tert-Butyl(2-oxo-2H-pyran-5-yl)carbamate as the First Chameleon Diene Bearing an Electron-Donating Substituent
Abstract
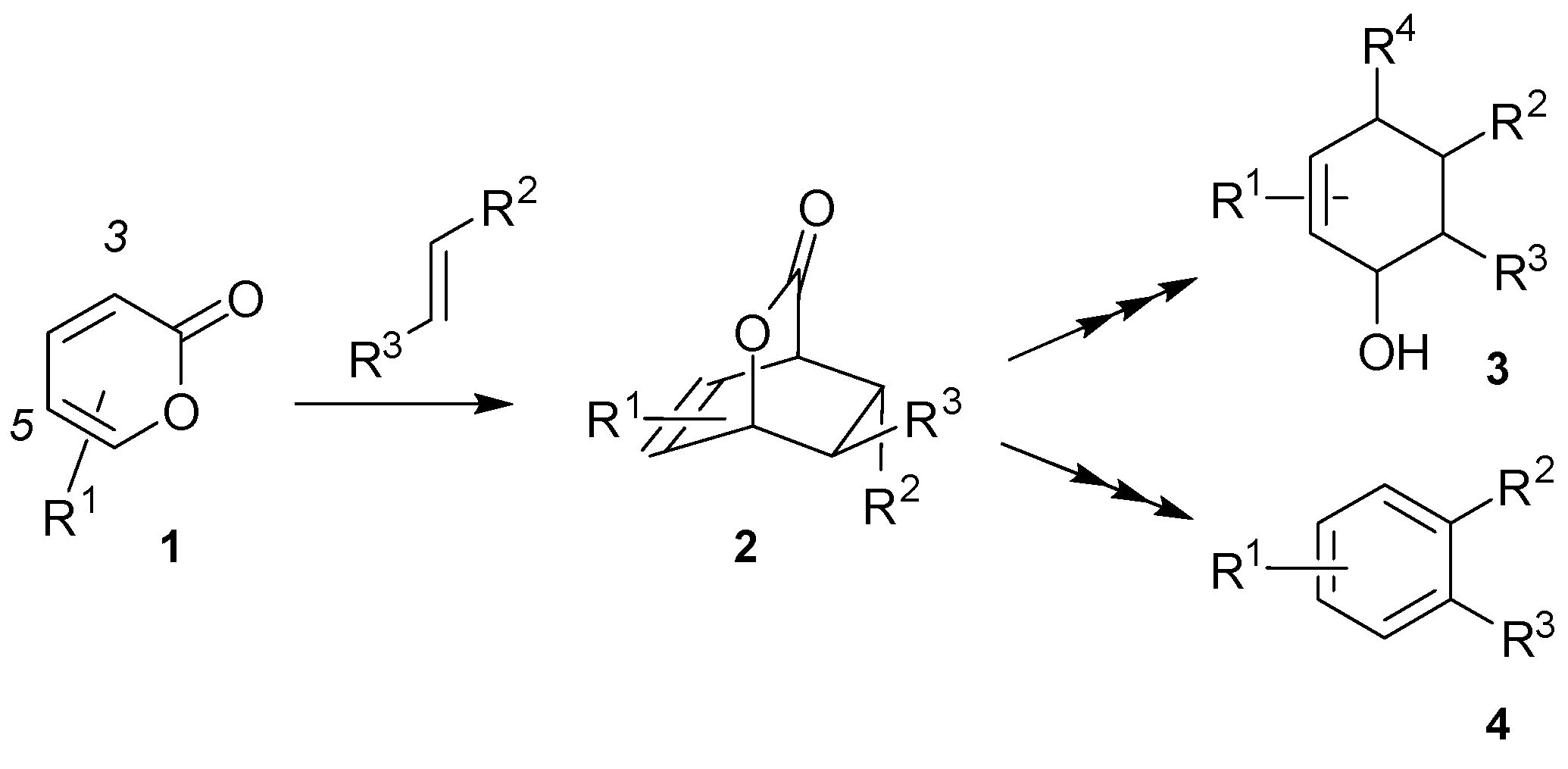
:1. Introduction
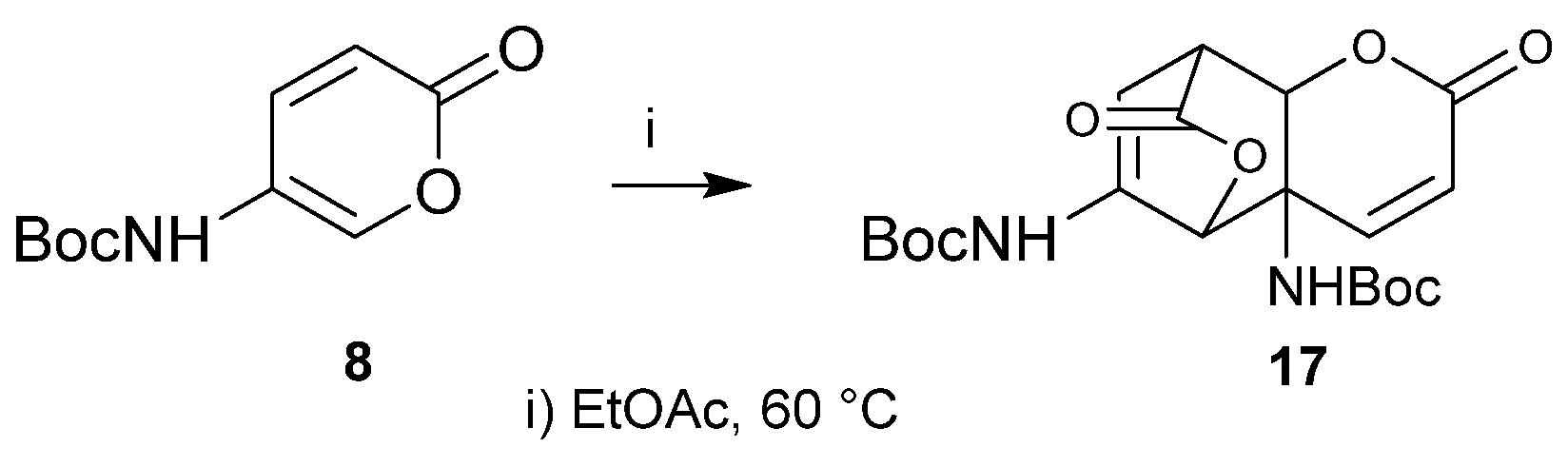
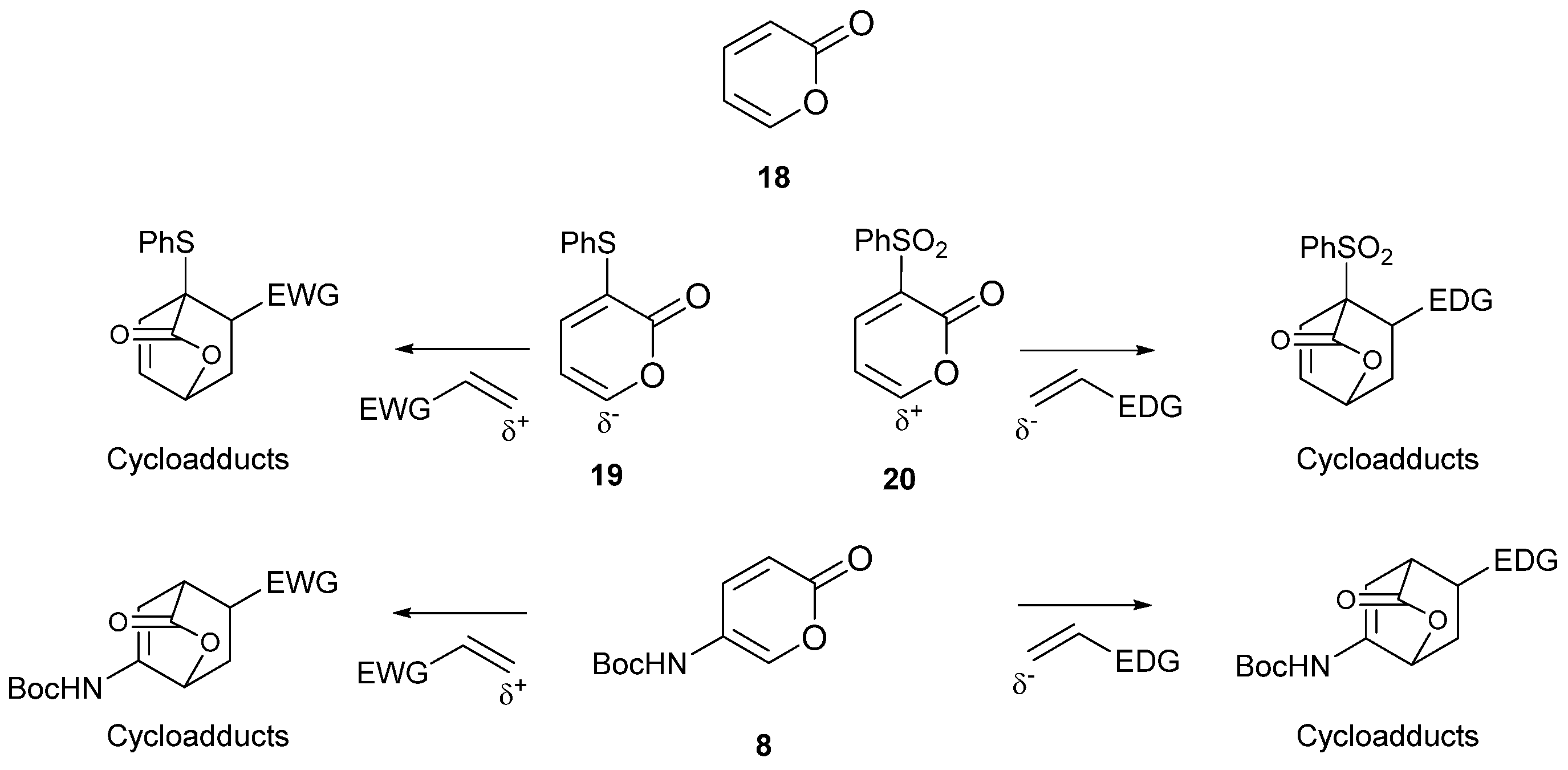
2. Results
3. Discussions and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afarinkia, K.; Nelson, T.D.; Vinader, M.V.; Posner, G.H. Diels-Alder cycloadditions of 2-pyrones and 2-pyridones. Tetrahedron 1992, 48, 9111–9171. [Google Scholar] [CrossRef]
- Woodward, B.T.; Posner, G.H. Recent Advances in Diels—Alder Cycloadditions of 2-Pyrones. Adv. Cyloaddition 1999, 5, 47–83. [Google Scholar]
- Goel, J.; Ram, V.J. Natural and synthetic 2H-pyran-2-ones and their versatility in. Tetrahedron 2009, 65, 7865–7913. [Google Scholar] [CrossRef]
- Cai, Q. The [4+ 2] Cycloaddition of 2-Pyrone in Total Synthesis. Chin. J. Chem. 2019, 37, 946–976. [Google Scholar] [CrossRef]
- Huang, G.; Kouklovsky, C.; de la Torre, A. Inverse-Electron-Demand Diels–Alder Reactions of 2-Pyrones: Bridged Lactones and Beyond. Chem. Eur. J. 2021, 27, 4760–4788. [Google Scholar] [CrossRef] [PubMed]
- Afarinkia, K.; Mahmood, F. A novel and concise synthesis of (±) 2-epi-Validamine. Tetrahedron 1999, 55, 3129–3140. [Google Scholar] [CrossRef]
- Vinader, V.; Haji Abdullahi, M.; Patterson, L.H.; Afarinkia, K. Synthesis of a pseudo-disaccharide library and its application to the characterisation of the heparanase catalytic site. PLoS ONE 2013, 8, e82111. [Google Scholar]
- Vinader, V.; Afarinkia, K. Carbasugar Probes to Explore the Enzyme Binding Pocket at the Anomeric Position: Application to the Design of Golgi Mannosidase II Inhibitors. Curr. Med. Chem. 2013, 20, 3797–3801. [Google Scholar] [CrossRef]
- Afarinkia, K.; Haji Abdullahi, M.; Scowen, I. A new, general method for the synthesis of carbasugar-sugar pseudodisaccharides. J. Org. Lett. 2009, 11, 5182–5184. [Google Scholar] [CrossRef]
- Afarinkia, K.; Haji Abdullahi, M.; Scowen, I. Synthesis of Carbasugar−Sugar Pseudodisaccharides via a Cycloaddition− Cycloreversion Reaction of 2 H-Pyran-2-ones. J. Org. Lett. 2010, 12, 5564–5566. [Google Scholar] [CrossRef]
