Hyaluronic Acid Oligosaccharide Derivatives Alleviate Lipopolysaccharide-Induced Inflammation in ATDC5 Cells by Multiple Mechanisms
Abstract
:1. Introduction
2. Results
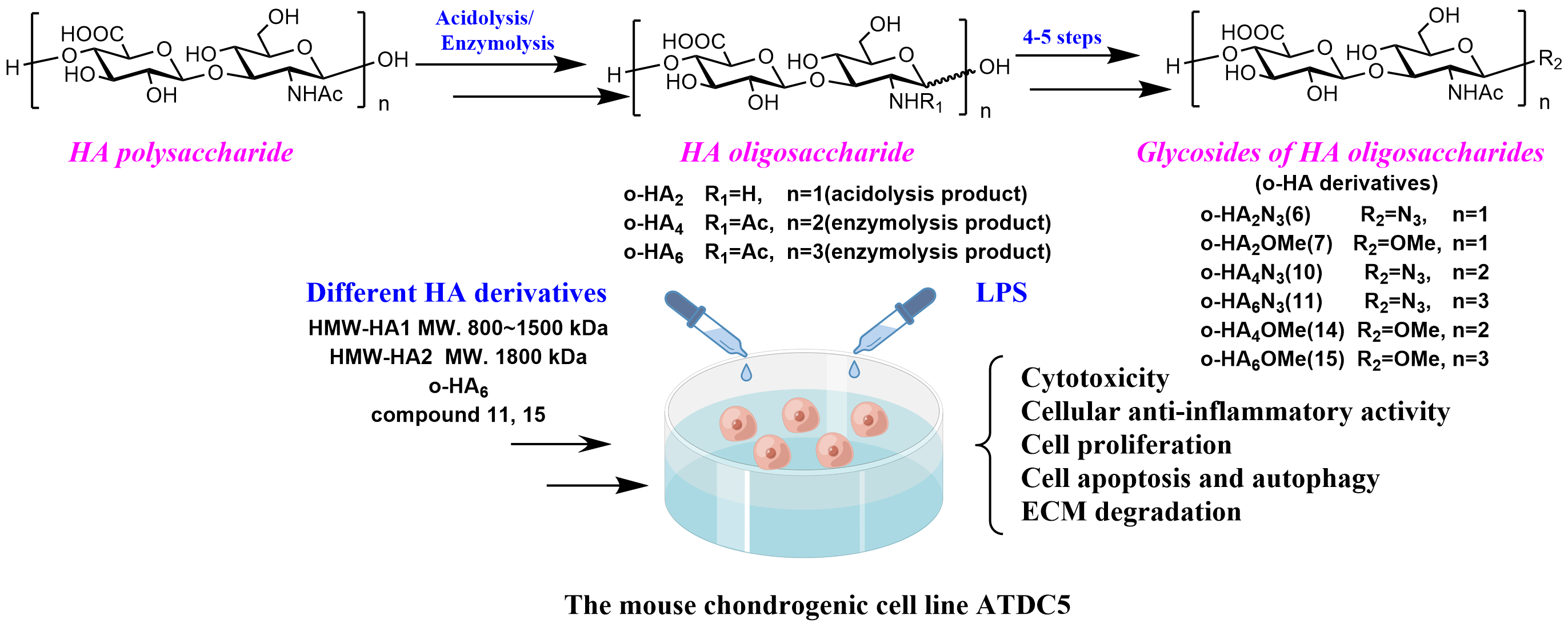
2.1. Study Design
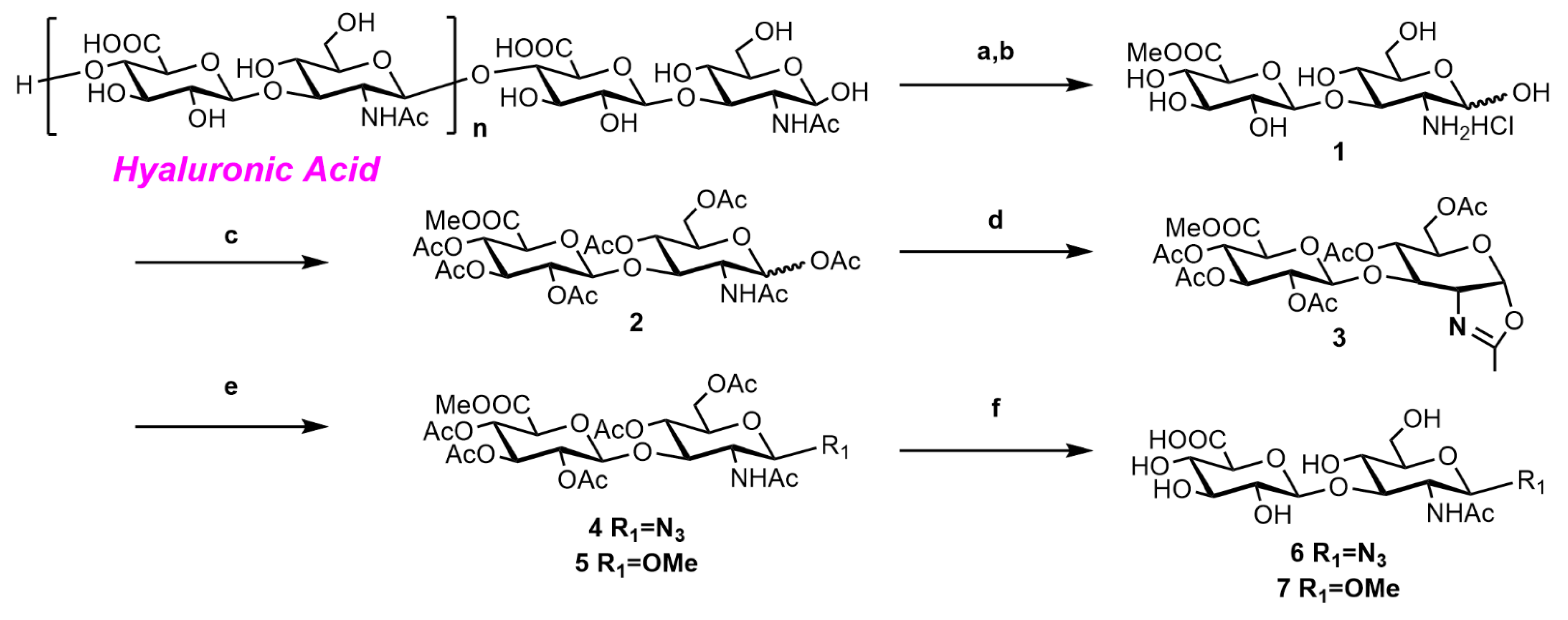
2.2. Synthesis of o-HA Derivatives
2.2.1. Synthesis of o-HA2OMe (6) and o-HA2N3 (7)
2.2.2. Synthesis of o-HA4N3 (11) and o-HA6N3 (12)
2.2.3. Synthesis of o-HA4OMe (14) and o-HA6OMe (15)
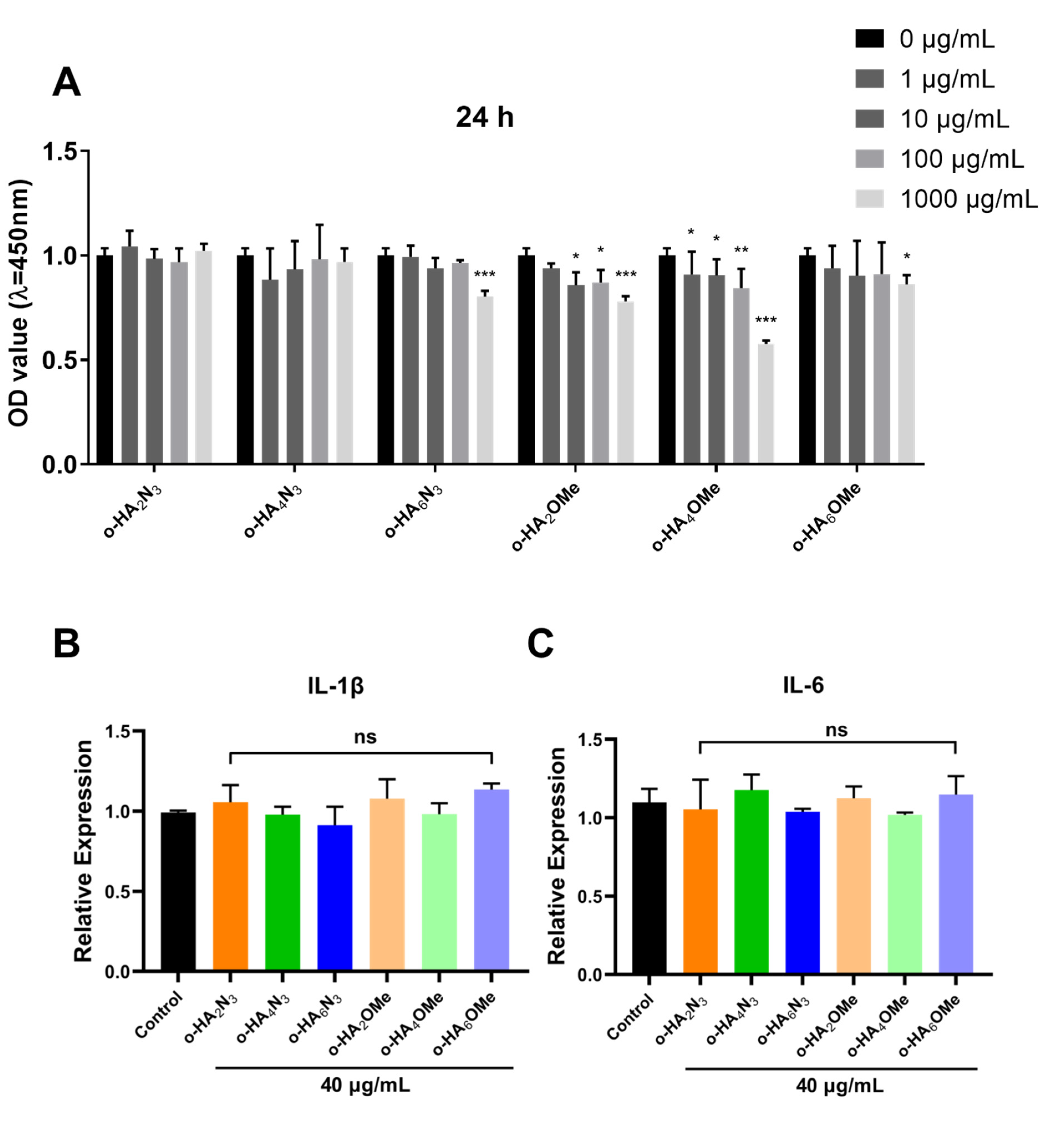
2.3. Evaluation of the Cytotoxicity and Pro-Inflammatory Activity of o-HA Derivatives in ATDC5 Cells
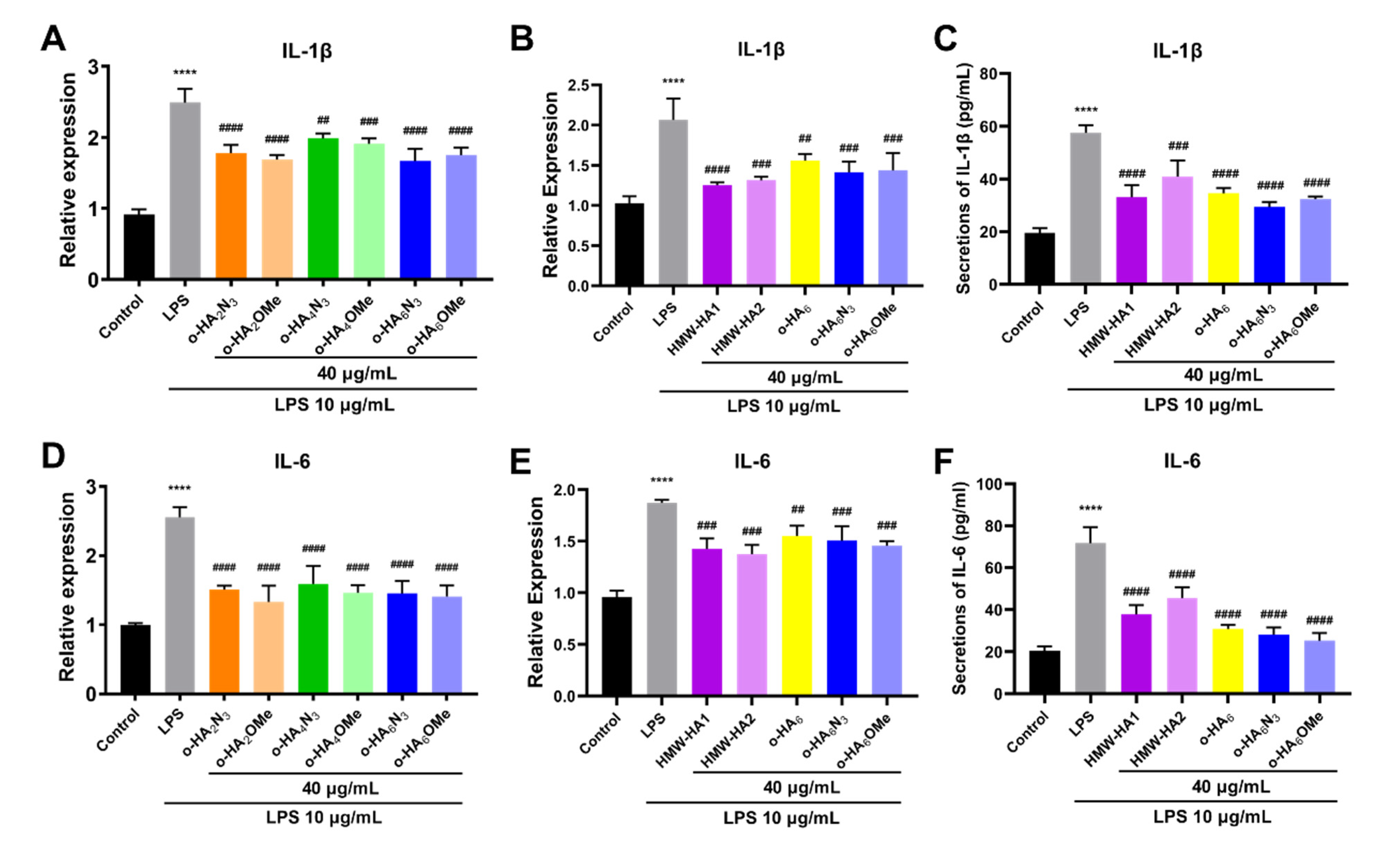
2.4. Evaluation of the Protective Effect of Different HA Derivatives on LPS-Induced Inflammatory Injury of ATDC5 Cells
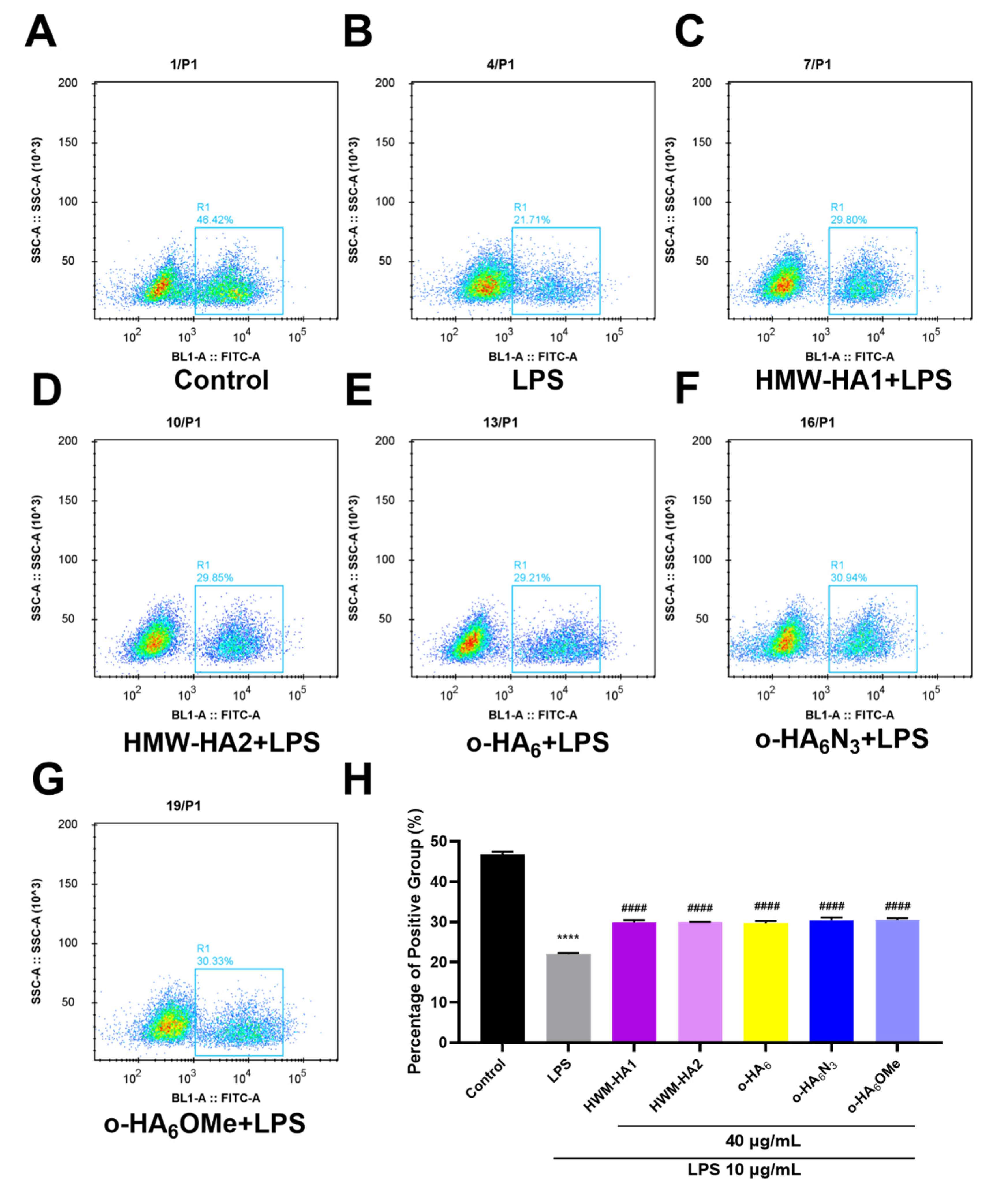
2.5. The Proliferation Effect of Different HA Derivatives on LPS Challenged ATDC5 Cells
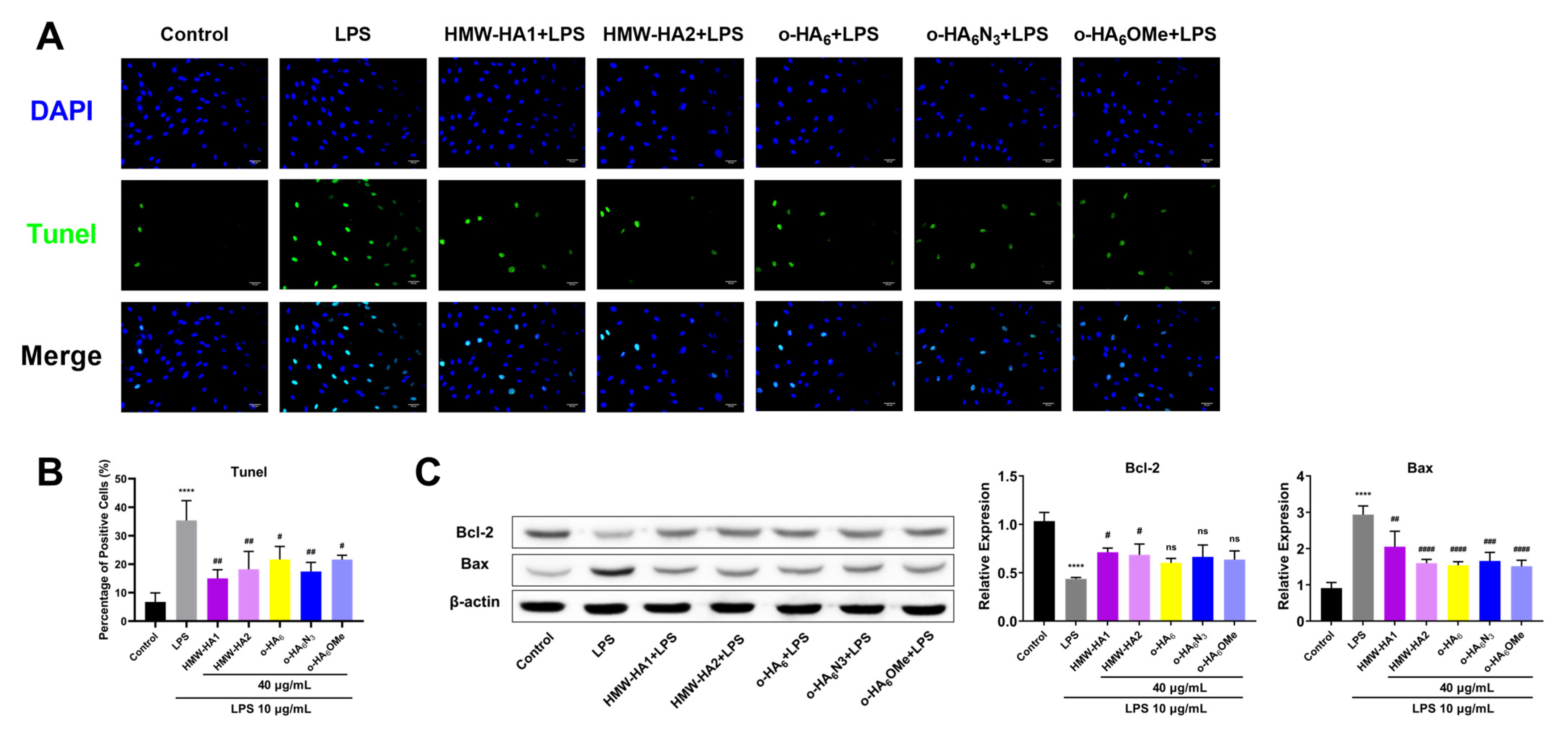
2.6. Anti-Apoptotic Effect of Different HA Derivatives on LPS Challenged ATDC5 Cells
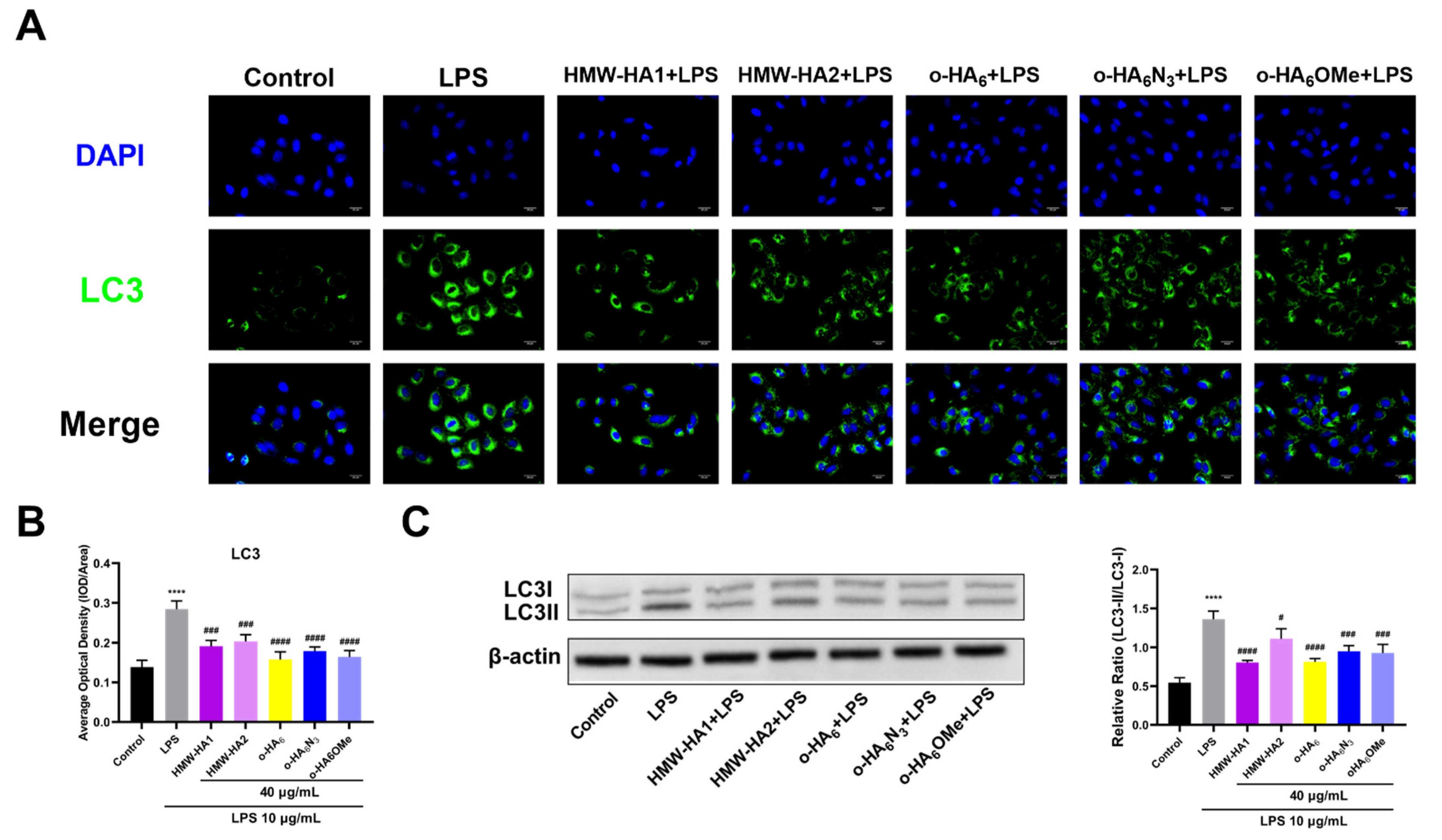
2.7. Anti-Autophagy Effect of Different HA Derivatives on LPS Challenged ATDC5 Cells
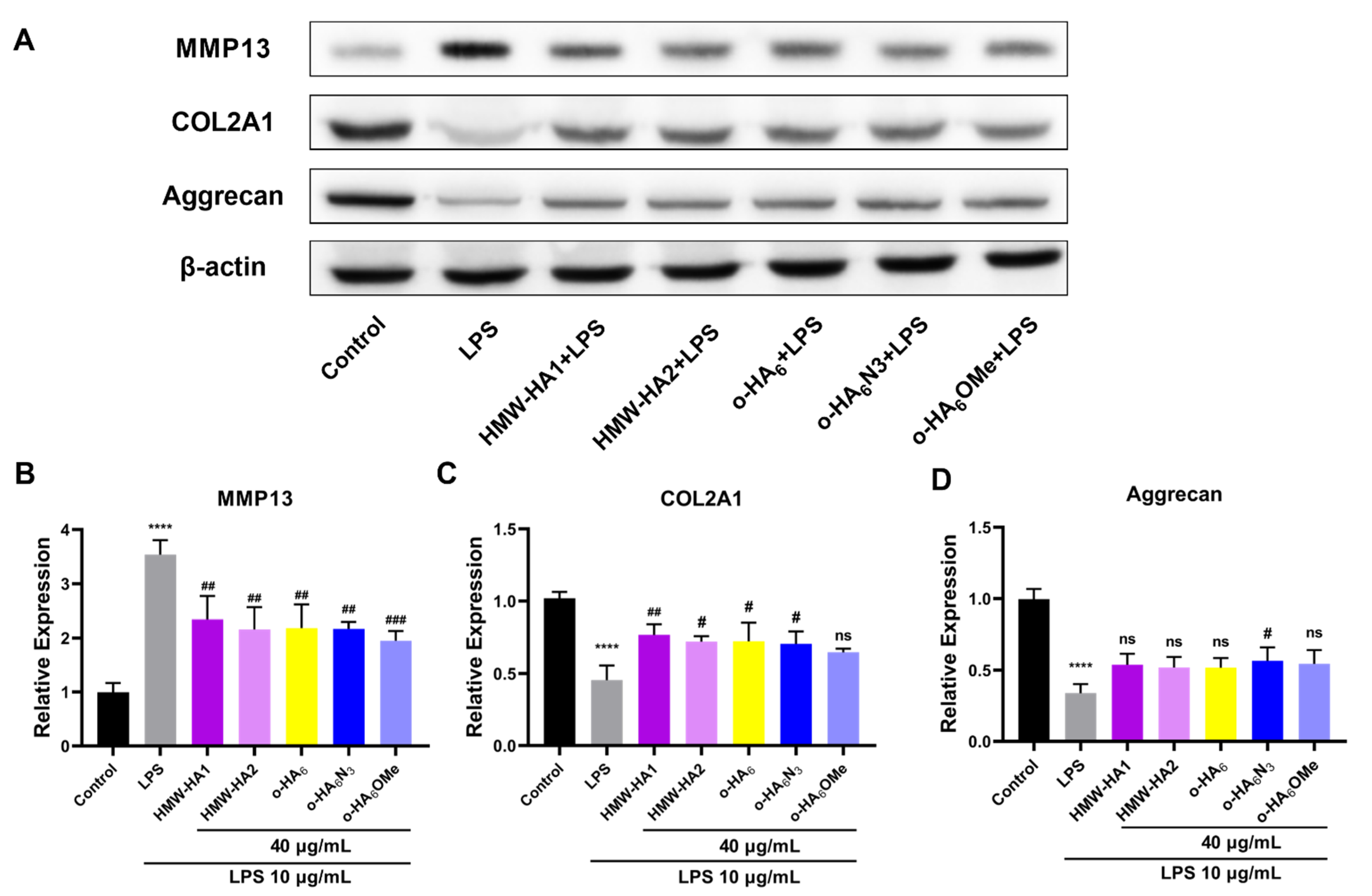
2.8. Alleviation of the ECM Degradation by Different HA Derivatives
3. Discussion
4. Materials and Methods
4.1. Material and Reagents
4.2. Chemical Synthesis
4.3. Cell Culture and Treatment
4.4. CCK-8 Assay
4.5. RT-qPCR Assay
4.6. ELISA
4.7. Western Blot Assay
4.8. TUNEL Assay
4.9. Immunofluorescence (IF) Assay
4.10. EdU Flow Cytometry Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Sofat, N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int. J. Exp. Pathol. 2009, 90, 463–479. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- From the Centers for Disease Control and Prevention. Prevalence and impact of arthritis among women—United States, 1989–1991. JAMA 1995, 273, 1820. [Google Scholar] [CrossRef]
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [CrossRef]
