Progress of Microencapsulated Phycocyanin in Food and Pharma Industries: A Review
Abstract
:1. Introduction
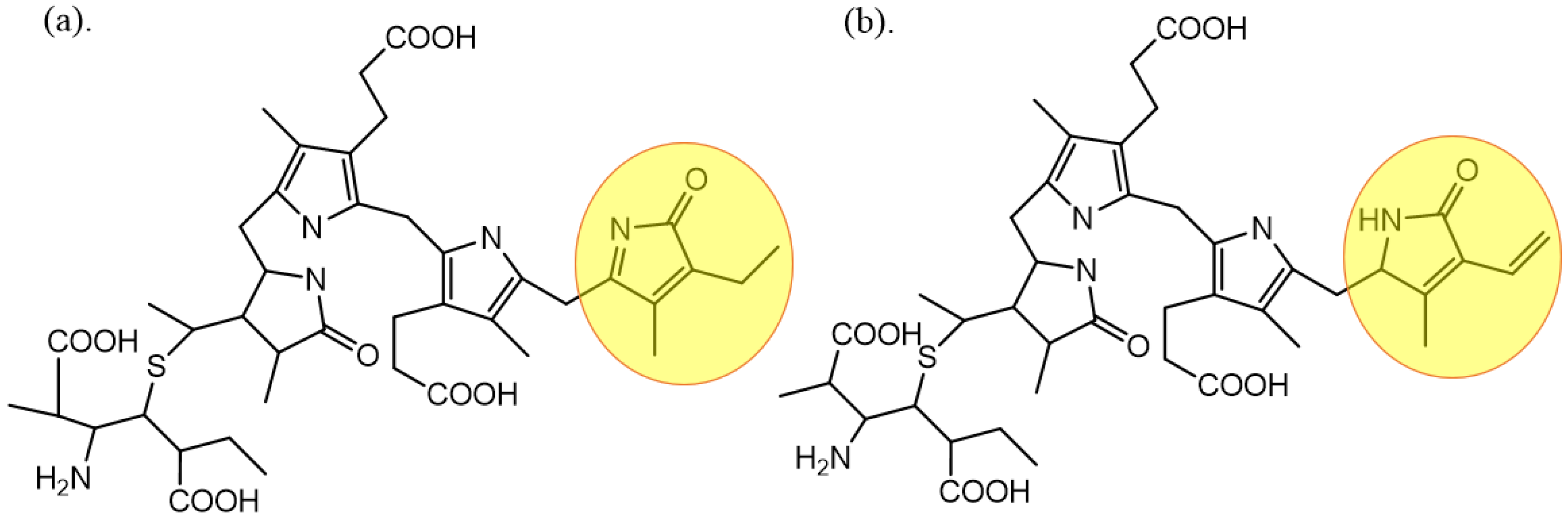
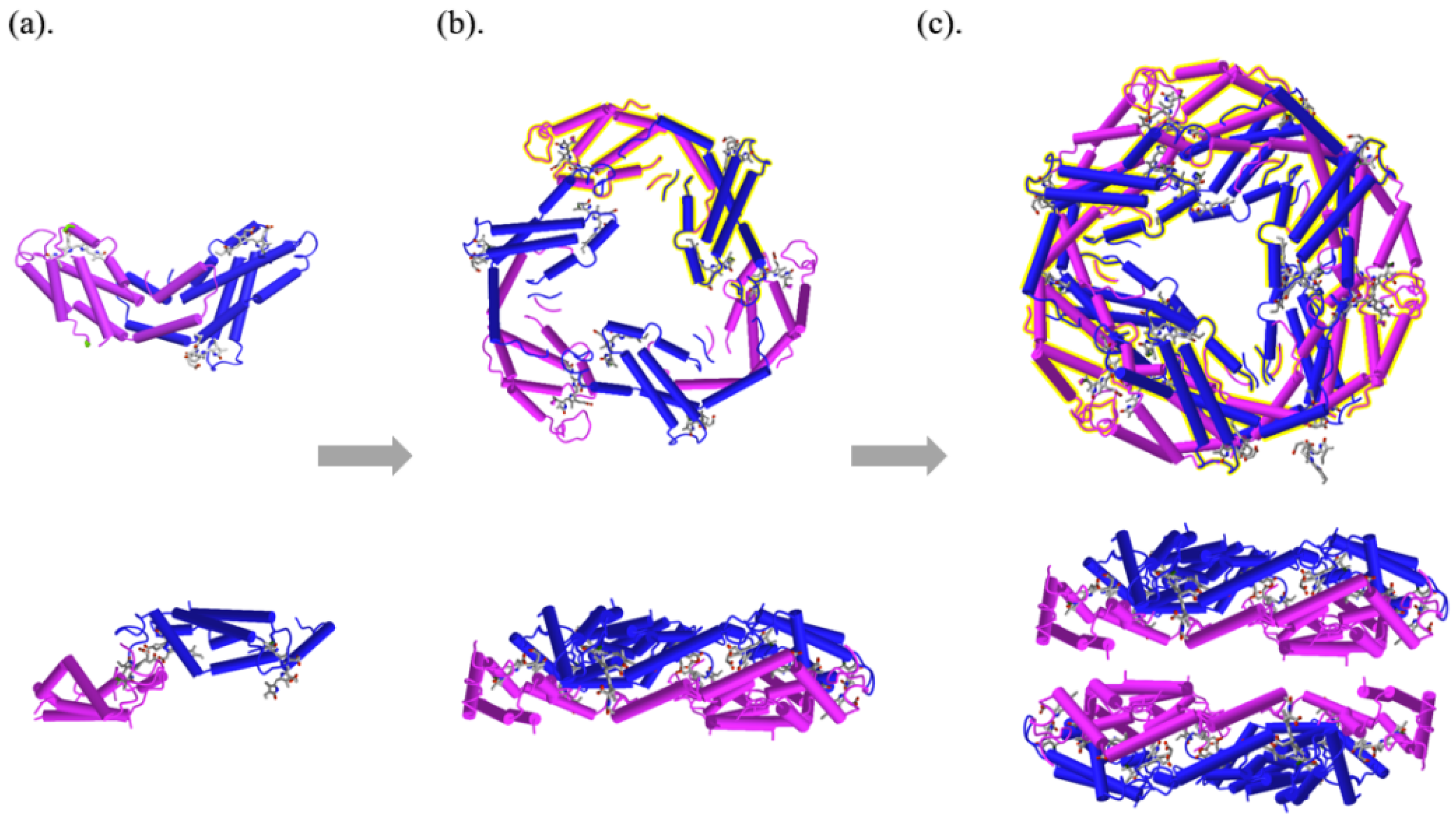
2. The Structure, Properties, and Functions of PC
3. Microencapsulated PC Wall Materials
3.1. Natural Polymer Materials
3.2. Synthetic Material
4. Microencapsulation Technology of PC
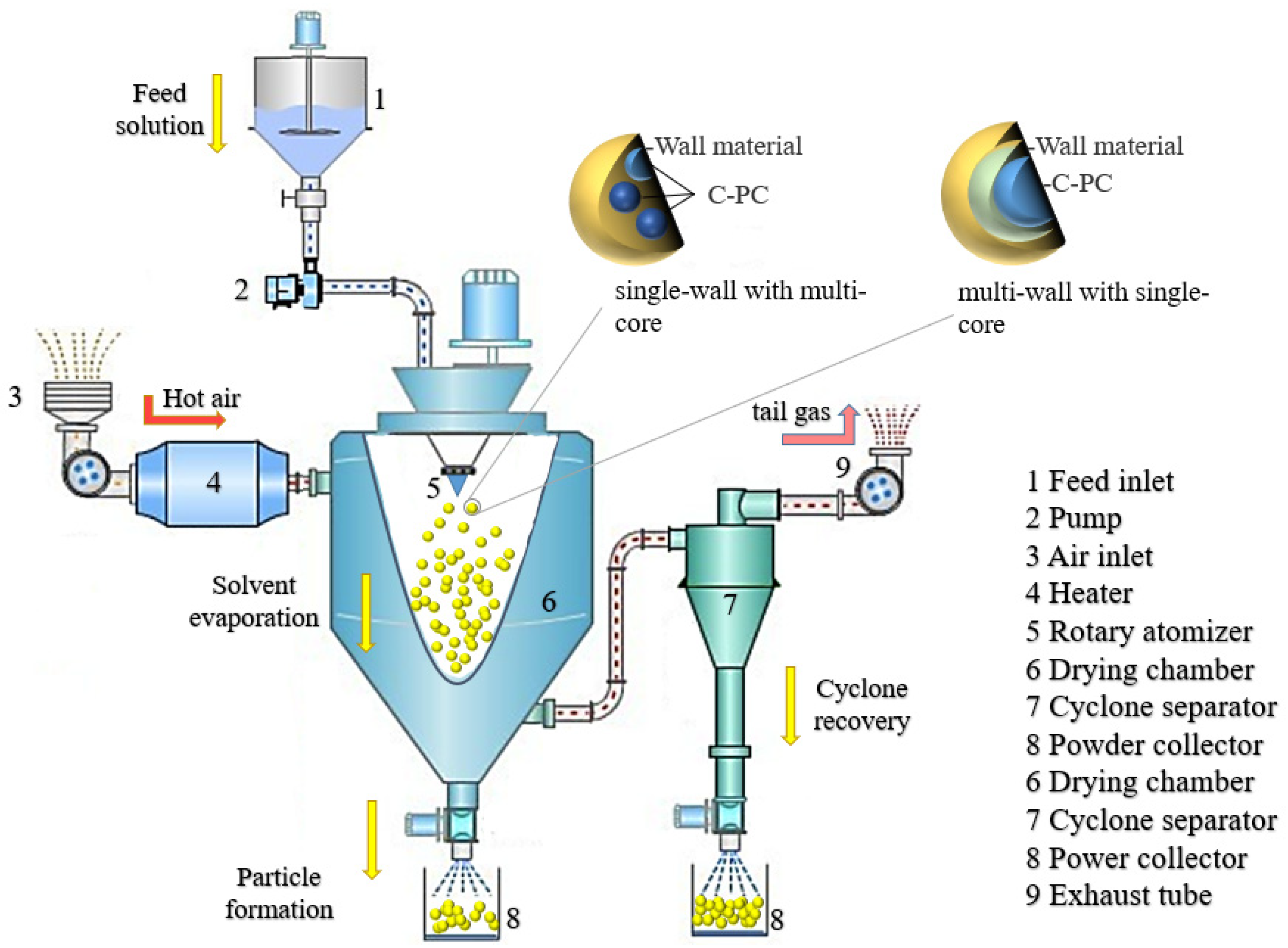
4.1. Spray Drying
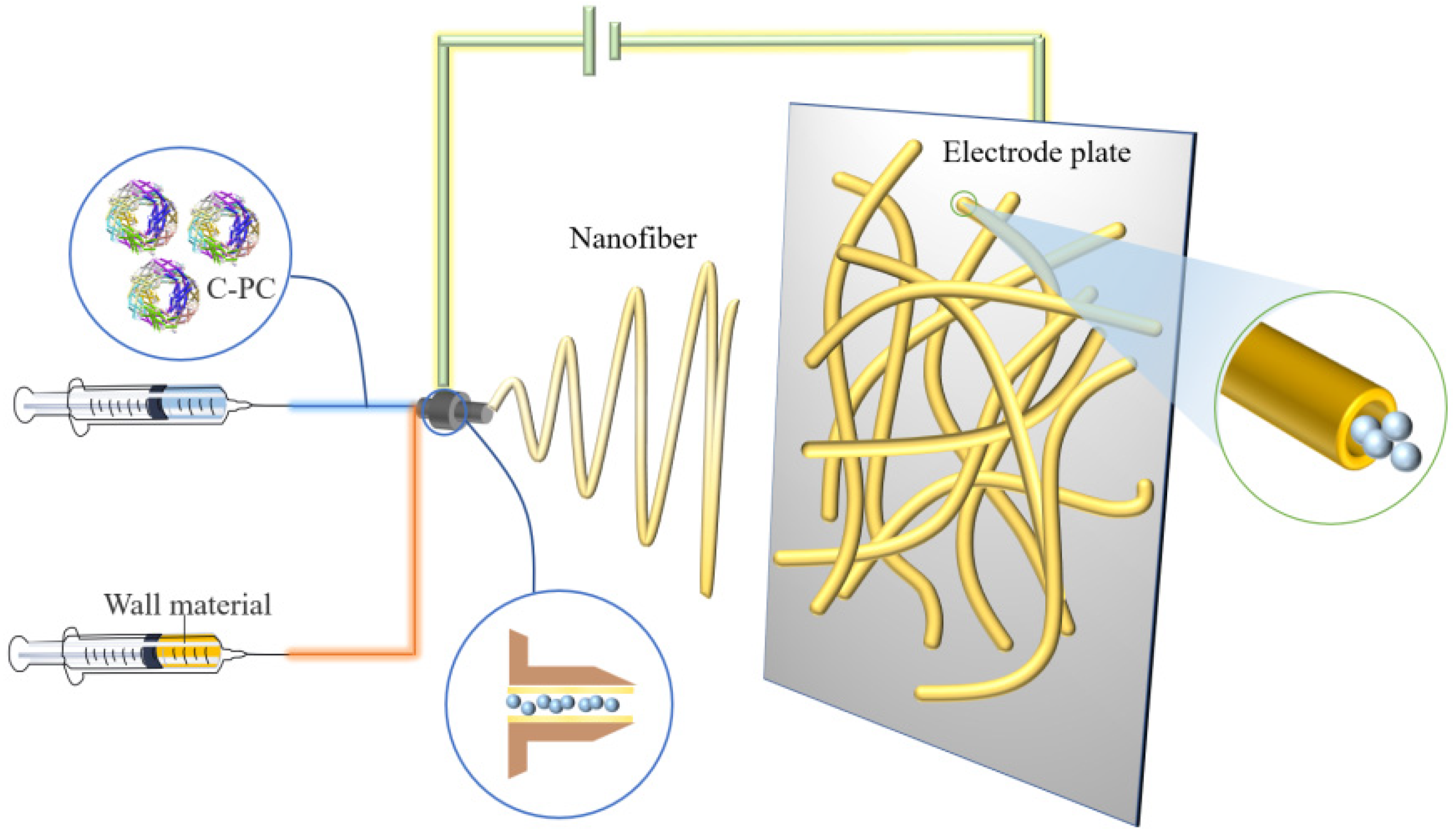
4.2. Electrospinning Technology
4.3. Electrospraying Technology
4.4. Liposome Delivery
4.5. Sharp-Hole Coagulation Bath
4.6. Ion Gelation
5. Prospect of PC Microencapsulation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Baky, H.H.A.; El-Baroty, G.S. Characterization and bioactivity of phycocyanin isolated from Spirulina maxima grown under salt stress. Food Funct. 2012, 3, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Meticulous Research (2020). January 30, 2021. Phycocyanin Market Worth $245.5 Million by 2027. Available online: https://www.meticulousresearch.com/pressrelease/30/phycocyanin-market-2027 (accessed on 30 January 2021).
- Deng, W.; Wang, X.Q. Progress of anti-tumor activity of phycocyanin and its enzymolied products. Mar. Sci. Bull. 2010, 29, 357–360. [Google Scholar] [CrossRef]
- Böcker, L.; Ortmann, S.; Surber, J.; Leeb, E. Biphasic short time heat degradation of the blue microalgae protein phycocyanin from Arthrospira platensis. Innov. Food Sci. Emerg. Technol. 2018, 52, 116–121. [Google Scholar] [CrossRef]
- Pleonsil, P.; Soogarun, S.; Suwanwong, Y. Anti-oxidant activity of holo- and apo-C-phycocyanin and their protective effects on human erythrocytes. Int. J. Biol. Macromol. 2013, 60, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Tanaka, M.; Ooike, M.; Sakaguchi, T.T.M. Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J. Appl. Phycol. 2000, 12, 435–439. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Lu, L.; Liu, B.; Du, Z.N.; Qin, S. Phycocyanin attenuates X-ray-induced pulmonary inflammation via the TLR2-MyD88-NF-kappa B signaling pathway. J. Oceanol. Limnol. 2019, 37, 1678–1685. [Google Scholar] [CrossRef]
- Alzokaky, A.A.; Abdelkader, E.M.; El-Dessouki, A.M.; Khaleel, S.A.; Raslan, N.A. C-phycocyanin protects against ethanol-induced gastric ulcers in rats: Role of HMGB1/NLRP3/NF-kappa B pathway. Basic Clin. Pharmacol. Toxicol. 2020, 127, 265–277. [Google Scholar] [CrossRef]