- Afarinkia, K.; Ndibwami, A. A general synthesis of phenanthridinone alkaloids. Synlett 2007, 2007, 1940–1944. [Google Scholar] [CrossRef]
- Posner, G.H.; Kinter, C.M. Asymmetric total synthesis of an A-ring precursor to hormonally active 1. alpha., 25-dihydroxyvitamin D3. J. Org. Chem. 1990, 55, 3967–3969. [Google Scholar] [CrossRef]
- Posner, G.H.; Nelson, T.D. Stereocontrolled synthesis of highly functionalized cyclohexenes. A short synthesis of a chorismic acid precursor. Tetrahedron 1990, 46, 4573–4586. [Google Scholar] [CrossRef]
- Whitney, J.G.; Gregory, W.A.; Kauer, J.C.; Roland, J.R.; Snyder, J.A.; Benson, R.E.; Hermann, E.C. Antiviral agents. I. Bicyclo[2.2.2]octan- and -oct-2-enamines. J. Med. Chem. 1970, 13, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Watt, D.S. Total synthesis of (+−)- alpha-and (+−)- beta-copaenes and ylangenes. J. Am. Chem. Soc. 1973, 95, 2303–2311. [Google Scholar] [CrossRef]
- Ciganek, E. 2, 3, 4, 4a, 5, 6, 7, 7a-Octahydro-1H-benzofuro [3, 2-e] isoquinoline: A new morphine fragment. J. Am. Chem. Soc. 1981, 103, 6261–6262. [Google Scholar] [CrossRef]
- Martin, S.F.; Rüeger, H.; Williamson, S.A.; Grzejsczak, S. General strategies for the synthesis of indole alkaloids. Total synthesis of (+−)-reserpine and (+−)- alpha-yohimbine. J. Am. Chem. Soc. 1987, 109, 6124–6134. [Google Scholar] [CrossRef]
- Noguchi, M.; Kakimoto, S.; Kajigaeshi, S. Regio-and Stereoselective Introduction of Functional Groups into 1-Isoindolinone and 1 (2 H)-Isoquinolone Systems. Bull. Chem. Soc. Jpn. 1987, 60, 3261–3267. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Bardshiri, E.; Simpson, T.J. A convenient synthesis of isotopically labelled anthraquinones, chrysophanol, islandicin, and emodin. Incorporation of [methyl-2H3]chrysophanol into tajixanthone in Aspergillus variecolor. J. Chem. Soc. Chem. Commun. 1987, 1995, 883–884. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Otsuji, A.; Utimoto, K.; Kozima, S. 1, 2-Addition of Allyl and 2-Oxo-2 H-pyran-6-ylcarbonyl Groups to Cyclic C=N Double Bonds by Means of Organotin Reagent for Alkaloids Synthesis; A Facile Synthesis of 8-Oxoprotoberberine and Norketoyobirine (Demethoxycarbonyloxogambirtannine). Bull. Chem. Soc. Jpn. 1992, 65, 298–300. [Google Scholar] [CrossRef]
