Spin-Dependent First-Principles Study on Optoelectronic Properties of Neodymium Zirconates Pyrochlores Nd2Zr2O7 in Fd-3m and Pmma Phases
Abstract
:1. Introduction
2. Materials and Methods
Computational Methodology
3. Results and Discussion
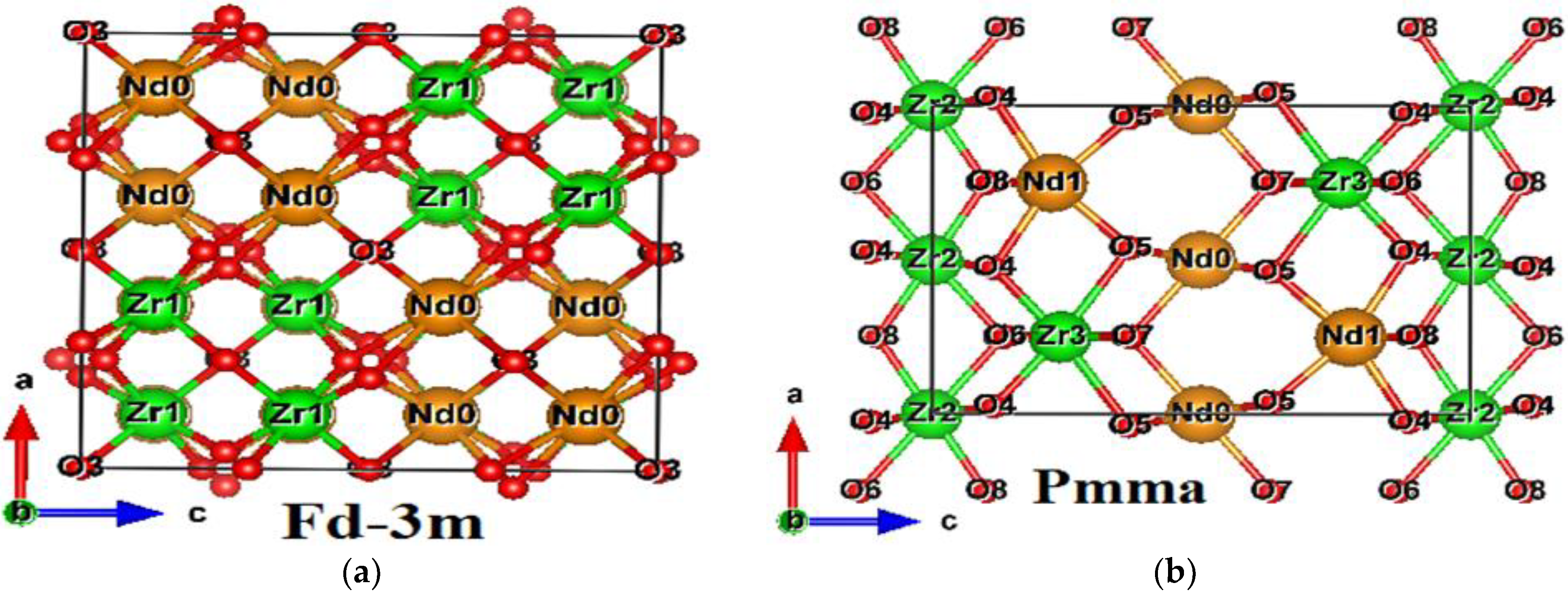
3.1. Crystallographic Structure
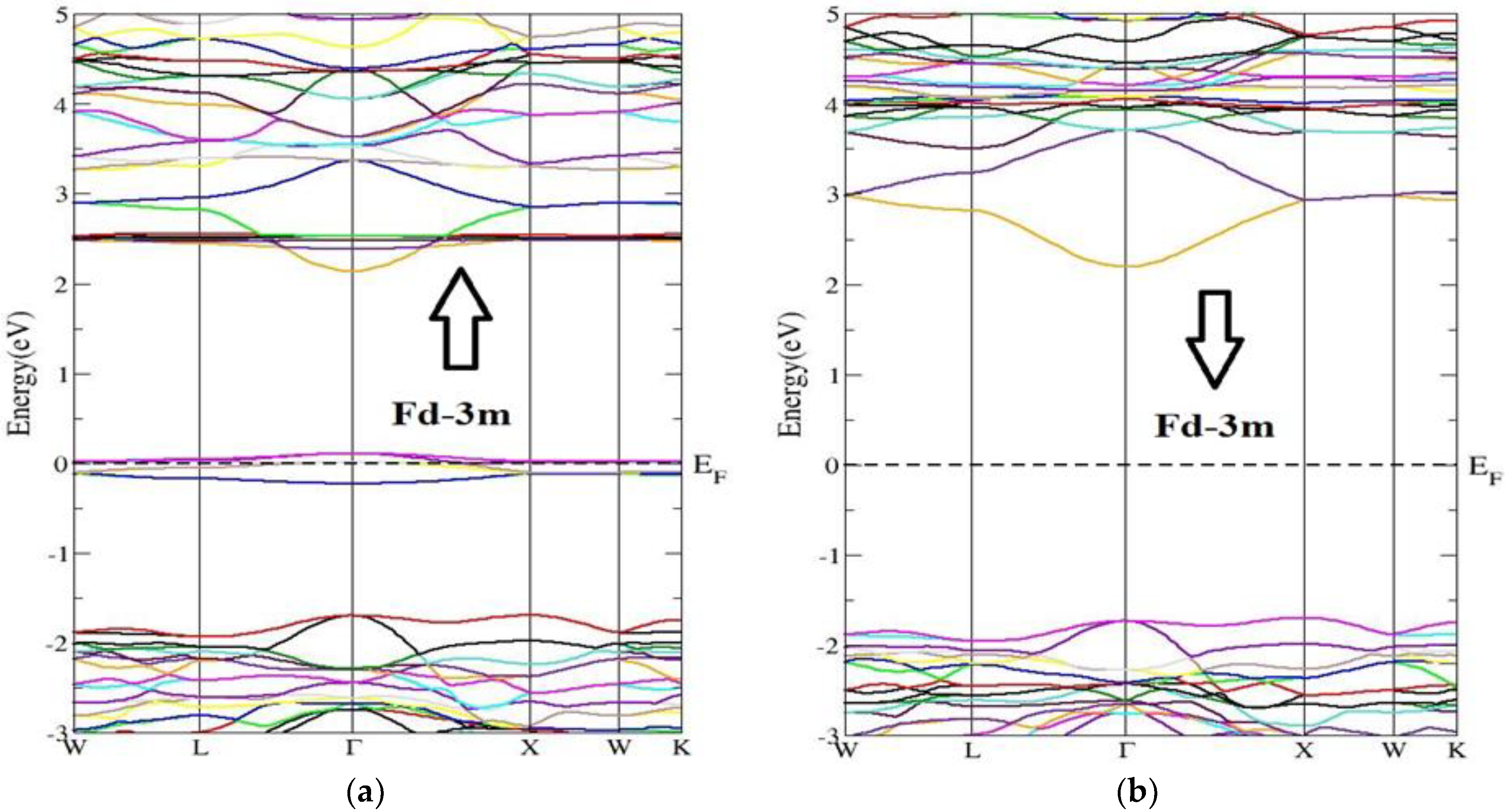
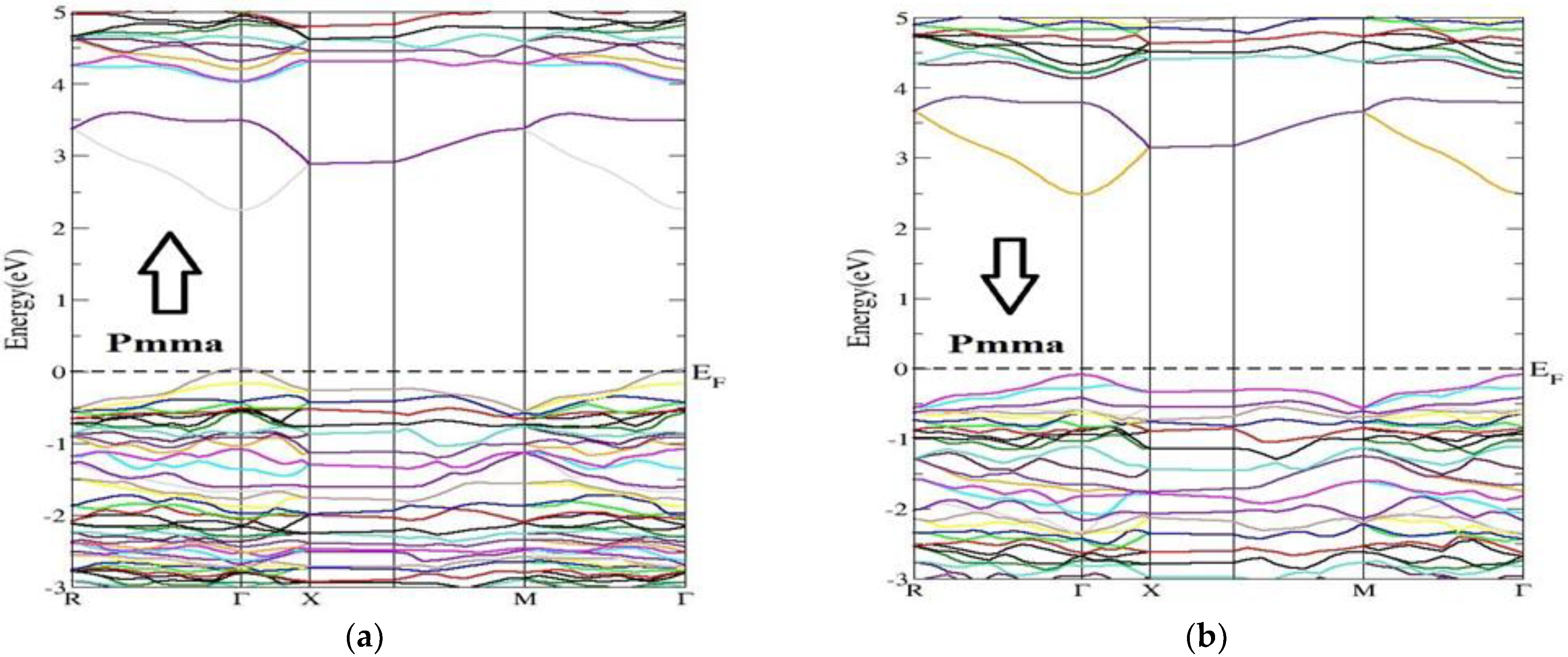
3.2. Electronic Band Structure
3.3. Electronic Density of States
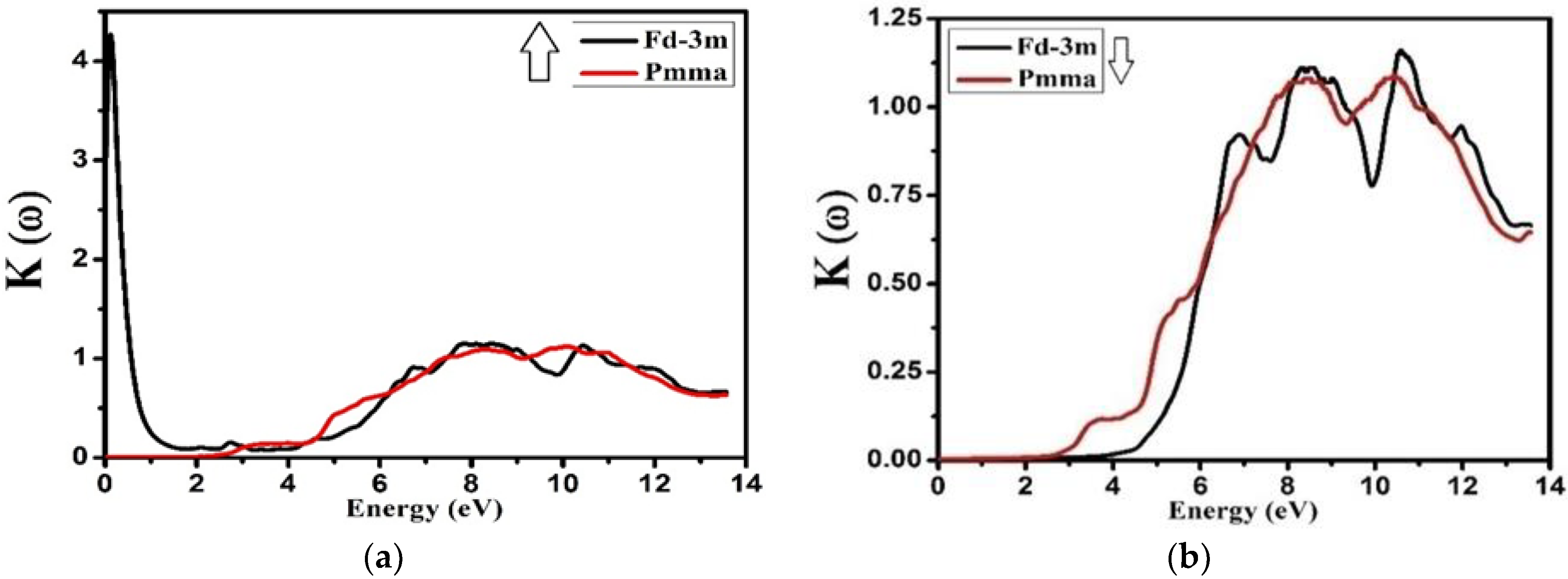
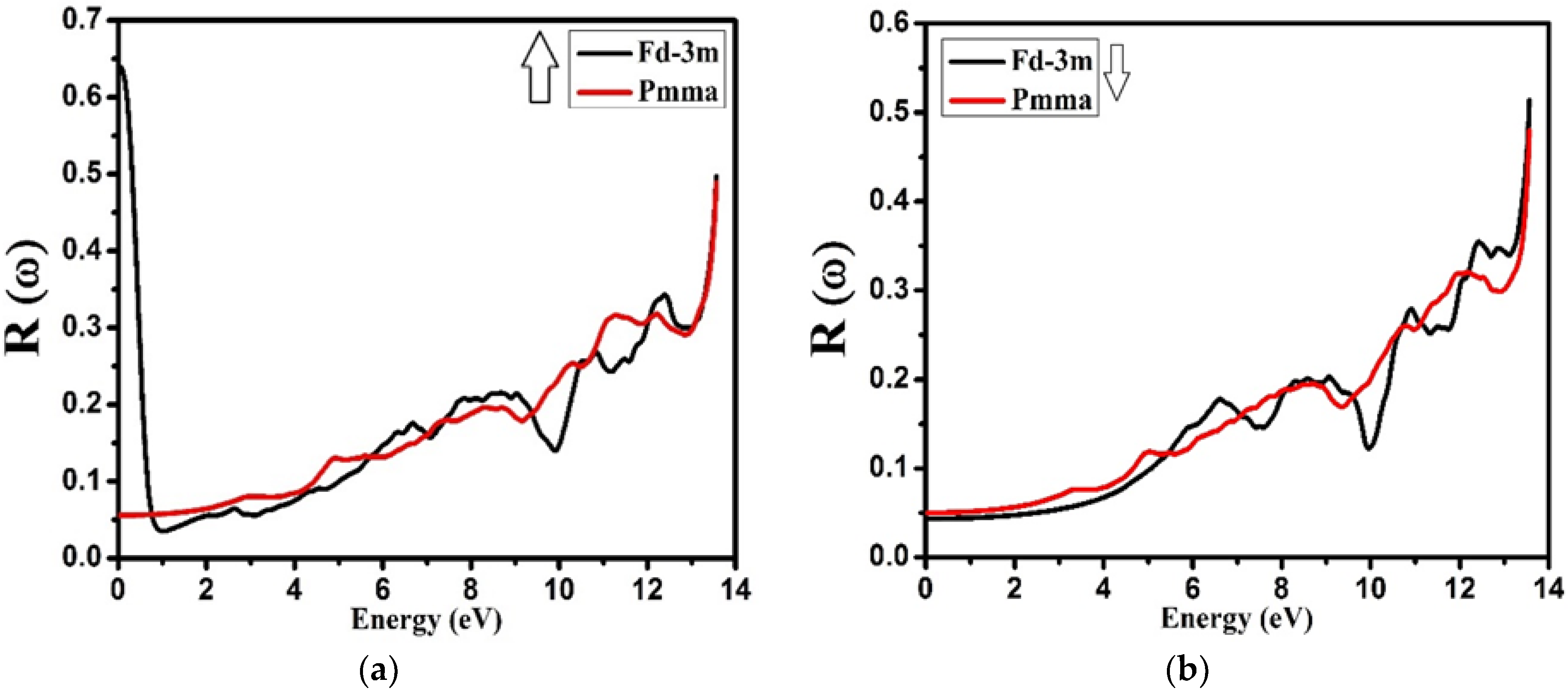
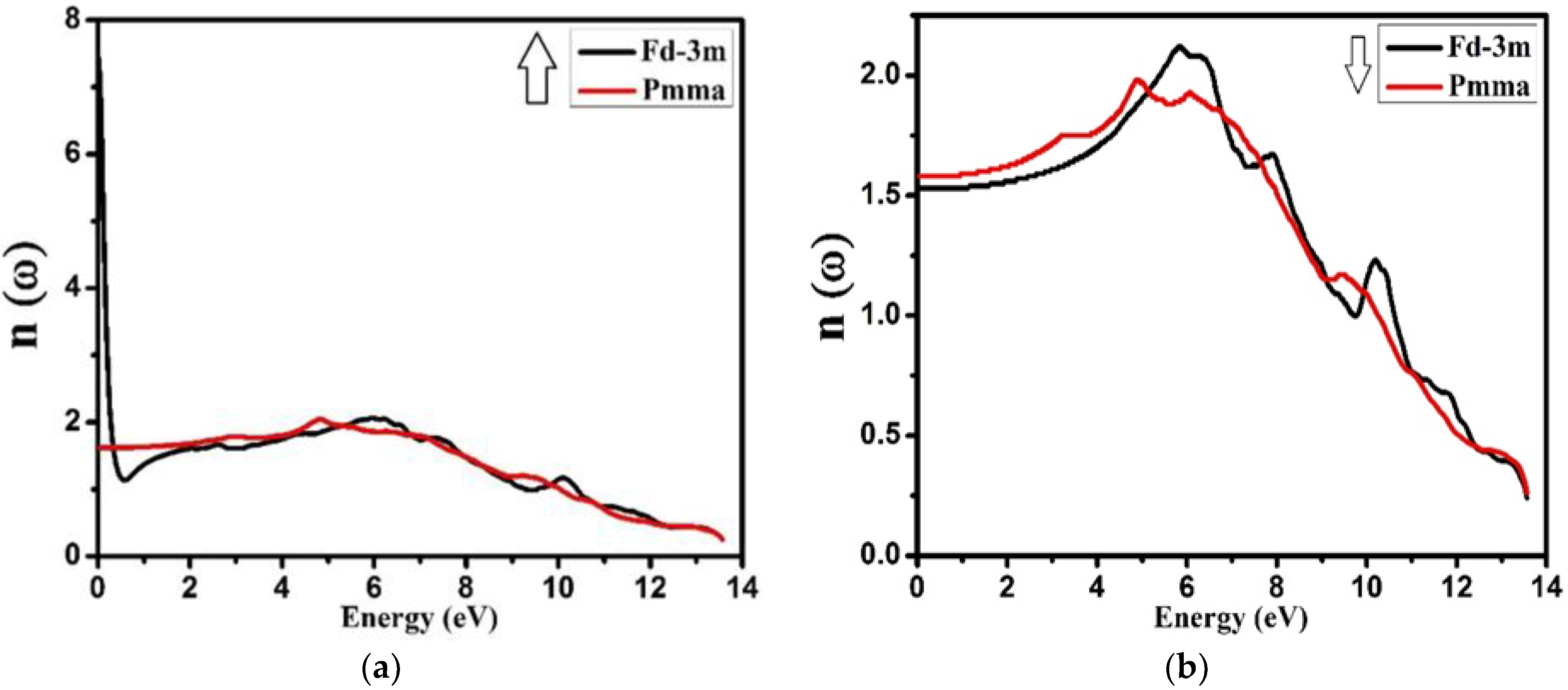
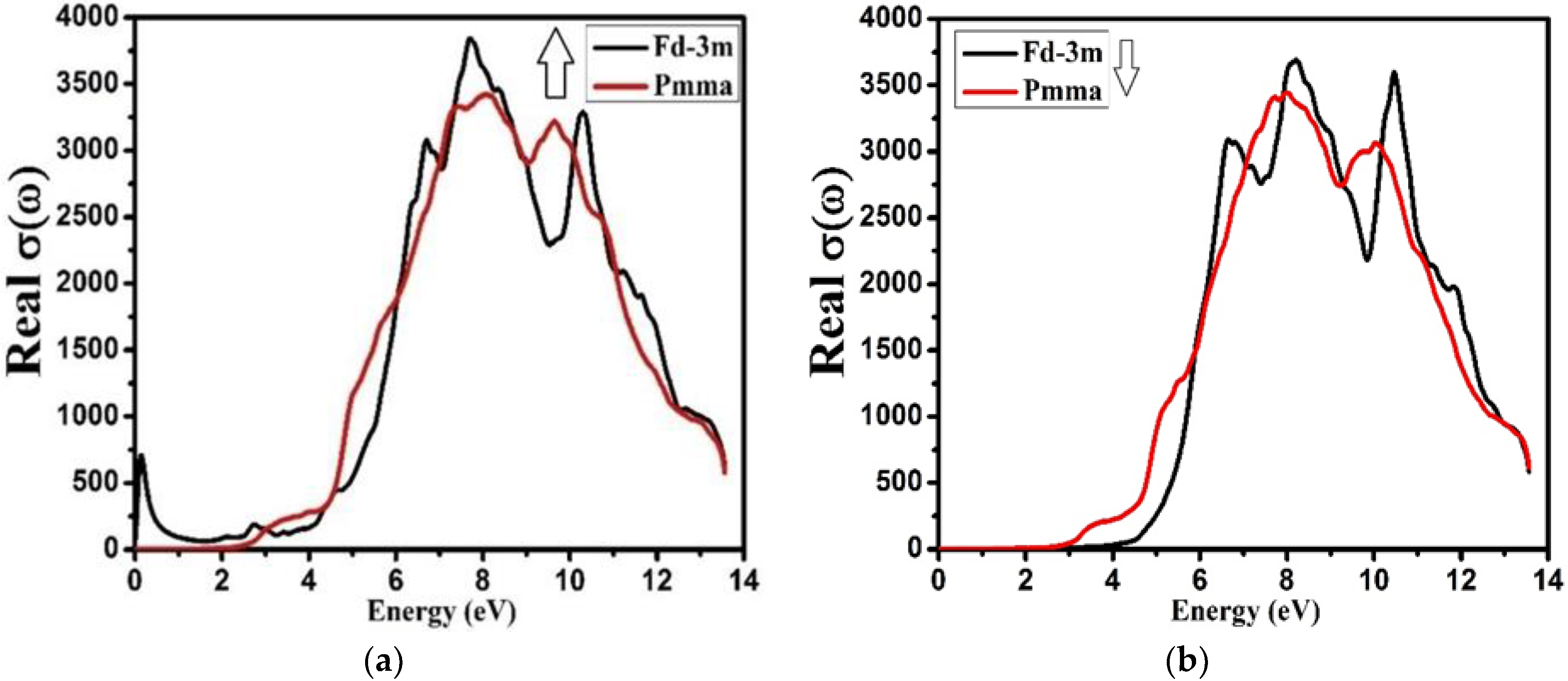
3.4. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55. [Google Scholar] [CrossRef]
- Kramer, S.A.; Tuller, H.L. A novel titanate-based oxygen ion conductor: Gd2Ti2O7. Solid State Ionics 1995, 82, 15. [Google Scholar] [CrossRef]
- Kutty, K.V.G.; Asuvatharaman, E.; Madhavan, R.R.; Jena, H. Impedance spectroscopy of unetched CdTe/CdSCdTe/CdS solar cells—equivalent circuit analysis. J. Phys. Chem. Solids 2005, 66, 296. [Google Scholar]
- Banerji, A.; Mandal, B.P.; Sairam, T.N.; Tyagi, A.K. Pressure induced structural stability studies on pyrochlore. Solid State Commun. 2011, 151, 321–325. [Google Scholar] [CrossRef]
- Wang, Y.F. The improvement of thermal and mechanical properties of La2Zr2O7 based pyrochlores as high temperature thermal barrier coatings. J. For. Res. 2013, 24, 143–147. [Google Scholar]
- Meng, C.; Ding, X.; Li, W.; Zh ao, J.; Yang, H. Phase structure evolution and chemical durability studies of Ce-doped zirconolite pyrochlore synroc for radioactive waste storage. J. Mater. Sci. 2016, 51, 5207–5215. [Google Scholar] [CrossRef]
- Patwe, S.J.; Ambekar, B.R.; Tyagi, A.K. Synthesis, characterization and lattice thermal expansion of some compounds in the system Gd2CexZr2-xO7. J. Alloys Compd. 2005, 389, 243–246. [Google Scholar] [CrossRef]
- Fan, L.; Shu, X.Y.; Ding, Y.; Duan, T.; Song, M.X.; Lu, X.R. Fabrication and phase transition of Gd2Zr2O7 ceramics immobilized various simulated radionuclides. J. Nucl. Mater. 2015, 456, 467–470. [Google Scholar] [CrossRef]
