Abstract
Hiyama cross-coupling is a versatile reaction in synthetic organic chemistry for the construction of carbon–carbon bonds. It involves the coupling of organosilicons with organic halides using transition metal catalysts in good yields and high enantioselectivities. In recent years, hectic progress has been made by researchers toward the synthesis of diversified natural products and pharmaceutical drugs using the Hiyama coupling reaction. This review emphasizes the recent synthetic developments and applications of Hiyama cross-coupling.
1. Introduction
Biaryl scaffolds are the prevailing structures that exist in numerous natural products [1,2,3], sensors [4], pharmaceuticals [5,6], ligands [7,8], polymers [9], agrochemicals [10], organocatalysts [11], fine chemical industries [12,13,14,15] and are scrutinized as the valuable intermediates in organic synthesis [16]. Several transition metal-catalyzed coupling protocols for the generation of the C-C bond have been reported in this regard, including Suzuki, Stille, Negishi, Kumada, and Hiyama coupling [17,18,19,20]. Among different protocols, Hiyama cross-coupling achieved remarkable attention by researchers for attaining biaryl moieties possessing a broad spectrum of pharmacological activities. For example, valsartan and losartan are used to treat high blood pressure and diabetic kidney disease. Felbinac is a non-steroidal anti-inflammatory drug (NSAID) used to cure muscular pain, inflammation, and sprains. Telmisartan is utilized as an alternative source for treating COVID-19 patients, and imatinib is a tyrosine kinase inhibitor (TKI) to inhibit cancer cell growth [21,22,23]. Hiyama coupling reveals to be an effective and convenient method of synthesizing stilbenoids. Stilbenoids have been found in medicinal plants and foods and exhibit potent biological activities such as antiviral, antifungal activity [24], anti-inflammatory property [25], neuroprotection [26], antioxidative property [27], and anticarcinogenic effect [28,29].
Aryl C-glucosides belong to a substantial class of natural compounds [30] and synthesized drugs [31,32]. Access to C-glycosides, which is an inhibitor of sodium–glucose cotransporter-2, via protecting a group-free Hiyama coupling reaction, has been developed. This strategy has been reported to synthesize pharmaceutically significant dapagliflozin compound that is useful to cure type 2 diabetes mellitus [33]. Hiyama cross-coupling offers 2-functionalized indoles formation by means of rhodium metal catalyst [34]. Furthermore, Hiyama coupling methodologies have been employed for the synthesis of diarylmethanes. Diarylmethane scaffolds containing natural products and drugs include segontin 1 (to cure the coronary heart disease) [35], benadryl 2 and tolpropamine 3 (antiallergic) [36,37], bifemelane 4 (antidepressant) [38] and piritrexim 5 (anticancer agent) [39]. Likewise, the diarylmethane containing marine natural product avrainvilleol 6 exhibits both antibacterial [40] and antioxidant [41] activities (Figure 1).
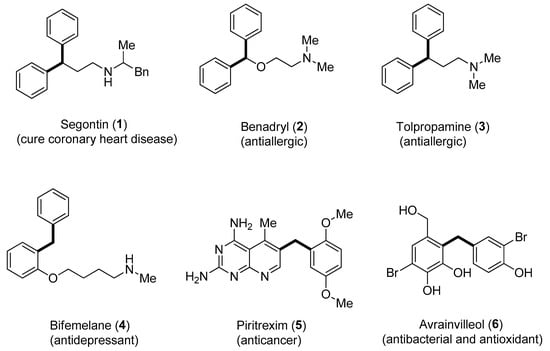
Figure 1.
Structures of bioactive natural products and drugs containing diarylmethane units.
Hiyama cross-coupling finds applications in synthesizing retinoids, benzofuran derivatives, benzoxocane, and picropodophyllin analogs, which impart a critical role in cell growth, embryo development, vision, immune response, and high affinity for tubulin [42,43,44,45,46,47,48].
Keeping the wide applications of the Hiyama coupling reaction in mind, here we have provided a comprehensive compilation of recent methodologies of the Hiyama coupling reaction.
2. Mechanistic Consideration
The mechanism of the Hiyama coupling reaction was proposed by Foubelo et al. in detail. The initial step of the mechanism involves oxidative addition of halide to Pd metal, resultantly converting palladium(0) to palladium(II). The transmetalation step leads to the splitting of the C-Si bond, and the cycle moves towards the construction of a new bond between carbon and palladium. In reductive elimination, the C-C bond is established, and a zero-valent state of palladium is achieved to restart a new cycle (Scheme 1) [49].
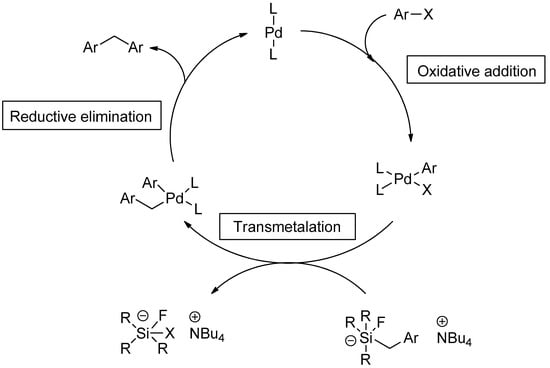
Scheme 1.
Mechanistic approach for Hiyama cross-coupling.
3. Palladium-Catalyzed Hiyama Cross-Coupling
Palladium Acetate as Catalyst
Palladium-catalyzed Hiyama cross-coupling reactions have extensively been employed for the synthesis of biaryl derivatives due to characteristic features of organosilane reagents such as non-toxicity, ease of access, high sustainability, and low cost [50,51,52]. Li and coworkers evaluated the catalytic efficacy of Pd(OAc)2/DABCO for the reaction of aryl halides and aryltrimethoxysilanes. This methodology covered a wide substrate scope by the addition of aryl bromides and iodides to aryl trialkoxysilanes giving biaryl derivatives in a moderate to excellent yield range (20–100%). Different solvents, including dioxane, MeCN, THF, acetone, and DMF, were investigated, and dioxane gave the highest yield. Out of the three different bases (KF, TBAF, and K2CO3), TBAF was selected as a suitable base for this reaction. A reference example is highlighted in Scheme 2. p-Iodonitrobenzene 7 was coupled with phenyl trimethoxysilane 8, affording the corresponding cross-coupled product 9 with quantitative yield. The coupling proceeded at 80 °C for 1 h using Pd(OAc)2 (3 mol%), DABCO (6 mol%), and tetrabutylammonium fluoride (TBAF) in dioxane [53].
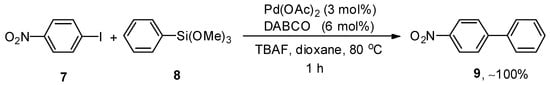
Scheme 2.
Pd(OAc)2 catalyzed cross-coupling of p-iodonitrobenzene 7 and phenyl trimethoxysilane 8.
A facile and effective protocol for the synthesis of symmetrically and asymmetrically functionalized (E)-1,2-diarylethenes by sequential one-pot Hiyama–Heck reactions was disclosed by Gordillo et al. The reaction proceeded in aqueous and ligand-free conditions. The symmetric (E)-1,2-diarylethene 12 was synthesized via one-pot Hiyama vinylation and Heck arylation of triethoxy(vinyl)silane 10 and aryl bromide 11 using Pd(OAc)2 (0.3 mol%) and NaOH in 98% yield with selectivities (100:0), respectively (Scheme 3). For the synthesis of asymmetric (E)-1,2-diarylethene via one-pot Hiyama vinylation reaction, a different strategy was developed in which treatment of 3-bromopyridine 13 with triethoxy(vinyl)silane 10 by using Pd(OAc)2 aqueous NaOH and PEG followed by the Heck arylation in H2O afforded 100% conversion with 91% yield of corresponding asymmetric (E)-1,2-diarylethene 14 (Scheme 4). Bromoarenes containing electron-donating and electron-withdrawing groups provided asymmetric (E)-1,2-diarylethenes in a moderate to excellent (71–91%) yield range [54].
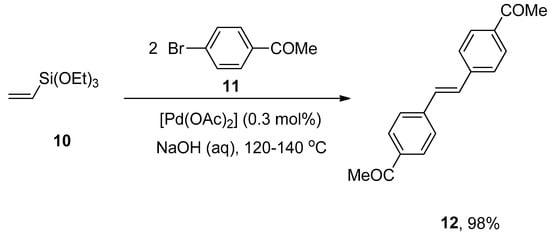
Scheme 3.
Formation of symmetric (E)-1,2-diarylethene 12 by sequential Hiyama vinylation and Heck arylation.
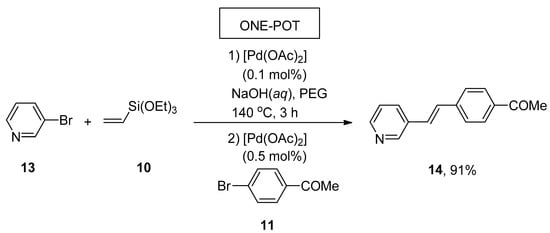
Scheme 4.
Synthesis of asymmetric (E)-1,2-diarylethene 14 using one-pot Hiyama vinylation and Heck arylation.
The combination of palladium acetate with XPhos exhibits high efficacy in Hiyama cross-coupling of aryl mesylates and arylsilanes to generate corresponding biaryl derivatives in a 40–97% yield range. Wu and coworkers developed a general and efficient synthetic route for synthesizing the substituted biaryl compounds by palladium-catalyzed Hiyama cross-coupling reaction of unactivated aryl mesylates with arylsilanes. Various substituted aryl mesylates containing either electron-rich or electron-poor groups furnished the corresponding biaryl products in 40–97% yields. Maximum yield (97%) was attained in the case of coupling of methoxy substituted aryl mesylate 15 with triethoxy(phenyl)silane 16 using 4 mol% of Pd(OAc)2 as a catalyst, 10 mol% of XPhos 17 as ligand, and 2.0 equivalents of tetra-n-butylammonium fluoride (TBAF) as an additive in THF/t-BuOH mixture at 90 °C (Scheme 5) [55].
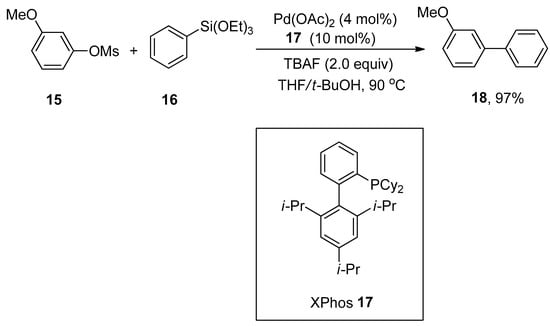
Scheme 5.
Use of Pd(OAc)2 and XPhos 17 in coupling of aryl mesylate 15 and triethoxy(phenyl)silane 16.
Molander and Iannazzo outlined an impressive synthetic approach toward the synthesis of biaryls and heterobiaryl derivatives by Hiyama cross-coupling reactions of aryltrifluorosilanes with aryl and heteroaryl chlorides using a palladium catalyst. Aryl chlorides, bearing both electron-donating and withdrawing substituents, afforded corresponding derivatives in 70–98% yields. Phenyltrifluorosilane 19 was allowed to couple with methyl 3-chlorobenzoate 20 in the presence of 2.5 mol% palladium acetate and 5 mol% XPhos 17 using TBAF (2.5 equiv) as fluoride activator and t-BuOH as solvent. The reaction proceeded at 60 °C, providing the targeted product 21 in 98% yield (Scheme 6). The coupling of a wide range of substituted heteroaryl chlorides with aryltrifluorosilanes provided the desired products in good to excellent yields (71–94%) [56].
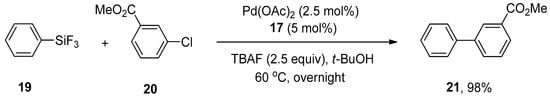
Scheme 6.
Pd(OAc)2 catalyzed coupling in the presence of XPhos 17.
Arenediazonium salts are the most reactive and efficient electrophiles for palladium-catalyzed synthetic organic chemistry [57,58,59,60,61,62]. Considering their importance, Qi and coworkers achieved the Hiyama coupling of dimethoxydiphenylsilane with mono or disubstituted arenediazonium tetrafluoroborate salts in a 65–89% yield range under mild reaction conditions. The dimethoxydiphenylsilane 22 was allowed to couple with bromo substituted arenediazonium tetrafluoroborate salt 23 using 5 mol% Pd(OAc)2 in methanol at room temperature for 6 h to obtain the targeted bromo substituted biaryl derivative 24 in 89% yield (Scheme 7). The cross-coupling of 4-methylbenzenediazonium tetrafluoroborate salt with various organosilanes yielded the desired cross-coupling products in 78–87% yields [63].
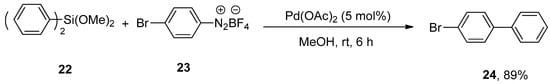
Scheme 7.
Reaction of dimethoxydiphenylsilane 22 with arenediazonium tetrafluoroborate salt 23 using Pd(OAc)2 as catalyst.
Yuen et al. carried out a facile synthetic protocol for the formation of biaryl derivatives by Hiyama coupling reaction of aryl and heteroaryl chlorides with phenyl trimethoxysilane catalyzed by 0.2 mol% Pd(OAc)2/26 to attain the cross-coupling products in moderate to good yield range (44–99%). The phenyl(trimethoxy)silane 8 was coupled with aryl chlorides 25 and 28 under H2O and solventless conditions, respectively, to obtain the desired products 27 and 29 in 97% and 99% yields, respectively. The reaction proceeded smoothly by using a 1:4 mixture of highly efficient Pd(OAc)2/26 and TBAF·3H2O as the base at 110 °C under an N2 atmosphere (Scheme 8). The aryl chlorides having electron-withdrawing groups (F, CF3, CO2Me, and CN) afforded a 47–99% yield range. Under solvent-free conditions, the reaction of heteroaryl chlorides and alkenyl chlorides with aryl trialkoxysilanes gave a 63–99% yield range, while in the case of H2O, a 36–97% yield range was attained. On the other hand, a 54–81% yield range was observed in the case of heteroaryl trialkoxysilanes [64].
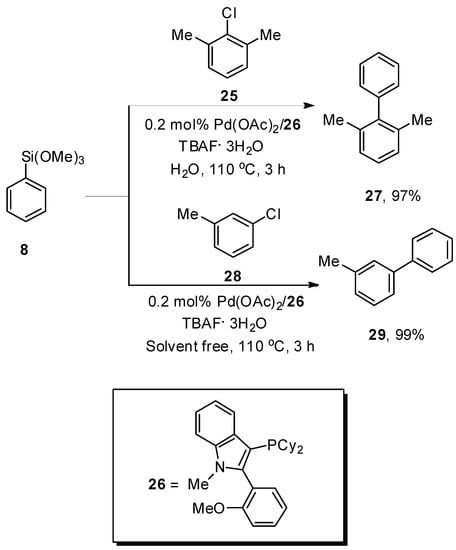
Scheme 8.
Hiyama cross-coupling reaction by using H2O and solvent free condition.
Azetidines are distinctly comprised of four-membered azaheterocycle motifs due to possessing numerous chemical properties and ring strain [65,66]. Arylazetidine scaffolds are significant building modules and exhibit a diverse range of biological activities [67,68,69,70,71,72]. A convenient approach toward the synthesis of a variety of 3-arylazetidines derivatives through Hiyama coupling of 3-iodoazetidines with arylsilanes in mild reaction conditions was reported by Zou and coworkers. Triethoxy(phenyl)silane with electron-donating and -withdrawing substituents afforded the products moderate to excellent yields (30–88%). 1-Boc-3-iodoazetidine 30 was allowed to couple with 4-methylphenyltriethoxysilane 31 in the presence of Pd(OAc)2 catalyst (5 mol%), phosphine ligand (10 mol%) 32, tetra-n-butylammonium fluoride (TBAF in THF) and dioxane under argon atmosphere to obtain the targeted product 33 in an excellent (88%) yield along with 34 in 99:1 ratio, respectively (Scheme 9). The substrate scope of heterocycloalkyl iodides was observed under optimized conditions. The desired coupling products were obtained in a 33–85% yield range [73].
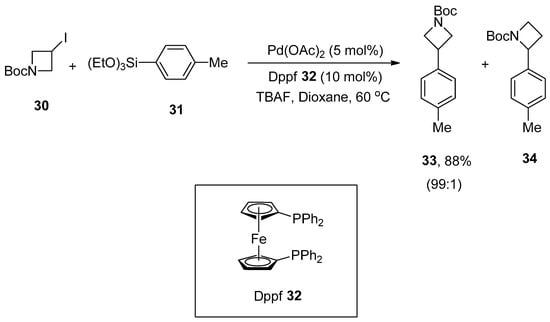
Scheme 9.
Synthesis of 3-arylazetidine derivative 33 from 3-iodoazetidine 30.
Dihetaryl disulfides possessing pyrimidine rings exhibit diversified biological and pharmacological activities [74,75,76], such as antifungal, antibacterial, and calcium-channel modulation [77]. An interesting and modular strategy for the formation of carbon–carbon bonds to produce potent biologically active compounds through palladium-catalyzed copper-promoted Hiyama cross-coupling reaction has been reported by Liu et al. Dihetaryl disulfides containing electron-rich and electron-poor substituents in the para-positions of benzene rings afforded corresponding products in moderate yield range. A maximum yield (78%) of 36 was observed in the case of cross-coupling of dihetaryl disulfide 35 with trimethoxy(phenyl)silane 8 using 3 mol% Pd(OAc)2 catalyst, an efficient activator copper (I) thiophene-2-carboxylate (CuTC), TBAF and 6 mol% PCy3 ligand using THF as the only solvent (Scheme 10) [78].
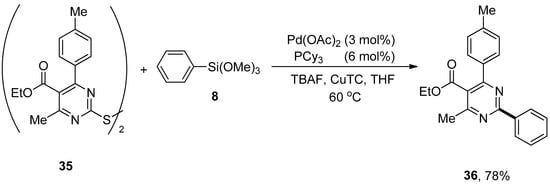
Scheme 10.
Dihetaryl disulfides 35 and trimethoxy(phenyl)silane 8 using PCy3 as ligand.
The palladium NNC-pincer complex was found to be an efficient catalyst precursor for the generation of monomeric palladium(0) species. The applications of palladium NNC-pincer complex as a catalyst in the allylic arylation of allyl acetates with sodium tetraarylboronates [79] and in the Heck reaction of aryl halides with activated alkenes [80] gained tremendous importance in recent years. Keeping its efficiency under consideration, Ichii et al. synthesized corresponding biaryls via palladium NNC-pincer complex catalyzed Hiyama coupling reactions of aryl bromides with aryl(trialkoxy)silanes. Bromobenzenes having electron-donating and electron-withdrawing substituents resulted in a moderate to excellent yield range (39–99%) of biaryl products. The substituted aryl bromide 11 was allowed to couple with phenyl trimethoxysilane 8 using 5 mol ppm of palladium complex 37. KF was selected as a suitable base for the corresponding reaction. The maximum yield (99%) of biaryl derivative 38 was observed using propylene glycol as an effective solvent (Scheme 11) [81].
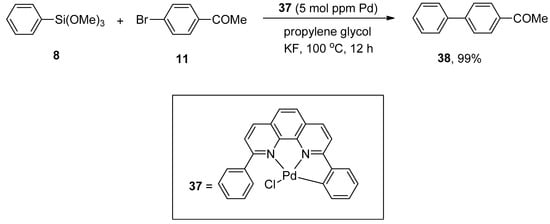
Scheme 11.
Aryl bromide 11 and phenyl trimethoxysilane 8 coupling reaction using palladium NNC-pincer complex as catalyst.
Amide is the versatile and valuable functionality widely utilized as a building block in biologically important compounds such as proteins and enzymes due to the sturdy nature of the amide C-N bond [82,83]. Highlighting the significance of the amide C-N bond, Idris and Lee devised a route for the Pd-catalyzed transformation of amides to corresponding aryl ketones. A number of different substituted N-acylglutarimides were reacted with phenyl triethoxysilanes to give targeted products in (0–98%) yield. The highest yield (98%) was observed by coupling of N-4-fluorobenzoylglutarimide 39 and phenyltriethoxysilane 16 using palladium acetate (2 mol%), PCy3 (4 mol%) in 1,4-dioxane/H2O, Et3N.3HF and LiOAc at 90 °C for 6 h (Scheme 12). N-Acylglutarimide bearing sterically bulky tert-butyl group provided no desired coupling product. The coupling of substituted N-acylglutarimides with a broad range of arylsiloxanes afforded corresponding coupled products up to 93% yield [84].
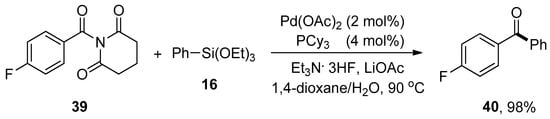
Scheme 12.
Synthesis of ketone 40 from N-4-fluorobenzoylglutarimide 39.
The trapping of σ-alkylpalladium intermediate using arylsilanes under palladium-catalyzed Domino Heck/Hiyama coupling was disclosed by Wu and coworkers. This approach demonstrated the broad substrate scope and compatibility with various functional groups. Different aryl-tethered activated or unactivated alkenes were treated with substituted arylsilanes by means of palladium acetate catalyst in MeCN at 80 °C under argon atmospheric conditions. Consequently, the corresponding products were obtained in 62–88% and 53–81% yields, respectively. Excellent results were observed in the case of coupling of N-(2-iodo-4-methoxyphenyl)-N-methylmethacrylamide 41 and methoxy substituted phenyl triethoxysilane 42 using an effective ligand PPh3 (10 mol%) and Bu4NF giving 88% yield to furnish the respective product 43 (Scheme 13). This methodology was employed to yield the ezetimibe, a cholesterol absorption inhibitor, analogs using N-(2-iodophenyl)-N-methylmethacrylamide and ezetimibe-derived arylsilane [85].
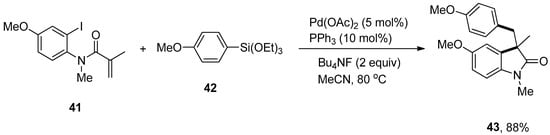
Scheme 13.
Synthesis of 43 by coupling of N-(2-iodo-4-methoxyphenyl)-N-methylmethacrylamide 41 and arylsilane 42.