- Varela-Eirin, M.; Loureiro, J.; Fonseca, E.; Corrochano, S.; Caeiro, J.R.; Collado, M.; Mayan, M.D. Cartilage regeneration and ageing: Targeting cellular plasticity in osteoarthritis. Ageing Res. Rev. 2018, 42, 56–71. [Google Scholar] [CrossRef]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Stabler, T.; Pei, F.X.; Kraus, V.B. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr. Cartil. 2016, 24, 1769–1775. [Google Scholar] [CrossRef] [Green Version]
- Binvignat, M.; Sokol, H.; Mariotti-Ferrandiz, E.; Berenbaum, F.; Sellam, J. Osteoarthritis and gut microbiome. Jt. Bone Spine 2021, 88, 105203. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, Y. ATDC5: An excellent in vitro model cell line for skeletal development. J. Cell. Biochem. 2013, 114, 1223–1229. [Google Scholar] [CrossRef]
- Wilhelm, D.; Kempf, H.; Bianchi, A.; Vincourt, J.B. ATDC5 cells as a model of cartilage extracellular matrix neosynthesis, maturation and assembly. J. Proteom. 2020, 219, 103718. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Conde, J.; Scotece, M.; Abella, V.; López, V.; Pino, J.; Gómez, R.; Gómez-Reino, J.J.; Gualillo, O. Choosing the right chondrocyte cell line: Focus on nitric oxide. J. Orthop. Res. 2015, 33, 1784–1788. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, X.; Wang, R.; Zhao, H.; Wang, L.; Liu, J.; Li, M.; Chen, Z.; Wang, Z.; Li, L.; et al. 4-octyl Itaconate inhibits lipopolysaccharide (LPS)-induced osteoarthritis via activating Nrf2 signalling pathway. J. Cell Mol. Med. 2022, 26, 1515–1529. [Google Scholar] [CrossRef]
- Conde, J.; Gomez, R.; Bianco, G.; Scotece, M.; Lear, P.; Dieguez, C.; Gomez-Reino, J.; Lago, F.; Gualillo, O. Expanding the adipokine network in cartilage: Identification and regulation of novel factors in human and murine chondrocytes. Ann. Rheum. Dis. 2011, 70, 551–559. [Google Scholar] [CrossRef]
- Weindl, G.; Schaller, M.; Schäfer-Korting, M.; Korting, H.C. Hyaluronic acid in the treatment and prevention of skin diseases: Molecular biological, pharmaceutical and clinical aspects. Ski. Pharmacol. Physiol. 2004, 17, 207–213. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Ghavami, A.; Crosby, M.A. The role of hyaluronic acid fillers (Restylane) in facial cosmetic surgery: Review and technical considerations. Plast Reconstr. Surg. 2007, 120, 41s–54s. [Google Scholar] [CrossRef]
