Abstract
Gastric cancer is a common type of cancer that poses a serious threat to human health. Polysaccharides are important functional phytochemicals, and research shows that polysaccharides have good anti-gastric cancer effects. We collated all relevant literature published from 2000 to 2020 and found that more than 60 natural polysaccharides demonstrate anti-gastric cancer activity. At the present, the sources of these polysaccharides include fungi, algae, tea, Astragalus membranaceus, Caulis Dendrobii, and other foods and Chinese herbal medicines. By regulating various signaling pathways, including the PI3K/AKT, MAPK, Fas/FasL, Wnt/β-catenin, IGF-IR, and TGF-β signaling pathways, polysaccharides induce gastric cancer cell apoptosis, cause cell cycle arrest, and inhibit migration and invasion. In addition, polysaccharides can enhance the immune system and killing activity of immune cells in gastric cancer patients and rats. This comprehensive review covers the extraction, purification, structural characterization, and mechanism of plant and fungal polysaccharides against gastric cancer. We hope this review is helpful for researchers to design, research, and develop plant and fungal polysaccharides.
1. Introduction
Bray et al. evaluated global cancer incidence and mortality according to GLOBOCAN 2018, provided by the International Agency for Research on Cancer. Research showed that gastric cancer is still a serious threat to human health. There are more than one million new cases of gastric cancer and more than 700,000 deaths reported worldwide every year. Gastric cancer has become the fifth most frequently diagnosed cancer, and it is the third leading cause of cancer-related death [1]. At present, the clinical treatment of gastric cancer is largely chemotherapy, but these medicines cause adverse reactions. Fluorouracil can cause a loss of appetite, nausea, vomiting, and diarrhea [2]. Nitrosourea can damage liver and kidney functions [3]. Mitomycin and adriamycin are cardiotoxic [4,5]. Cytarabine can cause myelosuppression and adverse gastrointestinal reactions [6]. Therefore, researchers are looking towards natural medicines as potential medicines with higher anti-gastric cancer activity and lower toxicity. Currently, polysaccharides are used in biochemistry and medicine due to their remarkable therapeutic effects and low toxicity [7]. Moreover, due to these polysaccharides’ significant anti-gastric cancer activity, they have been used as food additives or medicines [8,9].
In this review, we summarize the anti-gastric mechanisms of polysaccharides, including their ability to induce apoptosis; inhibit proliferation, migration and invasion, and angiogenesis; regulate immune response; and enhance the anticancer effect of anticancer medicines. Sources of these polysaccharides include fungi, algae, Astragalus membranaceus, tea, Caulis Dendrobii, and other foods and Chinese herbal medicines. In addition, we briefly introduce the extraction, purification, and structural characterization of these polysaccharides. Of these polysaccharides, clinical trials show that the polysaccharide PSK (from the Basidiomycete Coriolus versicolor) can significantly improve the therapeutic effect of chemotherapy drugs in gastric cancer patients. However, other polysaccharides are still in the cell experiment or animal experiment phases. We believe that it is necessary to carry out major clinical research to further explore the anti-gastric cancer activity of these polysaccharides. In addition, exploring the relationship between structural characteristics and anti-gastric cancer activity may also be a focus of future research. In short, we hope this review provides guidance for researchers to design, research, and develop polysaccharides.
2. Search Strategy
Literature searches were performed using four databases: PubMed, Web of Science (WOS/SCI), Google Scholar, and CNKI (China National Knowledge Infrastructure). Three were English databases (PubMed, Web of Science, and Google Scholar), and one was a Chinese database (CNKI). The keywords used were “polysaccharides”, “plants,” “fungi”, “gastric cancer”, “Anti-gastric cancer”, and “mechanism”. We searched all of the relevant literature published in the last 20 years from 2000 to 2020 that was published in English. The review was completed by searching for bibliographic references and definitions of the topic described above.
3. Extraction of Polysaccharides
Polysaccharides are polar macromolecules, which are usually soluble in water but not soluble in organic solvents. Therefore, water is currently used as the extraction medium. As they need to be extracted multiple times using high-temperature distilled water, the water extraction method is simple and easy to operate. In addition, research shows that the use of acid or alkaline solutions instead of distilled water can improve the extraction rate of polysaccharides under certain circumstances [10]. However, although the water extraction method is suitable for almost all polysaccharides, the extraction time can be as long as 2–4 h, which causes the method to take a long time [11]. According to the basic principle of polysaccharide extraction, that is, destroying the cell wall and causing the polysaccharides to enter the solvent, many new extraction techniques have begun to appear.
3.1. Ultrasonic Extraction Method
This method uses cavitation to destroy the cell wall to accelerate the dissolution of polysaccharides. Using this method can increase the yield of polysaccharides and decrease the extraction time [12]. By summarizing the research progress on the ultrasonic extraction of Astragalus polysaccharides, Wang et al. found that ultrasonic power had the highest impact on ultrasonic extraction, followed by extraction temperature and extraction time [13]. Therefore, many researchers will screen for the best extraction conditions when using ultrasonic extraction methods [14]. However, it should be noted that exposure to an ultra-sonic environment for a long duration changes the structure of polysaccharides and affects their biological activity [15].
3.2. Microwave Extraction Method
When the energy carried by microwaves continues to act on cells, it can increase the intracellular pressure break the cells within a short period of time, and active ingredients such as polysaccharides can flow into the solvent [16]. However, rapid temperature change is very likely to change the molecular weight distribution and the structure of thermally unstable polysaccharides. Research on the microwave extraction of seaweed polysaccharides confirms that this method degrades polysaccharides, resulting in changes in their molecular weight and viscosity [17]. However, microwave extraction is also useful. By changing the microwave power, extraction time, and other factors, researchers can control the degradation rate, sulfate content, viscosity, and molecular weight of seaweed polysaccharides within the required range to obtain the required seaweed polysaccharides [18].
3.3. Enzyme-Assisted Extraction
This method destroys the cell wall and intracellular structure through enzymatic hydrolysis to obtain more polysaccharides. The main enzymes that are currently used include Viscozyme, Cellucast, Termamyl, Ultraflo, carragenanase, agarase, amyloglucosidase, xylanase, Kojizyme, Protamex, Neutrase, Flavourzyme, and Alcalase [19,20]. At present, this method is often used in combination with other methods, such as microwave extraction and ultrasonic extraction. Since enzymes are selective to the environment, ensuring enzyme activity is one of the key points to consider when using different enzymes together.
3.4. Other Extraction Methods
In addition to the extraction methods mentioned above, there are many new extraction methods that can be applied to polysaccharide extraction, including supercritical CO2 extraction [21], subcritical water extraction [22], ionic liquids extraction [23], and dynamic high-pressure micro-jet technology [24]. In general, there are no absolute advantages and disadvantages between different extraction technologies. Choosing a suitable extraction technology is not only related to the characteristics of the polysaccharides, but is also inseparable from the extraction conditions controlled by the researchers.
4. Purification of Polysaccharides
Extracted crude polysaccharides contain impurities such as inorganic salts, proteins, and pigments. Impurities seriously affect the evaluation of the relationship between the structure and biological activity of polysaccharides; as such, they need to be removed. In most cases, ethanol precipitation is the first step in polysaccharide purification, as it can remove low-molecular-weight impurities from polysaccharides [25]. In addition, membrane separation technologies, such as diafiltration and ultrafiltration, are also widely used to remove impurities [26]. Conventional methods of removing protein use Sevag reagent or trichloroacetic acid to denature and precipitate the protein [27]. Methods to remove pigments include the resin method, the activated carbon method, and the hydrogen peroxide oxidation method [15].
To explore the relationship between structure and biological activity, the polysaccharides obtained after removing impurities require further deep purification. At present, the most commonly used method is chromatographic separation. Ion-exchange chromatography is suitable for separating neutral or acidic polysaccharides via gradient salt elution or pH adjustment [28]. For most anti-gastric cancer polysaccharides, dicthylaminoethyl-cellulose anion exchange column chromatography is used for deep purification. Size exclusion chromatography (also known as gel chromatography) is based on the principle of different molecular weights or molecular size for separation [29]. Affinity chromatography uses the adsorption difference between different substances and stationary phases for separation [30].
5. Structural Characterization of Polysaccharides
The physical, chemical, and biological properties of polysaccharides mainly depend on the type, ratio, and sequence of monosaccharides; their molecular weight; the configuration of the glycosidic bonds; the types of glycosidic bonds; and the positions of the glycosidic bonds [31]. High-performance gel permeation chromatography can not only be used to determine the homogeneity of polysaccharides, but can also be used to determine the molecular weight [32]. Partial acid hydrolysis, periodic acid oxidation, Smith degradation, high-performance liquid chromatography, gas chromatography, and high-performance thin-layer chromatography are also used to determine the composition of monosaccharides [33]. Nuclear magnetic resonance spectroscopy is used to determine the ratio of monosaccharides and anomeric bonds [34]. Gas chromatography-mass spectrometry is used to determine linkage positions [33]. We summarize the extraction, purification, and characterization steps in Figure 1.
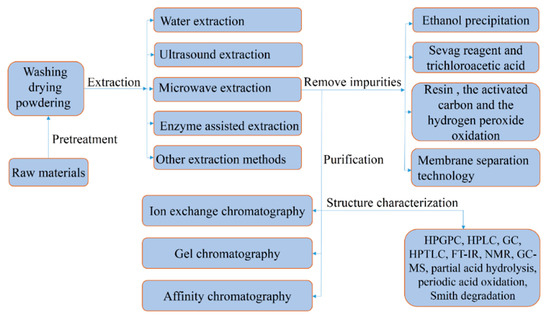
Figure 1.
Schematic representation of the extraction, purification, and characterization of polysaccharides against gastric cancer.
6. Polysaccharides with Anti-Gastric Cancer Effect
6.1. Protein-Bound Polysaccharide K (PSK)
PSK was obtained from the Basidiomycete Coriolus Versicolor, had a molecular weight of 100 kDa, and contained 18–38% protein [35]. It was composed of protein and polysaccharides, had a β-1,4 glucan structure [36], and was made from polysaccharides covalently bonded to peptides through O- or N-glycosidic bonds [37]. PSK has anti-tumor ability and has been used as a nonspecific immunostimulant to treat cancer patients. Additionally, PSK has been combined with various chemotherapeutic agents to act as a novel therapeutic regimen in animal models. In clinical settings, PSK is effective for the treatment of a variety of cancers, including gastric cancer [38]. We summarized the research progress of PSK for gastric cancer treatment in the past 20 years, and research showed that the actions of PSK on anti-gastric cancer include inhibiting the expression of immunosuppressive factors such as TGF-β; activating immune responses, such as promoting the maturation of dendritic cells and correcting Th1/Th2 imbalances; and enhancing the activity of anticancer drugs (Figure 2) [39].
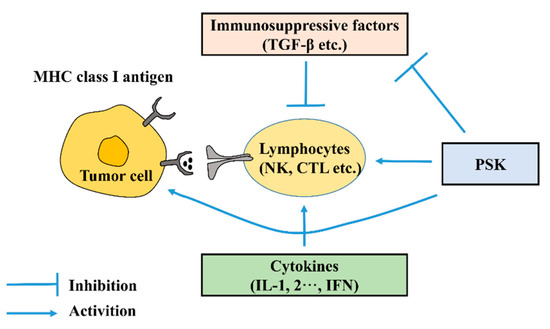
Figure 2.
Tumor microenvironment and actions of PSK.
Cell experiments and animal experiments have revealed the mechanism of PSK against gastric cancer (Table 1). The immunomodulatory effect of PSK is related to its anticancer activity. PSK increases the expression of HLA-B27 and β2-microglobulin and increases the expression of HLA-ABC, HLA-A2/A28, and HLA-DR in KATO-3 cells. This suggests that PSK can enhance the immune response against gastric cancer [40]. Research has shown that PSK is a selective TLR2 agonist that modulates immune response by activating dendritic cells and T cells in tumor-bearing neu transgenic mice [41,42]. Research has also shown that PSK induces the activation of natural killer cells as well as of cytotoxicity by enhancing the binding activity of AP-1 and CRE through promoting the expression of PKCδ, PKCε, and ERK3 [43,44]. In addition, PSK can prevent the functional inhibition of dendritic cells caused by the tumor-derived factors of MKN-45P cells, which include increasing phagocytic activity, inducing Th1-type immune regulation, and preventing the apoptosis of dendritic cells [45].

Table 1.
Direct actions of PSK on gastric cancer cells.
Transforming growth factor-β (TGF-β) is related to immune regulation and cancer development. In a mouse fibrotic tumor model (human peritoneal mesothelial cells and human gastric cancer cell line OCUM-2MD3), PSK inhibited the proliferation and the epithelial-mesenchymal transition by inhibiting the TGF-β-induced overexpression of α-SMA. This suggests that PSK can prevent the migration of gastric cancer to form peritoneal cancer [46]. In addition, PSK inhibits the invasion of MK-1P3 cells by down-regulating the expression of several invasion-related factors, including uPA, MMP-2, and MMP-9 by decreasing TGF-β1 expression [47]. By inhibiting the TGF-β signaling pathway, increasing the expression of E-cadherin, decreasing the expression of vimentin, and inhibiting the epithelial-mesenchymal transition-mediated generation of CD44+/CD24 cells, PSK suppresses the migration and invasion of MKN45 cells [48].