- Lu, X.R.; Ding, Y.; Shu, X.Y.; Mao, X.L.; Wang, X. Preparation and heavy-ion irradiation effects of Gd2CexZr2-xO7 ceramics. RSC Adv. 2015, 5, 64247–64253. [Google Scholar] [CrossRef]
- Lu, X.R.; Fan, L.; Shu, X.Y.; Su, S.J.; Ding, Y.; Yi, F.C. Phase evolution and chemical durability of co-doped Gd2Zr2O7 ceramics for nuclear waste forms. Ceram. Int. 2015, 41, 6344–6349. [Google Scholar] [CrossRef]
- Rao, K.K.; Banu, T.; Vithal, M.; Swamy, G.Y.S.K.; Kumar, K.R. Preparation and characterization of bulk and nano particles of La2Zr2O7 and Nd2Zr2O7 by sol–gel method. Mater. Lett. 2002, 54, 205–210. [Google Scholar]
- Kong, L.; Karatchevtseva, I.; Gregg, D.J.; Blackford, M.G.; Holmes, R.; Triani, G. Gd2Zr2O7 and Nd2Zr2O7 pyrochlore prepared by aqueous chemical synthesis. J. Eur. Ceram. Soc. 2013, 33, 3273–3285. [Google Scholar] [CrossRef]
- Lu, X.R.; Dong, F.Q.; Song, G.B.; Ning, M.J.; Su, S.J.; Li, W.M.; Wu, Y.L. Synthesis, phase and structural analysis of compounds inthe system Gd2−xNdxZr2O7 (0.0 ≤ x ≤ 2.0). Energy Procedia 2013, 39, 403–407. [Google Scholar]
- Sickafus, K.E.; Hartmann, T. Radiation tolerance of complex oxides. Science 2000, 289, 748. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Vijayakumar, V.; Avasthi, D.K.; Kailaset, S.; Pivin, J.C.; Grover, V.; Mandal, B.P.; Tyagial, A.K. Effect of swift heavy ion irradiation in pyrochlores. Nucl. Instrum. Meth. B 2008, 266, 2898–2901. [Google Scholar] [CrossRef]
- Sattonnay, G.; Grygiel, C.; Monnet, I.; Legros, C.; Herbst-Ghysel, M.; Thomé, L. Phenomenological model for the formation of heterogeneous tracks in pyrochlores irradiated with swift heavy ions. Acta Mater. 2012, 60, 22–34. [Google Scholar] [CrossRef]
- Lian, J.; Weber, W.J.; Jiang, W.; Wang, L.M.; Boatner, L.A.; Ewing, R.C. Radiation induced effects in pyrochlores and nanoscale materials engineering. Nucl. Instrum. Meth. 2006, 250, 128–136. [Google Scholar] [CrossRef]