- Neustadt, D.H. Intra-articular injections for osteoarthritis of the knee. Cleve Clin. J. Med. 2006, 73, 897–911. [Google Scholar]
- Am McGrath, A.F.M. A Comparison of Intra-Articular Hyaluronic Acid Competitors in the Treatment of Mild to Moderate Knee Osteoarthritis. J. Arthritis 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Belcher, C.; Yaqub, R.; Fawthrop, F.; Bayliss, M.; Doherty, M. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann. Rheum. Dis. 1997, 56, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis. Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Rannou, F.; Richette, P.; Bruyère, O.; Al-Daghri, N.; Altman, R.D.; Brandi, M.L.; Collaud Basset, S.; Herrero-Beaumont, G.; Migliore, A.; et al. Use of Intraarticular Hyaluronic Acid in the Management of Knee Osteoarthritis in Clinical Practice. Arthritis Care Res. 2017, 69, 1287–1296. [Google Scholar] [CrossRef]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. Febs J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Aggarwal, A.; Sempowski, I.P. Hyaluronic acid injections for knee osteoarthritis. Systematic review of the literature. Can. Fam. Physician 2004, 50, 249–256. [Google Scholar]
- Ghosh, P.; Guidolin, D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: Are the effects molecular weight dependent? Semin Arthritis Rheum 2002, 32, 10–37. [Google Scholar] [CrossRef]
- Barreto, R.B.; Sadigursky, D.; de Rezende, M.U.; Hernandez, A.J. Effect of hyaluronic acid on chondrocyte apoptosis. Acta Ortop Bras 2015, 23, 90–93. [Google Scholar] [CrossRef]
- Patti, A.M.; Gabriele, A.; Vulcano, A.; Ramieri, M.T.; Della Rocca, C. Effect of hyaluronic acid on human chondrocyte cell lines from articular cartilage. Tissue Cell 2001, 33, 294–300. [Google Scholar] [CrossRef]
