Underestimation about the Contribution of Nitrate Reducers to Iron Cycling Indicated by Enterobacter Strain
Abstract
:1. Introduction
2. Results
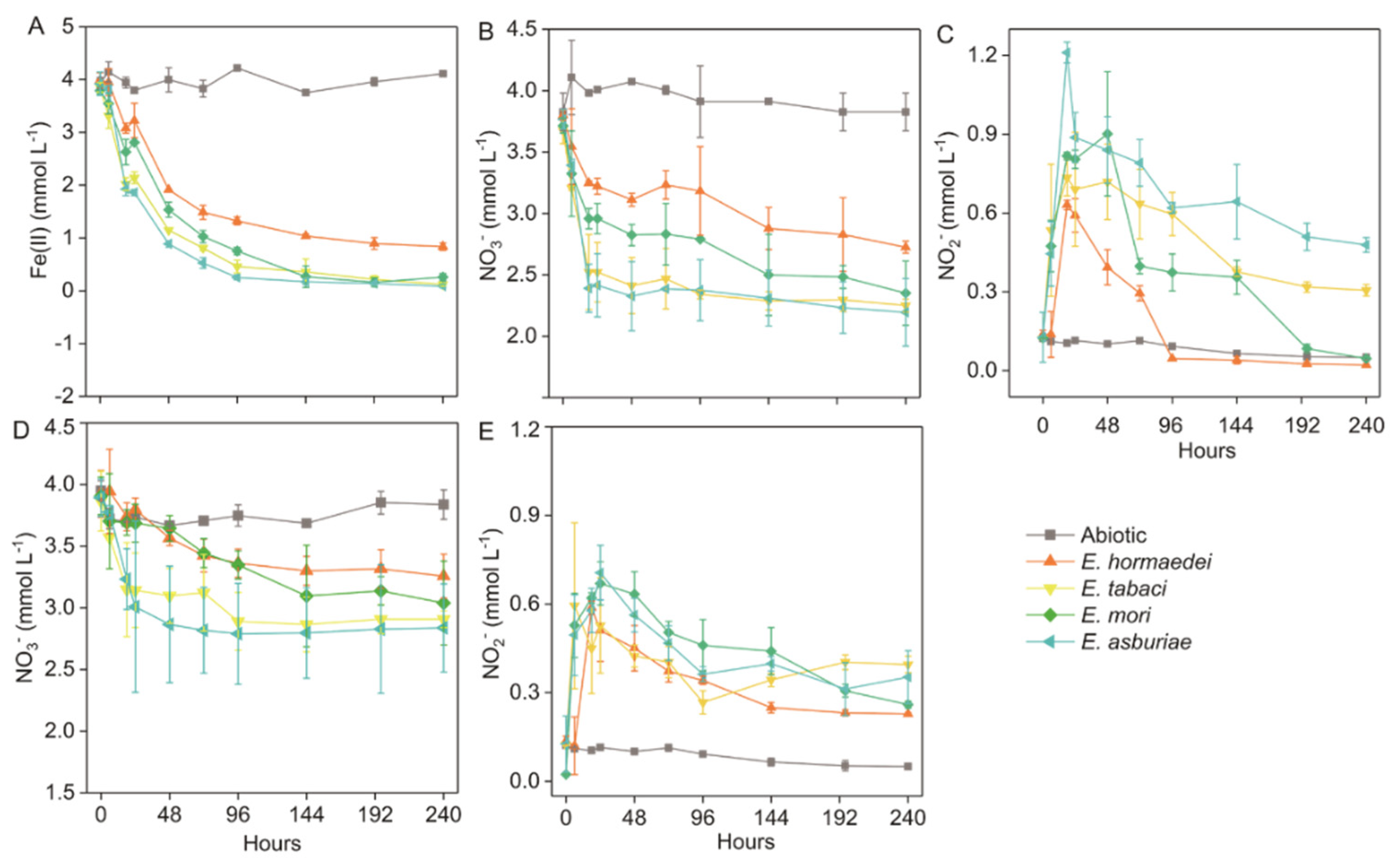
2.1. Nitrate Reduction and Iron(II) Oxidation by Enterobacter Strains
2.2. Morphological Characteristics of Enterobacter Strains after Iron(II) Oxidation and Nitrate Reduction
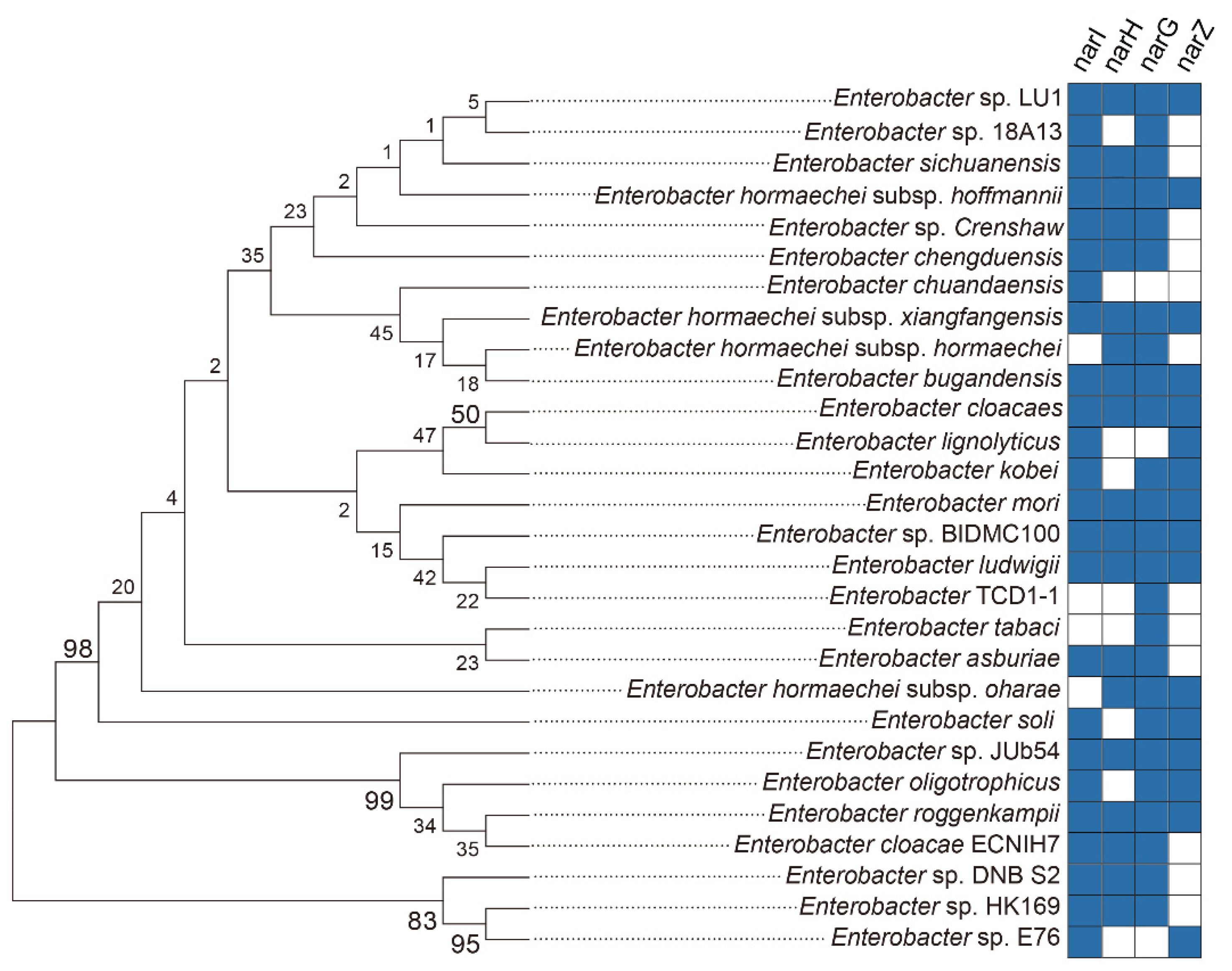
2.3. Nitrate Reductase Contained in the Enterobacteriaceae Strains
3. Discussion
3.1. Microbial-Mediated Nitrate-Dependent Fe(II) Oxidation by Enterobacter Strains
3.2. Cell Encrustation and Physical Inactivity after NRFO Process by Enterobacter Strains
3.3. The Potential Contribution of Nitrate Reducers to Iron Cycling Implicated by Enterobacter Strains
4. Materials and Methods
4.1. Isolation of Nitrate-Dependent Fe(II)-Oxidizing Bacterium
4.2. Experimental Setup
4.3. PCR
4.4. Chemical Analyses
4.5. Phenotypic Analysis
4.6. Raman Spectroscopy
4.7. Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nordhoff, M.; Tominski, C.; Halama, M.; Byrne, J.; Obst, M.; Kleindienst, S.; Behrens, S.; Kappler, A. Insights into nitrate-reducing Fe (II) oxidation mechanisms through analysis of cell-mineral associations, cell encrustation, and mineralogy in the chemolithoautotrophic enrichment culture KS. Appl. Environ. Microbiol. 2017, 83, e00752-17. [Google Scholar] [CrossRef]
- Jamieson, J.; Prommer, H.; Kaksonen, A.H.; Sun, J.; Siade, A.J.; Yusov, A.; Bostick, B. Identifying and quantifying the intermediate processes during nitrate-dependent iron (II) oxidation. Environ. Sci. Technol. 2018, 52, 5771–5781. [Google Scholar] [CrossRef]
- Zhang, M.; Zhangzhu, G.; Wen, S.; Lu, H.; Wang, R.; Li, W.; Ding, S.; Ghulam, A.; Zheng, P. Chemolithotrophic denitrification by nitrate-dependent anaerobic iron oxidizing (NAIO) process: Insights into the evaluation of seeding sludge. Chem. Eng. J. 2018, 345, 345–352. [Google Scholar] [CrossRef]
- Klueglein, N.; Picardal, F.; Zedda, M.; Zwiener, C.; Kappler, A. Oxidation of Fe(II)-EDTA by nitrite and by two nitrate-reducing Fe(II)-oxidizing Acidovorax strains. Geobiology 2015, 13, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Roden, E.E.; Schieber, J.; Picardal, F. Enhanced growth of Acidovorax sp. strain 2AN during nitrate-dependent Fe(II) oxidation in batch and continuous-flow systems. Appl. Environ. Microbiol. 2011, 77, 8548–8556. [Google Scholar] [CrossRef] [PubMed]