In addition, PSK can improve the therapeutic effect of chemotherapy drugs. PSK enhances docetaxel’s induction of apoptosis and growth inhibition on MK-1 cells. Moreover, PSK can inhibit the invasion of MK-1 cells invasion caused by docetaxel. In a xenograft rat model of MK-1 cells, PSK enhanced docetaxel-mediated tumor suppression by inhibiting the activation of NF-κB and survivin expression [49,50]. In addition, PSK can induce blood mononuclear cells to express IFN-α, inhibiting dihydropyrimidine dehydrogenase expression, and consequently augmenting the antitumor effect of 5-fluorouracil or 5′-deoxy-5-fluorouridine on GCIY and MKN45 cells [36].
On the basis of cell experiments, clinical research also showed that PSK was extremely useful as an adjuvant immunochemotherapy to conventional anticancer drugs [51,52]. PSK prolonged the survival of gastric cancer patients, mainly by regulating immune function, and no serious adverse reactions was reported [53,54,55]. For 8009 gastric cancer curative resection patients, a meta-analysis showed that PSK immunochemotherapy could significantly improve survival rate (P = 0.018) [38]. In 31 advanced gastric cancer patients, the plasma TGF-β levels of the PSK therapy group (n = 17) decreased compared to the no PSK group (n = 14), thereby antagonizing immune evasion and improving patient prognosis (P = 0.019) [56]. In 310 gastric cancer patients (stage IB: n = 19, II: n = 65, IIIA: n = 58, IIIB: n = 28, IV: n = 37), the 5-year survival rate increased in the treatment group that received 5-fluorouracil, mitomycin-C, and PSK (84.4%) compared to the treatment group that only received 5-fluorouracil and mitomycin-C (67.6%) (P = 0.019). However, a significant difference was only observed from between stages IIIA and IV (P = 0.019) [57,58,59].
In 918 gastric curative gastrectomy patients (stages II and III), patients with death-1 ligand 1-negative expression in the PSK plus 5-fluorouracil group had longer survival when compared to the 5-fluorouracil-only group (P = 0.033). It is worth noting that the use of PSK was only valid for stages IIIA and IIIB (P = 0.031) and not for stage II or stage IIIC. By examining the patients’ immunological cells, it was observed that the number of natural killer and natural killer T cells increased [60]. For the 14 gastric cancer patients who received tegafur/uracil plus PSK treatment, FACS tests showed that the Th1/Th2 balance shifted to Th1 dominance (the Th1/Th2 ratio from 9.36 to 10.93, P = 0.074), and the DC1/DC2 balance shifted to DC1 dominance (the DC1/DC2 ratio from 1.25 to 1.62, P = 0.090). In addition, ELISA tests showed that IL-10 production significantly decreased (P = 0.015) [61]. In 21 stage III gastric cancer patients, the 3-year overall survival of the PSK plus tegafur/uracil group (62.2%) was better than that of the tegafur/uracil-only group (12.5%) (P = 0.038). FACS tests showed that this was related to decreasing the number of CD57(+) T cells (P = 0.0486) [62]. For 136 stage II or III advanced gastric cancer patients, S-1 (tegafur, gimeracil, and oltipraz) plus PSK treatment reduced the recurrence of patients with advanced non-T4 (P < 0.01) or N0-1 gastric cancer (P = 0.03) [63]. Further testing of the patients’ immunological parameters showed that this was related to decreasing the neutrophil: lymphocyte ratio (P = 0.043) [64], which decreased the expression of the Foxp3 gene (4.26% to 3.11%) and increased natural killer cell activity (27% to 47%) [65], preventing the T-cell apoptosis induced by S-1 by decreasing caspase-3 activity an Bax expression [66].
In the early stages of cancer development, primary tumors are composed of homogeneous HLA class I-positive cancer cells, and then a Darwinian type of T-cell-mediated immune selection results in a tumor solely composed of MHC class I-negative cells, which can be highly diverse and are not necessarily the same as the cells in the primary tumor, and research has shown that cancer cells can avoid macrophage phagocytosis by increasing MHC class I expression [67]. In 349 curative gastric cancer surgery patients, treatment with PSK plus fluoropyrimidine agents significantly improved the 5-year relapse-free survival of patients (MHC class I-positive). Although this treatment method had no effect on MHC class I-negative patients, it had a significant therapeutic effect on other patients (multiple lymph node metastasis and MHC class I-negative). These patients’ 5-year relapse-free survival was also significantly improved [68]. Similarly, in 254 gastric curative resection patients, there was no significant difference in the 8-year overall survival and relapse-free survival between the anti-metabolites group and anti-metabolite plus PSK group. However, the 8-year overall survival rate of the patients with pN3 lymph node metastasis was improved in the anti-metabolites plus PSK group (P = 0.002) [69]. In 349 gastric cancer curative resection patients, the 3-year recurrence-free survival rate was 65% in the PSK plus fluoropyrimidine group and 47% in the fluoropyrimidine-only group. For patients with pN3 lymph node metastasis, the recurrence-free survival rates were 68% in the PSK plus fluoropyrimidine group and 28% in the fluoropyrimidine-only group [70]. In addition, for patients with early tumor recurrence, the therapeutic effect of PSK was obvious. In 254 gastric curative resection patients, the overall survival of patients with early tumor recurrence was better in the fluorouracil plus PSK group than in fluorouracil-only group (P = 0.023) [71]. We summarized the clinical research of PSK as a postoperative adjuvant treatment for patients with gastric cancer in Table 2.

Table 2.
Postoperative adjuvant chemotherapy with PSK for resected gastric cancer.
6.2. Fungi Polysaccharides
Current research has shown that various fungal polysaccharides (mainly edible mushrooms) have significant anti-gastric cancer effects. For instance, lentinan is a biological response modulator of gastric cancer and demonstrates immunomodulatory activity. It has a (1→3)-β-D-glucan with two (1→6)-β-glucopyranoside branches for every five (1→3)-β-glucopyranoside linear linkages, and its molecular weight is between 304 kDa and 1832 kDa. In Japan, two types of β-glucans, krestin and lentinan, have been licensed as drugs for gastric cancer treatment [72]. β glucans stimulate the immune system through the activation of different immune cells including macrophages, dendritic cells, neutrophils, natural killer (NK), and lymphocytes [73] and are used for cancer therapy in association with cytotoxic chemotherapeutic agents [38]. Additionally, lentinan can activate non-specific cytotoxicity in vivo; enhance helper T cell-mediated cytotoxic T cell activity, NK cell activity, and humoral immune response; induce Th1 polarization; and improve the balance between Th1 and Th2 [72,74].
The anti-gastric cancer effects of fungal polysaccharides are discussed from a variety of different perspectives. (1) Proliferation inhibition: The Phellinus gilvus polysaccharide fraction inhibits the proliferation of TMK-1 cells [75]. The polysaccharides HEG-5 and HEP-1 were extracted from Hericium erinaceus. HEG-5 inhibited the proliferation of GES-1 cells by blocking cell cycle arrest during the S phase, and HEP-1 inhibited the proliferation of GES-1 cells by blocking the cell cycle during the G0/G1 phase [76,77]. The polysaccharides LSMS-1 and LSMS-2 were also extracted from Hericium erinaceus and the average molecular weights were 6842 kDa and 2154 kDa, respectively. MTT assays showed that LSMS-1 and LSMS-2 significantly inhibited the growth of SGC7901 cells [78]. A water-insoluble polysaccharide from Grifola frondosa was sulfated to obtain a sulfated polysaccharide S-GAP-P with a molecular weight of 28 kDa and a sulfate content of 16.4%. MTT assays showed that S-GAP-P could inhibit the proliferation of gastric cancer SGC-7901 cells [79]. Yang et al. isolated two polysaccharides FVP-1 and FVP-2 from Flammulina velutipes. FVP-1 was composed of glucose, fucose, mannose, and galactose in a ratio of 81.3:3.0:3.6:12.1 and had a molecular weight of 28 kDa, and the FVP-2 was composed of glucose, fucose, xylose, mannose, and galactose in a ratio of 57.9:5.5:9.5:15.1:12.0 and had a molecular weight of 268 kDa. Although MTT assays showed that both FVP-1 and FVP-2 could inhibit the proliferation of gastric cancer BGC-823 cells, the inhibitory strength of FVP-2 was higher than that of FVP-1, with this higher strength being attributed to structural differences [12]. Luo et al. extracted a homogeneous polysaccharide SP1 from Trametes robiniophila Murr, which had a molecular weight of 56 kDa [80]. Chen et al. found that SP1 inhibited the proliferation of MGC-803 cells by inhibiting the expression of SOX4, ZEB2, MMP9, Snail, and Slug through promoting SMAD7 expression and inhibiting the activation of the TGF-β/SMAD signaling pathway [81]. The polysaccharide RPS from Rhizopus nigricans was composed of mannose, rhamnose, glucuronic acid, glucose, galactose, and fucose, with a molar ratio of 5.1:1.0:1.6:92.2:1.3:2.3. RPS could inhibit the proliferation of BGC-823 cells by blocking the cell cycle during the G2/M phase [82]. (2) Apoptosis induction: The Phellinus gilvus polysaccharide fraction induced the apoptosis of TMK-1 cells and inhibited tumor growth in an orthotopic transplantation mouse model [75]. The polysaccharide POMP2 from Pleurotus ostreatus significantly reduced the weight and volume of the tumor in BGC-823 cells xenograft-bearing mice [83]. The polysaccharide HEG-5 induced the apoptosis of GES-1 cells by reducing the expression of Bcl-2 and increasing the expression of caspase-8, caspase-3, p53, Bax, and Bad, and the polysaccharide HEP-1 induced the apoptosis of GES-1 cells by promoting the expression of Bax and caspase-3 and inhibiting the expression of Bcl-2 [76,77]. The protein of the polysaccharide conjugates GFG-3a from Grifola frondosa and induced the apoptosis of SGC-7901 cells by increasing the expression of caspase-8, caspase-3, p53, Bax, and Bad, and reducing Bcl-2 and Bcl-xl expression through inhibiting the PI3K/AKT signaling pathway [84]. The sulfated polysaccharide S-GAP-P could induce apoptosis of SGC-7901 cells [79]. The Ganoderma lucidum polysaccharide fraction induced the apoptosis of gastric cancer SGC-7901 cells via down-regulating Bcl-2 protein expression and increasing Bax expression [85]. The polysaccharide RPS induced the apoptosis of BGC-823 cells by inducing the production of intracellular ROS and increasing the level of intracellular Ca2+ and the loss of mitochondrial membrane potential, and then activated the expression of caspase-9 and caspase-3 [82]. SP1 induced the apoptosis of MGC-803 cells by inhibiting the expression of SOX4, ZEB2, MMP9, Snail, and Slug through promoting SMAD7 expression and inhibiting the TGF-β/SMAD signaling pathway [81]. (3) Regulating immune activity: The Ganoderma lucidum polysaccharide fraction enhanced the immunity and antioxidant activity of Nmethyl-N9-nitro-Nnitrosoguanidine-induced gastric cancer rats by reducing IL-6 and TNF-α levels and increasing levels of IL-2, IL-4, IL-10, SOD, CAT, and GSH-Px [86]. (4) Enhancing the anticancer effect of anticancer medicines: The polysaccharide HEP from Hericium erinaceus, which has a molecular weight of 13 kDa, reduced the IC50 value of the doxorubicin-induced apoptosis in SGC7901 cells from 10 μg/mL to 5 μg/mL by increasing doxorubicin-induced ROS production and down-regulating HIF-1α expression [87]. In 39 patients who had been diagnosed with unresectable gastric cancer, the incidence of adverse events during treatment was reduced in comparison to 20 patients who only received S-1 treatment, and quality of life was improved in the 19 patients who were treated with S-1 plus lentinan [88].
6.3. Algae Polysaccharides
Current research shows that various algae polysaccharides (mainly brown algae polysaccharides) have significant anti-gastric cancer effects. For instance, laminaran is a water-soluble polysaccharide from the brown alga Eisenia bicyclis that has a molecular weight of 5 kDa and a glucan with β-(1→6) side chains linked to a β-(1→3) backbone [89]. Currently, a large number of studies shows the antitumor effects of QSC, with laminaran activating apoptotic cell death, inhibiting the colony formation in cancer cells, and inhibiting the angiogenesis potential of cancer [90]. Regarding the treatment of gastric cancer, oral supplementation with laminaran can attenuate the gross and microscopic signs of gastric dysplasia and can substantially counterbalance the increased induction of the expression of protein markers reminiscent of proliferative and angiogenic processes. Laminaran treatment can effectively overcome A4gnt KO-induced alteration in the gene expression profiles of selected cytokines; thus, β glucan can effectively restrain the progressive development of precancerous lesions and gastric dysplasia in A4gnt KO mice [91].
