Comprehensive Analysis of the Structure and Allergenicity Changes of Seafood Allergens Induced by Non-Thermal Processing: A Review
Abstract
:1. Introduction
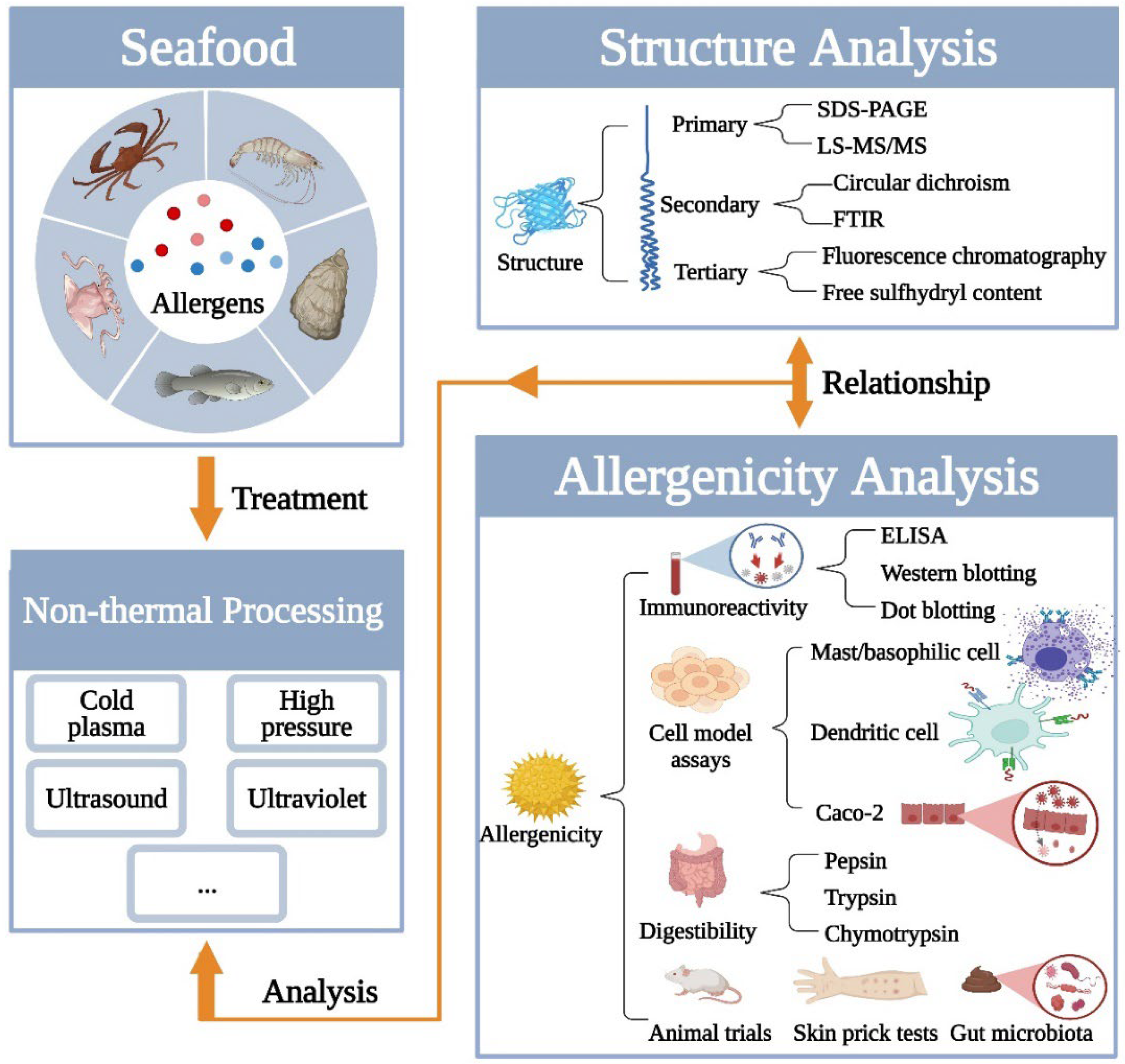
2. Analysis of Structure Changes of Seafood Allergens Induced by Non-Thermal Processing
2.1. Primary Structure Changes
2.2. Secondary Structure Changes
2.3. Tertiary Structure Changes
3. Analysis of Allergenic Changes of Seafood Allergens Induced by Non-Thermal Processing
3.1. IgE-Binding Capacities/Immunoreactivity Analysis
3.1.1. ELISA Analysis
3.1.2. Western Blotting Analysis
3.1.3. Dot Blotting Analysis
3.2. Allergenicity Analysis
3.2.1. Cell Model Assay Analysis
3.2.2. Animal Trials Analysis
3.2.3. Skin Prick Tests (SPT) Analysis
3.3. In Vitro Digestibility Analysis
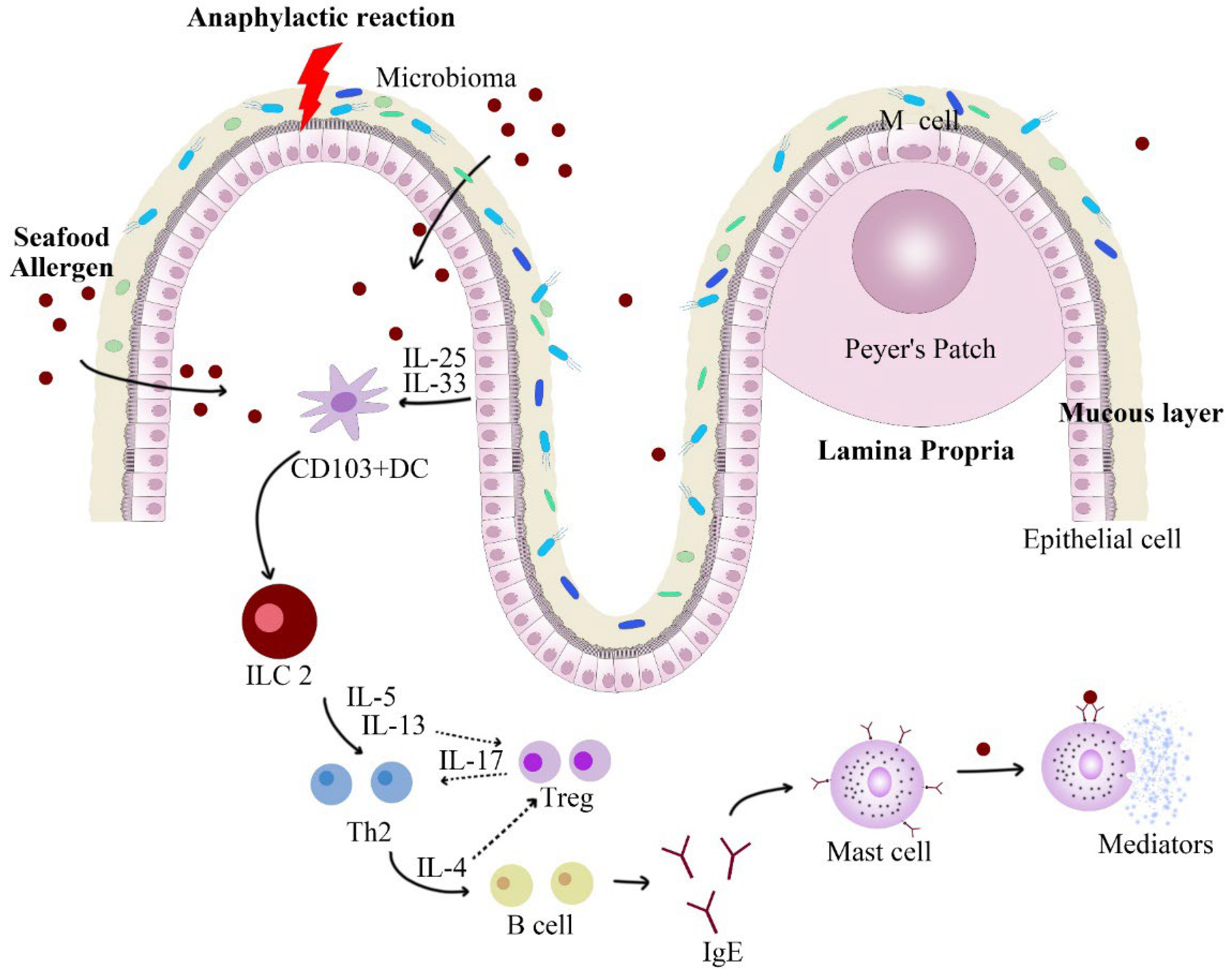
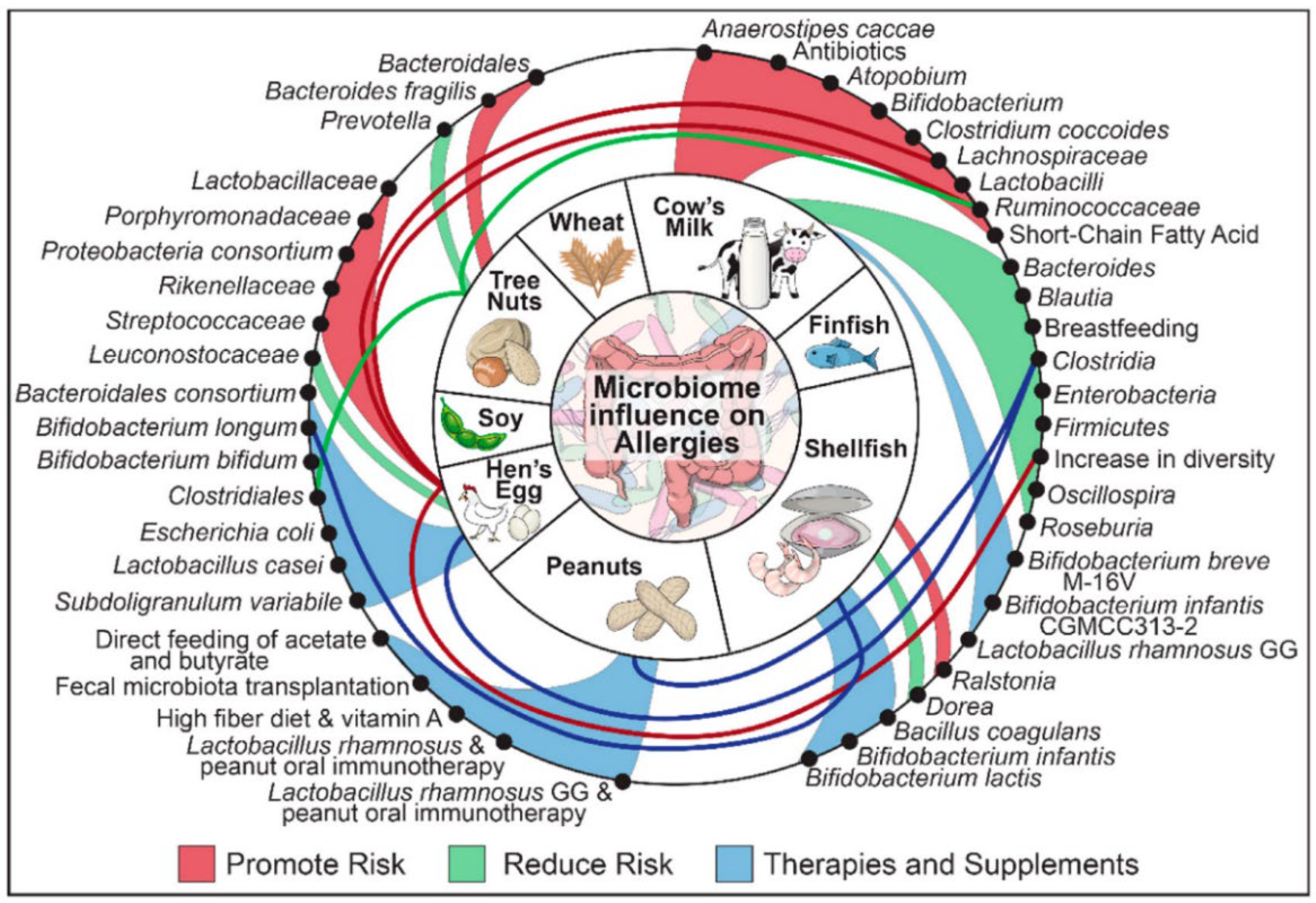
3.4. Gut Microbiota Analysis
4. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruethers, T.; Taki, A.C.; Johnston, E.B.; Nugraha, R.; Le, T.T.K.; Kalic, T.; McLean, T.R.; Kamath, S.D.; Lopata, A.L. Seafood allergy: A comprehensive review of fish and shellfish allergens. Mol. Immunol. 2018, 100, 28–57. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N.; Nagashima, T. Antioxidative activities and angiotensin I-converting enzyme inhibitory activities of enzymatic hydrolysates from commercial kamaboko type samples. Food Sci. Technol. Int. 2006, 12, 335–346. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.H.; Chen, S.J. Comparison of active non-volatile taste components in the viscera and adductor muscles of oyster (Ostrea rivularis Gould). Food Sci. Technol. Res. 2013, 19, 417–424. [Google Scholar] [CrossRef]
- Zheng, L.F.; Yu, H.C.; Wei, H.K.; Xing, Q.; Zou, Y.; Zhou, Y.F.; Peng, J. Antioxidative peptides of hydrolysate prepared from fish skin gelatin using ginger protease activate antioxidant response element-mediated gene transcription in IPEC-J2 cells. J. Funct. Foods 2018, 51, 104–112. [Google Scholar] [CrossRef]
- Burks, A.W.; Tang, M.; Sicherer, S.; Muraro, A.; Eigenmann, P.A.; Ebisawa, M.; Fiocchi, A.; Chiang, W.; Beyer, K.; Wood, R.; et al. Icon: Food allergy. J. Allergy Clin. Immunol. 2012, 129, 906–920. [Google Scholar] [CrossRef]
- Ochfeld, E.N.; Pongracic, J.A. Food allergy: Diagnosis and treatment. Allergy Asthma Proc. 2019, 40, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, J.; Selvasekaran, P.; Lokanadham, M.; Chidambaram, R. Food and food products associated with food allergy and food intolerance An overview. Food Res. Int. 2020, 138, 109780. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Cheng, J.H. DNA, protein and aptamer-based methods for seafood allergens detection: Principles, comparisons and updated applications. Crit. Rev. Food Sci. Nutr. 2021, 1–14. [Google Scholar] [CrossRef]
- Shek, L.P.C.; Cabrera-Morales, E.A.; Soh, S.E.; Gerez, I.; Ng, P.Z.; Yi, F.C.; Ma, S.; Lee, B.W. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J. Allergy Clin. Immunol. 2010, 126, 324–331.E7. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Zhao, Y. Structure-based modelling of hemocyanin allergenicity in squid and its response to high hydrostatic pressure. Sci. Rep. 2017, 7, 40021. [Google Scholar] [CrossRef] [PubMed]
- Sena-Torralba, A.; Pallas-Tamarit, Y.; Morais, S.; Maquieira, A. Recent advances and challenges in food-borne allergen detection. Trac, Trends Anal. Chem. 2020, 132, 116050. [Google Scholar] [CrossRef]
- Costa, J.; Villa, C.; Verhoeckx, K.; Cirkovic-Velickovic, T.; Schrama, D.; Roncada, P.; Rodrigues, P.M.; Piras, C.; Martin-Pedraza, L.; Monaci, L.; et al. Are Physicochemical Properties Shaping the Allergenic Potency of Animal Allergens? Clin. Rev. Allergy Immunol. 2022, 62, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.D.; Rahman, A.M.A.; Komoda, T.; Lopata, A.L. Impact of heat processing on the detection of the major shellfish allergen tropomyosin in crustaceans and molluscs using specific monoclonal antibodies. Food Chem. 2013, 141, 4031–4039. [Google Scholar] [CrossRef]