- Marko, I.E.; Seres, P.; Evans, G.R.; Swarbrick, T.M. Tandem pericylic reactions (TPR). A simple construction of functionalised [3n, 1] bicycles and a ready entry into the core of gibberellic acid and zizaene. Tetrahedron Lett. 1993, 34, 7305–7308. [Google Scholar] [CrossRef]
- Komiyama, T.; Takaguchi, Y.; Tsuboi, S. One-Pot Synthesis of 2-Arylthio-2-cyclohexenone Derivatives by the Diels–Alder Reaction of 4-Arylthio-3-hydroxy-2-pyrones. Synth. Commun. 2007, 37, 2131–2136. [Google Scholar] [CrossRef]
- Birch, A.M.; Birtles, S.; Buckett, L.K.; Kemmitt, P.D.; Smith, G.J.; Smith, T.J.D.; Turnbull, A.V.; Wang, S.J.Y. Discovery of a potent, selective, and orally efficacious pyrimidinooxazinyl bicyclooctaneacetic acid diacylglycerol acyltransferase-1 inhibitor. J. Med. Chem. 2009, 52, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Posner, G.H.; Jeon, H.B. New A-ring analogs of the hormone 1α, 25-dihydroxyvitamin D3:(2’-hydroxymethyl) tetrahydrofuro [1, 2-a]-25-hydroxyvitamin D3. Tetrahedon 2009, 65, 1235–1240. [Google Scholar]
- Larsson, R.; Scheeren, H.W.; Aben, R.W.M.; Johansson, M.; Sterner, O. Total Synthesis of Transtaganolide E and F: Insight in the Biosynthesis of the Transtaganolides. Eur. J. Org. Chem. 2013, 2013, 6955–6960. [Google Scholar] [CrossRef]
- Jung, Y.G.; Lee, S.C.; Cho, H.K.; Darvatkar, N.B.; Song, J.Y.; Cho, C.G. Total syntheses of (±)-α-lycorane and (±)-1-deoxylycorine. Org. Lett. 2013, 15, 132–135. [Google Scholar] [CrossRef]
- Slack, R.D.; Siegler, M.A.; Posner, G.H. Highly stereocontrolled and regiocontrolled syntheses of polyoxygenated [2.2. 2] oxabicyclic synthons. Tetrahedron Lett. 2013, 54, 6267–6270. [Google Scholar] [CrossRef]
- Cho, H.K.; Lim, H.Y.; Cho, C.G. (E)-β-Borylstyrene in the Diels–Alder Reaction with 3, 5-Dibromo-2-pyrone for the Syntheses of (±)-1-epi-Pancratistatin and (±)-Pancratistatin. Org. Lett. 2013, 15, 5806–5809. [Google Scholar] [CrossRef]
- Guney, T.; Lee, J.L.; Kraus, G.A. First Inverse Electron-Demand Diels–Alder Methodology of 3-Chloroindoles and Methyl Coumalate to Carbazoles. Organic Lett. 2014, 16, 1124–1127. [Google Scholar] [CrossRef]
