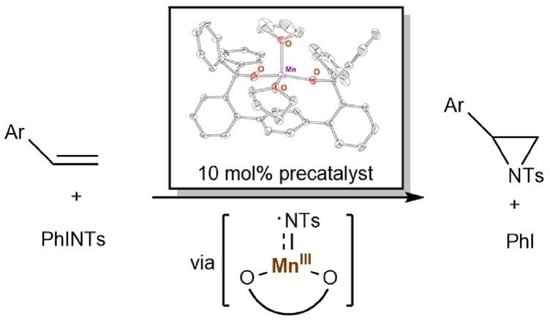
Aziridination Reactivity of a Manganese(II) Complex with a Bulky Chelating Bis(Alkoxide) Ligand
Abstract
:1. Introduction
2. Results and Discussion
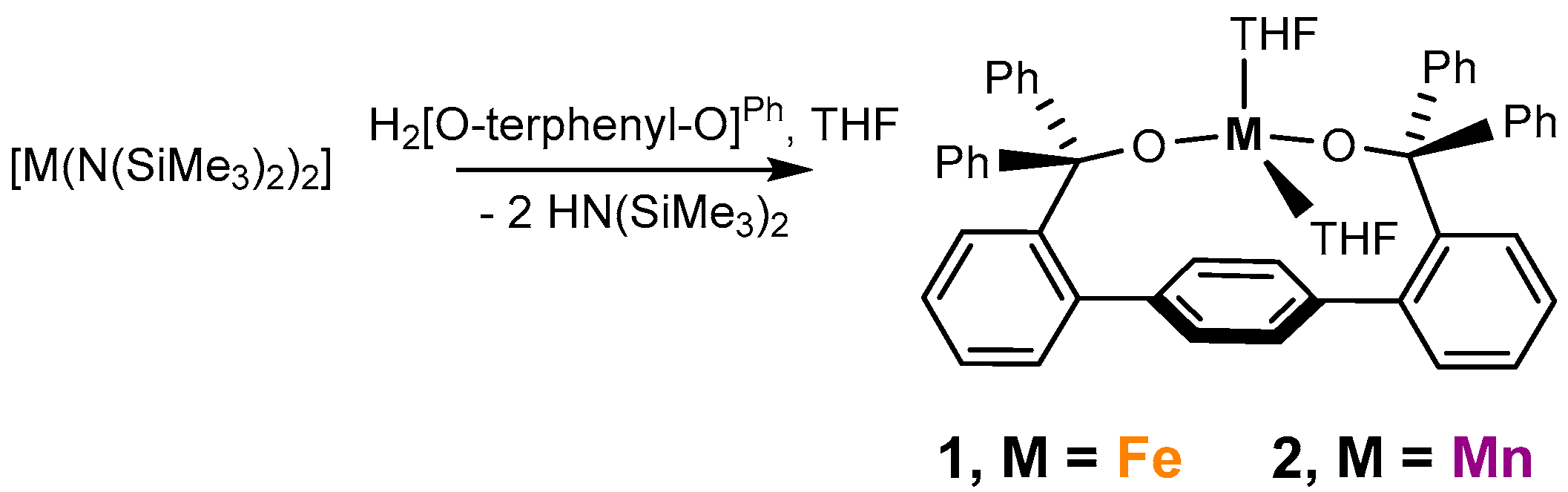
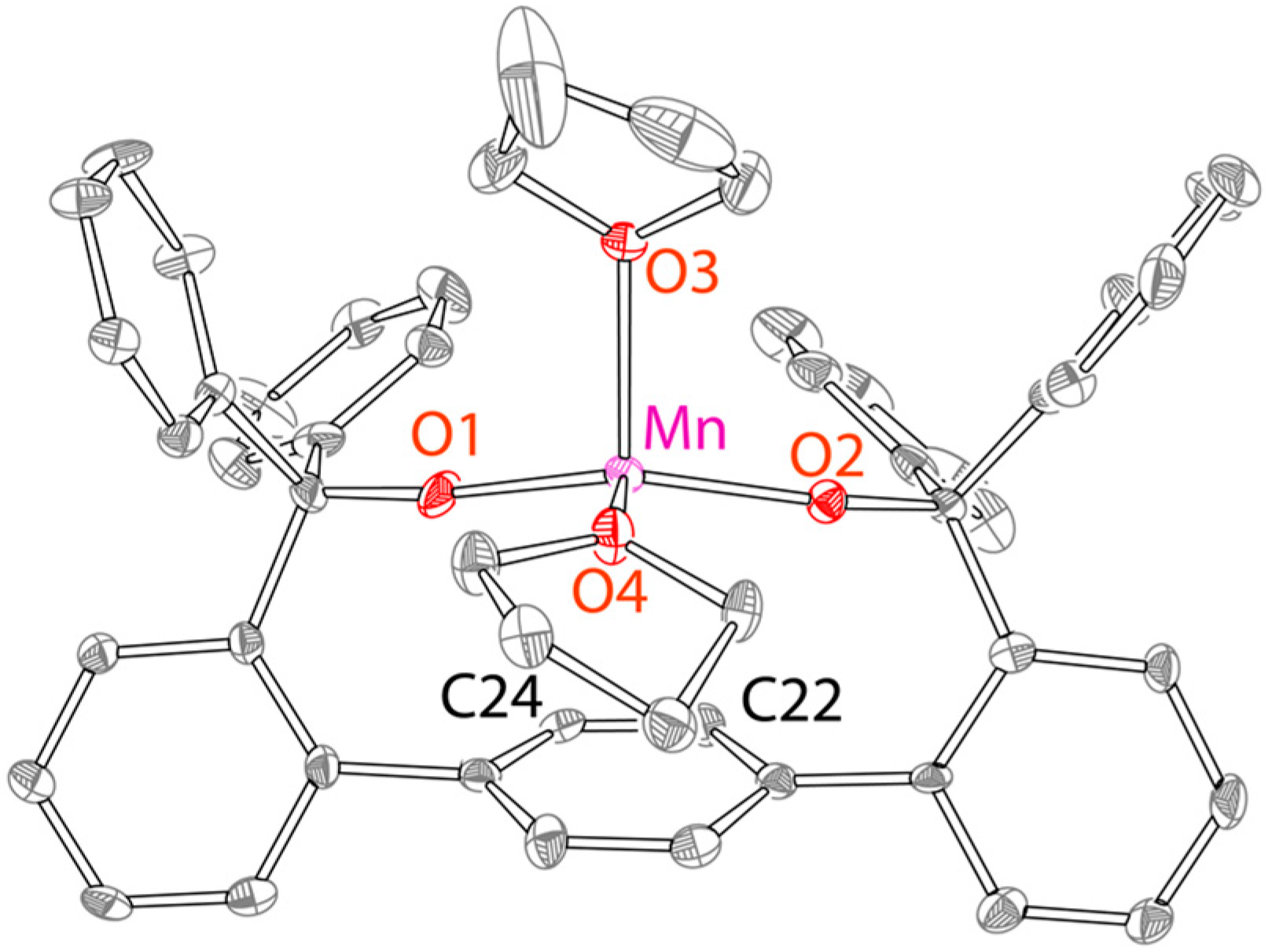
2.1. Reactivity of Fe[O-terphenyl-O]Ph(THF)2 (1) and Synthesis and Characterization of Mn[O-terphenyl-O]Ph(THF)2 (2)
2.2. Reactivity of 2 in Aziridination
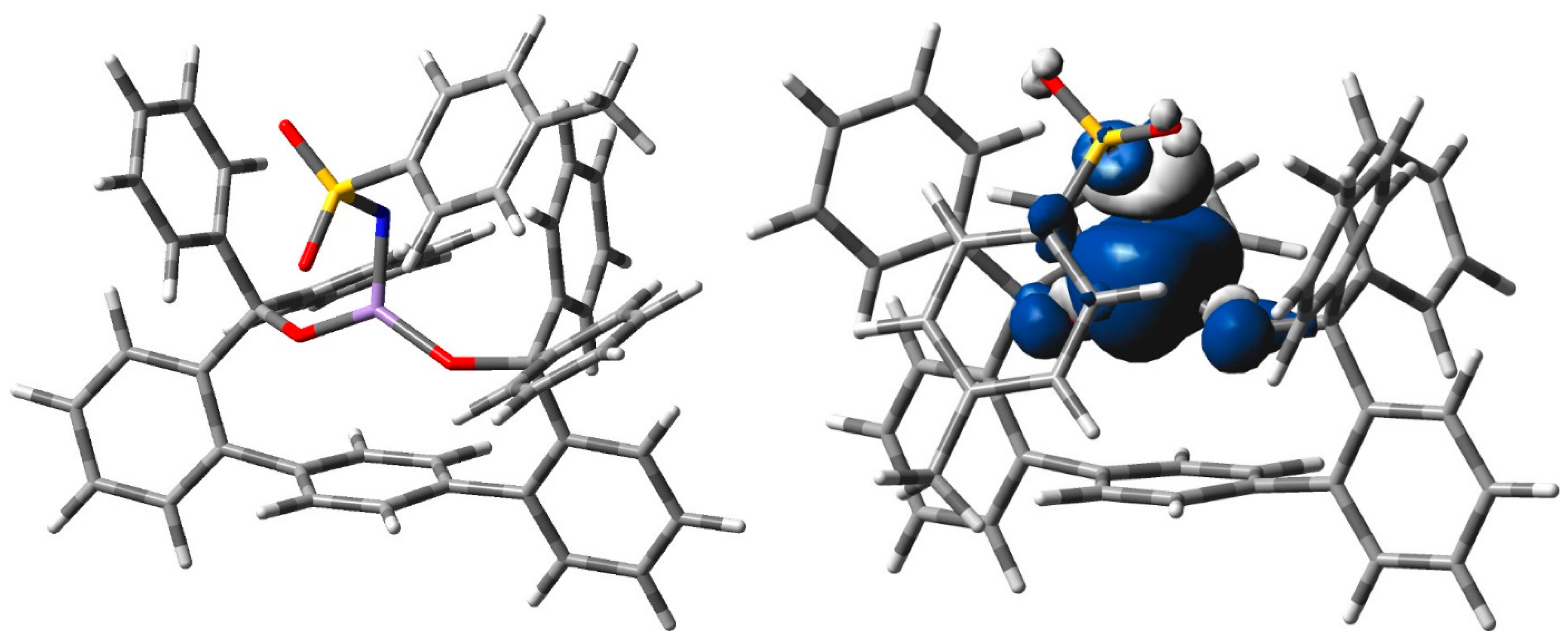
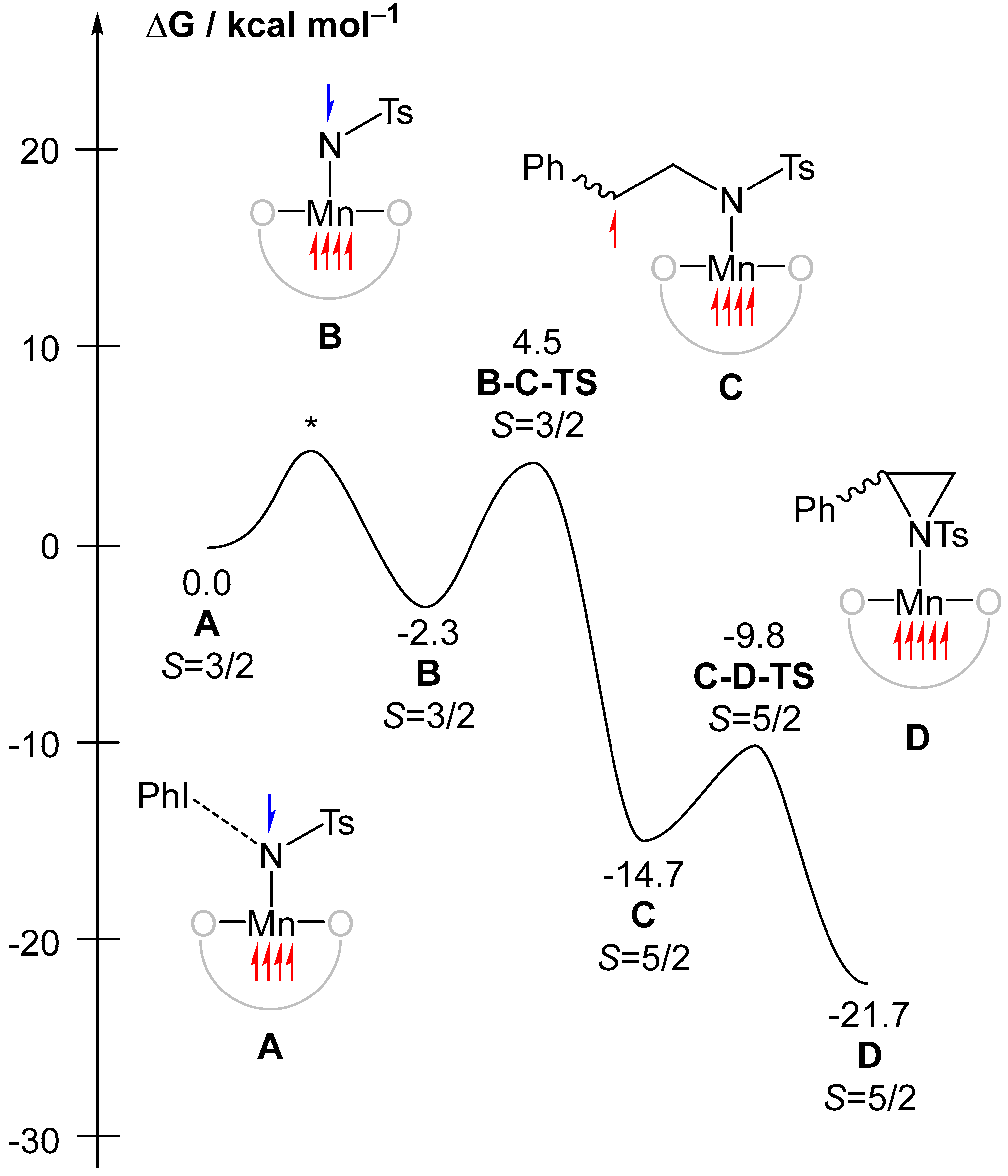
2.3. Spectroscopic and Computational Probing of the Reaction Mechanism
3. Summary and Conclusions
4. Materials and Methods
4.1. General Methods and Characterization
4.2. Synthesis of Mn[O-terphenyl-O]Ph(THF)2 (2)
4.3. General Procedure for Catalytic Formation of Aziridines
4.4. Computational Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sweeney, J.B. Aziridines: Epoxides’ Ugly Cousins? Chem. Soc. Rev. 2002, 31, 247–258. [Google Scholar] [PubMed]
- Paike, V.V.; Kauthale, S.S.; Kumar, R.C.; Katariya, M.V.; More, R.R.; Ameta, K.L.; Mane, S.B. Aziridines: Synthesis and bioactivity. In Bioactive Heterocycles; Ameta, K.L., Pawar, R.P., Domb, A.J., Eds.; Nova Scientific Publishers: Hauppauge, NY, USA, 2013; pp. 41–68. [Google Scholar]
- Kametani, T.; Honda, T. Application of Aziridines to the Synthesis of Natural Products. Adv. Heterocycl. Chem. 1986, 39, 181–236. [Google Scholar]
- Werner, L.; Machara, A.; Sullivan, B.; Carrera, I.; Moser, M.; Adams, D.R.; Hudlicky, T. Several Generations of Chemoenzymatic Synthesis of Oseltamivir (Tamiflu): Evolution of Strategy, Quest for a Process-Quality Synthesis, and Evaluation of Efficiency Metrics. J. Org. Chem. 2011, 76, 10050–10067. [Google Scholar]
- Hanessian, S.; Guesne, S.; Chenard, E. Total Synthesis of “Aliskiren”: The First Renin Inhibitor in Clinical Practice for Hypertension. Org. Lett. 2010, 12, 1816–1819. [Google Scholar] [PubMed]
- Nikitjuka, A.; Jirgensons, A. Synthesis, Chemical And Biological Properties Of Aziridine-1-Carbaldehyde Oximes. Chem. Heterocycl. Compd. 2014, 49, 1669–1684. [Google Scholar]
- Kinoshita, S.; Uzu, K.; Nakano, K.; Shimizu, M.; Takahashi, T.; Matsui, M. Mitomycin Derivatives. Preparation of Mitosane and Mitosene Compounds and Their Biological Activities. J. Med. Chem. 1971, 14, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lu, C.; Zhao, B.; Wang, Q.; Yao, Y. Cycloaddition of Aziridine with CO2/CS2 Catalyzed by Amidato Divalent Lanthanide Complexes. J. Org. Chem. 2019, 84, 1951–1958. [Google Scholar]
- Zhu, C.; Feng, J.; Zhang, J. Rhodium-catalyzed intermolecular [3+3] cycloaddition of vinyl aziridines with C,N-cyclic azomethine imines: Stereospecific Synthesis Of Chiral fused tricyclic 1,2,4-hexahydrotriazines. Chem. Commun. 2017, 53, 4688–4691. [Google Scholar]
- Degennaro, L.; Trinchera, P.; Luisi, R. Recent Advances in the Stereoselective Synthesis of Aziridines. Chem. Rev. 2014, 114, 7881–7929. [Google Scholar]
- Zhu, Y.; Wang, Q.; Cornwall, R.G.; Shi, Y. Organocatalytic Asymmetric Epoxidation and Aziridination of Olefins and Their Synthetic Applications. Chem. Rev. 2014, 114, 8199–8256. [Google Scholar]
- Wenker, H. The Preparation of Ethylene Imine from Monoethanolamine. J. Am. Chem. Soc. 1935, 57, 2328. [Google Scholar] [CrossRef]
