid Chromatography Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Glivar, T.; Eržen, J.; Kreft, S.; Zagožen, M.; Čerenak, A.; Čeh, B.; Tavčar Benković, E. Cannabinoid Content in Industrial Hemp (Cannabis sativa L.) Varieties Grown in Slovenia. Ind. Crops Prod. 2020, 145, 112082. [Google Scholar] [CrossRef]
- Hillig, K.W.; Mahlberg, P.G. A Chemotaxonomic Analysis of Cannabinoid Variation in Cannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Fischedick, J.T.; Glas, R.; Hazekamp, A.; Verpoorte, R. A Qualitative and Quantitative HPTLC Densitometry Method for the Analysis of Cannabinoids in Cannabis sativa L. Phytochem. Anal. 2009, 20, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Bruci, Z.; Papoutsis, I.; Athanaselis, S.; Nikolaou, P.; Pazari, E.; Spiliopoulou, C.; Vyshka, G. First Systematic Evaluation of the Potency of Cannabis sativa Plants Grown in Albania. Forensic Sci. Int. 2012, 222, 40–46. [Google Scholar] [CrossRef]
- Baron, E.P. Comprehensive Review of Medicinal Marijuana, Cannabinoids, and Therapeutic Implications in Medicine and Headache: What a Long Strange Trip It’s Been…. Headache 2015, 55, 885–916. [Google Scholar] [CrossRef]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (Hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef]
- Hazekamp, A.; Bastola, K.; Rashidi, H.; Bender, J.; Verpoorte, R. Cannabis Tea Revisited: A Systematic Evaluation of the Cannabinoid Composition of Cannabis Tea. J. Ethnopharmacol. 2007, 113, 85–90. [Google Scholar] [CrossRef]
- Citti, C.; Ciccarella, G.; Braghiroli, D.; Parenti, C.; Vandelli, M.A.; Cannazza, G. Medicinal Cannabis: Principal Cannabinoids Concentration and Their Stability Evaluated by a High Performance Liquid Chromatography Coupled to Diode Array and Quadrupole Time of Flight Mass Spectrometry Method. J. Pharm. Biomed. Anal. 2016, 128, 201–209. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/food/plants/plant-reproductive-material/plant-variety-catalogues-databases-information-systems_en (accessed on 1 October 2021).
- Zachwieja, J. The Content of Tetrahydrocannbinol in Polish Cultivars of Fibrous Hemp in 2004–2011. J. Nat. Fibers 2013, 10, 297–308. [Google Scholar] [CrossRef]
- Makowiecka, J.; Wielgus, K. Therapeutic Potential of Cannabinoids—Retrospective and Historical Developments. J. Nat. Fibers 2014, 11, 185–198. [Google Scholar] [CrossRef]
- Makowiecka, J.; Wielgus, K. Therapeutic Potential of Cannabinoids—Perspectives for the Future. J. Nat. Fibers 2014, 11, 283–311. [Google Scholar] [CrossRef]
- Zielonka, D.M.; Kiraga, Ł.; Kozłowski, R.M. Medical potential of cannabis: An overview. In Handbook of Natural Fibres: Volume 2: Processing and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 419–448. ISBN 9780128187821. [Google Scholar]
- Analytical Cannabis. Available online: https://www.analyticalcannabis.com/articles/advances-in-cannabis-extraction-techniques-311772 (accessed on 22 October 2021).
