Controllable Construction of Amino-Functionalized Dynamic Covalent Porous Polymers for High-Efficiency CO2 Capture from Flue Gas
Abstract
:1. Introduction
2. Results and Discussion
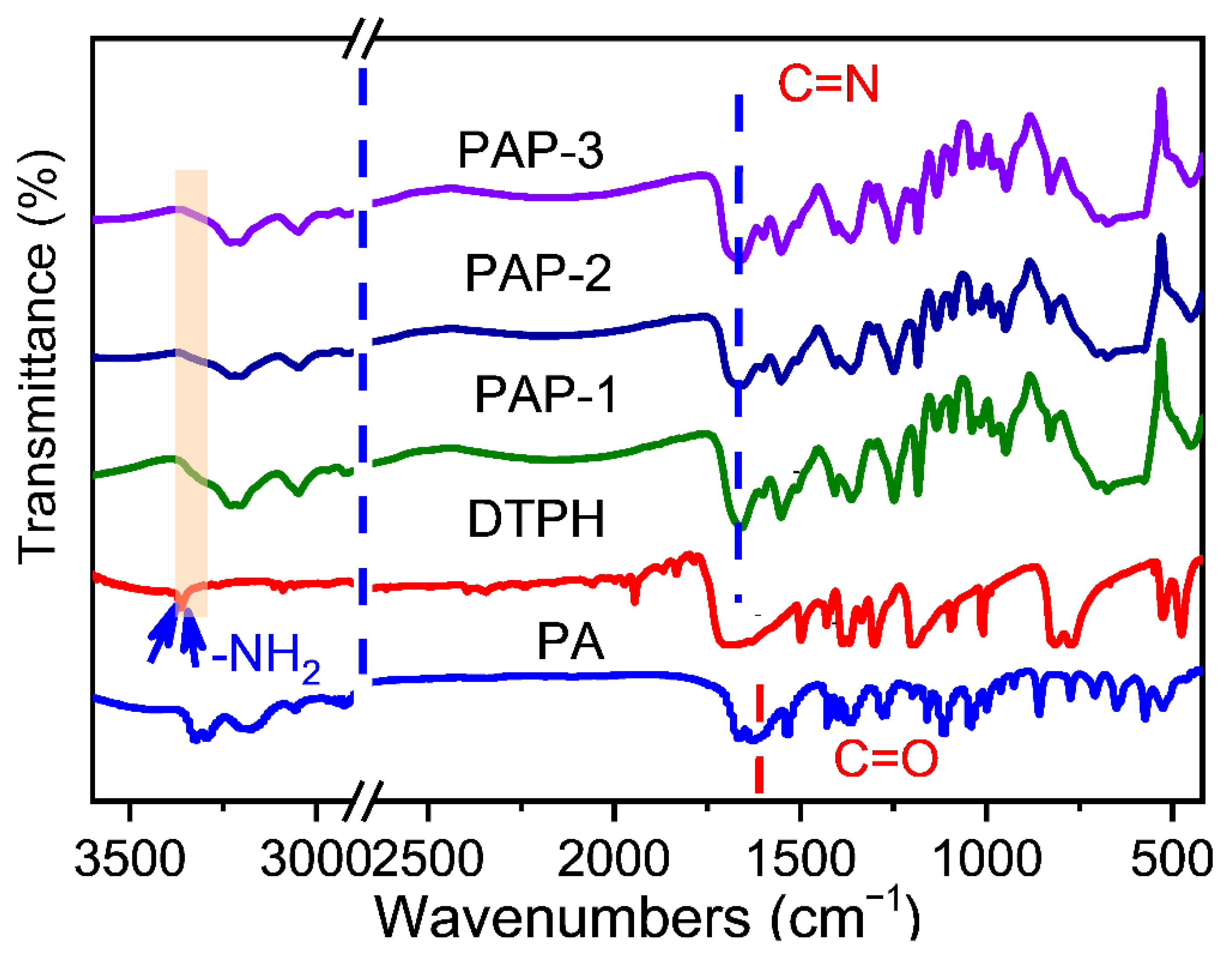
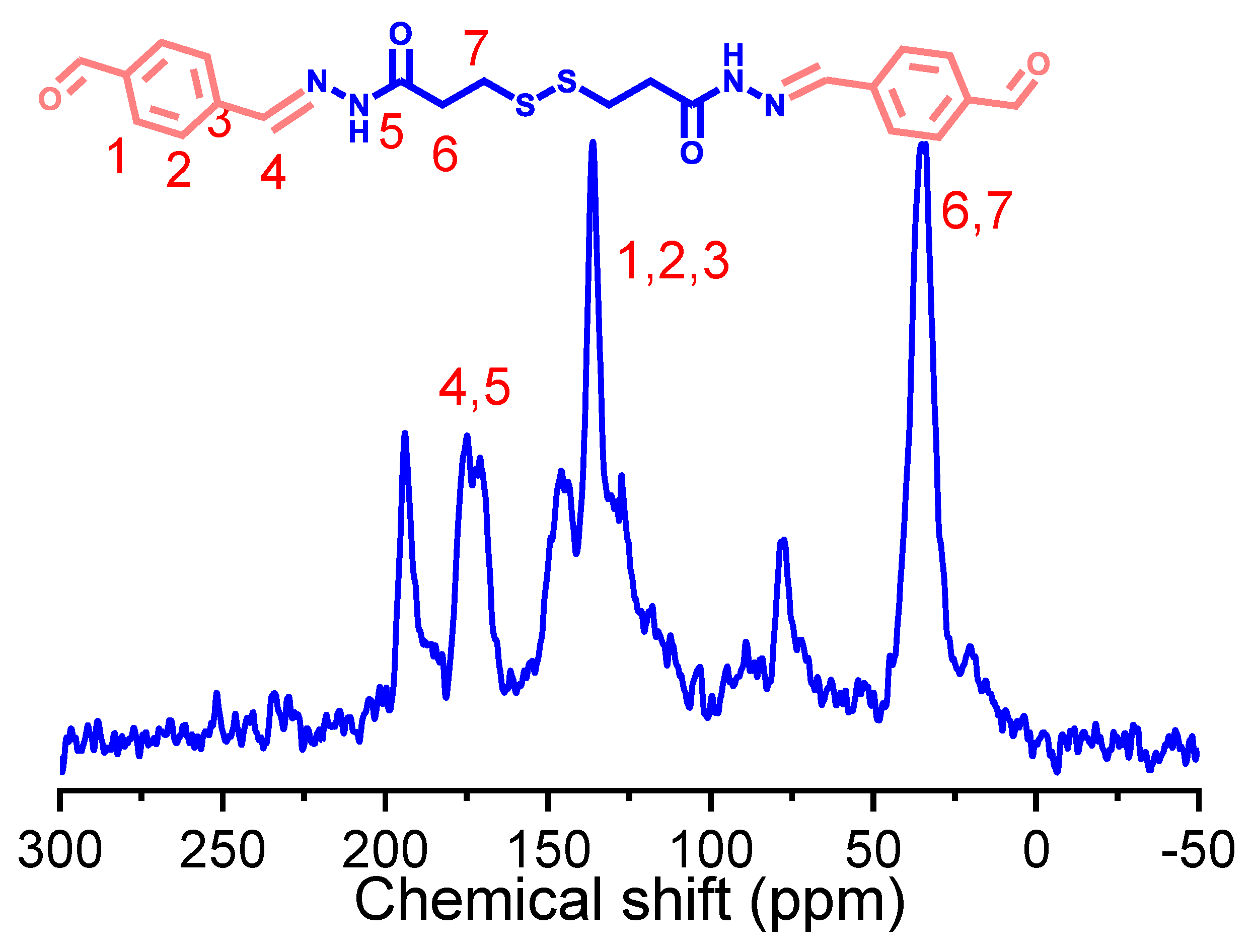
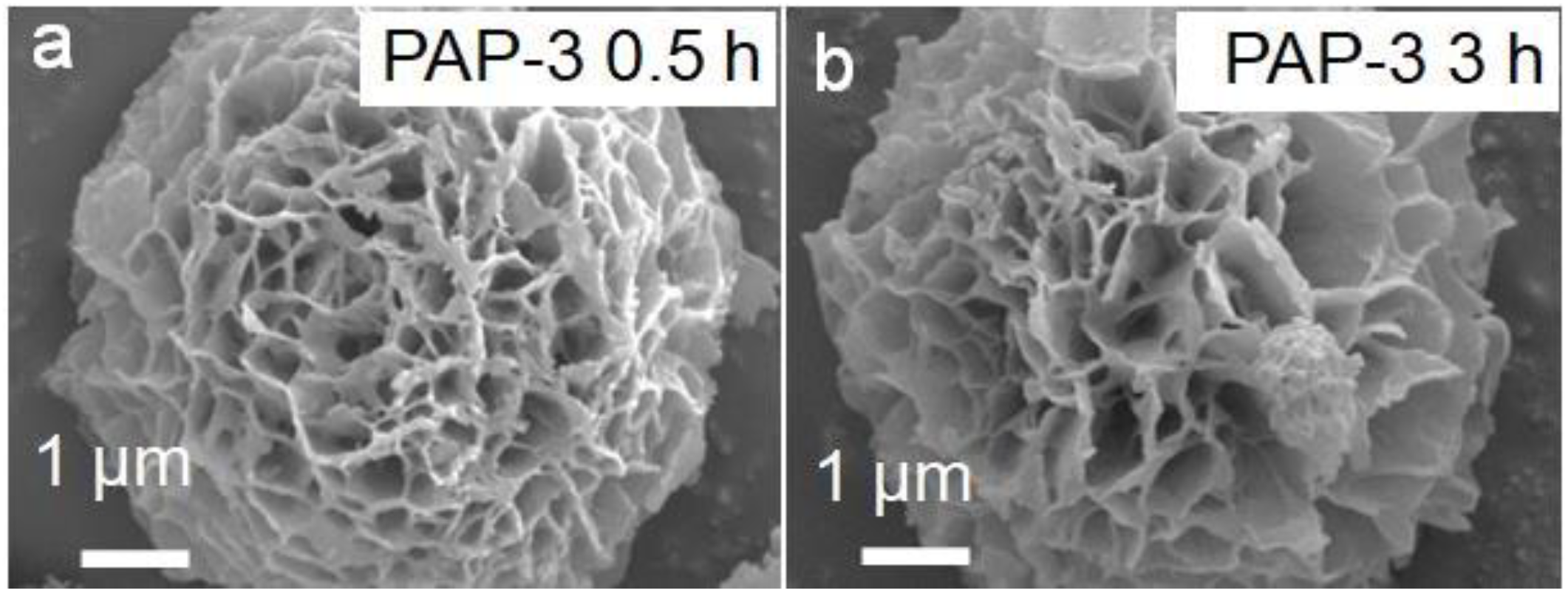
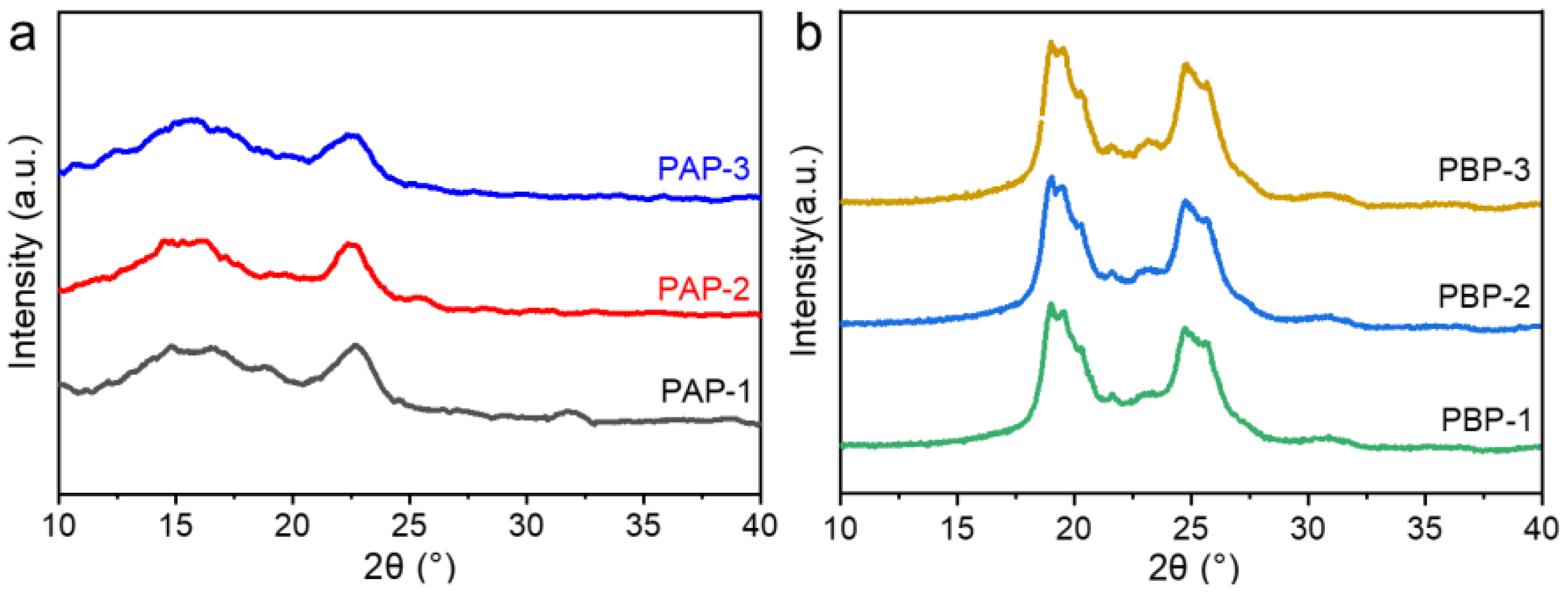
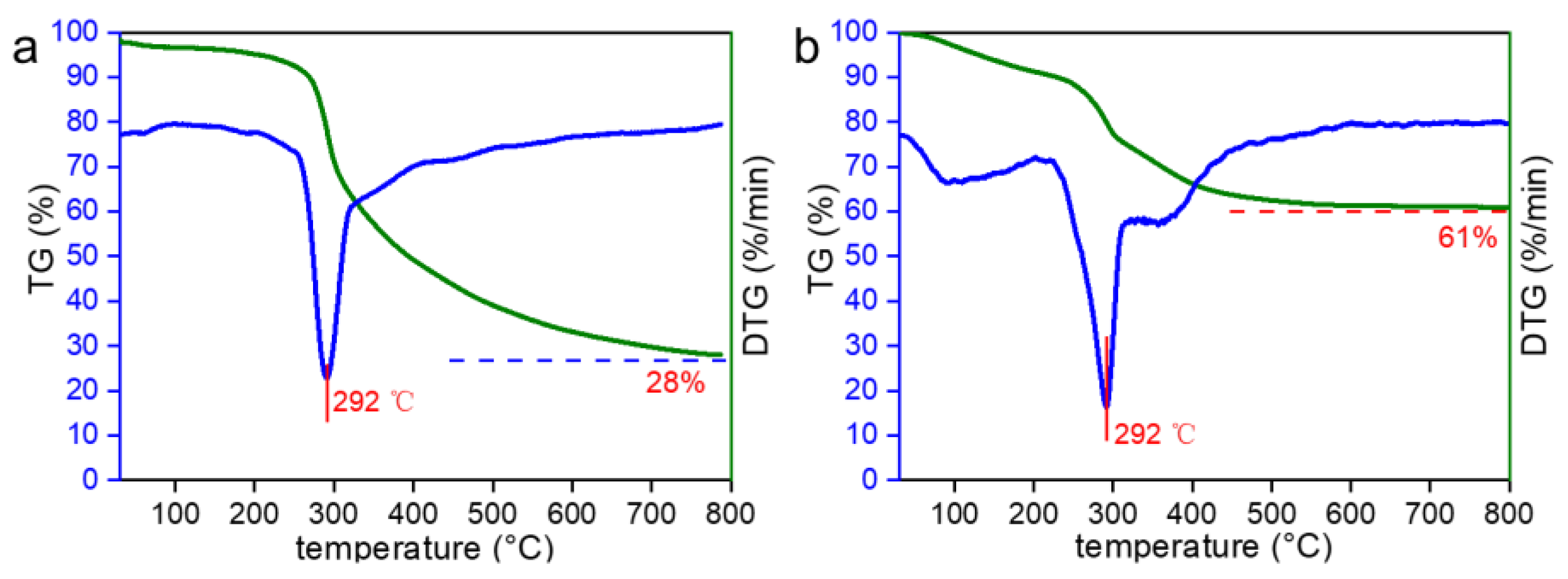
2.1. Characterization of Dynamic Covalent Materials
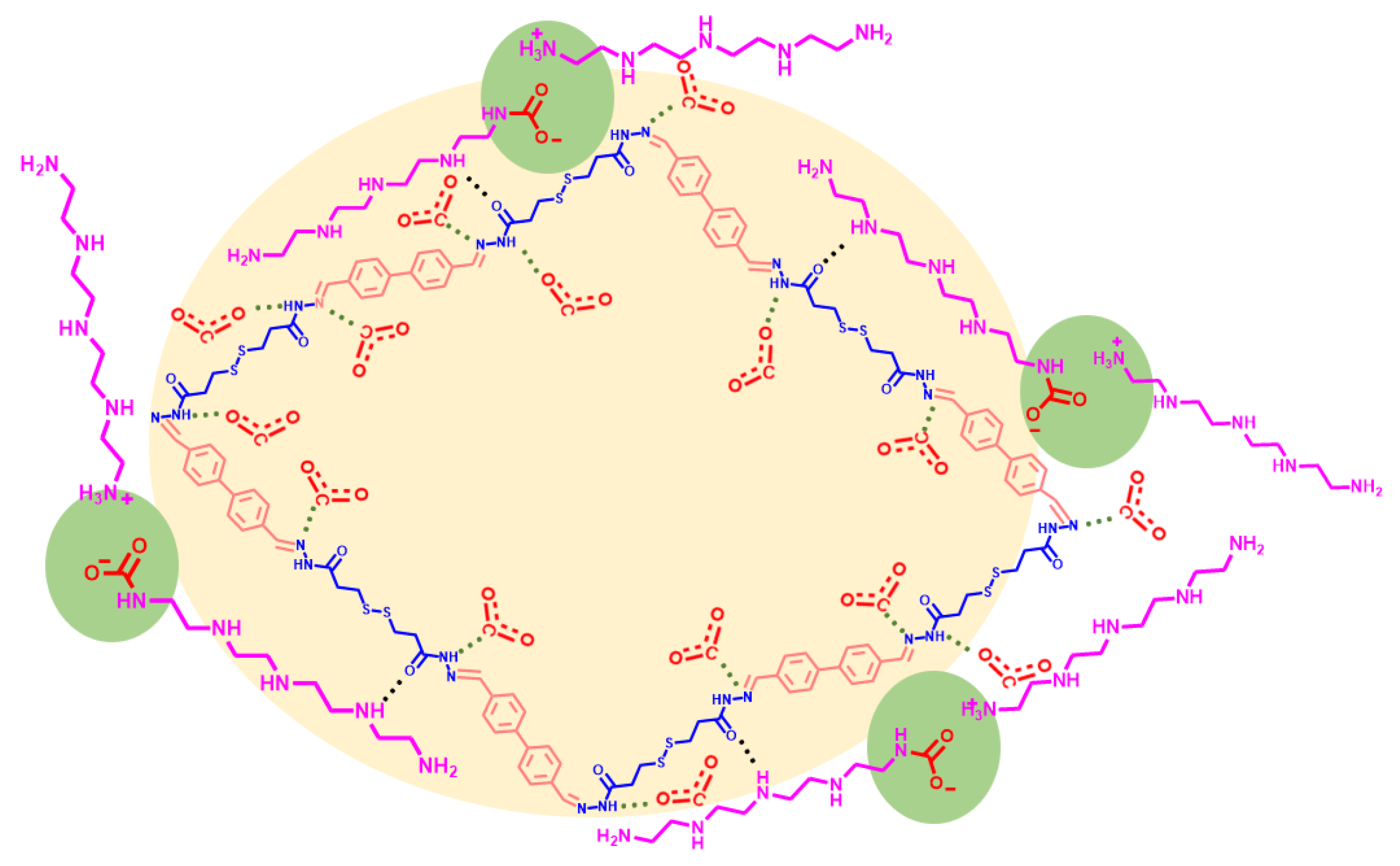
2.2. CO2 Adsorption Performance
3. Materials and Methods
3.1. Materials
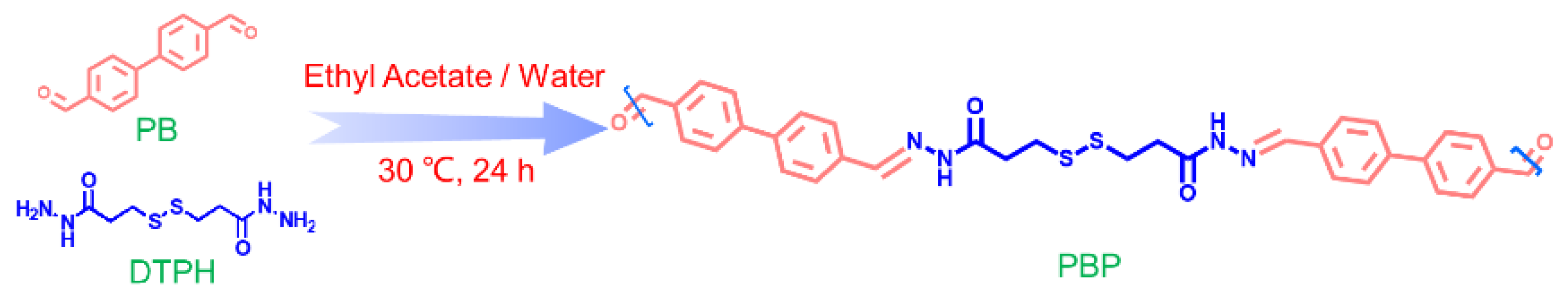
3.2. Synthesis of Materials
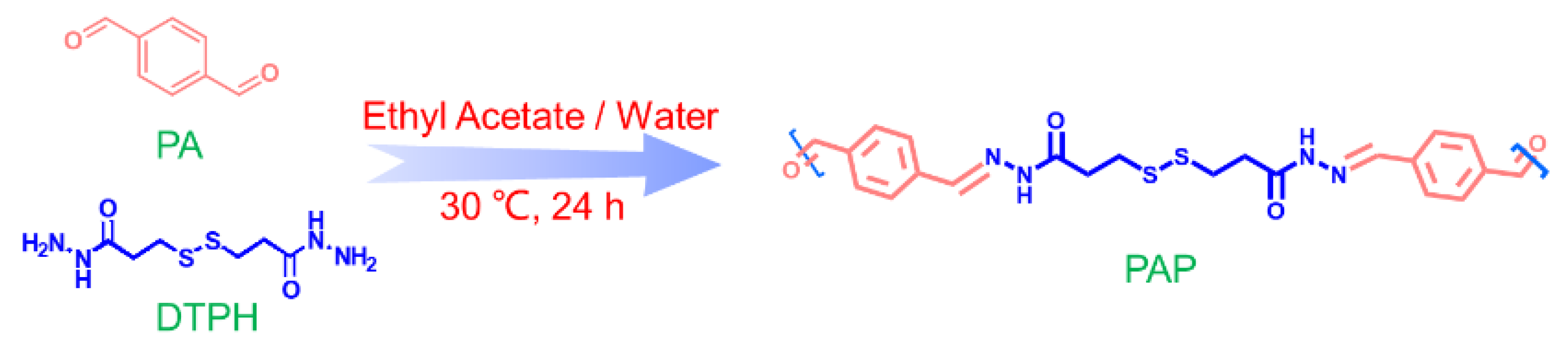
3.2.1. Synthesis of 3,3′-Dithiobis(Propionyl Hydrazine)
3.2.2. Synthesis of PAP-X
3.2.3. Synthesis of PBP-X
3.2.4. Synthesis of TEPA-PBP
3.3. Characterizations
3.4. CO2 Adsorption Capacity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Doney, S.C.; Busch, D.S.; Cooley, S.R.; Kroeker, K.J. The Impacts of Ocean Acidification on Marine Ecosystems and Reliant Human Communities. Annu. Rev. Environ. Resour. 2020, 45, 83–112. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Yang, R.; Liu, Y.; Jafari, M. CO2 Capture and Storage Monitoring Based on Remote Sensing Techniques: A Review. J. Clean. Prod. 2021, 281, 124409. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New Eco-Friendly Phosphorus Organic Polymers as Gas Storage Media. Polymers 2017, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.G.; Jawad, K.; Yousif, E.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S. Synthesis of Telmisartan Organotin(IV) Complexes and their use as Carbon Dioxide Capture Media. Molecules 2019, 24, 1631. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, S.; Li, Q.; Zhang, S.; Wang, L.; An, S. CO2 Capture Performance and Mechanism of Blended Amine Solvents Regulated By N-methylcyclohexyamine. Energy 2021, 215, 119209. [Google Scholar] [CrossRef]