- Okura, K.; Tamura, R.; Shigehara, K.; Masai, E.; Nakamura, M.; Otsuka, Y.; Katayama, Y.; Nakao, Y. Synthesis of Polysubstituted benzenes from 2-Pyrone-4, 6-dicarboxylic acid. Chem. Lett. 2014, 43, 1349–1351. [Google Scholar] [CrossRef]
- Min, L.; Zhang, Y.; Liang, X.F.; Huang, J.R.; Bao, W.L.; Lee, C.S. A Biomimetic Synthesis of (±)-Basiliolide B. Angew. Chem. Int. Edit. 2014, 53, 11294–11297. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Liu, B. A Diels–Alder Approach toward the Scaffolds of Polycyclic Sesquiterpenoids with 2-Pyrone. Synlett 2014, 25, 681–686. [Google Scholar]
- Kondratov, I.S.; Tolmachova, N.A.; Dolovanyuk, V.G.; Gerus, I.I.; Daniliuc, C.G.; Haufe, G. Eur. Synthesis of isomeric (3,3,3-trifluoropropyl)anilines. J. Org. Chem. 2015, 11, 2482–2491. [Google Scholar]
- Usachev, B.I. 6-(Trifluoromethyl)-2H-pyran-2-ones: Promising CF3-bearing conjugated cyclic diene and electrophilic building-blocks. J. Fluor. Chem. 2015, 175, 36–46. [Google Scholar] [CrossRef]
- Zhao, P.; Beaudry, C.M. Total Synthesis of (+)-Cavicularin: The Pyrone Diels–Alder Reaction in Enantioselective Cyclophane Synthesis. Synlett 2015, 26, 1923–1929. [Google Scholar]
- Lee, J.H.; Cho, C.G. Total synthesis of (−)-Neocosmosin A via intramolecular Diels–Alder reaction of 2-Pyrone. Org. Lett. 2016, 18, 5126–5129. [Google Scholar] [CrossRef]
- Gan, P.; Smith, M.W.; Braffman, N.R.; Snyder, S.A. Pyrone Diels–Alder Routes to Indolines and Hydroindolines: Syntheses of Gracilamine, Mesembrine, and Δ7-Mesembrenone. Angew. Chem. Int. Ed. 2016, 55, 3625–3630. [Google Scholar] [CrossRef]
- Lee, J.-H.; Cho, C.-G. H-Bonding Mediated Asymmetric Intramolecular Diels–Alder Reaction in the Formal Synthesis of (+)-Aplykurodinone-1. Org. Lett. 2018, 20, 7312–7316. [Google Scholar]
- Wang, C.; Chen, Q.; Shin, S.; Cho, C. Total Synthesis of (±)-Clivonine via Diels–Alder Reactions of 3,5-Dibromo-2-Pyrone. J. Org. Chem. 2020, 85, 10035–10049. [Google Scholar] [CrossRef]
- Pfennig, T.; Chemburkar, A.; Cakolli, S.; Neurock, M.; Shanks, B.H. Improving Selectivity of Toluic Acid from Biomass-Derived Coumalic Acid. ACS Sustain. Chem. Eng. 2018, 6, 12855–12864. [Google Scholar] [CrossRef]
