Detailed Structural Analysis of the Immunoregulatory Polysaccharides from the Mycobacterium Bovis BCG
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Purification of Polysaccharides
2.3. Homogeneity and Molecular Weight Determination
2.4. Qualitative Analysis of Monosaccharide Composition
2.5. Methylation and GC-MS Analysis
2.6. UV, IR, and NMR Analysis
2.7. In Vitro Immunomodulatory Activity Assay
2.7.1. Cell Culture
2.7.2. Cell Viability Assays
2.7.3. NO and Cytokine Production
2.7.4. RT-qPCR Analysis
2.8. Statistical Analysis
3. Results
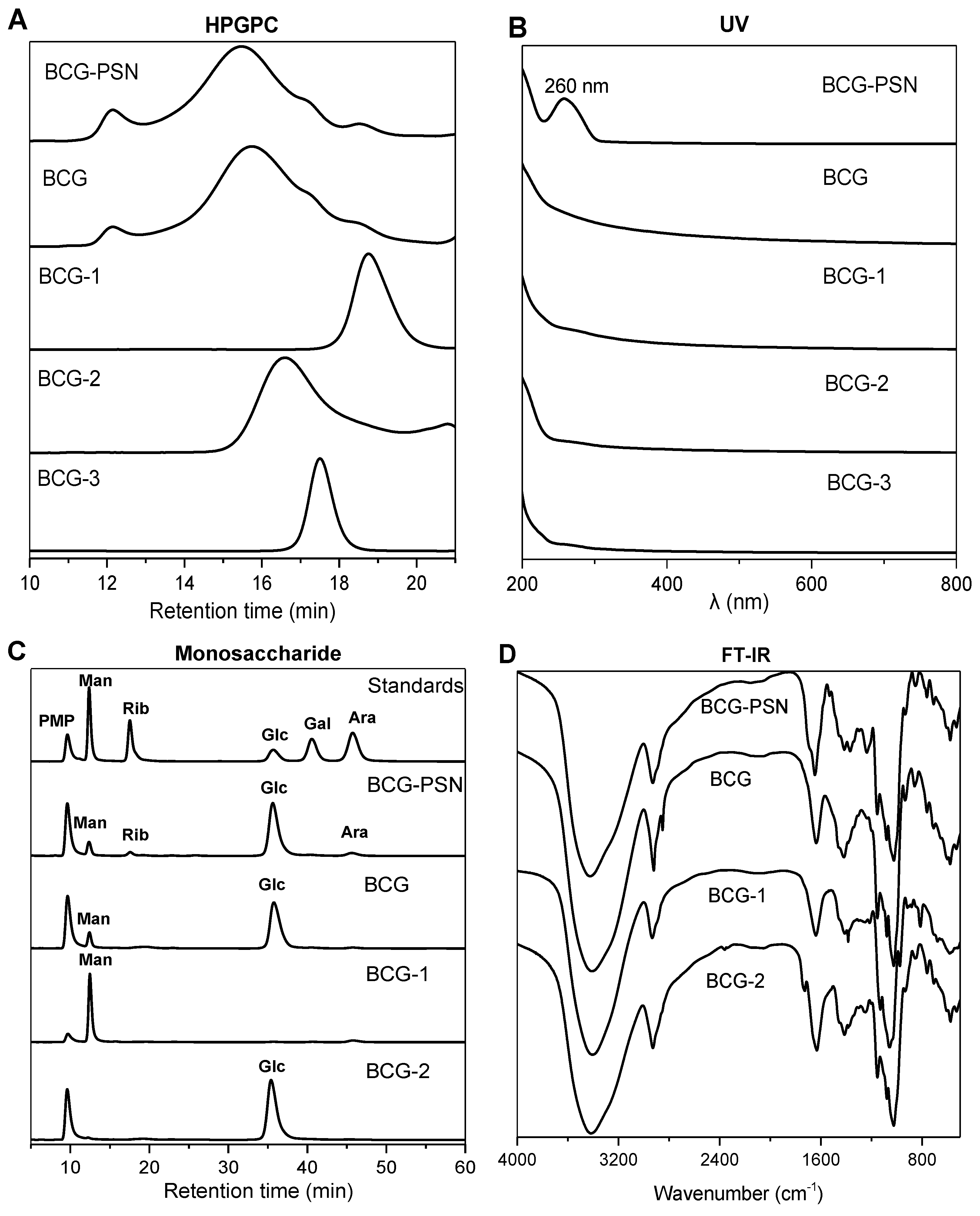
3.1. Isolation and Purification
3.2. Molecular Weight and Monosaccharide Composition Analysis
3.3. FT-IR Analysis
3.4. Methylation Analysis
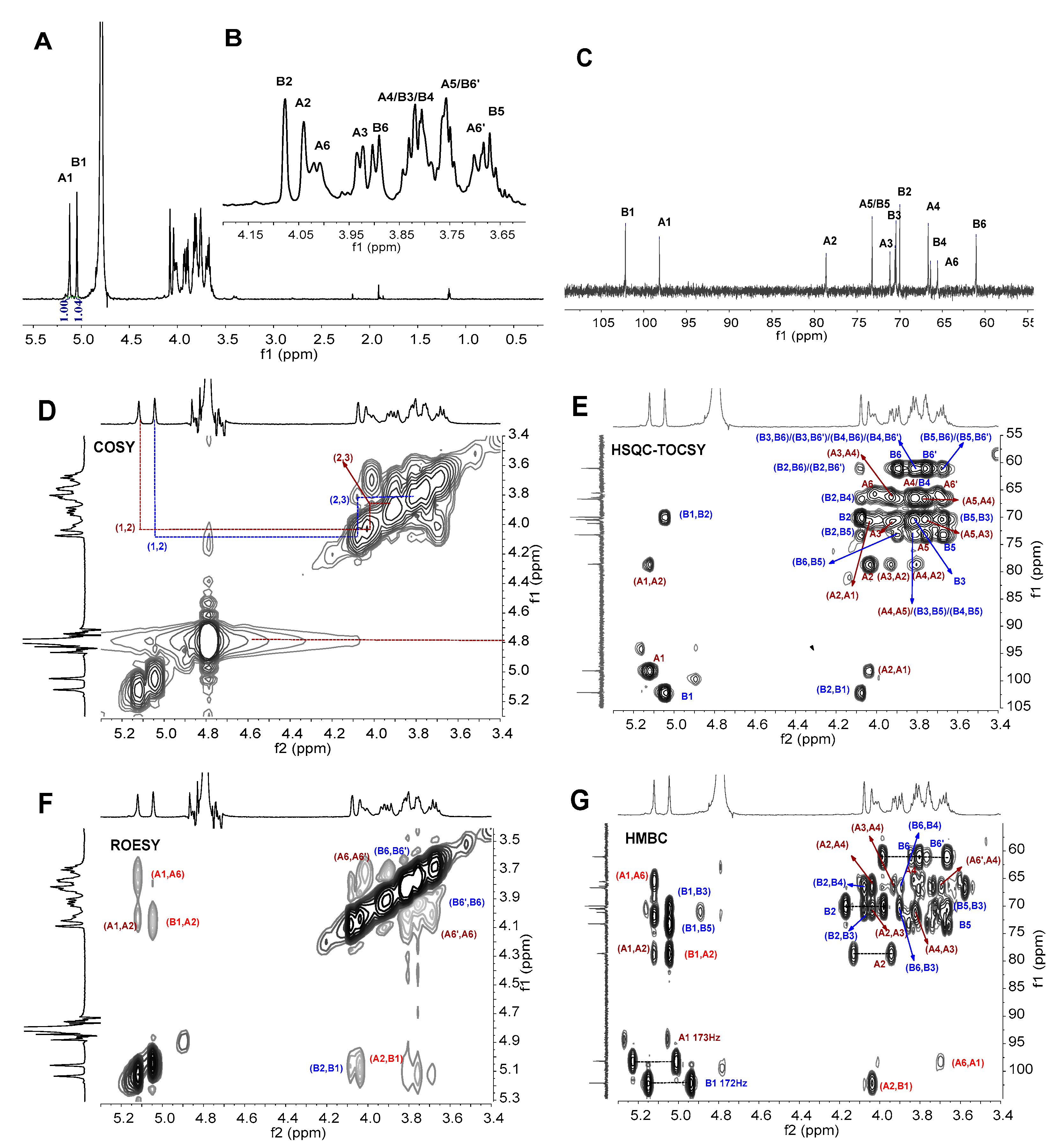
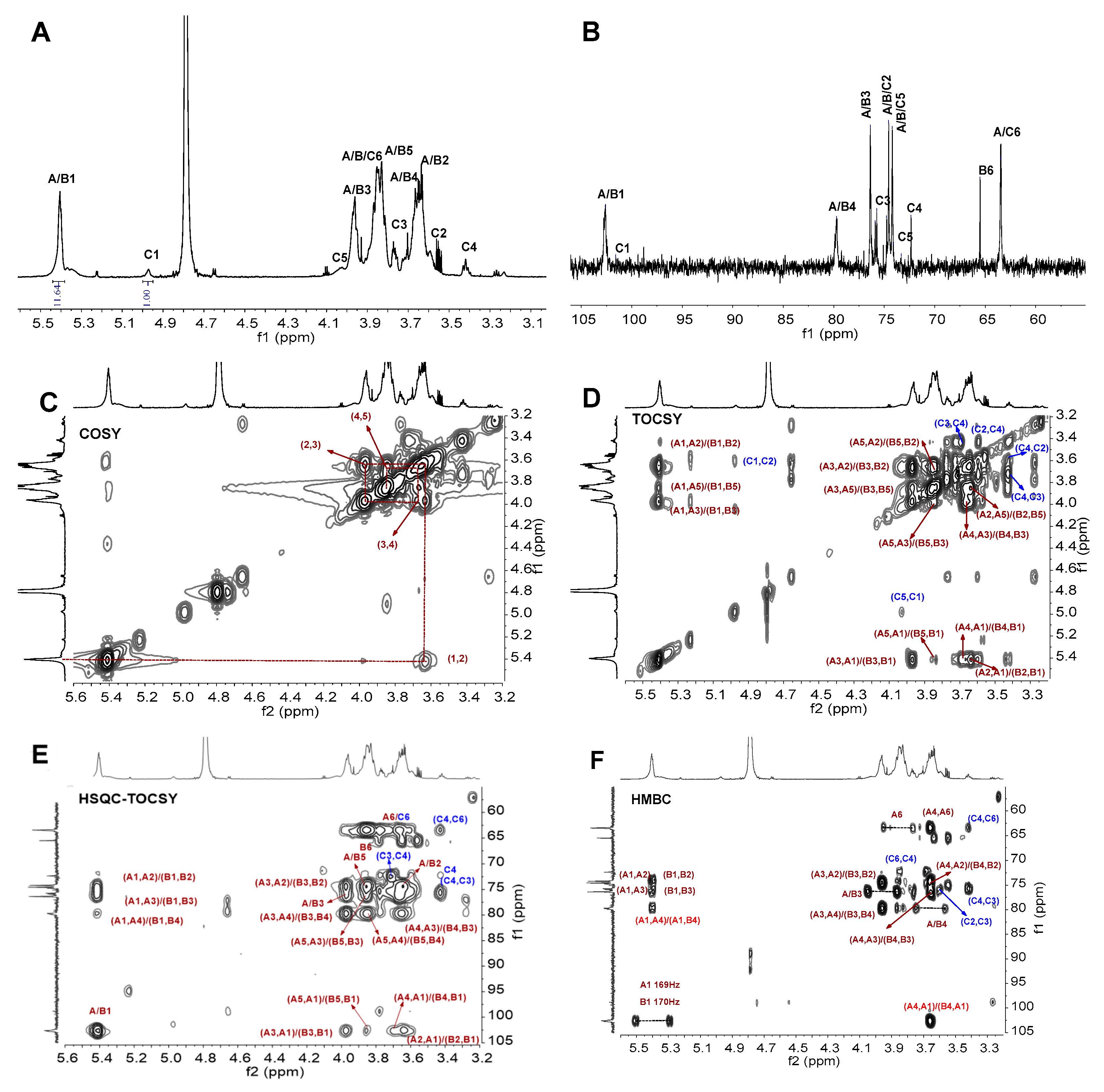
3.5. NMR Analysis
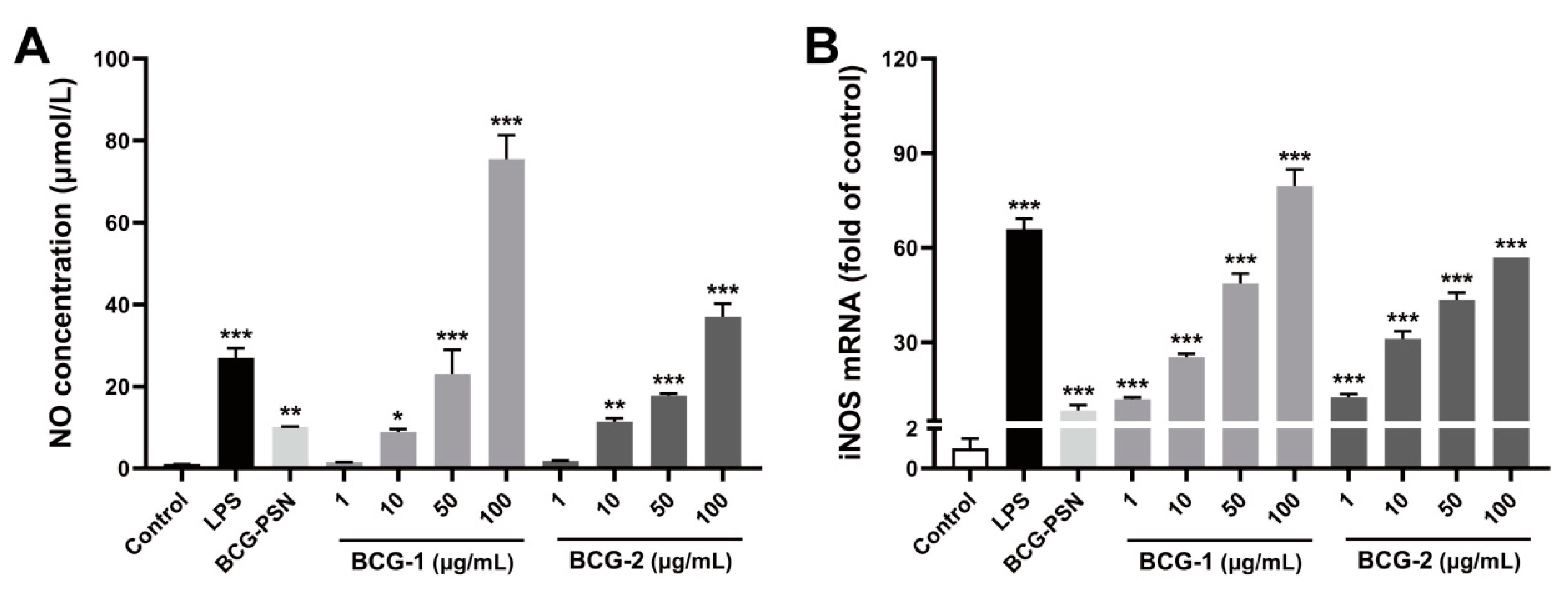
3.6. Effects of BCG-1 and BCG-2 on the NO Stimulation
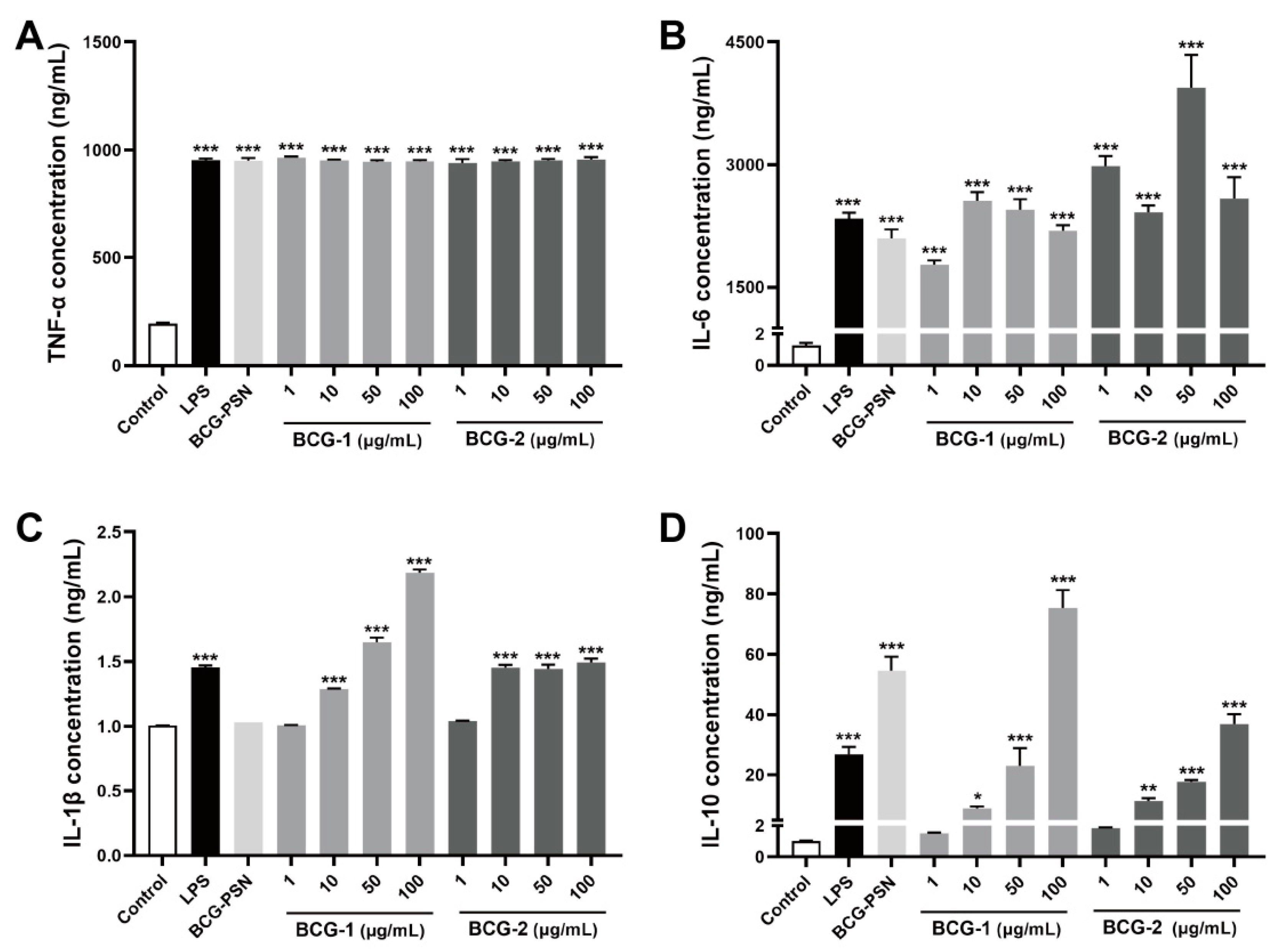
3.7. Effects of BCG-1 and BCG-2 on the Production of Cytokines
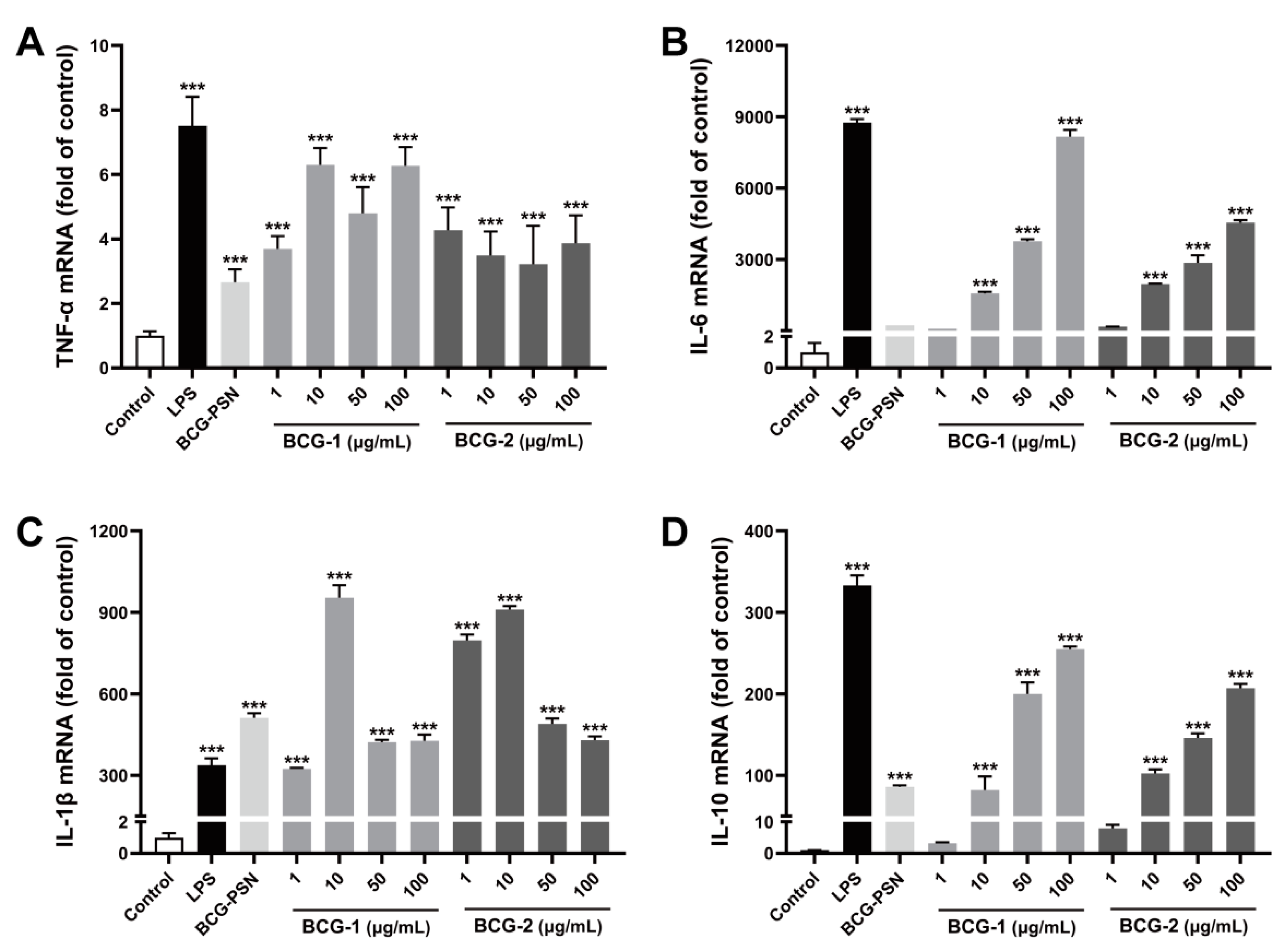
3.8. Effects of BCG-1 and BCG-2 on the Expression of Inflammation Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sun, J.; Hou, J.; Li, D.; Liu, Y.; Hu, N.; Hao, Y.; Fu, J.; Hu, Y.; Shao, Y. Enhancement of HIV-1 DNA vaccine immunogenicity by BCG-PSN, a novel adjuvant. Vaccine 2013, 31, 472–479. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Yu, J.; Liu, Y.; Lu, W.; Chai, Y.; Liu, C.; Pan, C.; Yao, W.; Gao, X. Structure, chain conformation, and immunomodulatory activity of the polysaccharide purified from Bacillus Calmette Guerin formulation. Carbohydr. Polym. 2016, 150, 149–158. [Google Scholar]
- Sharquie, K.E.; Hayani, R.K. BCG as a new therapeutic and prophylactic agent in patients with severe oral aphthosis. Clin. Exp. Rheumatol. 2005, 23, 914. [Google Scholar]