- Pliego, E.G.; Franco-Colin, M.; Franco, P.R.; Blas-Valdivia, V. Phycocyanobilin is the molecule responsible for the nephroprotective action of phycocyanin in acute kidney injury caused by mercury. Food Funct. 2021, 12, 2985–2994. [Google Scholar] [CrossRef]
- Guo, W.; Zeng, M.Y.; Zhu, S.Q.; Li, S.Y.; Qian, Y.L.; Wu, H.H. Phycocyanin ameliorates mouse colitis via phycocyanobilin-dependent antioxidant and anti-inflammatory protection of intestinal epithelial barrier. Food Funct. 2022, 13, 3294–3307. [Google Scholar] [CrossRef]
- Bhat, V.B.; Madyastha, K.M. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: Protection against oxidative damage to DNA. Biochem. Biophys. Res. Commun. 2001, 285, 262–266. [Google Scholar] [CrossRef]
- Abeliovich, A.; Shilo, M. Photooxidative reactions of c-phycocyanin. Biochim Biophys Acta. 1972, 283, 483–491. [Google Scholar] [CrossRef]
- Liang, X.; Wang, C.H.; Lu, X.; Pang, Y.Y.; Jiang, H.R.; Liu, X.L. Stability and fluorescence characteristics of C-phycocyanin in Spirulina platensis. Mod. Food Sci. Technol. 2020, 36, 89–96. [Google Scholar] [CrossRef]
- Kaya-Celiker, H.; Mallikarjunan, K. Better nutrients and therapeutics delivery in food through nanotechnology. Food Eng. Rev. 2012, 4, 114–123. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Kurek, M.A. Microencapsulation of anthocyanins—Critical review of techniques and wall materials. Appl. Sci. 2021, 11, 3936. [Google Scholar] [CrossRef]
- Jiang, T.; Wei, L.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Chu, C.C.; Chew, S.C.; Nyam, K.L. Recent advances in encapsulation technologies of kenaf (Hibiscus cannabinus) leaves and seeds for cosmeceutical application. Food Bioprod. Process. 2021, 127, 99–113. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Redha, A.A.; Esmaeili, Y.; Mehdizadeh, M. Novel insights on extraction and encapsulation techniques of elderberry bioactive compounds. Crit. Rev. Food Sci. Nutr. 2022, 12, 1–16. [Google Scholar] [CrossRef]
- İlter, I.; Koç, M.; Demirel, Z.; Dalay, M.C. Improving the Stability of Phycocyanin by Spray Dried Microencapsulation. J. Food Processing Preserv. 2021, 45, 15646. [Google Scholar] [CrossRef]
- Ilter, I.; Akyil, S.; Koc, M.; Demirel, Z.; Dalay, M.C.; Ertekin, F.K. Microencapsulation of phycocyanin by spray drying technique. J. Biotechnol. 2017, 256, S26. [Google Scholar] [CrossRef]
- Iqbal, M.N.; Hadiyanto, H. Experimental investigation of phycocyanin microencapsulation using maltodextrin as a coating material with spray drying method. AIP Conf. Proc. 2020, 2197, 100002. [Google Scholar] [CrossRef]