- Gambarotti, C.; Lauria, M.; Righetti, G.I.C.; Leonardi, G.; Sebastiano, R.; Citterio, A.; Truscello, A. Synthesis of Functionalized Aromatic Carboxylic Acids from Biosourced 3-Hydroxy-2-pyrones through a Base-Promoted Domino Reaction. ACS Sustain. Chem. Eng. 2020, 8, 11152–11161. [Google Scholar]
- Zhang, X.; Beaudry, C.M. Regioselective Synthesis of Benzofuranones and Benzofurans. J. Org. Chem. 2021, 86, 6931–6936. [Google Scholar] [CrossRef] [PubMed]
- Points, G.L., III; Stout, K.T.; Beaudry, C.M. Regioselective Formation of Substituted Indoles: Formal Synthesis of Lysergic Acid. Chem. Eur. J. 2020, 26, 16655–16658. [Google Scholar] [CrossRef] [PubMed]
- Points, G.L., III; Beaudry, C.M. Regioselective Synthesis of Substituted Carbazoles, Bicarbazoles, and Clausine C. Org. Lett. 2021, 23, 6882–6885. [Google Scholar] [CrossRef]
- Xu, M.-M.; You, X.-Y.; Zhang, Y.-Z.; Lu, Y.; Tan, K.; Yang, L.; Cai, Q. Enantioselective synthesis of axially chiral biaryls by Diels–Alder/Retro-Diels–Alder reaction of 2-pyrones with alkynes. J. Am. Chem. Soc. 2021, 143, 8993–9001. [Google Scholar] [CrossRef]
- Watanabe, S.; Nishikawa, T.; Nakazaki, A. Synthesis of Oxy-Functionalized Steroidal Skeletons via Mizoroki–Heck and Intramolecular Diels–Alder Reactions. Org. Lett. 2019, 21, 7410–7414. [Google Scholar] [CrossRef]
- Yu, H.; Kraus, G.A. Divergent pathways to isophthalates and naphthalate esters from methyl coumalate. Tetrahedron Lett. 2018, 59, 4008–4010. [Google Scholar] [CrossRef]
- Afarinkia, K.; Berna-Canovas, J. Diels–Alder cycloaddition of 5-aryl-2-pyrones. Tetrahedron Lett. 2000, 41, 4955–4958. [Google Scholar] [CrossRef]
- Afarinkia, K.; Daly, N.T.; Gomez-Farnos, S.; Joshi, S. Unusual stereoselectivity in Diels-Alder cycloadditions of 5-bromopyrone. Tetrahedron Lett. 1997, 38, 2369–2372. [Google Scholar] [CrossRef]
- Afarinkia, K.; Bearpark, M.; Ndibwami, A. Computational and Experimental Investigation of the Diels− Alder Cycloadditions of 4-Chloro-2 (H)-pyran-2-one. J. Org. Chem. 2003, 68, 7158–7166. [Google Scholar] [CrossRef]
- Afarinkia, K.; Bearpark, M.; Ndibwami, A. An Experimental and Computational Investigation of the Diels− Alder Cycloadditions of Halogen-Substituted 2 (H)-Pyran-2-ones. J. Org. Chem. 2005, 70, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Afarinkia, K.; Posner, G.H. 5-Bromo-2-pyrone: An easily prepared ambiphilic diene and a synthetic equivalent of 2-pyrone in mild, thermal, Diels-Alder cycloadditions. Tetrahedron Lett. 1992, 33, 7839–7843. [Google Scholar] [CrossRef]