Its anti-gastric cancer effects are mainly reflected in the following aspects: (1) Apoptosis induction: The polysaccharide fraction from the red alga Gracilariopsis lemaneiformis can induce the apoptosis of MKN28 cells by activating the Fas/FasL signaling pathway [92]. The sulfated polysaccharides from the red alga Porphyra yezoensis induced AGS cells apoptosis by increasing PARP cleavage and promoting caspase-3 activation through decreasing insulin-like growth factor I receptor (IGF-IR) phosphorylation [93]. Xie et al. extracted a polysaccharide with a molecular weight of 11.68 kDa from a brown alga that induced the apoptosis of MKN45 cells by inducing ROS production, JNK phosphorylation, and the activation of p53, caspase-9, and caspase-3 expression [94,95]. Similarly, the polysaccharide SFPS-B2 extracted from the brown alga Sargassum fusiforme induced the apoptosis of SGC-7901 cells by activating the intracellular mitochondrion permeability transition pore, caspase-9, and caspase-3, decreasing the mitochondrial membrane potential and the expression of Bcl-2, up-regulating the expression of Bax, and inducing the release of cytochrome [96]. Cf-PS is a sulfated polysaccharide derived from the brown alga Capsosiphon fulvescens. The monosaccharide composition included xylose and mannose at a ratio of 17:3, and the sulfate content was 28.7%. By decreasing the phosphorylation of IGF-IR and the expression of Bcl-2 and increasing the expression of caspase-3, Cf-PS significantly induced the apoptosis of AGS cells [97]. Fucoidan is from the brown alga Cladosiphon okamuranus, carries substantial levels of 2-O-α-D-glucuronopyranosyl branches in the linear (1→3)-linked poly-α-fucopyranoside chain, and has a molecular weight of 400 kDa [98]. By downregulating the expression of Bcl-2 and Bcl-xL, inducing the loss of mitochondrial membrane potential and activating the expression of caspase-3, fucoidan induces the apoptosis of AGS cells. Moreover, the conversion of microtubule-associated proteins from LC3-I to LC3-II and the increased accumulation of beclin 1 indicated that fucoidan induces the autophagy of AGS cells [99]. (2) Proliferation activity: By inhibiting the ASK1/p38 signaling pathway, fucoidan inhibits proliferation and DNA synthesis in MKN45 cells [91]. Xie et al. extracted a polysaccharide with a molecular weight of 11.68 kDa from brown alga that inhibited the proliferation of MKN45 cells by blocking the cell cycle during the G2/M phase [94,95]. (3) Angiogenesis inhibition: In A4gnt-KO mice, laminaran inhibited angiogenesis by reducing the expression of IL-10, IL-11, Hgf, Ccl2, IL-1B, Ptgs2, Egf, Fgf7, and Cxcl1 [100]. (4) Immune activity regulation: Glycosyl linkages from the extremely complex sulfated fucoidan SHPPB2 from brown alga Sargassum henslowianum improved the immune function of N-methyl-N’-nitro-nitrosoguanidine-induced gastric cancer rats by promoting spleen cell proliferation, increasing the secretion of anti-inflammatory cytokines, and reducing the expression of pro-inflammatory cytokines [101].
6.4. Astragalus Membranaceus Polysaccharides
Astragalus membranaceus is a widely used Chinese medicine, and the polysaccharides that have been extracted from it have been proven to have anti-gastric cancer effects [102]. (1) Apoptosis induction: The water-soluble polysaccharide APS4 was found to induce the apoptosis of MGC-803 cells by activating the expression of caspase-9/-3 and promoting PARP cleavage through intracellular ROS accumulation, the loss of mitochondrial membrane potential, the release of cytochrome c, the increased expression of Bax, and the reduced expression of Bcl-2 [103]. The water-soluble polysaccharide APS is an α-(1→4)-D-glucan with a single α-D-glucose at the C-6 position every nine residues and a molecular weight of 36 kDa. APS induced the apoptosis of SGC-7901 cells and adriamycin-resistant SGC-7901 cells by enhancing the expression of caspase-3, causing DNA fragmentation and increasing the expression of tumor suppressor genes (SEMA3F, P21WAF1/CIP1, and FBXW7) by activating the MAPK signaling pathway [104]. (2) Proliferation inhibition: The polysaccharide fraction of Astragalus membranaceus can inhibit the proliferation of SCG-7901 cells by blocking the cell cycle during the G0/G1 phase [105]. (3) Enhancement of the anticancer effect of anticancer medicines: Research showed that the polysaccharide fraction of Astragalus membranaceus enhanced the antitumor effects of Apatinib in AGS cells by inhibiting the proliferation of cancer cells, causing cancer cell apoptosis and autophagy through inhibiting the AKT signaling pathway [106]. (4) Regulation of immune activity: Li et al. isolated a glucan (α-(1→4)-d-glucan with α-(1→6)-linked branches attaching to the O-6 of branch points) that demonstrated an antitumor effect on 1-Methyl-2-nitro-1-nitrosoguanidine-induced gastric cancer mice by promoting spleen lymphocyte proliferation and enhancing natural killer cell activity, raising blood LgA, LgG, and LgM levels and the expression of CD4+ and CD4+/CD8+ [107]. Finally, clinical trials showed that the polysaccharide fraction of Astragalus membranaceus reduced gastrointestinal reactions such as nausea, vomiting, and the incidence of leukopenia induced by FOLFOX (composed of oxaliplatin, calcium folinate, and 5-fluorouracil) [108,109].
6.5. Tea Polysaccharides
Tea is a popular drink all over the world. Current research shows that tea polysaccharides have an anti-gastric effect [110,111]. (1) Apoptosis induction: Shao et al. found that all three polysaccharides (TSCR, TSCP-1, and TSCP-2) extracted from tea seeds could induce the apoptosis of MKN45 cells, with TSCP-1 demonstrating the strongest anti-tumor activity as well as the highest sulfate content [112]. (2) Proliferation inhibition: Three polysaccharides (TFPS-1, TFPS-2, and TFPS-3) from tea flower were found to inhibit the proliferation of BGC-823 cells. Preliminary characterization showed that TFPS-1 had a sulfate content of 2.63% and a monosaccharide composition that included arabinose, fucose, xylose, mannose, glucose, and galactose, with a molar ratio of 14.84:2.64:12.16:6.87:45.39:18.08. The sulfate contents of TFPS-2 and TFPS-3 were 0.84% and 1.76%, respectively. Both the monosaccharide compositions of TFPS-2 and TFPS-3 contained rhamnose, arabinose, and galactose, and the molar ratios were 11.19:55.16:33.65 and 20.95:53.34:25.71, respectively [113]. (3) Improvement of oxidative stress and inflammation: Three polysaccharides (TF-1, TF-2, and TF-3) from tea were found to protect mice with gastric cancer induced by N-methyl-N’-nitro-nitrosoguanidine from oxidative stress damage and inflammation by increasing the expression of SOD, CAT, and GSH-Px; decreasing the levels of IL-6 and TNF-α; and increasing the levels of immunoglobulin A, immunoglobulin G, immunoglobulin M, IL-2, IL-4, and IL-10. Preliminary characterization showed that the molecular weights of TF-1, TF-2, and TF-3 were 231.58 kDa, 46.27 kDa, and 7.25 kDa, respectively. The monosaccharide composition of TF-1 contained glucose, mannose, and xylose, with a molar ratio of 1:3.2:1.4. The monosaccharide composition of TF-2 contained glucose and xylose, with a molar ratio of 1:1.7. The monosaccharide composition of TF-3 contained glucose, xylose, and arabinose, with a molar ratio of 1:2.5:0.9 [114].
6.6. Caulis Dendrobii Polysaccharides
Caulis Dendrobii is a precious Chinese herbal medicine, and research has shown that Caulis Dendrobii polysaccharides have a therapeutic effect on gastric cancer [115]. (1) Suppression of precancerous lesions: The polysaccharide fraction of Caulis Dendrobii inhibited 1-Methyl-2-nitro-1-nitrosoguanidine-induced precancerous lesions in gastric cancer rats by downregulating the gene expression of Wnt2β, Gsk3β, PCNA, CyclinD1, and β-catenin through inhibiting the Wnt/β-catenin signaling pathway [116]. Subsequent research found that it was also related to reducing 8-OHdG levels and activating the NRF2 pathway and the related antioxidant enzymes HO-1 and NQO-1 [117]. (2) Apoptosis induction: The polysaccharide fraction of Caulis Dendrobii suppressed the growth of SGC-7901 cell xenografts in nude mice by increasing serum TNF-α and IL-2 levels, upregulating Bax protein expression, and downregulating Bcl-2 protein expression [118]. Liu et al. isolated four polysaccharides, cDHPS, cDHPR, cDHPL, and cDHPF, from Caulis Dendrobii. By upregulating the expression of the p53 gene and downregulating the expression of the c-myc gene, cDHPS, cDHPR, cDHPL, and cDHPF could induce the apoptosis of MFC cells [119]. (3) Immune activity regulation: Patients infected with H. pylori have a risk of developing gastric cancer [120]. Studies have shown that in patients with GI cancer receiving anti-PD-1/PD-L1 therapy, regardless of their clinical responses, the gut microbiomes predominantly consisted of Bacteroidetes and Firmicutes, with a 2.5-fold elevation in the Prevotella/Bacteroides ratio in patients with favorable outcomes, with a subgroup of responders showing a higher abundance of Prevotella [121]. Additionally, animal tests indicated that the Dendrobium officinale polysaccharide (DOW-5B) increased the diversity of the gut microbiota in mice, with levels of beneficial microbes such as Ruminococcus, Eubacterium, Clostridium, Bifidobacterium, Parabacteroides, and Akkermansia muciniphila increasing while the levels of harmful Proteobacteria decreased, improving the health of the large intestine as well as the immunity response of the mice [122]. Therefore, we speculate that the Dendrobium officinale polysaccharide may inhibit gastric cancer development by improving the gut microbiome, thus improving immune activity.
6.7. Other Polysaccharides Extracted from Foods
In addition to the various polysaccharides mentioned above, polysaccharides extracted from some foods have also shown anti-gastric cancer effects. (1) Proliferation inhibition: MTT assays have shown that CFPS-2, CSPS, and LRPs can inhibit the proliferation of SGC-7901 cells. Preliminary characterization showed the average molecular weight of CFPS-2 (from the fresh water clam Corbicula fluminea) was about 22 kDa and that the monosaccharide composition contained glucosamine, galactosamine, glucose, galactose, and fucose, with a molar ratio of 0.22:0.15:0.68:0.25:0.86 [123]. CSPS is a selenized polysaccharide obtained from Capparis spionosa L. [124]. The α-(1→6)-D-heteroglycans LRPs were extracted from lotus root, and their molecular weights were in the range of 1.33–5.30 kDa. LRPs were mainly composed of Glc-(1→, →6)-Glc-(1→, →6)-Gal-(1→, →4,6)-Gal-(1→ and →3,6)-Glc-(1→ at a molar ratio of 1.00:4.33:0.83:0.13:1.14, and the main monosaccharide composition contained mannose, rhamnose, galacturonic acid, glucose, galactose, and arabinose at a molar ratio of 0.19:0.14:0.17:6.49:1.00:0.16 [125]. In addition, through regulating the expression of cell cycle-associated proteins, cyclins, and CDKs, the polysaccharide fraction of Lycium barbarum inhibited the proliferation of MGC-803 and SGC-7901 cells by blocking the cell cycle during the G0/G1 and S phases, respectively [126]. (2) Regulation of immune activity: The α-(1→6)-D-heteroglycans LRPs were extracted from lotus root and were found to significantly enhance the NO production and TNF-α secretion of RAW264.7 macrophages [125]. (3) Migration and invasion suppression: By reducing the expression of MMP2, MMP9, Snail, and vimentin and upregulating the expression of E-cadherin through inhibiting the AKT/PI3K signaling pathway, the polysaccharide fraction of Lycium barbarum inhibited the migration and invasion of SGC-7901 cells [127]. (3) Apoptosis induction: Clinical trials showed that after the oral administration of the exocarp polysaccharide fraction of Ginkgo biloba in 30 gastric cancer patients, cancer cells appeared to undergo apoptosis and differentiation, and the tumor area was reduced. MTT experiments showed that the exocarp polysaccharide fraction of Ginkgo biloba induced the apoptosis of SGC-7901 cells by increasing the expression of the c-fos gene and reducing the expression of the c-myc and Bcl-2 genes [128].
