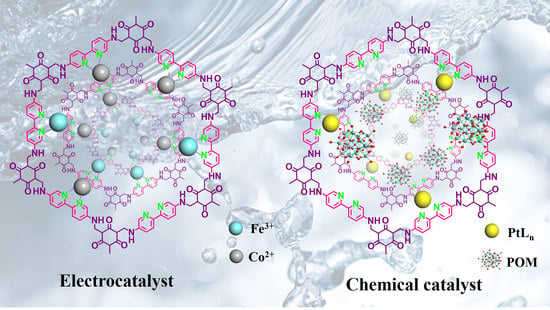
Covalent Organic Frameworks Composites Containing Bipyridine Metal Complex for Oxygen Evolution and Methane Conversion
Abstract
:1. Introduction
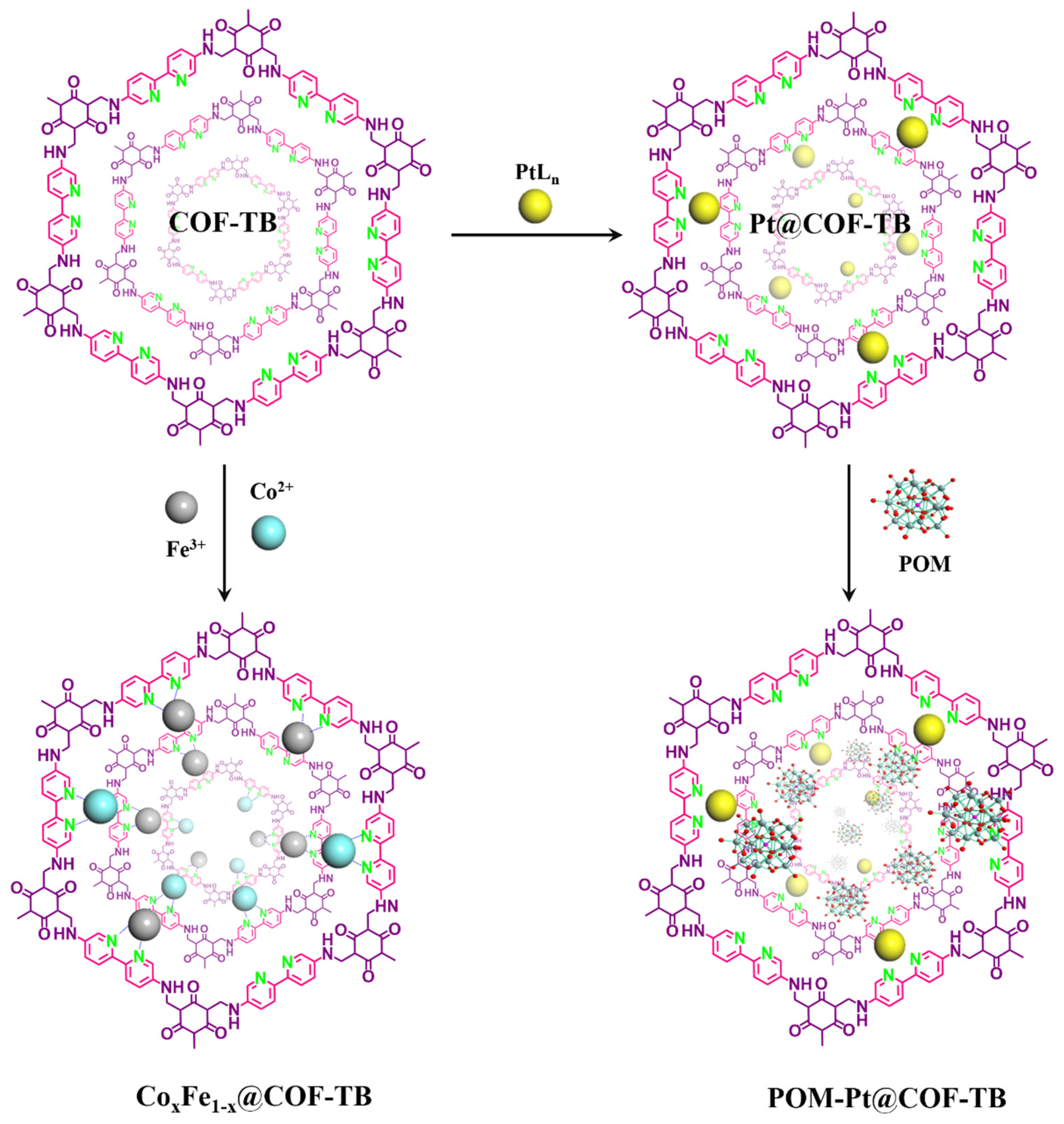
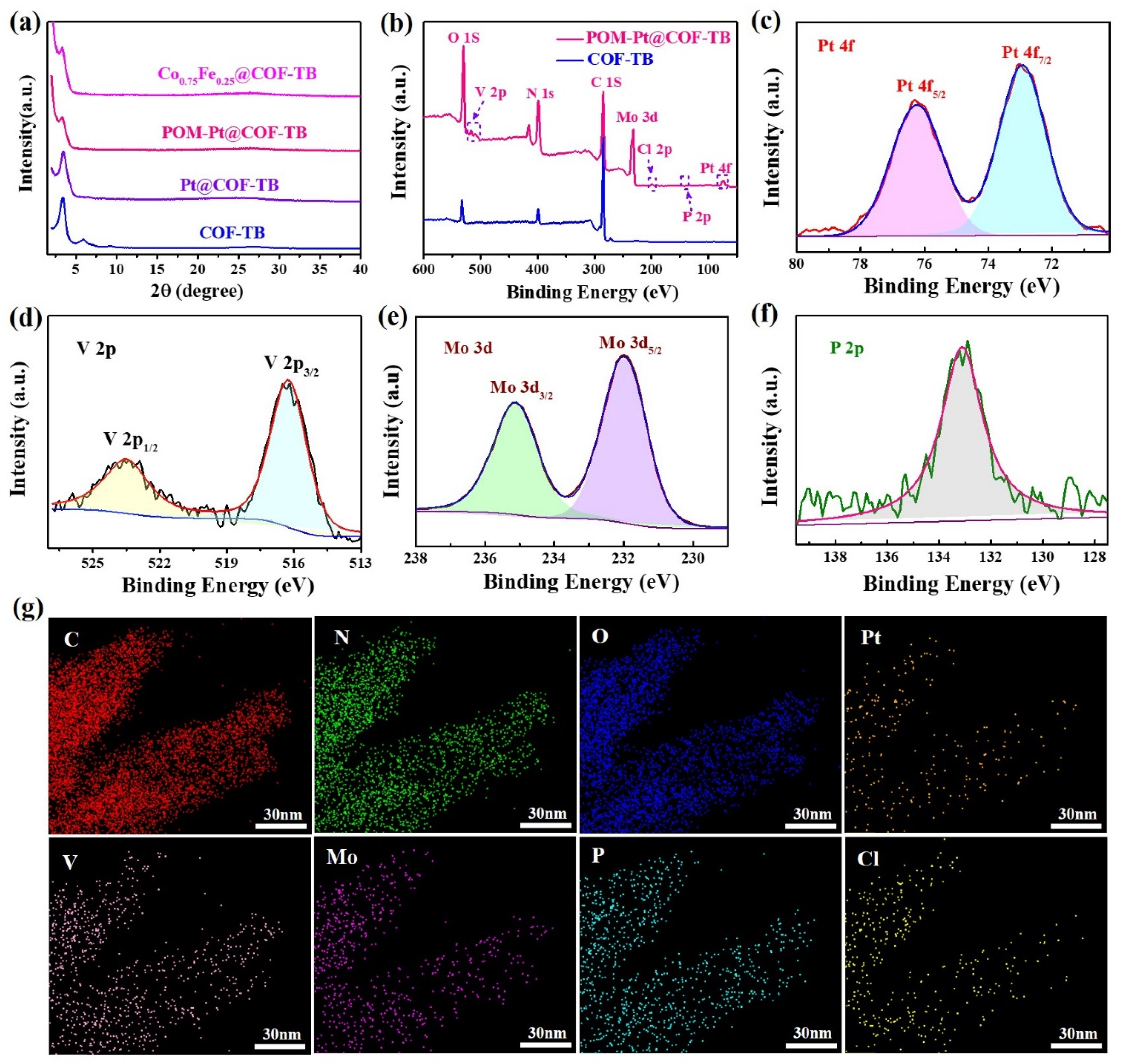
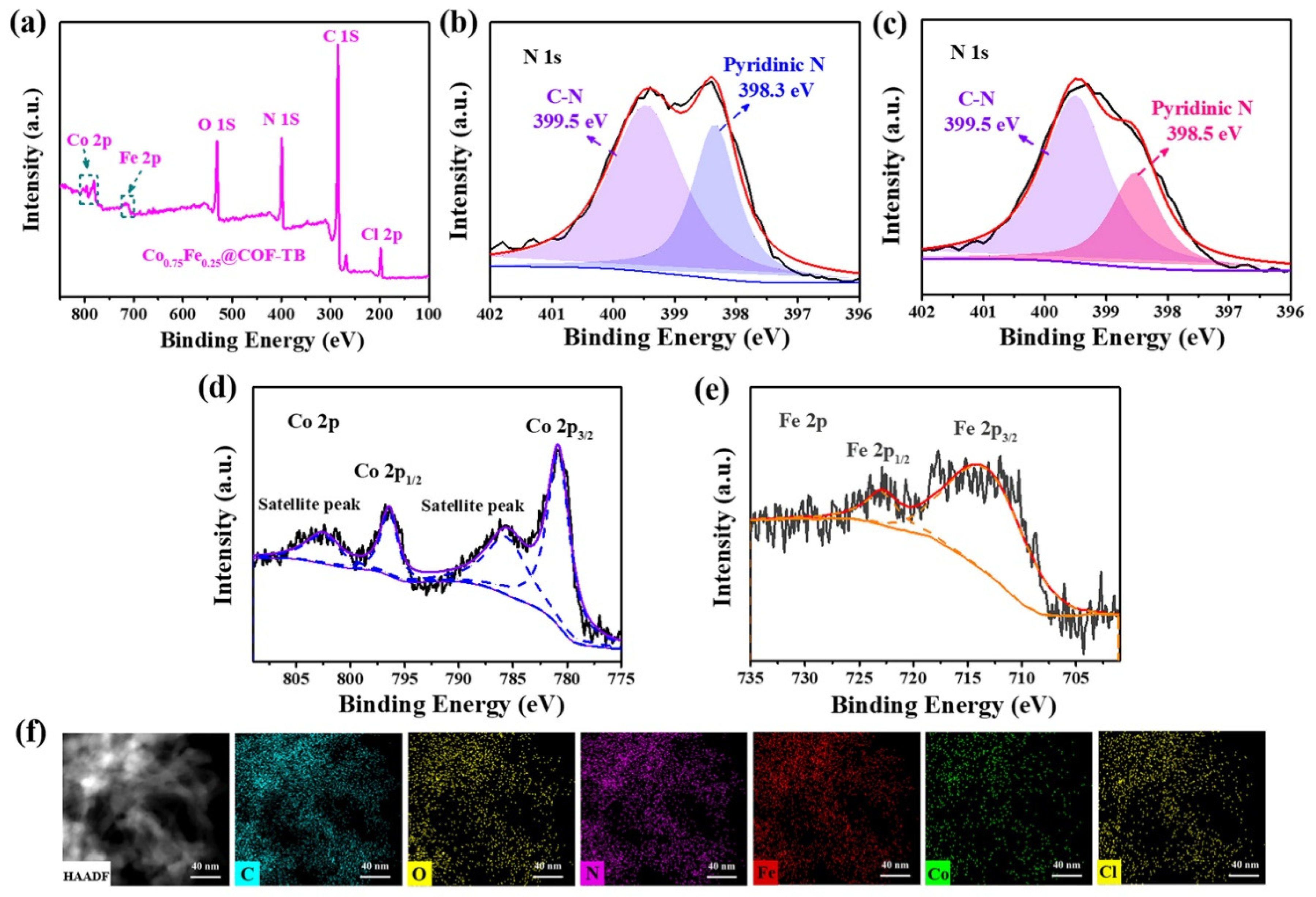
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of CoxFe1-x@COF–TB (x = 0, 0.25, 0.5, 0.75, 1)
3.3. Synthesis of Pt@COF–TB and POM–Pt@COF–TB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Losantos, R.; Sampedro, D. Design and tuning of photoswitches for solar energy storage. Molecules 2021, 26, 3796. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, L.; Pasini, M.; Menna, E. Organic functionalized carbon nanostructures for solar energy conversion. Molecules 2021, 26, 5286. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zeng, L.; Lei, A. Oxidative R1–H/R2–H cross-coupling with hydrogen evolution. J. Am. Chem. Soc. 2018, 140, 13128–13135. [Google Scholar] [CrossRef]
- Pradeep, N.; Tamil Selvi, G.; Venkatraman, U.; Van Le, Q.; Jeong, S.K.; Pandiaraj, S.; Alodhayb, A.; Muthuramamoorthy, M.; Grace, A.N. Development and investigation of the flexible hydrogen sensor based on ZnO-decorated Sb2O3 nanobelts. Mater. Today Chem. 2021, 22, 100576. [Google Scholar] [CrossRef]
- Sorcar, S.; Das, J.; Komarala, E.P.; Fadeev, L.; Rosen, B.A.; Gozin, M. Design of coke-free methane dry reforming catalysts by molecular tuning of nitrogen-rich combustion precursors. Mater. Today Chem. 2022, 24, 100765. [Google Scholar] [CrossRef]