- Posner, G.H.; Nelson, T.D.; Kinter, C.M.; Afarinkia, K. 3-bromo-2-pyrone: An easily prepared chameleon diene and a synthetic equivalent of 2-pyrone in thermal Diels-Alder cycloadditions. Tetrahedron Lett. 1991, 32, 5295–5298. [Google Scholar] [CrossRef]
- Feng, M.; Jiang, X. Stereoselective construction of a key hydroindole precursor of epidithiodiketopiperazine (ETP) natural products. Chem. Commun. 2014, 50, 9690–9692. [Google Scholar] [CrossRef]
- Leonard, M.S.; Carroll, P.J.; Joullie, M.M. Synthesis of a pondaplin dimer and trimer. Aromatic interactions in novel macrocycles. J. Org. Chem. 2004, 69, 2526–2531. [Google Scholar] [CrossRef]
- Kirkham, J.D.; Leach, A.G.; Row, E.C.; Harrity, J.P.A. Investigation of the Origins of Regiochemical Control in [4+ 2] Cycloadditions of 2-Pyrones and Alkynylboronates. Synthesis 2012, 44, 1964–1973. [Google Scholar]
- Kirkham, J.D.; Delaney, P.M.; Ellames, G.J.; Row, E.C.; Harrity, J.P.A. An alkynylboronate cycloaddition strategy to functionalised benzyne derivatives. Chem. Commun. 2010, 46, 5154–5156. [Google Scholar] [CrossRef]
- Patrick, T.B.; Li, H. Cycloaddition reactions of 3-fluorobutenone. J. Fluor. Chem. 2009, 130, 544–546. [Google Scholar] [CrossRef]
- Danieli, B.; Lesma, G.; Martinelli, M.; Passarella, D.; Peretto, I.; Silvani, A. Application of the Pd-catalyzed heteroarylation to the synthesis of 5-(indol-2′-yl) pyridin-2-one and 5-(indol-2′-yl) pyran-2-one. Tetrahedron 1998, 54, 14081–14088. [Google Scholar] [CrossRef]
- Reus, C.; Liu, N.-W.; Bolte, M.; Lerner, H.-W.; Wagner, M. Synthesis of Bromo-, Boryl-, and Stannyl-Functionalized 1, 2-Bis (trimethylsilyl) benzenes via Diels–Alder or C–H Activation Reactions. J. Org. Chem. 2012, 77, 3518–3523. [Google Scholar] [CrossRef]
- Liu, C.-T. Studies toward the Synthesis of AB Ring System of Nagilactone. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, 1980. Available online: http://digitalcommons.unl.edu/dissertations/AAI8101220 (accessed on 1 August 2022).