6.8. Other Chinese Herbal Medicine Polysaccharides
In addition to the various polysaccharides mentioned above, polysaccharides extracted from certain Chinese herbal medicines have also shown anti-gastric cancer effects. (1) Proliferation inhibition: The polysaccharide AMPS-a (Abelmoschus manihot (Linn.) Medicus) has an average molecular weight of 8.8 kDa, and is mainly composed of glucose, mannose, galactose, and fucose, with a molar ratio of 1.00:0.91:2.14:1.09. AMPS-a contains a backbone composed of repeating units of →6)α-D-Galp-(1→6)α-D-Manp-(1→6)α-D-Galp-(1→with β-D-Glcp (1→3)α-Fucp-(1→ branching at the O-3 of mannose and can inhibit the proliferation of MGC-803 and MKN-45 cells [129]. The polysaccharide pMTPS-3 from Melia toosendan Sieb. Et Zucc has a molecular weight of 26.1 kDa and contains arabinose, glucose, mannose, and galactose in a molar ratio of 17.3:28.3:41.6:12.6. MTT assays showed that pMTPS-3 could significantly inhibit the growth of BGC-823 cells [130]. The polysaccharide CNP-1-2 from Clinacanthus nutans Lindau leaves has a molecular weight of 91.7 kDa and contains rhamnose, arabinose, mannose, glucose, and galactose, with a molar ratio of 1.30:1.00:2.56:4.95:5.09. CNP-1-2 has a backbone consisting of 1,4-linked Glcp, 1,3-linked Glcp, 1,3-linked Manp, 1,4-linked Galp, 1,2,6-linked Galp, and 1,2,6-linked Galp, and it showed a significant growth inhibitory effect on SGC-7901 cells [131]. Polysaccharide P-3 has a molecular weight of 7.8 kDa and was extracted from the leaves of Magnolia kwangsiensis Figlar and Noot. It consists of xylose and rhamnose in a ratio of 1:4. MTT assays showed that P-3 could inhibit the growth of SGC7901 cells [132]. MTT assays showed that the polysaccharide fraction of Radix ranunculi ternati significantly inhibited the growth and colony formation of BGC823 cells [133]. The α-(1→4)-D-glucan HPS-1 was extracted from the roots of Hedysarum polybotrys Hand.-Mazz and was found to have a molecular weight of 94 kDa and a single α-D-glucose at the C-6 position every nine residues. MTT analysis showed that HPS-1 significantly inhibited the proliferation of MGC-803 cells [134]. The polysaccharide CPP was extracted from Cyclocarya paliurus (Batal.) Iljinskaja and was found to have a molecular weight of 900 kDa, with a monosaccharide composition containing glucose, rhamnose, arabinose, xylose, mannose, and galactose with molar percentages of 32.7%, 9.33%, 30.6%, 3.48%, 10.4%, and 13.5%. MTT assays have shown that HPS-1 significantly inhibits the proliferation of MGC-803 cells [135]. The polysaccharide PVP from Prunella vulgaris is composed of rhamnose, arabinose, xylose, mannose, glucose, and galactose, with a molar ratio of 2.8:28.2:38.5:11.0:3.0:16.5. MTT assays have shown that PVP can inhibit the growth of SGC 901 cells [136]. By blocking the cell cycle at the G1 phase by down-regulating the expression of phosphorylated p-Cdk2T160 and p-Rb and up-regulating the expression of cyclin-dependent kinase2 inhibitor, the polysaccharide fraction of bamboo shavings significantly inhibited the proliferation of six gastric cancer cell lines (AGS, BGC-823, MGC-803, HGC-27, and MKN-45) [137]. (2) Immune activity regulation. By decreasing CD11c+ dendritic cells and CD11b+F4/80+ macrophages in peripheral blood and the spleen, and increasing the levels of CD3+, CD4+, CD8+, and NK1.1+ cells in peripheral blood and the levels of CD19+ and CD11b+ cells in peripheral blood and the spleen, the polysaccharide fraction of bamboo shaving has been shown to inhibit tumor growth and to prolong the survival of forestomach carcinoma tumor-bearing mice [137]. The polysaccharide SMPA extracted from the roots of Salvia miltiorrhiza has a molecular weight of 43 kDa and a monosaccharide composition comprising galactose, glucose, rhamnose, mannose, and galacturonic acid in a molar ratio of 2.14:1.42:1.16:2.15:1. By inhibiting the inflammatory response (promoting the production of IL-2, IL-4 and IL-10 and inhibiting the secretion of IL-6 and TNF-α), enhancing the killing activity of natural killer cells and cytotoxic T lymphocytes and the phagocytic function of macrophages, and increasing the levels of total intracellular granzyme-B and IFN-γ, SMPA significantly inhibited tumor growth in N-methyl-N’-nitro-nitrosoguanidine-induced gastric cancer rats [138]. By increasing the peripheral white blood cells count, thymus and spleen indexes, the production of serum cytokines such as IL-2, IL-4, and TNF-α, and promoting splenocytes proliferation, the polysaccharide fraction of Portulaca oleracea L. inhibited tumor growth in N-methyl-N’-nitro-N-nitrosoguanidine-induced gastric cancer rats [139]. (3) Migration and invasion suppression: The polysaccharide PGPW1 was extracted from root of Panax ginseng and was found to have a monosaccharide composition that contained glucose, galactose, mannose, and arabinose in a molar ratio of 3.3:1.2:0.5:1.1 [140]. By inhibiting the expression of Twist, AKR1C2, vimentin, and N-cadherin, and upregulating the expression of NF1 and E-cadherin, PGPW1 inhibited the invasion and metastasis of HGC-27 cells [141]. Similarly, the polysaccharide PGP2a was extracted from the root of Panax ginseng. The molecular weight of PGP2a was 32 kDa and the monosaccharide composition contained galactose, arabinose, glucose, and galacturonic acid in a molar ratio of 3.7:1.6:0.5:5.4. By inhibiting the protein expression of Twist and AKR1C2, PGP2a inhibits the migration and invasion of HGC-27 cell [142]. (4) Apoptosis induction: By decreasing the expression of c-myc and bcl-2 and increasing the expression of p53, fas, fas-L, and the cell factor TGF-β1, the polysaccharide fraction of Acanthopanax giraldii Harms Var. Hispidus Hoo induced the apoptosis of SGC-7901 cells [143]. The polysaccharide WATP was extracted from Aster tataricus and was found to have a molecular weight of 91.7 kDa, and a monosaccharide composition that contained galactose, glucose, fucose, rhamnose, arabinose, and mannose in a molar ratio of 2.1:1.3:0.9:0.5:0.3:0.6. By increasing intracellular Ca2+ level and the loss of mitochondrial membrane potential, WATP induced the apoptosis of SGC-7901 cells [144].
7. Correlation of Structure and Anti-Gastric-Cancer Activities
Polysaccharides are a class of polymer compounds that are have monosaccharides as their basic constituent units. Structural differences in polysaccharides, including the type and composition ratio of the monosaccharides; the type, position, and number of glycosidic linkages; and the spatial configuration formed by folding, affect the anti-gastric cancer activity of polysaccharides [145,146]. At present, there is not enough research on the structure–function relationship of polysaccharides, so it is difficult to effectively link the structure of polysaccharides with their anti-gastric cancer activity. However, some relationships can be inferred. Shao et al. extracted three kinds of polysaccharides from tea seeds (TSCR, TSCP-1, and TSCP-2) with similar monosaccharide compositions. MTT assays showed that the activity of the three polysaccharides in inducing the apoptosis of MKN45 gastric cancer cells was positively correlated with sulfate content [112]. Similarly, the inhibitory effects of three polysaccharides (TFPS-1, TFPS-2 and TFPS-3) from Camellia sinensis on BGC-823 gastric cancer cells were also positively correlated with sulfate content [113]. In addition, Yang et al. isolated two polysaccharides, FVP-1 and FVP-2, from Flammulina velutipes. FVP-1 is composed of glucose, fucose, mannose, and galactose in a ratio of 81.3:3.0:3.6:12.1 with a molecular weight of 28 kDa. The FVP-2 is composed of glucose, fucose, xylose, mannose, and galactose in a ratio of 57.9:5.5:9.5:15.1:12.0 with a molecular weight of 268 kDa. FVP-2 can inhibit the proliferation of BGC-823 gastric cancer cells more strongly than FVP-1. Based on an analysis of the related research, Yang et al. believes that the monosaccharide type, molecular weight, and sulfate and uronic acid content are positively correlated with inhibitory effect of polysaccharides on gastric cancer cells [12]. Liu et al. isolated four polysaccharides: cDHPS, cDHPR, cDHPL, and cDHPF, from Dendrobium officinale. cDHPS (259 kDa) was composed of→4)-β-D-Glcp-(1→, →4)-β-D-Manp-(1→, →4)-3-O-acetyl-β-D-Manp-(1→, and had a monosaccharide composition that included mannose and glucose with a molar ratio of 3.04:1.00. cDHPL (209 kDa) was composed of→4)-β-D-Glcp-(1→, →4)-β-D-Manp-(1→, →4)-3-O-acetyl-β-D-Manp-(1→, →3,6)-β-D-Manp-(1→ and terminal α-D-Galp and had a monosaccharide composition that included mannose, glucose, and galactose with a molar ratio of 19.15:1.32:1.00. cDHPF (478 kDa) was composed of→4)-β-D-Glcp-(1→, →4)-β-D-Manp-(1→, →3,6)-β-D-Manp-(1→ and terminal α-D-Galp and had a monosaccharide composition that included mannose, glucose, and galactose with a molar ratio of 9.68:3.26:1.00. cDHPR (14.1 kDa) was composed of →3,5)-α-L-Araf-(1→, →4)-β-D-Glcp-(1→, →4)-β-D-Manp-(1→, →4,6)-β-D-Manp-(1→, →6)-α-D-Galp-(1→ and terminal β-L-Araf and had a monosaccharide composition that included mannose, glucose, galactose, and arabinose at a molar ratio of 2.38:1.00:8.49:5.23. Among these four polysaccharides, cDHPS demonstrated the strongest activity in proliferation inhibition and in inducing apoptosis, followed by cDHPL, cDHPF, and cDHPR. By comparing the differences between the structures, it can be seen that glycosidic linkage types and sequences also greatly affect the activity of polysaccharides against gastric cancer. Moreover, different from the previous research conclusions of Yang et al., although cDHPS had the fewest types of monosaccharide, and a molecular weight that was not the largest, it had the highest anti-gastric cancer activity. In addition, although types of monosaccharide composition were the same and the ratios of the monosaccharide composition were close, cDHPF had a larger molecular weight than cDHPF and cDHPL, which meant that the influence caused by the glycosidic linkage types and sequences made the anti-cancer activity of cDHPF lower than that of cDHPL [119]. Since the 1930s, studies in gastric cancer patients, melanoma, and leukemia treated with BCG showed disease remission or a non-relapsing disease [147]. In addition, what stands out is that PSK, lentinan, and laminaran, all derived from different biological sources, are beta glucans having 1-6 and 1-3 linkages, and many of the other anti-cancer polysaccharides described in the review are alpha glucans. This suggests that one possible origin of the immunomodulatory activity of glucans may be defense against mycobacteria, since the surface integument of M. bovis BCG consists largely of an alpha glucan that may account for most of BCG’s anti-cancer activity, possibly as a T-cell stimulant [148,149,150]. In short, the influence of the polysaccharide structure on anti-gastric cancer activity needs to be the subject of major research in the future. This is of great significance for modifying polysaccharides to improve their anti-gastric cancer activity.
8. Impacting on the Signaling Pathways and Immune System
By reviewing the above literature, it can be seen that there are more than 60 polysaccharides (including single polysaccharide components and polysaccharide fractions) that have the potential to be used in the treatment of gastric cancer. Despite the large number of studies, most research shows that these polysaccharides have anti-gastric cancer effects that regulate different signal pathways, including the inhibition of the TGF-β, AKT/PI3K, IGF/IR, and Wnt/β-catenin signaling pathways, and activate the MAPK and Fas/FasL signaling pathways (Figure 3). The anti-gastric cancer signaling mechanisms of polysaccharides can be summarized as follows: They inactivate Smad2 signaling and inhibit the expression of SOX4, ZEB2, MMP9, Snail, and Slug by inhibiting the activation of the TGF-β signaling pathway [81]. Additionally, they also reduce the expression of MMP-2, MMP-9, Snail, and vimentin and upregulate the expression of E-cadherin through inhibiting the AKT/PI3K signaling pathway and inducing the apoptosis of SGC-7901 cells by increasing the expression of caspase-8, caspase-3, p53, Bax, and Bad and by reducing Bcl-2 and Bcl-xl expression through inhibiting the PI3K/AKT signaling pathway [84]. Polysaccharides also induce apoptosis in AGS gastric cancer cells via an IGF-IR-mediated PI3K/Akt pathway or by increasing PARP cleavage and Bcl-2 and by promoting caspase-3 and Cf-PS activation by decreasing the phosphorylation of IGF-IR; they also induce the apoptosis of MKN28 cells by activating the Fas/FasL signaling pathway and induce the apoptosis of SGC-7901 cells by decreasing the expression of c-myc and bcl-2 and increasing the expression of p53, fas, fas-L, and the cell factor TGF-β1 [97]. In addition, polysaccharides also inhibit 1-methyl-2-nitro-1-nitrosoguanidine-induced precancerous lesions in gastric cancer rats by downregulating the gene expression of Wnt2β, Gsk3β, PCNA, CyclinD1, and β-catenin by inhibiting the Wnt/β-catenin signaling pathway [116]. They also regulate the expression of cell cycle-associated proteins, cyclins, and CDKs by inhibiting the proliferation of MGC-803 and SGC-7901 cells by blocking the cell cycle during the G0/G1 and S phases, respectively; downregulating the expression of phosphorylated p-Cdk2T160 and p-Rb [126] and upregulating the expression of the CDK2 inhibitor by blocking the cell cycle at the G1 phase [137]; and inhibiting the proliferation of BGC-823 cells and MKN45 cells by blocking the cell cycle at the G2/M phase [82]. Moreover, polysaccharides induce the apoptosis of SGC-7901 cells and adriamycin-resistant SGC-7901 cells by enhancing the expression of caspase-3, causing DNA fragmentation and increasing the expression of tumor suppressor genes (SEMA3F, P21WAF1/CIP1, and FBXW7) by activating the MAPK signaling pathway [104]. They can also induce the apoptosis of GES-1 cells by reducing the expression of Bcl-2 and increasing the expression of caspase-8, caspase-3, p53, Bax, and Bad, and the polysaccharide HEP-1, as well as by inhibiting the expression of Bcl-2 [76,77]. Polysaccharides can also inhibit the proliferation and DNA synthesis in MKN45 cells by inhibiting the ASK1-p38 signaling pathway and inducing ROS production and JNK phosphorylation and by activating the expression of p53, caspase-9, and caspase-3 [94,95].