- Connolly, B.M.; Aragones-Anglada, M.; Gandara-Loe, J.; Danaf, N.A.; Lamb, D.C.; Mehta, J.P.; Vulpe, D.; Wuttke, S.; Silvestre-Albero, J.; Moghadam, P.Z.; et al. Tuning porosity in macroscopic monolithic metal-organic frameworks for exceptional natural gas storage. Nat. Commun. 2019, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; He, Q.; Yang, G.; Zhou, W.; Shao, Z.; Ni, M. Recent advances and prospective in ruthenium-based materials for electrochemical water splitting. ACS Catal. 2019, 9, 9973–10011. [Google Scholar] [CrossRef]
- Bai, L.; Duan, Z.; Wen, X.; Si, R.; Zhang, Q.; Guan, J. Highly dispersed ruthenium-based multifunctional electrocatalyst. ACS Catal. 2019, 9, 9897–9904. [Google Scholar] [CrossRef]
- Jensen, A.W.; Sievers, G.W.; Jensen, K.D.; Quinson, J.; Arminio-Ravelo, J.A.; Brüser, V.; Arenz, M.; Escudero-Escribano, M. Self-supported nanostructured iridium-based networks as highly active electrocatalysts for oxygen evolution in acidic media. J. Mater. Chem. A 2020, 8, 1066–1071. [Google Scholar] [CrossRef]
- Xie, P.; Pu, T.; Nie, A.; Hwang, S.; Purdy, S.C.; Yu, W.; Su, D.; Miller, J.T.; Wang, C. Nanoceria-supported single-atom platinum catalysts for direct methane conversion. ACS Catal. 2018, 8, 4044–4048. [Google Scholar] [CrossRef]
- Tomkins, P.; Ranocchiari, M.; van Bokhoven, J.A. Direct conversion of methane to methanol under mild conditions over cu-zeolites and beyond. Acc. Chem. Res. 2017, 50, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Gao, Z.; Xiao, L.; Lai, Z.; Luo, F. A Ni/Fe complex incorporated into a covalent organic framework as a single-site heterogeneous catalyst for efficient oxygen evolution reaction. Inorg. Chem. Front. 2020, 7, 3925–3931. [Google Scholar] [CrossRef]