- He, X. Polyvinylamine-Based Facilitated Transport Membranes for Post-Combustion CO2 Capture: Challenges and Perspectives from Materials to Processes. Engineering 2021, 7, 124–131. [Google Scholar] [CrossRef]
- Li, Z.L.; Zhou, Y.L.; Yan, W.; Luo, L.; Su, Z.Z.; Fan, M.Z.; Wang, S.R.; Zhao, W.G. Cost-Effective Monolithic Hierarchical Carbon Cryogels with Nitrogen Doping and High-Performance Mechanical Properties for CO2 Capture. ACS. Appl. Mater. Inter. 2020, 12, 21748–21760. [Google Scholar] [CrossRef]
- Xu, L.; Xing, C.Y.; Ke, D.; Chen, L.; Qiu, Z.J.; Zeng, S.L.; Li, B.J.; Zhang, S. Amino-Functionalized beta-Cyclodextrin to Construct Green Metal-Organic Framework Materials for CO2 Capture. ACS Appl. Mater. Inter. 2020, 12, 3032–3041. [Google Scholar] [CrossRef]
- Faria, A.C.; Trujillano, R.; Rives, V.; Miguel, C.V.; Rodrigues, A.E.; Madeira, L.M. Alkali metal (Na, Cs and K) Promoted Hydrotalcites for High Temperature CO2 Capture from Flue Gas in Cyclic Adsorption Processes. Chem. Eng. J. 2022, 427, 131502. [Google Scholar] [CrossRef]
- Omer, R.M.; Al-Tikrity, E.T.B.; El-Hiti, G.A.; Alotibi, M.F.; Ahmed, D.S.; Yousif, E. Porous Aromatic Melamine Schiff Bases as Highly Efficient Media for Carbon Dioxide Storage. Processes 2019, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the Direct Capture of CO2 from Ambient Air. Angew. Chem. Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef]
- Choi, H.J.; Min, J.G.; Ahn, S.H.; Shin, J.; Hong, S.B.; Radhakrishnan, S.; Chandran, C.V.; Bell, R.G.; Breynaert, E.; Kirschhock, C.E.A. Framework Flexibility-Driven CO2 Adsorption on a Zeolite. Mater. Horiz. 2020, 7, 1528–1532. [Google Scholar] [CrossRef]
- Rattanaphan, S.; Rungrotmongkol, T.; Kongsune, P. Biogas Improving by Adsorption of CO2 on Modified Waste Tea Activated Carbon. Renew. Energy 2020, 145, 622–631. [Google Scholar] [CrossRef]
- Chen, C.; Xu, H.; Jiang, Q.; Lin, Z. Rational Design of Silicas with Meso-Macroporosity as Supports for High-Performance Solid Amine CO2 Adsorbents. Energy 2021, 214, 119093. [Google Scholar] [CrossRef]
