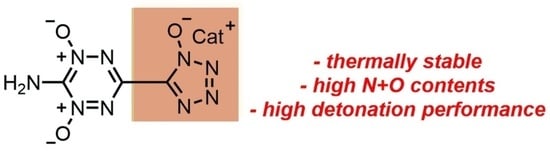
An Alliance of Polynitrogen Heterocycles: Novel Energetic Tetrazinedioxide-Hydroxytetrazole-Based Materials
Abstract
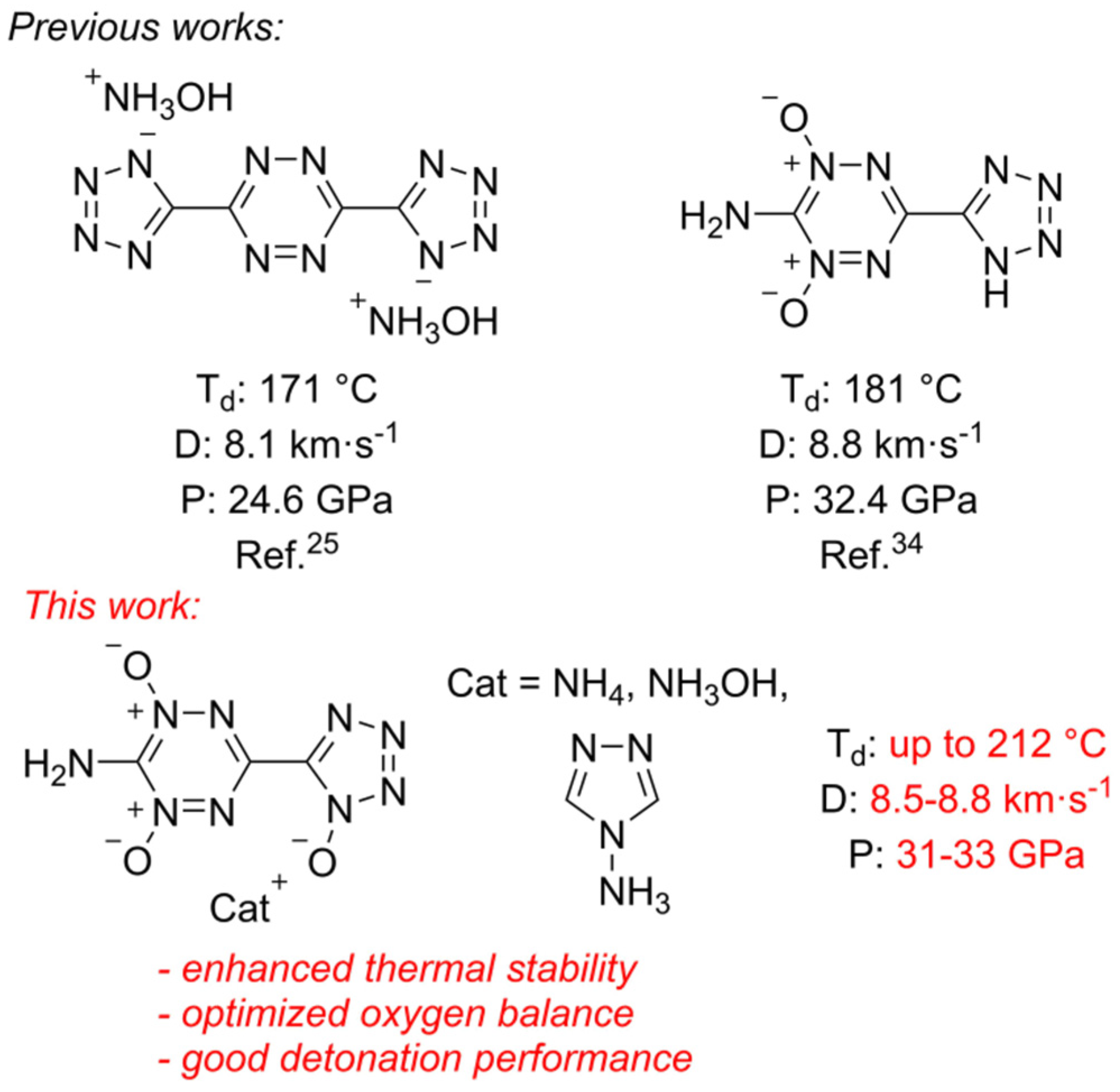
:1. Introduction
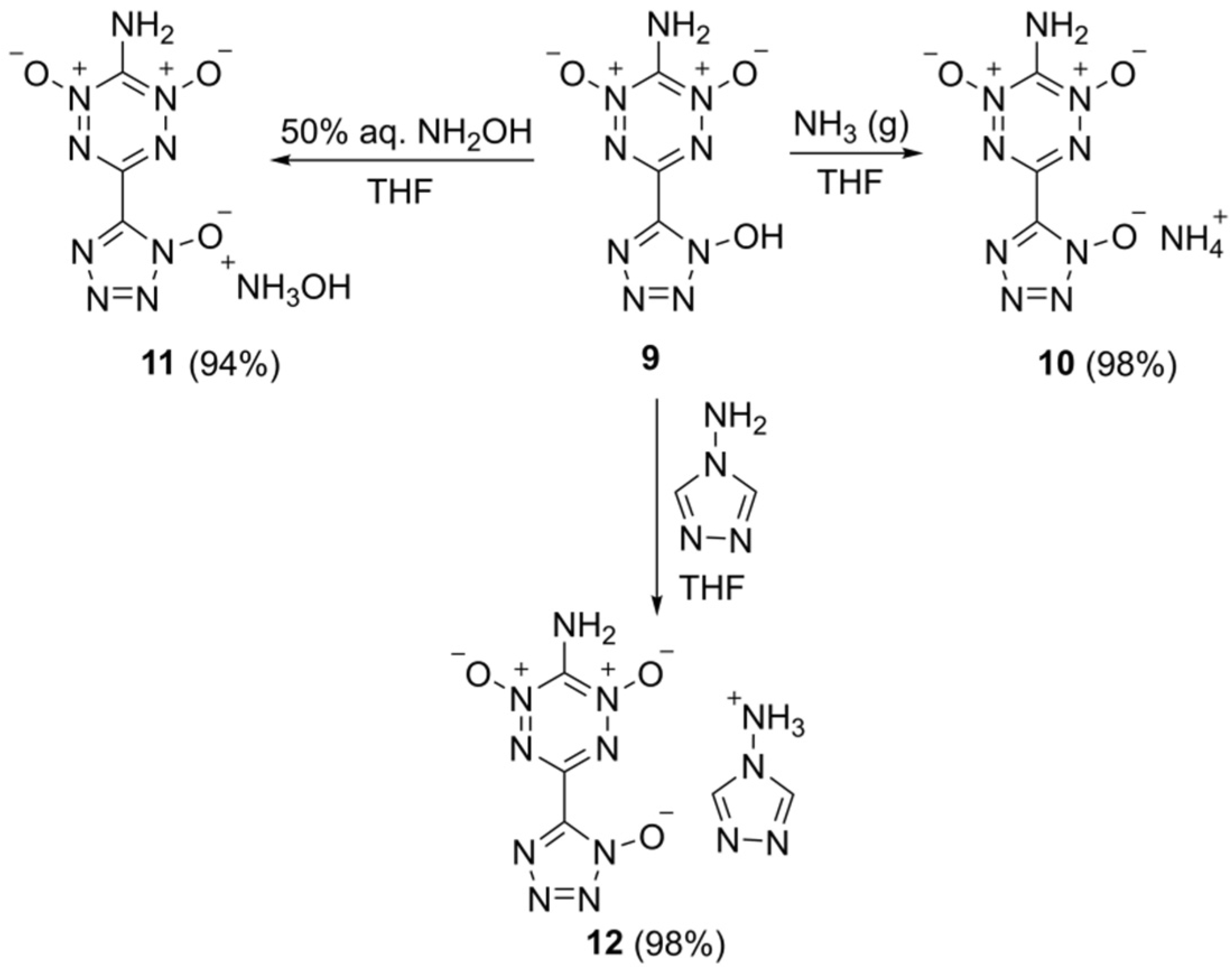
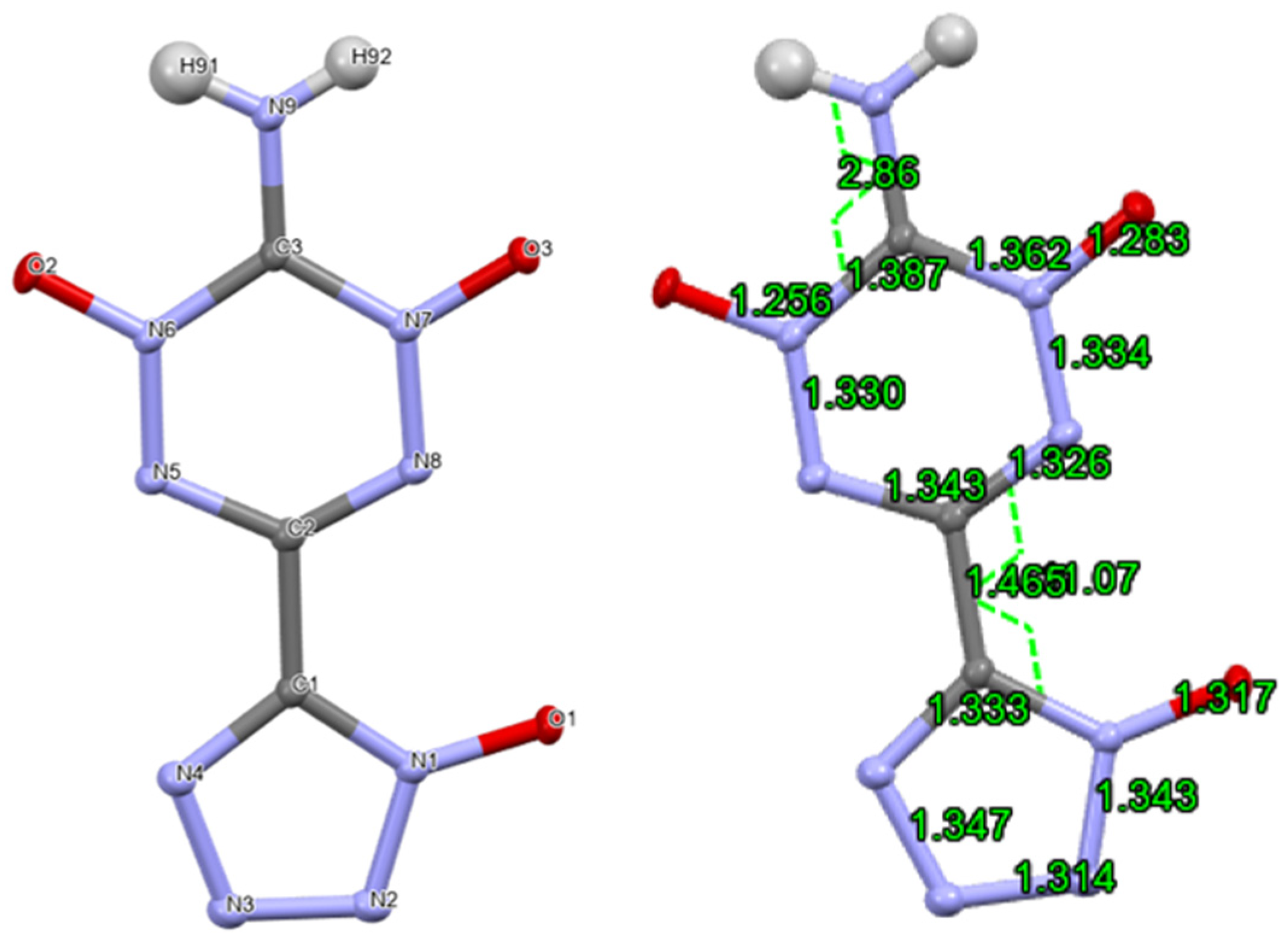
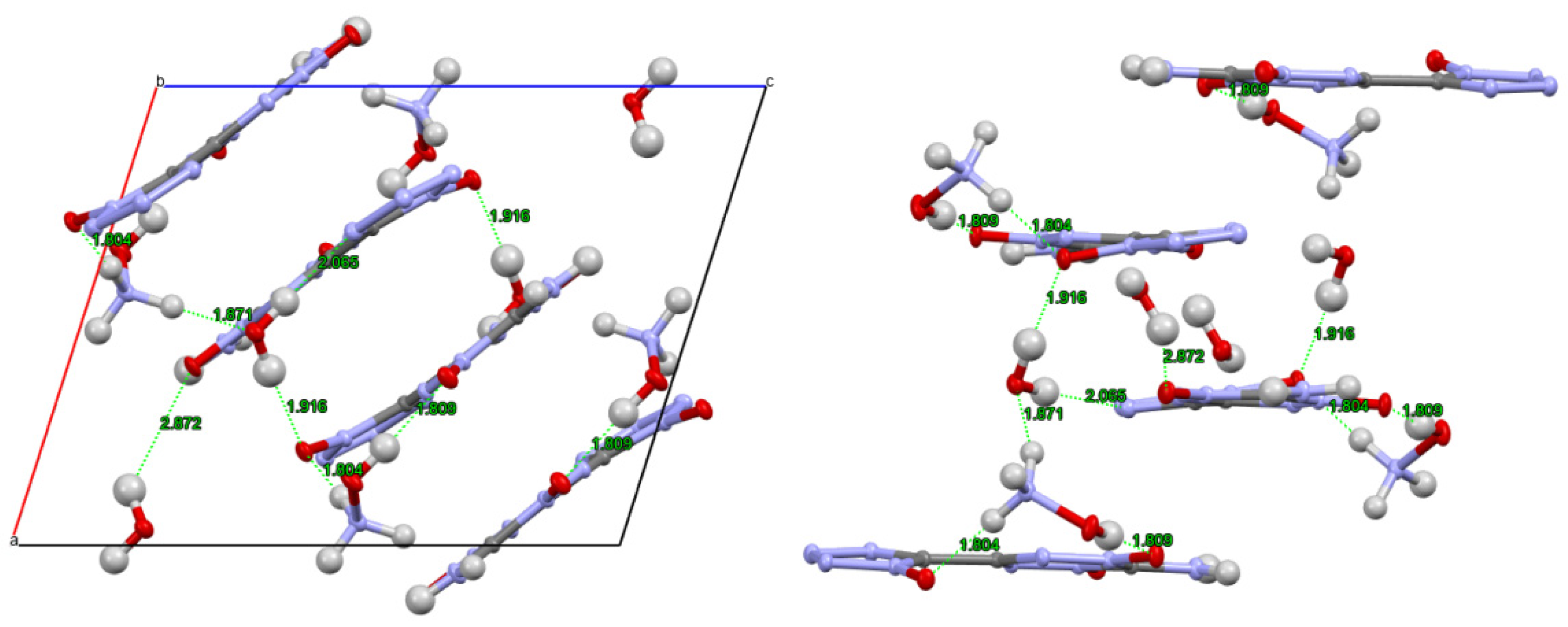
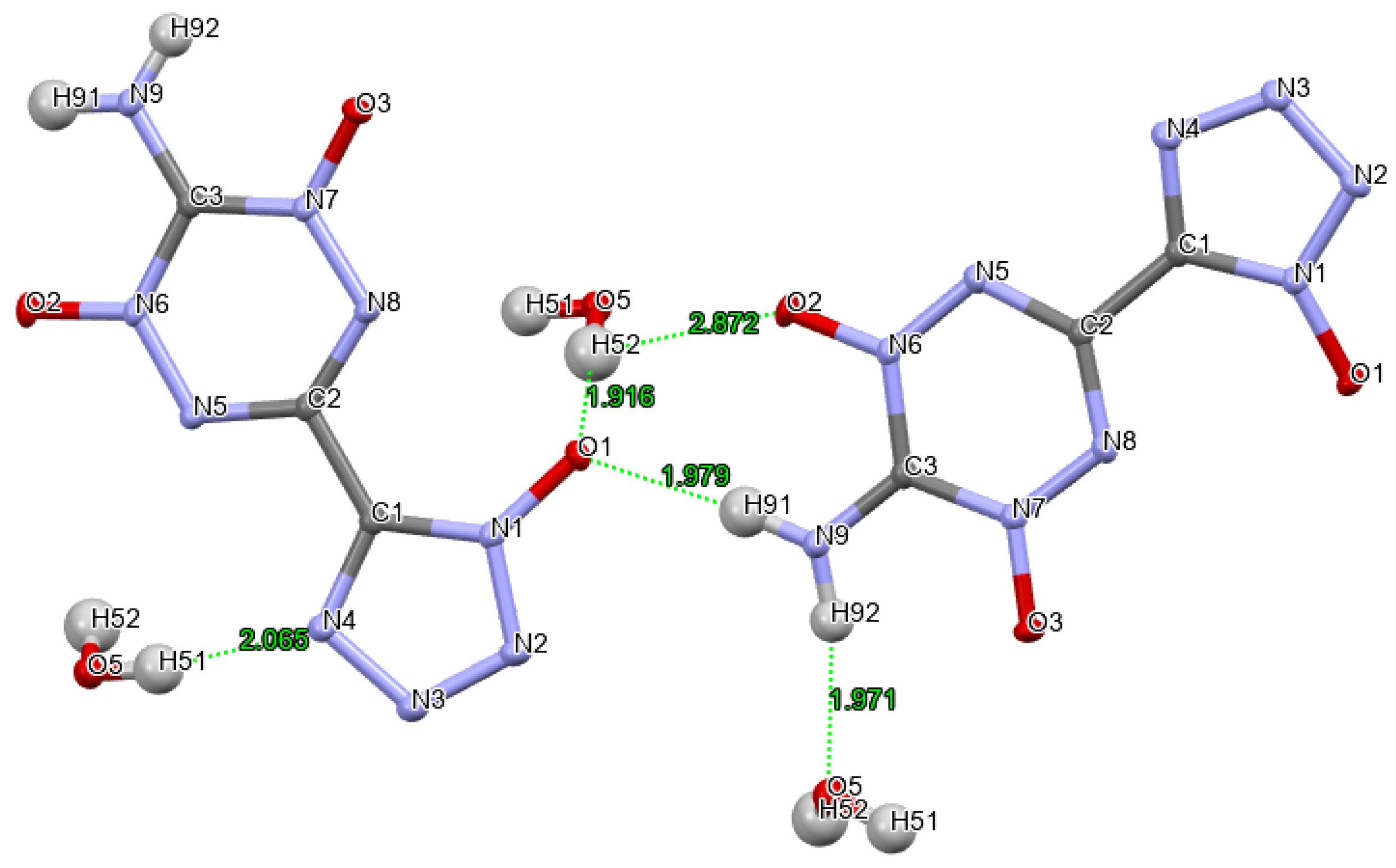
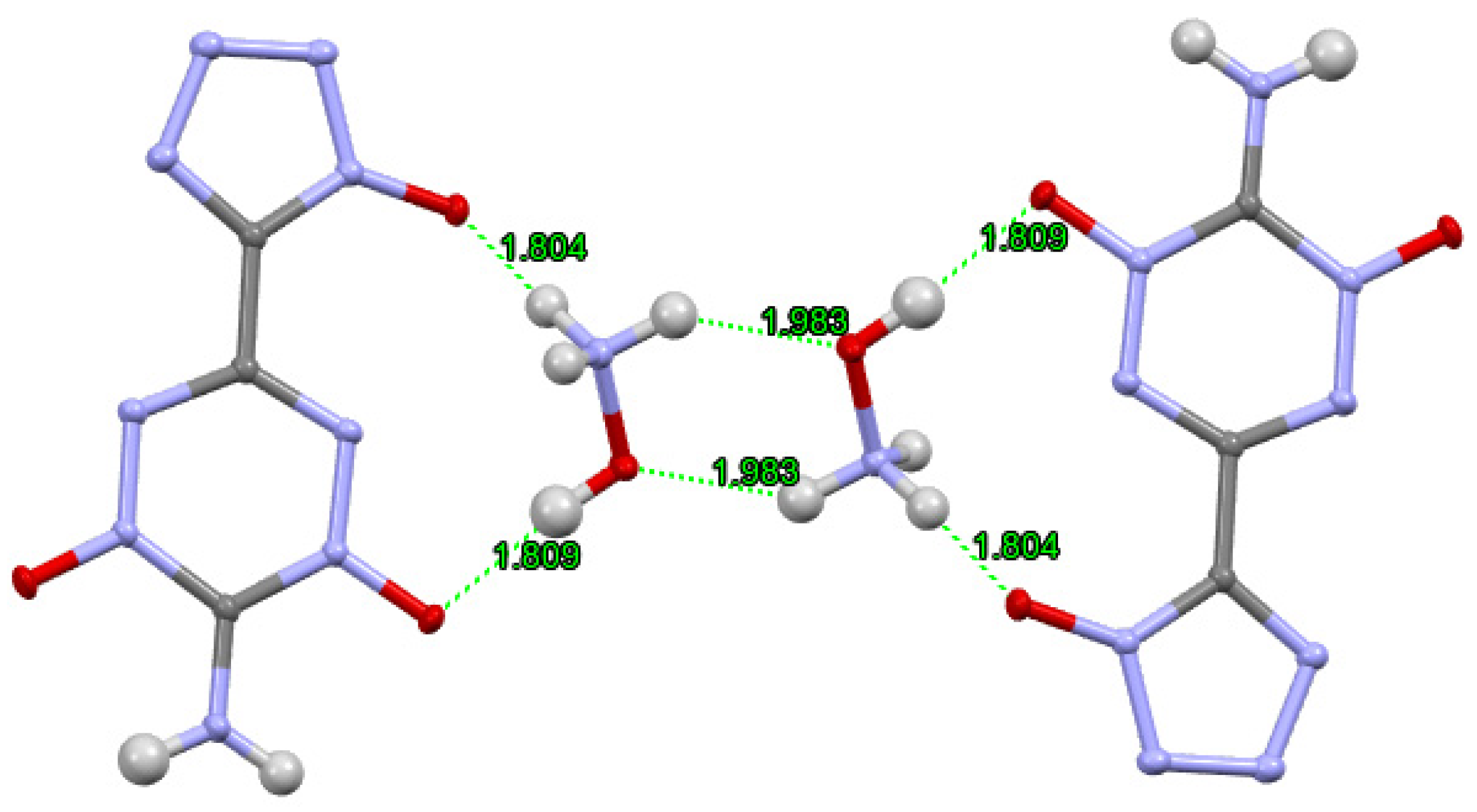
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. X-ray Crystallography
3.3. Computational Methods
3.4. Thermal Analysis and Sensitivity Measurements
3.5. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, D.; Yu, G. Innovation of Materials, Devices, and Functionalized Interfaces in Organic Spintronics. Adv. Funct. Mater. 2021, 31, 2100550. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Lin, P.-H.; Lee, K.-M. Development of Step-Saving Alternative Synthetic Pathways for Functional π-Conjugated Materials. Chem. Rec. 2021, 21, 3498–3508. [Google Scholar] [CrossRef] [PubMed]
- Sathiyan, G.; Wang, H.; Chen, C.; Miao, Y.; Zhai, M.; Cheng, M. Impact of fluorine substitution in organic functional materials for perovskite solar cell. Dye. Pigment. 2022, 198, 110029. [Google Scholar] [CrossRef]
- Yang, X.-D.; Tan, L.; Sun, J.-K. Encapsulation of Metal Clusters within Porous Organic Materials: From Synthesis to Catalysis Applications. Chem. Asian J. 2022, 17, e202101289. [Google Scholar] [CrossRef]
- Roy, S.; Das, S.K.; Khatua, H.; Das, S.; Chattopadhyay, B. Road Map for the Construction of High-Valued N-Heterocycles via Denitrogenative Annulation. Acc. Chem. Res. 2021, 54, 4395–4409. [Google Scholar] [CrossRef]