- Fernandes, S.P.S.; Mellah, A.; Kovář, P.; Sárria, M.P.; Pšenička, M.; Djamila, H.; Salonen, L.M.; Espiña, B. Extraction of ibuprofen from natural waters using a covalent organic framework. Molecules 2020, 25, 3132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, S.; Jin, T.; Gao, F.; Wang, M.; Gao, Y.; Zhang, W.; Ouyang, Y.; Ye, G. Selective entrapment of thorium using a three-dimensional covalent organic framework and its interaction mechanism study. Sep. Purif. Technol. 2022, 296, 121413. [Google Scholar] [CrossRef]
- Zhao, Y.; Sui, Z.; Chang, Z.; Wang, S.; Liang, Y.; Liu, X.; Feng, L.; Chen, Q.; Wang, N. A trifluoromethyl-grafted ultra-stable fluorescent covalent organic framework for adsorption and detection of pesticides. J. Mater. Chem. A 2020, 8, 25156–25164. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Liang, Y.; Zhao, Y.; Yuan, N.; Sui, Z.; Chen, Q. Adenine-bearing covalent organic frameworks via one-pot tandem reaction for selective adsorption of Ag+. Microporous Mesoporous Mater. 2021, 315, 110923. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, D.; Wang, G. Covalent organic frameworks for chemical and biological sensing. Molecules 2022, 27, 2586. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liang, Y.; Zhao, Y.; Xia, M.; Liu, X.; Shen, T.; Feng, L.; Yuan, N.; Chen, Q. Fluorescent test paper via the in situ growth of COFs for rapid and convenient detection of Pd(II) ions. ACS Appl. Mater. Interfaces 2021, 13, 1644–1650. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent organic polymers and frameworks for fluorescence-based sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef]
- Baldwin, L.A.; Crowe, J.W.; Shannon, M.D.; Jaroniec, C.P.; McGrier, P.L. 2D covalent organic frameworks with alternating triangular and hexagonal pores. Chem. Mater. 2015, 27, 6169–6172. [Google Scholar] [CrossRef]