- Li, N.; Cao, N.; Niu, Y.-D.; Bai, X.-H.; Lu, J.; Sun, Y.; Yu, M.; Sun, L.-X.; Duan, X.-S. Effects of the polysaccharide nucleic acid fraction of bacillus Calmette-Guérin on the production of interleukin-2 and interleukin-10 in the peripheral blood lymphocytes of patients with chronic idiopathic urticaria. Biomed. Rep. 2013, 1, 713–718. [Google Scholar] [PubMed]
- Sloot, S.; Rashid, O.M.; Sarnaik, A.A.; Zager, J.S. Developments in intralesional therapy for metastatic melanoma. Cancer Control 2016, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gharib, K.; Kandil, A.; Marie, A.; Mounir, H. Efficacy of intralesional Bacillus Calmette-Guérin polysaccharide nucleic acid and vitamin D injections in the treatment of Lichen Planus. J. Clin. Aesthet. Dermatol. 2021, 14, 40–45. [Google Scholar]
- Zhang, F.; Shi, L.; Liu, P.; Zhang, L.; Wu, Q.; Wang, B.; Zhang, G.; Wang, P.; Zhou, F.; Chen, W.R.; et al. A novel cosmetic and clinically practicable laser immunotherapy for facial verruca plana: Intense pulsed light combined with BCG-PSN. Photodiagnosis Photodyn. Ther. 2018, 22, 86–90. [Google Scholar] [CrossRef]
- Yan, S.; Liu, R.; Mao, M.; Liu, Z.; Zhang, W.; Zhang, Y.; Li, J.; Peng, C.; Chen, X. Therapeutic effect of Bacillus Calmette—Guérin polysaccharide nucleic acid on mast cell at the transcriptional level. PeerJ 2019, 7, e7404. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; Zhang, H.S.; Tang, W.X.; Deng, F. Effects of BCG-PSN on pulmonary alveolar macrophage function in pulmonary tuberculosis patients. Acta Acad. Med. Mil. Tertiae 2004, 26, 1762–1764. [Google Scholar]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef]