- Wang, M.; Wang, Y.H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; van Antwerp, J.; Parcher, J.F.; Elsohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef]
- De Vita, D.; Madia, V.N.; Tudino, V.; Saccoliti, F.; de Leo, A.; Messore, A.; Roscilli, P.; Botto, A.; Pindinello, I.; Santilli, G.; et al. Comparison of Different Methods for the Extraction of Cannabinoids from Cannabis. Nat. Prod. Res. 2020, 34, 2952–2958. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán Zuazo, V.H.; Sánchez-Carnenero, C.; Hernández, A.; Ferreiro-Vera, C.; Casano, S. Seeking Suitable Agronomical Practices for Industrial Hemp (Cannabis sativa L.) Cultivation for Biomedical Applications. Ind. Crops Prod. 2019, 139, 111524. [Google Scholar] [CrossRef]
- Loflin, M.; Earleywine, M. A New Method of Cannabis Ingestion: The Dangers of Dabs? Addict. Behav. 2014, 39, 1430–1433. [Google Scholar] [CrossRef]
- Schnelle, M.; Grotenhermen, F.; Reif, M.; Gorter, R.W. Ergebnisse Einer Standardisierten Umfrage Zur Medizinischen Verwendung von Cannabisprodukten Im Deutschen Sprachraum. Forschende Komplementärmedizin 1999, 6, 28–36. [Google Scholar] [CrossRef]
- Borodovsky, J.T.; Crosier, B.S.; Lee, D.C.; Sargent, J.D.; Budney, A.J. Smoking, Vaping, Eating: Is Legalization Impacting the Way People Use Cannabis? Int. J. Drug Policy 2016, 36, 141–147. [Google Scholar] [CrossRef]
- Available online: https://www.emcdda.europa.eu/publications/edr/trends-developments/2020_en (accessed on 1 October 2021).
- Verhoeckx, K.C.M.; Korthout, H.A.A.J.; van Meeteren-Kreikamp, A.P.; Ehlert, K.A.; Wang, M.; van der Greef, J.; Rodenburg, R.J.T.; Witkamp, R.F. Unheated Cannabis sativa Extracts and Its Major Compound THC-Acid Have Potential Immuno-Modulating Properties Not Mediated by CB1 and CB2 Receptor Coupled Pathways. Int. Immunopharmacol. 2006, 6, 656–665. [Google Scholar] [CrossRef]
- Citti, C.; Braghiroli, D.; Vandelli, M.A.; Cannazza, G. Pharmaceutical and Biomedical Analysis of Cannabinoids: A Critical Review. J. Pharm. Biomed. Anal. 2018, 147, 565–579. [Google Scholar] [CrossRef]
- Leghissa, A.; Hildenbrand, Z.L.; Schug, K.A. A Review of Methods for the Chemical Characterization of Cannabis Natural Products. J. Sep. Sci. 2018, 41, 398–415. [Google Scholar] [CrossRef]
- Brighenti, V.; Protti, M.; Anceschi, L.; Zanardi, C.; Mercolini, L.; Pellati, F. Emerging Challenges in the Extraction, Analysis and Bioanalysis of Cannabidiol and Related Compounds. J. Pharm. Biomed. Anal. 2021, 192, 113633. [Google Scholar] [CrossRef] [PubMed]
- Corroon, J.; Kight, R. Regulatory Status of Cannabidiol in the United States: A Perspective. Cannabis Cannabinoid Res. 2018, 3, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.; Nahtigal, I. The Evolving Landscape of Cannabis Edibles. Curr. Opin. Food Sci. 2019, 28, 25–31. [Google Scholar] [CrossRef]
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture 2021, 11, 384. [Google Scholar] [CrossRef]
- Nahar, L.; Uddin, S.J.; Alam, M.A.; Sarker, S.D. Extraction of Naturally Occurring Cannabinoids: An Update. Phytochem. Anal. 2021, 32, 228–241. [Google Scholar] [CrossRef]
- The Economics of Extraction. Available online: https://www.cannabisbusinesstimes.com/article/the-economics-of-extraction/ (accessed on 22 October 2021).