- Liang, Y.; Xia, M.; Zhao, Y.; Wang, D.; Li, Y.; Sui, Z.; Xiao, J.; Chen, Q. Functionalized triazine-based covalent organic frameworks containing quinoline via aza-Diels-Alder reaction for enhanced lithium-sulfur batteries performance. J. Colloid Interface Sci. 2022, 608, 652–661. [Google Scholar] [CrossRef]
- Liu, X.; Xia, M.; Zhao, Y.; Xia, T.; Li, Y.; Xiao, J.; Sui, Z.; Chen, Q. Cationic covalent organic framework via cycloaddition reactions as sulfur-loaded matrix for lithium-sulfur batteries. Mater. Today Chem. 2022, 23, 100664. [Google Scholar] [CrossRef]
- Liang, Y.; Xia, T.; Wu, Z.; Yang, Y.; Li, Y.; Sui, Z.; Li, C.; Fan, R.; Tian, X.; Chen, Q. Tetrazole-functionalized two-dimensional covalent organic frameworks coordinated with metal ions for electrocatalytic oxygen evolution reaction. Mater. Today Chem. 2022, 24, 100777. [Google Scholar] [CrossRef]
- Xie, M.; Li, C.; Ren, S.; Ma, Y.; Chen, X.; Fan, X.; Han, Y.; Shi, Z.; Feng, S. Ultrafine Sb nanoparticles in situ confined in covalent organic frameworks for high-performance sodium-ion battery anodes. J. Mater. Chem. A 2022, 28, 15089–15100. [Google Scholar] [CrossRef]
- Calik, M.; Auras, F.; Salonen, L.M.; Bader, K.; Grill, I.; Handloser, M.; Medina, D.D.; Dogru, M.; Löbermann, F.; Trauner, D.; et al. Extraction of photogenerated electrons and holes from a covalent organic framework integrated heterojunction. J. Am. Chem. Soc. 2014, 136, 17802–17807. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Furukawa, K.; Gao, J.; Nagai, A.; Nakamura, T.; Dong, Y.; Jiang, D. Photoelectric covalent organic frameworks: Converting open lattices into ordered donor–acceptor heterojunctions. J. Am. Chem. Soc. 2014, 136, 9806–9809. [Google Scholar] [CrossRef]
- Ren, X.; Liao, G.; Li, Z.; Qiao, H.; Zhang, Y.; Yu, X.; Wang, B.; Tan, H.; Shi, L.; Qi, X.; et al. Two-dimensional MOF and COF nanosheets for next-generation optoelectronic applications. Coord. Chem. Rev. 2021, 435, 213781. [Google Scholar] [CrossRef]