- Cowman, M.K.; Shortt, C.; Arora, S.; Fu, Y.; Villavieja, J.; Rathore, J.; Huang, X.; Rakshit, T.; Jung, G.I.; Kirsch, T. Role of Hyaluronan in Inflammatory Effects on Human Articular Chondrocytes. Inflammation 2019, 42, 1808–1820. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front Vet Sci 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Evanko, S.P.; Tammi, M.I.; Tammi, R.H.; Wight, T.N. Hyaluronan-dependent pericellular matrix. Adv. Drug Deliv. Rev. 2007, 59, 1351–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Cao, M.; Liu, H.; He, Y.; Xu, J.; Du, Y.; Liu, Y.; Wang, W.; Cui, L.; Hu, J.; et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 2012, 287, 43094–43107. [Google Scholar] [CrossRef] [PubMed]
- Knudson, W.; Ishizuka, S.; Terabe, K.; Askew, E.B.; Knudson, C.B. The pericellular hyaluronan of articular chondrocytes. Matrix Biol. 2019, 78–79, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; D’Ascola, A.; Scuruchi, M.; Prestipino, V.; Calatroni, A.; Campo, S. 6-Mer hyaluronan oligosaccharides increase IL-18 and IL-33 production in mouse synovial fibroblasts subjected to collagen-induced arthritis. Innate Immun 2012, 18, 675–684. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; D’Ascola, A.; Prestipino, V.; Scuruchi, M.; Nastasi, G.; Calatroni, A.; Campo, S. Hyaluronan differently modulates TLR-4 and the inflammatory response in mouse chondrocytes. Biofactors 2012, 38, 69–76. [Google Scholar] [CrossRef]
- Taylor, K.R.; Yamasaki, K.; Radek, K.A.; Nardo, A.D.; Goodarzi, H.; Golenbock, D.; Beutler, B.; Gallo, R.L. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J. Biol. Chem. 2007, 282, 18265–18275. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; D’Ascola, A.; Scuruchi, M.; Calatroni, A.; Campo, S. Beta-arrestin-2 negatively modulates inflammation response in mouse chondrocytes induced by 4-mer hyaluronan oligosaccharide. Mol. Cell Biochem. 2015, 399, 201–208. [Google Scholar] [CrossRef]
- D’Ascola, A.; Scuruchi, M.; Ruggeri, R.M.; Avenoso, A.; Mandraffino, G.; Vicchio, T.M.; Campo, S.; Campo, G.M. Hyaluronan oligosaccharides modulate inflammatory response, NIS and thyreoglobulin expression in human thyrocytes. Arch. Biochem. Biophys. 2020, 694, 108598. [Google Scholar] [CrossRef]