- Marsini, M.A.; Reeves, J.T.; Desrosiers, J.N.; Herbage, M.A.; Savoie, J.; Li, Z.; Fandrick, K.R.; Sader, C.A.; McKibben, B.; Gao, D.A.; et al. Diastereoselective Synthesis of α-Quaternary Aziridine-2-Carboxylates via Aza-Corey-Chaykovsky Aziridination of N-Tert-Butanesulfinyl Ketimino Esters. Org. Lett. 2015, 17, 5614–5617. [Google Scholar] [CrossRef] [PubMed]
- Dokli, I.; Matanovic, I.; Hamersak, Z. Sulfur ylide promoted synthesis of N-protected aziridines: A combined experimental and computational approach. Chem. Eur. J. 2010, 16, 11744–11752. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.W.; Ton, T.M.U.; Chan, P.W.H. Transition-Metal-Catalyzed Aminations and Aziridinations of C-H and C=C Bonds with Iminoiodinanes. Chem. Rec. 2011, 11, 331–357. [Google Scholar] [PubMed]
- Darses, B.; Rodrigues, R.; Neuville, L.; Mazurais, M.; Dauban, P. Transition metal-catalyzed iodine(III)-mediated nitrene transfer reactions: Efficient tools for challenging syntheses. Chem. Commun. 2017, 53, 493–508. [Google Scholar]
- Zhdankin, V.V.; Protasiewicz, J.D. Development of New Hypervalent Iodine Reagents with Improved Properties and Reactivity by Redirecting Secondary Bonds at Iodine Center. Coord. Chem. Rev. 2014, 275, 54–62. [Google Scholar] [CrossRef]
- Karila, D.; Dodd, R.H. Recent Progress in Iminoiodane-Mediated Aziridination of Olefins. Curr. Org. Chem. 2011, 15, 1507–1538. [Google Scholar]
- Uchida, T.; Katsuki, T. Asymmetric Nitrene Transfer Reactions: Sulfimidation, Aziridination and C-H Amination Using Azide Compounds as Nitrene Precursors. Chem. Rec. 2014, 14, 117–129. [Google Scholar]
- Aujla, P.S.; Baird, C.P.; Taylor, P.C.; Mauger, H.; Vallée, Y. Can chloramine-T be a nitrene transfer agent? Tetrahedron Lett. 1997, 38, 7453–7456. [Google Scholar]
- Cano, I.; Nicasio, M.C.; Pérez, P.J. Nitrene transfer reactions catalysed by copper(I) complexes in ionic liquid using chloramine-T. Dalton Trans. 2009, 4, 730–734. [Google Scholar] [CrossRef]
- Antunes, A.M.M.; Marto, S.J.L.; Branco, P.S.; Prabhakar, S.; Lobo, A.M. Palladium(II)-promoted Aziridination of Olefins with Bromamine T as the Nitrogen Transfer Reagent. Chem. Commun. 2001, 5, 405–406. [Google Scholar]
- Elkoush, T.; Mak, C.L.; Paley, D.W.; Campbell, M.G. Silver(II) and Silver(III) Intermediates in Alkene Aziridination with a Dinuclear Silver(I) Nitrene Transfer Catalyst. ACS Catal. 2020, 10, 4820–4826. [Google Scholar]
- Mat Lani, A.S.; Schomaker, J.M. Site-Selective, Catalyst-Controlled Alkene Aziridination. Synthesis 2018, 50, 4462–4470. [Google Scholar]
- Ju, M.; Weatherly, C.D.; Guzei, I.A.; Schomaker, J.M. Chemo- and Enantioselective Intramolecular Silver-Catalyzed Aziridinations. Angew. Chem. Int. Ed. 2017, 56, 9944–9948. [Google Scholar] [CrossRef] [PubMed]
- Weatherly, C.; Alderson, J.M.; Berry, J.F.; Hein, J.E.; Schomaker, J.M. Catalyst-controlled nitrene transfer by tuning metal:ligand ratios: Insight into the mechanisms of chemoselectivity. Organometallics 2017, 36, 1649–1661. [Google Scholar] [CrossRef]
- Safin, D.A.; Pialat, A.; Korobkov, I.; Murugesu, M. Unprecedented Trinuclear AgI Complex with 2,4,6-Tris (2-pyrimidyl)-1,3,5-triazine as an Efficient Catalyst for the Aziridination of Olefins. Chem. Eur. J. 2015, 21, 6144–6149. [Google Scholar] [CrossRef]
- Maestre, L.; Sameera, W.M.C.; Diaz-Requejo, M.M.; Maseras, F.; Perez, P.J. A General Mechanism for the Copper- and Silver-Catalyzed Olefin Aziridination Reactions: Concomitant Involvement of the Singlet and Triplet Pathways. J. Am. Chem. Soc. 2013, 135, 1338–1348. [Google Scholar]