- Sattonnay, G.; Sellami, N.; Thomé, L.; Legros, C.; Grygiel, C.; Monent, I.; Jagielski, J.; Jozwik-Biala, I.; Simon, P. Structural stability of Nd2Zr2O7 pyrochlore ion-irradiated in a broad energy range. Acta Mater. 2013, 61, 6492–6505. [Google Scholar] [CrossRef]
- Mustafa, G.M.; Atiq, S.; Abbas, S.K.; Riaz, S.; Naseem, S. Tunable structural and electrical impedance properties of pyrochlores based Nd doped lanthanum zirconate nanoparticles for capacitive applications. Ceram. Int. 2018, 44, 2170–2177. [Google Scholar] [CrossRef]
- Cepeda-Sánchez, N.M.; Díaz-Guillén, J.A.; Maczka, M.; Amador, U.; Fuentes, A.F. Mechanochemical synthesis, crystal structure and ion conduction in the Gd2Hf2−xTixO7 system. J. Mater. Sci. 2017, 52, 11933–11946. [Google Scholar] [CrossRef]
- Chun, J.; Reuvekamp, P.G.; Chen, D.; Lin, C.; Kremer, R.K. Promising high-k dielectric permittivity of pyrochlore-type crystals of Nd2Hf2O7. J. Mater. Chem. C 2015, 3, 491–494. [Google Scholar] [CrossRef]
- Lu, X.; Shu, X.; Shao, D.; Chen, S.; Zhang, H.; Yuan, X.; Chi, F. Radiation stability of Gd2Zr2O7 and Nd2Ce2O7 ceramics as nuclear waste forms. Ceram. Int. 2018, 44, 760–765. [Google Scholar] [CrossRef]
- Shamblin, J.; Tracy, C.L.; Palomares, R.I.; O’Quinn, E.C.; Ewing, R.C.; Neuefeind, J.; Feygenson, M.; Behrens, J.; Trautmann, C.; Lang, M. Similar local order in disordered fluorite and aperiodic pyrochlore structures. Acta Mater. 2018, 144, 60–67. [Google Scholar] [CrossRef]
- Gupta, S.K.; Zuniga, J.P.; Abdou, M.; Mao, Y. Thermal annealing effects on La2Hf2O7:Eu3+ nanoparticles: A curious case study of structural evolution and site-specific photoand radio-luminescence. Inorg. Chem. Front. 2018, 5, 2508–2521. [Google Scholar] [CrossRef]
- Gupta, S.K.; Zuniga, J.P.; Ghosh, P.S.; Abdou, M.; Mao, Y. Correlating structure and luminescence properties of undoped and La2Hf2O7:Eu3+NPs prepared with different coprecipitating pH Values through experimental and theoretical studies. Inorg. Chem. Front. 2018, 57, 11815–11830. [Google Scholar] [CrossRef]