- Xu, X.; Gu, Z.X.; Liu, S.; Gao, N.; He, X.Z.; Xin, X. Purification and characterization of a glucan from Bacillus Calmette Guérin and the antitumor activity of its sulfated derivative. Carbohydr. Polym. 2015, 128, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Z.; Luo, L.; Tao, M.; Chang, X.; Yang, L.; Huang, X.; Hu, L.; Wu, M. A non-anticoagulant heparin-like snail glycosaminoglycan promotes healing of diabetic wound. Carbohydr. Polym. 2020, 247, 116682. [Google Scholar] [CrossRef]
- Zaia, J. Mass spectrometry of oligosaccharides. Mass Spectrom. Rev. 2004, 23, 161–227. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-T.; Wang, S.-H.; Chen, Y.-T.; Yu, H.-M.; Chen, C.-H.; Yang, W.-B. MALDI-TOF MS analysis of native and permethylated or benzimidazole-derivatized polysaccharides. Molecules 2012, 17, 4950–4961. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Zhang, J.; Khoo, K.-H.; Wu, S.-W.; Chatterjee, D. Characterization of a distinct arabinofuranosyltransferase in Mycobacterium smegmatis. J. Am. Chem. Soc. 2007, 129, 9650–9662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; Luo, L.; Zhou, Z.; Wang, Y.; Gao, T.; Yang, L.; Peng, T.; Wu, M. Structures of fructan and galactan from Polygonatum cyrtonema and their utilization by probiotic bacteria. Carbohydr. Polym. 2021, 267, 118219. [Google Scholar] [CrossRef]
- López-García, M.; García, M.S.D.; Vilariño, J.M.L.; Rodríguez, M.V.G. MALDI-TOF to compare polysaccharide profiles from commercial health supplements of different mushroom species. Food Chem. 2016, 199, 597–604. [Google Scholar] [CrossRef]
- Fu, D.T.; ONeill, R.A. Monosaccharide composition analysis of oligosaccharides and glycoproteins by high-performance liquid chromatography. Anal. Biochem. 