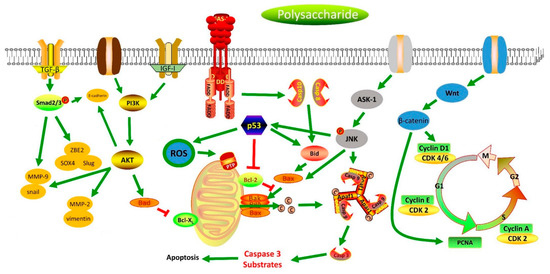
Figure 3.
Scheme summarizing the polysaccharides’ signaling mechanisms of anti-gastric-cancer.
Through above effects, polysaccharides can induce apoptosis of gastric cancer cells and inhibit the proliferation, migration, and invasion of gastric cancer. However, although research shows that the anti-gastric cancer effects of polysaccharides involve multiple mechanisms from a holistic perspective, we speculated that there are still some important signaling pathways and related proteins and nucleic acids that have not been revealed. Moreover, when focusing on each specific polysaccharide, it can be found that the anti-gastric cancer research on some polysaccharides is superficial, that is, it only shows the inhibitory effect on the growth of gastric cancer cells and does not reveal more mechanisms of action. In addition, some research shows that polysaccharides can kill gastric cancer cells by activating the immune system (Figure 4). However, in addition to PSK, only the polysaccharide fractions of Ganoderma lucidum, bamboo shavings, SHPPB2, LRPs, and a glucan from Astragalus membranaceus show immunomodulatory activity.
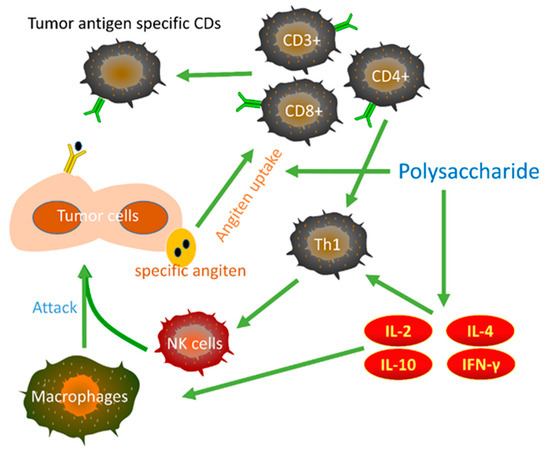
Figure 4.
Schematic representation of the antitumor immune responses and actions of polysaccharides.
9. Conclusions
At present, researchers are gradually paying more attention to the anti-gastric cancer effects of polysaccharides, and these polysaccharides also show great developmental potential. We summarized the mechanism of polysaccharides against gastric cancer (Table 3). Although the polysaccharide PSK has achieved significant clinical therapeutic effects, pharmacological research on other polysaccharides is still in the cell experiment or animal experiment stages. Moreover, the molecular mechanisms obtained from cell or animal experiments show a weak correlation with the actual role of polysaccharides in the human body. Therefore, we believe that more clinical trials are required in the future to determine the therapeutic effects of these polysaccharides. In addition, because the molecular structures of these polysaccharides are quite complex, the significant degradation caused by the first-pass effect needs to be considered. Therefore, when conducting clinical research in the future, the data obtained from cell experiments and animal experiments need to be used with caution. In addition, we recommend using omics technologies and bioinformatics to further explore the therapeutic mechanism of polysaccharides. In addition, the relationship between the biological activity of polysaccharides and their chemical structure needs to be explored. The biological characteristics of polysaccharides are closely related to their chemical structures, the main components of which are their monosaccharide composition, molecular weight, and type and location of their glycosidic bonds. The current literature research has initially shown that the structure has a non-negligible effect on anti-gastric cancer activity [12,112,119]. Finally, we speculate that polysaccharides may inhibit gastric cancer development by improving the gut microbiome, thus improving immune activity. Thus, there is much room to search for the intrinsic link between polysaccharides and intestinal microbiota for the treatment of gastric cancer.

Table 3.
Anti-gastric cancer effect of polysaccharides.
In short, we have detailed the anti-gastric cancer effects of plant and fungal polysaccharides, and hope that review is helpful for our researchers to design, research, and develop polysaccharides for the treatment of gastric cancer.
Author Contributions
Conceptualisation, L.C., C.H. and M.Z.; methodology, L.C. and J.L.; validation, L.L.; resources, L.C.; data curation, L.C; writing—original draft preparation, L.C. and C.H.; writing—review and editing, L.C. and C.H.; visualization, L.C., L.L. and M.Z.; supervision, J.L. and L.L.; project administration, L.L. and L.C.; funding acquisition, L.L. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by (1) Sichuan Science and Technology Planning Project: 2020YFS0463; (2) Sichuan Provincial Administration of Traditional Chinese Medicine Science and Technology Research Special Project: 2021MS115; (3) Sichuan University Students Innovation and Entrepreneurship Project: S202110650057.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors state that there are no conflicts of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide For 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Youker, S. Metallic Taste as a Side Effect of Topical Fluorouracil Use. J. Drugs Dermatol. 2011, 10, 1201–1203. [Google Scholar] [PubMed]
- Rösch, S.; Werner, C.; Müller, F.; Walter, P. Photoreceptor Degeneration by Intravitreal Injection of N-methyl-N-nitrosourea (MNU) in rabbits: A pilot Study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 255, 317–331. [Google Scholar] [CrossRef]
- Gallardo, A.C.; Rodríguez, R.M.; Pacheco, C.C.; Satue, C.G.; Servio, L.I. Dermatological Side Effects of Intravesical Mitomycin C: Delayed Hypersensitivity. Arch. Espanoles Urol. 2016, 69, 89–91. [Google Scholar]
- Hong, W.; Kim, K.; Jung, Y.; Kim, J.; Kang, S.; Chun, J.; Chun, M.; Yim, H.; Kang, D.; Kim, T. 432 Comparison of Efficiency and Side Effect of Adriamycin and Doxetaxel and Adriamycin, Cyclophosphamide and Paclitaxel in Patients with Locally Advanced Breast Cancer Receiving Neoadjuvant Chemotherapy. Eur. J. Cancer 2012, 48, S172–S173. [Google Scholar] [CrossRef]
- Doval, D.; Sharma, S.K.; Kumar, M.; Khandelwal, V.; Choudhary, D. Cytarabine Ears—A Side Effect of Cytarabine Therapy. J. Oncol. Pharm. Pract. 2019, 26, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage Immunomodulation and Therapeutic Potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Huh, S.; Lee, S.; Choi, S.J.; Wu, Z.; Cho, J.-H.; Kim, L.; Shin, Y.S.; Kang, B.W.; Kim, J.G.; Liu, K.; et al. Quercetin Synergistically Inhibit EBV-Associated Gastric Carcinoma with Ganoderma lucidum Extracts. Molecules 2019, 24, 3834. [Google Scholar] [CrossRef]
- Nie, X.; Shi, B.; Ding, Y.; Tao, W. Antitumor and Immunomodulatory Effects of Weikangfu Granule Compound in Tumor-Bearing Mice. Curr. Ther. Res. 2006, 67, 138–150. [Google Scholar] [CrossRef]
- Yue, H.; Liu, Y.; Qu, H.; Ding, K. Structure Analysis of a Novel Heteroxylan from the Stem of Dendrobium Officinale and Anti-Angiogenesis Activities of Its Sulfated Derivative. Int. J. Biol. Macromol. 2017, 103, 533–542. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the Polysaccharides from Five Algae and Their Potential Antioxidant Activity In Vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Yang, W.; Pei, F.; Shi, Y.; Zhao, L.; Fang, Y.; Hu, Q. Purification, Characterization and Anti-Proliferation Activity of Polysaccharides from Flammulina velutipes. Carbohydr. Polym. 2012, 88, 474–480. [Google Scholar] [CrossRef]
- Wang, J.; Jia, J.; Song, L.; Gong, X.; Xu, J.; Yang, M.; Li, M. Extraction, Structure, and Pharmacological Activities of Astragalus Polysaccharides. Appl. Sci. 2018, 9, 122. [Google Scholar] [CrossRef]
- Rahimi, F.; Tabarsa, M.; Rezaei, M. Ulvan from Green Algaeulva Intestinalis: Optimization of Ultrasound-Assisted Extraction and Antioxidant Activity. J. Appl. Phycol. 2016, 28, 2979–2990. [Google Scholar] [CrossRef]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef]
- Amanda, D.S.E.S.; Weuller, T.D.M.; Laís, M.M.a.; Maria, V.P.R.; Ana, K.P.B. Microwave-Assisted Extraction of Polysaccharides from Arthrospira (Spirulina) Platensis Using the Concept of Green Chemistry. Algal Res. 2018, 35, 178–184. [Google Scholar] [CrossRef]
- Tsubaki, S.; Oono, K.; Hiraoka, M.; Onda, A.; Mitani, T. Microwave-Assisted Hydrothermal Extraction of Sulfated Polysaccharides from Ulva spp. And Monostroma Latissimum. Food Chem. 2016, 210, 311–316. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-Assisted Extraction of Sulfated Polysaccharides (Fucoidan) from Brown Seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef]
- Baik, J.H.; Shin, K.-S.; Park, Y.; Yu, K.-W.; Suh, H.J.; Choi, H.-S. Biotransformation of Catechin and Extraction of Active Polysaccharide from Green Tea Leaves Via Simultaneous Treatment with Tannase and Pectinase. J. Sci. Food Agric. 2014, 95, 2337–2344. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Sun, A.-D.; Zhang, B.-L.; Qin, S.-G.; Zhang, Y.-Q. Supercritical CO2 Extraction and Pre-Column Derivatization of Polysaccharides from Artemisia Sphaerocephala Krasch. Seeds Via Gas Chromatography. Ind. Crop. Prod. 2014, 60, 138–143. [Google Scholar] [CrossRef]
- Zhao, T.; Luo, Y.; Zhang, X.; Zhang, W.; Qu, H.; Mao, G.; Zou, Y.; Wang, W.; Li, Q.; Chen, Y.; et al. Subcritical Water Extraction of Bioactive Compounds from Radix Puerariae and Optimization Study Using Response Surface Methodology. Chem. Eng. Commun. 2019, 206, 1218–1227. [Google Scholar] [CrossRef]
- Martins, M.; Vieira, F.A.; Correia, I.; Ferreira, R.A.S.; Abreu, H.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of Phycobiliproteins from the Red Macroalga Gracilaria sp. Using Ionic Liquid Aqueous Solutions. Green Chem. 2016, 18, 4287–4296. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.-C.; Wang, H.; Kou, Y.; Wen, Q.-H.; Fu, Z.-F.; Chang, H.-X. Response Surface Optimization and Physicochemical Properties of Polysaccharides from Nelumbo Nucifera Leaves. Int. J. Biol. Macromol. 2015, 74, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nie, W.; Yu, G.; Li, Y.; Hu, Y.; Lu, J.; Jin, L. Antitumor and Immunomodulatory Activity of Polysaccharides from Sargassum fusiforme. Food Chem. Toxicol. 2012, 50, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and Fractionation of Exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Zhang, D.-N.; Zhu, Q.; Yang, Q.-H.; Han, Y.-B. Purification, Preliminary Characterization And In Vitro Immunomodulatory Activity of Tiger Lily Polysaccharide. Carbohydr. Polym. 2014, 106, 217–222. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Zvyagintseva, T.N.; Ermakova, S.P. The Comparison of Structure and Anticancer Activity In Vitro Of Polysaccharides from Brown Algae Alaria marginata and A. Angusta. Carbohydr. Polym. 2016, 153, 258–265. [Google Scholar] [CrossRef]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. Antioxidant and Immunostimulating Activities In Vitro of Sulfated Polysaccharides Isolated from Gracilaria Rubra. J. Funct. Foods 2017, 28, 64–75. [Google Scholar] [CrossRef]