- Pirkle, W.; Eckert, C.A.; Turner, W.V.; Scott, B.A.; McKendry, L.H. High pressure thermal and the photosensitized dimerizations of 2-pyrones. J. Org. Chem. 1976, 41, 2945–2946. [Google Scholar] [CrossRef]
- White, D.L.; Seyfert, D. Diels-Alder dimerization of 2-pyrone. J. Org. Chem. 1972, 37, 3545–3546. [Google Scholar] [CrossRef]
- Williams, J.D.; Otake, Y.; Coussanes, G.; Saridakis, I.; Maulide, N.; Kappe, C.O. Towards a Scalable Synthesis of 2-Oxabicyclo [2.2. 0] hex-5-en-3-one Using Flow Photochemistry. ChemPhotoChem 2019, 3, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, E.; Plieninger, H. Synthese einer Reihe von 3-Oxo-2-oxabicyclo [2.2.2] oct-7-en-Derivaten und Versuche zu deren Umwandlung in 3-Oxo-2-oxabicyclo [2.2. 2] octa-5, 7-dien. Chem. Ber. 1982, 115, 1967–1981. [Google Scholar] [CrossRef]
- Marko, I.E.; Seres, P.; Swarbrick, T.M.; Staton, I.; Adams, H. Tandem pericyclic reactions. Novel and efficient methodology for the Rapid assembly of complex polycyclic systems. Tetrahedron Lett. 1991, 32, 2452–2549. [Google Scholar]
- Hashimoto, Y.; Abe, R.; Morita, N.; Tamura, O. Inverse-electron-demand Diels–Alder reactions of α, β-unsaturated hydrazones with 3-methoxycarbonyl α-pyrones. Org. Bio. Chem. 2018, 16, 8913–8916. [Google Scholar] [CrossRef]
- Posner, G.H.; Ishihara, Y. Lewis acid-catalyzed, high pressure, stereospecific, regiospecific, Diels-Alder cycloaddition of unsubstituted 2-pyrone: Short synthesis of a racemic A-ring precursor to physiologically active 1-hydroxyvitamin D3 steroids. Tetrahedron Lett. 1994, 35, 7545–7548. [Google Scholar] [CrossRef]
- Chen, C.; Liao, C. One-pot stereoselective synthesis of tricyclic bislactones from 2-pyrones and 2-methoxyfuran. Org. Lett. 2000, 2, 2049–2052. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Gultekin, Z.; Grainger, R.S.; Adams, H.; Spargo, P.L. (1 R, 3 R)-2-Methylene-1, 3-dithiolane 1, 3-dioxide: A highly reactive and highly selective chiral ketene equivalent in cycloaddition reactions with a broad range of dienes. J. Chem. Soc. Perkin Trans. 1 1998, 17, 2771–2782. [Google Scholar] [CrossRef]
- Posner, G.H.; Nelson, T.D.; Kinter, C.M.; Johnson, N. Diels-Alder cycloadditions using nucleophilic 3-(p-tolylthio)-2-pyrone. Regiocontrolled and stereocontrolled synthesis of unsaturated, bridged, bicyclic lactones. J. Org. Chem. 1992, 57, 4083–4088. [Google Scholar] [CrossRef]
- Posner, G.H.; Wettlaufer, D.G. Asymmetric Diels-Alder cycloadditions using chiral alkyl vinyl ethers and a dienyl sulfone. Tetrahedron Lett. 1986, 27, 667–670. [Google Scholar]
- Kraus, G.A.; Wang, S. Synthesis of isophthalates from methyl coumalate. RSC Adv. 2017, 7, 56760–56763. [Google Scholar]