- Faieta, M.; Corradini, M.G.; Michele, A.D.; Ludescher, R.D. Effect of encapsulation process on technological functionality and stability of Spirulina platensis extract. Food Biophys. 2020, 15, 50–63. [Google Scholar] [CrossRef]
- Silva, S.C.; Fernandes, I.P.; Barros, L.; Fernandes, A.; Alves, M.J.; Calhelha, R.C.; Pereira, C.; Barreira, J.C.M.; Manrique, Y.; Colla, E.; et al. Spray-dried Spirulina platensis as an effective ingredient to improve yogurt formulations: Testing different encapsulating solutions. J. Funct. Foods 2019, 60, 103427. [Google Scholar] [CrossRef]
- Ayekpam, C.; Hs, P.P.; Tavanandi, H.A.; Raghavarao, K. A novel method for double encapsulation of C-phycocyanin using aqueous two phase systems for extension of shelf life. J. Food Sci. Technol. 2020, 57, 3545–3555. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Figueira, F.D.S.; Silveira, J.T.D.; Morais, M.G.D.; Costa, J.A.V.; Kalil, S.J. Improvement of thermal stability of C-phycocyanin by nanofiber and preservative agents. J. Food Process. Preserv. 2016, 40, 1264–1269. [Google Scholar] [CrossRef]
- Moreira, J.B.; Lim, L.T.; Zavareze, E.D.R.; Dias, A.R.G.; Costa, J.A.V.; Morais, M.G.D. Antioxidant ultrafine fibers developed with microalga compounds using a free surface electrospinning. Food Hydrocoll. 2019, 93, 131–136. [Google Scholar] [CrossRef]
- Wen, Y.; Wen, P.; Hu, T.G.; Linhardt, R.J.; Zong, M.H.; Wu, H.; Chen, Z.Y. Encapsulation of phycocyanin by prebiotics and polysaccharides-based electrospun fibers and improved colon cancer prevention effects. Int. J. Biol. Macromol. 2020, 149, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Hu, T.G.; Wen, Y.; Linhardt, R.J. Targeted delivery of phycocyanin for the prevention of colon cancer using electrospun fibers. Food Funct. 2019, 10, 1816–1825. [Google Scholar] [CrossRef]
- Terra, A.; Moreira, J.B.; Costa, J.; Morais, M.G.D. Development of time-pH indicator nanofibers from natural pigments: An emerging processing technology to monitor the quality of foods. LWT Food Sci. Technol. 2021, 142, 111020. [Google Scholar] [CrossRef]
- González, Y.V.; Prieto, C.; Filizoglu, M.F.; Ragazzo-Sanchez, J.A.; Calderon-Santoyo, M.; Furtado, R.F.; Chen, H.N.; Biswas, A.; Lagaron, J.M. Electrosprayed cashew gum microparticles for the encapsulation of highly sensitive bioactive materials. Carbohydr. Polym. 2021, 264, 118060. [Google Scholar] [CrossRef]
