Biopreservation of Chocolate Mousse with Lactobacillus helveticus 2/20: Microbial Challenge Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Nutrient Media
2.2.1. Normal Saline Solution (0.9% (w/v) NaCl)
2.2.2. MRS Broth
2.2.3. LAPTg10 Agar
2.2.4. LBG Broth
2.2.5. LBG Agar
2.3. Experimental Procedures and Methods of Analysis
2.3.1. Determination of Biochemical Profile of Lactobacillus 2/20
2.3.2. Molecular-Genetic Identification of Lactobacillus 2/20: Sequencing of the 16S rRNA Gene of Lactobacillus 2/20
2.3.3. Biopreservation Assay
Encapsulation of Lactic Acid Bacteria in Calcium Alginate Particles [24]
Preparation of Chocolate Mousse
Investigation of the Capacity of Lb. helveticus 2/20 to Control Unwanted Bacteria
- A.
- Investigation in MRS Broth
- B.
- Determination of the Antimicrobial Activity Against Pathogenic Microorganisms by Co-cultivation in Chocolate Mousse (Microbial Challenge Test)
2.4. Processing of the Results
3. Results and Discussion
3.1. Identification of Lactobacillus 2/20
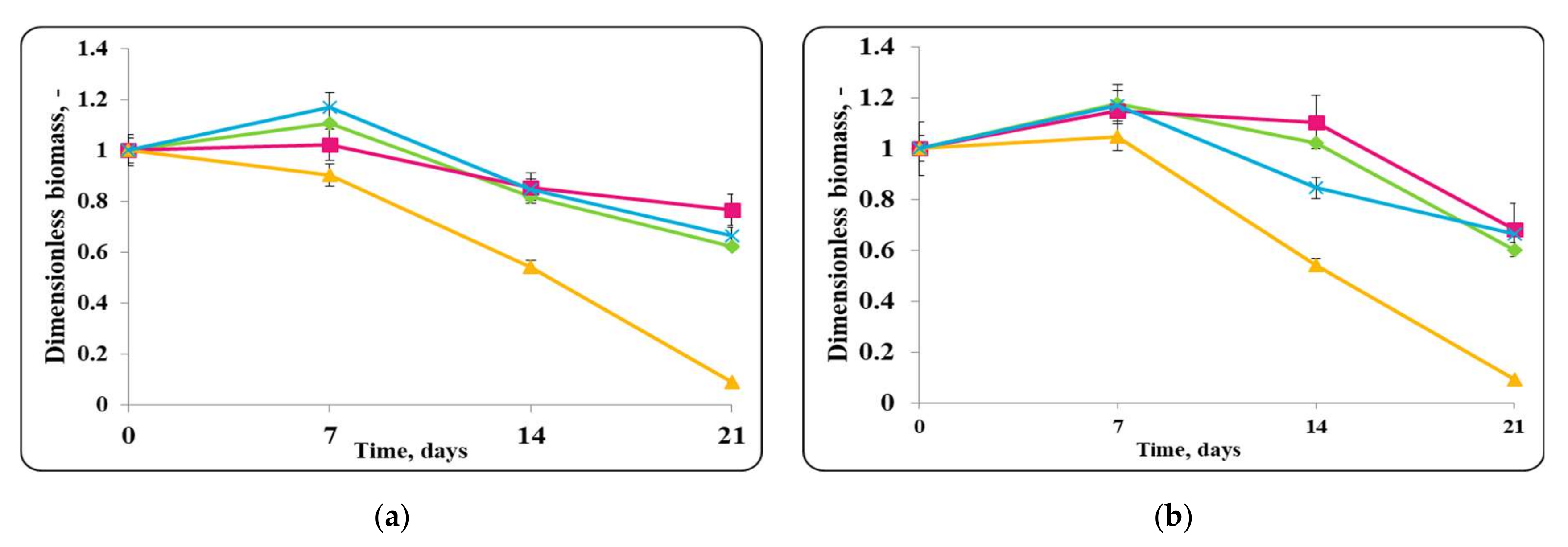
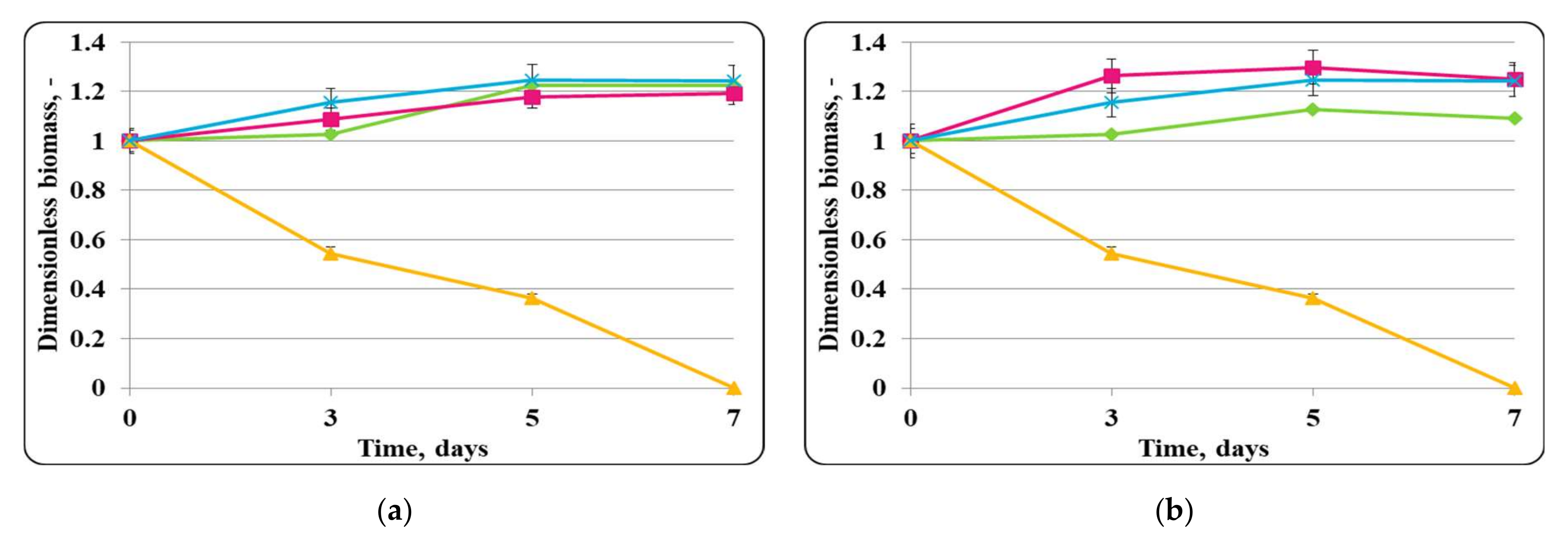
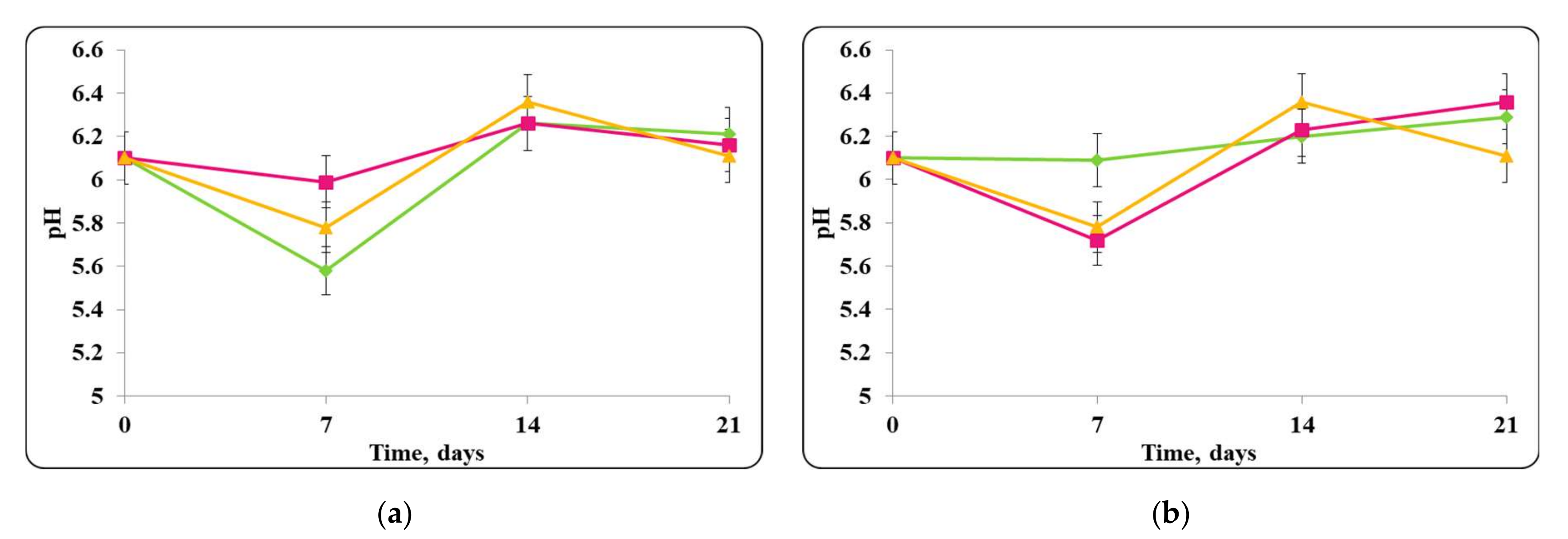
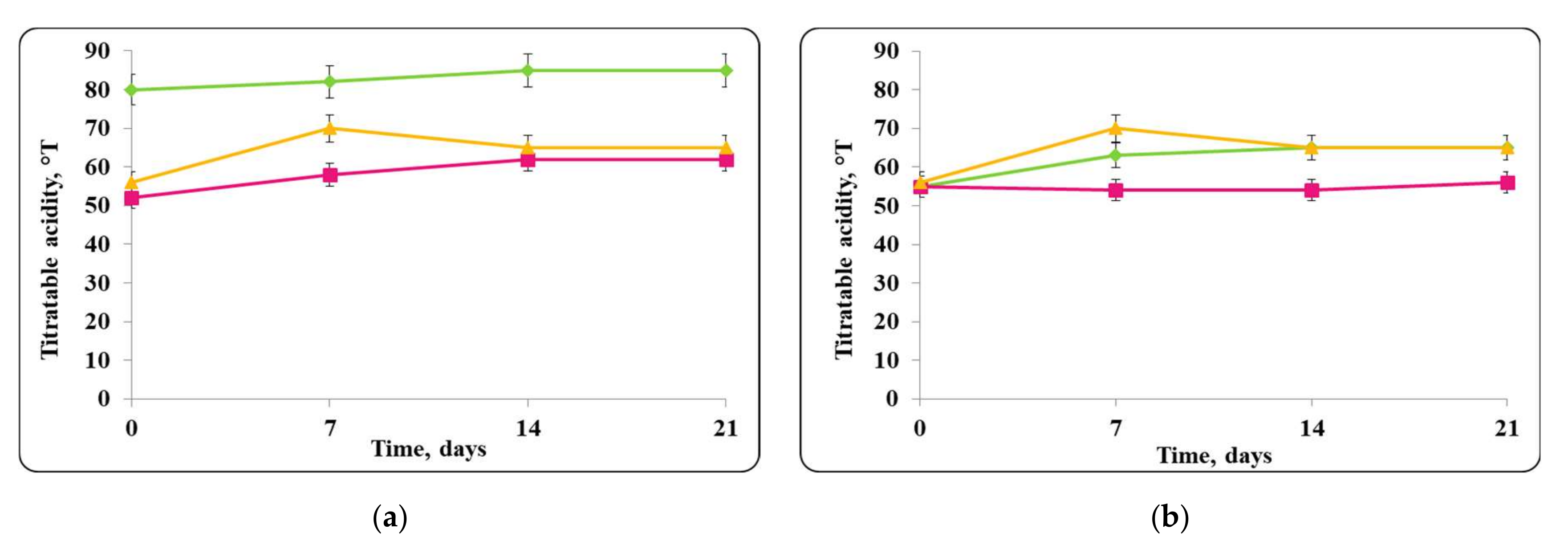
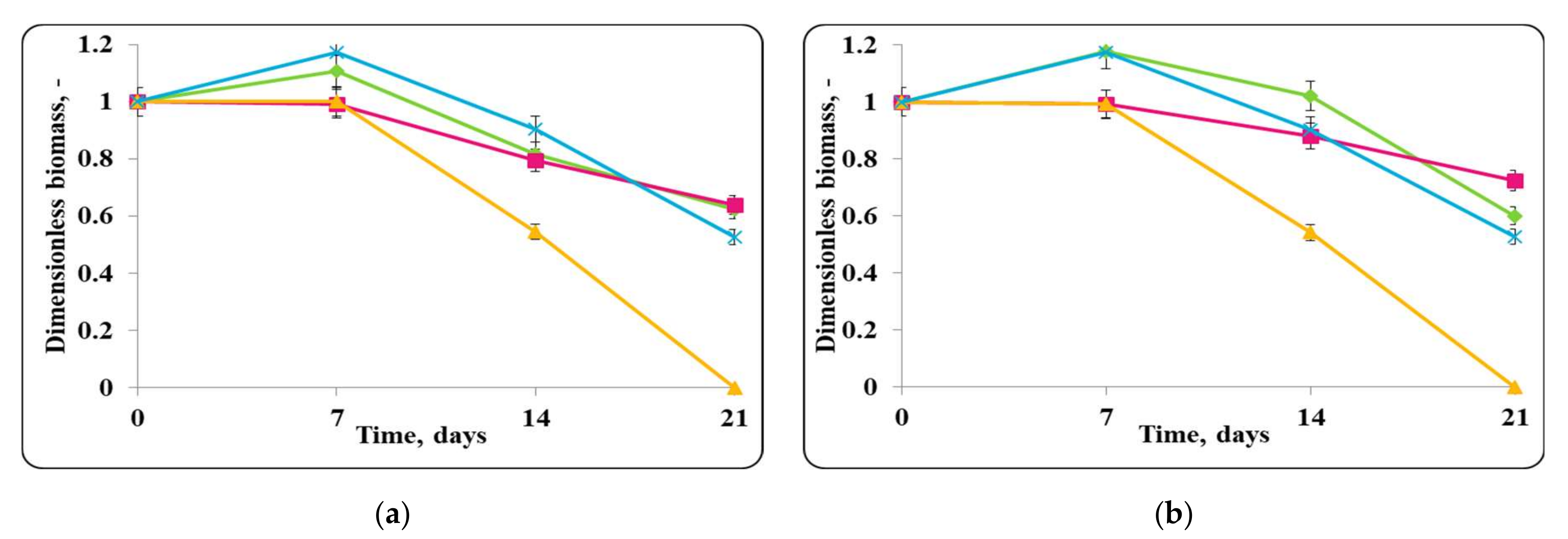
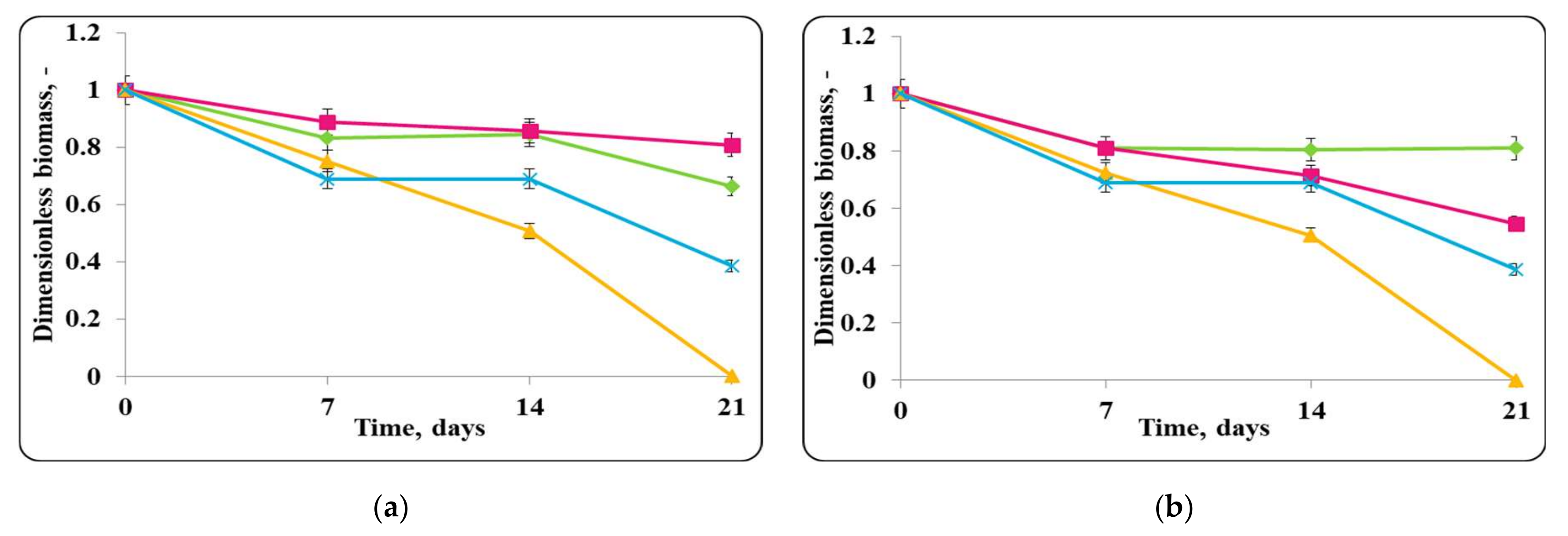
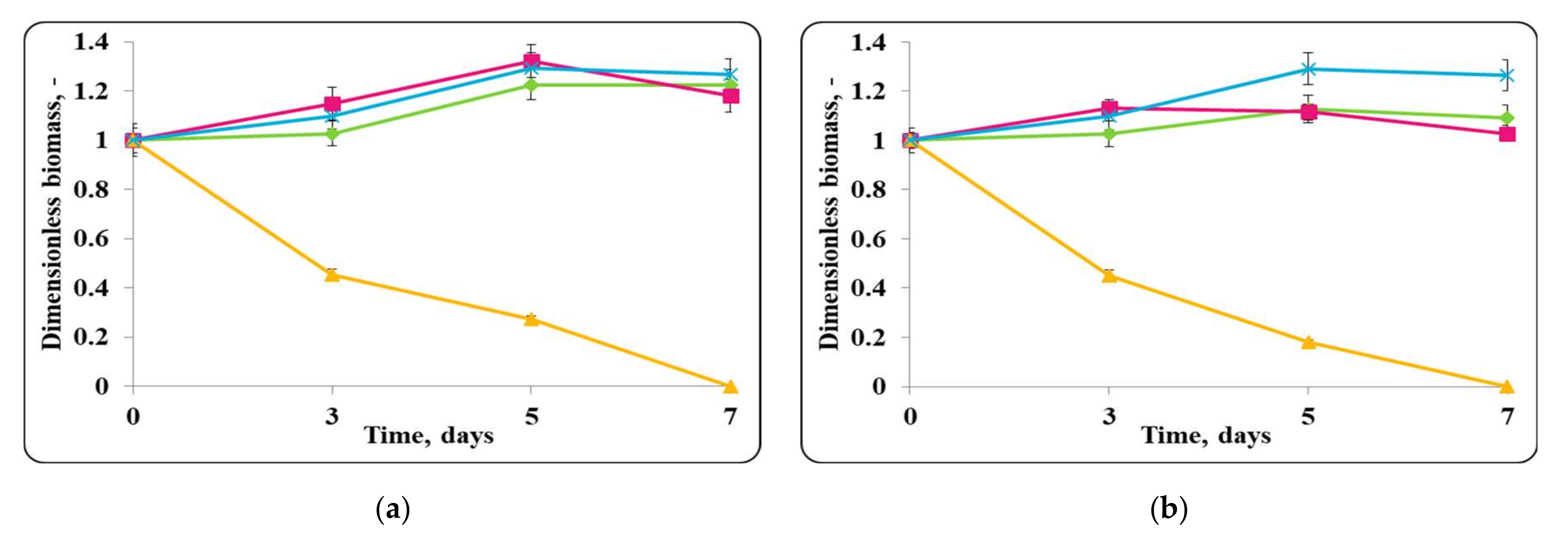