- Benz, M.; Brune, A.; Schink, B. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch. Microbiol. 1998, 169, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Brons, H.J.; Hagen, W.R.; Zehnder, A.J.B. Ferrous iron dependent nitric oxide production in nitrate reducing cultures of Escherichia coli. Arch. Microbiol. 1991, 155, 341–347. [Google Scholar] [CrossRef]
- Ishii, S.; Joikai, K.; Otsuka, S.; Senoo, K.; Okabe, S. Denitrification and nitrate-dependent Fe(II) oxidation in various Pseudogulbenkiania strains. Microbes Environ. 2016, 31, 293–298. [Google Scholar] [CrossRef]
- Carlson, H.K.; Clark, I.C.; Melnyk, R.A.; Coates, J.D. Toward a mechanistic understanding of anaerobic nitrate-dependent iron oxidation: Balancing electron uptake and detoxification. Front. Microbiol. 2012, 3, 57. [Google Scholar] [CrossRef]
- Kappler, A.; Schink, B.; Newman, D.K. Fe(III) mineral formation and cell encrustation by nitrate dependent Fe(II) oxidizers the nitrate dependent Fe(II) oxidizer strain BoFeN1. Geobiology 2005, 3, 235–245. [Google Scholar] [CrossRef]
- Zhou, G.W.; Yang, X.R.; Ronn, R.; Su, J.Q.; Cui, L.; Zheng, B.X.; Zhu, Y.G. Metabolic Inactivity and Re-awakening of a Nitrate Reduction Dependent Iron(II)-Oxidizing Bacterium Bacillus ferrooxidans. Front. Microbiol. 2019, 10, 1494. [Google Scholar] [CrossRef]
- Klueglein, N.; Zeitvogel, F.; Stierhof, Y.D.; Floetenmeyer, M.; Konhauser, K.O.; Kappler, A. Potential role of nitrite for abiotic Fe(II) oxidation and cell encrustation during nitrate reduction by denitrifying bacteria. Appl. Environ. Microbiol. 2014, 80, 1051–1061. [Google Scholar] [CrossRef]
- Miot, J.; Remusat, L.; Duprat, E.; Gonzalez, A.; Pont, S.; Poinsot, M. Fe biomineralization mirrors individual metabolic activity in a nitrate-dependent Fe (II)-oxidizer. Front. Microbiol. 2015, 6, 879. [Google Scholar] [CrossRef]
- Philippot, L. Tracking nitrate reducers and denitrifiers in the environment. Biochem. Soc. Trans. 2005, 33, 200–204. [Google Scholar] [CrossRef]
- Brenner, D.J.; Farmer, J.J., III. Enterobacteriaceae. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–24. [Google Scholar]