- Cuadrado, C.; Cabanillas, B.; Pedrosa, M.M.; Varela, A.; Guillamon, E.; Muzquiz, M.; Crespo, J.F.; Rodriguez, J.; Burbano, C. Influence of thermal processing on IgE reactivity to lentil and chickpea proteins. Mol. Nutr. Food Res. 2009, 53, 1462–1468. [Google Scholar] [CrossRef]
- Lasekan, A.O.; Nayak, B. Effects of buffer additives and thermal processing methods on the solubility of shrimp (Penaeus monodon) proteins and the immunoreactivity of its major allergen. Food Chem. 2016, 200, 146–153. [Google Scholar] [CrossRef]
- Nakamura, A.; Watanabe, K.; Ojima, T.; Ahn, D.H.; Saeki, H. Effect of Maillard reaction on allergenicity of scallop tropomyosin. J. Agric. Food Chem. 2005, 53, 7559–7564. [Google Scholar] [CrossRef]
- Fu, L.; Wang, C.; Wang, J.; Ni, S.; Wang, Y. Maillard reaction with ribose, galacto-oligosaccharide or chitosan-oligosaccharide reduced the allergenicity of shrimp tropomyosin by inducing conformational changes. Food Chem. 2019, 274, 789–795. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Cheng, J.H.; Sun, D.W. Effects of nonthermal food processing technologies on food allergens: A review of recent research advances. Trends Food Sci. Technol. 2018, 74, 12–25. [Google Scholar] [CrossRef]
- Dasanayaka, B.P.; Li, Z.; Pramod, S.N.; Chen, Y.; Khan, M.U.; Lin, H. A review on food processing and preparation methods for altering fish allergenicity. Crit. Rev. Food Sci. Nutr. 2020, 62, 1951–1970. [Google Scholar] [CrossRef]
- Prieto, N.; Burbano, C.; Iniesto, E.; Rodriguez, J.; Cabanillas, B.; Crespo, J.F.; Pedrosa, M.M.; Muzquiz, M.; Del Pozo, J.C.; Linacero, R.; et al. A novel proteomic analysis of the modifications induced by high hydrostatic pressure on hazelnut water-soluble proteins. Foods 2014, 3, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Maleki, S.J.; Rodriguez, J.; Cheng, H.; Teuber, S.S.; Wallowitz, M.L.; Muzquiz, M.; Pedrosa, M.M.; Linacero, R.; Burbano, C.; et al. Allergenic properties and differential response of walnut subjected to processing treatments. Food Chem. 2014, 157, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Bai, Y.; Gao, J.; Li, X.; Chen, H. Effects of high hydrostatic pressure on the structure and potential allergenicity of the major allergen bovine beta-lactoglobulin. Food Chem. 2017, 219, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Reineke, K.; Schlüter, O.; Schweiggert-Weisz, U.; Eisner, P. The effects of pulsed ultraviolet light, cold atmospheric pressure plasma, and gamma-irradiation on the immunoreactivity of soy protein isolate. Innov. Food Sci. Emerg. Technol. 2016, 38, 374–383. [Google Scholar] [CrossRef]
- Tammineedi, C.V.R.K.; Choudhary, R.; Perez-Alvarado, G.C.; Watson, D.G. Determining the effect of UV-C, high intensity ultrasound and nonthermal atmospheric plasma treatments on reducing the allergenicity of α-casein and whey proteins. LWT-Food Sci. Technol. 2013, 54, 35–41. [Google Scholar] [CrossRef]
- Nooji, J.K. Reduction of Wheat Allergen Potency by Pulsed Ultraviolet Light, High Hydrostatic Pressure, and Non-Thermal Plasma. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2011. [Google Scholar]
- Jin, Y.; Deng, Y.; Qian, B.; Zhang, Y.; Liu, Z.; Zhao, Y. Allergenic response to squid (Todarodes pacificus) tropomyosin Tod p1 structure modifications induced by high hydrostatic pressure. Food Chem. Toxicol. 2015, 76, 86–93. [Google Scholar] [CrossRef]
- Ekezie, F.C.; Sun, D.W.; Cheng, J.H. Altering the IgE binding capacity of king prawn (Litopenaeus vannamei) tropomyosin through conformational changes induced by cold argon-plasma jet. Food Chem. 2019, 300, 125143. [Google Scholar] [CrossRef]