- Lee, S.; Jang, Y.J.; Phipps, E.J.T.; Lei, H.; Rovis, T. Rhodium(III)-Catalyzed Three-Component 1,2-Diamination of Unactivated Terminal Alkenes. Synthesis 2020, 52, 1247–1252. [Google Scholar] [CrossRef]
- Azek, E.; Spitz, C.; Ernzerhof, M.; Lebel, H. A Mechanistic Study of the Stereochemical Outcomes of Rhodium-Catalysed Styrene Aziridinations. Adv. Syn. Catal. 2020, 362, 384–397. [Google Scholar]
- Lee, S.; Lei, H.; Rovis, T. A Rh(III)-Catalyzed Formal [4+1] Approach to Pyrrolidines from Unactivated Terminal Alkenes and Nitrene Sources. J. Am. Chem. Soc. 2019, 141, 12536–12540. [Google Scholar] [CrossRef]
- Sabir, S.; Pandey, C.B.; Yadav, A.K.; Tiwari, B.; Jat, J.L. Direct N-H/N-Me Aziridination of Unactivated Olefins Using O-(Sulfonyl)hydroxylamines as Aminating Agents. J. Org. Chem. 2018, 83, 12255–12260. [Google Scholar] [PubMed]
- Su, J.Y.; Olson, D.E.; Ting, S.I.; Du Bois, J. Synthetic Studies Toward Pactamycin Highlighting Oxidative C-H and Alkene Amination Technologies. J. Org. Chem. 2018, 83, 7121–7134. [Google Scholar]
- Ciesielski, J.; Dequirez, G.; Retailleau, P.; Gandon, V.; Dauban, P. Rhodium-Catalyzed Alkene Difunctionalization with Nitrenes. Chem. Eur. J. 2016, 22, 9338–9347. [Google Scholar] [PubMed]
- Olson, D.E.; Su, J.Y.; Roberts, D.A.; Du Bois, J. Vicinal Diamination of Alkenes under Rh-Catalysis. J. Am. Chem. Soc. 2014, 136, 13506–13509. [Google Scholar]
- Smith, D.T.; Njardarson, J.T. A scalable rhodium-catalyzed intermolecular aziridination reaction. Angew. Chem. Int. Ed. 2014, 53, 4278–4280. [Google Scholar]
- Jat, J.L.; Paudyal, M.P.; Gao, H.; Xu, Q.-L.; Yousufuddin, M.; Devarajan, D.; Ess, D.H.; Kurti, L.; Falck, J.R. Direct Stereospecific Synthesis of Unprotected N-H and N-Me Aziridines from Olefins. Science 2014, 343, 61–65. [Google Scholar] [PubMed]
- Wu, K.; Zhou, C.-Y.; Che, C.-M. Perfluoroalkyl Aziridines with Ruthenium Porphyrin Carbene Intermediates. Org. Lett. 2019, 21, 85–89. [Google Scholar]
- Sengupta, G.; Pandey, P.; De, S.; Ramapanicker, R.; Bera, J.K. A bromo-capped diruthenium(II) N-heterocyclic carbene compound for in situ bromine generation with NBS: Catalytic olefin aziridination reactions. Dalton Trans. 2018, 47, 11917–11924. [Google Scholar]
- Kim, C.; Uchida, T.; Katsuki, T. Asymmetric olefin aziridination using a newly designed Ru(CO)(salen) complex as the catalyst. Chem. Commun. 2012, 48, 7188–7190. [Google Scholar]
- Tso, K.C.-H.; Chan, S.L.-F.; Huang, J.-S.; Che, C.-M. Wheel-to-rhomboid isomerization as well as nitrene transfer catalysis of ruthenium-thiolate wheels. Chem. Commun. 2017, 53, 2419–2422. [Google Scholar]
- Rossi, S.; Puglisi, A.; Benaglia, M.; Carminati, D.M.; Intrieri, D.; Gallo, E. Synthesis in mesoreactors: Ru(porphyrin)CO-catalyzed aziridination of olefins under continuous flow conditions. Catal. Sci. Technol. 2016, 6, 4700–4704. [Google Scholar] [CrossRef]
- Zardi, P.; Pozzoli, A.; Ferretti, F.; Manca, G.; Mealli, C.; Gallo, E. A mechanistic investigation of the ruthenium porphyrin catalyzed aziridination of olefins by aryl azides. Dalton Trans. 2015, 44, 10479–10489. [Google Scholar] [CrossRef] [PubMed]
- Law, S.-M.; Chen, D.; Chan, S.L.-F.; Guan, X.; Tsui, W.-M.; Huang, J.-S.; Zhu, N.; Che, C.-M. Ruthenium Porphyrins with Axial π-Conjugated Arylamide and Arylimide Ligands. Chem. Eur. J. 2014, 20, 11035–11047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Origins of the enantioselectivity of a palladium catalyst with BINOL-phosphoric acid ligands. Org. Biomol. Chem. 2018, 16, 8064–8071. [Google Scholar] [CrossRef]