- Abdou, M.; Gupta, S.K.; Zuniga, J.P.; Mao, Y. On structure and phase transformation of uranium doped La2Hf2O7 nanoparticles as an efficient nuclear waste host. Mater. Chem. Front. 2018, 2, 2201–2211. [Google Scholar] [CrossRef]
- Zuniga, J.P.; Gupta, S.K.; Pokhrel, M.; Mao, Y. Exploring the optical properties of La2Hf2O7:Pr3+ nanoparticles under UV and X-ray excitation for potential lighting and scintillating applications. N. J. Chem. 2018, 42, 9381–9392. [Google Scholar] [CrossRef]
- Gupta, S.K.; Ghosh, P.; Reghukumar, C.; Pathak, N.; Kadam, R. Experimental and theoretical approach to account for green luminescence from Gd2Zr2O7 pyrochlore: Exploring the site occupancy and origin of host-dopant energy transfer in Gd2Zr2O7:Eu3+. RSC Adv. 2016, 6, 44908–44920. [Google Scholar] [CrossRef]
- Gupta, S.K.; Reghukumar, C.; Kadam, R. Eu3+ local site analysis and emission characteristics of novel Nd2Zr2O7: Eu phosphor: Insight into the effect of europium concentration on its photoluminescence properties. RSC Adv. 2016, 6, 53614–53624. [Google Scholar] [CrossRef]
- Gupta, S.K.; Reghukumar, C.; Keskar, M.; Kadam, R. Revealing the oxidation number and local coordination of uranium in Nd2Zr2O7 pyrochlore: A photoluminescence study. J. Lumin. 2016, 177, 166–171. [Google Scholar] [CrossRef]
- Gupta, S.K.; Reghukumar, C.; Pathak, N.; Sudarshan, K.; Tyagi, D.; Mohapatra, M.; Pujari, P.; Kadam, R. Speciation of uranium and doping induced defects in Gd1.98U0.02Zr2O7: Photoluminescence, X-ray photoelectron and positron annihilation lifetime spectroscopy. Chem. Phys. Lett. 2017, 669, 245–250. [Google Scholar] [CrossRef]
- Feng, T.; Clarke, D.R.; Jiang, D.; Xia, J.; Shi, J. Neodymium zirconate (Nd2Zr2O7) transparent ceramics as a solid state laser material. Appl. Phys. Lett. 2011, 98, 151105. [Google Scholar] [CrossRef]