- Scuruchi, M.; D’Ascola, A.; Avenoso, A.; Campana, S.; Abusamra, Y.A.; Spina, E.; Calatroni, A.; Campo, G.M.; Campo, S. 6-Mer Hyaluronan Oligosaccharides Modulate Neuroinflammation and α-Synuclein Expression in Neuron-Like SH-SY5Y Cells. J. Cell Biochem. 2016, 117, 2835–2843. [Google Scholar] [CrossRef]
- Dong, Y.; Arif, A.; Olsson, M.; Cali, V.; Hardman, B.; Dosanjh, M.; Lauer, M.; Midura, R.J.; Hascall, V.C.; Brown, K.L.; et al. Endotoxin free hyaluronan and hyaluronan fragments do not stimulate TNF-α, interleukin-12 or upregulate co-stimulatory molecules in dendritic cells or macrophages. Sci. Rep. 2016, 6, 36928. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Bremer, L.; Aulin, C.; Harris, H.E. Fragmented hyaluronan has no alarmin function assessed in arthritis synovial fibroblast and chondrocyte cultures. Innate Immun. 2018, 24, 131–141. [Google Scholar] [CrossRef]
- Lu, X.; Kamat, M.N.; Huang, L.; Huang, X. Chemical synthesis of a hyaluronic acid decasaccharide. J. Org. Chem. 2009, 74, 7608–7617. [Google Scholar] [CrossRef] [PubMed]
- Blatter, G.; Jacquinet, J.-C. The use of 2-deoxy-2-trichloroacetamido-d-glucopyranose derivatives in syntheses of hyaluronic acid-related tetra-, hexa-, and octa-saccharides having a methyl β-d-glucopyranosiduronic acid at the reducing end. Carbohydr. Res. 1996, 288, 109–125. [Google Scholar] [CrossRef]
- Furukawa, T.; Hinou, H.; Shimawaki, K.; Nishimura, S.-I. A potential glucuronate glycosyl donor with 2-O-acyl-6,3-lactone structure: Efficient synthesis of glycosaminoglycan disaccharides. Tetrahedron Lett. 2011, 52, 5567–5570. [Google Scholar] [CrossRef]
- Tokita, Y.; Okamoto, A. Hydrolytic degradation of hyaluronic acid. Polym. Degrad. Stab. 1995, 48, 269–273. [Google Scholar] [CrossRef]
- Song, Z.; Meng, L.; Xiao, Y.; Zhao, X.; Fang, J.; Zeng, J.; Wan, Q. Calcium hypophosphite mediated deiodination in water: Mechanistic insights and applications in large scale syntheses of d-quinovose and d-rhamnose. Green Chem. 2019, 21, 1122–1127. [Google Scholar] [CrossRef]