- Smalley, A.P.; Gaunt, M.J. Mechanistic Insights into the Palladium-Catalyzed Aziridination of Aliphatic Amines by C-H Activation. J. Am. Chem. Soc. 2015, 137, 10632–10641. [Google Scholar] [CrossRef]
- Han, J.; Li, Y.; Zhi, S.; Pan, Y.; Timmons, C.; Li, G. Palladium-catalyzed aziridination of alkenes using N,N-dichloro-p-toluenesulfonamide as nitrogen source. Tetrahedron Lett. 2006, 47, 7225–7228. [Google Scholar] [CrossRef]
- Fingerhut, A.; Serdyuk, O.V.; Tsogoeva, S.B. Non-heme iron catalysts for epoxidation and aziridination reactions of challenging terminal alkenes: Towards sustainability. Green Chem. 2015, 17, 2042–2058. [Google Scholar] [CrossRef]
- Jenkins, D.M. Atom-economical C2 + N1 aziridination: Progress towards catalytic intermolecular reactions using alkenes and aryl azides. Synlett 2012, 23, 1267–1270. [Google Scholar] [CrossRef]
- Breslow, R.; Gellman, S.H. Tosylamidation of Cyclohexane by a Cytochrome P-450 Model. J. Chem. Soc. Chem. Commun. 1982, 24, 1400–1401. [Google Scholar] [CrossRef]
- Coin, G.; Patra, R.; Rana, S.; Biswas, J.P.; Dubourdeaux, P.; Clemancey, M.; de Visser, S.P.; Maiti, D.; Maldivi, P.; Latour, J.-M. Fe-Catalyzed Aziridination Is Governed by the Electron Affinity of the Active Imido-Iron Species. ACS Catal. 2020, 10, 10010–10020. [Google Scholar] [CrossRef]
- Du, Y.-D.; Zhou, C.-Y.; To, W.-P.; Wang, H.-X.; Che, C.-M. Iron porphyrin catalysed light driven C-H bond amination and alkene aziridination with organic azides. Chem. Sci. 2020, 11, 4680–4686. [Google Scholar] [CrossRef] [PubMed]
- Damiano, C.; Gadolini, S.; Intrieri, D.; Lay, L.; Colombo, C.; Gallo, E. Iron and Ruthenium Glycoporphyrins: Active Catalysts for the Synthesis of Cyclopropanes and Aziridines. Eur. J. Inorg. Chem. 2019, 41, 4412–4420. [Google Scholar] [CrossRef]
- Shehata, M.F.; Ayer, S.K.; Roizen, J.L. Iron(MCP) Complexes Catalyze Aziridination with Olefins As Limiting Reagents. J. Org. Chem. 2018, 83, 5072–5081. [Google Scholar] [CrossRef] [PubMed]
- Damiano, C.; Intrieri, D.; Gallo, E. Aziridination of Alkenes Promoted by Iron or Ruthenium Complexes. Inorg. Chim. Acta 2018, 470, 51–67. [Google Scholar] [CrossRef]
- Hennessy, E.T.; Liu, R.Y.; Iovan, D.A.; Duncan, R.A.; Betley, T.A. Iron-mediated intermolecular N-group transfer chemistry with olefinic substrates. Chem. Sci. 2014, 5, 1526–1532. [Google Scholar] [CrossRef]
- Liang, L.; Lv, H.; Yu, Y.; Wang, P.; Zhang, J.-L. Iron(III) Tetrakis(pentafluorophenyl)Porpholactone Catalyzes Nitrogen Atom Transfer to C:C and C-H Bonds with Organic Azides. Dalton Trans. 2012, 41, 1457–1460. [Google Scholar] [CrossRef]
- Iovan, D.A.; Betley, T.A. Characterization of Iron-Imido Species Relevant for N-Group Transfer Chemistry. J. Am. Chem. Soc. 2016, 138, 1983–1993. [Google Scholar] [CrossRef]
- Liu, Y.; Che, C.-M. [FeIII(F20-tpp)Cl] Is an Effective Catalyst for Nitrene Transfer Reactions and Amination of Saturated Hydrocarbons with Sulfonyl and Aryl Azides as Nitrogen Source under Thermal and Microwave-Assisted Conditions. Chem. Eur. J. 2010, 16, 10494–10501. [Google Scholar] [CrossRef]
- Klotz, K.L.; Slominski, L.M.; Riemer, M.E.; Phillips, J.A.; Halfen, J.A. Mechanism of the Iron-Mediated Alkene Aziridination Reaction: Experimental and Computational Investigations. Inorg. Chem. 2009, 48, 801–803. [Google Scholar] [CrossRef]