- Grimont, P.A.; Grimont, F. Enterobacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–17. [Google Scholar]
- Mojarad, M.; Alemzadeh, A.; Ghoreishi, G.; Javaheri, M. Kerosene biodegradation ability and characterization of bacteria isolated from oil-polluted soil and water. J. Environ. Chem. Eng. 2016, 4, 4323–4329. [Google Scholar] [CrossRef]
- Luang-In, V.; Saengha, W.; Deeseenthum, S.; Maneewan, K.; Udomwong, P. Identification of soil bacteria isolated from Nasinuan community forest with potential application in agriculture. J. Sustain. Sci. Manag. 2021, 16, 153–165. [Google Scholar] [CrossRef]
- Abdullahi, S.; Haris, H.; Zarkasi, K.Z.; Amir, H.G. Complete genome sequence of plant growth-promoting and heavy metal-tolerant Enterobacter tabaci 4M9 (CCB-MBL 5004). J. Basic Microbiol. 2021, 61, 293–304. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Parekh, L.; Archana, G.; Poole, P.; Collins, M.; Hutson, R.; Kumar, G.N. Involvement of a phosphate starvation inducible glucose dehydrogenase in soil phosphate solubilization by Enterobacter asburiae. FEMS Microbiol. Lett. 1999, 171, 223–229. [Google Scholar] [CrossRef]
- Reasoner, D.J.; Geldreich, E.E. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 1985, 49, 1–7. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [CrossRef] [Green Version]
- Amstaetter, K.; Borch, T.; Kappler, A. Influence of humic acid imposed changes of ferrihydrite aggregation on microbial Fe(III) reduction. Geochim. Cosmochim. Acta 2012, 85, 326–341. [Google Scholar] [CrossRef]
- Yang, X.R.; Li, H.; Nie, S.A.; Su, J.Q.; Weng, B.S.; Zhu, G.B.; Yao, H.Y.; Gilbert, J.A.; Zhu, Y.G. Potential contribution of anammox to nitrogen loss from paddy soils in Southern China. Appl. Environ. Microbiol. 2015, 81, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Ratering, S.; Schnell, S. Nitrate-dependent iron (II) oxidation in paddy soil. Environ. Microbiol. 2001, 3, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hegler, F.; Posth, N.R.; Jiang, J.; Kappler, A. Physiology of phototrophic iron(II)-oxidizing bacteria: Implications for modern and ancient environments. FEMS Microbiol. Ecol. 2008, 66, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Klueglein, N.; Kappler, A. Abiotic oxidation of Fe(II) by reactive nitrogen species in cultures of the nitrate-reducing Fe(II) oxidizer Acidovorax sp. BoFeN1-questioning the existence of enzymatic Fe(II) oxidation. Geobiology 2013, 11, 180–190. [Google Scholar] [CrossRef]