- Vadillo, J.M.; Gómez-Coma, L.; Garea, A.; Irabien, A. Hollow Fiber Membrane Contactors in CO2 Desorption: A Review. Energy Fuels 2020, 35, 111–136. [Google Scholar] [CrossRef]
- Lee, R.J.; Jawad, Z.A.; Ahmad, A.L.; Chua, H.B. Incorporation of Functionalized Multi-Walled Carbon Nanotubes (MWCNTs) into Cellulose Acetate Butyrate (CAB) Polymeric Matrix to Improve the CO2/N2 Separation. Process Saf. Environ. 2018, 117, 159–167. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; You, Z.; Dong, Y.; Yuan, H.; Yin, J.; Zhou, X. Synergetic Effect of Liquid/Plasma on Wet Wheat Straw Porous Carbon: Pore Structure, Heteroatom Doping and CO2 adsorption. Mater. Lett. 2020, 262, 127185. [Google Scholar] [CrossRef]
- Mutyala, S.; Jonnalagadda, M.; Ibrahim, S.M. Effect of Modification of UiO-66 for CO2 Adsorption and Separation of CO2/CH4. J. Mol. Struct. 2021, 1227, 129506. [Google Scholar] [CrossRef]
- Gautam, S.; Cole, D. CO2 Adsorption in Metal-Organic Framework Mg-MOF-74: Effects of Inter-Crystalline Space. Nanomaterials 2020, 10, 2274. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Duan, H.; Li, Y.; Xu, J.; Huang, Y.; Liu, B.; Zhang, T. Polyvinyl Chloride-Derived Carbon Spheres for CO2 Adsorption. ChemSusChem 2020, 13, 6426–6432. [Google Scholar]
- Avci, G.; Erucar, I.; Keskin, S. Do New MOFs Perform Better for CO2 Capture and H2 Purification? Computational Screening of the Updated MOF Database. ACS. Appl. Mater. Inter. 2020, 12, 41567–41579. [Google Scholar] [CrossRef] [PubMed]
- Hisaki, I.; Xin, C.; Takahashi, K.; Nakamura, T. Designing Hydrogen-Bonded Organic Frameworks (HOFs) with Permanent Porosity. Angew. Chem. Int. Ed. 2019, 58, 11160–11170. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zou, R.; Zhao, Y. Covalent Organic Frameworks for CO2 Capture. Adv. Mater. 2016, 28, 2855–2873. [Google Scholar] [CrossRef] [PubMed]