The azaindoline framework is a nitrogen-bearing heterocycle that exists in several complex compounds and exhibits numerous biological and pharmaceutical attributes [86]. Keeping these characteristics in mind, Ye et al. disclosed the formation of azaindoline derivatives, which was carried out by the Domino Heck cyclization/Hiyama coupling protocol. A number of functionalized azaindoline derivatives were achieved in 46–85% yields. A series of ligands (PPh3, P(2-furyl)3, XPhos, SPhos, P(4-MeOC6H4)3 were screened, and the electron-rich P(4-MeOC6H4)3 was declared as a suitable ligand for the corresponding reaction. The reaction of Bn-protected aminopyridine 44 and 4-methoxy substituted phenyltriethoxysilane 42 was progressed in 1,4-dioxane using 5 mol% Pd(OAc)2 catalyst and 2.0 equivalents of Bu4NF. The targeted coupling product 45 was furnished in 85% yield by maintaining the temperature at 80 °C (Scheme 14). Excellent results (46–85%) were obtained in the case of electron-donating (phenyl, OMe, CH3) and -withdrawing (F, CF3, Cl) substituents. Moreover, heteroarylsilanes, including 2-thienylsilane and 3-thienylsilane, provided access to targeted products in 77% and 82% yields, respectively [87].
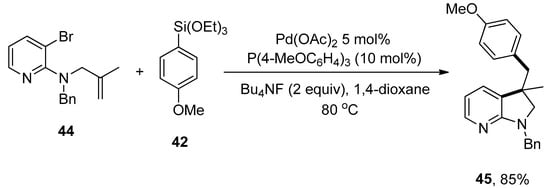
Scheme 14.
Pd(OAc)2 catalyzed formation of (hetero)aryl-functionalized azaindoline-derived compounds.
4. Palladium Chloride as Catalyst
Clark, in 2005, reported the Hiyama cross-coupling under microwave irradiation for the first time. The microwave-assisted reaction of 3-bromotoluene 46 was accomplished with phenyl trimethoxysilane 8 in the presence of 1.25% [Pd(allyl)Cl]2, ligand 47/N-mepip solution and tetra n-butylammonium fluoride (TBAF) promoter (1 M soln. in THF) to achieve the targeted coupling product 48 in >95% yield with >99% conversion (Scheme 15). A fascinating application of Hiyama coupling involves the synthesis of arylalkenes. In this regard, para-chloroacetophenone 49 underwent cross-coupling with vinyltrimethoxysiloxane 50 utilizing catalyst derived from 1.25% [Pd(allyl)Cl2] and ligand 47/N-mepip solution using TBAF in THF at 110 °C under microwave irradiation to afford colorless styrene derivative 51 in 95% yield (Scheme 16) [88].
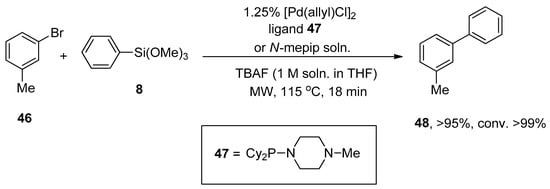
Scheme 15.
Synthesis of 3-methyl-1,1′-biphenyl 48 under microwave irradiation.
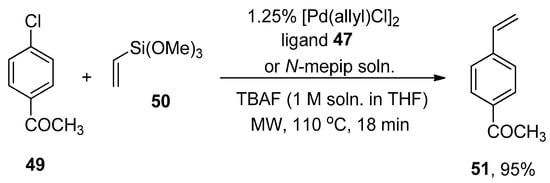
Scheme 16.
[Pd(allyl)Cl2] catalyzed synthesis of styrene derivative 51.
Organosilanes have attracted the attention of research groups due to their assorted benefits (such as low cost, ease of availability, nontoxic byproducts, and stability) as compared to the other organometallic precursors (organoboron, organostannane, organozinc, organotin). Unsuccessful attempts to couple 2-trimethylsilylpyridine with 4-iodoanisole diverted the attention of researchers towards sustainable liquids, i.e., chloropyridyltrimethylsilanes for Hiyama cross-coupling reaction. Chloropyridyltrimethylsilanes could be afforded either by halogen or hydrogen lithium exchange of chloropyridines followed by the reaction with chlorotrimethylsilane. Pierrat et al., in 2005, disclosed the Hiyama cross-coupling of chloro-substituted pyridyltrimethylsilanes with aryl halides. 1-Fluoro-4-iodobenzene 52 was allowed to react with chloro substituted pyridyltrimethylsilanes 53 using 5% PdCl2(PPh3)2, 10% PPh3, and copper iodide (1 equiv) to carry out the coupling in DMF solvent. The reaction was conducted using two equivalents of tetra-n-butylammonium fluoride (TBAF in THF) as a suitable base at room temperature for 12 h, affording the targeted biaryl product 54 in 95% yield (Scheme 17) [89].
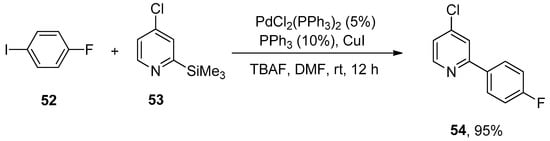
Scheme 17.
Reaction of 1-fluoro-4-iodobenzene 52 with chloro substituted pyridyltrimethylsilane 53 using PdCl2(PPh3)2.
The phosphine-free palladium-catalyzed synthesis of para-substituted biaryl scaffold using air-insensitive PdCl2/hydrazone ligand was reported by Mino and coworkers. Several functionalized substituted biaryls were obtained in a 50–90% yield range via the reaction of aryl bromides with different siloxanes. An excellent result (90%) was attained by the reaction of phenyl bromide 55 with para-substituted phenyl triethoxysilane 56 in the presence of air-stable phosphine-free PdCl2/hydrazone ligand 57 using TBAF in THF under argon atmosphere. After screening a variety of solvents (DMSO, dioxane, t-BuOH, toluene, and DMF), toluene was selected as a suitable solvent for the corresponding reaction (Scheme 18) [90].
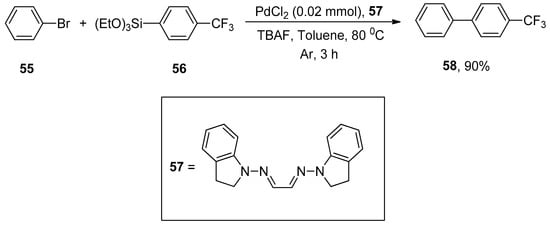
Scheme 18.
PdCl2 catalyzed Hiyama cross-coupling using phosphine-free hydrazone ligand.
Multicomponent assembly protocol fascinates synthetic chemists due to its efficacy in the synthesis of molecular complexity and is acceptable for diversity-oriented synthesis [91,92]. An efficient approach for the synthesis of multisubstituted unsymmetrical biaryls was reported by Akai and coworkers in 2008. The reaction of substituted 8-TBDMS-1-naphthols with a wide range of aryl iodides afforded the biaryl derivatives moderate to excellent yields (46–81%). The fluoride-free Hiyama coupling of 59 was accomplished with 4-cyanoiodobenzene 60 giving a multisubstituted asymmetrical biaryl through the formation of pentacoordinate silicate 61 as an intermediate. [(allyl)PdCl]2, AsPh3, Cs2CO3, and dimethoxyethane (DME) were selected as an appropriate catalyst, ligand, base, and solvent, respectively, to afford the maximum yield (81%) of targeted asymmetrical biaryl derivative 62 under argon atmosphere (Scheme 19) [93].
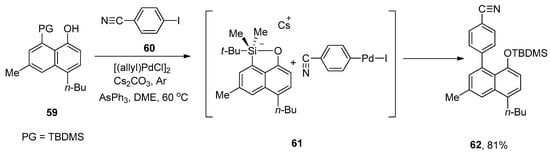
Scheme 19.
Synthesis of asymmetrical biaryl derivative 62 by Hiyama cross-coupling reaction.
Pincer-type palladium complexes are intriguing due to their promising reactivity and stability. Inés et al. synthesized the non-symmetric PCN pincer-type palladium complex by the reduction of 1-(3-nitrophenyl)pyrazole to amine, which upon treatment with ClPPh2 provided an unreliable and air-sensitive ligand. The ligand further reacted with Pd(COD)Cl2 to yield a targeted non-symmetric pincer-type complex that efficiently catalyzed the Hiyama cross-coupling reaction in eco-friendly reaction media. Excellent results were achieved using two methods in the presence of an efficient catalyst. The first pathway was concerned with the reaction of phenyl trimethoxysilane 8 with 11 catalyzed by 2 mol% 63 using NaOH in H2O at 140 °C for 3 h to obtain the corresponding biaryl derivative 38 in 82% yield. The alternative developed method involved the coupling of phenyl trimethoxysilane 8 with 11 in the presence of 4 mol% of catalyst 63 using n-Bu4NF and o-xylene at 80 °C for 4 h to afford the required biaryl product 38 in 61% yield (Scheme 20) [94].
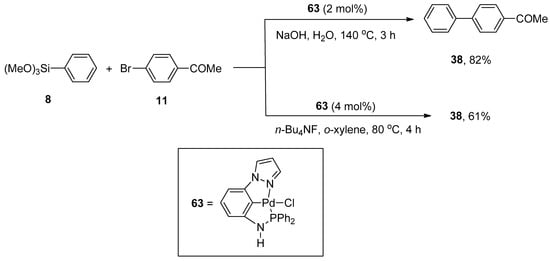
Scheme 20.
Non-symmetric PCN pincer-type palladium catalyzed coupling of phenyl trimethoxysilane 8 with 11.
Chen et al. synthesized the sustainable cationic bipyridyl ligand by reacting 4,4′-bis(bromomethyl)-2,2′-bipyridine with 50% aqueous solution of trimethylamine in CH2Cl2 that catalyzed the Hiyama cross-coupling reaction efficiently. 4-Bromoanisole 64 was reacted with phenyl triethoxysiloxane 16 in H2O using Pd(NH3)2Cl2/65 (0.1 mol%) as a catalyst. NaOH was screened as a suitable base to attain the desired biaryl derivative 66 in 99% yield (Scheme 21). The Hiyama cross-coupling of several aryl bromides with a variety of triethoxy(aryl)silanes gave substituted biaryl derivatives in (35–99%) yield range [95].
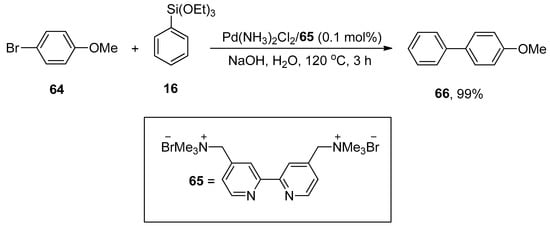
Scheme 21.
Use of Pd(NH3)2Cl2 catalyst in Hiyama cross-coupling reaction with bipyridyl ligand 65.
Aryl imidazol-1-ylsulfonates are the electron-deficient substrates that promote the palladium-catalyzed Hiyama and Sonogashira cross-coupling under copper-free conditions, as reported by Williams and coworkers. Several functionalized biaryl derivatives were obtained by the reaction of aryl imidazylates with (2-hydroxymethylphenyl) dimethyl silane (HOMSi) reagent in 72–99% yields. The 2-naphthyl imidazol-1-ylsulfonate 67 was coupled with 4-methoxyphenyl HOMSi reagent 68 in the presence of Pd(dppf)Cl2 (0.05 equiv) in dry DMSO with K2CO3 at 65 °C giving the cross-coupling product 69 in 99% yield (Scheme 22) [96].
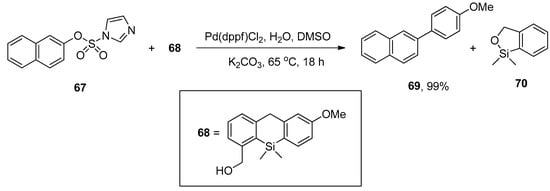
Scheme 22.
Hiyama cross-coupling of 2-naphthyl imidazole-1-ylsulfonate 67 and 4-methoxyphenyl HOMSi reagent 68 using K2CO3 as base.
A research group by Hughes also reported a facile and convenient methodology for the palladium-catalyzed synthesis of biphenyls in 2011 using the HOMSi® reagent. In this methodology, the aryl bromides and iodides were proved to be more efficient substrates to couple with the HOMSi® reagent in DMF solvent. Excellent (>98%) conversion was achieved by the reaction of HOMSi® reagent 71 with 72 using PdCl2 as the catalyst, 73 as ligand using CuI, and K2CO3 as the base at 80 °C for 15 h. DMF was selected as the optimal solvent by observing the results with THF, MeOH, 1,4-dioxane, and DMSO (Scheme 23). Compatibility with functional groups, reactions under fluoride-free conditions, and recycling of organosilicon byproducts are the salient features of the HOMSi® reagent [97].
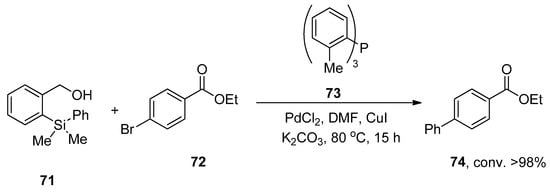
Scheme 23.
Synthesis of biphenyl ester 74 via Hiyama cross-coupling.
Stilbenes and their hydroxylated derivatives are of tremendous interest as they exhibit diversified biological activities such as fungistatic, antibacterial and anticancer [98,99,100,101]. Due to their extended conjugation system, stilbenes have been utilized to assemble electronic and optoelectrical devices, i.e., solar cells, LEDs, and dye lasers [102,103]. Resveratrol and combretastatin A-4 are a few bioactive derivatives of stilbene that can be obtained naturally from the extraction of plants. An effective catalytic system was established for the stereoselective formation of substituted E-stilbenes using Heck cross-coupling reaction while studying the catalytic activity of Pd complexes with H-spirophosphane ligands. Hiyama coupling reaction emerged to be a more effective route to synthesize substituted E-stilbenes. Keeping these considerations in mind, Skarżyńska and coworkers, in 2011, proposed an efficient synthesis of E-stilbenes via Hiyama coupling reactions of substituted styrylsilanes with aryl halides catalyzed by an efficient and stereoselective precatalyst. The styrylsilanes possessing either electron-deficient groups or para-substituted electron-rich groups resulted in an excellent yield range (87–99%). A 100% conversion of iodobenzene and 99% yield of bromo substituted E-stilbene 77 was obtained by the addition of 75 to iodobenzene 76 using palladium complex with H-spirophosphorane ligand [PdCl2P(OCH2Cme2NH)OCH2Cme2NH2] as precatalyst in the presence of [nBu4N]F additive. The reaction progressed in tetrahydrofuran solvent at 60 °C for 12 h (Scheme 24) [104].
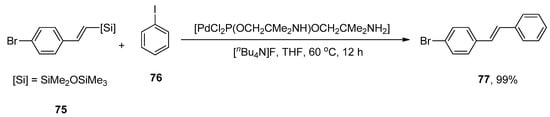
Scheme 24.
Synthesis of bromo substituted E-stilbene 77 using palladium complex with H-spirophosphorane ligand.
Cheng et al. disclosed a PdCl2-catalyzed Hiyama-type coupling of arenesulfinates and organosilanes in mild reaction conditions. A wide range of substituted biaryls was obtained in 73–94% yield by coupling various arenesulfinates with aryl trialkoxysilanes. The coupling of p-methylbenzenesulfinate 78 with phenyl triethoxysilane 16 was carried out in the presence of PdCl2 (5 mol%) and TBAF (additive) in the THF solvent. The reaction progressed well under aerobic conditions at 70 °C giving the corresponding biaryl product 79 in 94% yield (Scheme 25) [105].
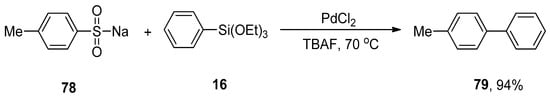
Scheme 25.
P-Methylbenzenesulfinate 78 and phenyl triethoxysilane 16 coupling reaction using PdCl2.
Chang et al. reported a facile protocol for synthesizing the biaryl ketones via carbonylative Hiyama coupling reaction. In this regard, the phenyl triethoxysilane 16 was allowed to react with 4-bromophenyl iodide 80 using PdCl2(MeCN)2 catalyst and cesium fluoride (CsF) promoter to afford the corresponding ketone 81 in 94% yield (Scheme 26). The reaction temperature was maintained at 80 °C. Different solvents (DMSO, toluene, DMF, CH3CN, and 1,4-dioxane) were screened, and NMP (N-methyl-2-pyrrolidone) was selected as the optimal solvent for the corresponding reaction. Aryl halides having electron-withdrawing (-Cl, -F, -NO2, CN) and electron-donating substituent (-CH3) gave moderate to excellent yields (68–94%) [106].
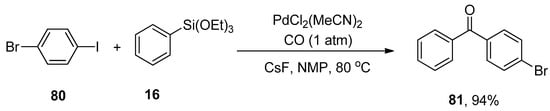
Scheme 26.
Synthesis of ketones by using carbonylative Hiyama cross-coupling.
Diarylmethanes have attained huge interest as they exist in biologically active natural compounds and drugs [107,108]. For example, avrainvilleol, a marine natural product, possesses a diarylmethane motif and exhibits antibacterial [40] and antioxidant [41] activities. Several drugs containing diarylmethanes demonstrate a wide range of biological activities such as segontin (used to cure coronary heart disease) [35], bifemelane, tolpropamine, and piritrexim act as an antidepressant, antiallergic and anticancer agents, respectively [36,37,38,39]. Functionalized diarylmethane derivatives were achieved by coupling a variety of benzylic ammonium salts with phenyl trimethoxysilane, as elaborated by Zhao and coworkers in 2019. Methoxy substituted benzyltrimethylammonium salt 82 was treated with phenyl trimethoxysilane 8 to acquire the desired diarylmethane derivative 83 in 92% yield. The reaction proceeded well at 120 °C in the presence of 5 mol% PdCl2(CH3CN)2 catalyst, 20 mol% PPh2Cy ligands, and TBAF in EtOH (Scheme 27). In the case of aryl trimethoxysilanes, the substrate containing electron-donating groups afforded corresponding diarylmethane derivatives in (57–97%) yield range. Moreover, the substrate-bearing heterocycles, including thiophene and furan, gave coupling products a 94% yield [109].
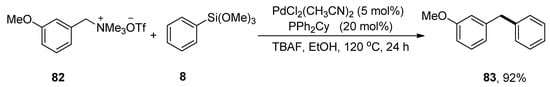
Scheme 27.
Synthesis of diarylmethane derivative 83 from substituted benzyltrimethylammonium salt 82.
Synthesis of vinylsilanes is a very promising task in research due to their spacious applications in organic syntheses, such as the Hiyama coupling reaction [110]. Wisthoff et al. designed a facile protocol to accomplish the synthesis of cis- or trans-tetrasubstituted vinylsilanes via carbosilylation of three components, including symmetrical alkynes, alkyl zinc iodides, and iodosilanes using a palladium catalyst. The authors also reported conditions for tetrasubstituted vinylsilanes via the Hiyama coupling reaction that facilitated the synthesis of tetrasubstituted alkenes. The cis- or trans-tetrasubstituted vinylsilanes were synthesized in a 32–97% yield range by the addition of iodosilane to the solution of alkyl zinc iodide and alkyne using (Ph3P)2PdCl2 (2 mol%) catalyst in dioxane. Triethylamine (Et3N) was reported as a suitable base for this reaction. Tetrasubstituted vinylsilanes underwent Hiyama cross-coupling reactions to achieve the stereodefined tetrasubstituted alkenes. The reaction of 84 with 1-bromo-3-methybenzene 46 was conducted in the presence of KOSiMe3, and 18-crown-6 in THF at 65 °C, followed by the treatment with 2.5 mol% [(allyl)PdCl2] catalyst and 5 mol% SPhos ligands at 65 °C afforded the desired geometrically defined tetrasubstituted alkene 85 in 62% yield (Scheme 28). The effect of ligands on the selectivity of alkyl-substituted tetrasubstituted vinylsilanes was also observed. It was found that the use of DrewPhos ligand 86 (Figure 2) gave results in (54–92%) yield of alkyl-substituted tetrasubstituted vinylsilanes with excellent syn-selectivity while using JessePhos 87 (Figure 2) as ligand resulted in 61–92% yield range of alkyl-substituted tetrasubstituted vinylsilanes with excellent anti-selectivity [111].
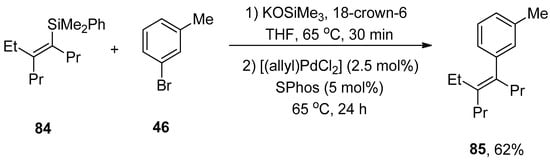
Scheme 28.
Synthesis of tetrasubstituted alkene 85 from tetrasubstituted vinylsilane 84.
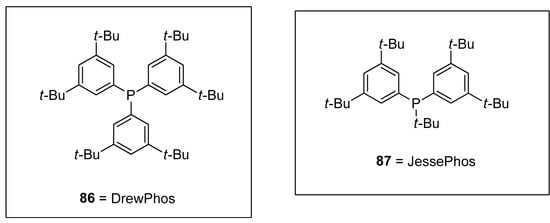
Figure 2.
Structures of DrewPhos 86 and JessePhos 87 ligands.
γ-Valerolactone-based tetrabutylphosphonium 4-ethoxyvalerate are effective and versatile biomass-derived ionic liquids and have been extensively utilized in synthesis protocols due to their tunability. In order to elaborate on the effect of ionic liquid, Orha et al. outlined the efficient synthesis of substituted biaryl derivatives by the addition of iodoaromatic substrates to triethoxyphenylsilane. In this methodology, reactions of several aryl iodides were performed with triethoxyphenylsilane to give numerous substituted biaryl structures in good yields (45–72%). 1-Iodo-4-methylbenzene 88 was coupled with triethoxyphenylsilane 16 in tetrabutylphosphonium 4-ethoxyvalerate [TBA][4EtOV] afforded the desired biaryl derivative 79 in 72% yield. The reaction was carried out at 130 °C using Pd(PPh3)2Cl2 as catalyst precursor and a fluoride source (TBAF) to activate the transmetalation reagent (Scheme 29) [112].
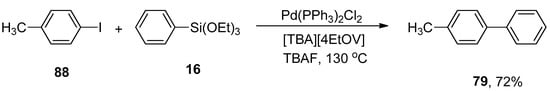
Scheme 29.
Pd(PPh3)2Cl2 catalyzed coupling reaction using tetrabutylphosphonium 4-ethoxyvalerate as ionic liquid.