- Stubner, S. Enumeration of 16S rDNA of Desulfotomaculum lineage 1 in rice field soil by real-time PCR with SybrGreen™ detection. J. Microbiol. Methods 2002, 50, 155. [Google Scholar] [CrossRef]
- Christopher, M.D.; Paul, B.; Blackall, L.L.; Mcewan, A.G. Aerobic nitrate respiration in a nitrite-oxidising bioreactor. FEMS Microbiol. Lett. 2000, 184, 113–118. [Google Scholar]
- Laurent, P.; Séverine, P.; Fabrice, M.L.; Stéphanie, H.; Jean Claude, G. Molecular analysis of the nitrate-reducing community from unplanted and maize-planted soils. Appl. Environ. Microbiol. 2002, 68, 6121–6128. [Google Scholar]
- Allen, A.E.; Booth, M.G.; Frischer, M.E.; Verity, P.G.; Zehr, J.P.; Zani, S. Diversity and Detection of Nitrate Assimilation Genes in Marine Bacteria. Appl. Environ. Microbiol. 2001, 67, 5343. [Google Scholar] [CrossRef]
- Braker, G.; Fesefeldt, A.; Witzel, K.P. Witzel Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 1998, 64, 3769–3775. [Google Scholar] [CrossRef]
- Paolina, G.; Baggs, E.M.; Prosser, J.I. Phylogeny of nitrite reductase (nirK) and nitric oxide reductase (norB) genes from Nitrosospira species isolated from soil. FEMS Microbiol. Lett. 2010, 266, 83–89. [Google Scholar]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Chen, P.; Chen, S.; Yuan, Z.; Yu, C.; Ren, B.; Zhang, K. In situ study of the antibacterial activity and mechanism of action of silver nanoparticles by surface-enhanced Raman spectroscopy. Anal. Chem. 2013, 85, 5436–5443. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, L.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Felsenstein, J. Phylogenies from Gene Frequencies: A Statistical Problem. Syst. Zool. 1985, 34, 300–311. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Freeman, C.; Lock, M.A. The biofilm polysaccharide matrix: A buffer against changing organic substrate supply? Limnol. Oceanogr. 1995, 40, 273–278. [Google Scholar] [CrossRef]