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a New Extraction Technique and HPLC Method for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis sativa L. (Hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical Constituents, Advanced Extraction Technologies and Techno-Functional Properties of Selected Mediterranean Plants for Use in Meat Products. A Comprehensive Review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of Cannabinoids Concentration and Stability in Standardized Preparations of Cannabis Tea and Cannabis Oil by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1555–1563. [Google Scholar] [CrossRef]
- Politi, M.; Peschel, W.; Wilson, N.; Zloh, M.; Prieto, J.M.; Heinrich, M. Direct NMR Analysis of Cannabis Water Extracts and Tinctures and Semi-Quantitative Data on Δ9-THC and Δ9-THC-Acid. Phytochemistry 2008, 69, 562–570. [Google Scholar] [CrossRef]
- Seltenrich, N. Cannabis Contaminants: Regulating Solvents, Microbes, and Metals in Legal Weed. Environ. Health Perspect. 2019, 127, 082001. [Google Scholar] [CrossRef]
- Pinkhasova, D.V.; Jameson, L.E.; Conrow, K.D.; Simeone, M.P.; Davis, A.P.; Wiegers, T.C.; Mattingly, C.J.; Leung, M.C.K. Regulatory Status of Pesticide Residues in Cannabis: Implications to Medical Use in Neurological Diseases. Curr. Res. Toxicol. 2021, 2, 140–148. [Google Scholar] [CrossRef]
- Nuapia, Y.; Maraba, K.; Tutu, H.; Chimuka, L.; Cukrowska, E. In Situ Decarboxylation-Pressurized Hot Water Extraction for Selective Extraction of Cannabinoids from Cannabis sativa. Chemometric Approach. Molecules 2021, 26, 3343. [Google Scholar] [CrossRef]
- Segelman, A.B.; Sofia, R.D.; Segelman, F.P.; Harakal, J.J.; Knobloch, L.C. Cannabis sativa L. (marijuana) V: Pharmacological evaluation of marijuana aqueous extract and volatile oil. J. Pharm. Sci. 1974, 63, 962–964. [Google Scholar] [CrossRef]
- Segelman, A.B.; Sofia, R.D. Cannabis sativa L. (Marijuana) IV: Chemical Basis for Increased Potency Related to Novel Method of Preparation. J. Pharm. Sci. 1973, 62, 2044–2046. [Google Scholar] [CrossRef]
- Giroud, C.; Menetrey, A.; Augsburger, M.; Buclin, T.; Sanchez-Mazas, P.; Mangin, P. Hemp Tea versus Hemp Milk: Behavioral, Physiological Effects, Blood, Urine, Saliva and Sweat Cannabinoids Levels Following Ingestion by Two Groups of Six Healthy Volunteers. Probl. Forensic Sci. 2000, 42, 102–110. [Google Scholar]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and Extraction Methods of Medicinal Cannabis: A Narrative Review. J. Cannabis Res. 2021, 3. [Google Scholar] [CrossRef]
- Moreno, T.; Dyer, P.; Tallon, S. Cannabinoid Decarboxylation: A Comparative Kinetic Study. Ind. Eng. Chem. Res. 2020, 59, 20307–20315. [Google Scholar] [CrossRef]