5. Pd/C as Catalyst
A heterogeneous catalytic system proved to be an effective system over a homogeneous catalytic system in the organic synthetic field regarding their stability, recoverability, and ease of handling [113]. Considering the efficacy of heterogeneous catalysts, Yanase et al., in 2011, reported the synthesis of biaryl derivatives via water-mediated Hiyama cross-coupling reaction using Pd/C, a heterogeneous catalyst [114] with electron-deficient phosphine ligand [(4-FC6H4)3P]. Several functionalized biaryl derivatives were attained in a 47–90% yield range by cross-coupling of aryl halides with aryltrialkoxysilanes. The reaction was progressed at 120 °C using 0.5 mol% of 10% Pd/C catalyst, 1 mol% of tris(4-fluorophenyl)phosphine as ligand, TBAF.3H2O as an activator, and 4.8% aqueous toluene. A 90% yield of targeted biaryl product 18 was obtained by coupling 3-methoxyphenylbromide 89 with phenyl triethoxysilane 16 (Scheme 30) [115]. Later on, in a 2013 research group by Monguchi, they developed a facile and efficient methodology to synthesize the biaryl derivatives in (47–90%) yield range using (4-C6H4)3P (1 mol%) ligand [116].
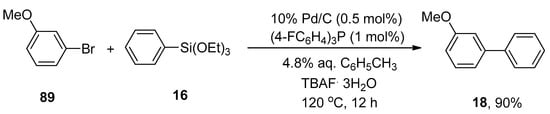
Scheme 30.
Hiyama cross-coupling in the presence of tris(4-fluorophenyl)phosphine ligand.
The very first ligand-free Pd/C catalyzed Hiyama cross-coupling reaction for the construction of a variety of biphenyl derivatives was developed by Sajiki and coworkers in 2012. A series of solvents (DMF, THF, CH3CN, EtOH, toluene) and fluoride sources (TBAF, LiF, CsF, KF) were screened, and among them, toluene and TBAF·3H2O were selected as a suitable solvent, and fluoride source for corresponding Pd/C catalyzed Hiyama cross-coupling reaction. Aryl bromide 11 was reacted with methoxy substituted phenyl triethoxysilane 42 to afford a maximum (90%) yield of desired biphenyl derivative 90. The reaction was refluxed for 24 h in toluene containing 0.5 mol% of 5% Pd/C catalyst, TBAF·3H2O as an activator, and acetic acid (Scheme 31) [117].
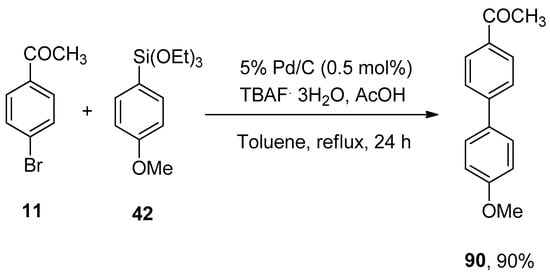
Scheme 31.
Synthesis of biphenyl derivative 90 by ligand-free Pd/C catalyzed Hiyama coupling reaction.
γ-Valerolactone (GVL), a safe and sustainable bio-based chemical obtained from lignocellulosic biomass [118], was found to be an efficient media for Hiyama coupling reactions. The use of highly effective and non-expensive heterogeneous catalyst Pd/C with γ-valerolactone competently promotes the eco-friendly Hiyama cross-coupling reaction under practical and mild conditions without using any ligand or additive as reported by Ismalaj et al. A number of aryl halides were treated with phenyl triethoxysilanes using 1 M GVL as an efficient biomass-based solvent for Hiyama coupling reaction. Tetrabutylammonium fluoride (TBAF) was found to be the best activator of organosilanes, while CsF and KF were not such efficient activators. An excellent result (94%) of desired biaryl product 92 was obtained by carrying out the reaction of bromobenzene 55 with phenyl triethoxysilane 16 using Pd/C (0.5 mol%) as catalyst in 1M GVL 91 solvent and TBAF for 24 h at 130 °C (Scheme 32) [119].
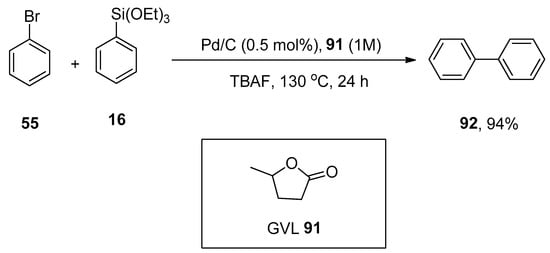
Scheme 32.
Reaction of bromobenzene 55 with phenyl triethoxysilane 16 in the presence of γ-valerolactone (GVL) solvent.
6. Pd/Fe3O4 as Catalyst
An efficient methodology for the synthesis of a diverse range of biaryl derivatives via Hiyama cross-coupling reaction of aryl bromides with aryl siloxanes using a highly efficient, easily recoverable, and eco-friendly Pd/Fe3O4 catalytic system was proposed by Sreedhar and coworkers. Fe3O4 supported palladium catalyst shows superparamagnetic behavior, and its catalytic activity remains unchanged after recycling five times. The mechanistic studies showed that Pd/Fe3O4 catalyzed Hiyama cross-coupling reaction proceeds through oxidative addition and transmetalation followed by reductive elimination [120]. The effect of the base is more pronounced in achieving the maximum yield. Sodium hydroxide (NaOH) was investigated as an appropriate base for this reaction. In this methodology, the substituted aryl bromide 93 was treated with aryl siloxanes 94 by using magnetically recoverable Pd/Fe3O4 catalyst and NaOH in H2O at 90 °C to obtain the excellent yield (92%) of desired coupling product 95 (Scheme 33) [121].
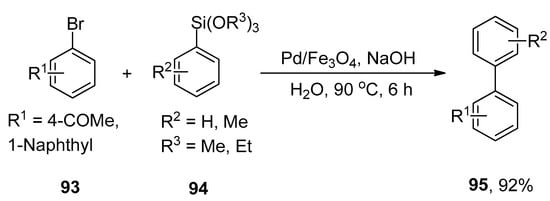
Scheme 33.
Pd/Fe3O4 catalyzed Hiyama cross-coupling to attain biaryl compound 95.
Pd-Fe3O4 Heterodimeric nanocrystals have been found to be effective and recyclable catalysts to achieve a successful Hiyama cross-coupling under ligand-free conditions. Keeping the effectiveness of catalyst in view, Lee et al. proposed a methodology for the synthesis of diversified biaryl derivatives. The protocol involved the treatment of 96 with phenyl trimethoxysilane 8 under ligand-free conditions. The reaction was proceeded in DMA (dimethylacetamide) using 1 mol% of Pd-Fe3O4 heterodimeric nanocrystals as a catalyst, KF as a base, and tetra-n-butylammonium iodide (TBAI) as an additive at high temperature of 150 °C. Consequently, the desired biphenyl derivative 97 was afforded in 94% yield (Scheme 34). Electron-donating and -withdrawing groups (-Me, -OMe, -F, -CF3, -NO2) bearing aryl halides provided the corresponding biaryl derivatives in an excellent yield range (70–94%) [122].
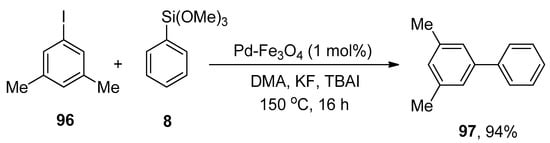
Scheme 34.
Use of Pd-Fe3O4 catalyst in Hiyama coupling reaction under ligand-free conditions.
7. N-Heterocyclic Carbenes (NHCs) Palladium Complexes
NHCs are nucleophilic in nature as they exhibit σ-electron donating attributes. The application of N-heterocyclic carbene (NHC) ligands in transition metal-catalyzed cross-coupling acquired notable interest recently [123,124,125,126]. Peñafiel et al. synthesized [(NHC)2PdCl2] complex through direct metalation of 0.2% hydroxy-functionalized imidazolium salt and 0.1% palladium acetate to efficiently catalyze the Hiyama cross-coupling of aryl chlorides and bromides with arylsiloxanes under microwave irradiation and fluoride free conditions. The fluoride-free Hiyama reaction of 4-bromoanisole 64 was carried out with phenyl trimethoxysilane 8 using [(NHC)2PdCl2] complex 98 to catalyze the reaction under microwave irradiation (80 W, 120 °C) for 1 h. NaOH was screened to be a suitable base for the corresponding reaction to afford the targeted derivative 66 in 95% yield (Scheme 35). The coupling of aryl and heteroaryl bromides with siloxanes resulted in biaryl products in a 5–88% yield range. The required products were obtained in lower yields with aryl chlorides than with aryl bromides [127]. Another research group of Pastor and coworkers disclosed the efficient synthesis of biaryl derivatives using 0.2 or 0.5% of imidazolium salt in 2013 [128].
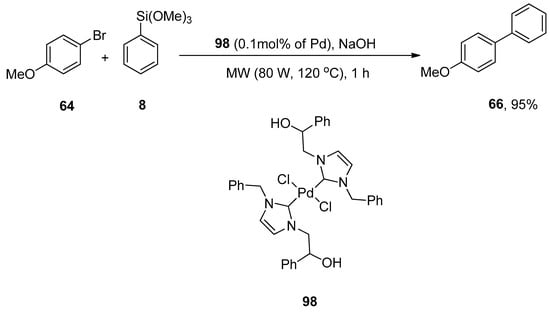
Scheme 35.
Fluoride-free Hiyama cross-coupling of 4-bromoanisole 64 under microwave irradiation.
The synthesis of four linear dinuclear N-heterocyclic carbene palladium complexes was reported by Yang and Wang. The structures were characterized by NMR, FT-IR, and elemental analysis. The synthesis of NHC-palladium complexes was achieved by a one-pot reaction of imidazolium salts, PdCl2, and bidentate N-heterocycles in the presence of potassium carbonate (K2CO3) with the formula [PdCl2(NHC)]2(μ-L) (L = DABCO, pyrazine). The catalytic effect of N-heterocyclic carbene palladium complexes was investigated in the Hiyama coupling reaction of aryl trialkoxysilanes with aryl chlorides. Among four dinuclear N-heterocyclic carbenes, 100 expressed excellent catalytic activity in the corresponding Hiyama coupling reaction. Both electron-donating and electron-withdrawing groups substituted aryl chlorides afforded biphenyl products in (54–92%) yield range. The reaction of 99 with phenyl trimethoxysilane 8 was conducted at 120 °C for 5 h using 0.5 mol% of NHC-Pd 100, TBAF in toluene to achieve a 92% yield of corresponding product 9 (Scheme 36) [129].
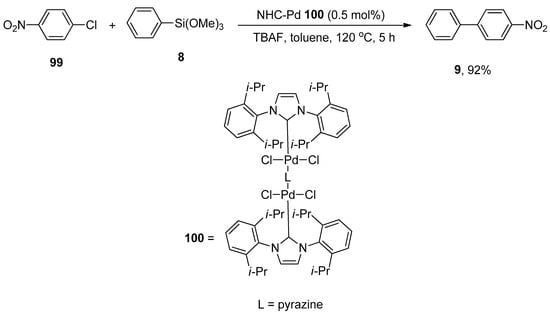
Scheme 36.
Synthesis of biaryl derivative 9 using dinuclear NHC palladium complexes.
The application of homoleptic chelating N-heterocyclic carbene palladium complexes immobilized within the pores of SBA-15/IL (NHC-Pd@SBA-15/IL) as heterogeneous catalyst in the Hiyama coupling reaction was reported by Rostamnia and coworkers. The desired biaryl derivatives were achieved in an excellent yield range (82–95%). The catalytic activity of the catalyst was analyzed by treating phenyl trimethoxysilane 8 with phenyl iodide 76 using 0.8 mol% (NHC-Pd@SBA-15/IL) 101. The reaction proceeded in H2O/dioxane (1:2) at 80 °C using Cs2CO3 as a suitable base and TBAF that increased the catalytic activity of (NHC-Pd@SBA-15/IL). As a result, 92 was obtained in an excellent (95%) yield (Scheme 37). Chemoselectivity of (NHC-Pd@SBA-15/IL) for the Hiyama coupling reaction was analyzed by treating p-bromobenzaldehyde with phenyl trimethoxysilane under the optimized reaction conditions giving the corresponding biaryl product. Reusability of catalyst for five successful runs was observed [130].
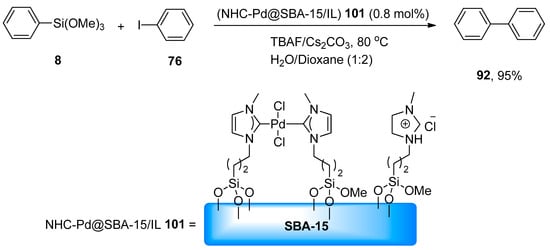
Scheme 37.
NHC-Pd@SBA-15/IL catalyzed synthesis of biphenyl 92.
Yang synthesized the tetrazole ligand stabilized NHC-Pd complexes by reacting dimeric compounds [Pd(μ-Cl)(Cl)(NHC)]2 and tetrazole ligands that catalyzed the Hiyama coupling reaction very well. The catalytic activity of mono and dinuclear Pd(II) complexes were analyzed. Among them, the mononuclear palladium complex [PdCl2(SIPr)(1-phenyl-1H-tetrazole)] proved to be the most effective one giving a maximum yield (88%) of biaryl product 9. For this purpose, compound 99 was refluxed with phenyl trimethoxysilane 8 for 8 h using 0.5 mol% of [PdCl2(SIPr)(1-phenyl-1H-tetrazole)] 102 as catalyst and TBAF in toluene (Scheme 38) [131].
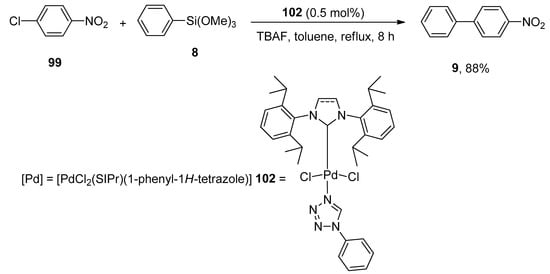
Scheme 38.
Use of [PdCl2(SIPr)(1-phenyl-1H-tetrazole)] palladium complex as catalyst in Hiyama coupling reaction.
A very interesting application of palladium PEPPSI (Pyridine Enhanced Precatalyst Preparation Stabilization and Initiation) complexes with non-bulky NHC ligands as a catalyst precursor in cross-coupling reactions was disclosed by Osińska et al. for the synthesizing the non-symmetric biaryl scaffolds. The application of PEPPSI complexes in Suzuki–Miyaura was investigated, in which their catalytic activity was clearly evidenced [132,133,134,135,136,137], while the use of PEPPSI complexes in the Hiyama coupling reaction is rare [138,139,140]. The PEPPSI complexes were synthesized by reacting a palladium dimer [Pd(bmim-y)X2]2 and N-ligand. Hiyama coupling worked efficiently in ethylene glycol, and 97% conversion was achieved in the case of coupling of 2-bromotoluene with phenyl trimethoxysilane using Pd(IPr)Cl2(Cl-py) 103 (Figure 3) as a catalyst in ethylene glycol solvent. The reaction did not work efficiently in water. Pd(bmim)Br2(CN-py) complex 104 catalyzed Hiyama coupling of substituted bromobenzenes and chlorobenzenes with phenyl trimethoxysilane afforded cross-coupled products in (50–93%) yields using NaOH base and ethylene glycol as solvent at 110 °C for 24 h with the exception of 1-chloro-3-nitrobenzene and 4-chloro-2-nitrobenzene giving no results [141].
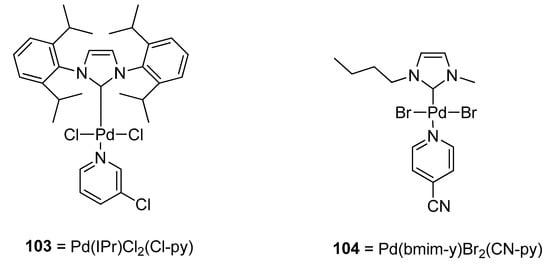
Figure 3.
Pd(IPr)Cl2(Cl-py) 103 and Pd(bmim)Br2(CN-py) 104 complexes (catalyst).
8. Nanoparticles as Catalyst
The substantial application of transition metal nanoparticles in catalysis has remained an area of interest for research groups due to their harmless characteristic features, easy preparation methods, and large surface area [142,143]. Srimani et al. synthesized the palladium nanoparticles by the dropwise addition of metal acylate salt (CO)5W=C(CH3)O(−)NEt4(+) solution to the PEG-6000 containing an aqueous solution of K2PdCl4 to catalyze the Hiyama coupling reactions [144]. It was noticed that the increased amount of PEG-6000 stabilizer decreases the size of nanoparticles. The substituted aryl bromides 105 were coupled with phenyl triethoxysilane 8 using palladium nanoparticles as catalyst 106 and an appropriate base NaOH in the air at 90 °C to achieve the desired biaryls 107 in 98% yield (Scheme 39). H2O was utilized as an effective solvent in place of THF or DME [145].
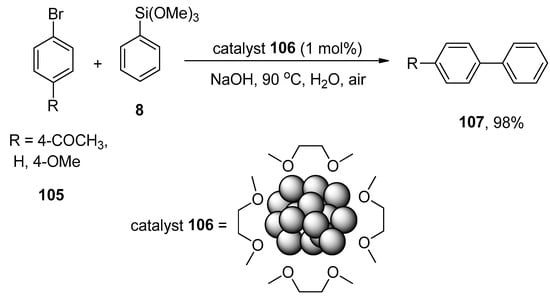
Scheme 39.
Hiyama coupling reaction of substituted aryl bromide 105 to attain biaryl derivative 107.
A simple, convenient, and rapid one-pot Hiyama coupling of aryl bromides or iodides with arylsilanes under fluoride-free conditions was reported by the research group of Ranu and coworkers. The corresponding reaction was catalyzed by using palladium nanoparticles that were generated in situ from sodium dodecyl sulfate (Na2PdCl4/SDS) in H2O. SDS stabilizes the formation of palladium nanoparticles. The reaction of phenyl bromide 55 with phenyl trimethoxysilane 8 was carried out using palladium nanoparticles that efficiently catalyzed the reaction. Excellent yield (96%) of desired biphenyl product 92 was attained using water as solvent. A series of bases (KOH, Na2CO3, NaOAc, and NaHCO3) was screened, and NaOH was investigated as a suitable base for this reaction (Scheme 40). Environmentally friendly solvent, no utilization of ligand, and good yields are the salient features of this synthetic protocol [146].
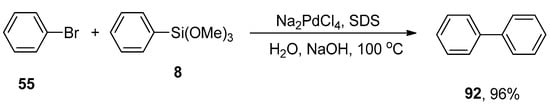
Scheme 40.
Palladium nanoparticles catalyzed Hiyama coupling reaction using NaOH as base.
Diarylmethanes have received appreciable attention from researchers due to their pharmacological activities [147,148,149,150,151]. They are regarded as distinct components of supramolecular structures, including catenanes, macrocycles, and rotaxanes [152,153,154,155]. Focusing on the significance of diarylmethanes, Sarkar and colleagues designed a successful protocol for the synthesis of diarylmethane derivatives using palladium nanoparticles in THF. Palladium nanoparticles were formed by the reaction of 4 mol% K2PdCl4 with PEG-600 at 70 °C. PEG-600 was used as a reducing and stabilizing agent for the preparation of nanoparticles [156]. Numerous diarylmethane derivatives were formed by Hiyama cross-coupling of benzyl halides with phenyl trialkoxysilanes in a 78–95% yield range. The 108 was coupled with phenyl trimethoxysilane 8 in the presence of 4 mol% K2PdCl4, PEG-600 using TBAF in tetrahydrofuran at 70 °C under argon environment to afford the desired coupling product 109 in 95% yield (Scheme 41). They applied the same methodology to synthesize diarylmethanes 111 in 95% yield using allyl halides 110 and phenyl trimethoxysilane 8 as reacting substrates (Scheme 42). The naturally occurring 2,4-bis(4-hydroxybenzyl)phenol 112 was also synthesized by the same research group through multiple steps reactions starting from anisole using the same methodology (Figure 4) [157].
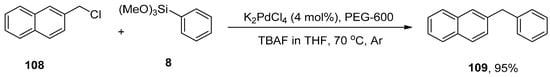
Scheme 41.
Synthesis of diarylmethane 109 using nano K2PdCl4 as catalyst.
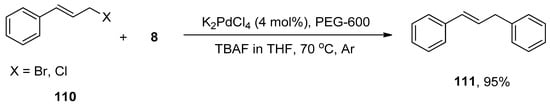
Scheme 42.
Hiyama cross-coupling of allyl halides 110 with 8 to attain diarylmethane derivative 111.
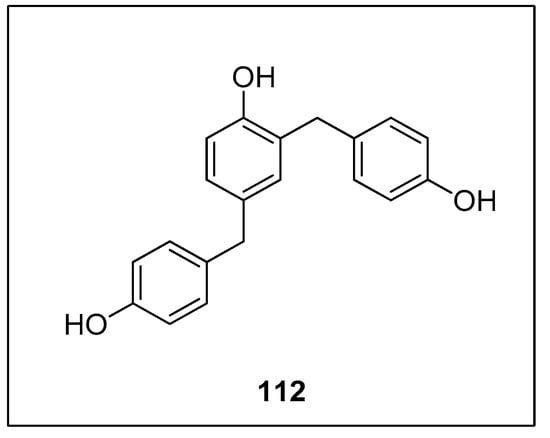
Figure 4.
Structure of 2,4-bis(4-hydroxybenzyl)-phenol 112.
Zhang et al. synthesized a silica-coated SiO2@Fe3O4-Pd catalyst that efficiently catalyzed the Hiyama coupling of 113 with phenyl trimethoxysilane 8. The catalytic efficacy of the supported catalyst remains even after 10 times of recycling. The reaction proceeded by using SiO2@Fe3O4 supported palladium catalyst (0.5 mol%) using TBAF as a suitable base in THF at 60 °C under an N2 atmosphere to obtain desired products 114 in 99% yield (Scheme 43). It was noticed that either electron-donating or electron-withdrawing groups containing aryl halides gave a good to excellent yield range (81–99%) [158].
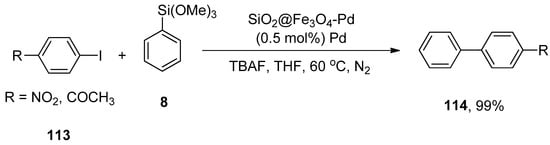
Scheme 43.
SiO2@Fe3O4-Pd catalyzed coupling of aryl iodides 113 and phenyl trimethoxysilane 8.