- Schmatz, D.; Mastrantonio, D.J.D.S.; Costa, J.A.V.; Morais, M.G.D. Encapsulation of phycocyanin by electrospraying: A promising approach for the protection of sensitive compounds. Food Bioprod. Process. 2020, 119, 206–215. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Rostamabadi, M.M.; Assadpour, E.; Jafari, S.M. Electrospraying as a novel process for the synthesis of particles/nanoparticles loaded with poorly water-soluble bioactive molecules. Adv. Colloid Interface Sci. 2021, 290, 102384. [Google Scholar] [CrossRef] [PubMed]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef] [PubMed]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnurch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 6, 114097. [Google Scholar] [CrossRef] [PubMed]
- Yagoubi, A.S.; Shahidi, F.; Mohebbi, M.; Varidi, M.; Golmohammadzadeh, S. Preparation, characterization and evaluation of physicochemical properties of phycocyanin-loaded solid lipid nanoparticles and nanostructured lipid carriers. J. Food Meas. Charact. 2018, 12, 378–385. [Google Scholar] [CrossRef]
- Hardiningtyas, S.D.; Wakabayashi, R.; Kitaoka, M.; Tahara, Y.; Minamihata, K.; Goto, M.; Kamiya, N. Mechanistic investigation of transcutaneous protein delivery using solid-in-oil nanodispersion: A case study with phycocyanin. Eur. J. Pharm. Biopharm. 2018, 127, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, L.M.; Latorre, A.C.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Phycocyanin-encapsulating hyalurosomes as carrier for skin delivery and protection from oxidative stress damage. J. Mater. Sci. Mater. Med. 2016, 27, 75. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Ma, Z.J.; Kong, L.Z.; He, Y.H.; Chan, H.F.; Li, H.Y. Modulation of macrophages by bioactive glass/sodium alginate hydrogel is crucial in skin regeneration enhancement. Biomaterials 2020, 256, 120216. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Iamtham, S. Physical extraction and extrusion entrapment of C-phycocyanin from Arthrospira platensis. J. King Saud Univ.-Sci. 2018, 31, 1535–1542. [Google Scholar] [CrossRef]
- Pradeep, H.; Nayak, C. Enhanced stability of C-phycocyanin colorant by extrusion encapsulation. J. Food Sci. Technol. 2019, 56, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Pan-Utai, W.; Kahapana, W.; Iamtham, S. Extraction of C-phycocyanin from Arthrospira (Spirulina) and its thermal stability with citric acid. J. Appl. Phycol. 2017, 30, 231–242. [Google Scholar] [CrossRef]