- Shriver, S.; Yang, W.; Chung, S.Y.; Percival, S. Pulsed ultraviolet light reduces immunoglobulin E binding to Atlantic white shrimp (Litopenaeus setiferus) extract. Int. J. Environ. Res. Public Health 2011, 8, 2569–2583. [Google Scholar] [CrossRef]
- Zhou, A.; Lin, L.; Liang, Y.; Benjakul, S.; Shi, X.; Liu, X. Physicochemical properties of natural actomyosin from threadfin bream (Nemipterus spp.) induced by high hydrostatic pressure. Food Chem. 2014, 156, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Ekezie, F.G.; Cheng, J.H.; Sun, D.W. Effects of mild oxidative and structural modifications induced by argon plasma on physicochemical properties of actomyosin from king prawn (Litopenaeus vannamei). J. Agric. Food Chem. 2018, 66, 13285–13294. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, Z.X.; Lin, H.; Samee, H. Effect of power ultrasound on the immunoactivity and texture changes of shrimp (Penaeus vannamei). Czech J. Food Sci. 2011, 29, 508–514. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, B.; Deng, Y.; Zhao, Y. In vitro anti-inflammatory and antioxidant activities and protein quality of high hydrostatic pressure treated squids (Todarodes pacificus). Food Chem. 2016, 203, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Kulawik, P.; Ozogul, Y.; Ozogul, F.; Bekhit, A.E.A. Recent developments in non-thermal processing for seafood and seafood products: Cold plasma, pulsed electric field and high hydrostatic pressure. Int. J. Food Sci. Technol. 2021, 57, 774–790. [Google Scholar] [CrossRef]
- Grigera, J.R.; McCarthy, A.N. The behavior of the hydrophobic effect under pressure and protein denaturation. Biophys. J. 2010, 98, 1626–1631. [Google Scholar] [CrossRef]
- De Oliveira, F.A.; Neto, O.C.; doc Santos, L.M.R.; Ferreira, E.H.R.; Rosenthal, A. Effect of high pressure on fish meat quality—A review. Trends Food Sci. Technol. 2017, 66, 1–19. [Google Scholar] [CrossRef]
- Kurpiewska, K.; Biela, A.; Loch, J.I.; Lipowska, J.; Siuda, M.; Lewinski, K. Towards understanding the effect of high pressure on food protein allergenicity: Beta-lactoglobulin structural studies. Food Chem. 2019, 270, 315–321. [Google Scholar] [CrossRef]
- Sathe, S.K.; Liu, C.Q.; Zaffran, V.D. Food Allergy. Annu. Rev. Food Sci. Technol. 2016, 7, 191–220. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Chen, W.; Zhou, P. Conformation stability, in vitro digestibility and allergenicity of tropomyosin from shrimp (Exopalaemon modestus) as affected by high intensity ultrasound. Food Chem. 2018, 245, 997–1009. [Google Scholar] [CrossRef]
- Cianci, M. Introduction to proteins, introduction to proteins: Structure, function, and motion, 2nd edition. Crystallogr. Rev. 2020, 27, 47–50. [Google Scholar] [CrossRef]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Zhou, F.L.; He, S.D.; Sun, H.J.; Wang, Y.F.; Zhang, Y. Advances in epitope mapping technologies for food protein allergens: A review. Trends Food Sci. Technol. 2021, 107, 226–239. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Bi, Y.; Wang, Q.; Cheng, K.-W.; Chen, F. Review: Seafood allergy and potential application of high hydrostatic pressure to reduce seafood allergenicity. Int. J. Food Eng. 2019, 15, 20180392. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, Y.; Wang, Q.; Cheng, K.W.; Chen, F. Application of high pressure processing to improve digestibility, reduce allergenicity, and avoid protein oxidation in cod (Gadus morhua). Food Chem. 2019, 298, 125087. [Google Scholar] [CrossRef]
- Zhang, H.E.; Liao, H.Q.; Lu, Y.B.; Hu, Y.H.; Yang, H.; Cao, S.Q.; Qi, X.Y. Effects of high hydrostatic pressure on the structural characteristics of parvalbumin of cultured large yellow croaker (Larimichthys crocea). J. Food Process. Preserv. 2020, 44, e14911. [Google Scholar] [CrossRef]