Premi and Jain, in 2013, carried out the synthesis of substituted biphenyl derivatives, aromatic heterocycles, and substituted styrene derivatives by phosphane-free Hiyama cross-coupling reaction of aryl and heterocyclic halides with aryl and vinyltrimethoxysilane using palladium nanoparticles in ionic liquids. Palladium nanoparticles were generated by adding the solution of palladium acetate in acetonitrile to 3-(3-cyanopropyl)-1-methyl-1H-imidazol-3-ium hexafluorophosphate {[CN-bmim]PF6}, an ionic liquid, that stabilizes synthesis of palladium nanoparticles. It was observed that the electron-donating and -withdrawing substituents on arylhalides resulted in an excellent yield range (76–98%). The reaction of iodobenzene 76 with phenyl trimethoxysilane 8 using 4 mol% Pd(OAc)2 in 115 and CH3CN and 1-butyl-3-methylimidazolium fluoride [bmim]F, an organosilane activator, at 70–120 °C for 8 h to afford the desired biphenyl derivative in 98% yield (Scheme 44). The substituted styrene derivatives were also synthesized in (75–98%) yields by the Hiyama cross-coupling of a broad range of aryl iodides with vinyltrimethoxysilane catalyzed by Pd-NPs at 60–70 °C for 15–30 min [159].
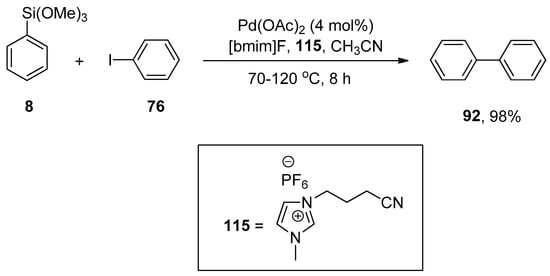
Scheme 44.
Pd-NP catalyzed cross-coupling of iodobenzene 76 and phenyl trimethoxysilane 8.
In Heck and copper-free Sonogashira reactions, palladium nanocatalysts synthesized by hydrogenation of Pd(dba)2 using tris-imidazolium iodide that stabilizes the synthesis of nanocatalysts are substantially used. The catalytic efficacy of the corresponding nanocatalyst regarding Hiyama coupling reaction under fluoride-free conditions was reported by Planellas et al. in 2014. The authors synthesized the nanoparticles by hydrogenation of Pd(dba)2 [160] using tris-imidazolium iodide [161] and adopted an impressive approach for the synthesis of substituted styrene derivatives through Hiyama coupling reaction of substituted aryl iodides 116 with triethoxy(vinyl)silane 10 using nanocatalyst 117 (0.25 mol% Pd catalyst loading). NaOH was used as a suitable base in a 1:1 mixture of MeOH/H2O. The reaction worked effectively at 100 °C to attain 118 in 97% yields (Scheme 45). The synthesis of unsymmetrically-substituted stilbene 120 was achieved in 82% yield through a one-pot Hiyama–Heck reaction of 1-iodo-4-nitrobenzene 7, triethoxy(vinyl)silane 10, and 1-iodo-4-methoxybenzene 119 catalyzed by 117 in the presence of NaOH in MeOH/H2O (1:1) (Scheme 46) [162].
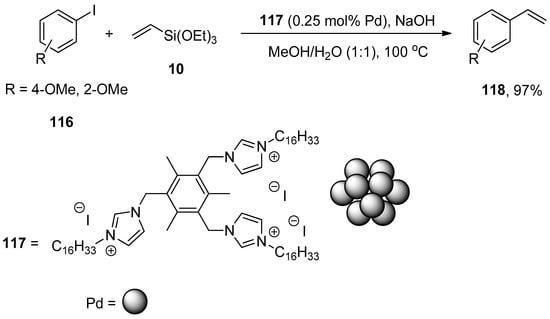
Scheme 45.
Synthesis of styrene derivatives 118 using palladium nanocatalyst 117.
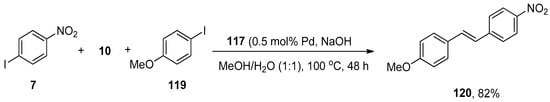
Scheme 46.
One-pot Hiyama–Heck coupling reaction to attain styrene derivative 120.
The catalysis of a facile, cost-effective, and ligand-free Hiyama cross-coupling by functionalized SBA-15 palladium nanoparticles was reported by Huang et al. The Pd catalysts (Pd@M-SBA-15 and Pd@P-SBA-15) gave (13–92%) yield range for the Hiyama coupling reaction of a variety of aryltriethoxysilanes with haloaryls. The catalytic efficacy of Pd@M-SBA-15 and Pd@P-SBA-15 is associated with the presence of TMS or TPS groups on mesopores. The reaction of 11 with phenyltriethoxysilane 16 was carried out by utilizing Pd@M-SBA-15 (0.5 mol%) catalyst, acetic acid, and TBAF.3H2O in toluene at 100 °C in the air for 24 h resultantly afforded the corresponding biphenyl derivative 38 in 92% yield (Scheme 47). Between 38–89% yields were obtained in the case of coupling of various aryl bromides with aryltriethoxysilanes [163].
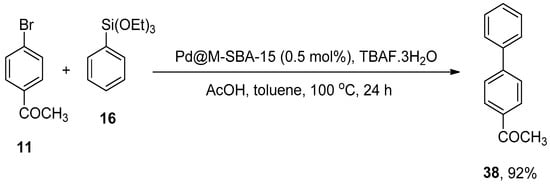
Scheme 47.
Use of Pd@M-SBA-15 catalyst in Hiyama cross-coupling of 11 with phenyltriethoxysilane 16.
Euphorbia thymifolia L. belongs to the family of Euphorbiaceae and is an apparently small and branched medicinal herb. It exhibits numerous pharmaceutical applications against dysentery, venereal diseases, and diarrhea. Focusing on the importance of Pd NPs, Nasrollahzadeh and colleagues developed a sustainable protocol for the formation of Pd NPs using an aqueous leaf extract of Euphorbia thymifolia L. due to its reducing and stabilizing abilities and observed its effectiveness in ligand-free Stille and Hiyama cross-coupling reactions in a green solvent. The Pd-NPs were distinguished by TEM, powder XRD, and UV-visible techniques. The catalytic efficacy of Pd NPs was evaluated for the Hiyama coupling reaction of 121 with phenyl trimethoxysilane 8 to afford the targeted biaryl products 122 in 96% yields (Scheme 48). The optimized reaction conditions (1 mol% of Pd NPs, NaOH in H2O as solvent at 90 °C, under air) were utilized for the corresponding reaction. Green solvent, high yields, substrate scope, cost-effectiveness, mild reaction conditions, and ease of handling are certain salient features of the corresponding methodology [164].
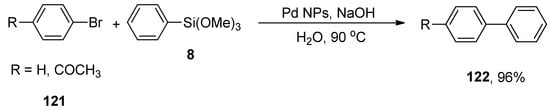
Scheme 48.
Pd-NPs catalyzed synthesis of biaryl derivatives 122 using green solvent.
A homogeneous catalytic system is frequently used in metal-catalyzed C-C coupling reactions. However, due to some drawbacks of a homogeneous catalytic system, including availability, stability, difficult separation, and recycling, diverted the attention of researchers toward the synthesis of heterogeneous catalytic systems. Nanoscale palladium supported on zinc oxide was formed by a coprecipitation method and characterized by XRD, XPS, SEM, TEM, and thermogravimetric analysis. The application of palladium supported on zinc oxide nanoparticles as a novel heterogeneous catalyst for the formation of unsymmetrical biaryl derivatives by Suzuki–Miyaura and Hiyama coupling reaction was reported by Hosseini-Sarvari and colleagues in 2015. Suzuki–Miyaura coupling of aryl halides and aryl boronic acid catalyzed by Pd/ZnO nanoparticles afforded biaryl derivatives in an 84–97% yield range. Hiyama coupling reaction of iodobenzene 76 and phenyl trimethoxysilane 8 resulted in 96% yield of biaryl product 92. The reaction was catalyzed by Pd/ZnO nanoparticles using K2CO3 as the base in ethylene glycol under an air atmosphere by maintaining a temperature of 100 °C (Scheme 49). Aryl iodides having electron-rich and poor groups gave the desired products in good to excellent yields. Electron deficient aryl bromides and chlorides resulted in biaryl products in short times (40–70 min) [165].

Scheme 49.
Nano Pd/ZnO catalyzed Hiyama coupling of iodobenzene 76 and phenyl trimethoxysilane 8.
Ohtaka and coworkers developed a pathway for the synthesis of substituted biphenyl derivatives by fluoride-free Hiyama coupling reaction of aryl bromides with aryl trimethoxysilanes and observed the catalytic effectiveness of linear polystyrene-stabilized PdO nanoparticles [PS-PdONPs] and polystyrene-stabilized Pd nanoparticles [PS-PdNPs] under green conditions. For example, the reaction of 4-methylphenylbromide 123 was performed with phenyl trimethoxysilane 8 using PS-PdONPs (1.5 mol%) catalyst, TBAC in aqueous NaOH solution at 80 °C under aerobic conditions for 3 h, which afforded 4-methylbiphenyl 79 in 88% yield. The catalytic effect of PS-PdNPs on the Hiyama coupling reaction was observed, and it was noticed that the desired coupled product was not attained, but 4,4′-dimethylbiphenyl 124, the Ullmann coupling product, was afforded in 99% yield (Scheme 50). PS-PdONPs showed higher catalytic efficacy in contrast to PS-PdNPs. The catalyst was retrieved and successively subjected to four runs of cross-coupling reaction [166].
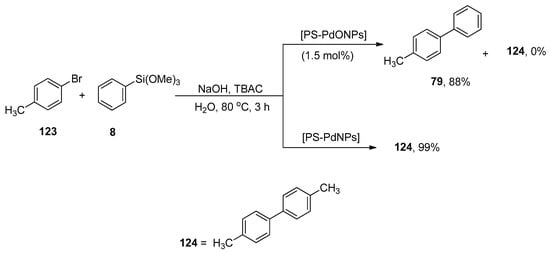
Scheme 50.
Synthesis of 4-methylbiphenyl 79 using PS-PdONPs as catalyst.
The Heck and Hiyama coupling reactions clasp a pronounced emplacement due to their implementation in the synthesis of complex organic structures [167,168,169,170]. Focusing on the green methodology, Gaikwad et al. utilized Triton X-100, a non-ionic surfactant, as a stabilizer [171,172] for the generation of palladium nanoparticles and executed an appreciable protocol for the synthesis of symmetrical stilbenes via one-pot sequential Hiyama–Heck coupling reactions. For this purpose, the research group adopted arenediazonium salt and triethoxy(vinyl)silane as substrates catalyzed by Triton X-100 stabilized palladium nanoparticles. The arenediazonium tetrafluoroborate 125 was allowed to couple with triethoxy(vinyl)silane 10 to afford the symmetrical trans-stilbene derivative 126 in excellent yield (95%) (Scheme 51). The potency of other surfactants, including cetyltrimethylammonium bromide (CTAB) and sodium dodecyl sulfate (SDS), was examined and found Triton X-100 an appropriate non-ionic surfactant for the respective reaction. The reaction was conducted at room temperature by using Triton X-100 (5 mol%) with Pd(OAc)2 (2 mol%) in H2O [173].
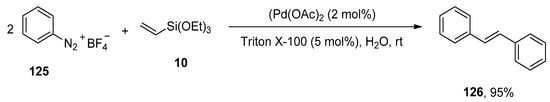
Scheme 51.
Synthesis of symmetrical stilbene derivative 126 using Triton X-100 stabilized Pd-NPs as catalyst.
Black pepper (piper nigrum) belongs to the Piperaceae family. Black pepper extract contains a variety of phytochemicals such as piperine, phenols, ethyl piperonyl cyanoacetate, N-isobutyl-tetradeca-2,4-dienamide that help to achieve the reduction of Pd(II) to Pd(0). It is used as an important component in traditional medicines [174]. The formation of green nanocatalyst (Pd NPs) using aqueous ethanolic extract of black pepper was carried out by Kandathil et al. in 2018, and its catalytic activity in the cyanation and Hiyama cross-coupling was observed. The cyanation of aryl halides was carried out in the presence of cyanating reagent K4Fe(CN)6. Either electron-donating or electron-withdrawing groups substituted on aryl halides gave moderate to excellent yields. The ligand-free Hiyama cross-coupling reaction of various aryl halides with aryl trimethoxysilane was carried out under fluoride-free conditions to afford the biphenyl derivatives in an 87–98% yield range. The reaction of iodobenzene 76 and phenyl trimethoxysilane 8 proceeded at 100 °C in the presence of NaOH, ethylene glycol, and Pd nanoparticles with 0.2 mol% Pd loading, which catalyzed the reaction efficiently, to obtain the cross-coupling product 92 in 98% yield (Scheme 52) [175].
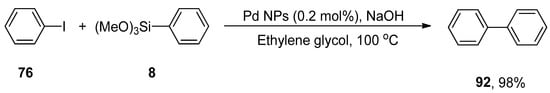
Scheme 52.
Synthesis of biphenyl 92 in ethylene glycol using Pd NPs as catalyst.
9. Pd(PPh3)4 as Catalyst
Cyclopropane motifs have gained huge interest due to their unique features and immense applications in chemical transformations [176,177]. These motifs exist in numerous biologically active natural products and synthetic drugs [178,179,180]. The contribution of the silanol group in the cyclopropanation and Hiyama–Denmark cross-coupling reaction was described by Beaulieu et al. Di-tert-butoxy(cyclopropyl)silanol serves as a substrate for Hiyama–Denmark cross-coupling reaction was synthesized by Simmons–Smith via cyclopropanation reaction of di-tert-butoxy(alkenyl)silanol. (Cyclopropyl)silanol 127 was treated with BF3.OEt2 followed by the reaction with 128, 5 mol% Pd(PPh3)4, and TBAF using THF solvent. The reaction worked well at 100 °C to afford the desired cross-coupling product 129 in 92% yield (Scheme 53) [181].
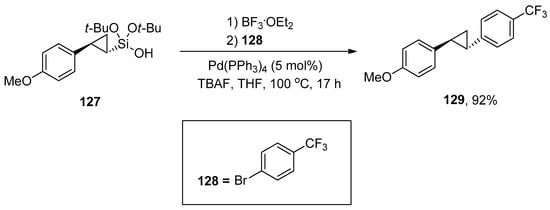
Scheme 53.
Hiyama–Denmark coupling of (cyclopropyl)silanol 127.
In 2015, the synthesis of unsymmetrical biaryl derivatives through the Hiyama cross-coupling protocol in the presence of Cu(I) and H2O was reported by Delpiccolo and coworkers. Cu(I) salts have been proven to improve the efficacy of palladium-catalyzed cross-coupling reactions [182]. A series of biaryl derivatives was achieved in a 62–98% yield range. Maximum yield (98%) of 131 was observed in the case of coupling of 130 with 31 using Pd(PPh3)4 (0.025 equiv.), TBAF as fluoride source and CuI to improve the catalytic reaction in THF (5% H2O). The reaction proceeded well at 80 °C for 18 h (Scheme 54) [183].
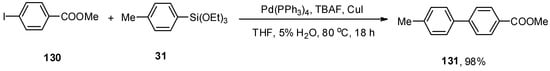
Scheme 54.
Pd(PPh3)4 catalyzed Hiyama coupling of 130 and 31.
10. Copper Catalyzed Hiyama Cross-Coupling Reactions
Hiyama cross-coupling remained a valuable tool for the construction of carbon–carbon bonds in diversified natural products, pharmaceutical compounds [184,185,186,187,188,189], and complex structures. Hiyama coupling reaction is predominantly conducted with palladium catalysts. In 2013, Gurung et al. reported the first CuI-catalyzed Hiyama cross-coupling reaction of aryl- and heteroaryl halides with aryl- and heteroaryltriethoxysilane in the presence or absence of PN-1 bidentate ligand. The electron-rich and electron-deficient groups substituted on aryl halides and aryltriethoxysilanes gave moderate to excellent yields. The coupling reaction of heteroaryl triethoxysilanes with aryl iodides in the presence of PN-1 ligand afforded the desired biaryl derivatives in a 40–74% yield range. An excellent result (94%) of 134 was obtained by the addition of 132 to 133 using 10 mol% CuI and CsF in the absence of PN-1 ligand 135. The reaction was conducted in DMF by maintaining the temperature at 120 °C for 24 h (Scheme 55). CsF stabilizes the formation of monomeric [CuAr] intermediate and acts as a fluoride source for the corresponding reaction [190].
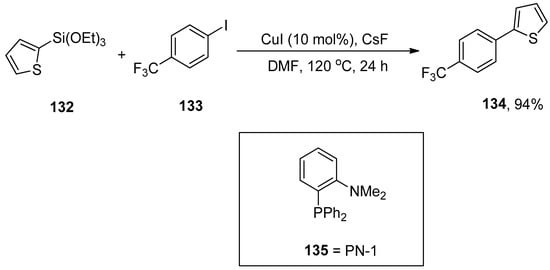
Scheme 55.
Hiyama cross-coupling of aryl iodide with heteroaryl triethoxysilane using copper catalyst.
Allylbenzenes have acquired an eminent interest in organic synthesis due to their pharmacological activities. The research group of Cornelissen carried out the synthesis to afford allylbenzene derivatives via copper-catalyzed Hiyama cross-coupling of vinylalkoxysilanes in the presence of an activating agent, TBAT (tetrabutylammonium difluorotriphenylsilicate) without using any ligand. Various allylbenzene derivatives were achieved in an 83–99% yield range by reaction of vinyl silane with substituted benzyl halides. The substrate 136 was allowed to couple with 137 to afford the desired product 138 in maximum (99%) yield. The optimization reaction conditions were Cu[MeCN]4PF6 (10%), TBAT, MeCN solvent, 40 °C, 16 h (Scheme 56). The benzylation of various vinylsilanes gave coupling products in a 51–92% yield range. Moreover, Z-alkenes were synthesized using a copper-catalyzed Hiyama cross-coupling reaction. The substrate β-(Z)-vinylsilane 139 was coupled with 140 to acquire the desired Z-alkene 141 in 92% yield under optimized reaction conditions (Scheme 57) [191].
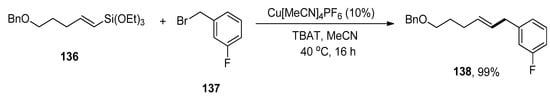
Scheme 56.
Cu[MeCN]4PF6 catalyzed Hiyama coupling reaction of substrate 136 with 137.
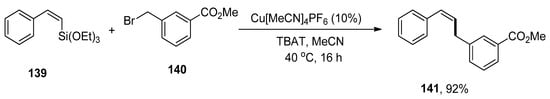
Scheme 57.
Benzylation of a β-(Z)-vinylsilane 141 by Hiyama coupling reaction.
11. Nickel-Catalyzed Hiyama Cross-Coupling Reaction
Bimetallic nanoparticles have attained substantial attention as an efficient catalytic system for Hiyama cross-coupling reactions. Rothenberg and coworkers synthesized the core–shell Ni-Pd nanoclusters by combining electrochemical and wet chemical methods that efficiently catalyzed Hiyama coupling reactions in contrast to bimetallic alloy clusters and monometallic clusters. Aryl iodides with electron-donating and electron-withdrawing substituents gave substituted biaryl derivatives in a 6->99% yield range. A competent reaction of haloaryls 142, phenyl trimethoxysilane 8, and 143 afforded the corresponding biaryl product 144 in good (>99%) yield along with homocoupling side-product (<2%). The reaction worked efficiently at 65 °C in the presence of core–shell Ni-Pd cluster (1 mol%) catalyst and tetrabutylammonium fluoride (TBAF) under an N2 atmosphere. THF was utilized as the only solvent for the corresponding reaction (Scheme 58) [192].
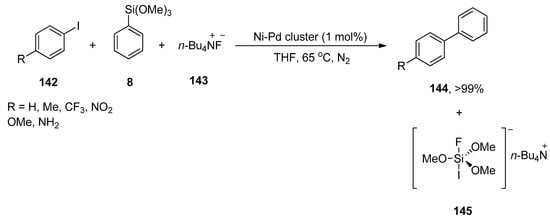
Scheme 58.
Synthesis of biaryl derivatives 144 using Ni-Pd cluster as catalyst.
The nickel/bathophenanthroline catalyzed Hiyama coupling reaction of unactivated secondary alkyl halides has been recently reported, but it was not found to be an efficient catalyst for activated secondary alkyl halides. The activity of amino alcohol ligand was also observed in the case of the Hiyama coupling reaction. Later, in 2007, the catalytic effect of nickel/norephedrine in Hiyama coupling reactions of several unactivated alkyl halides was reported by Strotman et al. The cyclohexyl iodide 146 was treated with phenyl trifluorosilane 147 to achieve the desired cross-coupling product 148 in 94% yield. The reaction worked efficiently at 60 °C in the presence of 10% NiCl2.glyme with 15% norephedrine as a catalyst, using 12% lithium hexamethyldisilazide (LiHMDS) as the base, H2O and CsF in DMA (N,N-dimethylacetamide) (Scheme 59). In the case of nickel-catalyzed Hiyama coupling reactions of activated secondary alkyl halides, excellent results were obtained in a 60–92% yield range under the same reaction conditions mentioned above [193].
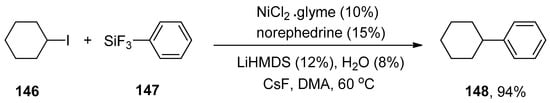
Scheme 59.
NiCl2.glyme catalyzed Hiyama cross-coupling of cyclohexyl iodide 146 with phenyl trifluorosilane 147.
Dai et al. reported a convenient protocol for Ni-catalyzed asymmetric Hiyama cross-coupling reactions of racemic α-bromo esters. On varying reaction conditions, several α-aryl esters were obtained in a low to excellent yield range (<2–92%) with enantioselectivities (13–99%), respectively. The phenylation of α-bromo ester 149 was carried out with phenyl trimethoxysilane 8 at room temperature using 10% NiCl2.glyme, 12% 150, and TBAT in dioxane yielded the corresponding coupling product 151 in 80% yield with 99% ee enantioselectivity (Scheme 60). The addition of substituted aryl silanes to α-bromo esters resulted in 64–76% yields of respective cross-coupling products and 87–94% ee enantioselective values, while α-bromo esters underwent alkenylation through catalytic asymmetric Hiyama coupling reactions afforded the coupling products in 66–72% yield range with 91–93% ee [194].