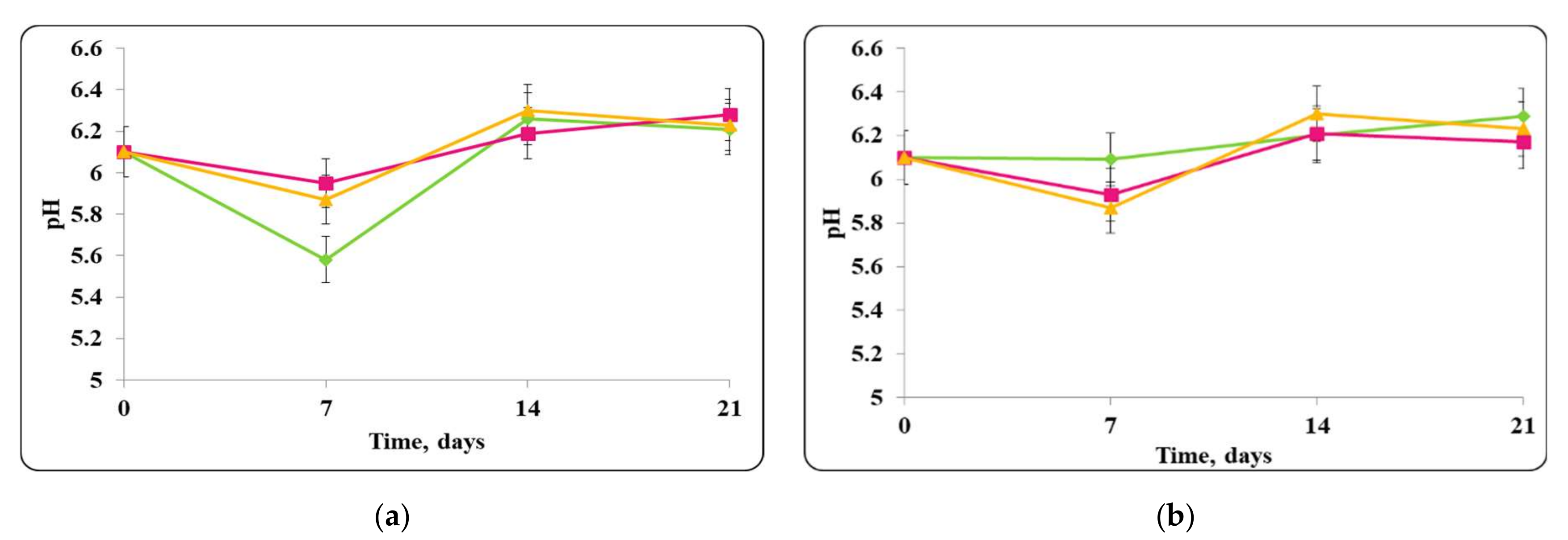
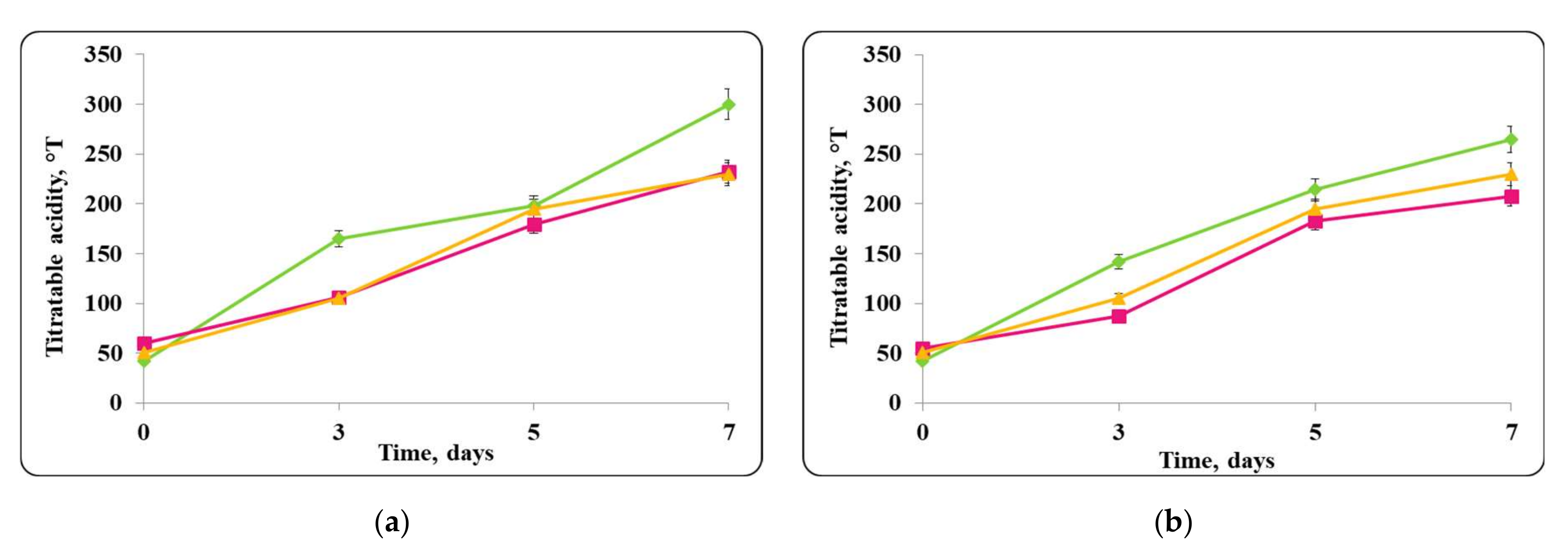
3.2. Biopreservation of Chocolate Mousse: Determination of the Survival of Lactobacilli and Pathogenic Bacteria during Storage at Temperatures of 4 ± 2 °C and 20 ± 2 °C
- Biopreservation of Chocolate Mousse with Free or Encapsulated Lb. helveticus 2/20 Cells
- B.