- Liu, R.; Xue, W.T. High-pressure treatment with silver carp (Hypophthalmichthys molitrix) protein and its allergic analysis. High Press. Res. 2010, 30, 438–442. [Google Scholar] [CrossRef]
- Long, F.Y.; Yang, X.; Wang, R.R.; Hu, X.S.; Chen, F. Effects of combined high pressure and thermal treatments on the allergenic potential of shrimp (Litopenaeus vannamei) tropomyosin in a mouse model of allergy. Innov. Food Sci. Emerg. Technol. 2015, 29, 119–124. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Effects of high-intensity ultrasound processing on the physiochemical and allergenic properties of shrimp. Innov. Food Sci. Emerg. Technol. 2020, 65, 102441. [Google Scholar] [CrossRef]
- Li, Z.X.; Lin, H.; Cao, L.M.; Jameel, K. Effect of high intensity ultrasound on the allergenicity of shrimp. J. Zhejiang Univ. Sci. B 2006, 7, 251–256. [Google Scholar] [CrossRef]
- Ma, W.; Wang, J.; Xu, X.; Qin, L.; Wu, C.; Du, M. Ultrasound treatment improved the physicochemical characteristics of cod protein and enhanced the stability of oil-in-water emulsion. Food Res. Int. 2019, 121, 247–256. [Google Scholar] [CrossRef]
- Yang, W.W.; Shriver, S.K.; Chung, S.Y.; Percival, S.; Correll, M.J.; Rababah, T.M. In vitro gastric and intestinal digestions of pulsed light-treated shrimp extracts. Appl. Biochem. Biotechnol. 2012, 166, 1409–1422. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Critical reviews and recent advances of novel non-thermal processing techniques on the modification of food allergens. Crit. Rev. Food Sci. Nutr. 2021, 61, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikawa, S.; Yoshizawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J. Phys. D Appl. Phys. 2014, 47, 285403. [Google Scholar] [CrossRef]
- Liu, Z.W.; Liu, L.J.; Zhou, Y.X.; Tan, Y.C.; Cheng, J.H.; Bekhit, A.E.; Inam-Ur-Raheem, M.; Aadil, R.M. Dielectric-barrier discharge (DBD) plasma treatment reduces IgG binding capacity of beta-lactoglobulin by inducing structural changes. Food Chem. 2021, 358, 129821. [Google Scholar] [CrossRef]
- Faisal, M.; Vasiljevic, T.; Donkor, O.N. A review on methodologies for extraction, identification and quantification of allergenic proteins in prawns. Food Res. Int. 2019, 121, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Shriver, S.K.; Yang, W.W. Thermal and nonthermal methods for food allergen control. Food Eng. Rev. 2011, 3, 26–43. [Google Scholar] [CrossRef]
- Sharafodin, H.; Soltanizadeh, N. Potential application of DBD plasma technique for modifying structural and physicochemical properties of Soy Protein Isolate. Food Hydrocoll. 2022, 122, 107077. [Google Scholar] [CrossRef]
- Lv, L.; Lin, H.; Li, Z.; Wang, J.; Ahmed, I.; Chen, H. Changes of structure and IgE binding capacity of shrimp (Metapenaeus ensis) tropomyosin followed by acrolein treatment. Food Funct. 2017, 8, 1028–1036. [Google Scholar] [CrossRef]
- Vanga, S.K.; Singh, A.; Kalkan, F.; Gariepy, Y.; Orsat, V.; Raghavan, V. Effect of thermal and high electric fields on secondary structure of peanut protein. Int. J. Food Prop. 2015, 19, 1259–1271. [Google Scholar] [CrossRef]
- Tong, P.; Gao, J.Y.; Chen, H.B.; Li, X.; Zhang, Y.; Jian, S.; Wichers, H.; Wu, Z.H.; Yang, A.S.; Liu, F.H. Effect of heat treatment on the potential allergenicity and conformational structure of egg allergen ovotransferrin. Food Chem. 2012, 131, 603–610. [Google Scholar] [CrossRef]
- Bußler, S.; Steins, V.; Ehlbeck, J.; Schlüter, O. Impact of thermal treatment versus cold atmospheric plasma processing on the techno-functional protein properties from Pisum sativum ‘Salamanca’. J. Food Eng. 2015, 167, 166–174. [Google Scholar] [CrossRef]