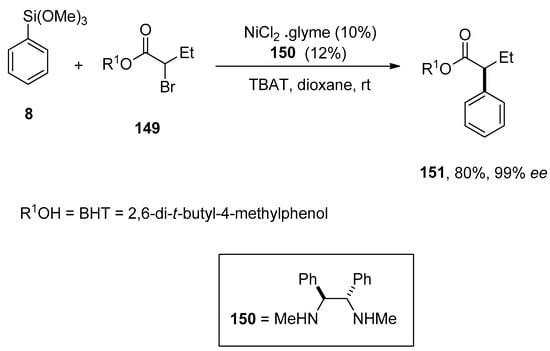
Scheme 60.
Nickel catalyzed phenylation of α-bromo ester.
The environmentally benign Hiyama cross-coupling of tetrafluoroethylene (TFE) and perfluoroarenes via C-F bond activation was developed by Ogoshi and coworkers in 2014. An excellent yield (90%) of 154 was obtained when 152 was treated with 153 using 5 mol% [Ni2(iPr2lm)4(cod)] catalyst in tetrahydrofuran at 100 °C for 10 h (Scheme 61). The palladium-catalyzed base-free Hiyama coupling of tetrafluoroethylene with substituted aryl trimethoxysilane yielding α,β,β-trifluorostyrene derivatives in 40–94% yield range. The reaction was carried out using 2.5 mol% Pd2(dba)3(C6H6) as the catalyst, 5 mol% PCyp3, and 10 mol% FSi(OEt)3 in THF at 100 °C [195].
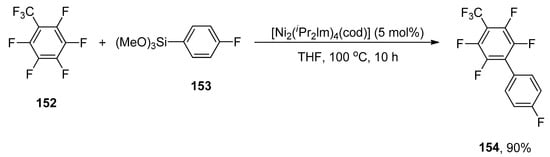
Scheme 61.
Base free nickel catalyzed Hiyama coupling reaction.
Wang and coworkers carried out the fluoroalkylation of arylsilanes via nickel-catalyzed Hiyama coupling reactions. The arylsilanes bearing electron-rich substituents underwent monofluoroalkylation smoothly to afford excellent yields (74–94%). The arylsilane 155 was coupled with 156 using a catalytic amount of Ni(dme)Cl2 (10 mol%), 157 (12 mol%) CsF as an activator to achieve the desired monofluoroalkylated product 158 in 94% yields. 1,4-Dioxane was the only solvent used for this reaction, and the temperature was maintained at 80 °C (Scheme 62). Ezetimibe is a cholesterol absorption inhibiting drug [196]. The monofluoroalkylation of ezetimibe-derived arylsilane proceeded under optimized reaction conditions to afford the monofluoroalkylated ezetimibe 159 in 91% yield (Figure 5) [197].
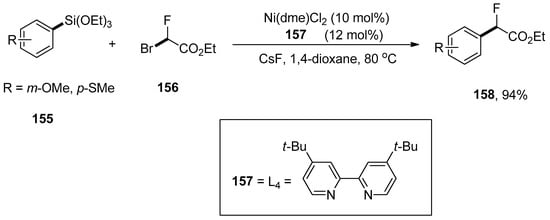
Scheme 62.
Use of Ni(dme)Cl2 catalyst Hiyama cross-coupling reaction for monofluoroalkylation of arylsilanes 155.
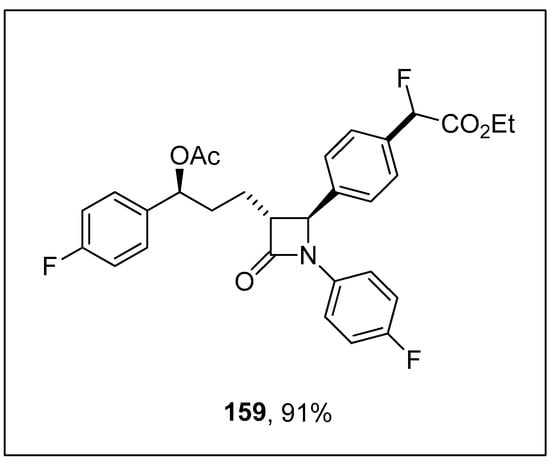
Figure 5.
Structure of monofluoroalkylated ezetimibe 159.
The palladium-catalyzed decarboxylative coupling reactions have been reported by many research groups in the past. The nickel-catalyzed decarboxylative Hiyama coupling reactions of alkynyl carboxylic acids with organosilanes were introduced for the first time by Raja et al. The reaction of 160 was carried out with phenyl triethoxysilane 16 using a catalytic amount of Ni(acac)2 (10 mol%), affording the corresponding decarboxylative product 161 in 90% yield along with undesired homocoupling product 162 in low yield. The application of 1,10-phenanthroline as a ligand provided good results by using CsF as an activator of organosilanes and CuF2 as an oxidant. The reaction worked well in DMSO at 120 °C for 12 h (Scheme 63). Various aryl alkynyl carboxylic acids were allowed to couple with substituted phenyl triethoxysilanes, resulting in decarboxylative products in a 74–90% yield range. A 90% yield of the desired decarboxylative product was also obtained in the case of p-chloro phenyl triethoxysilane. The symmetrical diarylacetylenes were synthesized in a 21–45% yield range under optimized reaction conditions. It was noticed that variation in reaction conditions (in the presence of TEMPO and the absence of CuF2, CsF, and Ni ligands) resulted in relatively low yields of corresponding products [198].
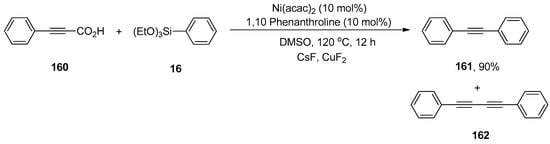
Scheme 63.
Decarboxylative coupling of alkynyl carboxylic acid 160 with triethoxyphenylsilane 16.
12. Applications of Hiyama Coupling Reactions for the Synthesis of Biologically Active Scaffolds
Retinoids, chemically related to vitamin A, have been the subject of tremendous endorsement due to their activities in biological processes, including cell growth, vision, embryonic development, immune response, and reproduction [199]. The transition metal-catalyzed Suzuki and Stille cross-coupling reactions have been extensively utilized to obtain retinoids. However, drawbacks to the above reactions, including low stability of organoboranes, toxicity, and high molecular weight of organostannanes, diverted the attention of researchers towards the highly efficient Hiyama coupling reaction. The total synthesis of retinoids was first outlined by Montenegro et al., employing the Hiyama coupling reaction as a key step. To the solution of organosilane reagents 164 and 167 in THF, TBAF was added, followed by the addition of trienyl iodide 163 and Pd2(dba)3.CHCl3 to afford retinyl ethers 165 and 168, which underwent deprotection using TMSCl, H2O, and methanol to attain trans-retinol 166 and 11-cis-retinol 169 in 74% and 83% yields, respectively. The oxidation of 11-cis-retinol in the presence of BaMnO4 in dichloromethane solvent afforded the synthesis of 11-cis-retinal 170 in 90% yield (Scheme 64) [200].
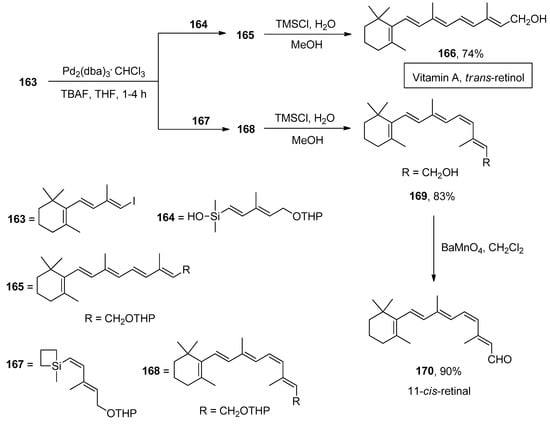
Scheme 64.
Synthesis of trans-retinol 166 and 11-cis-retinal 170 via Hiyama cross-coupling reaction.
Heliannuol A, isolated from sunflower Helianthus annuus [201,202,203,204,205,206], is regarded as the first member of the family of allelopathic [207,208] sesquiterpenoids holding benzoxocane moiety. Heliannuols have attained eminent attention from synthetic chemists due to their possessing unusual structures and biological activity. Vyvyan and coworkers described the synthesis of benzoxocane using trisubstituted Z-styrene derivatives synthesized by Hiyama coupling of oxasilacycloalkenes with aryl iodides. The substituted Z-styrene derivative, obtained by Hiyama coupling of 171 and 172 using Pd2(dba)3 (2–3 mol%) catalyst and tetrabutylammonium fluoride (TBAF) in THF solvent, underwent intramolecular Buchwald–Hartwig etherification using 10 mol% Pd2(dba)3 catalyst and 10 mol% Q-Phos ligands at 80 °C for 24 h. NaOt-Bu was selected as a suitable base in toluene for corresponding etherification reaction to afford the maximum (10%) yield of benzoxocane 174 along with 175 as the major product (Scheme 65). The hydrogenation of benzoxocane 174 using Pd/C in ethanol gave 176 in 44% yield [209].
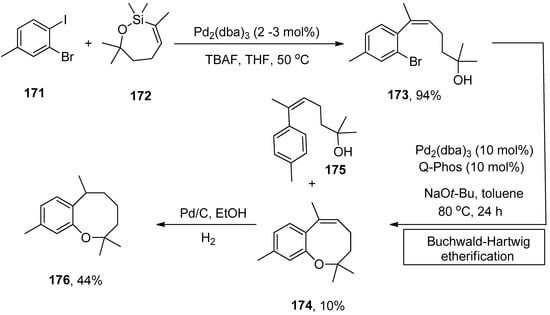
Scheme 65.
Synthesis of benzoxocane 174 via Hiyama coupling followed by Buchwald–Hartwig etherification.
The synthesis of biologically relevant benzofuran scaffolds by a convenient route is of huge interest because benzofuran compounds account for important bioactive molecules, including amiodarone [210], BNC105 [211], cytotoxic flavonoids [212], and natural products such as egonol [213,214], daphnodorin A and B [215] and moracin O and P [216,217]. The application of palladium(II) acyclic diaminocarbene (ADC) complexes in one-pot tandem Hiyama alkynylation/cyclization for synthesizing biologically relevant benzofuran derivatives was disclosed by Singh et al. The palladium ADC complexes were synthesized via nucleophilic addition of secondary amines such as morpholine, pyrrolidine, and piperidine to metal precursor cis-{(2,4,6(CH3)3C6H2)NC}2PdCl2 at room temperature that efficiently catalyzed synthesis of benzofuran derivatives. Iodophenol and triethoxysilylalkynes underwent Hiyama alkynylation followed by cyclization catalyzed by Pd ADC complex to afford benzofuran derivatives in (14–57%) yield. Excellent result (57%) of benzofuran derivative 180 was obtained by Hiyama alkynylation/cyclization of 177 and 178 using 2 mol% palladium ADC complex 179 as catalyst and NaOH as the base. The reaction proceeded in the 4:2 mixture of 1,4-dioxane/H2O by maintaining the temperature at 80 °C for 4 h. The Hiyama cross-coupling of iodobenzene with triethoxysilylalkynes catalyzed by palladium ADC complex 179 gave alkyne derivatives in (35–76%) yield range using optimized reaction conditions (Scheme 66) [218].
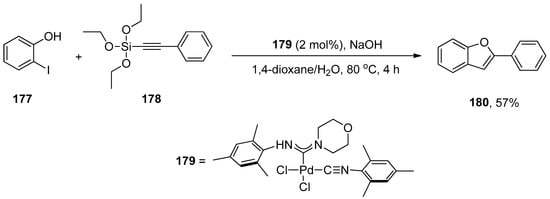
Scheme 66.
Synthesis of benzofuran derivative 180 via Hiyama alkynylation followed by cyclization.
Triflate derivatives have remained an area of interest for researchers due to their portfolio in catalytic transformation, i.e., one-pot tandem Heck alkynylation/cyclization reactions [219], and in biomedical applications as potent anticancer agents [220]. The utility of triflate derivatives of palladium acyclic diaminocarbene (ADC) complexes as effective precatalysts for Hiyama alkynylation/cyclization reaction in the synthesis of benzofuran derivative was reported by Ghosh and coworkers. The complexes cis-[(R1NH)(R2)methylidene]Pd(OCOCF3)2(CNR1) [R1 = 2,4,6-(CH3)3C6H2: R2 = NC4H8 (181); NC5H10 (182)] were synthesized by the treatment of chloro derivatives cis-[(R1 NH)(R2)methylidene]PdCl2(CNR1)[R1 = 2,4,6- (CH3)3C6H2: R2 = NC5H10; NC4H8] with AgOCOCF3 in excellent yields (84–94%) under ambient conditions. One-pot tandem Hiyama alkynylation/cyclization reaction of iodophenol with a range of triethoxysilylalkynes catalyzed by palladium acyclic diaminocarbene triflate complexes gave low to moderate yields of corresponding benzofuran derivatives. It was observed that the application of precatalyst 182 exhibited a comparatively higher yield range (15–52%) than precatalyst 181 (10–49%). The essential parameters for the smooth functioning of the corresponding reaction comprised 2 mol% precatalysts 181 and 182, NaOH in a 4:2 mixture of 1,4-dioxane:H2O at 80 °C for 4 h. Maximum yields of 49% and 52% were obtained in case of coupling of 177 with 178 catalyzed by palladium acyclic diaminocarbene (ADC) complexes 181 and 182 precatalysts, respectively (Figure 6) [221].
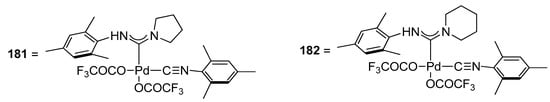
Figure 6.
Palladium acyclic diaminocarbene triflate complexes 181 and 182.
13. Miscellaneous
The Hiyama coupling of unactivated alkyl bromides and iodides in mild conditions was achieved by Lee et al. A series of cross-coupled products were achieved in a 65–81% yield range by Hiyama coupling of functionalized alkyl bromides with phenyl trimethoxysilanes using PdBr2 catalyst, P(t-Bu)2Me and Bu4NF. The reaction was carried out in THF at room temperature, while in the case of [HP(t-Bu)2Me]BF4, 42–88% yields of cross-coupled products were achieved. The 183 was allowed to couple with 184 using 4% PdBr2/P(t-Bu)2Me and Bu4NF in the presence of THF solvent at room temperature to afford the desired coupling product 185 in 84% yield (Scheme 67). The same Hiyama coupling reaction also proved to be effective for functionalized alkyl iodides [222].
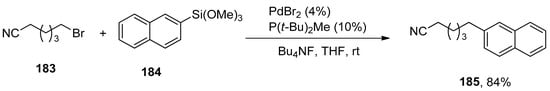
Scheme 67.
Hiyama cross-coupling reaction of unactivated alkyl bromides.
Alacid and Nájera reported the NaOH promoted Hiyama coupling reactions of aryl halides with vinyltrialkoxysilanes using fluoride-free conditions. The Hiyama coupling of vinyltrimethoxysilane with aryl bromides and chlorides afforded styrene derivatives in 47–99% yields. The reaction of 4-bromo acetophenone 11 was carried out with vinyltrimethoxysilane 50 using 4-hydroxyacetophenone oxime-derived palladacycle (0.1 mol% Pd), 2.5 equivalent NaOH promotor, and 1 equivalent of tetra-n-butylammonium bromide (TBAB) additive under microwave irradiation for 10 min. In the case of styryltriethoxysilane, stereospecific coupling with aryl or vinyl bromides provided the corresponding stilbenes or dienes, respectively. Moreover, the undesirable polymerization of products is prevented by using mild reaction conditions in this protocol (Scheme 68) [223].
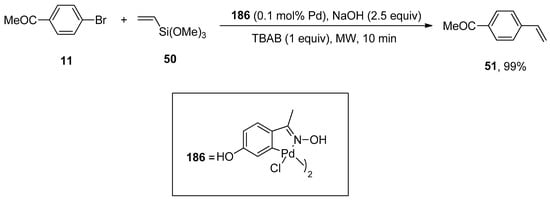
Scheme 68.
Oxime palladacycle catalyzed synthesis of 4-acetoxystyrene 51.
Bhaumik and coworkers synthesized the palladium containing periodic mesoporous organosilica (PMO) and analyzed their catalytic efficacy in Hiyama coupling, Sonogashira coupling, and cyanation reaction. Despite Sonogashira coupling and cyanation reaction, the focus was being placed on environment-friendly Hiyama coupling to achieve unsymmetrical biphenyls. The substituted benzonitriles and disubstituted alkynes were achieved in 68–95%, and 72–90% yield ranges, respectively. Hiyama coupling reaction proceeded under green conditions, using a Pd-containing (PMO) catalytic system giving 60–95% yield of the respective coupled products. The best results were obtained in the case of coupling reaction of p-iodonitrobenzene 7 with trimethoxy(vinyl)silane 50 to attain a 95% yield of the targeted product 187. The essential parameters utilized in the reaction comprised of Pd-LHMS-3 catalytic system and NaOH at 100 °C in water (Scheme 69). The excellent yields of products, reusability, and easy work-up made Pd-grafted PMO an effective catalyst for synthesizing benzonitrile derivatives, disubstituted alkynes, and unsymmetrical biphenyls [224].
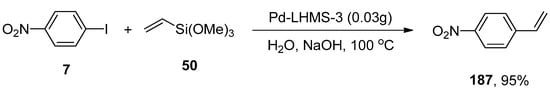
Scheme 69.
Pd-grafted PMO catalyzed Hiyama coupling reaction of p-iodonitrobenzene 7 with 50.
The catalytic activity of thermally stable and oxygen insensitive dimeric ortho-palladated complex [Pd{C6H4(CH2N(CH2Ph)2)}(µ-Br)]2 homogeneous catalysts [225] in Hiyama coupling was investigated by Hajipour and colleagues. The substituted biphenyls were obtained in a 68–97% yield range along with homocoupling products. Maximum yield (97%) of 92 was observed in the case of coupling of aryl halides (bromides and iodides) 188 with phenyl triethoxysilane 16 using efficient [Pd{C6H4(CH2N(CH2Ph)2)}(µ-Br)]2 catalysts under microwave irradiation at 90 °C and 500W. Among different solvents (THF, DMF, Dioxane, EtOH, MeOH, p-Xylene), DMF was screened to be a microwave-active polar solvent and TBAF.3H2O as an efficient additive to improve the yield within short times (Scheme 70) [226].
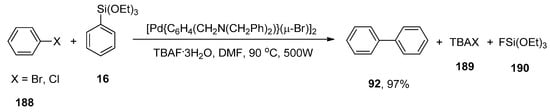
Scheme 70.
[Pd{C6H4(CH2N(CH2Ph)2)}(µ-Br)]2 catalyzed Hiyama coupling of aryl halides 188 with 16 under microwave irradiation.
Arylsulfonyl chlorides have been used for many years in manufacturing pesticides, dyes, drugs, polymers [227,228], etc., due to their affordability and ease of availability. The application of arylsulfonyl chlorides in palladium-catalyzed desulfitative Hiyama coupling to afford biphenyl derivatives under mild reaction conditions was outlined by Zhang et al. The reaction of phenylsulfonyl chloride 191 with 4-methoxyphenyltrimethoxysilane 192 underwent palladium-catalyzed desulfitative Hiyama coupling by using Pd2(dba)3 (3 mol%) catalyst and TBAF.3H2O as an additive to obtain the desired biaryl derivative 66 in 92% yield (Scheme 71). The 1:4 mixture of DMF/CH3CN was utilized as a suitable solvent for the corresponding reaction by observing the results of DMSO, DMF/1,4-dioxane, NMP, THF, and toluene. The reaction worked well at 100 °C for 3 h under an N2 atmosphere [229].
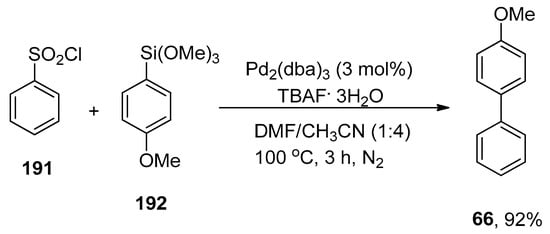
Scheme 71.
Synthesis of biaryl derivative 66 using Pd2(dba)3 as catalyst.
A novel method for the formation of unsymmetrical biaryls using arylsulfonyl hydrazides by Hiyama cross-coupling was developed by Miao et al. The arylsulfonyl hydrazides containing electron-rich and -poor groups smoothly underwent cross-coupling with phenyl trimethoxysilane providing a series of biaryl derivatives in a 72–95% yield range. The coupling of 193 with phenyl trimethoxysilane 8 catalyzed by 5 mol% Pd(TFA)2 gave the desired biaryl product 66 in 95% yield under desulfitative and denitrificative reaction. The reaction worked efficiently in DMI solvent using a TBAT (tetrabutylammoniumdifluorotriphenylsilicate) activator at 60 °C for 12 h under an O2 atmosphere (Scheme 72). Between 74–94% of yields of desired cross-coupled products were obtained by the addition of phenylsulfonyl hydrazide with a wide range of aryl trimethoxysilanes under optimized conditions [230].
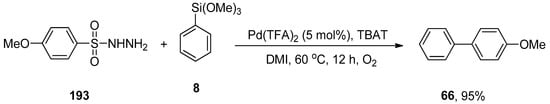
Scheme 72.
Hiyama coupling reaction of 193 with phenyl trimethoxysilane 8 using Pd(TFA)2 as catalyst.
In 2016, the catalysis of Suzuki–Miyaura and Hiyama–Denmark coupling of aryl sulfamates using (1-tBu-indenyl)Pd(L)(Cl) precatalysts was reported by Hazari and coworkers. Hiyama–Denmark coupling of substituted aryl silanolates and aryl chlorides afforded the desired coupled products in maximum yields (85–97%). A total of 2.5 mol% Pd-PtBu3 precatalysts, toluene, 70 °C, and 4 h were the standard reaction conditions (Scheme 73). The same research group described the first Hiyama–Denmark coupling of aryl sulfamates using Pd-RuPhos precatalyst. Several additives (NaOMe, NaOtBu, and RuPhos) were screened to improve the yields of Hiyama–Denmark reactions. An excellent result (91%) was obtained in the case of coupling of 197 with 198 in the presence of 5 mol% Pd-RuPhos precatalyst and 5 mol% RuPhos as an additive in toluene at 110 °C for 8 h (Scheme 74) [231].
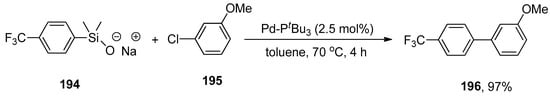
Scheme 73.
Use of Pd-PtBu3 catalyst in Hiyama–Denmark coupling of aryl sulfamate.
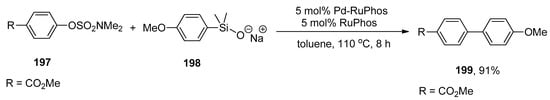
Scheme 74.