- Heins, S.P.; Morris, W.D.; Wolczanski, P.T.; Lobkovsky, E.B.; Cundari, T.R. Nitrene Insertion into C-C and C-H Bonds of Diamide Diimine Ligands Ligated to Chromium and Iron. Angew. Chem. Int. Ed. 2015, 54, 14407–14411. [Google Scholar] [CrossRef]
- van Leest, N.P.; Tepaske, M.A.; Venderbosch, B.; Oudsen, J.-P.H.; Tromp, M.; van der Vlugt, J.I.; de Bruin, B. Electronically Asynchronous Transition States for C–N Bond Formation by Electrophilic [CoIII(TAML)]-Nitrene Radical Complexes Involving Substrate-to-Ligand Single-Electron Transfer and a Cobalt-Centered Spin Shuttle. ACS Catal. 2020, 10, 7449–7463. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lang, K.; Tao, J.; Marshall, M.K.; Cheng, Q.; Cui, X.; Wojtas, L.; Zhang, X.P. Next-Generation D2-Symmetric Chiral Porphyrins for Cobalt(II)-Based Metalloradical Catalysis: Catalyst Engineering by Distal Bridging. Angew. Chem. Int. Ed. 2019, 58, 2670–2674. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lang, K.; Lu, H.; Wojtas, L.; Zhang, X.P.J. Asymmetric Radical Bicyclization of Allyl Azidoformates via Cobalt(II)-Based Metalloradical Catalysis. J. Am. Chem. Soc. 2017, 139, 9164–9167. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jiang, H.; Hu, Y.; Wojtas, L.; Zhang, X.P. Chemoselective intramolecular allylic C-H amination versus C:C aziridination through Co(II)-based metalloradical catalysis. Chem. Sci. 2011, 2, 2361–2366. [Google Scholar] [CrossRef]
- Kalra, A.; Bagchi, V.; Paraskevopoulou, P.; Das, P.; Ai, L.; Sanakis, Y.; Raptopoulos, G.; Mohapatra, S. Choudhury, A.; Sun, Z.; et al. Is the Electrophilicity of the Metal Nitrene the Sole Predictor of Metal-Mediated Nitrene Transfer to Olefins? Secondary Contributing Factors as Revealed by a Library of High-Spin Co(II) Reagents. Organometallics 2021, 40, 1974–1996. [Google Scholar] [CrossRef]
- Coin, G.; Patra, R.; Clemancey, M.; Dubourdeaux, P.; Pecaut, J.; Lebrun, C.; Castro, L.; Maldivi, P.; Chardon-Noblat, S.; Latour, J.-M. Fe-based Complexes as Styrene Aziridination Catalysts: Ligand Substitution Tunes Catalyst Activity. ChemCatChem 2019, 11, 5296–5299. [Google Scholar] [CrossRef]
- Patra, R.; Coin, G.; Castro, L.; Dubourdeaux, P.; Clemancey, M.; Pecaut, J.; Lebrun, C.; Maldivi, P.; Latour, J.-M. Rational design of Fe catalysts for olefin aziridination through DFT-based mechanistic analysis. Catal. Sci. Technol. 2017, 7, 4388–4400. [Google Scholar] [CrossRef]
- Blatchford, K.M.; Mize, C.J.; Roy, S.; Jenkins, D.M. Toward Asymmetric Aziridination with an Iron Complex Supported by a D2-symmetric Tetra-NHC. Dalton Trans. 2022, 51, 6153–6156. [Google Scholar] [CrossRef]
- Isbill, S.B.; Chandrachud, P.P.; Kern, J.L.; Jenkins, D.M.; Roy, S. Elucidation of the Reaction Mechanism of C2 + N1 Aziridination from Tetracarbene Iron Catalysts. ACS Catal. 2019, 9, 6223–6233. [Google Scholar] [CrossRef]
- Crandell, D.W.; Munoz, S.B.; Smith, J.M.; Baik, M.-H. Mechanistic study of styrene aziridination by iron(IV) nitrides. Chem. Sci. 2018, 9, 8542–8552. [Google Scholar] [CrossRef]
- Chandrachud, P.P.; Bass, H.M.; Jenkins, D.M. Synthesis of Fully Aliphatic Aziridines with a Macrocyclic Tetracarbene Iron Catalyst. Organometallics 2016, 35, 1652–1657. [Google Scholar] [CrossRef]