- Ra, C.; Lo, K.; Shin, J.; Oh, J.; Hong, B. Biological nutrient removal with an internal organic carbon source in piggery wastewater treatment. Water Res. 2000, 34, 965–973. [Google Scholar] [CrossRef]
- Silverstein, J.; Schroeder, E.D. Performance of SBR activated sludge processes with nitrification/denitrification. J. Water Pollut. Control. Fed. 1983, 55, 377–384. [Google Scholar]
- Oh, J.; Silverstein, J. Aceate limitation and nitrite accumulation during denitrification. J. Environ. Eng. 1999, 125, 234–242. [Google Scholar] [CrossRef]
- Wang, R.; Xu, S.Y.; Zhang, M.; Ghulam, A.; Dai, C.L.; Zheng, P. Iron as electron donor for denitrification: The efficiency, toxicity and mechanism. Ecotoxicol. Environ. Saf. 2020, 194, 110343. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Picardal, F. Neutrophilic, nitrate-dependent, Fe(II) oxidation by a Dechloromonas species. World J. Microbiol. Biotechnol. 2013, 29, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol.-Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed]
- Miot, J.; Benzerara, K.; Morin, G.; Kappler, A.; Bernard, S.; Obst, M.; Férard, C.; Skouri-Panet, F.; Guigner, J.-M.; Posth, N.; et al. Iron biomineralization by anaerobic neutrophilic iron-oxidizing bacteria. Geochim. Cosmochim. Acta 2009, 73, 696–711. [Google Scholar] [CrossRef]
- Dippon, U.; Pantke, C.; Porsch, K.; Larese-Casanova, P.; Kappler, A. Potential function of added minerals as nucleation sites and effect of humic substances on mineral formation by the nitrate-reducing Fe(II)-oxidizer Acidovorax sp. BoFeN1. Environ. Sci. Technol. 2012, 46, 6556–6565. [Google Scholar] [CrossRef]
- Schädler, S.; Burkhardt, C.; Hegler, F.; Straub, K.L.; Miot, J.; Benzerara, K.; Kappler, A. Formation of Cell-Iron-Mineral Aggregates by Phototrophic and Nitrate-Reducing Anaerobic Fe(II)-Oxidizing Bacteria. Geomicrobiol. J. 2009, 26, 93–103. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Yang, K.; Ji, B.; Chen, D.; Zhang, H.; Sun, Y.; Tian, J. Autotrophic denitrification by nitrate-dependent Fe(II) oxidation in a continuous up-flow biofilter. Bioprocess Biosyst. Eng. 2016, 39, 277–284. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.; Zhou, J.; Chen, M.; Wang, X.; Shi, Z. Fe(II)EDTA-NO reduction coupled with Fe(II)EDTA oxidation by a nitrate- and Fe(III)-reducing bacterium. Bioresour. Technol. 2013, 138, 339–344. [Google Scholar] [CrossRef]