- Kobayashi, S.; Fujikawa, S.-i.; Ohmae, M. Enzymatic Synthesis of Chondroitin and Its Derivatives Catalyzed by Hyaluronidase. J. Am. Chem. Soc. 2003, 125, 14357–14369. [Google Scholar] [CrossRef]
- Wipf, P.; Eyer, B.R.; Yamaguchi, Y.; Zhang, F.; Neal, M.D.; Sodhi, C.P.; Good, M.; Branca, M.; Prindle, T.; Lu, P.; et al. Synthesis of anti-inflammatory α-and β-linked acetamidopyranosides as inhibitors of toll-like receptor 4 (TLR4). Tetrahedron Lett. 2015, 56, 3097–3100. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Yao, W.; Meng, X.; Li, Z. Semisynthesis of Chondroitin Sulfate Oligosaccharides Based on the Enzymatic Degradation of Chondroitin. J. Org. Chem. 2019, 84, 7418–7425. [Google Scholar] [CrossRef]
- Sha, M.; Yao, W.; Zhang, X.; Li, Z. Synthesis of structure-defined branched hyaluronan tetrasaccharide glycoclusters. Tetrahedron Lett. 2017, 58, 2910–2914. [Google Scholar] [CrossRef]
- Tanaka, T.; Nagai, H.; Noguchi, M.; Kobayashi, A.; Shoda, S.-I. One-step conversion of unprotected sugars to β-glycosyl azides using 2-chloroimidazolinium salt in aqueous solution. Chem. Commun. 2009, 3378–3379. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xing, D.; Zhang, Q.; Li, H.; Lin, J.; He, Z.; Lin, J. LncRNAs as a new regulator of chronic musculoskeletal disorder. Cell Prolif 2021, 54, e13113. [Google Scholar] [CrossRef]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, S.; Huang, J.; Guo, W.; Chen, J.; Zhang, L.; Zhao, B.; Peng, J.; Wang, A.; Wang, Y.; et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed. Res. Int. 2014, 2014, 648459. [Google Scholar] [CrossRef]
- Shahid, M.; Manchi, G.; Slunsky, P.; Naseer, O.; Fatima, A.; Leo, B.; Raila, J. A systemic review of existing serological possibilities to diagnose canine osteoarthritis with a particular focus on extracellular matrix proteoglycans and protein. Pol. J. Vet. Sci. 2017, 20, 189–201. [Google Scholar] [CrossRef]