- Hahn, T.; Zayed, A.; Kovacheva, M.; Stadtmüller, R.; Lang, S.; Muffler, K.; Ulber, R. Dye Affinity Chromatography for Fast and Simple Purification of Fucoidan from Marine Brown Algae. Eng. Life Sci. 2015, 16, 78–87. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N.; Zhao, Z.; Chang, Y.; Ning, N.; Guo, H.; et al. Structures, Bioactivities and Future Prospective of Polysaccharides from Morus Alba (White Mulberry): A Review. Food Chem. 2018, 245, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, C.-F.; Feng, X.; Cheng, L.; Ibrahim, S.A.; Wang, C.-T.; Huang, W. Isolation, Characterization and Antioxidant of Polysaccharides from Stropharia Rugosoannulata. Int. J. Biol. Macromol. 2019, 155, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lin, H.; Li, J.; Wang, Y.; Cui, S.W.; Nie, S.; Xie, M. Structural Characterization of a Highly Branched Polysaccharide from the Seeds of Plantago Asiatica L. Carbohydr. Polym. 2012, 87, 2416–2424. [Google Scholar] [CrossRef]
- Hu, J.-L.; Nie, S.-P.; Wu, Q.-M.; Li, C.; Fu, Z.-H.; Gong, J.; Cui, S.W.; Xie, M.-Y. Polysaccharide from Seeds of Plantago asiatica L. Affects Lipid Metabolism and Colon Microbiota of Mouse. J. Agric. Food Chem. 2013, 62, 229–234. [Google Scholar] [CrossRef]
- Holt, S. The Mushroom Extract Polysaccharide K (PSK) Is a Promising Adjuvant Cancer Therapy. Focus Altern. Complement. Ther. 2015, 20, 166–167. [Google Scholar] [CrossRef]
- Mekata, E.; Murata, S.; Sonoda, H.; Shimizu, T.; Umeda, T.; Shiomi, H.; Naka, S.; Yamamoto, H.; Abe, H.; Edamatsu, T.; et al. Protein-Bound Polysaccharide-K Augments the Anticancer Effect of Fluoropyrimidine Derivatives Possibly by Lowering Dihydropyrimidine Dehydrogenase Expression in Gastrointestinal Cancers. Oncol. Rep. 2013, 30, 2845–2851. [Google Scholar] [CrossRef][Green Version]
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Oba, K.; Teramukai, S.; Kobayashi, M.; Matsui, T.; Kodera, Y.; Sakamoto, J. Efficacy of Adjuvant Immunochemotherapy with Polysaccharide K For Patients with Curative Resections of Gastric Cancer. Cancer Immunol. Immunother. 2006, 56, 905–911. [Google Scholar] [CrossRef]
- Maehara, Y.; Tsujitani, S.; Saeki, H.; Oki, E.; Yoshinaga, K.; Emi, Y.; Morita, M.; Kohnoe, S.; Kakeji, Y.; Yano, T.; et al. biological Mechanism and Clinical Effect of Protein-Bound Polysaccharide K (KRESTIN®): Review of Development and Future Perspectives. Surg. Today 2011, 42, 8–28. [Google Scholar] [CrossRef]
- Iguchi, C.; Nio, Y.; Takeda, H.; Yamasawa, K.; Hirahara, N.; Toga, T.; Itakura, M.; Tamura, K. Plant polysaccharide PSK: Cytostatic Effects on Growth and Invasion; Modulating Effect on The Expression of HLA And Adhesion Molecules on Human Gastric and Colonic Tumor Cell Surface. Anticancer Res. 2001, 21, 1007–1013. [Google Scholar]
- Lu, H.; Yang, Y.; Gad, E.; Wenner, C.A.; Chang, A.; Larson, E.R.; Dang, Y.; Martzen, M.; Standish, L.J.; Disis, M.L. Polysaccharide Krestin Is a Novel TLR2 Agonist that Mediates Inhibition of Tumor Growth via Stimulation of CD8 T Cells and NK Cells. Clin. Cancer Res. 2011, 17, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Quayle, K.; Coy, C.; Standish, L.; Lu, H. The TLR2 Agonist in Polysaccharide-K Is a Structurally Distinct Lipid Which Acts Synergistically with the Protein-Bound Β-Glucan. J. Nat. Med. 2014, 69, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lora, A.; Pedrinaci, S.; Garrido, F. Protein-bound polysaccharide K and interleukin-2 regulate different nuclear transcription factors in the NKL human natural killer cell line. Cancer Immunol. Immunother. 2001, 50, 191–198. [Google Scholar] [CrossRef] [PubMed]
- García-Lora, A.; Martinez, M.; Pedrinaci, S.; Garrido, F. Different Regulation of PKC Isoenzymes and MAPK by PSK and IL-2 in the Proliferative and Cytotoxic Activities of the NKL Human Natural Killer Cell Line. Cancer Immunol. Immunother. 2003, 52, 59–64. [Google Scholar] [CrossRef]
- Okuzawa, M.; Shinohara, H.; Kobayashi, T.; Iwamoto, M.; Toyoda, M.; Tanigawa, N. PSK, a Protein-Bound Polysaccharide, Overcomes Defective Maturation of Dendritic Cells Exposed to Tumor-Derived Factors In Vitro. Int. J. Oncol. 2002, 20, 1189–1195. [Google Scholar] [CrossRef]
- Shinbo, T.; Fushida, S.; Tsukada, T.; Harada, S.; Kinoshita, J.; Oyama, K.; Okamoto, K.; Ninomiya, I.; Takamura, H.; Kitagawa, H.; et al. Protein-Bound Polysaccharide K Suppresses Tumor Fibrosis in Gastric Cancer by Inhibiting the TGF-Β Signaling Pathway. Oncol. Rep. 2014, 33, 553–558. [Google Scholar] [CrossRef]
- Zhang, H.; Morisaki, T.; Matsunaga, H.; Sato, N.; Uchiyama, A.; Hashizume, K.; Nagumo, F.; Tadano, J.; Katano, M. Protein-Bound Polysaccharide PSK Inhibits Tumor Invasiveness by Down-Regulation of TGF-Β1 and Mmps. Clin. Exp. Metastasis 2000, 18, 345–351. [Google Scholar] [CrossRef]
- Yoshihiro, O.; Tetsu, H.; Ayano, K.; Hiroshi, O.; Kazuhiro, M.; Shigeyuki, K.; Minoru, T.; Masahiro, S.; Hiromitsu, J.; Hirotoshi, H. Direct Inhibition of the Transforming Growth Factor-Β Pathway by Protein-Bound Polysaccharide Through Inactivation of Smad2 Signaling. Cancer Sci. 2012, 103, 317–324. [Google Scholar] [CrossRef]
- Yamasaki, A.; Shoda, M.; Iijima, H.; Nagai, S.; Wada, J.; Suzuki, H.; Chikazawa, N.; Tasaka, T.; Kameda, C.; Tanaka, H.; et al. A Protein-Bound Polysaccharide, PSK, Enhances Tumor Suppression Induced by Docetaxel in a Gastric Cancer Xenograft Model. Anticancer Res. 2009, 29, 843–850. [Google Scholar]
- Kinoshita, J.; Fushida, S.; Harada, S.; Makino, I.; Nakamura, K.; Oyama, K.; Fujita, H.; Ninomiya, I.; Fujimura, T.; Kayahara, M.; et al. PSK Enhances the Efficacy of Docetaxel in Human Gastric Cancer Cells Through Inhibition of Nuclear Factor-Κb Activation and Survivin Expression. Int. J. Oncol. 2010, 36, 593–600. [Google Scholar] [CrossRef]
- Kim, J.W. Complementary Therapies and Cancer Treatment. J. Korean Med. Assoc. 2008, 51, 427–434. [Google Scholar] [CrossRef][Green Version]
- Fisher, M.; Yang, L.-X. Anticancer Effects and Mechanisms of Polysaccharide-K (PSK): Implications of Cancer Immunotherapy. Anticancer Res. 2002, 22, 1737–1754. [Google Scholar] [PubMed]
- Toge, T.; Yamaguchi, Y. Protein-Bound Polysaccharide Increases Survival in Resected Gastric Cancer Cases Stratified with a Preoperative Granulocyte and Lymphocyte Count. Oncol. Rep. 2000, 7, 1157–1218. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, X.; Yu, J.; Zhu, J.; Pen, X.; Meng, X. Can polysaccharide K Improve Therapeutic Efficacy and Safety in Gastrointestinal Cancer? A Systematic Review and Network Meta-Analysis. Oncotarget 2017, 8, 89108–89118. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Fujimura, T.; Kinami, S.; Hirono, Y.; Yamaguchi, A.; Naitoh, H.; Tani, T.; Kaji, M.; Yamagishi, H.; Miwa, K. A Randomized Phase III Trial of Postoperative Adjuvant Therapy with S-1 Alone versus S-1 plus PSK for Stage II/IIIA Gastric Cancer: Hokuriku-Kinki Immunochemo-Therapy Study Group-Gastric Cancer (HKIT-GC). Jpn. J. Clin. Oncol. 2006, 36, 519–522. [Google Scholar] [CrossRef][Green Version]
- Yamashita, K.; Sakuramoto, S.; Mieno, H.; Nemoto, M.; Shibata, T.; Katada, N.; Ohtsuki, S.; Sakamoto, Y.; Hoshi, K.; Wang, G.; et al. Preoperative Administration of Polysaccharide Kureha and Reduced Plasma Transforming Growth Factor-Β in Patients with Advanced Gastric Cancer: A Randomized Clinical Trial. Mol. Clin. Oncol. 2015, 3, 471–478. [Google Scholar] [CrossRef][Green Version]
- Choi, J.-H.; Kim, Y.-B.; Lim, H.-Y.; Park, J.S.; Kim, H.C.; Cho, Y.K.; Han, S.W.; Kim, M.W.; Joo, H.J. 5-Fluorouracil, Mitomycin-C, And Polysaccharide-K Adjuvant Chemoimmunotherapy for Locally Advanced Gastric Cancer: The Prognostic Significance of Frequent Perineural Invasion. Hepatogastroenterology 2007, 54, 290–297. [Google Scholar]
- Choi, J.; Kang, S.; Park, J.; Lee, H.; Cho, Y.; Han, S.; Lim, H. A Prospective Randomized Trial Comparing 5-Fluorouracil (5-FU), Mitomycin-C (MMC), And Polysaccharide-K (PSK) Versus UFT And PSK As Adjuvant Chemoimmunotherapy (CITX) For Patients with Locally Advanced Gastric Cancer with Curative Resection. J. Clin. Oncol. 2007, 25 (Suppl. S18), 15074. [Google Scholar] [CrossRef]
- Lee, H.; Choi, J.; Kang, S.; Jeong, S.; Jung, J.; Choi, Y.; Han, S.; Lim, H.; Cho, Y. A Prospective Randomized Trial Comparing 5-Fluorouracil (5-FU), Mitomycin-C (MMC), And Polysaccharide-K (PSK) Versus UFT And PSK As Adjuvant Chemoimmunotherapy (CITX) For Patients with Locally Advanced Gastric Cancer with Curative Resection. J. Clin. Oncol. 2008, 26 (Suppl. 15), 4581. [Google Scholar] [CrossRef]
- Hsu, J.-T.; Hsu, C.-S.; Le, P.-H.; Chen, T.-C.; Chou, W.-C.; Lin, C.-Y.; Yeh, T.-S. Immunochemotherapy Benefits in Gastric Cancer Patients Stratified by Programmed Death-1 Ligand-1. J. Surg. Res. 2016, 211, 30–38. [Google Scholar] [CrossRef]
- Kanazawa, M.; Yoshihara, K.; Abe, H.; Iwadate, M.; Watanabe, K.; Suzuki, S.; Endoh, Y.; Takita, K.-I.; Sekikawa, K.; Takenoshita, S.; et al. Effects of PSK on T and Dendritic Cells Differentiation in Gastric or Colorectal Cancer Patients. Anticancer Res. 2005, 25, 443–449. [Google Scholar] [PubMed]
- Akagi, J.; Baba, H. PSK may suppress CD57+ T Cells to Improve Survival of Advanced Gastric Cancer Patients. Int. J. Clin. Oncol. 2010, 15, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Mochiki, E.; Ishiguro, T.; Ogura, T.; Sobajima, J.; Kumagai, Y.; Ishibashi, K.; Ishida, H. Improved Efficacy by Addition of Protein-bound Polysaccharide K to Adjuvant Chemotherapy for Advanced Gastric Cancer. Anticancer Res. 2016, 36, 4237–4241. [Google Scholar] [PubMed]
- Namikawa, T.; Fukudome, I.; Ogawa, M.; Munekage, E.; Shiga, M.; Maeda, H.; Kitagawa, H.; Kobayashi, M.; Hanazaki, K. Clinical Efficacy of Protein-Bound Polysaccharide K in Patients with Gastric Cancer Undergoing Chemotherapy with an Oral Fluoropyrimidine (S-1). Eur. J. Surg. Oncol. 2015, 41, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Shimada, M.; Kurita, N.; Sato, H.; Iwata, T.; Nishioka, M.; Morimoto, S.; Miyatani, T.; Komatsu, M. The Effect of Polysaccharide K With S-1 Based Chemotherapy in Advanced Gastric Cancer. Hepatogastroenterology 2013, 60, 1387–1390. [Google Scholar] [CrossRef]
- Kono, K.; Kawaguchi, Y.; Mizukami, Y.; Mimura, K.; Sugai, H.; Akaike, H.; Fujii, H. Protein-Bound Polysaccharide K Partially Prevents Apoptosis of Circulating T Cells Induced by Anti-Cancer Drug S-1 in Patients with Gastric Cancer. Oncology 2008, 74, 143–149. [Google Scholar] [CrossRef]
- Garrido, F.; Aptsiauri, N. Cancer Immune Escape:Mhcexpression in Primary Tumours Versus Metastases. Immunology 2019, 158, 255–266. [Google Scholar] [CrossRef]