- Odom, A.L.; McDaniel, T.J. Titanium-Catalyzed Multicomponent Couplings: Efficient One-Pot Syntheses of Nitrogen Heterocycles. Acc. Chem. Res. 2015, 48, 2822–2833. [Google Scholar] [CrossRef]
- Makhova, N.N.; Belen’kii, L.I.; Gazieva, G.A.; Dalinger, I.L.; Konstantinova, L.S.; Kuznetsov, V.V.; Kravchenko, A.N.; Krayushkin, M.M.; Rakitin, O.A.; Starosotnikov, A.M.; et al. Progress in the chemistry of nitrogen-, oxygen- and sulfur-containing heterocyclic systems. Russ. Chem. Rev. 2020, 89, 55–124. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of fluorescent sensors based on azaheterocyclic push-pull systems towards nitroaromatic explosives and related compounds: A review. Dye. Pigment. 2020, 180, 108414. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- O’Sullivan, O.T.; Zdilla, M.J. Properties and Promise of Catenated Nitrogen Systems As High-Energy-Density Materials. Chem. Rev. 2020, 120, 5682–5744. [Google Scholar] [CrossRef]
- Miomandre, F.; Audebert, P. 1,2,4,5-Tetrazines: An intriguing heterocycles family with outstanding characteristics in the field of luminescence and electrochemistry. J. Photochem. Photobiol. C Photochem. Rev. 2020, 44, 100372. [Google Scholar] [CrossRef]
- Lipunova, G.N.; Nosova, E.V.; Zyryanov, G.V.; Charushin, V.N.; Chupakhin, O.N. 1,2,4,5-Tetrazine derivatives as components and precursors of photo- and electroactive materials. Organ. Chem. Front. 2021, 8, 5182–5205. [Google Scholar] [CrossRef]
- Wilkovitsch, M.; Haider, M.; Sohr, B.; Herrmann, B.; Klubnick, J.; Weissleder, R.; Carlson, J.C.T.; Mikula, H. A Cleavable C2-Symmetric trans-Cyclooctene Enables Fast and Complete Bioorthogonal Disassembly of Molecular Probes. J. Am. Chem. Soc. 2020, 142, 19132–19141. [Google Scholar] [CrossRef]