- Sun, A.R.; Friis, T.; Sekar, S.; Crawford, R.; Xiao, Y.; Prasadam, I. Is Synovial Macrophage Activation the Inflammatory Link Between Obesity and Osteoarthritis? Curr. Rheumatol. Rep. 2016, 18, 57. [Google Scholar] [CrossRef]
- Bauer, C.; Niculescu-Morzsa, E.; Jeyakumar, V.; Kern, D.; Späth, S.S.; Nehrer, S. Chondroprotective effect of high-molecular-weight hyaluronic acid on osteoarthritic chondrocytes in a co-cultivation inflammation model with M1 macrophages. J. Inflamm. 2016, 13, 31. [Google Scholar] [CrossRef]
- Hiramitsu, T.; Yasuda, T.; Ito, H.; Shimizu, M.; Julovi, S.M.; Kakinuma, T.; Akiyoshi, M.; Yoshida, M.; Nakamura, T. Intercellular adhesion molecule-1 mediates the inhibitory effects of hyaluronan on interleukin-1beta-induced matrix metalloproteinase production in rheumatoid synovial fibroblasts via down-regulation of NF-kappaB and p38. Rheumatol 2006, 45, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Majumdar, T.; Kessler, P.; Ozhegov, E.; Zhang, Y.; Chattopadhyay, S.; Barik, S.; Sen, G.C. STING Requires the Adaptor TRIF to Trigger Innate Immune Responses to Microbial Infection. Cell Host Microbe 2016, 20, 329–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, Y.; Wu, B.; Xia, F. Semaphorin 3A mitigates lipopolysaccharide-induced chondrocyte inflammation, apoptosis and extracellular matrix degradation by binding to Neuropilin-1. Bioengineered 2021, 12, 9641–9654. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, I.; Ibori, N.; Kojima, K.; Yamaguchi, M.; Endo, M. Mechanism for the hydrolysis of hyaluronan oligosaccharides by bovine testicular hyaluronidase. FEBS J. 2010, 277, 1776–1786. [Google Scholar] [CrossRef]
- Kobayashi, S.; Morii, H.; Itoh, R.; Kimura, S.; Ohmae, M. Enzymatic Polymerization to Artificial Hyaluronan: A Novel Method to Synthesize a Glycosaminoglycan Using a Transition State Analogue Monomer. J. Am. Chem. Soc. 2001, 123, 11825–11826. [Google Scholar] [CrossRef]
- Han, W.; Lv, Y.; Sun, Y.; Wang, Y.; Zhao, Z.; Shi, C.; Chen, X.; Wang, L.; Zhang, M.; Wei, B.; et al. The anti-inflammatory activity of specific-sized hyaluronic acid oligosaccharides. Carbohydr Polym 2022, 276, 118699. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef]