- Gentaro, I.; Hiroaki, T.; Mami, Y.; Takehiro, I.; Kazuya, M.; Kubo, N.; Hisashi, N.; Kenjiro, K.; Ryosuke, A.; Eiji, N.; et al. Abstract 2687: Immunopotentiator, Protein-Bound Polysaccharide K, Is Efficient for Advanced Gastric Cancer with Negative Expression of MHC Class I. Cancer Res. 2011, 71, 2687. [Google Scholar] [CrossRef]
- Tanaka, H.; Ohira, M.; Muguruma, K.; Kubo, N.; Nagahara, H.; Kimura, K.; Amano, R.; Noda, E.; Yamada, N.; Yashiro, M.; et al. Abstract 1796: The Impact of Adjuvant Immunochemotherapy Using Protein-Bound Polysaccharide-K on Overall Survival of Gastric Cancer. Cancer Res. 2011, 71, 1796. [Google Scholar] [CrossRef]
- Ito, G.; Tanaka, H.; Ohira, M.; Yoshii, M.; Muguruma, K.; Kubo, N.; Yashiro, M.; Yamada, N.; Maeda, K.; Sawada, T.; et al. Correlation Between Efficacy of PSK Postoperative Adjuvant Immunochemotherapy for Gastric Cancer and Expression of MHC class I. Exp. Ther. Med. 2012, 3, 925–930. [Google Scholar] [CrossRef]
- Tanaka, H.; Muguruma, K.; Ohira, M.; Kubo, N.; Yamashita, Y.; Maeda, K.; Sawada, T.; Hirakawa, K. Impact of Adjuvant Immunochemotherapy Using Protein-Bound Polysaccharide-K on Overall Survival of Patients with Gastric Cancer. Anticancer Res. 2012, 32, 3427–3433. [Google Scholar] [PubMed]
- Kenji, I.; Takae, K.; Takafumi, A. The Use of Lentinan for Treating Gastric Cancer. Anti-Cancer Agents in Medicinal Chemistry. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2013, 13, 681–688. [Google Scholar]
- Del Buono, A.; Di Martino, S.; D’Orta, A.; De Monaco, A.; Simona, D.; Santillo, M.R.; Montanino, C.; De Felice, B. Action of Low Molecular Weight Glucan on Trascriptome of Neoplastic Cell Lines. World Cancer Res. J. 2020, 7, e1460. [Google Scholar]
- Li, Y.; Wang, X.; Ma, X.; Liu, C.; Wu, J.; Sun, C. Natural Polysaccharides and Their Derivates: A Promising Natural Adjuvant for Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 621813. [Google Scholar] [CrossRef]
- Bae, J.-S.; Jang, K.-H.; Jin, H.K. Effects of Natural Polysaccharides on the Growth and Peritoneal Carcinomatosis of Human Gastric Adenocarcinoma in a Nude Mouse Model. Cancer Lett. 2006, 235, 60–68. [Google Scholar] [CrossRef]
- Zan, X.; Cui, F.; Li, Y.; Yang, Y.; Wu, D.; Sun, W.; Ping, L. Hericium Erinaceus Polysaccharide-Protein HEG-5 Inhibits SGC-7901 Cell Growth Via Cell Cycle Arrest and Apoptosis. Int. J. Biol. Macromol. 2015, 76, 242–253. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Xiao, X.; Xu, D.; Gao, Y.; Gao, Q.; Mingxing, W. A Polysaccharide Isolated from Mycelia of the Lion’s Mane Medicinal Mushroom Hericium erinaceus (Agaricomycetes) Induced Apoptosis in Precancerous Human Gastric Cells. Int. J. Med. Mushrooms 2017, 19, 1053–1060. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Xu, C.; Huang, W.; He, P. Characterization and Antiproliferative Effect of Novel Acid Polysaccharides from the Spent Substrate of Shiitake Culinary-Medicinal Mushroom Lentinus edodes (Agaricomycetes) Cultivation. Int. J. Med. Mushrooms 2017, 19, 395–403. [Google Scholar] [CrossRef]
- Shi, B.J.; Nie, X.-H.; Chen, L.-Z.; Liu, Y.-L.; Tao, W.-Y. Anticancer Activities of a Chemically Sulfated Polysaccharide Obtained from Grifola Frondosa and Its Combination With 5-Fluorouracil Against Human Gastric Carcinoma Cells. Carbohydr. Polym. 2007, 68, 687–692. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, X.; Xiong, H.; Qiu, H.; Yuan, X.; Zhu, F.; Wang, Y.; Zou, Y. A Polysaccharide from Huaier Induced Apoptosis In MCF-7 Breast Cancer Cells Via Down-Regulation of MTDH Protein. Carbohydr. Polym. 2016, 151, 1027–1033. [Google Scholar] [CrossRef]
- Chen, M.; Lu, Y.; Zhang, R.; Bi, T.; Zhou, S. Effects of Huaier Polysaccharide SP1 on Gastric Cancer Cell Proliferation, Apoptosis, Migration, and Invasion by Regulating TGF-β/SMAD Signaling Pathway. Adv. Polym. Technol. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Chen, G.C.; Zhang, P.Y.; Huang, T.T.; Yu, W.Q.; Lin, J.; Li, P.; Chen, K.S. Polysaccharides from Rhizopus Nigricans Mycelia Induced Apoptosis and G2/M Arrest In BGC-823 Cells. Carbohydr. Polym. 2013, 97, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-Y.; Liu, J.-L.; Yang, W.; Hou, X.; Li, Q.-J. Antitumor Activity of Polysaccharide Extracted from Pleurotus Ostreatus Mycelia Against Gastric Cancer In Vitro and In Vivo. Mol. Med. Rep. 2015, 12, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zan, X.; Li, Y.; Sun, W.; Yang, Y.; Ping, L. Grifola frondosa Glycoprotein GFG-3a Arrests S phase, Alters Proteome, and Induces Apoptosis in Human Gastric Cancer Cells. Nutr. Cancer 2016, 68, 267–279. [Google Scholar] [CrossRef]
- Hui, T.W. Apoptosis Induced by Ganopoly in Human Gastric Cancer Cell Line SGC-7901 Cells. Afr. J. Biotechnol. 2011, 10, 18279–18284. [Google Scholar] [CrossRef]
- Pan, K.; Jiang, Q.; Liu, G.; Miao, X.; Zhong, D. Optimization Extraction of Ganoderma Lucidum Polysaccharides and Its Immunity and Antioxidant Activities. Int. J. Biol. Macromol. 2013, 55, 301–306. [Google Scholar] [CrossRef]
- Yang, W.; Han, D.; Wu, L.; Huang, Y.; Li, J.; Guo, H.; Liu, Y. Hericium Erinaceus Synergizing with Doxorubicin Induced SGC7901 Cell Apoptosis. Int. J. Clin. Exp. Med. 2016, 9, 1447–1457. [Google Scholar]
- Higashi, D.; Seki, K.; Ishibashi, Y.; Egawa, Y.; Koga, M.; Sasaki, T.; Hirano, K.; Mikami, K.; Futami, K.; Maekawa, T.; et al. The Effect of Lentinan Combination Therapy for Unresectable Advanced Gastric Cancer. Anticancer Res. 2012, 32, 2365–2368. [Google Scholar]
- Men’Shova, R.V.; Ermakova, S.P.; Um, B.H.; Zvyagintseva, T.N. The Composition and Structural Characteristics of Polysaccharides of the Brown Alga Eisenia bicyclis. Russ. J. Mar. Biol. 2013, 39, 208–213. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Lee, J.-S.; Kim, W.-S.; Jeon, Y.-J. The Potential of Brown-Algae Polysaccharides for The Development of Anticancer Agents: An Update on Anticancer Effects Reported for Fucoidan and Laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Higaki, K.; Nanba, E.; Ikeguchi, M. Anti-Proliferation Activity of Fucoidan in MKN45 Gastric Cancer Cells and Downregulation of Phosphorylated ASK1, a Cell Cycle-Regulated Kinase. Yonago Acta Med. 2015, 58, 1–7. [Google Scholar] [PubMed]
- Kang, Y.; Wang, Z.-J.; Xie, D.; Sun, X.; Yang, W.; Zhao, X.; Xu, N. Characterization and Potential Antitumor Activity of Polysaccharide from Gracilariopsis lemaneiformis. Mar. Drugs 2017, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.-J.; Nam, T.-J. Porphyran Induces Apoptosis Related Signal Pathway in AGS Gastric Cancer Cell Lines. Life Sci. 2006, 79, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Fujii, I.; Zhao, J.; Shinohara, M.; Matsukura, M. A novel Polysaccharide Derived from Algae Extract Induces Apoptosis and Cell Cycle Arrest in Human Gastric Carcinoma MKN45 Cells Via ROS/JNK Signaling Pathway. Int. J. Oncol. 2016, 49, 1561–1568. [Google Scholar] [CrossRef]
- Xie, P.; Horio, F.; Fujii, I.; Zhao, J.; Shinohara, M.; Matsukura, M. A Novel Polysaccharide Derived from Algae Extract Inhibits Cancer Progression Via JNK, Not Via the P38 MAPK Signaling Pathway. Int. J. Oncol. 2018, 52, 1380–1390. [Google Scholar] [CrossRef]
- Ji, Y.-B.; Ji, C.-F.; Yue, L. Human Gastric Cancer Cell Line SGC-7901 Apoptosis Induced By SFPS-B2 Via a Mitochondrial-Mediated Pathway. Bio-Medical Mater. Eng. 2014, 24, 1141–1147. [Google Scholar] [CrossRef]
- Kwon, M.-J.; Nam, T.-J. A Polysaccharide of the Marine Alga Capsosiphon Fulvescens Induces Apoptosis in AGS Gastric Cancer Cells Via An IGF-IR-Mediated PI3K/Akt Pathway. Cell Biol. Int. 2007, 31, 768–775. [Google Scholar] [CrossRef]
- Albana, C.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Armida, D.I.; Antonio, P.; Licia, T.; Nicola, T.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I. A Comparative Study of the Anti-Inflammatory, Anticoagulant, Antiangiogenic, and Antiadhesive Activities of Nine Different Fucoidans from Brown Seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar]
- Park, H.S.; Kim, G.-Y.; Nam, T.-J.; Kim, N.D.; Choi, Y.H. Antiproliferative Activity of Fucoidan Was Associated with the Induction of Apoptosis and Autophagy in AGS Human Gastric Cancer Cells. J. Food Sci. 2011, 76, T77–T83. [Google Scholar] [CrossRef]
- Desamero, M.J.; Kakuta, S.; Chambers, J.K.; Uchida, K.; Hachimura, S.; Takamoto, M.; Nakayama, J.; Nakayama, H.; Kyuwa, S. Orally Administered Brown Seaweed-Derived Β-Glucan Effectively Restrained Development of Gastric Dysplasia in A4gnt KO Mice That Spontaneously Develop Gastric Adenocarcinoma. Int. Immunopharmacol. 2018, 60, 211–220. [Google Scholar] [CrossRef]
- Han, M.; Sun, P.; Li, Y.; Wu, G.; Nie, J. Structural Characterization of a Polysaccharide from Sargassum Henslowianum, and Its Immunomodulatory Effect on Gastric Cancer Rat. Int. J. Biol. Macromol. 2018, 108, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Xie, S.R.; Zhao, S.; Zhang, G.X.; Liu, Z.W.; Zheng, K.; Chao, Y.; Shi, R.H.; Sun, W.H. Effects of Astragalus Polysaccharides on Proliferation and Apoptosis of Gastric Cancer Cell. J. Gastroenterol. Hepatol. 2007, 22, 176. [Google Scholar]
- Yu, J.; Ji, H.; Dong, X.; Feng, Y.; Liu, A. Apoptosis of Human Gastric Carcinoma MGC-803 Cells Induced by a Novel Astragalus Membranaceus Polysaccharide Via Intrinsic Mitochondrial Pathways. Int. J. Biol. Macromol. 2018, 126, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Peng, L. IDDF2019-ABS-0248 Astragalus polysaccharide Promotes Adriamycin-Induced Apoptosis in Gastric Cancer Cells. Gut 2019, 68, A25–A26. [Google Scholar] [CrossRef]
- Liu, X.-N.; Zhang, C.-Y.; Jin, X.-D.; Li, Y.-Z.; Zheng, X.-Z.; Li, L. Inhibitory Effect of Schisandrin B on Gastric Cancer Cells In Vitro. World J. Gastroenterol. 2007, 13, 6506–6511. [Google Scholar] [CrossRef]
- Wu, J.; Yu, J.; Wang, J.; Zhang, C.; Shang, K.; Yao, X.; Cao, B. Astragalus Polysaccharide Enhanced Antitumor Effects of Apatinib in Gastric Cancer AGS Cells by Inhibiting AKT Signalling Pathway. Biomed. Pharmacother. 2018, 100, 176–183. [Google Scholar] [CrossRef]
- Li, R.; Chen, W.-C.; Wang, W.-P.; Tian, W.-Y.; Zhang, X.-G. Extraction, Characterization of Astragalus Polysaccharides and Its Immune Modulating Activities in Rats with Gastric Cancer. Carbohydr. Polym. 2009, 78, 738–742. [Google Scholar] [CrossRef]