- Biopreservation of chocolate mousse variants with Lb. helveticus 2/20. Monitoring of lactobacilli and S. aureus ATCC 25923 populations, pH, and titratable acidity of chocolate mousse and MRS broth during storage at 4 ± 2 °C and 20 ± 2 °C.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Salas, M.L.; Lemaitre, M.; Garric, G.; Harel-Oger, M.; Lê, S.; Mounier, J.; Valence-Bertel, F.; Coton, E.; Thierry, A. Antifungal Lactic Acid Bacteria Combinations as Biopreservation Tool in Dairy Products. In 5ème Rencontre Nutrition Alimentation Métabolisme Santé; Rennes, France, 2019, Poster Communication. Available online: https://hal.archives-ouvertes.fr/hal-02437589 (accessed on 20 March 2022).
- Strack, L.; Carli, R.C.; Silva, R.V.; da Sartor, K.B.; Colla, L.M.; Reinehr, C.O. Food biopreservation using antimicrobials produced by lactic acid bacteria. Res. Soc. Dev. 2020, 9, e998986666. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef] [PubMed]
- Bourdichon, F.; Arias, E.; Babuchowski, A.; Buckle, A.; Dal Bello, F.; Dubois, A.; Fontana, A.; Fritz, D.; Kemperman, R.; Laulund, S.; et al. The forgotten role of food cultures. FEMS Microbiol. Lett. 2021, 368, fnab085. [Google Scholar] [CrossRef] [PubMed]
- Smit, G.; Smit, B.A.; Engels, W.J.M. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef]
- Sharma, H.; Ozogul, F.; Bartkiene, E.; Rocha, J.M. Impact of lactic acid bacteria and their metabolites on the techno-functional properties and health benefits of fermented dairy products. Crit. Rev. Food Sci. Nutr. 2021, 2007844. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Shushkovich, J.; Kos, B.; Beganovic, J.; Pavunc, A.L.; Habjanic, K.; Matoshic, S. Antimicrobial activity—The most important property of probiotic and starter lactic acid bacteria. A review. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2021, 32, 17–28. [Google Scholar] [CrossRef]
- Jones, R.J.; Wescombe, P.A.M.; Tagg, J.R. Identifying new protective cultures and culture components for food biopreservation. In Protective Cultures, Antimicrobial Metabolites and Bacteriophages for Food and Beverage Biopreservation, 1st ed.; Lacroix, C., Ed.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Cambridge, UK, 2011; Volume 201, pp. 3–26. [Google Scholar] [CrossRef]
- Barrios-Roblero, C.; Rosas-Quijano, R.; Salvador-Figueroa, M.; Gálvez-López, D.; Vázquez-Ovando, A. Antifungal lactic acid bacteria isolated from fermented beverages with activity against. Colletotrichum Gloeosporioides Food Biosci. 2019, 29, 47–54. [Google Scholar] [CrossRef]
- García-Cano, I.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Wang, K.; Kosmerl, E.; Jiménez-Flores, R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl. Microbiol. Biotechnol. 2019, 103, 5243–5257. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Chen, M.; Duo, L.; Wang, J.; Guo, S.; Sun, H.; Menghe, B.; Zhang, H. Characterization of potentially probiotic lactic acid bacteria and bifidobacteria isolated from human colostrum. J. Dairy Sci. 2020, 103, 4013–4025. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.L.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Brosnan, B.; Zannini, E.; Peyer, L.C.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal activities of three different Lactobacillus species and their production of antifungal carboxylic acids in wheat sourdough. Appl. Microbiol. Biotechnol. 2016, 100, 1701–1711. [Google Scholar] [CrossRef]
- Akbar, A.; Ali, I.; Anal, A.K. Industrial perspectives of lactic acid bacteria for biopreservation and food safety. J. Anim. Plant Sci. 2016, 26, 938–948. [Google Scholar]
- Singh, V. Recent approaches in food bio-preservation—A review. Open Vet. J. 2018, 8, 104–111. [Google Scholar] [CrossRef]
- Wiernasz, N.; Cornet, J.-M.; Cardinal, M.; Pilet, M.-F.; Passerini, D.; Leroi, F. Lactic acid bacteria selection for biopreservation as a part of hurdle technology approach applied on seafood. Front. Mar. Sci. 2017, 4, 119. [Google Scholar] [CrossRef]
- Luz, C.; D’Opazo, V.; Quiles, J.M.; Romano, R.; Mañes, J.; Meca, G. Biopreservation of tomatoes using fermented media by lactic acid bacteria. LWT Food Sci. Technol. 2020, 130, 109618. [Google Scholar] [CrossRef]
- Suo, B.; Chen, X.; Wang, Y. Recent research advances of lactic acid bacteria in sourdough: Origin, diversity, and function. Curr. Opin. Food Sci. 2021, 37, 66–75. [Google Scholar] [CrossRef]