- Geist, E.; Berneaud-Koetz, H.; Baikstis, T.; Draeger, G.; Kirschning, A. Toward chromanes by de novo construction of the benzene ring. Org. Lett. 2019, 21, 8930–8933. [Google Scholar] [PubMed]
- Jung, M.E.; Street, L.J.; Usui, J. Chemoselective cycloadditions of 3, 4-dialkoxyfurans and alkyl coumalates. Novel loss of aromaticity of two nonbenzenoid aromatic rings in a mild thermal process. J. Am. Chem. Soc. 1986, 108, 6810–6811. [Google Scholar]
- Lantos, I.; Sheldrake, P.W.; Wells, A.S. Novel cage compounds from inter-and intra-molecular Diels–Alder reactions of heteroaromatic azadienes and methyl coumalate with cyclo-octa-1, 5-diene. J. Chem. Soc. Perkin Trans. 1 1990, 7, 1887–1890. [Google Scholar]
- Hatsui, T.; Hashiguchi, T.; Takeshita, H. Total Synthesis of (+−)-Shizuka-Acoradienol. Chem. Express 1993, 8, 581–584. [Google Scholar]
- Smith, M.W.; Snyder, S.A. A concise total synthesis of (+)-scholarisine A empowered by a unique C–H arylation. J. Am. Chem. Soc. 2013, 135, 12964–12967. [Google Scholar]
- Saktura, M.; Grzelak, P.; Dybowska, J.; Albrecht, L. Asymmetric Synthesis of [2.2. 2]-Bicyclic Lactones via All-Carbon Inverse-Electron-Demand Diels–Alder Reaction. Org. Lett. 2020, 22, 1813–1817. [Google Scholar]
- Obata, T.; Shimo, T.; Suishu, T.; Somekawa, K. Stereoselective photo[4+2]cycloadditions of 2-pyrone-5-carboxylates with maleimides in the solid state and in solution. J. Het. Chem. 1998, 35, 1361–1364. [Google Scholar]

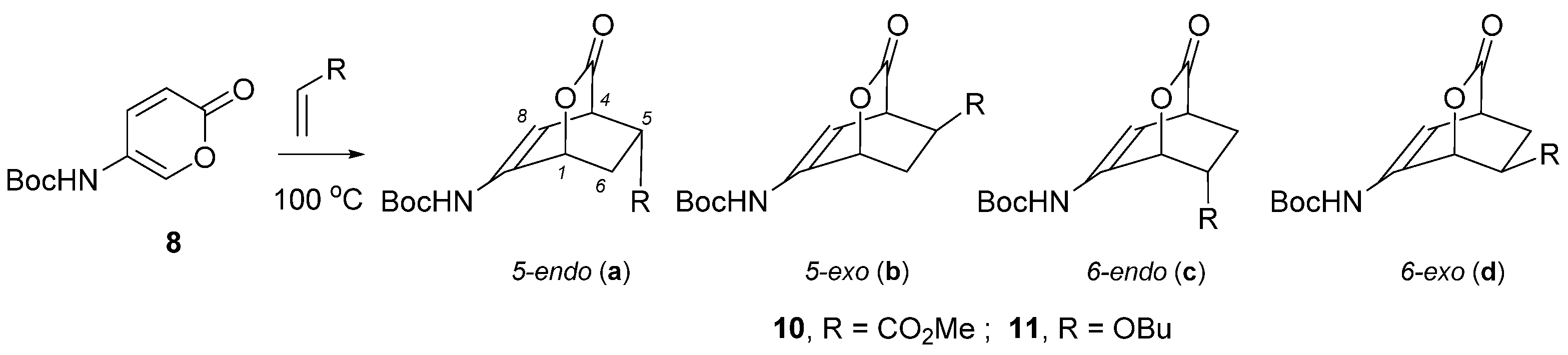

| Dienophile | Yield * (%) | Cycloadduct | 5-endo:5-exo:6-endo:6-exo |
|---|---|---|---|
| Methyl acrylate | 79 | 10 | 91:9:0:0 |
| Methyl metacrylate | 72 | 12 | 93:7:0:0 |
| Acrylonitrile | 76 | 13 | 50:50:0:0 |
| Styrene | 67 | 14 | 70:30:0:0 |
| Vinyl acetate | 65 | 15 | 25:63:12:0 |
| Vinylene carbonate | 51 | 16 | 67:33: 0:0 |
| Butyl vinyl ether | 54 | 11 | 80:20:0:0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, Y.M.; Santucci, G.; Afarinkia, K. tert-Butyl(2-oxo-2H-pyran-5-yl)carbamate as the First Chameleon Diene Bearing an Electron-Donating Substituent. Molecules 2022, 27, 5666. https://doi.org/10.3390/molecules27175666
Omar YM, Santucci G, Afarinkia K. tert-Butyl(2-oxo-2H-pyran-5-yl)carbamate as the First Chameleon Diene Bearing an Electron-Donating Substituent. Molecules. 2022; 27(17):5666. https://doi.org/10.3390/molecules27175666
Chicago/Turabian StyleOmar, Yasser M., Giulia Santucci, and Kamyar Afarinkia. 2022. "tert-Butyl(2-oxo-2H-pyran-5-yl)carbamate as the First Chameleon Diene Bearing an Electron-Donating Substituent" Molecules 27, no. 17: 5666. https://doi.org/10.3390/molecules27175666
APA StyleOmar, Y. M., Santucci, G., & Afarinkia, K. (2022). tert-Butyl(2-oxo-2H-pyran-5-yl)carbamate as the First Chameleon Diene Bearing an Electron-Donating Substituent. Molecules, 27(17), 5666. https://doi.org/10.3390/molecules27175666