- Zhou, G.W.; Yang, X.R.; Su, J.Q.; Zheng, B.X.; Zhu, Y.G. Bacillus ferrooxidans sp. nov., an iron(II)-oxidizing bacterium isolated from paddy soil. J. Microbiol. 2018, 56, 472–477. [Google Scholar] [CrossRef]
- Sparacino-Watkins, C.; Stolz, J.F.; Basu, P. Nitrate and periplasmic nitrate reductases. Chem. Soc. Rev. 2014, 43, 676–706. [Google Scholar] [CrossRef]
- Carlson, H.K.; Clark, I.C.; Blazewicz, S.J.; Iavarone, A.T.; Coates, J.D. Fe(II) oxidation is an innate capability of nitrate-reducing bacteria that involves abiotic and biotic reactions. J. Bacteriol. 2013, 195, 3260–3268. [Google Scholar] [CrossRef]


| Strain | Culture Preservation Organization | Isolation Source |
|---|---|---|
| E. hormaechei | CGMCC 1.10608T | Pig farm |
| E. tabaci | CGMCC 1.15707T | Stem of a tobacco plant |
| E. mori | CGMCC 1.10322T | Diseased mulberry roots |
| E. asburiae | JCM 6051 | Mulberry |
| Gene | PCR Products | E. hormaechei | E. tabaci | E. mori | E. asburiae |
|---|---|---|---|---|---|
| napA | 1040 bp | − | − | − | − |
| narG | 650 bp | + | + | + | + |
| nasA | 700 bp | + | + | + | + |
| nirK | 526 bp | + | + | + | + |
| nirS | 774 bp | + | + | + | + |
| norB | 669 bp | + | + | + | + |
| nosZ | 300 bp | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.-J.; Wei, M.-Y.; Fan, X.-T.; Zhou, G.-W. Underestimation about the Contribution of Nitrate Reducers to Iron Cycling Indicated by Enterobacter Strain. Molecules 2022, 27, 5581. https://doi.org/10.3390/molecules27175581
Li M-J, Wei M-Y, Fan X-T, Zhou G-W. Underestimation about the Contribution of Nitrate Reducers to Iron Cycling Indicated by Enterobacter Strain. Molecules. 2022; 27(17):5581. https://doi.org/10.3390/molecules27175581
Chicago/Turabian StyleLi, Ming-Jun, Meng-Yun Wei, Xiao-Ting Fan, and Guo-Wei Zhou. 2022. "Underestimation about the Contribution of Nitrate Reducers to Iron Cycling Indicated by Enterobacter Strain" Molecules 27, no. 17: 5581. https://doi.org/10.3390/molecules27175581
APA StyleLi, M.-J., Wei, M.-Y., Fan, X.-T., & Zhou, G.-W. (2022). Underestimation about the Contribution of Nitrate Reducers to Iron Cycling Indicated by Enterobacter Strain. Molecules, 27(17), 5581. https://doi.org/10.3390/molecules27175581