- Yan, M.Y.; Liu, B.; Jiao, X.D.; Qin, S. Preparation of phycocyanin microcapsules and its properties. Food Bioprod. Process. 2014, 92, 89–97. [Google Scholar] [CrossRef]
- Hashemikamangar, S.S.; Alsaedi, R.J.F.; Chiniforush, N.; Motevaselian, F. Effect of antimicrobial photodynamic therapy with different photosensitizers and adhesion protocol on the bond strength of resin composite to sound dentin. Clin. Oral Investig. 2022, 26, 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P. Chitosan nanoparticle-mediated delivery of curcumin and phycocyanin for photodynamic therapy against biofilm forming bacteria. Mater. Express 2020, 10, 1854–1870. [Google Scholar] [CrossRef]
- Zhang, B.C.; Zhang, X.W. Separation and nanoencapsulation of antitumor polypeptide from Spirulina platensis. Biotechnol. Prog. 2013, 29, 1230–1238. [Google Scholar] [CrossRef]
- Dev, A.; Mohanbhai, S.J.; Kushwaha, A.C.; Sood, A.; Sardoiwala, M.N.; Choudhury, S.R.; Karmakar, S. κ-carrageenan-C-phycocyanin based smart injectable hydrogels for accelerated wound recovery and real-time monitoring. Acta Biomater. 2020, 109, 121–131. [Google Scholar] [CrossRef]
- Yang, P.; Li, B.; Yin, Q.F.; Wang, Y.J. Carboxymethyl chitosan nanoparticles coupled with CD59-specific ligand peptide for targeted delivery of C-phycocyanin to HeLa cells. Tumor Biol. 2017, 39, 87–108. [Google Scholar] [CrossRef]
- Manconi, M.; Mura, S.; Manca, M.L.; Fadda, A.M.; Dolz, M.; Hernandez, M.J.; Casanovas, A.; Diez-Sales, O. Chitosomes as drug delivery systems for C-phycocyanin: Preparation and characterization. Int. J. Pharm. 2010, 392, 92–100. [Google Scholar] [CrossRef]
- Wang, X.Q.; Li, L.N.; Chang, W.R.; Zhang, J.P.; Gui, L.L.; Guo, B.J.; Liang, D.C. Structure of C-phycocyanin from Spirulina platensisat 2.2 Å resolution: A novel monoclinic crystal form for phycobiliproteins in phycobilisomes. Acta Crystallogr. 2001, 57, 784–792. [Google Scholar] [CrossRef]
- Ferraro, G.; Imbimbo, P.; Marseglia, A.; Lucignano, R.; Monti, D.M.; Merlino, A. X-ray structure of C-phycocyanin from Galdieria phlegrea: Determinants of thermostability and comparison with a C-phycocyanin in the entire phycobilisome. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148236. [Google Scholar] [CrossRef]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Böcker, L.; Hostettler, T.; Diener, M.; Eder, S.; Demuth, T.; Adamcik, J.; Reineke, K.; Leeb, E.; Nystrom, L.; Mathys, A. Time-temperature-resolved functional and structural changes of phycocyanin extracted from Arthrospira Platensis/Spirulina. Food Chem. 2020, 316, 126374. [Google Scholar] [CrossRef] [PubMed]