- Inukai, M.; Tamura, M.; Horike, S.; Higuchi, M.; Kitagawa, S.; Nakamura, K. Storage of CO2 into Porous Coordination Polymer Controlled by Molecular Rotor Dynamics. Angew. Chem. Int. Ed. 2018, 57, 8687–8690. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.; Yu, Z.; Huang, Z.; Lai, J.-C.; Tok, J.B.H.; Cui, Y.; Bao, Z. Reprocessable and Recyclable Polymer Network Electrolytes via Incorporation of Dynamic Covalent Bonds. Chem. Mater. 2022, 34, 2393–2399. [Google Scholar] [CrossRef]
- Afrose, S.P.; Mahato, C.; Sharma, P.; Roy, L.; Das, D. Nonequilibrium Catalytic Supramolecular Assemblies of Melamine- and Imidazole-Based Dynamic Building Blocks. J. Am. Chem. Soc. 2022, 144, 673–678. [Google Scholar] [CrossRef]
- Blanke, M.; Postulka, L.; Ciara, I.; D’Acierno, F.; Hildebrandt, M.; Gutmann, J.S.; Dong, R.Y.; Michal, C.A.; Giese, M. Manipulation of Liquid Crystalline Properties by Dynamic Covalent Chemistry horizontal line En Route to Adaptive Materials. ACS. Appl. Mater. Inter. 2022, 14, 16755–16763. [Google Scholar] [CrossRef]
- Chen, S.; Liu, M.; Zhang, J.; Zhang, Z.; Zhu, J.; Pan, X.; Zhu, X. Photoresponsive Dynamic Covalent Bond Based On Addition–Fragmentation Chain Transfer of Allyl Selenides. Polym. Chem. 2021, 12, 1622–1626. [Google Scholar] [CrossRef]
- Vo, T.K.; Kim, W.-S.; Kim, J. Ethylenediamine-incorporated MIL-101(Cr)-NH2 Metal-Organic Frameworks for Enhanced CO2 Adsorption. Korean J. Chem. Eng. 2020, 37, 1206–1211. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Lobato, B.; Lopez-Anton, M.A.; Arencibia, A.; Sanz, R.; Martínez-Tarazona, M.R. Effectiveness of Amino-Functionalized Sorbents for CO2 Capture in the Presence of Hg. Fuel 2020, 267, 117250. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, L.; Zheng, Y.; Zhu, T. Optimization of CO2 Adsorption on Solid-Supported Amines and Thermal Regeneration Mode Comparison. ACS Omega 2020, 5, 9641–9648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Xue, Y.; Wang, C.; Ji, J.; Zhou, Z.; Tang, C. Improved Capture of Carbon Dioxide and Methane via Adding Micropores Within Porous Boron Nitride Fibers. J. Mater. Sci. 2019, 54, 10168–10178. [Google Scholar] [CrossRef]