- Wu, H.; Devaraj, N.K. Advances in Tetrazine Bioorthogonal Chemistry Driven by the Synthesis of Novel Tetrazines and Dienophiles. Acc. Chem. Res. 2018, 51, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Schnell, S.D.; Schilling, M.; Sklyaruk, J.; Linden, A.; Luber, S.; Gademann, K. Nucleophilic Attack on Nitrogen in Tetrazines by Silyl-Enol Ethers. Organ. Lett. 2021, 23, 2426–2430. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Fang, Y.; Huang, Z.; Tallon, A.M.; am Ende, C.W.; Fox, J.M. Divergent Synthesis of Monosubstituted and Unsymmetrical 3,6-Disubstituted Tetrazines from Carboxylic Ester Precursors. Angew. Chem. Int. Ed. 2020, 59, 16967–16973. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xue, Q.; Zhang, C.; Wu, H.; Feng, P. Derivatization based on tetrazine scaffolds: Synthesis of tetrazine derivatives and their biomedical applications. Org. Chem. Front. 2022, 9, 481–498. [Google Scholar] [CrossRef]
- Zhu, Z.; Glinkerman, C.M.; Boger, D.L. Selective N1/N4 1,4-Cycloaddition of 1,2,4,5-Tetrazines Enabled by Solvent Hydrogen Bonding. J. Am. Chem. Soc. 2020, 142, 20778–20787. [Google Scholar] [CrossRef]
- Mittal, R.; Awasthi, S.K. Recent Advances in the Synthesis of 5-Substituted 1H-Tetrazoles: A Complete Survey (2013–2018). Synthesis 2019, 51, 3765–3783. [Google Scholar] [CrossRef]
- Popova, E.A.; Trifonov, R.E.; Ostrovskii, V.A. Tetrazoles for biomedicine. Russ. Chem. Rev. 2019, 88, 644–676. [Google Scholar] [CrossRef]
- Ostrovskii, V.A.; Popova, E.A.; Trifonov, R.E. Chapter One—Developments in Tetrazole Chemistry (2009–2016). Adv. Heterocycl. Chem. 2017, 123, 1–62. [Google Scholar] [CrossRef]