- Han, X.Y.; Yang, H.; Rao, S.T.; Liu, G.Y.; Hu, M.J.; Zeng, B.C.; Cao, M.J.; Liu, G.M. The maillard reaction reduced the sensitization of tropomyosin and arginine kinase from Scylla paramamosain, simultaneously. J. Agric. Food Chem. 2018, 66, 2934–2943. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Q.; Chen, H.B.; Gao, J.Y.; Luo, C.P.; Ma, X.J.; Tong, P. High-pressure microfluidisation-induced changes in the antigenicity and conformation of allergen Ara h 2 purified from Chinese peanut. J. Sci. Food Agric. 2011, 91, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Hazebrouck, S.; Guillon, B.; Drumare, M.F.; Paty, E.; Wal, J.M.; Bernard, H. Trypsin resistance of the major peanut allergen Ara h 6 and allergenicity of the digestion products are abolished after selective disruption of disulfide bonds. Mol. Nutr. Food Res. 2012, 56, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zheng, Y.; Liu, Z.; Deng, Y.; Zhao, Y. Structure and IgE-binding properties of alpha-casein treated by high hydrostatic pressure, UV-C, and far-IR radiations. Food Chem. 2016, 204, 46–55. [Google Scholar] [CrossRef]
- Shimakura, K.; Tonomura, Y.; Hamada, Y.; Nagashima, Y.; Shiomi, K. Allergenicity of crustacean extractives and its reduction by protease digestion. Food Chem. 2005, 91, 247–253. [Google Scholar] [CrossRef]
- Lv, L.T.; Lin, H.; Li, Z.X.; Ahmed, I.; Chen, G.Z. Determining the effect of malondialdehyde on the IgE-binding capacity of shrimp tropomyosin upon in vitro digestion. J. Sci. Food Agric. 2017, 97, 4588–4594. [Google Scholar] [CrossRef]
- Shriver, S.K. Effect of Selected Nonthermal Processing Methods on the Allergen Reactivity of Altantic White Shrimp (Litopenaeus setiferus). Master’s Thesis, University of Florida, Gainesville, FL, USA, 2011. [Google Scholar]
- Lee, J.W.; Kim, J.H.; Yook, H.S.; Kang, K.O.; Lee, S.Y.; Hwang, H.J.; Byun, M.W. Effects of gamma radiation on the allergenic and antigenic properties of milk proteins. J. Food Prot. 2001, 64, 272–276. [Google Scholar] [CrossRef]
- Wu, Y.T.; Lin, H.; Lu, Y.Y.; Huang, Y.H.; Dasanayaka, B.P.; Ahmed, I.; Chen, G.Z.; Chen, Y.; Li, Z.X. Allergenicity determination of Turbot parvalbumin for safety of fish allergy via dendritic cells, RBL-2H3 cell and mouse model. Eur. Food Res. Technol. 2021, 247, 1959–1974. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, H.; Zhou, P. Allergenicity suppression of tropomyosin from Exopalaemon modestus by glycation with saccharides of different molecular sizes. Food Chem. 2019, 288, 268–275. [Google Scholar] [CrossRef]
- Teodorowicz, M.; Fiedorowicz, E.; Kostyra, H.; Wichers, H.; Kostyra, E. Effect of Maillard reaction on biochemical properties of peanut 7S globulin (Ara h 1) and its interaction with a human colon cancer cell line (Caco-2). Eur. J. Nutr. 2013, 52, 1927–1938. [Google Scholar] [CrossRef] [Green Version]
- Loschko, J.; Schreiber, H.A.; Rieke, G.J.; Esterhazy, D.; Meredith, M.M.; Pedicord, V.A.; Yao, K.H.; Caballero, S.; Pamer, E.G.; Mucida, D.; et al. Absence of MHC class II on cDCs results in microbial-dependent intestinal inflammation. J. Exp. Med. 2016, 213, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.M.; Yang, Y.; Maleki, S.J.; Alcocer, M.; Xu, S.S.; Shi, C.L.; Cao, M.J.; Liu, G.M. Anti-Food allergic activity of sulfated polysaccharide from gracilaria lemaneiformis is dependent on immunosuppression and inhibition of p38 MAPK. J. Agric. Food Chem. 2016, 64, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Li, Z.X.; Wu, Y.T.; Guo, Y.M.; Pavase, T.R.; Chen, G.Z.; Zhang, Z.Y.; Lin, H. Comparison of immunological properties of recombinant and natural turbot (Scophthalmus maximus) parvalbumin. Eur. Food Res. Technol. 2021, 247, 2053–2065. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Chen, G.; Ahmed, I.; Sun, L.; Li, W.; Pavase, T.R.; Li, Z. Immunostimulatory and allergenic properties of emulsified and non-emulsified digestion products of parvalbumin (Scophthalmus maximus) in RBL-2H3 cells and BALB/c mouse models. Food Funct. 2021, 12, 5351–5360. [Google Scholar] [CrossRef] [PubMed]