- Keller, N.; Bein, T. Optoelectronic processes in covalent organic frameworks. Chem. Soc. Rev. 2021, 50, 1813–1845. [Google Scholar] [CrossRef]
- Li, J.; Zhao, D.; Liu, J.; Liu, A.; Ma, D. Covalent organic frameworks: A promising materials platform for photocatalytic CO2 reductions. Molecules 2020, 25, 2425. [Google Scholar] [CrossRef]
- Li, Y.; Zuo, K.; Gao, T.; Wu, J.; Su, X.; Zeng, C.; Xu, H.; Hu, H.; Zhang, X.; Gao, Y. Bimetallic docked covalent organic frameworks with high catalytic performance towards coupling/oxidation cascade reactions. RSC Adv. 2022, 12, 4874–4882. [Google Scholar] [CrossRef]
- Chai, Y.; Li, Y.; Hu, H.; Zeng, C.; Wang, S.; Xu, H.; Gao, Y. N-heterocyclic carbene functionalized covalent organic framework for transesterification of glycerol with dialkyl carbonates. Catalysts 2021, 11, 423. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, W.; Liang, L.; Xu, Z.; Li, X.; Qiao, S. Prevailing conjugated porous polymers for electrochemical energy storage and conversion: Lithium-ion batteries, supercapacitors and water-splitting. Coord. Chem. Rev. 2021, 436, 213782. [Google Scholar] [CrossRef]
- Vardhan, H.; Pan, Y.; Yang, Z.; Verma, G.; Nafady, A.; Al-Enizi, A.M.; Alotaibi, T.M.; Almaghrabi, O.A.; Ma, S. Iridium complex immobilization on covalent organic framework for effective C—H borylation. APL Mater. 2019, 7, 101111. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, H.-Y.; Zheng, H.; Zhang, W.; Cao, R. Porphyrin-based frameworks for oxygen electrocatalysis and catalytic reduction of carbon dioxide. Chem. Soc. Rev. 2021, 50, 2540–2581. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Lai, J.; Xu, G. Recent advances on electrocatalysis using pristinely conductive metal-organic frameworks and covalent organic frameworks. ChemElectroChem 2021, 8, 2764–2777. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, Z.; Huang, Y.; He, X.; Su, X.; Xiao, L.; Yu, Y.; Huang, X.; Luo, F. Flexible and robust bimetallic covalent organic frameworks for the reversible switching of electrocatalytic oxygen evolution activity. J. Mater. Chem. A 2020, 8, 5907–5912. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Perman, J.; Nguyen, N.; Ma, S. Flexibility matters: Cooperative active sites in covalent organic framework and threaded ionic polymer. J. Am. Chem. Soc. 2016, 138, 15790–15796. [Google Scholar] [CrossRef]
- Aiyappa, H.B.; Thote, J.; Shinde, D.B.; Banerjee, R.; Kurungot, S. Cobalt-modified covalent organic framework as a robust water oxidation electrocatalyst. Chem. Mater. 2016, 28, 4375–4379. [Google Scholar] [CrossRef]
- Barats-Damatov, D.; Shimon, L.J.W.; Feldman, Y.; Bendikov, T.; Neumann, R. Solid-state crystal-to-crystal phase transitions and reversible structure–temperature behavior of phosphovanadomolybdic acid, H5PV2Mo10O40. Inorg. Chem. 2015, 54, 628–634. [Google Scholar] [CrossRef]
- Predoeva, A.; Damyanova, S.; Gaigneaux, E.M.; Petrov, L. The surface and catalytic properties of titania-supported mixed PMoV heteropoly compounds for total oxidation of chlorobenzene. Appl. Catal. A 2007, 319, 14–24. [Google Scholar] [CrossRef]
- Chavan, L.D.; Shankarwar, S.G. KSF supported 10-molybdo-2-vanadophosphoric acid as an efficient and reusable catalyst for one-pot synthesis of 2,4,5-trisubstituted imidazole derivatives under solvent-free condition. Chin. J. Catal. 2015, 36, 1054–1059. [Google Scholar] [CrossRef]