Pd-RuPhos catalyzed synthesis of biaryl derivatives via reaction of 197 and 198.
The synthesis of valuable biarylboronates via photochemical gold-catalyzed Hiyama redox-neutral arylation of mechanistically preferential B,Si-bimetallic coupling reagents with substituted diazonium salts was reported by Hashmi and coworkers. The scope of Hiyama coupling with respect to boronic acid derivatives was investigated. It was observed that boronic acid derivatives (BMIDA, BPin, and Bnep) with substituted aryl silanes afforded desired cross-coupled products in reasonable (49–86%), (40–65%) and (42–60%) yield ranges, respectively. Excellent output (91%) of desired biofunctionalized biarylboronate derivative 202 was attained in case of coupling of B,Si-bimetallic reagent 201, and 4-CF3 substituted aryldiazonium salt 200 using a 10 mol% Ph3PAuNTf2 catalyst in acetonitrile solvent under irradiation of blue LEDs at room temperature (Scheme 75) [232].
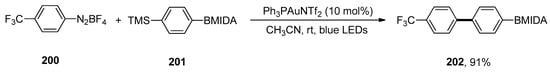
Scheme 75.
Synthesis of biarylboronates under blue LEDs irradiation.
A very interesting green procedure of Hiyama cross-coupling using a highly stable NCN-pincer palladium complex as catalyst was disclosed by Marset et al. The catalytic efficacy of the NCN-pincer palladium complex was assessed during the coupling of aryl iodides and bromides and phenyl trimethoxysilane. The aryl bromides and iodides with electron-donating or electron-withdrawing groups gave moderate to excellent yields. The NCN-pincer palladium complex catalyzed coupling of aryl iodides and bromides with phenyl trimethoxysilane in glycerol gave expected biaryl products in 40–99% yields while a 1:2 mixture of choline chloride:glycerol (ChCl:glycerol) facilitated the synthesis of targeted biaryl products in 1–85% yields. Maximum yield (99%) of biphenyl product 92 was obtained in the case of coupling phenyl iodide 76 with phenyl trimethoxysilane 8 using 1 mol% NCN-pincer palladium-catalyzed catalyst 203 in 0.5 M glycerol at 100 °C for 24 h. K2CO3 was screened as a suitable base for the corresponding reaction (Scheme 76). Isomerization was observed in the case of reaction with allyl trimethoxysilane. A mixture of isomers was formed, affording a highly stable internal trans double bond containing a major product in 83% yield. The recyclability of catalysts and the usage of biorenewable solvents are the salient features of this methodology [233].
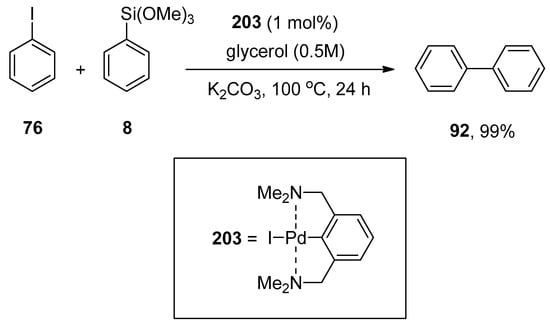
Scheme 76.
NCN-pincer palladium catalyzed Hiyama coupling of phenyl iodide 76 with phenyl trimethoxysilane 8.
Sobhani and coworkers synthesized a hydrophilic heterogeneous cobalt catalyst supported on chitosan [mTEG-CS-Co-Schiff-base]. The catalyst was identified by XRD, TEM, FT-IR, TGA, ICP, FE-SEM, and XPS analyses, and its implication as a heterogeneous catalyst in Hiyama, Heck, Suzuki, and Hirao cross-coupling was investigated. The application of the mTEG-CS-Co-Schiff base in fluoride-free Hiyama coupling reaction under green reaction conditions was reported for the first time. A series of biaryl derivatives having electron releasing and electron-withdrawing groups on aryl halides were synthesized in a good to excellent yield range (80–98%). The coupling reaction of iodobenzene 76 with triethoxyphenylsilane 16 worked well under green conditions using 0.5 mol% mTEG-CS-Co-Schiff-base (catalyst) 204 and NaOH as a base. Consequently, an excellent (98%) yield of desired biaryl product 92 was obtained (Scheme 77). The utilization of water as a green solvent, recovery, and scalability of catalyst for at least six successful runs without losing catalytic activity and inexpensive abundant cobalt catalyst make this reaction an environmentally and economically friendly protocol [234].
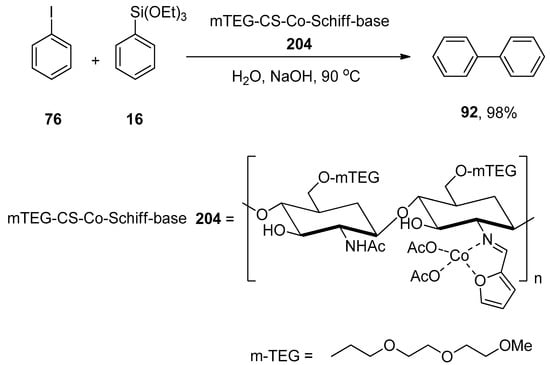
Scheme 77.
Hiyama cross-coupling of iodobenzene 76 with triethoxyphenylsilane 16 catalyzed by mTEG-CS-Co-Schiff base 204.
The comparison of catalysts reported in the section above indicates that among the palladium-catalyzed reaction, several Pd based catalysts afforded the products in quantitative yields (Table 1, entries 1, 2, 3, 4, 5, 7, 11, 12, 22, 23, 24, 25, 40 and 41) [53,54,55,56,64,81,84,95,96,97,104,141]. Among these Pd catalyzed methodologies, [PdCl2P(OCH2CMe2NH)OCH2CMe2N2] at catalyst loading of 1.31 × 10−5 mol was found to afford a range of products in excellent yields (87–99%) at mild reaction conditions (Table 1, entry 25) [104]. Further, the catalyst Pd(dppf)Cl2 (0.05 equiv), Pd(OAc)2/17, Pd(OAc)2/26 afforded products in promising yields of 72–99%, using water as green solvent (Table 1, entry 23) [96] and 70–98%, and 44–99% under neat and mild reaction conditions, respectively (Table 1, entries 5 and 8) [56,64]. The catalyst Pd(allyl)Cl]2/47 at 1.25% catalyst loading efficiently yielded the products in an excellent to quantitative yield range of 90–95% at two different temperatures, 110 °C and 115 °C, using THF solvent (Table 1, entry 15) [88]. However, some catalysts, such as Pd(Oac)2 catalyst and Pd(Oac)2/26, showed considerable variability in the yield range of 20–100% and 36–97%, respectively (Table 1, entries 1 and 7) [53,64]. Affording the products in poor/moderate to quantitative yields suggested the limited applicability of the catalyst and narrow substrate scope. Among the nanoparticle-based catalysts, SiO2@Fe3O4-Pd at a low catalyst loading of 0.5 mol% of Pd and mild reaction conditions afforded the best yield range (81–99%) (Table 1, entry 56) [158]. While, the Pd NPs 106 (1 mol%) and Pd NPs 117 (0.25 mol% Pd) using green solvent water, readily available base NaOH afforded promising yields of 88–98% (at 90 °C) (Table 1, entry 53) [145] and 59–97% (100 °C) (Table 1, entry 58) [162], respectively. While, Pd NPs (4 mol%) derived from Pd(OAc)2 using also afforded promising yields of 76–98%, respectively. Among Copper catalysts, Cu[MeCN]4PF6 at (10% catalyst loading) yielded products in 83–99% (Table 1, entry 67) [191]. Furthermore, Ni(dme)Cl2/157 at 10 mol% loading and CsF obtained products in excellent yield range 74–94% (Table 1, entry 71) [197]. Overall, the palladium catalyst showed the best results, and due to the yield range, green reaction conditions, and substrate scope, they observed wide applicability for the synthesis of a range of scaffolds.

Table 1.
Comparison of transition metal-based catalysts in Hiyama cross-coupling.
14. Conclusions
In conclusion, we have reviewed a large number of strategic approaches published about Hiyama cross-coupling reactions during the last 15 years. Moreover, this review article highlights all major advances in Hiyama coupling reaction regarding different catalytic systems N-heterocyclic carbene (NHC)-Pd complexes, palladium-supported nickel catalysts, copper iodide, nanoparticles as catalysts, etc.), the effect of different substituents on product yields, enantioselectivity, and applications in the synthesis of valuable scaffolds. Transition metal-catalyzed coupling reactions have been emerging as efficient and reliable methodologies to realize the synthesis of a range of products. Transition metal-catalyzed Hiyama reaction is also a coupling reaction yielding scaffolds with potential applications in pharmaceutical and chemical industries. Although a number of methodologies highlighting different transition metal-based catalysts at different catalyst loadings have been reported in recent years, the area needs to be explored more and unbox certain limitations. Several nanocatalysts have been developed that realize the synthesis more efficiently due to higher surface-to-volume ratio and contact ratio with substrates; however, more efficient catalysts with environmentally benign methodologies, mild reaction conditions, and broad substrate scope are desired. Along with the monometallic catalytic systems, the focus could be placed on the development of bi-metallic and tri-metallic catalysts. The nanocatalysts are needed to be explored to develop cheap, reliable, and most efficient catalysts. The substrate scope of the reaction should be extended for the use of aryl chloride along with aryl bromide/iodide as coupling partners. We believe that this review will be a significant contribution to increasing the research interest in the respective field.
Author Contributions
Conceptualization: A.F.Z. and M.M.; Supervision: A.F.Z.; K.K.-M. and M.M.; writing—original draft preparation: R.N., M.I., S.M.H., S.A. and A.I.; writing—review and editing: A.F.Z., K.K.-M. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
The authors are thankful to Government College University Faisalabad and HEC, Pakistan for providing facilities to carry out this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Torssell, K.B.G. Natural Product Chemistry; Wiley: Chichester UK, 1983. [Google Scholar]
- Thomson, R.H. The Chemistry of Natural Products; Blackie and Son: Glasgow, UK, 1985. [Google Scholar]
- Baudoin, O.; Gueritte, F. Natural bridged biaryls with axial chirality and antimitotic properties. Stud. Nat. Prod. Chem. 2003, 29, 355–417. [Google Scholar] [CrossRef]
- Mei, X.; Wolf, C. Determination of enantiomeric excess and concentration of unprotected amino acids, amines, amino alcohols, and carboxylic acids by competitive binding assays with a chiral scandium complex. J. Am. Chem. Soc. 2006, 128, 13326–13327. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Gurjar, M.K.; Reddy, K.L.; Rao, A.S. Studies Directed toward the Synthesis of Vancomycin and Related Cyclic Peptides. Chem. Rev. 1995, 95, 2135–2167. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Boddy, C.N.; Bräse, S.; Winssinger, N. Chemistry, biology, and medicine of the glycopeptide antibiotics. Angew. Chem. Int. Ed. 1999, 38, 2096–2152. [Google Scholar] [CrossRef]
- Brunel, J.M. BINOL: A versatile chiral reagent. Chem. Rev. 2005, 105, 857–898. [Google Scholar] [CrossRef]
- Berthod, M.; Mignani, G.; Woodward, G.; Lemaire, M. Modified BINAP: The how and the why. Chem. Rev. 2005, 105, 1801–1836. [Google Scholar] [CrossRef]
- Roncali, J. Conjugated poly (thiophenes): Synthesis, functionalization, and applications. Chem. Rev. 1992, 92, 711–738. [Google Scholar] [CrossRef]
- Matheron, M.E.; Porchas, M. Activity of boscalid, fenhexamid, fluazinam, fludioxonil, and vinclozolin on growth of Sclerotinia minor and S. sclerotiorum and development of lettuce drop. Plant. Dis. 2004, 88, 665–668. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Venkatakrishnan, P.; Natarajan, P.; Huang, D.F.; Chow, T.J. De novo design for functional amorphous materials: Synthesis and thermal and light-emitting properties of twisted anthracene-functionalized bimesitylenes. J. Am. Chem. Soc. 2008, 130, 17320–17333. [Google Scholar] [CrossRef]
- Blaser, H.U.; Indolese, A.; Naud, F.; Nettekoven, U.; Schnyder, A. Industrial R&D on Catalytic C-C and C-N Coupling Reactions: A Personal Account on Goals, Approaches and Results. Adv. Synth. Catal. 2004, 346, 1583–1598. [Google Scholar] [CrossRef]
- Corbet, J.P.; Mignani, G. Selected patented cross-coupling reaction technologies. Chem. Rev. 2006, 106, 2651–2710. [Google Scholar] [CrossRef] [PubMed]
- Torborg, C.; Beller, M. Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Busacca, C.A.; Fandrick, D.R.; Song, J.J.; Senanayake, C.H. The growing impact of catalysis in the pharmaceutical industry. Adv. Synth. Catal. 2011, 353, 1825–1864. [Google Scholar] [CrossRef]
- Bringmann, G.; Günther, C.; Ochse, M.; Schupp, O.; Tasler, S. Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–249. [Google Scholar]
- Ayogu, J.I.; Onoabedje, E.A. Recent advances in transition metal-catalysed cross-coupling of (hetero) aryl halides and analogues under ligand-free conditions. Catal. Sci. Technol. 2019, 9, 5233–5255. [Google Scholar] [CrossRef]
- Komiyama, T.; Minami, Y.; Hiyama, T. Recent advances in transition-metal-catalyzed synthetic transformations of organosilicon reagents. ACS Catal. 2017, 7, 631–651. [Google Scholar] [CrossRef]
- Gil, A.; Albericio, F.; Alvarez, M. Role of the Nozaki–Hiyama–Takai–Kishi Reaction in the Synthesis of Natural Products. Chem. Rev. 2017, 117, 8420–8446. [Google Scholar] [CrossRef]
- Cherney, A.H.; Kadunce, N.T.; Reisman, S.E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 2015, 115, 9587–9652. [Google Scholar] [CrossRef]
- Markham, A.; Goa, K.L. Valsartan. Drugs 1997, 54, 299–311. [Google Scholar] [CrossRef]
- Croom, K.F.; Keating, G.M. Valsartan. Am. J. Cardiovasc. Drugs 2004, 4, 395–404. [Google Scholar] [CrossRef]
- Sharpe, M.; Jarvis, B.; Goa, K.L. Telmisartan. Drugs 2001, 61, 1501–1529. [Google Scholar] [CrossRef]
- Schultz, T.P.; Hubbard, T.F., Jr.; Jin, L.; Fisher, T.H.; Nicholas, D.D. Role of stilbenes in the natural durability of wood: Fungicidal structure-activity relationships. Phytochemistry 1990, 29, 1501–1507. [Google Scholar] [CrossRef]
- Yoshiyuki, K.; Hiromichi, O.; Shigeru, A. Effects of stilbenes on arachidonate metabolism in leukocytes. Biochim. Biophys Acta 1985, 834, 275–278. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Chaudhary, G.; Srivastava, A.K. Protective effect of resveratrol against pentylenetetrazole-induced seizures and its modulation by an adenosinergic system. Pharmacology 2002, 65, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Vannini, V. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Nagarathnam, D.; Gopal, D.; Chakraborti, A.K.; Lin, C.M.; Hamel, E. Synthesis and evaluation of stilbene and dihydrostilbene derivatives as potential anticancer agents that inhibit tubulin polymerization. J. Med. Chem. 1991, 34, 2579–2588. [Google Scholar] [CrossRef]
- Savouret, J.F.; Quesne, M. Resveratrol and cancer. Biomed. Pharmacother. 2002, 56, 84–87. [Google Scholar] [CrossRef]
- Kitamura, K.; Ando, Y.; Matsumoto, T.; Suzuki, K. Total synthesis of aryl C-glycoside natural products: Strategies and tactics. Chem. Rev. 2018, 118, 1495–1598. [Google Scholar] [CrossRef]
- De Clercq, E. C-Nucleosides to be revisited: Miniperspective. J. Med. Chem. 2016, 59, 2301–2311. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on SGLT2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef]
- Vaňková, K.; Rahm, M.; Choutka, J.; Pohl, R.; Parkan, K. Facile Approach to C-glucosides by Using a Protecting-Group-Free Hiyama Cross-Coupling Reaction: High-Yielding Dapagliflozin Synthesis. Chem. Eur. J. 2021, 27, 10583–10588. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, W.Q.; Lu, H.; Yu, T.Y.; Xu, W.H.; Wei, H. Rhodium-Catalyzed Hiyama Coupling Reaction of Unstrained Ketones via C− C Bond Cleavage. Asian J. Org. Chem. 2019, 8, 1358–1362. [Google Scholar] [CrossRef]
- Schütz, E. Longterm treatment of coronary heart disease with Segontin (author’s transl). MMW. Munch. Med. Wochenschr. 1976, 118, 1571–1574. [Google Scholar] [PubMed]
- Zolov, B. Benadryl intravenous use in allergy. Ann. Allergy 1949, 7, 254–257. [Google Scholar]
- Rische, T.; Eilbracht, P. One-pot synthesis of pharmacologically active secondary and tertiary 1-(3,3-diarylpropyl) amines via rhodium-catalysed hydroaminomethylation of 1, 1-diarylethenes. Tetrahedron 1999, 55, 1915–1920. [Google Scholar] [CrossRef]
- Tobe, A.; Yoshida, Y.; Ikoma, H.; Tonomura, S.; Kikumoto, R. Pharmacological evaluation of 2-(4-methylaminobutoxy) diphenylmethane hydrochloride (MCI-2016), a new psychotropic drug with antidepressant activity. Arzneim. Forsch. 1981, 31, 1278–1285. [Google Scholar]
- Zink, M.; Lanig, H.; Troschütz, R. Structural variations of piritrexim, a lipophilic inhibitor of human dihydrofolate reductase: Synthesis, antitumor activity and molecular modeling investigations. Eur. J. Med. Chem. 2004, 39, 1079–1088. [Google Scholar] [CrossRef]
- Sun, H.H.; Paul, V.J.; Fenical, W. Avrainvilleol, a brominated diphenylmethane derivative with feeding deterrent properties from the tropical green alga Avrainvillea longicaulis. Phytochemistry 1983, 22, 743–745. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef]
- Damayanthi, Y.; Lown, J.W. Podophyllotoxins: Current status and recent developments. Curr. Med. Chem. 1998, 5, 205–252. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Lee, K.H. Recent progress in the development of novel antitumor etoposide analogs. Chin. Pharm. J. 1994, 46, 319–369. [Google Scholar]
- Ramos, A.C.; Clairac, R.P.L.D.; Medarde, M.; Ramos, A.C.; Clairac, R.P.L.D.; Medarde, M. Heterolignans. Heterocycles 1999, 51, 1443–1470. [Google Scholar]
- Ward, R.S. The synthesis of lignans and neolignans. Chem. Soc. Rev. 1982, 11, 75–125. [Google Scholar] [CrossRef]
- Ward, R.S. Synthesis of podophyllotoxin and related compounds. Synthesis 1992, 1992, 719–730. [Google Scholar] [CrossRef]
- Sellars, J.D.; Steel, P.G. Advances in the synthesis of aryltetralin lignan lactones. Eur. J. Org. Chem. 2007, 2007, 3815–3828. [Google Scholar] [CrossRef]
- Gordaliza, M.; Garcıa, P.A.; Miguel del Corral, J.M.; Castro, M.A.; Gómez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Foubelo, F.; Nájera, C.; Yus, M. The Hiyama cross-coupling reaction: New discoveries. Chem. Rec. 2016, 16, 2521–2533. [Google Scholar] [CrossRef]
- Horn, K.A. Regio-and stereochemical aspects of the palladium-catalyzed reactions of silanes. Chem. Rev. 1995, 95, 1317–1350. [Google Scholar] [CrossRef]
- Hagiwara, E.; Gouda, K.I.; Hatanaka, Y.; Hiyama, T. NaOH-Promoted cross-coupling reactions of organosilicon compounds with organic halides: Practical routes to biaryls, alkenylarenes and conjugated dienes. Tetrahedron Lett. 1997, 38, 439–442. [Google Scholar] [CrossRef]
- Lee, H.M.; Nolan, S.P. Efficient cross-coupling reactions of aryl chlorides and bromides with phenyl-or vinyltrimethoxysilane mediated by a palladium/imidazolium chloride system. Org. Lett. 2000, 2, 2053–2055. [Google Scholar] [CrossRef]
- Li, J.H.; Deng, C.L.; Liu, W.J.; Xie, Y.X. Pd (OAc) 2/DABCO as an inexpensive and efficient catalytic system for Hiyama cross-coupling reactions of aryl halides with aryltrimethoxysilanes. Synthesis 2005, 2005, 3039–3044. [Google Scholar] [CrossRef]
- Gordillo, Á.; de Jesús, E.; López-Mardomingo, C. Consecutive palladium-catalyzed Hiyama–Heck reactions in aqueous media under ligand-free conditions. Chem. Commun. 2007, 39, 4056–4058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qing, J.; Yang, P.; Wu, J. Palladium-Catalyzed Hiyama Cross-Coupling Reactions of Aryl Mesylates. Org. Lett. 2008, 10, 4971–4974. [Google Scholar] [CrossRef] [PubMed]
- Molander, G.A.; Iannazzo, L. Palladium-catalyzed Hiyama cross-coupling of aryltrifluorosilanes with aryl and heteroaryl chlorides. J. Org. Chem. 2011, 76, 9182–9187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roglans, A.; Pla-Quintana, A.; Moreno-Manas, M. Diazonium Salts as Substrates in Palladium-Catalyzed Cross-Coupling Reactions. Chem. Rev. 2006, 106, 4622–4643. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, G.; Goggiamani, A.; Sferrazza, A.; Cacchi, S. Sonogashira Cross-Coupling of Arenediazonium Salts. Angew. Chem. Int. Ed. 2010, 49, 4067–4070. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Persiani, D. Palladium-Catalyzed Hydroarylation of Alkynes with Arenediazonium Salts. Org. Lett. 2008, 10, 1597–1600. [Google Scholar] [CrossRef]