- Duan, R.; Xie, H.; Liu, Z.Z. The Role of Autophagy in Osteoarthritis. Front Cell Dev. Biol. 2020, 8, 608388. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Reviews. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Peng, Z.; Sun, H.; Bunpetch, V.; Koh, Y.; Wen, Y.; Wu, D.; Ouyang, H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 2021, 268, 120555. [Google Scholar] [CrossRef]
- Sanchez, C.; Bay-Jensen, A.C.; Pap, T.; Dvir-Ginzberg, M.; Quasnichka, H.; Barrett-Jolley, R.; Mobasheri, A.; Henrotin, Y. Chondrocyte secretome: A source of novel insights and exploratory biomarkers of osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1199–1209. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Amorim, S.; Reis, C.A.; Reis, R.L.; Pires, R.A. Extracellular Matrix Mimics Using Hyaluronan-Based Biomaterials. Trends Biotechnol 2021, 39, 90–104. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Ding, X.; Xing, D.; Lin, J.; Li, Z.; Lin, J. Hyaluronic Acid Oligosaccharide Derivatives Alleviate Lipopolysaccharide-Induced Inflammation in ATDC5 Cells by Multiple Mechanisms. Molecules 2022, 27, 5619. https://doi.org/10.3390/molecules27175619
Huang H, Ding X, Xing D, Lin J, Li Z, Lin J. Hyaluronic Acid Oligosaccharide Derivatives Alleviate Lipopolysaccharide-Induced Inflammation in ATDC5 Cells by Multiple Mechanisms. Molecules. 2022; 27(17):5619. https://doi.org/10.3390/molecules27175619
Chicago/Turabian StyleHuang, Hesuyuan, Xuyang Ding, Dan Xing, Jianjing Lin, Zhongtang Li, and Jianhao Lin. 2022. "Hyaluronic Acid Oligosaccharide Derivatives Alleviate Lipopolysaccharide-Induced Inflammation in ATDC5 Cells by Multiple Mechanisms" Molecules 27, no. 17: 5619. https://doi.org/10.3390/molecules27175619
APA StyleHuang, H., Ding, X., Xing, D., Lin, J., Li, Z., & Lin, J. (2022). Hyaluronic Acid Oligosaccharide Derivatives Alleviate Lipopolysaccharide-Induced Inflammation in ATDC5 Cells by Multiple Mechanisms. Molecules, 27(17), 5619. https://doi.org/10.3390/molecules27175619