- Bar-Nahum, I.; Khenkin, A.M.; Neumann, R. Mild, aqueous, aerobic, catalytic oxidation of methane to methanol and acetaldehyde catalyzed by a supported bipyrimidinylplatinum-polyoxometalate hybrid compound. J. Am. Chem. Soc. 2004, 126, 10236–10237. [Google Scholar] [CrossRef]
- Shavi, R.; Ko, J.; Cho, A.; Han, J.W.; Seo, J.G. Mechanistic insight into the quantitative synthesis of acetic acid by direct conversion of CH4 and CO2: An experimental and theoretical approach. Appl. Catal. B 2018, 229, 237–248. [Google Scholar] [CrossRef]
- Saveant, J.-M.; Tard, C. Attempts to catalyze the electrochemical CO2-to-methanol conversion by biomimetic 2e− + 2H+ transferring molecules. J. Am. Chem. Soc. 2016, 138, 1017–1021. [Google Scholar] [CrossRef]
- Ghanghas, R.; Jindal, A.; Vasudevan, S. Geometry of hydrogen bonds in liquid ethanol probed by proton NMR experiments. J. Phys. Chem. B 2020, 124, 662–667. [Google Scholar] [CrossRef]
- Chandra, S.; Kundu, T.; Dey, K.; Addicoat, M.; Heine, T.; Banerjee, R. Interplaying intrinsic and extrinsic proton conductivities in covalent organic frameworks. Chem. Mater. 2016, 28, 1489–1494. [Google Scholar] [CrossRef]
- Elko-Hansen, T.D.M.; Ekerdt, J.G. XPS investigation of the atomic layer deposition half reactions of bis(N-tert-butyl-N′-ethylpropionamidinato) cobalt(II). Chem. Mater. 2014, 26, 2642–2646. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Xia, T.; Tian, H.; Li, Y.; Sui, Z.; Yuan, N.; Tian, X.; Chen, Q. Pyrimidine-functionalized covalent organic framework and its cobalt complex as an efficient electrocatalyst for oxygen evolution reaction. ChemSusChem 2021, 14, 4556–4562. [Google Scholar] [CrossRef]