- Ai, L.; Wang, Z.; Gao, Y.; Cui, C.; Wang, B.; Liu, W.; Wang, L. Effect of surface and bulk palladium doping on the catalytic activity of La2Sn2O7 pyrochlore oxides for diesel soot oxidation. J. Mater. Sci. 2019, 54, 4495–4510. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sudarshan, K.; Ghosh, P.S.; Srivastava, A.P.; Bevara, S.; Pujari, P.K.; Kadam, R.M. Role of various defects in the photoluminescence characteristics of nanocrystalline Nd2Zr2O7: An investigation through spectroscopic and DFT calculations. J. Mater. Chem. C 2016, 4, 4988–5000. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Salavati-Niasari, M. Photo-catalytic degradation of erythrosine and eriochrome black T dyes using Nd2Zr2O7 nanostructures prepared by a modified Pechini approach. Sep. Purif. Technol. 2017, 179, 77–85. [Google Scholar] [CrossRef]
- Lutique, S.; Konings, R.J.M.; Rondinella, V.V.; Somers, J.; Wiss, T. The thermal conductivity of Nd2Zr2O7 pyrochlore and the thermal behaviour of pyrochlore-based inert matrix fuel. J. Alloys Compd. 2003, 352, 1–5. [Google Scholar] [CrossRef]
- Guruciaga, P.C.; Tarzia, M.; Ferreyra, M.V.; Cugliandolo, L.F.; Grigera, S.A.; Borzi, R.A. Field-tuned, order by disorder in frustrated ising magnets with antiferromagnetic interactions. Phys. Rev. Lett. 2016, 117, 167203. [Google Scholar] [CrossRef]
- Wu, J.; Wei, X.; Padture, N.P.; Klemens, P.G.; Gell, M.; García, E.; Miranzo, P.; Osendi, M.I. Low-thermal-conductivity rare-earth zirconates for potential thermal-barrier-coating applications. J. Am. Ceram. Soc. 2002, 85, 3031–3035. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Zhang, F.X.; Gao, F.; Lang, M.; Ewing, R.C.; Weber, W.J. Zirconate pyrochlores under high pressure. Phys. Chem. Chem. Phys. 2010, 12, 12472–12477. [Google Scholar] [CrossRef]
- Qing, Q.; Shu, X.; Shao, D.; Zhang, H.; Chi, F.; Lu, X. Irradiation response of Nd2Zr2O7 under heavy ions irradiation. J. Eur. Ceram. Soc. 2018, 38, 2068–2073. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Hartridge, A.; Mallick, K.K.; Woodhead, J.L. Preparation and characterization of Ln2Zr2O7 microspheres by an inorganic sol–gel route. J. Mater. Sci. 1994, 29, 6076–6078. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, G.; Jiang, D.; Wang, S. Recent development of A2B2O7 system transparent ceramics. J. Adv. Ceram. 2018, 7, 289–306. [Google Scholar] [CrossRef]