- Wang, T.; Gao, H.; Shreeve, J.M. Functionalized Tetrazole Energetics: A Route to Enhanced Performance. Z. Anorg. Allg. Chem. 2021, 647, 157–191. [Google Scholar] [CrossRef]
- Chavez, D.E.; Hanson, S.K.; Veauthier, J.M.; Parrish, D.A. Electroactive Explosives: Nitrate Ester-Functionalized 1,2,4,5-Tetrazines. Angew. Chem. Int. Ed. 2013, 52, 6876–6879. [Google Scholar] [CrossRef] [PubMed]
- Rudakov, G.F.; Kalinichenko, A.I.; Nguyen, T.Q.; Zinchenko, S.S.; Cherkaev, G.V.; Fedyanin, I.V.; Sinditskii, V.P. Monosubstituted Polynitroalkoxy-1,2,4,5-Tetrazines: A New Family of Melt-Castable Energetic Materials. Propellants Explos. Pyrotech. 2022, 47, e202100262. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Piercey, D.G.; Rohrbacher, F.; Stierstorfer, J. Synthesis and Characterization of Energetic Salts of the (C4N122–) Dianion. Z. Anorg. Allg. Chem. 2012, 638, 2235–2242. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Kormanov, A.V.; Suponitsky, K.Y.; Muravyev, N.V.; Sheremetev, A.B. Pyrazole–Tetrazole Hybrid with Trinitromethyl, Fluorodinitromethyl, or (Difluoroamino) dinitromethyl Groups: High-Performance Energetic Materials. Chem. Asian J. 2018, 13, 1165–1172. [Google Scholar] [CrossRef]
- Chaplygin, D.A.; Larin, A.A.; Muravyev, N.V.; Meerov, D.B.; Kosareva, E.K.; Kiselev, V.G.; Pivkina, A.N.; Ananyev, I.V.; Fershtat, L.L. Nitrogen-Rich Metal-Free Salts: A New Look at 5-(Trinitromethyl) tetrazolate Anion as an Energetic Moiety. Dalton Trans. 2021, 50, 13778–13785. [Google Scholar] [CrossRef]
- Larin, A.A.; Muravyev, N.V.; Pivkina, A.N.; Suponitsky, K.Y.; Ananyev, I.V.; Khakimov, D.V.; Fershtat, L.L.; Makhova, N.N. Assembly of Tetrazolylfuroxan Organic Salts: Multipurpose Green Energetic Materials with High Enthalpies of Formation and Excellent Detonation Performance. Chem. Eur. J. 2019, 25, 4225–4233. [Google Scholar] [CrossRef]