- Robinson, M.K.; Kochurina, V.S.; Hanna, J.M., Jr. Palladium-catalyzed homocoupling of arenediazonium salts: An operationally simple synthesis of symmetrical biaryls. Tetrahedron Lett. 2007, 48, 7687–7690. [Google Scholar] [CrossRef]
- Panda, B.; Sarkar, T.K. Gold and palladium combined for the Sonogashira-type cross-coupling of arenediazonium salts. Chem. Commun. 2010, 46, 3131–3133. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Perboni, A.; Sferrazza, A.; Stabile, P. 2,3-Disubstituted indoles via palladium-catalyzed reaction of 2-alkynyltrifluoroacetanilides with arenediazonium tetrafluoroborates. Org. Lett. 2010, 12, 3279–3281. [Google Scholar] [CrossRef]
- Cheng, K.; Wang, C.; Ding, Y.; Song, Q.; Qi, C.; Zhang, X.M. Hiyama cross-coupling of arenediazonium salts under mild reaction conditions. J. Org. Chem. 2011, 76, 9261–9268. [Google Scholar] [CrossRef]
- Yuen, O.Y.; So, C.M.; Man, H.W.; Kwong, F.Y. A General Palladium-Catalyzed Hiyama Cross-Coupling Reaction of Aryl and Heteroaryl Chlorides. Chem. Eur. J. 2016, 22, 6471–6476. [Google Scholar] [CrossRef] [PubMed]
- Brandi, A.; Cicchi, S.; Cordero, F.M. Novel syntheses of azetidines and azetidinones. Chem. Rev. 2008, 108, 3988–4035. [Google Scholar] [CrossRef] [PubMed]
- Van Brabandt, W.; Mangelinckx, S.; D’hooghe, M.; Van Driessche, B.; De Kimpe, N. Synthesis and reactivity of 3-haloazetidines and 3-sulfonyloxyazetidines: A review. Curr. Org. Chem. 2009, 13, 829–853. [Google Scholar] [CrossRef]
- Secor, H.V.; Edwards III, W.B. Nicotine analogs: Synthesis of pyridylazetidines. J. Org. Chem. 1979, 44, 3136–3140. [Google Scholar] [CrossRef]
- Wang, D.X.; Booth, H.; Lerner-Marmarosh, N.; Osdene, T.S.; Abood, L.G. Structure–activity relationships for nicotine analogs comparing competition for [3H] nicotine binding and psychotropic potency. Drug Dev. Res. 1998, 45, 10–16. [Google Scholar] [CrossRef]
- Vagg, R.; Chapman, S. Nicotine analogues: A review of tobacco industry research interests. Addiction 2005, 100, 701–712. [Google Scholar] [CrossRef]
- Denton, T.T.; Zhang, X.; Cashman, J.R. 5-Substituted, 6-Substituted, and Unsubstituted 3-Heteroaromatic Pyridine Analogues of Nicotine as Selective Inhibitors of Cytochrome P-450 2A6. J. Med. Chem. 2005, 48, 224–239. [Google Scholar] [CrossRef]
- Barlow, R.B.; Hamilton, J.T. Effects of some isomers and analogues of nicotine on junctional transmission. Br. J. Pharmacol. Chemother. 1962, 18, 510–542. [Google Scholar] [CrossRef]
- Pogocki, D.; Ruman, T.; Danilczuk, M.; Danilczuk, M.; Celuch, M.; Wałajtys-Rode, E. Application of nicotine enantiomers, derivatives and analogues in therapy of neurodegenerative disorders. Eur. J. Pharmacol. 2007, 563, 18–39. [Google Scholar] [CrossRef]
- Liu, Z.; Luan, N.; Shen, L.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Palladium-Catalyzed Hiyama Cross-Couplings of Arylsilanes with 3-Iodoazetidine: Synthesis of 3-Arylazetidines. J. Org. Chem. 2019, 84, 12358–12365. [Google Scholar] [CrossRef]
- Kappe, C.O. 100 YEARS OF THE BlGlNELLl DIHYDROPYRIMIDINE SYNTHESIS. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Kappe, C.O. Synthesis of octahydroquinazolinone derivatives using silica sulfuric acid as an efficient catalyst. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Dallinger, D.; Stadler, A.; Kappe, C.O. Solid-and solution-phase synthesis of bioactive dihydropyrimidines. Pure Appl. Chem. 2004, 76, 1017–1024. [Google Scholar] [CrossRef][Green Version]
- Deres, K.; Schröder, C.H.; Paessens, A.; Goldmann, S.; Hacker, H.J.; Weber, O.; Rübsamen-Waigmann, H. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science 2003, 299, 893–896. [Google Scholar] [CrossRef]
- Liu, M.X.; Gong, H.P.; Quan, Z.J.; Wang, X.C. Palladium-Catalyzed Copper-Promoted Hiyama-Type Carbon–Carbon Cross-Coupling Reactions of Dihetaryl Disulfides as Electrophiles. Synlett 2018, 29, 330–335. [Google Scholar] [CrossRef]
- Hamasaka, G.; Sakurai, F.; Uozumi, Y.; Hamasaka, G.; Sakurai, F.; Uozumi, Y. A palladium NNC-pincer complex: An efficient catalyst for allylic arylation at parts per billion levels. Chem. Commun. 2015, 51, 3886–3888. [Google Scholar] [CrossRef]
- Hamasaka, G.; Ichii, S.; Uozumi, Y. A Palladium NNC-Pincer Complex as an Efficient Catalyst Precursor for the Mizoroki− Heck Reaction. Adv. Synth. Catal. 2018, 360, 1833–1840. [Google Scholar] [CrossRef]
- Ichii, S.; Hamasaka, G.; Uozumi, Y. The Hiyama Cross-Coupling Reaction at Parts Per Million Levels of Pd: In Situ Formation of Highly Active Spirosilicates in Glycol Solvents. Chem. Asian J. 2019, 14, 3850–3854. [Google Scholar] [CrossRef]
- Hughes, A.B. Amino Acids, Peptides and Proteins in Organic Chemistry, Analysis and Function of Amino Acids and Peptides; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Greenberg, A.; Breneman, C.M.; Liebman, J.F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Materials Science; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Idris, M.A.; Lee, S. Palladium-Catalyzed Amide N–C Hiyama Cross-Coupling: Synthesis of Ketones. Org. Lett. 2020, 22, 9190–9195. [Google Scholar] [CrossRef]
- Wu, X.X.; Ye, H.; Jiang, G.; Hu, L. Domino Heck/Hiyama cross-coupling: Trapping of the σ-alkylpalladium intermediate with arylsilanes. Org. Biomol. Chem. 2021, 19, 4254–4257. [Google Scholar] [CrossRef]
- Gonzalez-Lopez de Turiso, F.; Shin, Y.; Brown, M.; Cardozo, M.; Chen, Y.; Fong, D.; Cushing, T.D. Discovery and in vivo evaluation of dual PI3Kβ/δ inhibitors. J. Med. Chem. 2012, 55, 7667–7685. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhang, R.; Xia, X.; Ding, Y.; Sun, M.; Shi, L.; Wu, X.X. Synthesis of (Hetero) Aryl-Functionalized Azaindoline Derivatives by Palladium-Catalyzed Domino Heck Cyclization/Hiyama Cross-Coupling. Synthesis 2021, 53, 4079–4085. [Google Scholar] [CrossRef]
- Clarke, M.L. First Microwave-Accelerated Hiyama Coupling of Aryl-and Vinylsiloxane Derivatives: Clean Cross-Coupling of Aryl Chlorides within Minutes. Adv. Synth. Catal. 2005, 347, 303–307. [Google Scholar] [CrossRef]
- Pierrat, P.; Gros, P.; Fort, Y. Hiyama Cross-Coupling of Chloro-, Fluoro-, and Methoxypyridyltrimethylsilanes: Room-Temperature Novel Access to Functional Bi(het)aryl. Org. Lett. 2005, 7, 697–700. [Google Scholar] [CrossRef]
- Mino, T.; Shirae, Y.; Saito, T.; Sakamoto, M.; Fujita, T. Palladium-catalyzed Sonogashira and Hiyama reactions using phosphine-free hydrazone ligands. J. Org. Chem. 2006, 71, 9499–9502. [Google Scholar] [CrossRef] [PubMed]
- Tonogaki, K.; Itami, K.; Yoshida, J.I. Catalytic four-component assembly based on allenylboronate platform: New access to privileged allylic amine structures. J. Am. Chem. Soc. 2006, 128, 1464–1465. [Google Scholar] [CrossRef]
- Iftikhar, R.; Zahoor, A.F.; Ahmad, S.; Haq, A.U.; Naheed, S. Revisiting the Synthesis of Betti Bases: Facile, One-pot, and Efficient Synthesis of Betti Bases Promoted by FeCl3• 6H2O. Curr. Org. Syn. 2022, 19, 569–577. [Google Scholar] [CrossRef]
- Akai, S.; Ikawa, T.; Takayanagi, S.I.; Morikawa, Y.; Mohri, S.; Tsubakiyama, M.; Kita, Y. Synthesis of Biaryl Compounds through Three-Component Assembly: Ambidentate Effect of the tert-Butyldimethylsilyl Group for Regioselective Diels–Alder and Hiyama Coupling Reactions. Angew. Chem. Int. Ed. 2008, 47, 7673–7676. [Google Scholar] [CrossRef]
- Ines, B.; SanMartin, R.; Churruca, F.; Dominguez, E.; Urtiaga, M.K.; Arriortua, M.I. A nonsymmetric pincer-type palladium catalyst in Suzuki, Sonogashira, and Hiyama couplings in neat water. Organometallics 2008, 27, 2833–2839. [Google Scholar] [CrossRef]
- Chen, S.N.; Wu, W.Y.; Tsai, F.Y. Hiyama reaction of aryl bromides with arylsiloxanes catalyzed by a reusable palladium (II)/cationic bipyridyl system in water. Tetrahedron 2008, 64, 8164–8168. [Google Scholar] [CrossRef]
- Shirbin, S.J.; Boughton, B.A.; Zammit, S.C.; Zanatta, S.D.; Marcuccio, S.M.; Hutton, C.A.; Williams, S.J. Copper-free palladium-catalyzed Sonogashira and Hiyama cross-couplings using aryl imidazol-1-ylsulfonates. Tetrahedron Lett. 2010, 51, 2971–2974. [Google Scholar] [CrossRef]
- Marcuccio, S.M.; Epa, R.; Moslmani, M.; Hughes, A.B. Improved Hiyama cross-coupling reactions using HOMSi® reagents: A novel application of a palladacycle. Tetrahedron Lett. 2011, 52, 7178–7181. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Pezzuto, J.M. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Rhodes, M.R.; Herald, D.L.; Hamel, E.; Schmidt, J.M.; Pettit, R.K. Antineoplastic agents. 445. Synthesis and evaluation of structural modifications of (Z)-and (E)-combretastatin A-4. J. Med. Chem. 2005, 48, 4087–4099. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Pan, M.H.; Ghai, G.; Ho, C.T. Food bioactives, apoptosis, and cancer. Mol. Nutr. Food Res. 2008, 52, 43–52. [Google Scholar] [CrossRef]
- Segura, J.L.; Martín, N.; Guldi, D.M. Materials for organic solar cells: The C 60/π-conjugated oligomer approach. Chem. Soc. Rev. 2005, 34, 31–47. [Google Scholar] [CrossRef]
- Samuel, I.D.W.; Turnbull, G.A. Organic Semiconductor Lasers. Chem. Rev. 2007, 107, 1272–1295. [Google Scholar] [CrossRef]
- Skarżyńska, A.; Majchrzak, M.; Trzeciak, A.M.; Marciniec, B. An efficient synthesis of functional stilbenes in Hiyama coupling reaction catalysed by H-spirophosphorane palladium complex. J. Mol. Catal. A Chem. 2011, 351, 128–135. [Google Scholar] [CrossRef]
- Cheng, K.; Hu, S.; Zhao, B.; Zhang, X.M.; Qi, C. Palladium-catalyzed hiyama-type cross-coupling reactions of arenesulfinates with organosilanes. J. Org. Chem. 2013, 78, 5022–5025. [Google Scholar] [CrossRef]
- Chang, S.; Jin, Y.; Zhang, X.R.; Sun, Y.B. Carbonylative Hiyama coupling of aryl halides with arylsilanes under balloon pressure of CO. Tetrahedron Lett. 2016, 57, 2017–2020. [Google Scholar] [CrossRef]
- Cheltsov, A.V.; Aoyagi, M.; Aleshin, A.; Yu, E.C.W.; Gilliland, T.; Zhai, D.; Abagyan, R. Vaccinia virus virulence factor N1L is a novel promising target for antiviral therapeutic intervention. J. Med. Chem. 2010, 53, 3899–3906. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; Hamze, A.; Provot, O.; Tréguier, B.; Bignon, J.; Liu, J.M.; Alami, M. Discovery of isoerianin analogues as promising anticancer agents. ChemMedChem 2011, 6, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, Z.; Xu, S.; Wang, K.; Chen, K.; Zhao, J. Palladium-Catalyzed Hiyama Coupling of Benzylic Ammonium Salts via C–N Bond Cleavage. J. Org. Chem. 2019, 84, 16308–16313. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, T.; Oestreich, M. Organosilicon Chemistry—Novel Approaches and Reactions; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Wisthoff, M.F.; Pawley, S.B.; Cinderella, A.P.; Watson, D.A. Stereoselective synthesis of cis-and trans-tetrasubstituted vinyl silanes using a silyl-heck strategy and hiyama conditions for their cross-coupling. J. Am. Chem. Soc. 2020, 142, 12051–12055. [Google Scholar] [CrossRef]
- Orha, L.; Papp, Á.; Tukacs, J.M.; Kollár, L.; Mika, L.T. Tetrabutylphosphonium 4-ethoxyvalerate as a biomass-originated media for homogeneous palladium-catalyzed Hiyama coupling reactions. Chem. Pap. 2020, 74, 4593–4598. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon−Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef]
- Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Yanase, T.; Mori, S.; Monguchi, Y.; Sajiki, H. Pd/C-catalyzed and water-mediated Hiyama cross-coupling reaction using an electron-deficient phosphine ligand. Chem. Lett. 2011, 40, 910–912. [Google Scholar] [CrossRef]
- Monguchi, Y.; Yanase, T.; Mori, S.; Sajiki, H. A practical protocol for the hiyama cross-coupling reaction catalyzed by palladium on carbon. Synthesis 2013, 45, 40–44. [Google Scholar] [CrossRef][Green Version]
- Yanase, T.; Monguchi, Y.; Sajiki, H. Ligand-free Hiyama cross-coupling reaction catalyzed by palladium on carbon. RSC Adv. 2012, 2, 590–594. [Google Scholar] [CrossRef]
- Gu, Y.; Jerome, F. Bio-based solvents: An emerging generation of fluids for the design of eco-efficient processes in catalysis and organic chemistry. Chem. Soc. Rev. 2013, 42, 9550–9570. [Google Scholar] [CrossRef] [PubMed]
- Ismalaj, E.; Strappaveccia, G.; Ballerini, E.; Elisei, F.; Piermatti, O.; Gelman, D.; Vaccaro, L. γ-Valerolactone as a Renewable Dipolar Aprotic Solvent Deriving from Biomass Degradation for the Hiyama Reaction. ACS Sustain. Chem. Eng. 2014, 2, 2461–2464. [Google Scholar] [CrossRef]
- Diederich, F.; Stang, P.J. (Eds.) . Metal-Catalyzed Cross-Coupling Reactions; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Sreedhar, B.; Kumar, A.S.; Yada, D. Magnetically Recoverable Pd/Fe3O4-Catalyzed Hiyama Cross-Coupling of Aryl Bromides with Aryl Siloxanes. Synlett 2011, 2011, 1081–1084. [Google Scholar] [CrossRef]
- Lee, W.S.; Byun, S.; Kwon, J.; Kim, B.M. Magnetic Pd–Fe3O4 Heterodimer Nanocrystals as Recoverable Catalysts for Ligand-Free Hiyama Cross-Coupling Reactions. Bull. Korean Chem. Soc. 2016, 37, 1992–1997. [Google Scholar] [CrossRef]
- Kantchev, E.A.B.; O’Brien, C.J.; Organ, M.G. Palladium complexes of N-heterocyclic carbenes as catalysts for cross-coupling reactions—A synthetic chemist’s perspective. Angew. Chem. Int. Ed. 2007, 46, 2768–2813. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. Well-defined N-heterocyclic carbenes− palladium (II) precatalysts for cross-coupling reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef]
- Diez-Gonzalez, S.; Marion, N.; Nolan, S.P. N-heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef]
- Penafiel, I.; Pastor, I.M.; Yus, M.; Esteruelas, M.A.; Olivan, M.; Onate, E. (NHC) Palladium Complexes from Hydroxy-Functionalized Imidazolium Salts as Catalyst for the Microwave-Accelerated Fluorine-Free Hiyama Reaction. Eur. J. Org. Chem. 2011, 2011, 7174–7181. [Google Scholar] [CrossRef]
- Peñafiel, I.; Pastor, I.M.; Yus, M. NHC-Ligand Effectiveness in the Fluorine-Free Hiyama Reaction of Aryl Halides. Eur. J. Org. Chem. 2013, 2013, 1479–1484. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L. Synthesis and characterization of dinuclear NHC–palladium complexes and their applications in the Hiyama reactions of aryltrialkyoxysilanes with aryl chlorides. Dalton Trans. 2012, 41, 12031–12037. [Google Scholar] [CrossRef] [PubMed]
- Rostamnia, S.; Hossieni, H.G.; Doustkhah, E. Homoleptic chelating N-heterocyclic carbene complexes of palladium immobilized within the pores of SBA-15/IL (NHC–Pd@ SBA-15/IL) as heterogeneous catalyst for Hiyama reaction. J. Organomet. Chem. 2015, 791, 18–23. [Google Scholar] [CrossRef]
- Yang, J. Mono-and dinuclear palladium (ii) complexes containing both N-heterocyclic carbenes and tetrazole ligands as catalysts for Hiyama coupling. New J. Chem. 2016, 40, 9739–9745. [Google Scholar] [CrossRef]
- Yasar, S.; Sahin, C.; Arslan, M.; Ozdemir, I. Synthesis, characterization and the Suzuki-Miyaura coupling reactions of N-heterocyclic carbene-Pd (II)-pyridine (PEPPSI) complexes. J. Organomet. Chem. 2015, 776, 107–112. [Google Scholar] [CrossRef]
- Rajabi, F.; Thiel, W.R. An Efficient Palladium N-Heterocyclic Carbene Catalyst Allowing the Suzuki–Miyaura Cross-Coupling of Aryl Chlorides and Arylboronic Acids at Room Temperature in Aqueous Solution. Adv. Synth. Catal. 2014, 356, 1873–1877. [Google Scholar] [CrossRef]
- Dunsford1, J.J.; Cavell, K.J. Pd–PEPPSI-type expanded ring N-heterocyclic carbene complexes: Synthesis, characterization, and catalytic activity in Suzuki–Miyaura cross coupling. Organometallics 2014, 33, 2902–2905. [Google Scholar] [CrossRef]
- Chen, M.T.; Vicic, D.A.; Turner, M.L.; Navarro, O. (N-heterocyclic carbene) PdCl2 (TEA) complexes: Studies on the effect of the “throw-away” ligand in catalytic activity. Organometallics 2011, 30, 5052–5056. [Google Scholar] [CrossRef]
- Benhamou, L.; Besnard, C.; Kündig, E.P. Chiral PEPPSI Complexes: Synthesis, Characterization, and Application in Asymmetric Suzuki–Miyaura Coupling Reactions. Organometallics 2014, 33, 260–266. [Google Scholar] [CrossRef]
- Ray, L.; Shaikh, M.M.; Ghosh, P. Air-stable, convenient to handle Pd based PEPPSI (pyridine enhanced precatalyst preparation, stabilization and initiation) themed precatalysts of N/O-functionalized N-heterocyclic carbenes and its utility in Suzuki–Miyaura cross-coupling reaction. Dalton Trans. 2007, 40, 4546–4555. [Google Scholar] [CrossRef]
- Modak, S.; Gangwar, M.K.; Rao, M.N.; Madasu, M.; Kalita, A.C.; Dorcet, V.; Ghosh, P. Fluoride-free Hiyama coupling by palladium abnormal N-heterocyclic carbene complexes. Dalton Trans. 2015, 44, 17617–17628. [Google Scholar] [CrossRef]
- Dash, C.; Shaikh, M.M.; Ghosh, P. Fluoride-Free Hiyama and Copper-and Amine-Free Sonogashira Coupling in Air in a Mixed Aqueous Medium by a Series of PEPPSI-Themed Precatalysts. Eur. J. Inorg. Chem. 2009, 2009, 1608–1618. [Google Scholar] [CrossRef]
- Mitsui, T.; Sugihara, M.; Tokoro, Y.; Fukuzawa, S.I. Synthesis of adamantyl substituted 1, 2, 3-triazol-5-ylidene ligands and their PEPPSI-type palladium complexes. Tetrahedron 2015, 71, 1509–1514. [Google Scholar] [CrossRef]
- Osińska, M.; Gniewek, A.; Trzeciak, A.M. Suzuki–Miyaura and Hiyama coupling catalyzed by PEPPSI-type complexes with non-bulky NHC ligand. J. Mol. Catal. A Chem. 2016, 418, 9–18. [Google Scholar] [CrossRef]
- Astruc, D. Palladium Nanoparticles as Efficient Green Homogeneous and Heterogeneous Carbon−Carbon Coupling Precatalysts: A Unifying View. Inorg. Chem. 2007, 46, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Favier, I.; Pla, D.; Gómez, M. Palladium Nanoparticles in Polyols: Synthesis, Catalytic Couplings, and Hydrogenations. Chem. Rev. 2020, 120, 1146–1183. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Sawoo, S.; Sarkar, A. In situ generation of gold nanoparticles on a protein surface: Fischer carbene complex as reducing agent. Chem. Commun 2006, 32, 3438–3440. [Google Scholar] [CrossRef]
- Srimani, D.; Sawoo, S.; Sarkar, A. Convenient synthesis of palladium nanoparticles and catalysis of Hiyama coupling reaction in water. Org. Lett. 2007, 9, 3639–3642. [Google Scholar] [CrossRef]
- Ranu, B.C.; Dey, R.; Chattopadhyay, K. A one-pot efficient and fast Hiyama coupling using palladium nanoparticles in water under fluoride-free conditions. Tetrahedron Lett. 2008, 49, 3430–3432. [Google Scholar] [CrossRef]
- Leeson, P.D.; Emmett, J.C.; Shah, V.P.; Showell, G.A.; Novelli, R.; Prain, H.D.; Benson, M.G.; Ellis, D.; Pearce, N.J.; Underwood, A.H. Selective thyromimetics. Cardiac-sparing thyroid hormone analogs containing 3’-arylmethyl substituents. J. Med. Chem. 1989, 32, 320–336. [Google Scholar] [CrossRef]