1995, 227, 377–384. [Google Scholar] [CrossRef]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl-5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Liu, J.; Shang, F.; Yang, Z.; Wu, M.; Zhao, J. Structural analysis of a homogeneous polysaccharide from Achatina fulica. Int. J. Biol. Macromol. 2017, 98, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Sims, I.M.; Carnachan, S.M.; Bell, T.J.; Hinkley, S.F.R. Methylation analysis of polysaccharides: Technical advice. Carbohydr. Polym. 2018, 188, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Griess, P. bemerkungen zu der abhandlung der hh. weselsky und benediktueber einige azoverbindungen. Ber. Dtsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef] [Green Version]
- Angala, S.K.; Palčeková, Z.; Belardininelli, J.M.; Jackson, M. Covalent modifivations of polysaccharides in mycobacteria. Nat. Chem. Biol. 2018, 14, 193–198. [Google Scholar] [CrossRef]
- Venisse, A.; Rivière, M.; Vercauteren, J.; Puzo, G. Structural analysis of the mannan region of lipoarabinomannan from Mycobacterium bovis BCG: Heterogeneity in phosphorylation state. J. Biol. Chem. 1995, 270, 15012–15021. [Google Scholar] [CrossRef]
- Hung, W.-T.; Wang, S.-H.; Chen, C.-H.; Yang, W.-B. Structure determination of beta-glucans from Ganoderma lucidum with matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. Molecules 2008, 13, 1538–1550. [Google Scholar] [CrossRef]
- Luo, L.; Wu, M.; Xu, L.; Lian, W.; Xiang, J.; Lu, F.; Gao, N.; Xiao, C.; Wang, S.; Zhao, J. Comparison of physicochemical characteristics and anticoagulant activities of polysaccharides from three sea cucumbers. Mar. Drugs 2013, 11, 399–417. [Google Scholar] [CrossRef]
- Lijour, Y.; Gentric, E.; Deslandes, E.; Guezennec, J. Estimation of the sulfate content of hydrothermal vent bacterial polysaccharides by fourier-transform infrared-spectroscopy. Anal. Biochem. 1994, 220, 244–248. [Google Scholar] [CrossRef]