- Buecker, S.; Grossmann, L.; Loeffler, M.; Leeb, E.; Weiss, J. Thermal and acidic denaturation of phycocyanin from Arthrospira platensis: Effects of complexation with λ-carrageenan on blue color stability. Food Chem. 2022, 380, 132157. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Jespersen, L.; Stromdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Li, W.J.; Lu, C.Y.; Zhu, L.M.; Qin, S.; Du, Z.N. The effects of phycocyanin on bleomycin-induced pulmonary fibrosis and the intestinal microbiota in C57BL/6 mice. Appl. Microbiol. Biotechnol. 2019, 103, 8559–8569. [Google Scholar] [CrossRef]
- Bhat, V.B.; Madyastha, K.M. C-phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochem. Biophys. Res. Commun. 2000, 275, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.Y.; Gao, B.; Gao, Y.N.; Yang, X.G.; Cheng, X.D.; Ou, Y. Phycocyanin inhibits tumorigenic potential of pancreatic cancer cells: Role of apoptosis and autophagy. Sci. Rep. 2016, 6, 34564. [Google Scholar] [CrossRef]
- Imai, Y.; Koseki, Y.; Hirano, M.; Nakamura, S. Nutrigenomic studies on the ameliorative effect of enzyme-digested phycocyanin in alzheimer’s disease model mice. Nutrients 2021, 13, 4431. [Google Scholar] [CrossRef]
- Wang, C.Y. Mechanism study of apoptosis induced by microcystic phycocyanin-mediated photodynamic therapy in liver cancer. Anhui Univ. 2012, 04, 1–42. [Google Scholar] [CrossRef]
- Lu, L.; Li, W.J.; Sun, C.; Kang, S.H.; Li, J.; Luo, X.P.; Su, Q.; Liu, B.; Qin, S. Phycocyanin ameliorates radiation-induced acute intestinal toxicity by regulating the effect of the gut microbiota on the TLR4/Myd88/NF-κB pathway. J. Parenter. Enter. Nutr. 2019, 44, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Lu, L.; Liu, B.; Qin, S. Effects of phycocyanin on pulmonary and gut microbiota in a radiation-induced pulmonary fibrosis model. Biomed. Pharmacother. 2020, 132, 110826. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Enhanced microencapsulation of C-phycocyanin from arthrospira by freeze-drying with different wall materials. Food Technol. Biotechnol. 2020, 58, 423–432. [Google Scholar] [CrossRef]