- Munoz, S.B., III; Lee, W.-T.; Dickie, D.A.; Scepaniak, J.J.; Subedi, D.; Pink, M.; Johnson, M.D.; Smith, J.M. Styrene Aziridination by Iron(IV) Nitrides. Angew. Chem. Int. Ed. 2015, 54, 10600–10603. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.A.; Hernandez Sanchez, R.; Brakhage, D.F.; Jenkins, D.M. Probing the Role of an Fe(IV) Tetrazene in Catalytic Aziridination. Chem. Commun. 2014, 50, 13967–13970. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.A.; Jenkins, D.M. Synthesis of Aziridines from Alkenes and Aryl Azides with a Reusable Macrocyclic Tetracarbene Iron Catalyst. J. Am. Chem. Soc. 2011, 133, 19342–19345. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, L.; Zhang, H.; Chen, H.; Deng, L. Three-Coordinate Iron(IV) Bisimido Complexes with Aminocarbene Ligation: Synthesis, Structure, and Reactivity. J. Am. Chem. Soc. 2015, 137, 14196–14207. [Google Scholar] [CrossRef]
- Liu, Q.; Long, L.; Ma, P.; Ma, Y.; Leng, X.; Xiao, J.; Chen, H.; Deng, L. Synthesis, Structure, and C–H Bond Activation Reaction of An Iron(IV) Terminal Imido Complex Bearing Trifluoromethyl Groups. Cell. Rep. Phys. Sci. 2021, 2, 100454. [Google Scholar] [CrossRef]
- Abu-Omar, M.M. High-valent iron and manganese complexes of corrole and porphyrin in atom transfer and dioxygen evolving catalysis. Dalton Trans. 2011, 40, 3435–3444. [Google Scholar] [CrossRef]
- Fantauzzi, S.; Caselli, A.; Gallo, E. Nitrene transfer reactions mediated by metallo-porphyrin complexes. Dalton Trans. 2009, 28, 5434–5443. [Google Scholar] [CrossRef]
- Chinkov, N. Catalytic enantioselective reactions using organomanganese compounds. In The Chemistry of Organomanganese Compounds; Rappoport, Z., Marek, I., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2011. [Google Scholar]
- Mansuy, D.; Mahy, J.P.; Dureault, A.; Bedi, G.; Battioni, P. Iron- and Manganese-Porphyrin Catalysed Aziridination of Alkenes by Tosyl- and Acyl-Iminoiodobenzene. J. Chem. Soc. Chem. Commun. 1984, 17, 1161–1163. [Google Scholar] [CrossRef]
- Liang, J.-L.; Huang, J.-S.; Yu, X.-Q.; Zhu, N.; Che, C.-M. Metalloporphyrin-mediated asymmetric nitrogen-atom transfer to hydrocarbons: Aziridination of alkenes and amidation of saturated C-H bonds catalyzed by chiral ruthenium and manganese porphyrins. Chem. Eur. J. 2002, 8, 1563–1572. [Google Scholar] [CrossRef]
- Noda, K.; Hosoya, N.; Irie, R.; Ito, Y.; Katsuki, T. Asymmetric Aziridination by Using Optically Active (Salen) Manganese(III) Complexes. Synlett 1993, 7, 469–471. [Google Scholar] [CrossRef]
- O’Connor, K.J.; Wey, S.J.; Burrows, C. Alkene aziridination and epoxidation catalyzed by chiral metal salen complexes. Tetrahedron Lett. 1992, 33, 1001–1004. [Google Scholar] [CrossRef]
- Zdilla, M.J.; Abu-Omar, M.M. Mechanism of Catalytic Aziridination with Manganese Corrole: The Often Postulated High-Valent Mn(V) Imido Is Not the Group Transfer Reagent. J. Am. Chem. Soc. 2006, 128, 16971–16979. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.Y.; Wang, Y.; Shu, Y.J.; Liu, H.H.; Zhou, X.G. Nitrene Transfer Reaction Catalyzed by Substituted Metallophthalocyanines. J. Mol. Catal. A Chem. 2006, 248, 148–151. [Google Scholar] [CrossRef]
- Liang, S.; Jensen, M.P. Half-Sandwich Scorpionates as Nitrene Transfer Catalysts. Organometallics 2012, 31, 8055–8058. [Google Scholar] [CrossRef]
- Bagchi, V.; Kalra, A.; Das, P.; Paraskevopoulou, P.; Gorla, S.; Ai, L.; Wang, Q.; Mohapatra, S.; Choudhury, A.; Sun, Z.; et al. Comparative Nitrene-Transfer Chemistry to Olefinic Substrates Mediated by a Library of Anionic Mn(II) Triphenylamido-Amine Reagents and M(II) Congeners (M = Fe, Co, Ni) Favoring Aromatic over Aliphatic Alkenes. ACS Catal. 2018, 8, 9183–9206. [Google Scholar] [CrossRef]