- Faccinetto-Beltrán, P.; Gómez-Fernández, A.R.; Santacruz, A.; Jacobo-Velázquez, D.A. Chocolate as carrier to deliver bioactive ingredients: Current advances and future perspectives. Foods 2021, 10, 2065. [Google Scholar] [CrossRef]
- Pires, M.H. Sobremesas lacteas aeradas—Sistemas de estabilizacao e tecnologia de aeracao. Food Ingred. 2004, 6, 74–77. [Google Scholar]
- Aragon-Alegro, L.C.; Alegro, J.H.A.; Cardarelli, H.R.; Chiu, M.C.; Saad, S.M.I. Potentially probiotic and synbiotic chocolate mousse. LWT Food Sci. Technol. 2007, 40, 669–675. [Google Scholar] [CrossRef]
- Bigdelian, E.; Razavi, S.H. Evaluation of survival rate and physicochemical properties of encapsulated bacteria in alginate and resistant starch in mayonnaise sauce. J. Bioprocess. Biotechnol. 2014, 4, 166. [Google Scholar] [CrossRef] [Green Version]
- Totosaus, A.; Ariza-Ortega, T.D.J.; Pérez-Chabela, M.D.L. Lactic acid bacteria microencapsulation in sodium alginate and other gelling hydrocolloids mixtures. J. Food Nutr. Res. 2013, 52, 107–120. [Google Scholar]
- Corbo, M.R.; Bevilacqua, A.; Speranza, B.; Di Maggio, B.; Gallo, M.; Sinigaglia, M. Use of alginate beads as carriers for lactic acid bacteria in a structured system and preliminary validation in a meat product. Meat Sci. 2016, 111, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Tyl, C.; Sadler, G.D. pH and titratable acidity. In Food Analysis; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017; pp. 389–406. [Google Scholar] [CrossRef]
- Kostov, G.; Denkova-Kostova, R.; Shopska, V.; Goranov, B.; Denkova, Z. Comparative evaluation of Lactobacillus plantarum strains through microbial growth kinetics. In Proceedings of the ECMS 2021, Virtual Event, UK, 31 May–2 June 2021; Khalid Al-Begain, K., Iacono, M., Campanile, L., Bargiela, A., Eds.; European Council for Modeling and Simulation: Caserta, Italy, 2021; pp. 165–171. [Google Scholar] [CrossRef]
- Kostov, G.; Denkova-Kostova, R.; Shopska, V.; Denkova, Z.; Goranov, B.; Teneva, D. Kinetics of microbial inactivation of human pathogens by biological factors. In Proceedings of the ECMS 2020, Wildau, Germany, 9–12 June 2020; Steglich, M., Mueller, C., Neumann, G., Walter, M., Eds.; European Council for Modeling and Simulation: Caserta, Italy, 2020; pp. 153–160. [Google Scholar] [CrossRef]
- Valcheva, E.; Girginov, A.; Veleva, S.; Ivanov, T.; Lazarova, E.; Hristov, M. Physical Chemistry with Examples and Tasks; New Knowledge: Sofia, Bulgaria, 2001. [Google Scholar]
- NF V04-600, January 2001, Produits Laitiers Frais—Spécifications des Laits Fermentés et des Yaourts/Yoghourts. Available online: https://www.boutique.afnor.org/fr-fr/norme/nf-v04600/produits-laitiers-frais-specifications-des-laits-fermentes-et-des-yaourts-y/fa106733/18078 (accessed on 13 March 2022).
- Agiular, C.; Klotz, B. Effect of the temperature on the antagonistic activity of lactic acid bacteria against Escherichia coli and Listeria monocytogenes. J. Food Saf. 2010, 30, 996–1015. [Google Scholar] [CrossRef]
- Fooladi, A.I.; Forooshai, M.C.; Saffarian, P.; Mehrab, R. Antimicrobial effects of four lactobacilli strains isolated from yoghurt against Escherichia coli O157:H7. J. Food Saf. 2014, 34, 150–160. [Google Scholar] [CrossRef]
- Delhalle, L.; Daubé, G.; Adolphe, Y.; Crevecoeur, S.; Clinquart, A. Les modèles de croissance en microbiologie prévisionnelle pour la maîtrise de la sécurité des aliments (synthèse bibliographique). A review of growth models in predictive microbiology to ensure food safety. Biotechnol. Agron. Soc. Environ. 2012, 16, 369–381. [Google Scholar]








| № | Chocolate Mousse Composition |
|---|---|
| 1 | Chocolate mousse control |
| 2 | Chocolate mousse contaminated with S. aureus ATCC 25923 cells alone |
| 3 | Chocolate mousse contaminated with E. coli ATCC 25922 cells alone |
| 4 | Chocolate mousse inoculated with free Lb. helveticus 2/20 cells |
| 5 | Chocolate mousse inoculated with encapsulated Lb. helveticus 2/20 cells |
| 6 | Chocolate mousse inoculated with free Lb. helveticus 2/20 cells and contaminated with S. aureus ATCC 25923 cells |
| 7 | Chocolate mousse inoculated with encapsulated Lb. helveticus 2/20 cells and contaminated with S. aureus ATCC 25923 cells |