- Kath, F.; Kulicke, W.-M. Polymer analytical characterization of glucan and mannan from yeast Saccharomyces cerevisiae. Macromol. Mater. Eng. 1999, 268, 69–80. [Google Scholar]
- Liu, Y.; Huang, G.; Lv, M. Extraction, characterization and antioxidant activities of mannan from yeast cell wall. Int. J. Biol. Macromol. 2018, 118, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, M.; Nigou, J.; Cahuzac, B.; Puzo, G. Structural study of the lipomannans from Mycobacterium bovis BCG: Characterisation of multiacylated forms of the phosphatidyl-myo-inositol anchor. J. Mol. Biol. 1999, 285, 2147–2160. [Google Scholar] [CrossRef]
- Kosaka, A.; Aida, M.; Katsumoto, Y. Reconsidering the activation entropy for anomerization of glucose and mannose in water studied by NMR spectroscopy. J. Mol. Struct. 2015, 1093, 195–200. [Google Scholar] [CrossRef]
- Bubb, W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts Magn. Reson. Part A 2003, 19A, 1–19. [Google Scholar] [CrossRef]
- Dinadayala, P.; Lemassu, A.; Granovski, P.; Cérantola, S.; Winter, N.; Daffé, M. Revisiting the structure of the anti-neoplastic glucans of Mycobacterium bovis Bacille Calmette-Guérin: Structural analysis of the extracellular and boiling water extract-derived glucans of the vaccine substrains. J. Biol. Chem. 2004, 279, 12369–12378. [Google Scholar] [CrossRef] [Green Version]
- Jarlier, V.; Nikaido, H. Mycobacterial cell wall: Structure and role in natural resistance to antibiotics. FEMS Microbiol. Lett. 1994, 123, 11–18. [Google Scholar] [CrossRef]
- Lemassu, A.; Daffé, M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem. J. 1994, 297, 351–357. [Google Scholar] [CrossRef]