- Stagg, A.J. Intestinal dendritic cells in health and gut inflammation. Front. Immunol. 2018, 9, 2883. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology 2008, 123, 326–338. [Google Scholar] [CrossRef]
- Summers, K.L.; Hock, B.D.; McKenzie, J.L.; Hart, D.N.J. Phenotypic characterization of five dendritic cell subsets in human tonsils. Am. J. Pathol. 2001, 159, 285–295. [Google Scholar] [CrossRef]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Fu, L.; Song, J.; Wang, C.; Fu, S.; Wang, Y. Bifidobacterium infantis potentially alleviates shrimp tropomyosin-induced allergy by tolerogenic dendritic cell-dependent induction of regulatory T Cells and alterations in gut microbiota. Front. Immunol. 2017, 8, 1536. [Google Scholar] [CrossRef]
- Ding, L.; Wang, L.; Yu, Z.; Zhang, T.; Liu, J. Digestion and absorption of an egg white ACE-inhibitory peptide in human intestinal Caco-2 cell monolayers. Int. J. Food Sci. Nutr. 2016, 67, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huan, F.; Li, M.; Han, T.; Xia, F.; Yang, Y.; Liu, Q.; Chen, G.; Cao, M.; Liu, G. Mapping and IgE-binding capacity analysis of heat/digested stable epitopes of mud crab allergens. Food Chem. 2021, 344, 128735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, H.; Zhang, X.; Zhou, P. Conformation, allergenicity and human cell allergy sensitization of tropomyosin from Exopalaemon modestus: Effects of deglycosylation and Maillard reaction. Food Chem. 2019, 276, 520–527. [Google Scholar] [CrossRef]
- Jimenez-Saiz, R.; Chu, D.K.; Mandur, T.S.; Walker, T.D.; Gordon, M.E.; Chaudhary, R.; Koenig, J.; Saliba, S.; Galipeau, H.J.; Utley, A.; et al. Lifelong memory responses perpetuate humoral Th2 immunity and anaphylaxis in food allergy. J. Allergy Clin. Immunol. 2017, 140, 1604–1615.e1605. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Fu, S.; Wang, C.; Xie, M.; Wang, Y. Yogurt-sourced probiotic bacteria alleviate shrimp tropomyosin-induced allergic mucosal disorders, potentially through microbiota and metabolism modifications. Allergol. Int. 2019, 68, 506–514. [Google Scholar] [CrossRef]
- Lam, Y.F.; Tong, K.K.; Kwan, K.M.; Tsuneyama, K.; Shu, S.A.; Leung, P.S.; Chu, K.H. Gastrointestinal immune response to the shrimp allergen tropomyosin: Histological and immunological analysis in an animal model of shrimp tropomyosin hypersensitivity. Int. Arch. Allergy Immunol. 2015, 167, 29–40. [Google Scholar] [CrossRef]
- Chokshi, N.Y.; Sicherer, S.H. Interpreting IgE sensitization tests in food allergy. Expert Rev. Clin. Immunol. 2016, 12, 389–403. [Google Scholar] [CrossRef]
- Fornadley, J.A. Skin testing for inhalant allergy. Int. Forum Allergy Rhinol. 2014, 4, S41–S45. [Google Scholar] [CrossRef]
- Cabanillas, B.; Cuadrado, C.; Rodriguez, J.; Dieguez, M.C.; Crespo, J.F.; Novak, N. Boiling and pressure cooking impact on IgE reactivity of soybean allergens. Int. Arch. Allergy Immunol. 2018, 175, 36–43. [Google Scholar] [CrossRef]
- Lavilla, M.; Orcajo, J.; Diaz-Perales, A.; Gamboa, P. Examining the effect of High Pressure Processing on the allergenic potential of the major allergen in peach (Pru p 3). Innov. Food Sci. Emerg. Technol. 2016, 38, 334–341. [Google Scholar] [CrossRef]