- Dussy, F.E.; Hamberg, C.; Luginbühl, M.; Schwerzmann, T.; Briellmann, T.A. Isolation of Δ9-THCA-A from Hemp and Analytical Aspects Concerning the Determination of Δ9-THC in Cannabis Products. Forensic Sci. Int. 2005, 149, 3–10. [Google Scholar] [CrossRef]
- Raharjo, T.J.; Verpoorte, R. Methods for the Analysis of Cannabinoids in Biological Materials: A Review. Phytochem. Anal. 2004, 15, 79–94. [Google Scholar] [CrossRef]
- Citti, C.; Russo, F.; Sgrò, S.; Gallo, A.; Zanotto, A.; Forni, F.; Vandelli, M.A.; Laganà, A.; Montone, C.M.; Gigli, G.; et al. Pitfalls in the Analysis of Phytocannabinoids in Cannabis Inflorescence. Anal. Bioanal. Chem. 2020, 412, 4009–4022. [Google Scholar] [CrossRef]
- Perrotin-Brunel, H.; Kroon, M.C.; van Roosmalen, M.J.E.; van Spronsen, J.; Peters, C.J.; Witkamp, G.J. Solubility of Non-Psychoactive Cannabinoids in Supercritical Carbon Dioxide and Comparison with Psychoactive Cannabinoids. J. Supercrit. Fluids 2010, 55, 603–608. [Google Scholar] [CrossRef]
- Knight, G.; Hansen, S.; Connor, M.; Poulsen, H.; McGovern, C.; Stacey, J. The Results of an Experimental Indoor Hydroponic Cannabis Growing Study, Using the “Screen of Green” (ScrOG) Method-Yield, Tetrahydrocannabinol (THC) and DNA Analysis. Forensic Sci. Int. 2010, 202, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, L.; Koziara, W. The Effect of Nitrogen Dose, Sowing Density and Time of Harvest on Development and Yields of Hemp Cultivar Bialobrzeskie. J. Nat. Fibers 2006, 2, 1–17. [Google Scholar] [CrossRef]
- Vera, C.L.; Malhi, S.S.; Raney, J.P.; Wang, Z.H. The Effect of N and P Fertilization on Growth, Seed Yield and Quality of Industrial Hemp in the Parkland Region of Saskatchewan. Can. J. Plant Sci. 2004, 84, 939–947. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis sativa L.). Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Landi, S.; Berni, R.; Capasso, G.; Hausman, J.F.; Guerriero, G.; Esposito, S. Impact of Nitrogen Nutrition on Cannabis sativa: An Update on the Current Knowledge and Future Prospects. Int. J. Mol. Sci. 2019, 20, 5803. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, C.L.; Fanovich, M.A.; Churio, M.S. Cannabinoids: Extraction Methods, Analysis, and Physicochemical Characterization. Stud. Nat. Prod. Chem. 2019, 61, 143–173. [Google Scholar]
- Available online: https://www.phytopharmaint.com/ (accessed on 1 October 2021).
| Variety | Plant Material | Sowing Density (kg⋅ha−1) | Nitrogen Dose (kg⋅ha−1) | Total CBD (%) | Total Δ9-THC (%) |
|---|---|---|---|---|---|
| Futura 75 | F/10/90 | 10 | 90 | 2.48 b | 0.06 b |
| F/30/30 | 30 | 30 | 2.43 b | 0.05 b | |
| KC Dora | K/30/30 | 30 | 30 | 3.60 a | 0.09 ab |
| K/30/90 | 30 | 90 | 3.63 a | 0.10 ab | |
| Tygra | T/10/30 | 10 | 30 | 2.26 b | 0.12 a |
| T/30/30 | 30 | 30 | 2.58 b | 0.15 a |
| Extraction Method | Variety | Plant Material | Total CBD (%) | Total Δ9-THC (%) |