- Lee, Y.H.; Sheu, H.S.; Kao, H.C.I. Preparation and characterization of Nd2Zr2O7 nanocrystals by a polymeric citrate precursor method. Mater. Chem. Phys. 2010, 124, 145–149. [Google Scholar] [CrossRef]
- Zhang, A.; Lü, M.; Yang, Z.; Zhou, G.; Zhou, Y. Systematic research on RE2Zr2O7 (RE = La, Nd, Eu and Y) nanocrystals: Preparation, structure and photoluminescence characterization. Solid State Sci. 2008, 10, 74–81. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Weber, W.J.; Zhang, Y.; Zu, X.T. Ab initio molecular dynamics simulations of ion–solid interactions in zirconate pyrochlores. Acta Mater. 2015, 87, 273–282. [Google Scholar] [CrossRef]
- Uno, M.; Kosuga, A.; Okui, M.; Horisaka, K.; Muta, H.; Kurosaki, K.; Yamanaka, S. Photoelectrochemical study of lanthanide zirconium oxides, Ln2Zr2O7 (Ln = La, Ce, Nd and Sm). J. Alloys Compd. 2006, 420, 291–297. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Wan, C.L.; Qu, Z.X.; Huang, Z.C.; Chen, J.C.; Zhou, R.; Pan, W. Electronic structure, mechanical properties and thermal conductivity of Ln2Zr2O7 (Ln = La, Pr, Nd, Sm, Eu and Gd) pyrochlore. Acta Mater. 2011, 59, 1742. [Google Scholar] [CrossRef]
- Mazed, M.A.; Mazed, S. Optical Biomodule for Detection of Diseases. U.S. Patent 2010/0021533, 28 January 2010. [Google Scholar]
- Grimvall, G. Thermophysical Properties of Materials; Elsevier: North-Holland, Amsterdam, 1999. [Google Scholar]
- Lan, G.; Ouyang, B.; Song, J. The role of low-lying optical phonons in lattice thermal conductance of rare-earth pyrochlores: A first-principle study. Acta Mater. 2015, 91, 304–317. [Google Scholar] [CrossRef]
- Groh, D.; Pandey, R.; Sahariah, M.; Amzallag, E.; Baraille, I.; Rérat, M. First-Principles Study of the Optical Properties of BeO in its Ambient and High-Pressure Phases. J. Phys. Chem. Solids 2009, 70, 789–801. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Madsen, G.; Kvasnicka, D.; Luitz, J. WIEN2k: An Augmented Plane Wave Plus Local Orbitals Program for Calculating Crystal Properties; Techn. Universitat Wien: Vienna, Austria, 2001. [Google Scholar]