- Gamez, C.; Zafra, M.P.; Sanz, V.; Mazzeo, C.; Ibanez, M.D.; Sastre, J.; del Pozo, V. Simulated gastrointestinal digestion reduces the allergic reactivity of shrimp extract proteins and tropomyosin. Food Chem. 2015, 173, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Lin, H.; Li, Z.; Nayak, B.; Ahmed, I.; Tian, S.; Chen, G.; Lin, H.; Zhao, J. Structural changes of 2,2’-azobis(2-amidinopropane) dihydrochloride (AAPH) treated shrimp tropomyosin decrease allergenicity. Food Chem. 2019, 274, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Perera, E.; Moyano, F.J.; Rodriguez-Viera, L.; Cervantes, A.; Martinez-Rodriguez, G.; Mancera, J.M. In vitro digestion of protein sources by crude enzyme extracts of the spiny lobster Panulirus argus (Latreille, 1804) hepatopancreas with different trypsin isoenzyme patterns. Aquaculture 2010, 310, 178–185. [Google Scholar] [CrossRef]
- Liu, G.M.; Cao, M.J.; Huang, Y.Y.; Cai, Q.F.; Weng, W.Y.; Su, W.J. Comparative study of in vitro digestibility of major allergen tropomyosin and other food proteins of Chinese mitten crab (Eriocheir sinensis). J. Sci. Food Agric. 2010, 90, 1614–1620. [Google Scholar] [CrossRef]
- FAO; WHO. Evaluation of Allergenicity of Genetically Modified Foods; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001; pp. 1–26. [Google Scholar]
- Huang, Y.Y.; Liu, G.M.; Cai, Q.F.; Weng, W.Y.; Maleki, S.J.; Su, W.J.; Cao, M.J. Stability of major allergen tropomyosin and other food proteins of mud crab (Scylla serrata) by in vitro gastrointestinal digestion. Food Chem. Toxicol. 2010, 48, 1196–1201. [Google Scholar] [CrossRef]
- Liu, G.M.; Huang, Y.Y.; Cai, Q.F.; Weng, W.Y.; Su, W.J.; Cao, M.J. Comparative study of in vitro digestibility of major allergen, tropomyosin and other proteins between Grass prawn (Penaeus monodon) and Pacific white shrimp (Litopenaeus vannamei). J. Sci. Food Agric. 2011, 91, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.J.; Abbott, U.R.; Hatzos, C. Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid-a comparative study. J. Agric. Food Chem. 2002, 50, 7154–7160. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Sawai, T.; Sawai, C.; Nakatani, M.; Romero-Perez, G.A.; Ozeki, M.; Nonomura, K.; Tsukahara, T. A preliminary study of gut dysbiosis in children with food allergy. Biosci. Biotechnol. Biochem. 2017, 81, 2396–2399. [Google Scholar] [CrossRef]
- Cianci, R.; Pagliari, D.; Piccirillo, C.A.; Fritz, J.H.; Gambassi, G. The microbiota and immune system crosstalk in health and disease. Mediat. Inflamm. 2018, 2018, 2912539. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307.e295. [Google Scholar] [CrossRef]
- Aitoro, R.; Paparo, L.; Amoroso, A.; Di Costanzo, M.; Cosenza, L.; Granata, V.; Di Scala, C.; Nocerino, R.; Trinchese, G.; Montella, M.; et al. Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients 2017, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Malago, J.J. Contribution of microbiota to the intestinal physicochemical barrier. Benef. Microbes 2015, 6, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef] [PubMed]
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M.; et al. The microbiota regulates type 2 immunity through ROR gamma t(+) T cells. Science 2015, 349, 989–993. [Google Scholar] [CrossRef]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hamalainen, A.M.; et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Feehley, T.; Plunkett, C.H.; Bao, R.; Hong, S.M.C.; Culleen, E.; Belda-Ferre, P.; Campbell, E.; Aitoro, R.; Nocerino, R.; Paparo, L.; et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat. Med. 2019, 25, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Yoon, W.; Lee, S.Y.; Shin, H.S.; Lim, M.Y.; Nam, Y.D.; Yoo, Y. Effects of lactobacillus pentosus in children with allergen-sensitized atopic dermatitis. J. Korean Med. Sci. 2020, 35, e128. [Google Scholar] [CrossRef]
- Muir, A.B.; Benitez, A.J.; Dods, K.; Spergel, J.M.; Fillon, S.A. Microbiome and its impact on gastrointestinal atopy. Allergy 2016, 71, 1256–1263. [Google Scholar] [CrossRef]
- Suther, C.; Moore, M.D.; Beigelman, A.; Zhou, Y. The gut microbiome and the big eight. Nutrients 2020, 12, 3728. [Google Scholar] [CrossRef]