- Ortalo-Magne, A.; Dupont, M.-A.; Lemassu, A.; Andersen, A.B.; Gounon, P.; Daffe, M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology 1995, 141, 1609–1620. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 2015, 36, 161–178. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Vongsawasdi, P.; Chao, L.K. Assessment of biochemical and immunomodulatory activity of sulphated polysaccharides from Ulva intestinalis. Int. J. Biol. Macromol. 2016, 91, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, J.-F.; Zha, X.-Q.; Cui, S.-H.; Cao, L.; Luo, J.-P. Immunomodulatory activity on macrophage of a purified polysaccharide extracted from Laminaria japonica. Carbohydr. Polym. 2015, 134, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Liu, Z.; Huang, H. Immune-enhancing activities of chondroitin sulfate in murine macrophage RAW 264.7 cells. Carbohydr. Polym. 2018, 198, 611–619. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Commins, S.P.; Borish, L.; Steinke, J.W. Immunologic messenger molecules: Cytokines, interferons, and chemokines. J. Allergy Clin. Immunol. 2010, 125, S53–S72. [Google Scholar] [CrossRef]
- Wang, L.-H.; Ye, Y.; Zhang, Y.-Q.; Xiao, T. Curative effect of BCG-polysaccharide nuceic acid on atopic dermatitis in mice. Asian Pac. J. Trop. Med. 2014, 7, 913–917. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Luo, Y.; Yamada, H.; Evanoff, D.P.; Chen, X. Role of Th1-stimulating cytokines in bacillus Calmette—Guérin (BCG)-induced macrophage cytotoxicity against mouse bladder cancer MBT-2 cells. Clin. Exp. Immunol. 2006, 146, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Kuroda, E.; Matsumoto, S.; Matsumoto, T.; Yamada, T.; Yamashita, U. Prostaglandin E2 down-regulates viable Bacille Calmette-Guérin-induced macrophage cytotoxicity against murine bladder cancer cell MBT-2 in vitro. Clin. Exp. Immunol. 2002, 128, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Cenci, E.; Romani, L.; Mencacci, A.; Spaccapelo, R.; Schiaffella, E.; Puccetti, P.; Bistoni, F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur. J. Immunol. 1993, 23, 1034–1038. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar] [PubMed]