- Larin, A.A.; Shaferov, A.V.; Kulikov, A.S.; Pivkina, A.N.; Monogarov, K.A.; Dmitrienko, A.O.; Ananyev, I.V.; Khakimov, D.V.; Fershtat, L.L.; Makhova, N.N. Design and Synthesis of Nitrogen-Rich Azo-Bridged Furoxanylazoles as High-Performance Energetic Materials. Chem. Eur. J. 2021, 27, 14628–14637. [Google Scholar] [CrossRef]
- Zhai, L.; Bi, F.; Luo, Y.; Wang, N.; Zhang, J.; Wang, B. New Strategy for Enhancing Energetic Properties by Regulating Trifuroxan Configuration: 3,4-Bis (3-nitrofuroxan-4-yl) furoxan. Sci. Rep. 2019, 9, 4321. [Google Scholar] [CrossRef] [Green Version]
- Larin, A.A.; Shaferov, A.V.; Epishina, M.A.; Melnikov, I.N.; Muravyev, N.V.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Pushing the Energy-Sensitivity Balance with High-Performance Bifuroxans. ACS Appl. Energy Mater. 2020, 3, 7764–7771. [Google Scholar] [CrossRef]
- Song, S.; Wang, Y.; He, W.; Wang, K.; Yan, M.; Yan, Q.-L.; Zhang, Q. Melamine N-oxide based self-assembled energetic materials with balanced energy & sensitivity and enhanced combustion behavior. Chem. Eng. J. 2020, 395, 125114. [Google Scholar] [CrossRef]
- Zlotin, S.G.; Churakov, A.M.; Egorov, M.P.; Fershtat, L.L.; Klenov, M.S.; Kuchurov, I.V.; Makhova, N.N.; Smirnov, G.A.; Tomilov, Y.V.; Tartakovsky, V.A. Advanced energetic materials: Novel strategies and versatile applications. Mendeleev Commun. 2021, 31, 731–749. [Google Scholar] [CrossRef]