| Non-Thermal Techniques | Target Allergen | Food Material | Treatment Conditions | Evaluations | Results | IgE-Binding Capacities/Immunoreactivity | References |
|---|---|---|---|---|---|---|---|
| High pressure processing (HPP) | Tropomyosin | Squid | 400 MPa, 20 °C, 10 min | SDS-PAGE | No changes | Decrease | [27] |
| CD | α-helix decrease, β-sheet increase | ||||||
| FSC | Decrease | ||||||
| SH | Increase | ||||||
| Hemocyanin | Squid | 200–600 MPa, 25 °C, 20 min | SDS-PAGE | Band intensities decrease | Decrease | [11] | |
| FTIR | α-helix decrease, β-sheet increase | ||||||
| FSC | Decrease | ||||||
| SH | Increase | ||||||
| SAXS | Rg decreased, 〈Nagg〉G and 〈Nagg〉Q increased | ||||||
| NS | Squid | 200–600 MPa, 20 min | SDS-PAGE | Band intensities decrease | NS | [33] | |
| NS | Fish | 200 MPa, 25 °C, 20 min | SDS-PAGE | New bands at 95 and 26 kDa | Decrease | [44] | |
| FSC | Decrease | ||||||
| SH | Increase | ||||||
| Parvalbumin | Fish | 300–600 MPa, 10 min | CD | α-helix decrease | NS | [45] | |
| FSC | Decrease | ||||||
| SH | Increase | ||||||
| Laser Raman spectroscopy | α-helix decrease, β-sheet increase | ||||||
| Actomyosin | Fish | 200–600 MPa, 10–50 min | SDS-PAGE | Band intensities decrease | NS | [30] | |
| FSC | Decrease | ||||||
| SH | Increase | ||||||
| NS | Fish | 100–300 MPa, 20 °C, 10–60 min | SDS-PAGE | No changes | No changes | [46] | |
| CD | Structure changes | ||||||
| Tropomyosin | Shrimp | 100–600MPa, 0–30 min | / | / | Decrease | [47] | |
| Cold plasma (CP) | Tropomyosin | Shrimp | 3–15 min | SDS-PAGE | No changes with DTT, slightly fade without DTT | Decrease | [28] |
| CD | α-helix decrease, β-sheet increase | ||||||
| FSC | Decrease | ||||||
| SH | Increase | ||||||
| Actomyosin | Shrimp | 1–5 min | SDS-PAGE | No changes | NS | [31] | |
| CD | α-helix decrease, β-turns increase | ||||||
| FSC | Decrease | ||||||
| SH | Increase | ||||||
| Ultrasound | Total proteins | Shrimp | 400 W, 20 min | SDS-PAGE | Band intensities decrease | Decrease | [48] |
| FTIR | α-helix increase, β-sheet increase, β-turns decrease | ||||||
| Tropomyosin | Shrimp | 800 W, 30–180 min | SDS-PAGE | Band intensities decrease | Decrease | [49] | |
| Tropomyosin | Shrimp | 100–800 W, 15 min | SDS-PAGE | Band intensities decrease | Decrease | [39] | |
| CD | α-helix decrease, β-sheet and β-turns increase | ||||||
| FSC | Decrease | ||||||
| SH | Increase | ||||||
| NS | Fish | 200–950 W, 60 min | SDS-PAGE | No changes | NS | [50] | |
| FSC | Decrease | ||||||
| SH | Increase | ||||||
| FS | Blue shifts | ||||||
| Ultraviolet (UV) | Tropomyosin | Shrimp | 4 min | SDS-PAGE | Band intensities decrease | Decrease | [51] |
| Tropomyosin | Shrimp | 0–6 min | SDS-PAGE | Band intensities decrease | Decrease | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhong, H.; Cheng, J.-H. Comprehensive Analysis of the Structure and Allergenicity Changes of Seafood Allergens Induced by Non-Thermal Processing: A Review. Molecules 2022, 27, 5857. https://doi.org/10.3390/molecules27185857
Wang F, Zhong H, Cheng J-H. Comprehensive Analysis of the Structure and Allergenicity Changes of Seafood Allergens Induced by Non-Thermal Processing: A Review. Molecules. 2022; 27(18):5857. https://doi.org/10.3390/molecules27185857
Chicago/Turabian StyleWang, Fengqi, Hangyu Zhong, and Jun-Hu Cheng. 2022. "Comprehensive Analysis of the Structure and Allergenicity Changes of Seafood Allergens Induced by Non-Thermal Processing: A Review" Molecules 27, no. 18: 5857. https://doi.org/10.3390/molecules27185857
APA StyleWang, F., Zhong, H., & Cheng, J.-H. (2022). Comprehensive Analysis of the Structure and Allergenicity Changes of Seafood Allergens Induced by Non-Thermal Processing: A Review. Molecules, 27(18), 5857. https://doi.org/10.3390/molecules27185857