- Luo, Y.; Yamada, H.; Chen, X.; Ryan, A.A.; Evanoff, D.P.; Triccas, J.A.; O’Donnell, M.A. Recombinant Mycobacterium bovis bacillus Calmette—Guérin (BCG) expressing mouse IL-18 augments Th1 immunity and macrophage cytotoxicity. Clin. Exp. Immunol. 2004, 137, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Han, R.; Evanoff, D.P.; Chen, X. Interleukin-10 inhibits Mycobacterium bovis bacillus Calmette—Guérin (BCG)-induced macrophage cytotoxicity against bladder cancer cells. Clin. Exp. Immunol. 2010, 160, 359–368. [Google Scholar] [CrossRef]
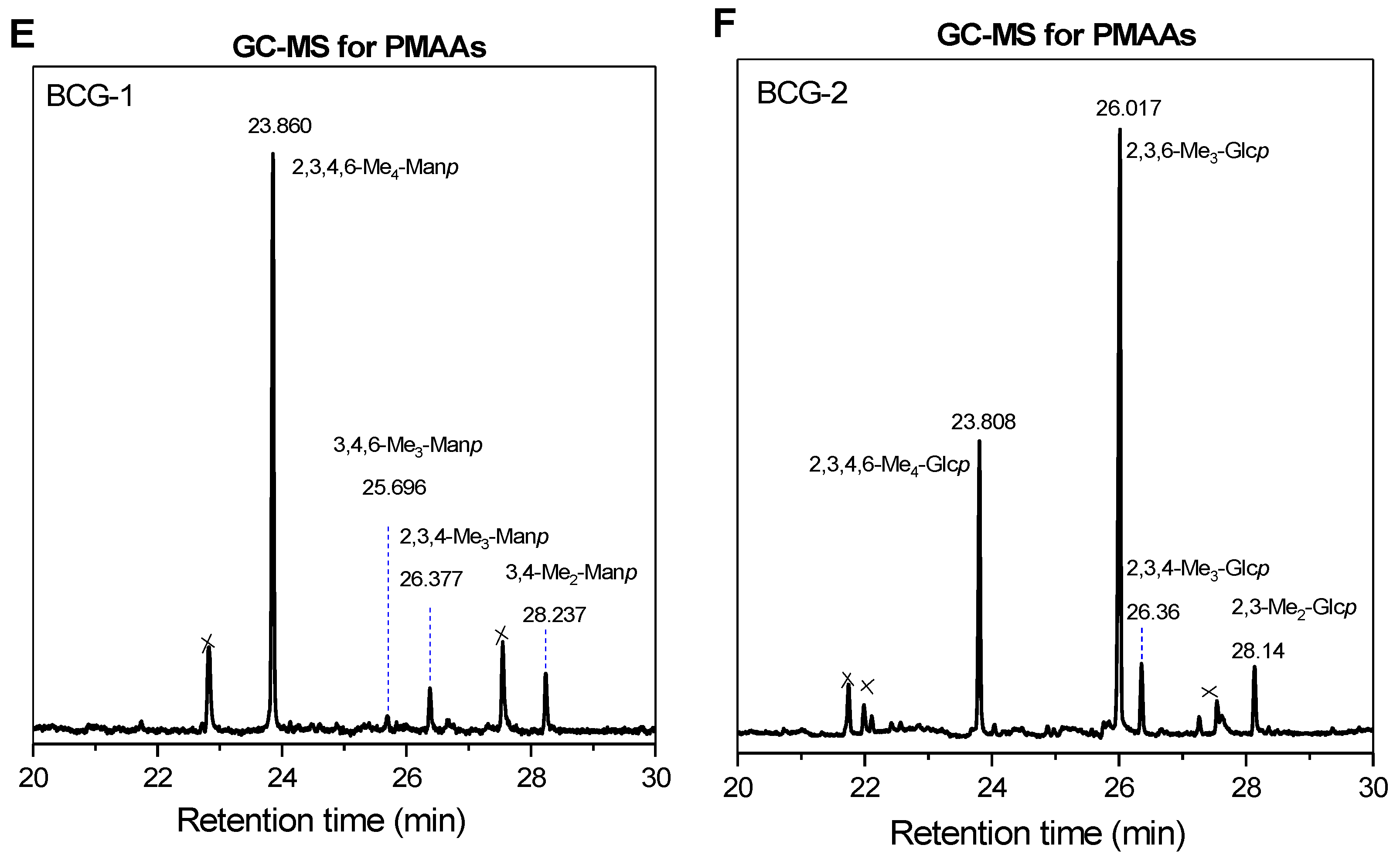
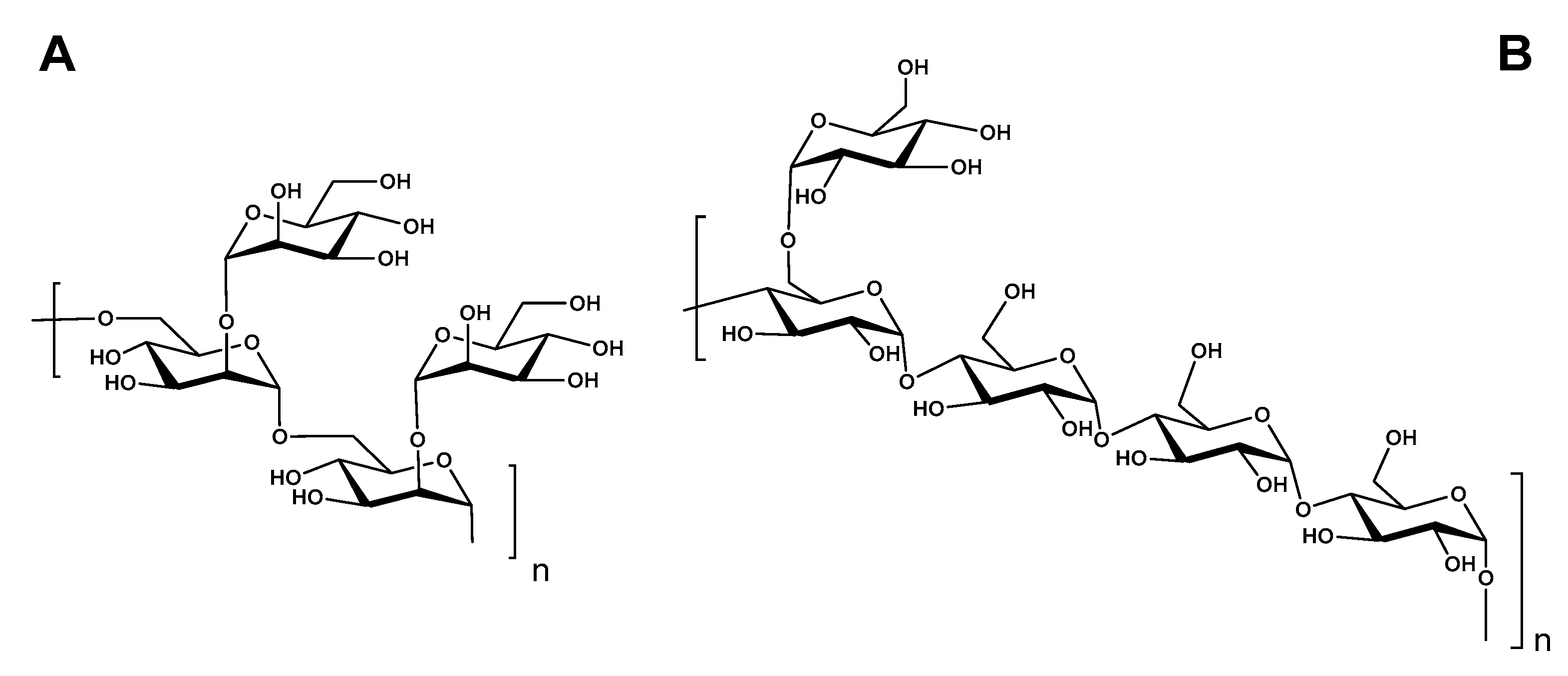
| PMAA Derivatives a | Type of Linkage | Relative Retention Time b | Molar Ratio | Mass Fragments (m/z) |
|---|---|---|---|---|
| BCG-1 | ||||
| 2,3,4,6-Me4- Manp | t-Manp | 1.00 | 35.86 | 71, 87, 101, 129, 145, 162, 205 |
| 3,4,6-Me3-Manp | 2- Manp | 1.08 | 1.00 | 87, 129, 161, 189 |
| 2,3,4-Me3-Manp | 6- Manp | 1.11 | 3.10 | 71, 87, 101, 118, 129, 161, 189 |
| 3,4-Me2- Manp | 2,6- Manp | 1.18 | 3.52 | 87, 99, 129, 189 |
| BCG-2 | ||||
| 2,3,4,6-Me4-Glcp | t-Glcp | 1.00 | 5.16 | 87, 102, 118, 129, 145, 162, 205 |
| 2,3,6- Me3- Glcp | 4- Glcp | 1.09 | 10.38 | 87, 100, 118, 129, 233 |
| 2,3,4-Me3- Glcp | 6- Glcp | 1.17 | 1.13 | 87, 100, 118, 129, 161, 189 |
| 2,3-Me2- Glcp | 4,6- Glcp | 1.18 | 1.00 | 100, 117, 129, 261 |
| Sugar Residues | Chemical Shifts (ppm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6a | 6b | ||
| BCG-1 | ||||||||
| 2,6-O-α-Manp (A) | H | 5.12 | 4.04 | 3.94 | 3.82 | 3.77 | 4.01 | 3.68 |