- Wei, H.; Gao, H.; Shreeve, J.M. N-Oxide 1,2,4,5-Tetrazine-Based High-Performance Energetic Materials. Chem. Eur. J. 2014, 20, 16943–16952. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, J.-G.; Yin, X.; Wu, J.-T.; Wu, L.; Zhou, Z.-N.; Zhang, T.-L. Energetic Salts Based on Tetrazole N-Oxide. Chem. Asian J. 2016, 22, 7670–7685. [Google Scholar] [CrossRef]
- Larin, A.A.; Fershtat, L.L. High-energy hydroxytetrazoles: Design, synthesis and performance. Energ. Mater. Front. 2021, 2, 3–13. [Google Scholar] [CrossRef]
- Coburn, M.D.; Buntain, G.A.; Harris, B.W.; Hiskey, M.A.; Lee, K.-Y.; Ott, D.G. An improved synthesis of 3,6-diamino-1,2,4,5-tetrazine. II. From triaminoguanidine and 2,4-pentanedione. J. Heterocycl. Chem. 1991, 28, 2049–2050. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, J.; He, C.; Shreeve, J.M. Energetic Salts Based on Furazan-Functionalized Tetrazoles: Routes to Boost Energy. Chem. Eur. J. 2015, 21, 8607–8612. [Google Scholar] [CrossRef]
- Ugrak, B.I.; Vinogradov, V.M.; Dalinger, I.L.; Shevelev, S.A. Nitropyrazoles 9.* Parameters of the 1H, 13C, and 15N (14N) spectra and the structures of N-aminonitropyrazoles. Russ. Chem. Bull. 1995, 44, 2087–2092. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Yu, Q.; Tang, Y.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Intermolecular Weak Hydrogen Bonding (Het-H-N/O): An Effective Strategy for the Synthesis of Monosubstituted 1,2,4,5-Tetrazine-Based Energetic Materials with Excellent Sensitivity. J. Organ. Chem. 2019, 84, 16019–16026. [Google Scholar] [CrossRef]
- Snyder, C.J.; Wells, L.A.; Chavez, D.E.; Imler, G.H.; Parrish, D.A. Polycyclic N-oxides: High performing, low sensitivity energetic materials. Chem. Commun. 2019, 55, 2461–2464. [Google Scholar] [CrossRef] [PubMed]