| 8 | Chocolate mousse inoculated with free Lb. helveticus 2/20 cells and contaminated with E. coli ATCC 25922 cells |
| 9 | Chocolate mousse inoculated with encapsulated Lb. helveticus 2/20 cells and contaminated with E. coli ATCC 25922 cells |
| # | Carbon Source | Lactobacillus 2/20 |
|---|---|---|
| 1 | Glycerol | - |
| 2 | Erythriol | - |
| 3 | D-arabinose | - |
| 4 | L-arabinose | - |
| 5 | Ribose | - |
| 6 | D-xylose | - |
| 7 | L-xylose | - |
| 8 | Adonitol | - |
| 9 | β-metil-D-xyloside | - |
| 10 | Galactose | + (90–100%) |
| 11 | D-glucose | + (90–100%) |
| 12 | D-fructose | + (90–100%) |
| 13 | D-mannose | + (90–100%) |
| 14 | L-sorbose | - |
| 15 | Rhamnose | - |
| 16 | Dulcitol | - |
| 17 | Inositol | - |
| 18 | Manitol | + (90–100%) |
| 19 | Sorbitol | + (90–100%) |
| 20 | α-methyl-D-mannoside | + (90–100%) |
| 21 | α-methyl-D-glucoside | + (90–100%) |
| 22 | N-acetyl-glucosamine | + (90–100%) |
| 23 | Amigdalin | + (90–100%) |
| 24 | Arbutin | + (90–100%) |
| 25 | Esculin | + (90–100%) |
| 26 | Salicin | + (90–100%) |
| 27 | Cellobiose | + (90–100%) |
| 28 | Maltose | + (90–100%) |
| 29 | Lactose | + (90–100%) |
| 30 | Melibiose | + (90–100%) |
| 31 | Saccharose | + (90–100%) |
| 32 | Trehalose | + (90–100%) |
| 33 | Inulin | + (90–100%) |
| 34 | Melezitose | + (90–100%) |
| 35 | D-raffinose | + (90–100%) |
| 36 | Amidon | + (90–100%) |
| 37 | Glycogen | - |
| 38 | Xylitol | - |
| 39 | β-gentiobiose | + (90–100%) |
| 40 | D-turanose | + (90–100%) |
| 41 | D-lyxose | - |
| 42 | D-tagarose | - |
| 43 | D-fuccose | - |
| 44 | L-fuccose | - |
| 45 | D-arabitol | - |
| 46 | L-arabitol | - |
| 47 | Gluconate | + (90–100%) |
| 48 | 2-keto-gluconate | - |
| 49 | 5-keto-gluconate | - |
| Microorganisms | Ea (kJ/mol) | Z (h−1) |
|---|---|---|
| Free Lb. helveticus 2/20 cells | 87 | 8.6 × 1013 |
| Encapsulated Lb. helveticus 2/20 cells | 85.9 | 5.9 × 1013 |
| Free Lb. helveticus 2/20 cells in co-culture with E. coli ATCC 25922 | 85.4 | 3.9 × 1013 |
| Encapsulated Lb. helveticus 2/20 cells in co-culture with E. coli ATCC 25922 | 86.1 | 6.4 × 1013 |
| E. coli ATCC 25922 | 116.2 | 2.7 × 1013 |
| Microorganisms | Ea k(J/mol) | Z (h−1) |
|---|---|---|
| Free Lb. helveticus 2/20 cells | 87 | 8.6 × 1013 |
| Encapsulated Lb. helveticus 2/20 cells | 85.9 | 5.9 × 1013 |
| Free Lb. helveticus 2/20 cells in co-culture with S. aureus ATCC 25923 | 171.7 | 8.6 × 1028 |
| Encapsulated Lb. helveticus 2/20 cells in co-culture with S. aureus ATCC 25923 | 182.7 | 1.1 × 1031 |
| S. aureus ATCC 25923 | 140.2 | 2.3 × 1023 |
| Strain/Mixture | Ea (kJ/mol) | Z (h−1) |
|---|---|---|
| E. coli ATCC 25922 in co-culture with Lb. helveticus 2/20 (free cells) | 50.9 | 3.0 × 108 |
| E. coli ATCC 25922 in co-culture with Lb. helveticus 2/20 (encapsulated cells) | 50.2 | 2.2 × 108 |
| S. aureus ATCC 25923 in co-culture with Lb. helveticus 2/20 (free cells) | 66.6 | 1.8 × 1011 |
| S. aureus ATCC 25923 in co-culture with Lb. helveticus 2/20 (encapsulated cells) | 65.9 | 1.4 × 1011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goranov, B.; Teneva, D.; Denkova-Kostova, R.; Shopska, V.; Oulahal, N.; Denkova, Z.; Kostov, G.; Degraeve, P.; Pagan, R. Biopreservation of Chocolate Mousse with Lactobacillus helveticus 2/20: Microbial Challenge Test. Molecules 2022, 27, 5631. https://doi.org/10.3390/molecules27175631
Goranov B, Teneva D, Denkova-Kostova R, Shopska V, Oulahal N, Denkova Z, Kostov G, Degraeve P, Pagan R. Biopreservation of Chocolate Mousse with Lactobacillus helveticus 2/20: Microbial Challenge Test. Molecules. 2022; 27(17):5631. https://doi.org/10.3390/molecules27175631
Chicago/Turabian StyleGoranov, Bogdan, Desislava Teneva, Rositsa Denkova-Kostova, Vesela Shopska, Nadia Oulahal, Zapryana Denkova, Georgi Kostov, Pascal Degraeve, and Rafael Pagan. 2022. "Biopreservation of Chocolate Mousse with Lactobacillus helveticus 2/20: Microbial Challenge Test" Molecules 27, no. 17: 5631. https://doi.org/10.3390/molecules27175631
APA StyleGoranov, B., Teneva, D., Denkova-Kostova, R., Shopska, V., Oulahal, N., Denkova, Z., Kostov, G., Degraeve, P., & Pagan, R. (2022). Biopreservation of Chocolate Mousse with Lactobacillus helveticus 2/20: Microbial Challenge Test. Molecules, 27(17), 5631. https://doi.org/10.3390/molecules27175631