|---|---|---|---|---|
| Cold water | Futura 75 | F/10/90 | 0.007 ± 0.000 a | 0.000 ± 0.000 |
| F/30/30 | 0.007 ± 0.002 a | 0.000 ± 0.000 | ||
| KC Dora | K/30/30 | 0.009 ± 0.002 a | 0.000 ± 0.000 | |
| K/30/90 | 0.007 ± 0.000 a | 0.000 ± 0.000 | ||
| Tygra | T/10/30 | 0.008 ± 0.001 a | 0.000 ± 0.000 | |
| T/30/30 | 0.005 ± 0.002 a | 0.000 ± 0.000 | ||
| Hot water | Futura 75 | F/10/90 | 0.063 ± 0.006 c | 0.000 ± 0.000 |
| F/30/30 | 0.048 ± 0.004 b | 0.002 ± 0.000 | ||
| KC Dora | K/30/30 | 0.054 ± 0.002 bc | 0.001 ± 0.000 | |
| K/30/90 | 0.051 ± 0.003 b | 0.001 ± 0.000 | ||
| Tygra | T/10/30 | 0.064 ± 0.003 c | 0.002 ± 0.000 | |
| T/30/30 | 0.049 ± 0.001 b | 0.002 ± 0.000 |
| Extraction Method (100 rpm, 72 h) | Variety | Plant Material | Total CBD (%) | Total Δ9-THC (%) |
|---|---|---|---|---|
| 20% EtOH | Futura 75 | F/10/90 | 0.081 ± 0.009 a | 0.000 ± 0.000 a |
| F/30/30 | 0.071 ± 0.005 a | 0.000 ± 0.000 a | ||
| KC Dora | K/30/30 | 0.072 ± 0.005 a | 0.000 ± 0.000 a | |
| K/30/90 | 0.064 ± 0.004 a | 0.000 ± 0.000 a | ||
| Tygra | T/10/30 | 0.070 ± 0.006 a | 0.000 ± 0.000 a | |
| T/30/30 | 0.063 ± 0.011 a | 0.000 ± 0.000 a | ||
| 40% EtOH | Futura 75 | F/10/90 | 0.581 ± 0.093 b | 0.004 ± 0.003 a |
| F/30/30 | 0.616 ± 0.041 b | 0.006 ± 0.002 ab | ||
| KC Dora | K/30/30 | 0.619 ± 0.021 b | 0.001 ± 0.000 a | |
| K/30/90 | 0.581 ± 0.005 b | 0.000 ± 0.000 a | ||
| Tygra | T/10/30 | 0.535 ± 0.037 b | 0.002 ± 0.000 a | |
| T/30/30 | 0.557 ± 0.021 b | 0.003 ± 0.001 a | ||
| 80% EtOH | Futura 75 | F/10/90 | 1.305 ± 0.000 ef | 0.045 ± 0.020 bc |
| F/30/30 | 1.393 ± 0.005 f | 0.048 ± 0.026 c | ||
| KC Dora | K/30/30 | 1.262 ± 0.037 ef | 0.057 ± 0.002 c | |
| K/30/90 | 1.169 ± 0.022 de | 0.026 ± 0.000 abc | ||
| Tygra | T/10/30 | 0.997 ± 0.040 cd | 0.045 ± 0.007 bc | |
| T/30/30 | 0.952 ± 0.160 c | 0.036 ± 0.025 abc |
| Extraction Method (45 Hz, 0.5 h) | Variety | Plant Material | Total CBD (%) | Total Δ9-THC (%) |
|---|---|---|---|---|
| 96% EtOH | Futura 75 | F/10/90 | 2.622 ± 0.141 | 0.039 ± 0.001 |
| F/30/30 | 2.682 ± 0.314 | 0.057 ± 0.011 | ||
| KC Dora | K/30/30 | 2.461 ± 0.009 | 0.029 ± 0.001 | |
| K/30/90 | 2.468 ± 0.598 | 0.022 ± 0.001 | ||
| Tygra | T/10/30 | 1.945 ± 0.137 | 0.045 ± 0.021 | |
| T/30/30 | 2.228 ± 0.246 | 0.035 ± 0.010 |
| Extraction Method | Plant Material | Total CBD (%) | Total Δ9-THC (%) |
|---|---|---|---|
| Cold water | F/10/90 | 0.007 ± 0.000 a | 0.000 ± 0.000 a |
| F/30/30 | 0.007 ± 0.002 a | 0.000 ± 0.000 a | |
| Hot water | F/10/90 | 0.063 ± 0.006 a | 0.000 ± 0.000 a |
| F/30/30 | 0.048 ± 0.004 a | 0.002 ± 0.000 a | |
| 20% EtOH | F/10/90 | 0.081 ± 0.009 a | 0.000 ± 0.000 a |
| F/30/30 | 0.071 ± 0.005 a | 0.000 ± 0.000 a | |
| 40% EtOH | F/10/90 | 0.581 ± 0.093 a | 0.004 ± 0.003 a |
| F/30/30 | 0.616 ± 0.041 a | 0.006 ± 0.002 a | |