- Park, S.; Jung, S.; Heo, J.; Koh, W.G.; Lee, S.M.; Hong, J.K. Chitosan/cellulose-based porous nanofilm delivering C-phycocyanin: A novel platform for the production of cost-effective cultured meat. ACS Appl. Mater. Interfaces 2021, 13, 32193–32204. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.V.F.; Opretzka, L.; Almeida, L.S.; Cardoso, L.G.; Sliva, J.B.A.D.; Souza, C.O.D.; Villarreal, C.F.; Druzian, J.I. Preparation and characterization of C-phycocyanin coated with STMP/STPP cross-linked starches from different botanical sources. Int. J. Biol. Macromol. 2020, 159, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.; Zhong, Z.; Li, Y.; Li, W.J.; Guo, Y.R.; Zhang, R.G.; Yang, X.B.; Peng, H.L. Load phycocyanin to achieve in vivo imaging of casein-porous starch microgels induced by ultra-high-pressure homogenization. Int. J. Biol. Macromol. 2021, 193, 127–136. [Google Scholar] [CrossRef]
- Zhang, Z.; Cho, S.; Dadmohammadi, Y.; Li, Y.; Abbaspourrad, A. Improvement of the storage stability of C-phycocyanin in beverages by high-pressure processing. Food Hydrocoll. 2020, 110, 106055. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Dadmohammadi, Y.; Li, Y.; Jaiswal, A.; Abbaspourrad, A. Whey protein improves the stability of C-phycocyanin in acidified conditions during light storage. Food Chem. 2020, 344, 128642. [Google Scholar] [CrossRef]
- Tong, F.; Tang, X.Y.; Liu, D.J. Phycocyanin/PEG-b-(PG-g-PEI) attenuated hepatic ischemia/reperfusion-induced pancreatic islet injury and enlarged islet functionality. Int. J. Nanomed. 2019, 14, 339–351. [Google Scholar] [CrossRef]
- Chen, D.Y.; Suo, M.; Guo, J.C.; Tang, W.X.; Jiang, W.; Liu, Y.; Duo, Y.H. Development of MOF “Armor-Plated” phycocyanin and synergistic inhibition of cellular respiration for hypoxic photodynamic therapy in patient-derived xenograft models. Adv. Healthc. Mater. 2020, 10, 2001577. [Google Scholar] [CrossRef]
- Munawaroh, H.; Gumilar, G.G.; Alifia, C.R.; Marthania, M.; Stellasary, B.; Yuliani, G.; Wulandari, A.P.; Kurniawan, I.; Hidayat, R.; Ningrum, A.; et al. Photostabilization of Phycocyanin from Spirulina platensis modified by Formaldehyde. Process Biochem. 2020, 94, 297–304. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Danielsen, S.P.O.; Panyukov, S.; Rubinstein, M. Ion pairing and the structure of gel coacervates. Macromolecules 2020, 53, 9420–9442. [Google Scholar] [CrossRef] [PubMed]
- McTigue, W.C.B.; Perry, S.L. Protein encapsulation using complex coacervates: What nature has to teach us. Small 2020, 16, 1907671. [Google Scholar] [CrossRef] [PubMed]
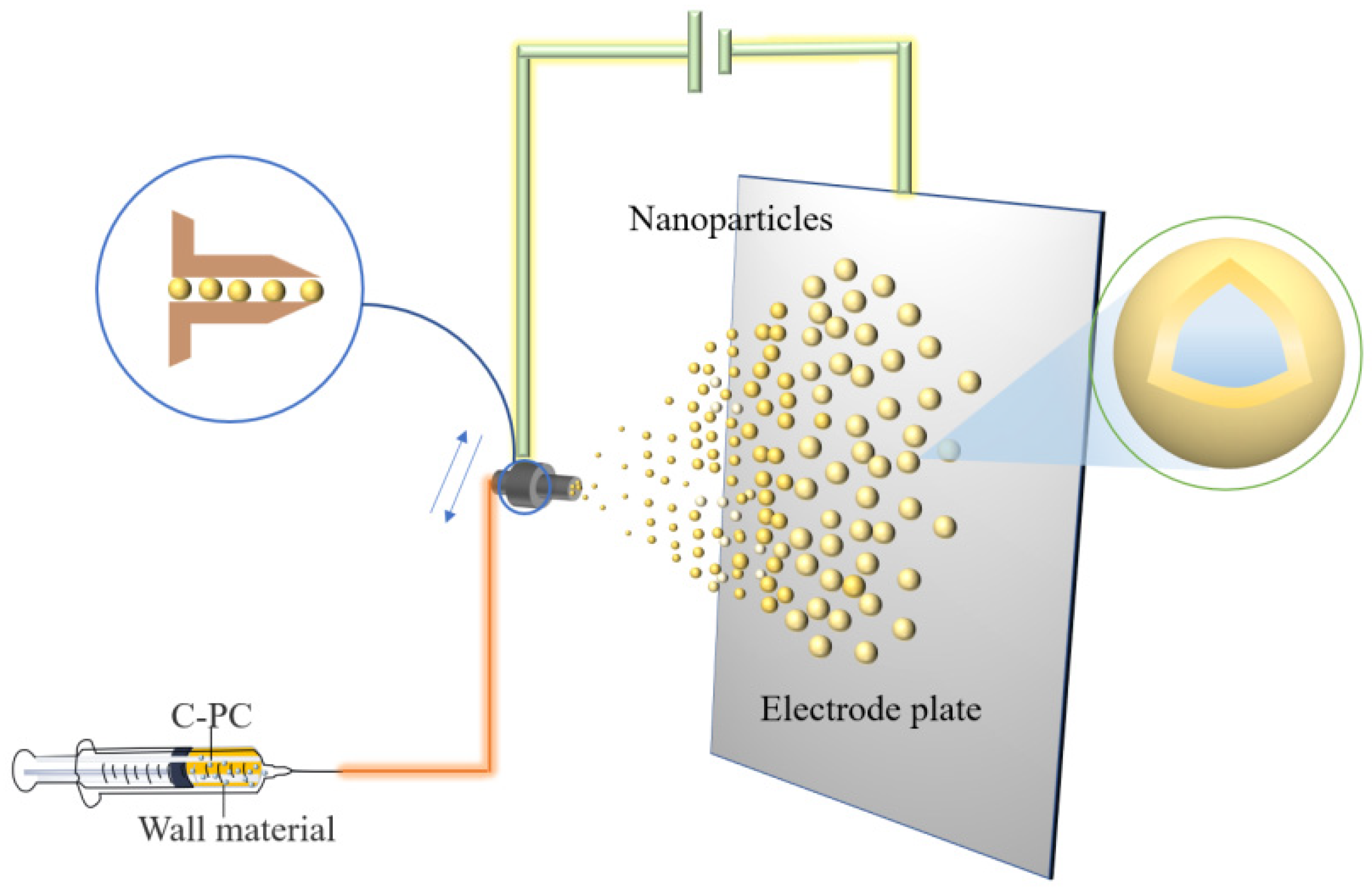
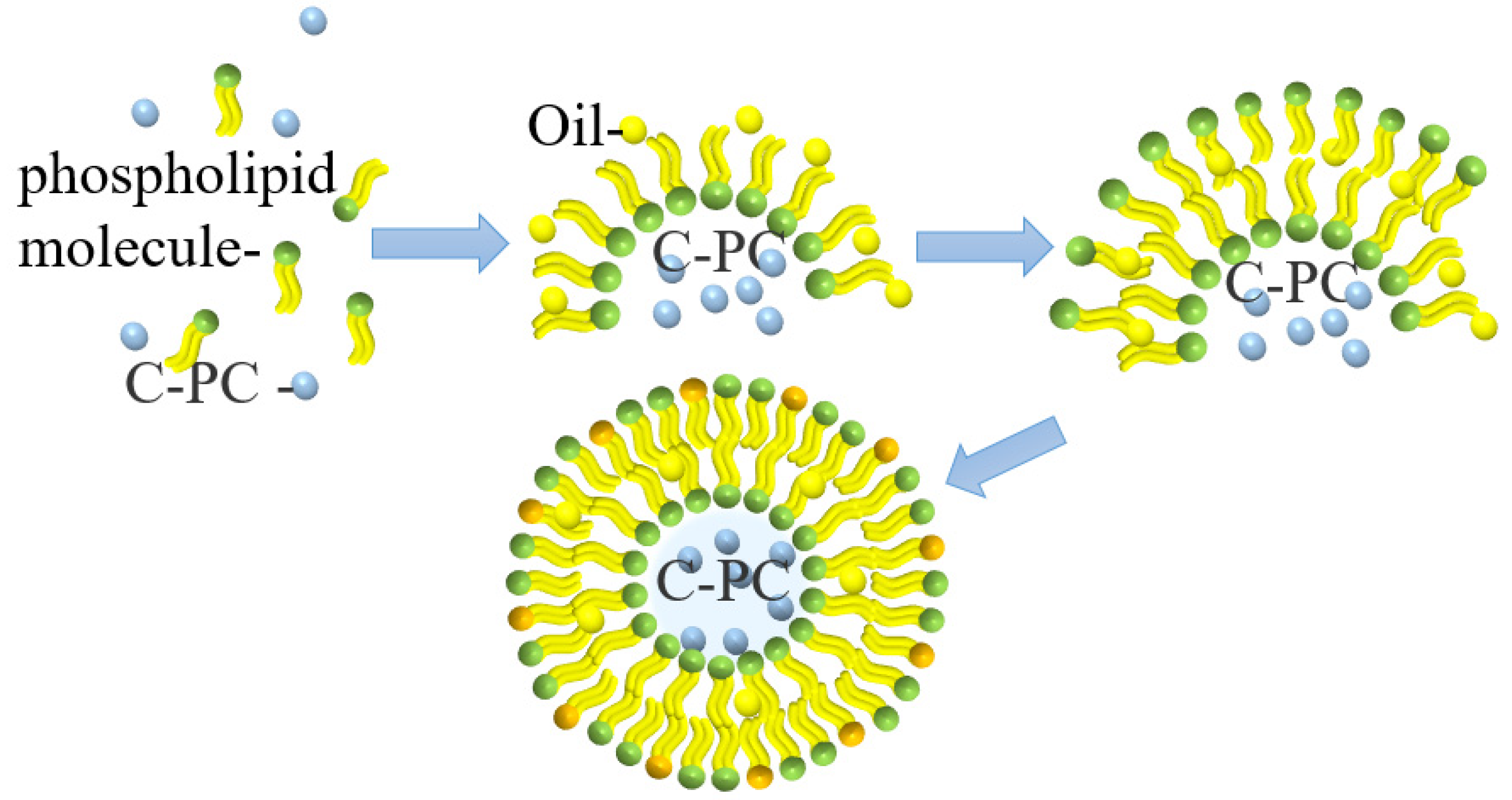
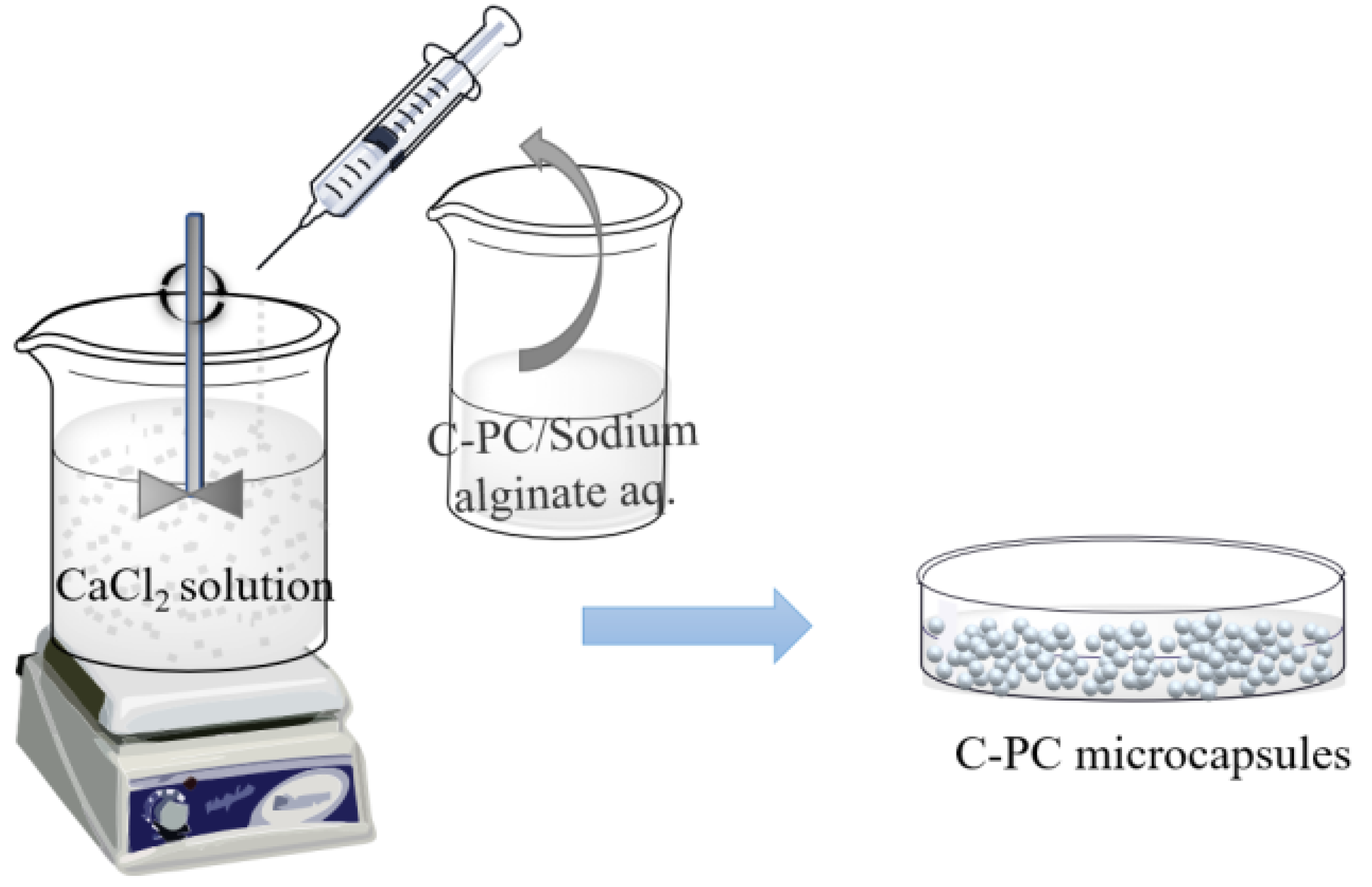
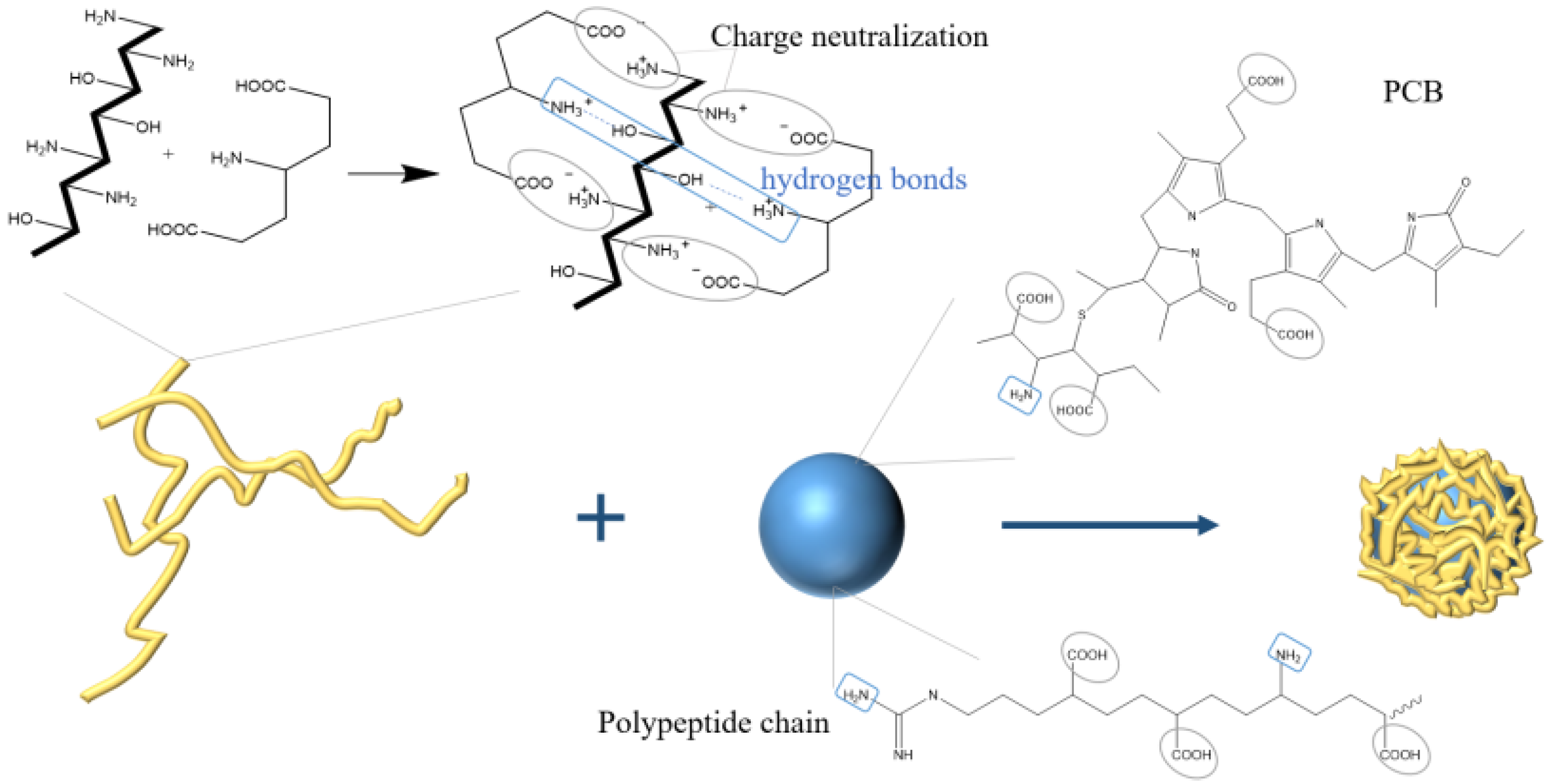
| Method | Benefits | Drawbacks | Reference |