- Muravyev, N.V.; Wozniak, D.R.; Piercey, D.G. Progress and performance of energetic materials: Open dataset, tool, and implications for synthesis. J. Mater. Chem. A 2022, 10, 11054–11073. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.41.106a; Rigaku Oxford Diffraction: Oxford, UK, 2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 229–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Montgomery, J.J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VII. Use of the minimum population localization method. J. Chem. Phys. 2000, 112, 6532–6542. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Momany, F.A.; Carruthers, L.M.; McGuire, R.F.; Scheraga, H.A. Intermolecular potentials from crystal data. III. Determination of empirical potentials and application to the packing configurations and lattice energies in crystals of hydrocarbons, carboxylic acids, amines, and amides. J. Phys. Chem. 1974, 78, 1595–1620. [Google Scholar] [CrossRef]
- Dzyabchenko, A.V. A multipole approximation of the electrostatic potential of molecules. Russ. J. Phys. Chem. A 2008, 82, 758–766. [Google Scholar] [CrossRef]
- Dzyabchenko, A.V. From molecule to solid: The prediction of organic crystal structures. Russ. J. Phys. Chem. A 2008, 82, 1663–1671. [Google Scholar] [CrossRef]
- Belsky, V.K.; Zorkaya, O.N.; Zorky, P.M. Structural Classes and Space Groups of Organic Homomolecular Crystals: New Statistical Data. Acta Crystallogr. Sect. A 1995, 51, 473–481. [Google Scholar] [CrossRef]
- Khakimov, D.V.; Pivina, T.S. New Method for Predicting the Enthalpy of Salt Formation. J. Phys. Chem. A 2022, 126, 5207–5214. [Google Scholar] [CrossRef] [PubMed]
- STANAG 4489; Explosives, Impact Sensitivity Tests. NATO: Brussels, Belgium, 1999.