- Rehman, A.; Farrukh, S.; Hussain, A.; Fan, X.; Pervaiz, E. Adsorption of CO2 on amine-functionalized green metal-organic framework: An interaction between amine and CO2 molecules. Environ. Sci. Pollut. Res. Int. 2019, 26, 36214–36225. [Google Scholar] [CrossRef] [PubMed]
- Putz, A.M.; Ciopec, M.; Negrea, A.; Grad, O.; Ianasi, C.; Ivankov, O.I.; Milanovic, M.; Stijepovic, I.; Almasy, L. Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods. Materials 2021, 14, 628. [Google Scholar] [CrossRef]
- Lawson, S.; Griffin, C.; Rapp, K.; Rownaghi, A.A.; Rezaei, F. Amine-Functionalized MIL-101 Monoliths for CO2 Removal from Enclosed Environments. Energy Fuels 2019, 33, 2399–2407. [Google Scholar] [CrossRef]
- De ON Ribeiro, J.; Nunes, E.H.M.; Vasconcelos, D.C.L.; Vasconcelos, W.L.; Nascimento, J.F.; Grava, W.M.; Derks, P.W.J. Role of the Type of Grafting Solvent and Its Removal Process on APTES Functionalization onto SBA-15 Silica for CO2 Adsorption. J. Porous Mater. 2019, 26, 1581–1591. [Google Scholar] [CrossRef]
- Ji, C.; Huang, X.; Li, L.; Xiao, F.; Zhao, N.; Wei, W. Pentaethylenehexamine-Loaded Hierarchically Porous Silica for CO2 Adsorption. Materials 2016, 9, 835. [Google Scholar] [CrossRef]
- Xie, W.; Huang, S.; Tang, D.; Liu, S.; Zhao, J. Biomass-derived Schiff Base Compound Enabled Fire-Safe Epoxy Thermoset with Excellent Mechanical Properties and High Glass Transition Temperature. Chem. Eng. J. 2020, 394, 123667. [Google Scholar] [CrossRef]
- Liu, X.; Liang, L.; Lu, M.; Song, X.; Liu, H.; Chen, G. Water-Resistant Bio-Based Vitrimers Based on Dynamic Imine Bonds: Self-healability, Remodelability and Ecofriendly Recyclability. Polymer 2020, 210, 123030. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, G.; Peng, Y.; Chen, L.; Fu, S. Effects of Cellulose Nanofibrils on Dialdehyde Carboxymethyl Cellulose Based Dual Responsive Self-Healing Hydrogel. Cellulose 2019, 26, 8813–8827. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Wu, H.; He, G.; Xu, Z.; Kong, Y.; Cao, L.; Shi, B.; Zhang, Z.; Tongsh, C.; et al. De Novo Design of Covalent Organic Framework Membranes toward Ultrafast Anion Transport. Adv. Mater. 2020, 32, e2001284. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wu, H.; Guan, J.; You, X.; Yang, C.; Wang, X.; Cao, L.; Shi, B.; Peng, Q.; Kong, Y.; et al. Scalable Fabrication of Crystalline COF Membranes from Amorphous Polymeric Membranes. Angew. Chem. Int. Ed. 2021, 60, 18051–18058. [Google Scholar] [CrossRef] [PubMed]
- Wehner, M.; Würthner, F. Supramolecular polymerization through kinetic pathway control and living chain growth. Nat. Rev. Chem. 2019, 4, 38–53. [Google Scholar] [CrossRef]
- Adelizzi, B.; Aloi, A.; Markvoort, A.J.; Ten Eikelder, H.M.M.; Voets, I.K.; Palmans, A.R.A.; Meijer, E.W. Supramolecular block copolymers under thermodynamic control. J. Am. Chem. Soc. 2018, 140, 7168–7175. [Google Scholar] [CrossRef] [PubMed]
- Vantomme, G.; Ter Huurne, G.M.; Kulkarni, C.; Ten Eikelder, H.M.M.; Markvoort, A.J.; Palmans, A.R.A.; Meijer, E.W. Tuning the length of cooperative supramolecular polymers under thermodynamic control. J. Am. Chem. Soc. 2019, 141, 18278–18285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jiang, C.; Fan, J.; Hong, S.; Mei, P.; Yao, R.; Liu, Y.; Zhang, S.; Li, H.; Zhang, H.; et al. Hydrophilicity gradient in covalent organic frameworks for membrane distillation. Nat. Mater. 2021, 20, 1551–1558. [Google Scholar] [CrossRef]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef]
- Alves, L.D.S.; Moreira, B.R.A.; Viana, R.D.S.; Dias, E.S.; Rinker, D.L.; Pardo-Gimenez, A.; Zied, D.C. Spent Mushroom Substrate Is Capable of Physisorption-Chemisorption of CO2. Environ. Res. 2022, 204, 111945. [Google Scholar] [CrossRef]
- Vitaku, E.; Dichtel, W.R. Synthesis of 2D Imine-Linked Covalent Organic Frameworks through Formal Transimination Reactions. J. Am. Chem. Soc. 2017, 139, 12911–12914. [Google Scholar] [CrossRef]