- Ambrosch-Draxl, C.; Sofo, J.O. Linear Optical Properties of Solids within the Full-Potential Linearized Augmented Planewave Method. Comput. Phys. Commun. 2006, 175, 1–14. [Google Scholar] [CrossRef]
- Singh, D.J.; Seo, S.S.A.; Lee, H.N. Optical Properties of Ferroelectric Bi4Ti3O12. Phys. Rev. B: Condens. Matter Mater. Phys. 2010, 82, 180103. [Google Scholar] [CrossRef]
- Rasukkannu, M.; Velauthapillai, D.; Vajeeston, P. A first-principle study of the electronic, mechanical and optical properties of inorganic perovskite Cs2SnI6 for intermediate-band solar cells. Mater. Lett. 2018, 218, 233–236. [Google Scholar] [CrossRef]







| Atoms | X | Y | Z |
|---|---|---|---|
| Nd | 0.12500000 | 0.12500000 | 0.12500000 |
| Nd | 0.62500000 | 0.12500000 | 0.12500000 |
| Nd | 0.12500000 | 0.12500000 | 0.62500000 |
| Nd | 0.12500000 | 0.62500000 | 0.12500000 |
| Zr | 0.62500000 | 0.62500000 | 0.62500000 |
| Zr | 0.12500000 | 0.62500000 | 0.62500000 |
| Zr | 0.62500000 | 0.62500000 | 0.12500000 |
| Zr | 0.62500000 | 0.12500000 | 0.62500000 |
| O | 0.00000000 | 0.00000000 | 0.00000000 |
| O | 0.25000000 | 0.25000000 | 0.25000000 |
| O | 0.28956700 | 0.28956700 | 0.71043300 |
| O | 0.53956700 | 0.53956700 | 0.96043300 |
| O | 0.71043300 | 0.28956700 | 0.71043300 |
| O | 0.71043300 | 0.28956700 | 0.28956700 |
| O | 0.28956700 | 0.71043300 | 0.71043300 |
| O | 0.28956700 | 0.71043300 | 0.28956700 |
| O | 0.71043300 | 0.71043300 | 0.28956700 |
| O | 0.96043300 | 0.53956700 | 0.53956700 |
| O | 0.53956700 | 0.96043300 | 0.96043300 |
| O | 0.96043300 | 0.96043300 | 0.53956700 |
| O | 0.96043300 | 0.53956700 | 0.96043300 |
| O | 0.53956700 | 0.96043300 | 0.53956700 |
| Atoms | X | Y | Z |
|---|---|---|---|
| Nd | 0.50000000 | 0.50000000 | 0.50000000 |
| Nd | 0.00000000 | 0.75000000 | 0.22231000 |
| Nd | 0.50000000 | 0.00000000 | 0.50000000 |
| Nd | 0.00000000 | 0.25000000 | 0.77769000 |
| Zr | 0.00000000 | 0.25000000 | 0.23880500 |