- Tsigdinos, G.A.; Hallada, C.J. Molybdovanadophosphoric acids and their salts. I. Investigation of methods of preparation and characterization. Inorg. Chem. 1968, 7, 437–441. [Google Scholar] [CrossRef]
- Xia, M.; Qiu, L.; Li, Y.; Shen, T.; Sui, Z.; Feng, L.; Chen, Q. A metal-organic frameworks composite catalyst containing platinum and polyoxometalate for direct conversion of methane. Mater. Lett. 2022, 307, 131078. [Google Scholar] [CrossRef]
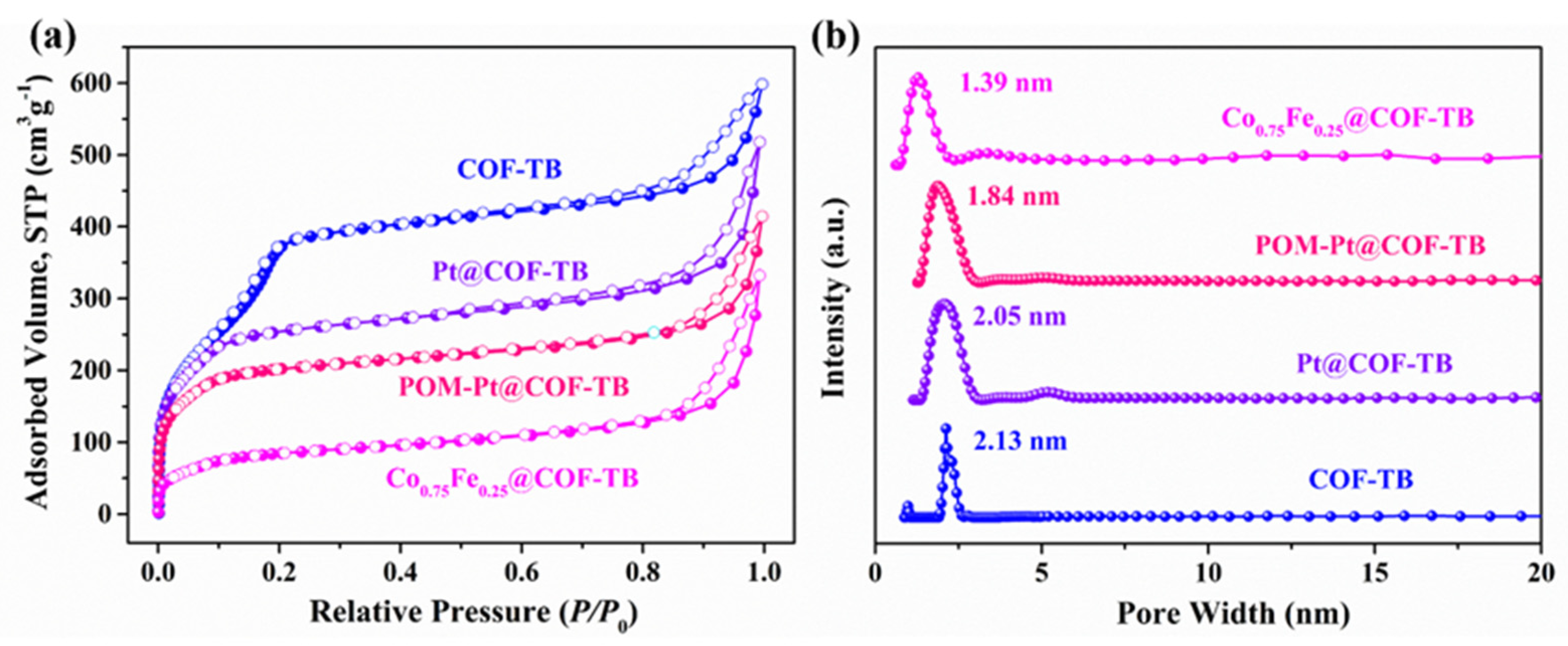

| COFs | SBET (m2 g−1) a | SL (m2 g−1) b | Vtotal (cm3 g−1) c | Dpore (nm) d |
|---|---|---|---|---|
| COF–TB | 1216 | 1263 | 1.002 | 2.13 |
| Pt@COF–TB | 965 | 1207 | 0.8008 | 2.05 |
| POM–Pt@COF–TB | 738 | 892 | 0.6408 | 1.84 |
| Co0.75Fe0.25@COF–TB | 319 | 369 | 0.5138 | 1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Feng, L.; Li, Y.; Xia, T.; Sui, Z.; Chen, Q. Covalent Organic Frameworks Composites Containing Bipyridine Metal Complex for Oxygen Evolution and Methane Conversion. Molecules 2022, 27, 5193. https://doi.org/10.3390/molecules27165193
Liu X, Feng L, Li Y, Xia T, Sui Z, Chen Q. Covalent Organic Frameworks Composites Containing Bipyridine Metal Complex for Oxygen Evolution and Methane Conversion. Molecules. 2022; 27(16):5193. https://doi.org/10.3390/molecules27165193
Chicago/Turabian StyleLiu, Xin, Lijuan Feng, Yongpeng Li, Tian Xia, Zhuyin Sui, and Qi Chen. 2022. "Covalent Organic Frameworks Composites Containing Bipyridine Metal Complex for Oxygen Evolution and Methane Conversion" Molecules 27, no. 16: 5193. https://doi.org/10.3390/molecules27165193
APA StyleLiu, X., Feng, L., Li, Y., Xia, T., Sui, Z., & Chen, Q. (2022). Covalent Organic Frameworks Composites Containing Bipyridine Metal Complex for Oxygen Evolution and Methane Conversion. Molecules, 27(16), 5193. https://doi.org/10.3390/molecules27165193