- Wang, J.-C.; Tian, J.-H.; Ge, L.; Gan, Y.-H.; Yang, K.-H. Which is the Best Chinese Herb Injection Based on the FOLFOX Regimen for Gastric Cancer? A Network Meta-analysis of Randomized Controlled Trials. Asian Pac. J. Cancer Prev. 2014, 15, 4795–4800. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, J.; Ni, M.; Wu, J.; Wang, K.; Duan, X.; Zhang, X.; Zhang, B. Comparative Efficacy and Safety of Chinese Herbal Injections Combined with the FOLFOX Regimen for Treating Gastric Cancer in China: A Network Meta-Analysis. Oncotarget 2017, 8, 68873–68889. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, M.; Zhao, L.; Ge, Y.-K.; Sheng, J.; Shi, W. Changes of Constituents and Activity to Apoptosis and Cell Cycle During Fermentation of Tea. Int. J. Mol. Sci. 2011, 12, 1862–1875. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Huang, Y.; Zhang, L.; Yang, J.; Xu, X.; Liu, Q. Study on the Anti-Tumor Ability of Niaowangzhong Green Tea. J. Food Biochem. 2016, 41, 1–13. [Google Scholar] [CrossRef]
- Shao, P.; Zhu, Y.Q.; Liu, Q.; Zhang, T.T.; Sun, P.L. Isolation, Antioxidant and Antitumor In Vitro Activities of Cold Pressed Green Tea Seed Cake Polysaccharide. Curr. Top. Nutraceutical Res. 2015, 13, 189–196. [Google Scholar]
- Xu, R.; Ye, H.; Sun, Y.; Tu, Y.; Zeng, X. Preparation, Preliminary Characterization, Antioxidant, Hepatoprotective and Antitumor Activities of Polysaccharides from the Flower of Tea Plant (Camellia sinensis). Food Chem. Toxicol. 2011, 50, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, B.; Gu, Y. Pharmacological Evaluation of Tea Polysaccharides with Antioxidant Activity in Gastric Cancer Mice. Carbohydr. Polym. 2012, 90, 943–947. [Google Scholar] [CrossRef]
- Yue, H.; Zeng, H.; Ding, K. A Review of Isolation Methods, Structure Features and Bioactivities of Polysaccharides from Dendrobium Species. Chin. J. Nat. Med. 2020, 18, 1–27. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Wang, G.; Ge, S.; Lan, X.; Xu, G.; Liu, H. Dendrobium Officinale Polysaccharides Inhibit 1-Methyl-2-Nitro-1-Nitrosoguanidine Induced Precancerous Lesions of Gastric Cancer in Rats through Regulating Wnt/β-Catenin Pathway and Altering Serum Endogenous Metabolites. Molecules 2019, 24, 2660. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Wang, G.; Ge, S.; Liu, H. Dendrobium Officinale Polysaccharides Protect against MNNG-Induced PLGC in Rats via Activating the NRF2 and Antioxidant Enzymes HO-1 and NQO-1. Oxidative Med. Cell. Longev. 2019, 2019, 9310245. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Ren, X. Inhibitory Effect of Dendrobium Officinale Polysaccharide on Human Gastric Cancer Cell Xenografts in Nude Mice. Food Sci. Technol. 2017, 38, 78–83. [Google Scholar] [CrossRef]
- Liu, B.; Shang, Z.-Z.; Li, Q.-M.; Zha, X.-Q.; Wu, D.-L.; Yu, N.-J.; Han, L.; Peng, D.-Y.; Luo, J.-P. Structural Features and Anti-Gastric Cancer Activity of Polysaccharides from Stem, Root, Leaf and Flower of Cultivated Dendrobium Huoshanense. Int. J. Biol. Macromol. 2019, 143, 651–664. [Google Scholar] [CrossRef]
- Yao, X.; Smolka, A.J. Gastric Parietal Cell Physiology and Helicobacter pylori–Induced Disease. Gastroenterology 2019, 156, 2158–2173. [Google Scholar] [CrossRef]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.-F.; Li, J.; Lu, M.; et al. The Gut Microbiome Is Associated with Clinical Response to Anti–PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yue, H.; Wang, Y.; Guo, C.; Du, Z.; Jin, C.; Ding, K. Intestinal Microbes Derived Butyrate Is Related to the Immunomodulatory Activities of Dendrobium Officinale Polysaccharide. Int. J. Biol. Macromol. 2020, 149, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Chen, S.; Ye, X.; Zhong, J.; Wu, N.; Dong, S.; Yang, B.; Liu, D. Antioxidant and Anti-Tumor Activity of a Polysaccharide from Freshwater Clam, Corbicula Fluminea. Food Funct. 2013, 4, 539–548. [Google Scholar] [CrossRef]
- Ji, Y.-B.; Dong, F.; Lang, L.; Zhang, L.-W.; Miao, J.; Liu, Z.-F.; Jin, L.-N.; Hao, Y. Optimization of Synthesis, Characterization and Cytotoxic Activity of Seleno-Capparis spionosa L. Polysaccharide. Int. J. Mol. Sci. 2012, 13, 17275–17289. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Huang, X.-Y.; Zhong, Z.-T.; Huang, F.; Li, S.-Y.; Wang, L.-M.; Min, T.; Wang, H.-X. Structural and Biological Properties of Polysaccharides from Lotus Root. Int. J. Biol. Macromol. 2019, 130, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Xiao, B.; Jiang, Z.; Guo, Y.; Mao, F.; Zhao, J.; Huang, X.; Guo, J. Growth Inhibition and Cell-Cycle Arrest of Human Gastric Cancer Cells by Lycium Barbarum Polysaccharide. Med. Oncol. 2009, 27, 785–790. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, R.; Jiang, D.; Liu, W.; Jia, Z. Lycium Barbarum Polysaccharide Inhibits Gastric Cancer Cell Proliferation, Migration and Invasion by Down-Regulation of Mmps and Suppressing Epithelial-Mesenchymal Transition. Int. J. Clin. Exp. Pathol. 2017, 10, 7369–7374. [Google Scholar]
- Xu, A.-H.; Chen, H.-S.; Sun, B.-C.; Xiang, X.-R.; Chu, Y.-F.; Zhai, F.; Jia, L.-C. Therapeutic Mechanism of Ginkgo Biloba Exocarp Polysaccharides on Gastric Cancer. World J. Gastroenterol. 2003, 9, 2424–2427. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Z.; Li, S.; Wang, L.; Lv, J.; Li, J.; Ma, X.; Fan, L.; Qian, F. Identification and Characterization of a Cytotoxic Polysaccharide from the Flower of Abelmoschus manihot. Int. J. Biol. Macromol. 2016, 82, 284–290. [Google Scholar] [CrossRef]
- He, L.; Ji, P.; Gong, X.; Li, W.; Cheng, J.; Qian, H.; Song, X. Physico-Chemical Characterization, Antioxidant and Anticancer Activities In Vitro Of a Novel Polysaccharide from Melia toosendan Sieb. Et Zucc fruit. Int. J. Biol. Macromol. 2011, 49, 422–427. [Google Scholar] [CrossRef]
- Huang, D.; Li, Y.; Cui, F.; Chen, J.; Sun, J. Purification and Characterization of a Novel Polysaccharide–Peptide Complex from Clinacanthus Nutans Lindau Leaves. Carbohydr. Polym. 2016, 137, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-F.; Zhang, Q.; Liu, X.-M.; Ma, L.; Lai, F. Extraction of Polysaccharides and Its Antitumor Activity on Magnolia Kwangsiensis Figlar & Noot. Carbohydr. Polym. 2016, 142, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhou, Y.; Sun, B.; Hu, J.; Kong, L.; Duan, S. Inhibitory Effect of Saponins and Polysaccharides from Radix Ranunculit Ternate on Human Gastric Cancer BGC823 Cells. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 561–566–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.-G.; Wang, D.-G.; Tian, W.; Wang, X.-X.; Zhao, J.-X.; Liu, Z.; Chen, R. Characterization and Anti-Tumor Activity of a Polysaccharide from Hedysarum Polybotrys Hand-Mazz. Carbohydr. Polym. 2008, 73, 344–350. [Google Scholar] [CrossRef]
- Xie, J.-H.; Liu, X.; Shen, M.-Y.; Nie, S.-P.; Zhang, H.; Li, C.; Gong, D.-M.; Xie, M.-Y. Purification, Physicochemical Characterisation and Anticancer Activity of a Polysaccharide from Cyclocarya Paliurus Leaves. Food Chem. 2012, 136, 1453–1460. [Google Scholar] [CrossRef]
- Li, C.; Fu, X.; Huang, Q.; Luo, F.; You, L. Ultrasonic Extraction and Structural Identification of Polysaccharides from Prunella Vulgaris and Its Antioxidant and Antiproliferative Activities. Eur. Food Res. Technol. 2014, 240, 49–60. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Xie, E.; Yang, X.; Wang, H.; Wang, X.; Li, W.; Song, Z.; Mu, Q.; Zhan, W.; et al. Functional Characterization of a Potent Anti-Tumor Polysaccharide in a Mouse Model of Gastric Cancer. Life Sci. 2019, 219, 11–19. [Google Scholar] [CrossRef]
- Wang, N.; Yang, J.; Lu, J.; Qiao, Q.; Wu, T.; Du, X.; Bao, G.; He, X. A Polysaccharide from Salvia Miltiorrhiza Bunge Improves Immune Function in Gastric Cancer Rats. Carbohydr. Polym. 2014, 111, 47–55. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Shi, S.; Jiang, L. Evaluation of Antioxidant and Immuno-Enhancing Activities of Purslane Polysaccharides in Gastric Cancer Rats. Int. J. Biol. Macromol. 2014, 68, 113–116. [Google Scholar] [CrossRef]
- Li, C.; Cai, J.; Geng, J.; Li, Y.; Wang, Z.; Li, R. Purification, Characterization and Anticancer Activity of a Polysaccharide from Panax Ginseng. Int. J. Biol. Macromol. 2012, 51, 968–973. [Google Scholar] [CrossRef]
- Cai, J.-P.; Wu, Y.-J.; Li, C.; Feng, M.-Y.; Shi, Q.-T.; Li, R.; Wang, Z.-Y.; Geng, J.-S. Panax Ginseng Polysaccharide Suppresses Metastasis Via Modulating Twist Expression in Gastric Cancer. Int. J. Biol. Macromol. 2013, 57, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tian, Z.-N.; Cai, J.-P.; Chen, K.-X.; Zhang, B.; Feng, M.-Y.; Shi, Q.-T.; Li, R.; Qin, Y.; Geng, J.-S. Panax Ginseng Polysaccharide Induces Apoptosis by Targeting Twist/AKR1C2/NF-1 Pathway in Human Gastric Cancer. Carbohydr. Polym. 2013, 102, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Su, M.; Li, Y.; Zeng, L.; Liu, X.; Li, J.; Zheng, B.; Wang, S. Effect of Acanthopanax Giraldii Harms Var. Hispidus Hoo Polysaccharides on the Human Gastric Cancer Cell Line SGC-7901 And Its Possible Mechanism. Chin. Med. J. 2002, 115, 716–721. [Google Scholar] [PubMed]
- Zhang, Y.; Wang, Q.; Wang, T.; Zhang, H.; Tian, Y.; Luo, H.; Yang, S.; Wang, Y.; Huang, X. Inhibition of Human Gastric Carcinoma Cell Growth In Vitro by a Polysaccharide from Aster tataricus. Int. J. Biol. Macromol. 2012, 51, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, Structures and Bioactivities of the Polysaccharides from Jujube Fruit (Ziziphus Jujuba Mill.): A Review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef]
- Xie, J.-H.; Jin, M.-L.; Morris, G.A.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56, S60–S84. [Google Scholar] [CrossRef]
- Noguera-Ortega, E.; Guallar-Garrido, S.; Julián, E. Mycobacteria-Based Vaccines as Immunotherapy for Non-urological Cancers. Cancers 2020, 12, 1802. [Google Scholar] [CrossRef]
- Wang, R.; Klegerman, M.E.; Marsden, I.; Sinnott, M.; Groves, M.J. An Anti-Neoplastic Glycan Isolated from Mycobacterium Bovis (BCG Vaccine). Biochem. J. 1995, 311, 867–872. [Google Scholar] [CrossRef][Green Version]
- Klegerman, M.E.; Devadoss, P.O.; Garrido, J.L.; Reyes, H.R.; Groves, M.J. Chemical and Ultrastructural Investigations Ofmycobacterium Bovisbcg: Implications for the Molecular Structure of the Mycobacterial Cell Envelope. FEMS Immunol. Med. Microbiol. 1996, 15, 213–222. [Google Scholar] [CrossRef]
- Garrido, J.L.; Klegerman, M.E.; Reyes, H.R.; Groves, M.J. Antineoplastic Activity of BCG: Location of Antineoplastic Glycans in the Cellular Integument of Mycobacterium Bovis, BCG Vaccine, Connaught Substrain. Cytobios 1997, 90, 47–65. [Google Scholar]
- Sun, C.; Rosendahl, A.; Wang, X.; Wu, D.; Andersson, R. Polysaccharide-K (PSK) in Cancer--Old Story, New Possibilities? Curr. Med. Chem. 2012, 19, 757–762. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).