- Yi-Yin, K.; Patel, R.R.; Sawick, D.P. A general, convenient and highly efficient synthesis of diarylmethanes by copper-catalyzed reaction. Tetrahedron Lett. 1996, 37, 1949–1952. [Google Scholar] [CrossRef]
- Gangjee, A.; Devraj, R.; Queener, S.F. Synthesis and dihydrofolate reductase inhibitory activities of 2, 4-diamino-5-deaza and 2, 4-diamino-5, 10-dideaza lipophilic antifolates. J. Med. Chem. 1997, 40, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Vasudevan, A.; Queener, S.F. Conformationally restricted analogues of trimethoprim: 2, 6-diamino-8-substituted purines as potential dihydrofolate reductase inhibitors from Pneumocystis carinii and Toxoplasma gondii. J. Med. Chem. 1997, 40, 3032–3039. [Google Scholar] [CrossRef] [PubMed]
- McPhail, K.L.; Rivett, D.E.; Lack, D.E.; Davies-Coleman, M.T. The structure and synthesis of tsitsikammafuran: A new furanosesquiterpene from a South African Dysidea sponge. Tetrahedron 2000, 56, 9391–9396. [Google Scholar] [CrossRef]
- Philp, D.; Stoddart, J.F. Self-Assembly in Natural and Unnatural Systems. Angew. Chem. Int. Ed. Engl. 1996, 35, 1154–1196. [Google Scholar] [CrossRef]
- Conn, M.M.; Rebek, J. Self-Assembling Capsules. Chem. Rev. 1997, 97, 1647–1668. [Google Scholar] [CrossRef]
- Jasat, A.; Sherman, J.C. Carceplexes and Hemicarceplexes. Chem Rev. 1999, 99, 931–968. [Google Scholar] [CrossRef]
- Ma, J.C.; Dougherty, D.A. The Cation−π Interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, Y.; Wang, Y. Palladium nanoparticles in poly (ethyleneglycol): The efficient and recyclable catalyst for Heck reaction. J. Mol. Catal. A Chem. 2005, 229, 7–12. [Google Scholar] [CrossRef]
- Srimani, D.; Bej, A.; Sarkar, A. Palladium nanoparticle catalyzed Hiyama coupling reaction of benzyl halides. J. Org. Chem. 2010, 75, 4296–4299. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; Li, H.; Wang, L. A recyclable magnetic nanoparticles supported palladium catalyst for the Hiyama reaction of aryltrialkoxysilanes with aryl halides. Catal. Sci. Technol. 2012, 2, 1859–1864. [Google Scholar] [CrossRef]
- Premi, C.; Jain, N. Phosphane-Free Hiyama Cross-Coupling of Aryl and Heteroaryl Halides Catalyzed by Palladium Nanoparticles in Ionic Liquids. Eur. J. Org. Chem. 2013, 2013, 5493–5499. [Google Scholar] [CrossRef]
- Rettig, M.F.; Maitlis, P.M.; Cotton, F.A.; Webb, T.R. Tetrakis (tert-Butyl Isocyanide) Di-μ-Chlorodipalladium (I). Inorganic Syntheses: Reagents for Transition Metal Complex and Organometallic Syntheses. Inorg. Synth. 1990, 28, 110–113. [Google Scholar] [CrossRef]
- Trilla, M.; Pleixats, R.; Parella, T.; Blanc, C.; Dieudonné, P.; Guari, Y.; Man, M.W.C. Ionic liquid crystals based on mesitylene-containing bis-and trisimidazolium salts. Langmuir 2008, 24, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Planellas, M.; Moglie, Y.; Alonso, F.; Yus, M.; Pleixats, R.; Shafir, A. Heck, Sonogashira, and Hiyama Reactions Catalyzed by Palladium Nanoparticles Stabilized by Tris-Imidazolium Salt. Eur. J. Org. Chem. 2014, 2014, 3001–3008. [Google Scholar] [CrossRef]
- Huang, S.H.; Liu, C.H.; Yang, C.M. Efficient ligand-free Hiyama cross-coupling reaction catalyzed by functionalized SBA-15-supported Pd nanoparticles. Green Chem. 2014, 16, 2706–2712. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Honarmand, E.; Maham, M. Preparation of palladium nanoparticles using Euphorbia thymifolia L. leaf extract and evaluation of catalytic activity in the ligand-free Stille and Hiyama cross-coupling reactions in water. New J. Chem. 2015, 39, 4745–4752. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Razmi, Z. Palladium Supported on Zinc Oxide Nanoparticles as Efficient Heterogeneous Catalyst for Suzuki Miyaura and Hiyama Reactions under Normal Laboratory Conditions. Helv. Chim. Acta 2015, 98, 805–818. [Google Scholar] [CrossRef]
- Ohtaka, A.; Kotera, T.; Sakon, A.; Ueda, K.; Hamasaka, G.; Uozumi, Y.; Nomura, R. Fluoride-Free Hiyama Coupling Reaction Catalyzed by Linear Polystyrene-Stabilized PdO Nanoparticles in Water: Specific Reactivity of PdO Nanoparticles over Pd nanoparticles. Synlett 2016, 27, 1202–1206. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S.; Hossieni, H.G.; Luque, R. Covalently Bonded PIDA on SBA-15 as Robust Pd Support: Water-Tolerant Designed Catalysts for Aqueous Suzuki Couplings. ChemistrySelect 2017, 2, 329–334. [Google Scholar] [CrossRef]
- Rostamnia, S.; Alamgholiloo, H.; Liu, X. Pd-grafted open metal site copper-benzene-1, 4-dicarboxylate metal organic frameworks (Cu-BDC MOF’s) as promising interfacial catalysts for sustainable Suzuki coupling. J. Colloid Interface Sci. 2016, 469, 310–317. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E.; Zeynizadeh, B. Cationic modification of SBA-15 pore walls for Pd supporting: Pd@ SBA-15/ILDABCO as a catalyst for Suzuki coupling in water medium. Microporous Mesoporous Mater. 2016, 222, 87–93. [Google Scholar] [CrossRef]
- Yousaf, M.; Zahoor, A.F.; Akhtar, R.; Ahmad, M.; Naheed, S. Development of green methodologies for Heck, Chan-Lam, Stille and Suzuki cross-coupling reactions. Mol. Divers. 2020, 24, 821–839. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.C.; Molina-Bolivar, J.A.; Aguiar, J.; MacIsaac, G.; Moroze, S.; Palepu, R. Thermodynamic and Structural Studies of Triton X-100 Micelles in Ethylene Glycol-Water Mixed Solvents. Langmuir 2001, 17, 6831–6840. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.H.; Wu, J. Effects of surfactants on the rate of chemical reactions. J. Chem. 2014, 2014, 908476. [Google Scholar] [CrossRef]
- Gaikwad, D.S.; Undale, K.A.; Patil, D.B.; Pore, D.M.; Kamble, A.A. Triton X-100 stabilized Pd nanoparticles and their catalytic application in one-pot sequential Heck and Hiyama coupling in water. Res. Chem. Intermed. 2018, 44, 265–275. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S.; Ali, M.; Khan, M.A. Biological role of Piper nigrum L. (Black pepper): A review. Asian Pac. J. Trop. Biomed. 2012, 2, S1945–S1953. [Google Scholar] [CrossRef]
- Kandathil, V.; Dateer, R.B.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. Green synthesis of palladium nanoparticles: Applications in aryl halide cyanation and hiyama cross-coupling reaction under ligand free conditions. Catal. Lett. 2018, 148, 1562–1578. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R.; Vivier-Bunge, A. Non-alkane behavior of cyclopropane and its derivatives: Characterization of unconventional hydrogen bond interactions. Theor. Chem. Acc. 2007, 118, 597–606. [Google Scholar] [CrossRef]
- Wiberg, K.B. Bent bonds in organic compounds. Acc. Chem. Res. 1996, 29, 229–234. [Google Scholar] [CrossRef]
- Pietruszka, J. Synthesis and properties of oligocyclopropyl-containing natural products and model compounds. Chem. Rev. 2003, 103, 1051–1070. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Brandt, W.; Thiemann, T. Biosynthesis and metabolism of cyclopropane rings in natural compounds. Chem. Rev. 2003, 103, 1625–1648. [Google Scholar] [CrossRef]
- Donaldson, W.A. Synthesis of cyclopropane containing natural products. Tetrahedron 2001, 57, 8589–8627. [Google Scholar] [CrossRef]
- Beaulieu, L.P.B.; Delvos, L.B.; Charette, A.B. Dual Role of Silanol Groups in Cyclopropanation and Hiyama− Denmark Cross-Coupling Reactions. Org. Lett. 2010, 12, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.Z.; Paone, D.V.; Ginnetti, A.T.; Kurihara, H.; Dreher, S.D.; Weissman, S.A.; Burgey, C.S. Copper-Facilitated Suzuki Reactions: Application to 2-Heterocyclic Boronates. Org. Lett. 2009, 11, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Traficante, C.I.; Mata, E.G.; Delpiccolo, C.M. Very efficient and broad-in-scope palladium-catalyzed Hiyama cross-coupling. The role of water and copper (i) salts. RSC Adv. 2015, 5, 26796–26800. [Google Scholar] [CrossRef]
- Chang, W.T.T.; Smith, R.C.; Regens, C.S.; Bailey, A.D.; Werner, N.S.; Denmark, S.E. Cross-coupling with organosilicon compounds. Org. React. 2004, 75, 213–746. [Google Scholar] [CrossRef]
- Sore, H.F.; Galloway, W.R.; Spring, D.R. Palladium-catalysed cross-coupling of organosilicon reagents. Chem. Soc. Rev. 2012, 41, 1845–1866. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, T. How I came across the silicon-based cross-coupling reaction. J. Organomet. Chem. 2002, 653, 58–61. [Google Scholar] [CrossRef]
- Denmark, S.E.; Liu, J.H.C. Silicon-Based Cross-Coupling Reactions in the Total Synthesis of Natural Products. Angew. Chem. Int. Ed. 2010, 49, 2978–2986. [Google Scholar] [CrossRef]
- Handy, C.J.; Manoso, A.S.; McElroy, W.T.; Seganish, W.M.; DeShong, P. Recent advances in siloxane-based aryl–aryl coupling reactions: Focus on heteroaromatic systems. Tetrahedron 2005, 52, 12201–12225. [Google Scholar] [CrossRef]
- Shukla, K.H.; DeShong, P. Advances in siloxane-based coupling reactions: Application of palladium-mediated allyl-aryl coupling to the synthesis of pancratistatin derivatives: The formal total synthesis of (±)-7-deoxypancratistatin. Heterocycles 2012, 86, 1055–1069. [Google Scholar] [CrossRef]
- Gurung, S.K.; Thapa, S.; Vangala, A.S.; Giri, R. Copper-catalyzed Hiyama coupling of (hetero) aryltriethoxysilanes with (hetero) aryl iodides. Org. Lett. 2013, 15, 5378–5381. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, L.; Cirriez, V.; Vercruysse, S.; Riant, O. Copper-catalyzed Hiyama cross-coupling using vinylsilanes and benzylic electrophiles. Chem. Commun. 2014, 50, 8018–8020. [Google Scholar] [CrossRef] [PubMed]
- Pachón, L.D.; Thathagar, M.B.; Hartl, F.; Rothenberg, G. Palladium-coated nickel nanoclusters: New Hiyama cross-coupling catalysts. Phys. Chem. Chem. Phys. 2006, 8, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Strotman, N.A.; Sommer, S.; Fu, G.C. Hiyama reactions of activated and unactivated secondary alkyl halides catalyzed by a nickel/norephedrine complex. Angew. Chem. 2007, 119, 3626–3628. [Google Scholar] [CrossRef]
- Dai, X.; Strotman, N.A.; Fu, G.C. Catalytic asymmetric Hiyama cross-couplings of racemic α-bromo esters. J. Am. Chem. Soc. 2008, 130, 3302–3303. [Google Scholar] [CrossRef] [PubMed]
- Saijo, H.; Sakaguchi, H.; Ohashi, M.; Ogoshi, S. Base-Free Hiyama Coupling Reaction via a Group 10 Metal Fluoride Intermediate Generated by C–F Bond Activation. Organometallics 2014, 33, 3669–3672. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.R.; Cao, Y.X.; Lan, Q.; Wang, X.S. Nickel-Catalyzed Monofluoroalkylation of Arylsilanes via Hiyama Cross-Coupling. Org. Lett. 2016, 18, 5564–5567. [Google Scholar] [CrossRef]
- Edwin Raja, G.C.; Irudayanathan, F.M.; Kim, H.S.; Kim, J.; Lee, S. Nickel-catalyzed Hiyama-type decarboxylative coupling of propiolic acids and organosilanes. J. Org. Chem. 2016, 81, 5244–5249. [Google Scholar] [CrossRef]
- Sporn, M.B.; Roberts, A.B.; Goodman, D.S. The Retinoids: Biology, Chemistry and Medicine; Raven: New York, NY, USA, 1993. [Google Scholar]
- Montenegro, J.; Bergueiro, J.; Saá, C.; Lopez, S. Hiyama cross-coupling reaction in the stereospecific synthesis of retinoids. Org. Lett. 2009, 11, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Fronczek, F.R. Novel sesquiterpene from bioactive fractions of cultivar sunflowers. Tetrahedron Lett. 1993, 34, 1999–2002. [Google Scholar] [CrossRef]
- Macias, F.A.; Molinillo, J.M.; Varela, R.M.; Torres, A.; Fronczek, F.R. Structural Elucidation and Chemistry of a Novel Family of Bioactive Sesquiterpenes: Heliannuols. J. Org. Chem. 1994, 59, 8261–8266. [Google Scholar] [CrossRef]
- Macías, F.A.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Heliannuol, E. A Novel Bioactive Sesquiterpene of the Heliannane Family. Tetrahedron Lett. 1999, 40, 4725–4728. [Google Scholar] [CrossRef]
- Macías, F.A.; Varela, R.M.; Torres, A.; Molinillo, J.M. New bioactive plant heliannuols from cultivar sunflower leaves. J. Nat. Prod. 1999, 62, 1636–1639. [Google Scholar] [CrossRef]
- Macías, F.A.; Torres, A.; Galindo, J.L.G.; Varela, R.M.; Álvarez, J.A.; Molinillo, J.M.G. Bioactive terpenoids from sunflower leaves cv. Peredovick®. Phytochemistry 2002, 61, 687–692. [Google Scholar] [CrossRef]
- El Marsni, Z.; Torres, A.; Varela, R.M.; Molinillo, J.M.; Casas, L.; Mantell, C.; Macias, F.A. Isolation of bioactive compounds from sunflower leaves (Helianthus annuus L.) extracted with supercritical carbon dioxide. J. Agric. Food Chem. 2015, 63, 6410–6421. [Google Scholar] [CrossRef]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Macías, F.A.; Varela, R.M.; Torres, A.; Molinillo, J.M.G. Potential allelopathic activity of natural plant heliannanes: A proposal of absolute configuration and nomenclature. J. Chem. Ecol. 2000, 26, 2173–2186. [Google Scholar] [CrossRef]
- Vyvyan, J.R.; Engles, C.A.; Bray, S.L.; Wold, E.D.; Porter, C.L.; Konev, M.O. Synthesis of substituted Z-styrenes by Hiyama-type coupling of oxasilacycloalkenes: Application to the synthesis of a 1-benzoxocane. Beilstein J. Org. Chem. 2017, 13, 2122–2127. [Google Scholar] [CrossRef]
- Singh, S.N.; Fletcher, R.D.; Fisher, S.G.; Singh, B.N.; Lewis, H.D.; Deedwania, P.C.; Lazzeri, D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. N. Engl. J. Med. 1995, 333, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Flynn, B.L.; Gill, G.S.; Grobelny, D.W.; Chaplin, J.H.; Paul, D.; Leske, A.F.; Kremmidiotis, G. Discovery of 7-hydroxy-6-methoxy-2-methyl-3-(3, 4, 5-trimethoxybenzoyl) benzo [b] furan (BNC105), a tubulin polymerization inhibitor with potent antiproliferative and tumor vascular disrupting properties. J. Med. Chem. 2011, 54, 6014–6027. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Q.; Fukai, T.; Sakagami, H.; Chang, W.J.; Yang, P.Q.; Wang, F.P.; Nomura, T. Cytotoxic flavonoids with isoprenoid groups from Morus mongolica. J. Nat. Prod. 2001, 64, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Naveen, M.; Reddy, C.U.; Hussain, M.M.; Chaitanya, M.; Narayanaswamy, G. Alternate and Efficient Method for the Total Synthesis of Egonol via Sonogashira Coupling Reaction. J. Heterocycl. Chem. 2013, 50, 1064–1066. [Google Scholar] [CrossRef]
- Choi, D.H.; Hwang, J.W.; Lee, H.S.; Yang, D.M.; Jun, J.G. Highly effective total synthesis of benzofuran natural product egonol. Bull. Korean Chem. Soc. 2008, 29, 1594–1596. [Google Scholar]
- Yuan, H.; Bi, K.J.; Li, B.; Yue, R.C.; Ye, J.; Shen, Y.H.; Zhang, W.D. Construction of 2-substituted-3-functionalized benzofurans via intramolecular heck coupling: Application to enantioselective total synthesis of daphnodorin B. Org. Lett. 2013, 15, 4742–4745. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, Y.; Kaur, N.; Choi, Y.; Lee, K. HIF-1α inhibitors: Synthesis and biological evaluation of novel moracin O and P analogues. Eur. J. Med. Chem. 2011, 46, 2386–2396. [Google Scholar] [CrossRef]
- Kaur, N.; Xia, Y.; Jin, Y.; Dat, N.T.; Gajulapati, K.; Choi, Y.; Lee, K. The first total synthesis of moracin O and moracin P, and establishment of the absolute configuration of moracin O. Chem. Commun. 2009, 14, 1879–1881. [Google Scholar] [CrossRef]
- Singh, C.; Prakasham, A.P.; Gangwar, M.K.; Butcher, R.J.; Ghosh, P. One-pot tandem hiyama alkynylation/cyclizations by palladium (II) acyclic diaminocarbene (ADC) complexes yielding biologically relevant benzofuran scaffolds. ACS Omega 2018, 3, 1740–1756. [Google Scholar] [CrossRef]
- Kumar, A.; Gangwar, M.K.; Prakasham, A.P.; Mhatre, D.; Kalita, A.C.; Ghosh, P. Accessing a Biologically Relevant Benzofuran Skeleton by a One-Pot Tandem Heck Alkynylation/Cyclization Reaction Using Well-Defined Palladium N-Heterocyclic Carbene Complexes. Inorg. Chem. 2016, 55, 2882–2893. [Google Scholar] [CrossRef]
- Kumar, A.; Naaz, A.; Prakasham, A.P.; Gangwar, M.K.; Butcher, R.J.; Panda, D.; Ghosh, P. Potent anticancer activity with high selectivity of a chiral palladium N-Heterocyclic Carbene Complex. ACS Omega 2017, 2, 4632–4646. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Prakasham, A.P.; Ghosh, P. Palladium Acyclic Diaminocarbene (ADC) Triflate Complexes as Effective Precatalysts for the Hiyama Alkynylation/Cyclization Reaction Yielding Benzofuran Compounds: Probing the Influence of the Triflate Co-Ligand in the One-Pot Tandem Reaction. ChemistrySelect. 2019, 4, 329–336. [Google Scholar] [CrossRef]
- Lee, J.Y.; Fu, G.C. Room-temperature Hiyama cross-couplings of arylsilanes with alkyl bromides and iodides. J. Am. Chem. Soc. 2003, 125, 5616–5617. [Google Scholar] [CrossRef]
- Alacid, E.; Najera, C. The first fluoride-free Hiyama reaction of vinylsiloxanes promoted by sodium hydroxide in water. Adv. Synth. Catal. 2006, 348, 2085–2091. [Google Scholar] [CrossRef]
- Modak, A.; Mondal, J.; Bhaumik, A. Pd-grafted periodic mesoporous organosilica: An efficient heterogeneous catalyst for Hiyama and Sonogashira couplings, and cyanation reactions. Green Chem. 2012, 14, 2840–2855. [Google Scholar] [CrossRef]
- Fuchita, Y.; Yoshinaga, K.; Hanaki, T.; Kawano, H.; Kinoshita-Nagaoka, J. Cyclopalladation of mono-, di-and tribenzylamine by palladium (II) acetate: Influence of bulkiness around the nitrogen atom of benzylamine upon internal metallation. J. Organomet. Chem. 1999, 580, 273–281. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F. Application of a dimeric ortho-palladated complex of tribenzylamine as an efficient catalyst in microwave-assisted Hiyama coupling reactions. Appl. Organomet. Chem. 2012, 26, 51–55. [Google Scholar] [CrossRef]
- Morrison, R.T.; Boyd, R.N. Structures and Properties. Organic Chemistry, 5th ed.; Allyn and Bacon: Boston, MA, USA, 1987. [Google Scholar]
- Kirk, R.E.; Othmer, D.F.; Grayson, M.; Eckroth, D. Kirk-Othmer Concise Encyclopedia of Chemical Technology; Wiley-VCH: New York, NY, USA, 1985. [Google Scholar]
- Zhang, W.; Liu, F.; Li, K.; Zhao, B. Pd-catalyzed desulfitative Hiyama coupling with sulfonyl chlorides. Appl. Organomet. Chem. 2014, 28, 379–381. [Google Scholar] [CrossRef]
- Miao, H.; Wang, F.; Zhou, S.; Zhang, G.; Li, Y. Palladium-catalyzed Hiyama coupling reaction of arylsulfonyl hydrazides under oxygen. Org. Biomol. Chem. 2015, 13, 4647–4651. [Google Scholar] [CrossRef]
- Melvin, P.R.; Hazari, N.; Beromi, M.M.; Shah, H.P.; Williams, M. Pd-catalyzed Suzuki–Miyaura and Hiyama–Denmark couplings of aryl sulfamates. J. Org. Lett. 2016, 18, 5784–5787. [Google Scholar] [CrossRef]
- Xie, J.; Sekine, K.; Witzel, S.; Krämer, P.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Light-Induced Gold-Catalyzed Hiyama Arylation: A Coupling Access to Biarylboronates. Angew. Chem. Int. Ed. 2018, 130, 16890–16895. [Google Scholar] [CrossRef]
- Marset, X.; De Gea, S.; Guillena, G.; Ramón, D. NCN–pincer–Pd complex as catalyst for the hiyama reaction in biomass-derived solvents. ACS Sustain. Chem. Eng. 2018, 6, 5743–5748. [Google Scholar] [CrossRef]
- Sobhani, S.; Moghadam, H.H.; Skibsted, J.; Sansano, J.M. A hydrophilic heterogeneous cobalt catalyst for fluoride-free Hiyama, Suzuki, Heck and Hirao cross-coupling reactions in water. Green Chem. 2020, 22, 1353–1365. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).