- Shan, Z.; Wu, X.; Xu, B.; Hong, Y.L.; Wu, M.; Wang, Y.; Nishiyama, Y.; Zhu, J.; Horike, S.; Kitagawa, S.; et al. Dynamic Transformation between Covalent Organic Frameworks and Discrete Organic Cages. J. Am. Chem. Soc. 2020, 142, 21279–21284. [Google Scholar] [CrossRef]
- Mao, H.Y.; Tang, J.; Day, G.S.; Peng, Y.C.; Wang, H.Z.; Xiao, X.; Yang, Y.F.; Jiang, Y.W.; Chen, S.; Halat, D.M.; et al. A scalable solid-state nanoporous network with atomic-level interaction design for carbon dioxide capture. Sci. Adv. 2022, 8, eabo6849. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, Y.; Wu, Q.; Liu, B.; Li, D.; Chen, R.; Wang, C.; Li, H.; Zeng, Z.; Li, L. Underlying Mechanism of CO2 Uptake onto Biomass-Based Porous Carbons: Do Adsorbents Capture CO2 Chiefly Through Narrow Micropores? Fuel 2020, 282, 118727. [Google Scholar] [CrossRef]
- Kong, W.; Liu, J. Ordered Mesoporous Carbon with Enhanced Porosity to Support Organic Amines: Efficient Nanocomposites for the Selective Capture of CO2. New J. Chem. 2019, 43, 6040–6047. [Google Scholar] [CrossRef]
- Alkadhem, A.M.; Elgzoly, M.A.A.; Onaizi, S.A. Novel Amine-Functionalized Magnesium Oxide Adsorbents for CO2 Capture at Ambient Conditions. J. Environ. Chem. Eng. 2020, 8, 103968. [Google Scholar] [CrossRef]
- Fang, Q.; Huang, W.; Wang, H. Role of Additives in Silica-Supported Polyethylenimine Adsorbents for CO2 Adsorption. Mater. Res. Express 2020, 7, 035026. [Google Scholar] [CrossRef]
- Mohamad, N.A.; Zubair, N.A.; Lotf, E.A.; Nasef, M.M.; Ahmad, A.; Ali, R.R.; Abdullah, T.A.T.; Ting, T.M. Effect of Ligand Type on CO2 Adsorption over Amine Functionalized Fibrous Adsorbents. IOP Conf. Ser. Mater. Sci. Eng. 2020, 808, 012009. [Google Scholar] [CrossRef]
- Rong, X.; Ettelaie, R.; Lishchuk, S.V.; Cheng, H.; Zhao, N.; Xiao, F.; Cheng, F.; Yang, H. Liquid Marble-Derived Solid-Liquid Hybrid Superparticles for CO2 Capture. Nat. Commun. 2019, 10, 1854. [Google Scholar] [CrossRef] [Green Version]