- Grass, A.; Wannipurage, D.; Lord, R.L.; Groysman, S. Group-transfer chemistry at transition metal centers in bulky alkoxide ligand environments. Coord. Chem. Rev. 2019, 400, 1–16. [Google Scholar] [CrossRef]
- Kurup, S.S.; Groysman, S. Catalytic Synthesis of Azoarenes via Metal-mediated Nitrene Coupling. Dalton Trans. 2022, 51, 4577–4589. [Google Scholar] [CrossRef]
- Yousif, M.; Tjapkes, D.J.; Lord, R.L.; Groysman, S. Catalytic Formation of Asymmetric Carbodiimides at Mononuclear Chromium (II/IV) Bis(alkoxide) Complexes. Organometallics 2015, 34, 5119–5128. [Google Scholar] [CrossRef]
- Yousif, M.; Wannipurage, D.; Huizenga, C.D.; Washnock-Schmid, E.; Peraino, N.J.; Ozarowski, A.; Stoian, S.A.; Lord, R.L.; Groysman, S. Catalytic Nitrene Homocoupling by an Iron(II) Bis(alkoxide) Complex: Bulking Up the Alkoxide Enables a Wider Range of Substrates and Provides Insight into the Reaction Mechanism. Inorg. Chem. 2018, 57, 9425–9438. [Google Scholar] [CrossRef]
- Bellow, J.A.; Yousif, M.; Cabelof, A.C.; Lord, R.L.; Groysman, S. Reactivity Modes of an Iron Bis(alkoxide) Complex with Aryl Azides: Catalytic Nitrene Coupling vs Formation of Iron(III) Imido Dimers. Organometallics 2015, 34, 2917–2923. [Google Scholar] [CrossRef]
- Wannipurage, D.; Kurup, S.S.; Groysman, S. Heterocoupling of Different Aryl Nitrenes to Produce Asymmetric Azoarenes Using Iron-Alkoxide Catalysis and Investigation of the Cis-Trans Isomerism of Selected Bulky Asymmetric Azoarenes. Organometallics 2021, 40, 3637–3644. [Google Scholar] [CrossRef]
- Grass, A.; Dewey, N.S.; Lord, R.L.; Groysman, S. Ketenimine Formation Catalyzed by a High Valent Cobalt Carbene in Bulky Alkoxide Ligand Environment. Organometallics 2019, 38, 962–972. [Google Scholar] [CrossRef]
- Grass, A.; Bellow, J.A.; Morrison, G.; zur Loye, H.-C.; Lord, R.L.; Groysman, S. One electron reduction transforms high-valent low-spin cobalt alkylidene into high-spin cobalt(II) carbene radical. Chem. Commun. 2020, 56, 8416–8419. [Google Scholar] [CrossRef] [PubMed]
- Kurup, S.S.; Wannipurage, D.; Lord, R.L.; Groysman, S. An iron complex with a new chelating bis(alkoxide) ligand leads to an active nitrene dimerization catalyst for a variety of para- and meta-substituted azide precursors. Chem. Commun. 2019, 55, 10780–10783. [Google Scholar] [CrossRef]
- Kurup, S.S.; Staples, R.J.; Lord, R.L.; Groysman, S. Synthesis of Chromium(II) Complexes with Chelating Bis(alkoxide) Ligand and Their Reactions with Organoazides and Diazoalkanes. Molecules 2020, 25, 373. [Google Scholar] [CrossRef]
- Bellow, J.A.; Yousif, M.; Fang, D.; Kratz, E.G.; Cisneros, G.A.; Groysman, S. Synthesis and reactivity of 3d metal complexes with the bulky alkoxide ligand [OCtBu2Ph]. Inorg. Chem. 2015, 54, 5624–5633. [Google Scholar] [CrossRef]
- Bellow, J.A.; Martin, P.D.; Lord, R.L.; Groysman, S. Reductive Coupling of Azides Mediated by an Iron(II) Bis(alkoxide) Complex. Inorg. Chem. 2013, 52, 12335–12337. [Google Scholar] [CrossRef]
- Eikey, R.A.; Khan, S.I.; Abu-Omar, M.M. The Elusive Terminal Imido of Manganese(V). Angew. Chem. Int. Ed. 2002, 41, 3591–3595. [Google Scholar] [CrossRef]
- Lansky, D.E.; Kosack, J.R.; Narducci Sarjeant, A.A.; Goldberg, D.P. An Isolable, Nonreducible High-Valent Manganese(V) Imido Corrolazine Complex. Inorg. Chem. 2006, 45, 8477–8479. [Google Scholar] [CrossRef]