| C | 98.2 | 78.6 | 70.5 | 66.4 | 73.1 | 65.6 | - | |
| t-α-Man (B) | H | 5.04 | 4.08 | 3.82 | 3.82 | 3.68 | 3.90 | 3.76 |
| C | 102.2 | 70.0 | 70.6 | 66.4 | 72.8 | 61.0 | - | |
| BCG-2 | ||||||||
| →4)-α-Glc→1 (A) | H | 5.41 | 3.63 | 3.96 | 3.66 | 3.86 | 3.88 | 3.76 |
| C | 102.6 | 74.5 | 76.3 | 79.8 | 74.1 | 63.4 | - | |
| →4,6)-α-Glc(1→ (B) | H | 5.39 | 3.63 | 3.96 | 3.66 | 3.83 | 3.86 | 3.85 |
| C | 102.6 | 74.5 | 76.3 | 79.8 | 74.1 | 65.5 | - | |
| t-α-Glc(1→ (C) | H | 4.96 | 3.61 | 3.71 | 3.41 | 4.04 | 3.85 | |
| C | 101.6 | 74.5 | 75.7 | 72.3 | 73.4 | 63.4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Song, X.; Chang, X.; Huang, S.; Zhou, Y.; Yang, S.; Zhu, Y.; Zhang, L.; Wu, Y.; Zhang, J.; et al. Detailed Structural Analysis of the Immunoregulatory Polysaccharides from the Mycobacterium Bovis BCG. Molecules 2022, 27, 5691. https://doi.org/10.3390/molecules27175691
Luo L, Song X, Chang X, Huang S, Zhou Y, Yang S, Zhu Y, Zhang L, Wu Y, Zhang J, et al. Detailed Structural Analysis of the Immunoregulatory Polysaccharides from the Mycobacterium Bovis BCG. Molecules. 2022; 27(17):5691. https://doi.org/10.3390/molecules27175691
Chicago/Turabian StyleLuo, Lan, Xuemei Song, Xiao Chang, Sheng Huang, Yunxi Zhou, Shengmei Yang, Yan Zhu, Lanyan Zhang, Yongsheng Wu, Junyin Zhang, and et al. 2022. "Detailed Structural Analysis of the Immunoregulatory Polysaccharides from the Mycobacterium Bovis BCG" Molecules 27, no. 17: 5691. https://doi.org/10.3390/molecules27175691
APA StyleLuo, L., Song, X., Chang, X., Huang, S., Zhou, Y., Yang, S., Zhu, Y., Zhang, L., Wu, Y., Zhang, J., Zhou, Z., & Wu, M. (2022). Detailed Structural Analysis of the Immunoregulatory Polysaccharides from the Mycobacterium Bovis BCG. Molecules, 27(17), 5691. https://doi.org/10.3390/molecules27175691