|---|---|---|---|
| Spray drying | Rapid, cost-effective, easy to scale up. C-PC retention, and relatively good storage stability. | Production of nonuniform particles with a wide size distribution. C-PC degradation and loss of product. | [19,20,21,22,23,24] |
| Electrospinning | Suitable for heat-sensitive compounds. | Slow, produces low encapsulation yield, and limited scope of application. | [25,26,27,28,29] |
| Electrospraying | Produces particles with a high surface-to-volume ratio, controlled release, improved functionality, and physical properties. | Time-consuming and hardly repeatable, especially at the industrial level. | [30,31,32] |
| Liposome delivery | Increased adsorption bioavailability, and nontoxic. | Low encapsulation efficiency. Lipid oxidation, poor physicochemical stability, and wide particle size distribution. Postprocess step is required. | [33,34,35,36,37] |
| Sharp-hole coagulation bath | High loading capacity, low temperature operating requirements, reduced evaporation losses of volatile compounds, and thermal degradation. Tailored release of active compounds. | Special instruments are required and are not suitable for large-scale industrial production. | [38,39,40,41,42] |
| Ion Gelation | Low cost and does not require advanced equipment, high temperatures, and organic solvents. | Produced particles are very sensitive to pH and ionic strength. Agglomeration of particles and particle size control. | [43,44,45,46,47,48,49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, X.; Liang, Z.-P.; Chang, X.-Y.; Li, F.-T.; Wang, X.-Q.; Lian, X.-J. Progress of Microencapsulated Phycocyanin in Food and Pharma Industries: A Review. Molecules 2022, 27, 5854. https://doi.org/10.3390/molecules27185854
Li Y, Li X, Liang Z-P, Chang X-Y, Li F-T, Wang X-Q, Lian X-J. Progress of Microencapsulated Phycocyanin in Food and Pharma Industries: A Review. Molecules. 2022; 27(18):5854. https://doi.org/10.3390/molecules27185854
Chicago/Turabian StyleLi, Yang, Xu Li, Zi-Peng Liang, Xin-Ying Chang, Fu-Tong Li, Xue-Qing Wang, and Xi-Jun Lian. 2022. "Progress of Microencapsulated Phycocyanin in Food and Pharma Industries: A Review" Molecules 27, no. 18: 5854. https://doi.org/10.3390/molecules27185854
APA StyleLi, Y., Li, X., Liang, Z.-P., Chang, X.-Y., Li, F.-T., Wang, X.-Q., & Lian, X.-J. (2022). Progress of Microencapsulated Phycocyanin in Food and Pharma Industries: A Review. Molecules, 27(18), 5854. https://doi.org/10.3390/molecules27185854