- Shi, H.; Xie, J.; Lam, W.W.Y.; Man, W.-L.; Mak, C.-K.; Yiu, S.-M.; Lee, H.K.; Lau, T.-C. Generation and Reactivity of a One-Electron-Oxidized Manganese(V) Imido Complex with a Tetraamido Macrocyclic Ligand. Chem. Eur. J. 2019, 25, 12895–12899. [Google Scholar] [CrossRef]
- Yamada, Y.; Yamamoto, T.; Okawara, M. Synthesis and Reaction of New Type I–N Ylide, N-Tosyliminoiodinane. Chem. Lett. 1975, 4, 361–362. [Google Scholar] [CrossRef]
- Bradley, D.C.; Hursthouse, M.B.; Ibrahim, A.A.; Malik, K.M.A.; Motevalli, M.; Möseler, R.; Powell, H.; Runnacles, J.D.; Sullivan, A.C. Synthesis and chemistry of the bis(trimethylsilyl)amido bis-tetrahydrofuranates of the group 2 metals magnesium, calcium, strontium and barium. X-ray crystal structures of Mg[N(SiMe3)2]2·2THF and related Mn[N(SiMe3)2]2·2THF. Polyhedron 1990, 9, 2959–2964. [Google Scholar] [CrossRef]
- Johnson, S.L.; Hilinski, M.K. Organocatalytic Olefin Aziridination via Iminium-Catalyzed Nitrene Transfer: Scope, Limitations, and Mechanistic Insight. J. Org. Chem. 2019, 84, 8589–8595. [Google Scholar] [CrossRef]
- Minakata, S.; Morino, Y.; Oderaotoshia, Y.; Komatsu, M. Novel aziridination of olefins: Direct synthesis from sulfonamides using t-BuOI. Chem. Commun. 2006, 31, 3337–3339. [Google Scholar] [CrossRef]
- Evans, D.A.; Faul, M.M.; Bilodeau, M.T. Development of the Copper-Catalyzed Olefin Aziridination Reaction. J. Am. Chem. Soc. 1994, 116, 2742–2753. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Schlegel, H.B. Geometry optimization. WIREs Comput. Mol. Sci. 2011, 1, 790–809. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Stability analysis for solutions of the closed shell Kohn-Sham equation. J. Chem. Phys. 1996, 104, 9047–9052. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence, and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coloumb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Bootsma, A.N.; Wheeler, S.E. Popular Integration Grids Can Result in Large Errors in DFT-Computed Free Energies. ChemRxiv 2019. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, 6th ed.; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]


 | |||||
|---|---|---|---|---|---|
| Entry | 2 (mol%) | Solvent | PhINTs (equiv) | Styrene (equiv) | Yield (%) |
| 1 | 10 | C6D6 | 1 | 4 | 78 |
| 2 | 10 | CD2Cl2 | 1 | 4 | 79 |
| 3 | 10 | CD3CN | 1 | 4 | 70 |
| 4 | 10 | C6D6 | 1 | 2 | 74 |
| 5 | 5 | C6D6 | 1 | 2 | 53 |
| 6 | 10 | C6D6 | 1 | 1 | 75 |
| 7 | 10 | CD2Cl2 | 1 | 1 | 17 |
| 8 | 10 | CD3CN | 1 | 1 | 43 |
| 9 | 10 | C6D6 | 2 | 1 | 74 |
| 10 | 10 | C6D6 | 1.5 | 1 | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurup, S.S.; Woodland, N.M.; Lord, R.L.; Groysman, S. Aziridination Reactivity of a Manganese(II) Complex with a Bulky Chelating Bis(Alkoxide) Ligand. Molecules 2022, 27, 5751. https://doi.org/10.3390/molecules27185751
Kurup SS, Woodland NM, Lord RL, Groysman S. Aziridination Reactivity of a Manganese(II) Complex with a Bulky Chelating Bis(Alkoxide) Ligand. Molecules. 2022; 27(18):5751. https://doi.org/10.3390/molecules27185751
Chicago/Turabian StyleKurup, Sudheer S., Natalie M. Woodland, Richard L. Lord, and Stanislav Groysman. 2022. "Aziridination Reactivity of a Manganese(II) Complex with a Bulky Chelating Bis(Alkoxide) Ligand" Molecules 27, no. 18: 5751. https://doi.org/10.3390/molecules27185751
APA StyleKurup, S. S., Woodland, N. M., Lord, R. L., & Groysman, S. (2022). Aziridination Reactivity of a Manganese(II) Complex with a Bulky Chelating Bis(Alkoxide) Ligand. Molecules, 27(18), 5751. https://doi.org/10.3390/molecules27185751