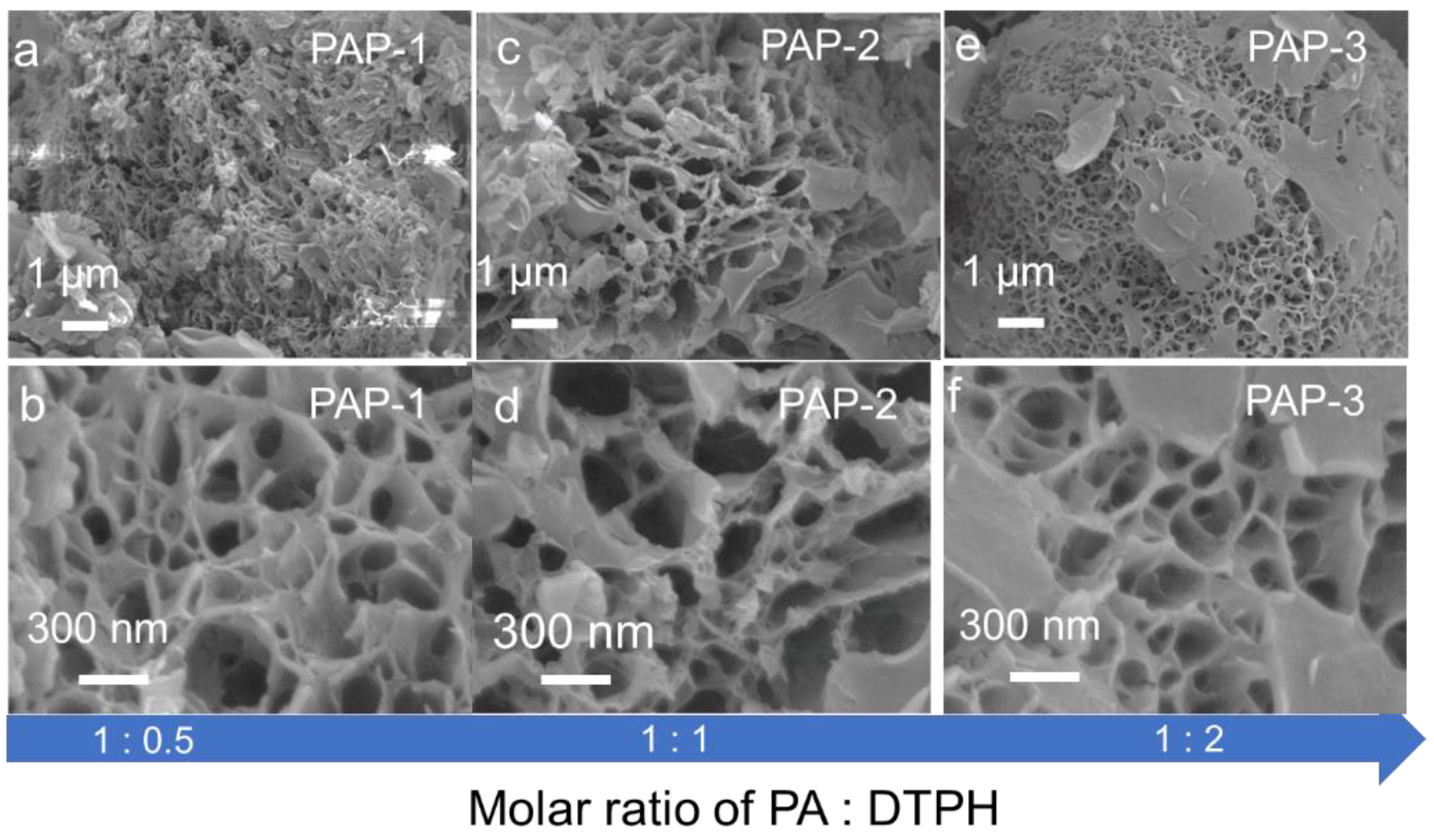
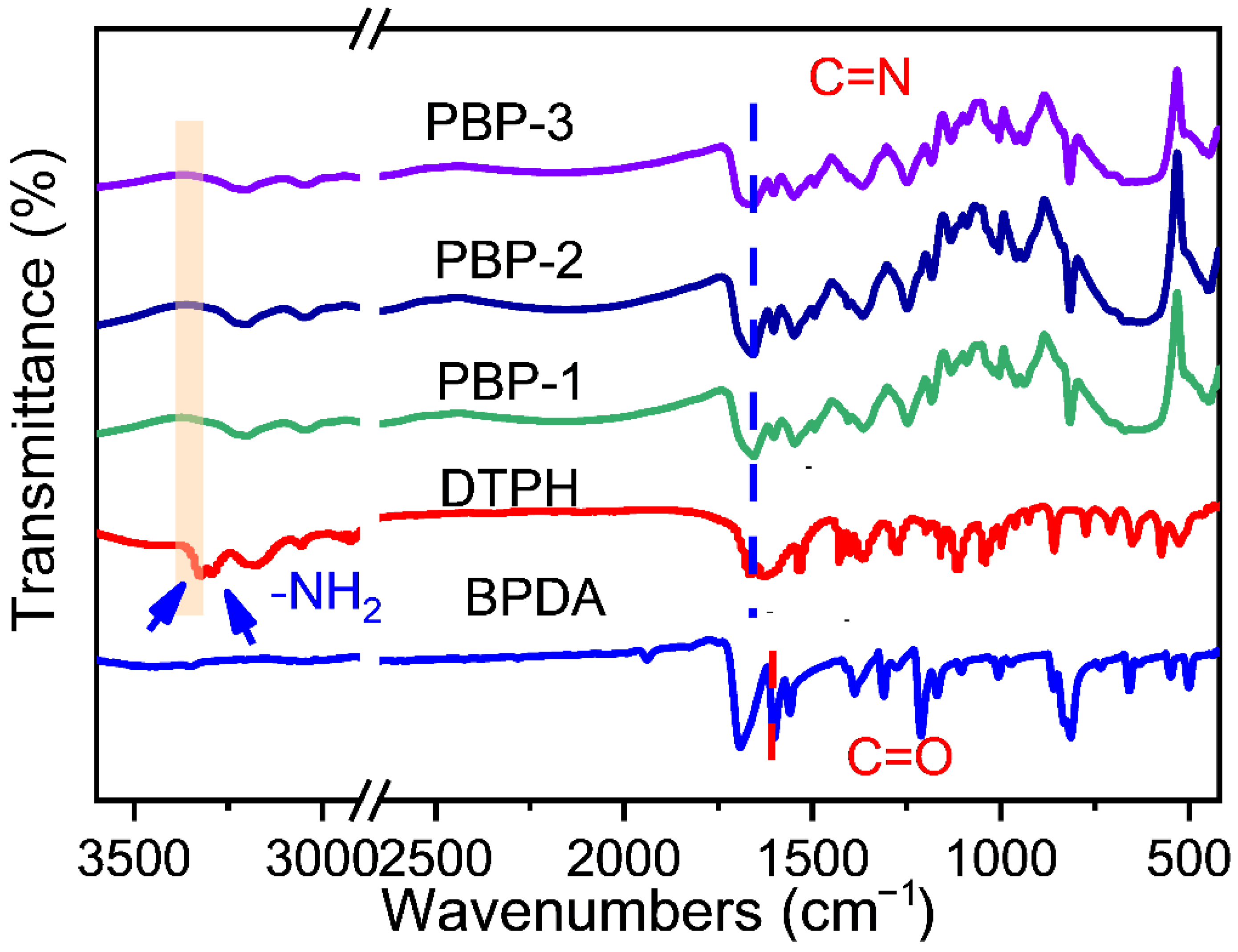
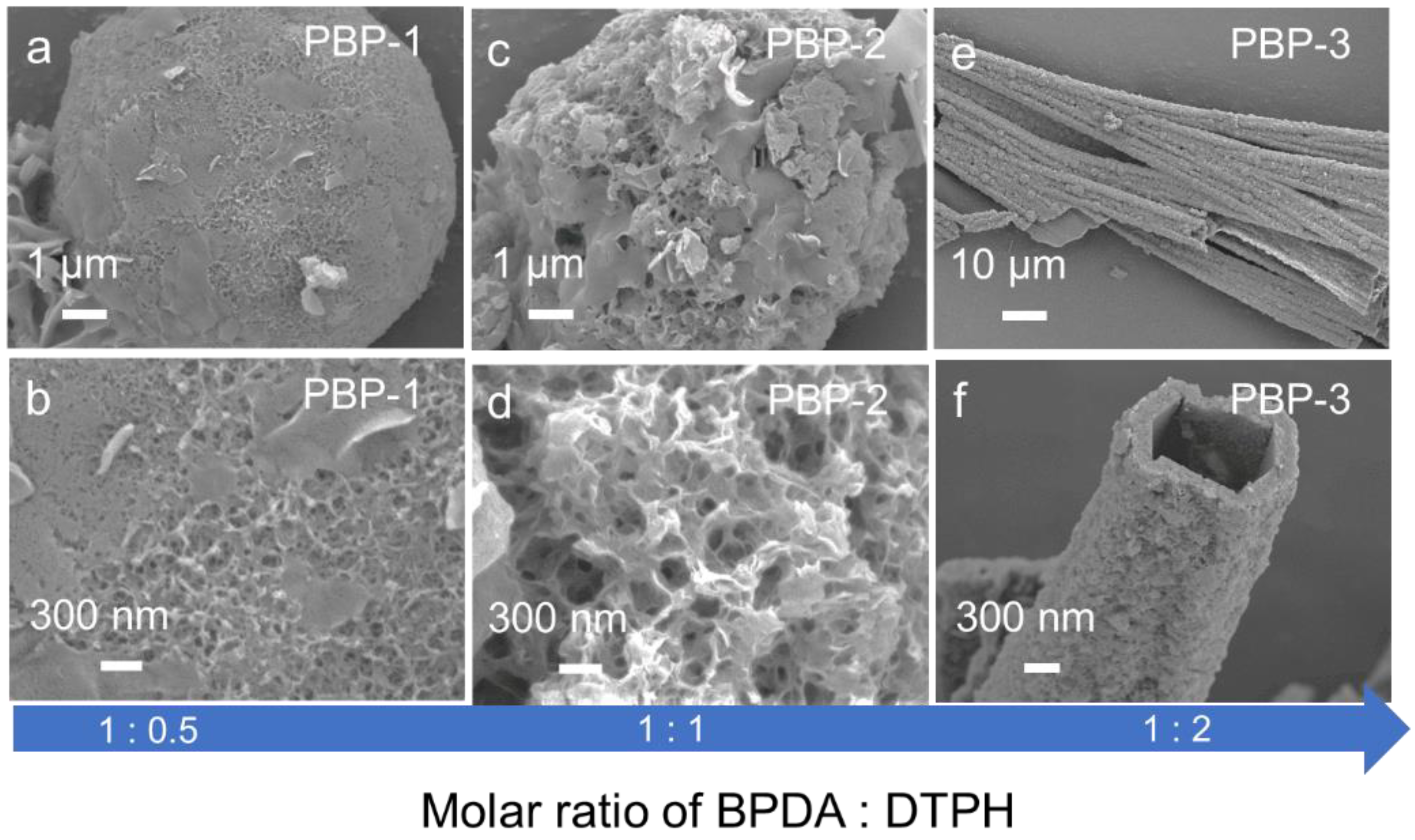
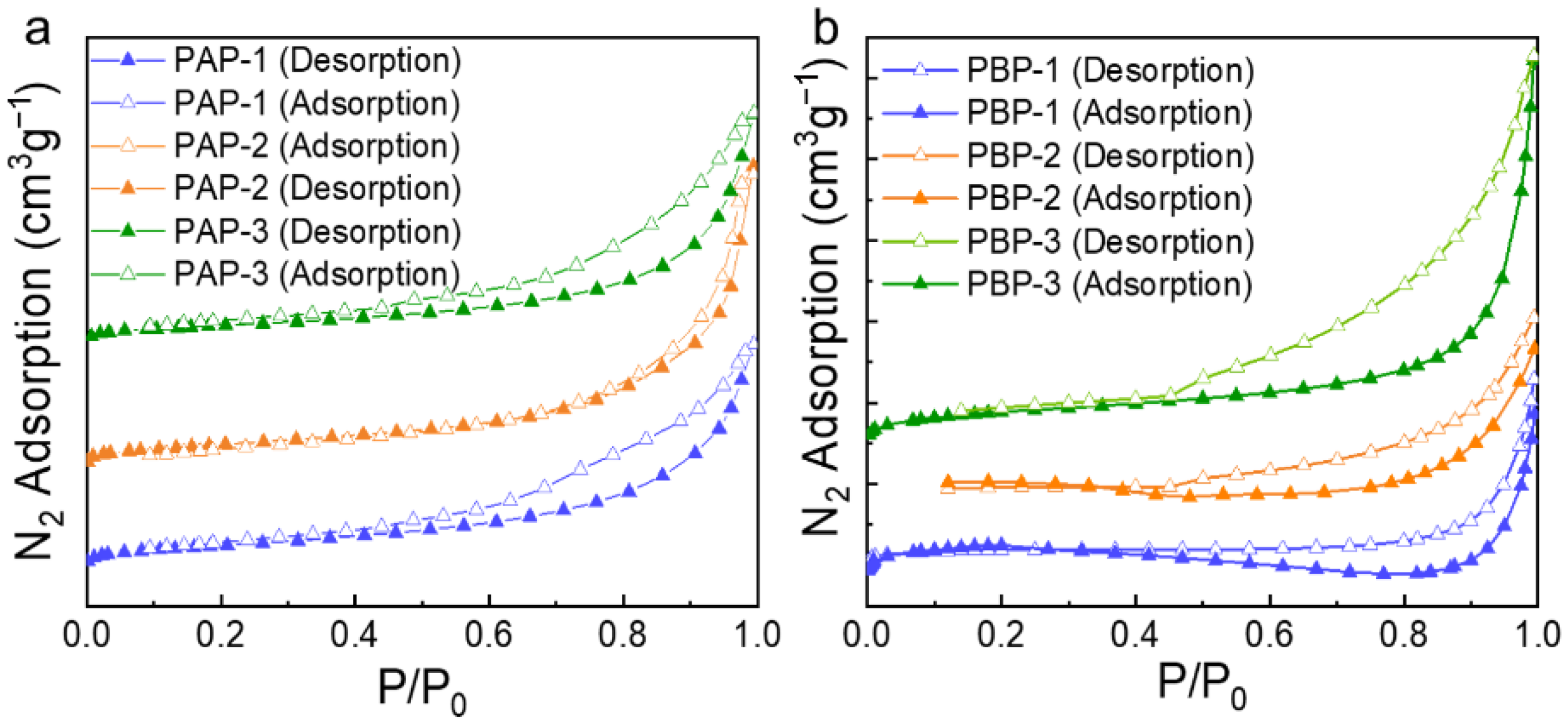
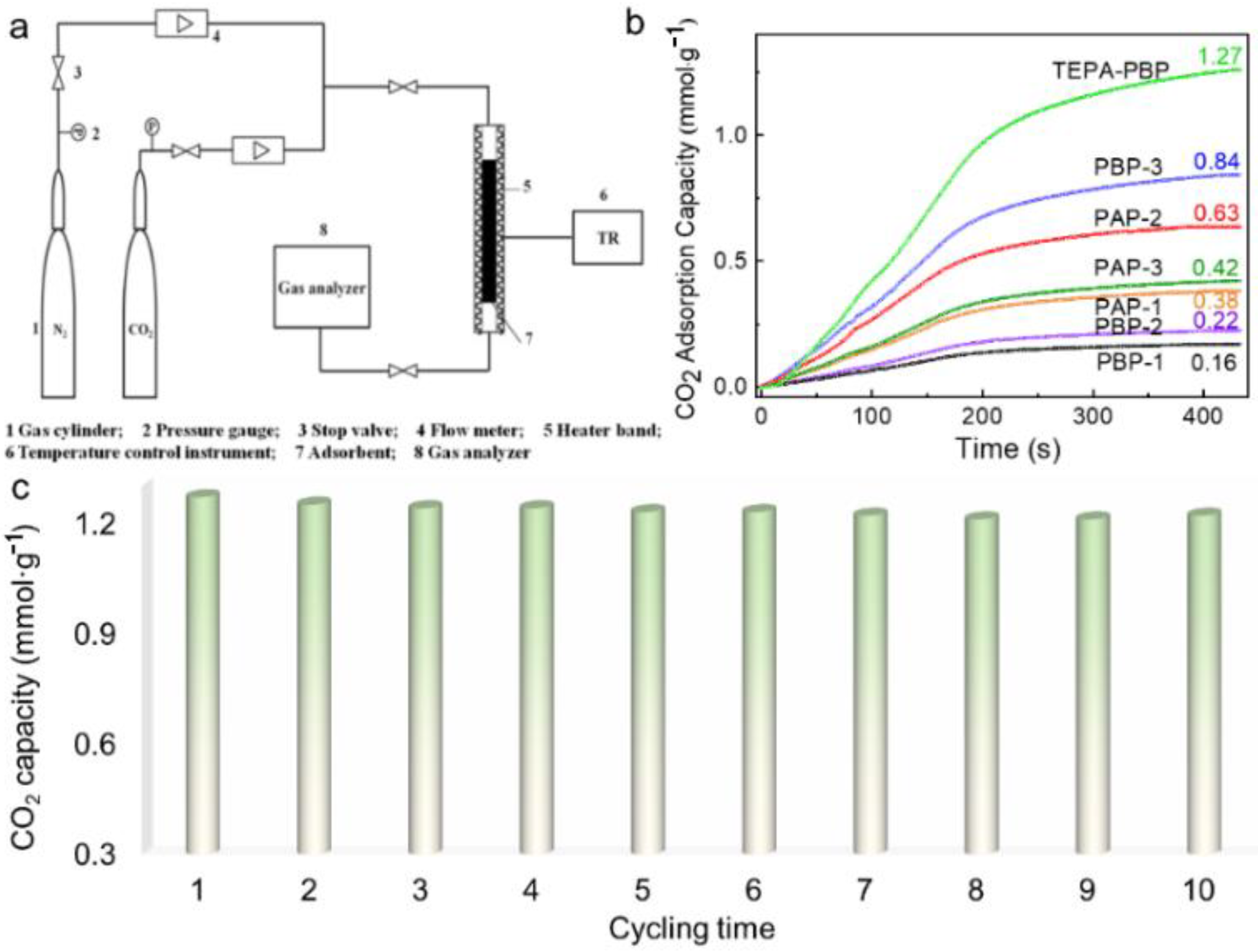
| Sample | SBET a (m2·g−1) | Vtotal b (cm3·g−1) | Vmeso c (cm3·g−1) |
|---|---|---|---|
| PAP-1 | 72.57 | 0.27 | 0.27 |
| PAP-2 | 77.91 | 0.36 | 0.36 |
| PAP-3 | 58.20 | 0.28 | 0.28 |
| PBP-1 | 17.27 | 0.09 | 0.19 |
| PBP-2 | 33.12 | 0.16 | 0.16 |
| PBP-3 | 63.12 | 0.32 | 0.32 |
| Entry | Adsorbent | CO2 Capacity (mmol·g−1) | CO2 Capacity (wt.%) |
|---|---|---|---|
| 1 | PAP-1 | 0.38 | 1.67 |
| 2 | PAP-2 | 0.63 | 2.77 |
| 3 | PAP-3 | 0.42 | 1.85 |
| 4 | PBP-1 | 0.16 | 0.70 |
| 5 | PBP-2 | 0.22 | 0.97 |
| 6 | PBP-3 | 0.84 | 3.70 |
| 7 | TEPA-PBP | 1.27 | 5.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, M.; Wu, H.; Huang, Y.; Guo, H.; Gao, D.; Pei, F.; Shi, L.; Yi, Q. Controllable Construction of Amino-Functionalized Dynamic Covalent Porous Polymers for High-Efficiency CO2 Capture from Flue Gas. Molecules 2022, 27, 5853. https://doi.org/10.3390/molecules27185853
Qiu M, Wu H, Huang Y, Guo H, Gao D, Pei F, Shi L, Yi Q. Controllable Construction of Amino-Functionalized Dynamic Covalent Porous Polymers for High-Efficiency CO2 Capture from Flue Gas. Molecules. 2022; 27(18):5853. https://doi.org/10.3390/molecules27185853
Chicago/Turabian StyleQiu, Mingyue, Haonan Wu, Yi Huang, Huijuan Guo, Dan Gao, Feng Pei, Lijuan Shi, and Qun Yi. 2022. "Controllable Construction of Amino-Functionalized Dynamic Covalent Porous Polymers for High-Efficiency CO2 Capture from Flue Gas" Molecules 27, no. 18: 5853. https://doi.org/10.3390/molecules27185853
APA StyleQiu, M., Wu, H., Huang, Y., Guo, H., Gao, D., Pei, F., Shi, L., & Yi, Q. (2022). Controllable Construction of Amino-Functionalized Dynamic Covalent Porous Polymers for High-Efficiency CO2 Capture from Flue Gas. Molecules, 27(18), 5853. https://doi.org/10.3390/molecules27185853