- STANAG 4487; Explosives, Friction Sensitivity Tests. NATO: Brussels, Belgium, 2002.
- Muravyev, N.V.; Meerov, D.B.; Monogarov, K.A.; Melnikov, I.N.; Kosareva, E.K.; Fershtat, L.L.; Sheremetev, A.B.; Dalinger, I.L.; Fomenkov, I.V.; Pivkina, A.N. Sensitivity of energetic materials: Evidence of thermodynamic factor on a large array of Chnofcl compounds. Chem. Eng. J. 2021, 421, 129804. [Google Scholar] [CrossRef]



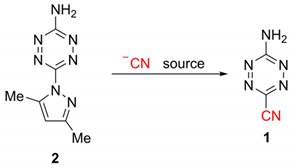 | |||||
|---|---|---|---|---|---|
| Entry | Cyanide Source | Base or Additive | Solvent | T, ◦C | Yield,b % |
| 1 | TMSCN (1 eq.) | NH4F (1 eq.) | MeCN | 82 | 0 |
| 2 | Acetone cyanohydrin (1.5 eq.) | Net3 (1.5 eq.), 3Å MS | MeCN | 82 | 31 |
| 3 | Acetone cyanohydrin (2 eq.) | KHCO3 (2 eq.) | MeCN | 82 | 48c |
| 4 | Acetone cyanohydrin (4 eq.) | Net3 (4 eq.), 3Å MS | dry dioxane | 101 | 71 |
| 5 | KCN (2 eq.) | 18-crown-6 (0.1 eq.) | HFIP | 58 | 0 |
| 6 | KCN (2 eq.) | - | MeCN | 82 | 35 c |
| 7 | KCN (2 eq.) | - | DMF | 85 | 61 |
| 8 | KCN (1.2 eq.) | - | DMF | 85 | 54 |
| 9 | KCN (2 eq.) | 3Å MS, Ar atm. | dry DMF | 20 | 84 |
| 10 | KCN (2 eq.) | 3Å MS, Ar atm. | dry DMF | 50 | 79 |
| Salt | Td,a °C | ρ,b g·cm−3 | N,c % | [N + O],d % | ΩCO,e % | ΔHof,f kJ·mol−1 | D,g km·s−1 | P,h GPa | IS,i J | FS,j N |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 212 | 1.75 | 60.9 | 81.7 | −20.9 | 417 | 8.5 | 31 | 9 | 265 |
| 11 | 155 | 1.78 | 56.9 | 82.9 | −13.0 | 413 | 8.8 | 33 | 10 | 190 |
| 12 | 206 | 1.77 | 61.3 | 77.4 | −29.6 | 779 | 8.5 | 32 | 15 | 260 |
| RDX | 204 | 1.81 | 37.8 | 81.1 | 0 | 68 | 8.8 | 34 | 10 | 130 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bystrov, D.M.; Pivkina, A.N.; Fershtat, L.L. An Alliance of Polynitrogen Heterocycles: Novel Energetic Tetrazinedioxide-Hydroxytetrazole-Based Materials. Molecules 2022, 27, 5891. https://doi.org/10.3390/molecules27185891
Bystrov DM, Pivkina AN, Fershtat LL. An Alliance of Polynitrogen Heterocycles: Novel Energetic Tetrazinedioxide-Hydroxytetrazole-Based Materials. Molecules. 2022; 27(18):5891. https://doi.org/10.3390/molecules27185891
Chicago/Turabian StyleBystrov, Dmitry M., Alla N. Pivkina, and Leonid L. Fershtat. 2022. "An Alliance of Polynitrogen Heterocycles: Novel Energetic Tetrazinedioxide-Hydroxytetrazole-Based Materials" Molecules 27, no. 18: 5891. https://doi.org/10.3390/molecules27185891
APA StyleBystrov, D. M., Pivkina, A. N., & Fershtat, L. L. (2022). An Alliance of Polynitrogen Heterocycles: Novel Energetic Tetrazinedioxide-Hydroxytetrazole-Based Materials. Molecules, 27(18), 5891. https://doi.org/10.3390/molecules27185891