| 80% EtOH | F/10/90 | 1.305 ± 0.000 b | 0.045 ± 0.020 b |
| F/30/30 | 1.393 ± 0.005 b | 0.048 ± 0.026 b | |
| 96% EtOH | F/10/90 | 2.622 ± 0.141 c | 0.039 ± 0.001 ab |
| F/30/30 | 2.682 ± 0.314 c | 0.057 ± 0.011 b |
| Extraction Method | Plant Material | Total CBD (%) | Total Δ9-THC (%) |
|---|---|---|---|
| Cold water | K/30/30 | 0.009 ± 0.002 a | 0.000 ± 0.000 a |
| K/30/90 | 0.007 ± 0.000 a | 0.000 ± 0.000 a | |
| Hot water | K/30/30 | 0.054 ± 0.002 a | 0.001 ± 0.000 a |
| K/30/90 | 0.051 ± 0.003 a | 0.001 ± 0.000 a | |
| 20% EtOH | K/30/30 | 0.072 ± 0.005 a | 0.000 ± 0.000 a |
| K/30/90 | 0.064 ± 0.004 a | 0.000 ± 0.000 a | |
| 40% EtOH | K/30/30 | 0.619 ± 0.021 ab | 0.001 ± 0.000 a |
| K/30/90 | 0.581 ± 0.005 ab | 0.000 ± 0.000 a | |
| 80% EtOH | K/30/30 | 1.262 ± 0.037 b | 0.057 ± 0.002 d |
| K/30/90 | 1.169 ± 0.022 b | 0.026 ± 0.000 bc | |
| 96% EtOH | K/30/30 | 2.461 ± 0.009 c | 0.029 ± 0.001 c |
| K/30/90 | 2.468 ± 0.598 c | 0.022 ± 0.001 b |
| Extraction Method | Plant Material | Total CBD (%) | Total Δ9-THC (%) |
|---|---|---|---|
| Cold water | T/10/30 | 0.008 ± 0.001 a | 0.000 ± 0.000 a |
| T/30/30 | 0.005 ± 0.002 a | 0.000 ± 0.000 a | |
| Hot water | T/10/30 | 0.064 ± 0.003 a | 0.002 ± 0.000 a |
| T/30/30 | 0.049 ± 0.001 a | 0.002 ± 0.000 a | |
| 20% EtOH | T/10/30 | 0.070 ± 0.006 a | 0.000 ± 0.000 a |
| T/30/30 | 0.063 ± 0.011 a | 0.000 ± 0.000 a | |
| 40% EtOH | T/10/30 | 0.535 ± 0.037 b | 0.002 ± 0.000 a |
| T/30/30 | 0.557 ± 0.021 b | 0.003 ± 0.001 a | |
| 80% EtOH | T/10/30 | 0.997 ± 0.040 c | 0.045 ± 0.007 b |
| T/30/30 | 0.952 ± 0.160 c | 0.036 ± 0.025 ab | |
| 96% EtOH | T/10/30 | 1.945 ± 0.137 d | 0.045 ± 0.021 b |
| T/30/30 | 2.228 ± 0.246 d | 0.035 ± 0.010 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szalata, M.; Dreger, M.; Zielińska, A.; Banach, J.; Szalata, M.; Wielgus, K. Simple Extraction of Cannabinoids from Female Inflorescences of Hemp (Cannabis sativa L.). Molecules 2022, 27, 5868. https://doi.org/10.3390/molecules27185868
Szalata M, Dreger M, Zielińska A, Banach J, Szalata M, Wielgus K. Simple Extraction of Cannabinoids from Female Inflorescences of Hemp (Cannabis sativa L.). Molecules. 2022; 27(18):5868. https://doi.org/10.3390/molecules27185868
Chicago/Turabian StyleSzalata, Milena, Mariola Dreger, Aleksandra Zielińska, Joanna Banach, Marlena Szalata, and Karolina Wielgus. 2022. "Simple Extraction of Cannabinoids from Female Inflorescences of Hemp (Cannabis sativa L.)" Molecules 27, no. 18: 5868. https://doi.org/10.3390/molecules27185868
APA StyleSzalata, M., Dreger, M., Zielińska, A., Banach, J., Szalata, M., & Wielgus, K. (2022). Simple Extraction of Cannabinoids from Female Inflorescences of Hemp (Cannabis sativa L.). Molecules, 27(18), 5868. https://doi.org/10.3390/molecules27185868