| Zr | 0.00000000 | 0.75000000 | 0.76119500 |
| Zr | 0.50000000 | 0.00000000 | 0.00000000 |
| Zr | 0.50000000 | 0.50000000 | 0.00000000 |
| O | 0.00000000 | 0.46145300 | 0.63341100 |
| O | 0.50000000 | 0.75000000 | 0.86688600 |
| O | 0.00000000 | 0.52504300 | 0.88566600 |
| O | 0.00000000 | 0.47495700 | 0.11433400 |
| O | 0.00000000 | 0.02504300 | 0.11433400 |
| O | 0.50000000 | 0.75000000 | 0.10071700 |
| O | 0.50000000 | 0.25000000 | 0.36987600 |
| O | 0.00000000 | 0.53854700 | 0.36658900 |
| O | 0.00000000 | 0.96145300 | 0.36658900 |
| O | 0.00000000 | 0.03854700 | 0.63341100 |
| O | 0.50000000 | 0.75000000 | 0.63012400 |
| O | 0.50000000 | 0.25000000 | 0.89928300 |
| O | 0.00000000 | 0.97495700 | 0.88566600 |
| O | 0.50000000 | 0.25000000 | 0.13311400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qayyum, A.; Azam, S.; Reshak, A.H.; Akbar, J.; Abbas, Z.; Ullah, H.; Ramli, M.M. Spin-Dependent First-Principles Study on Optoelectronic Properties of Neodymium Zirconates Pyrochlores Nd2Zr2O7 in Fd-3m and Pmma Phases. Molecules 2022, 27, 5711. https://doi.org/10.3390/molecules27175711
Qayyum A, Azam S, Reshak AH, Akbar J, Abbas Z, Ullah H, Ramli MM. Spin-Dependent First-Principles Study on Optoelectronic Properties of Neodymium Zirconates Pyrochlores Nd2Zr2O7 in Fd-3m and Pmma Phases. Molecules. 2022; 27(17):5711. https://doi.org/10.3390/molecules27175711
Chicago/Turabian StyleQayyum, Azhar, Sikander Azam, Ali H. Reshak, Jehan Akbar, Zeesham Abbas, Haseen Ullah, and Muhammad M. Ramli. 2022. "Spin-Dependent First-Principles Study on Optoelectronic Properties of Neodymium Zirconates Pyrochlores Nd2Zr2O7 in Fd-3m and Pmma Phases" Molecules 27, no. 17: 5711. https://doi.org/10.3390/molecules27175711
APA StyleQayyum, A., Azam, S., Reshak, A. H., Akbar, J., Abbas, Z., Ullah, H., & Ramli, M. M. (2022). Spin-Dependent First-Principles Study on Optoelectronic Properties of Neodymium Zirconates Pyrochlores Nd2Zr2O7 in Fd-3m and Pmma Phases. Molecules, 27(17), 5711. https://doi.org/10.3390/molecules27175711






