Ni(II) Ions May Target the Entire Melatonin Biosynthesis Pathway—A Plausible Mechanism of Nickel Toxicity
Abstract
:1. Introduction
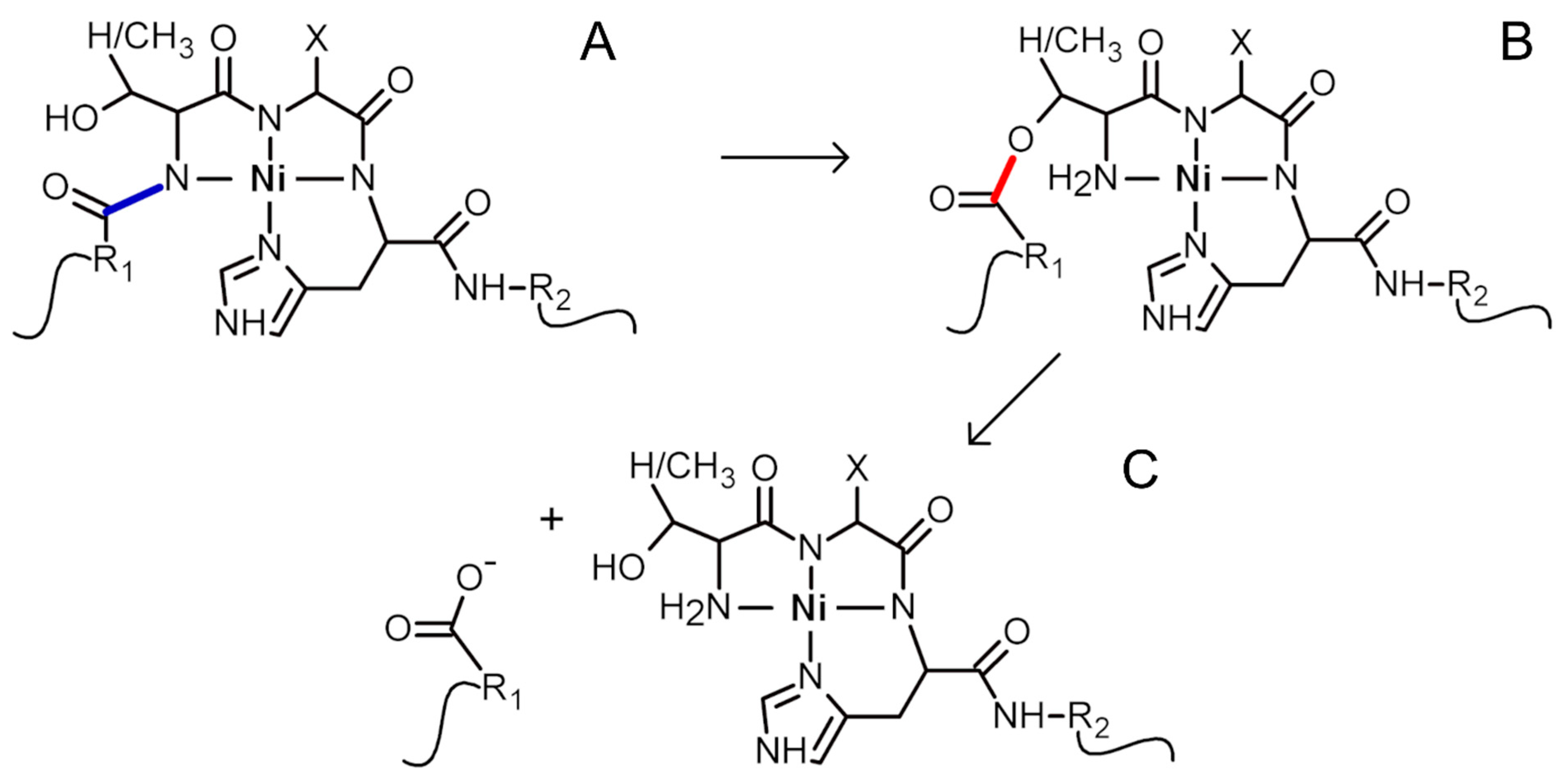
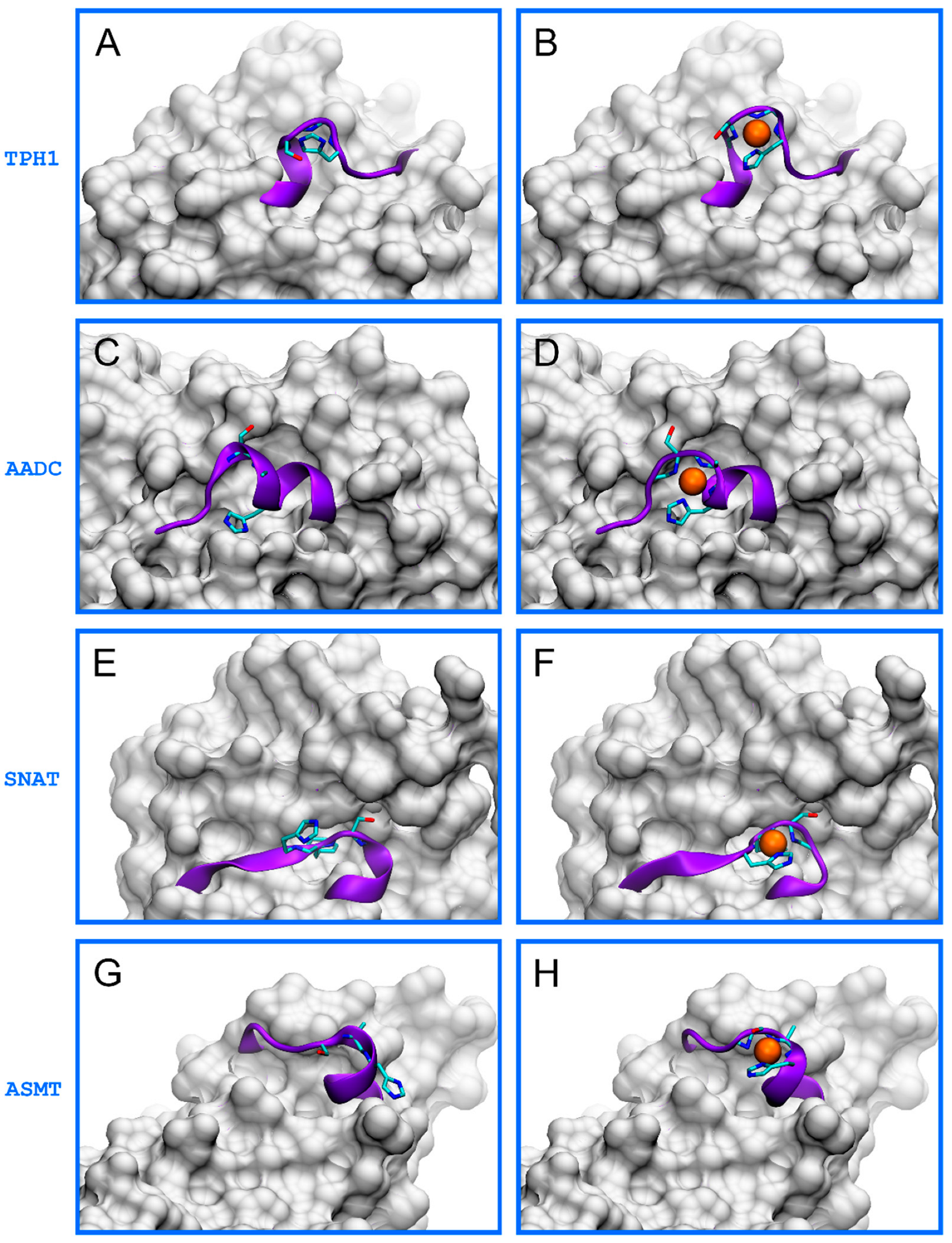
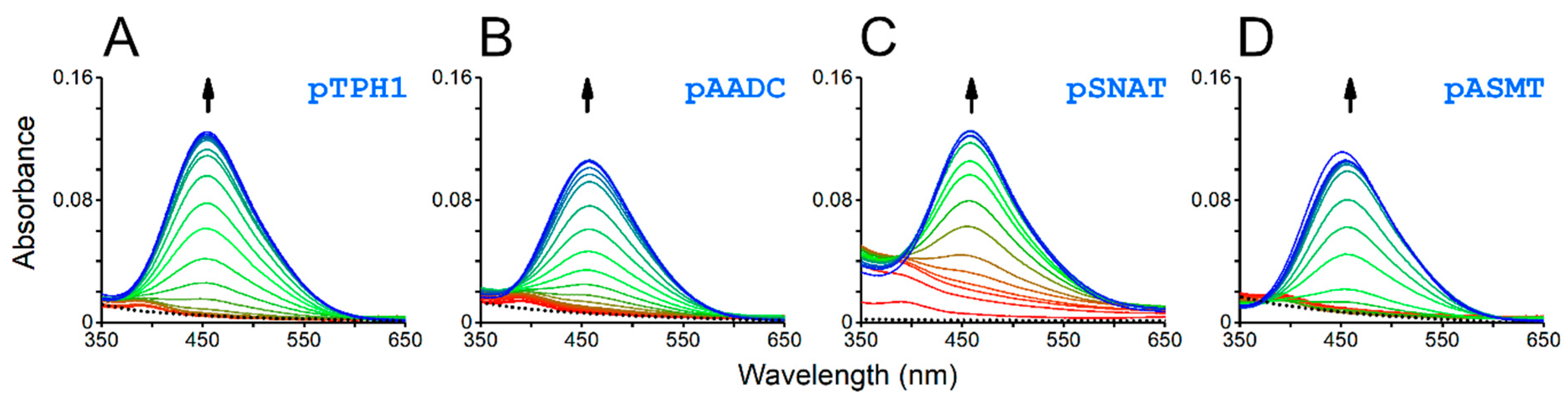
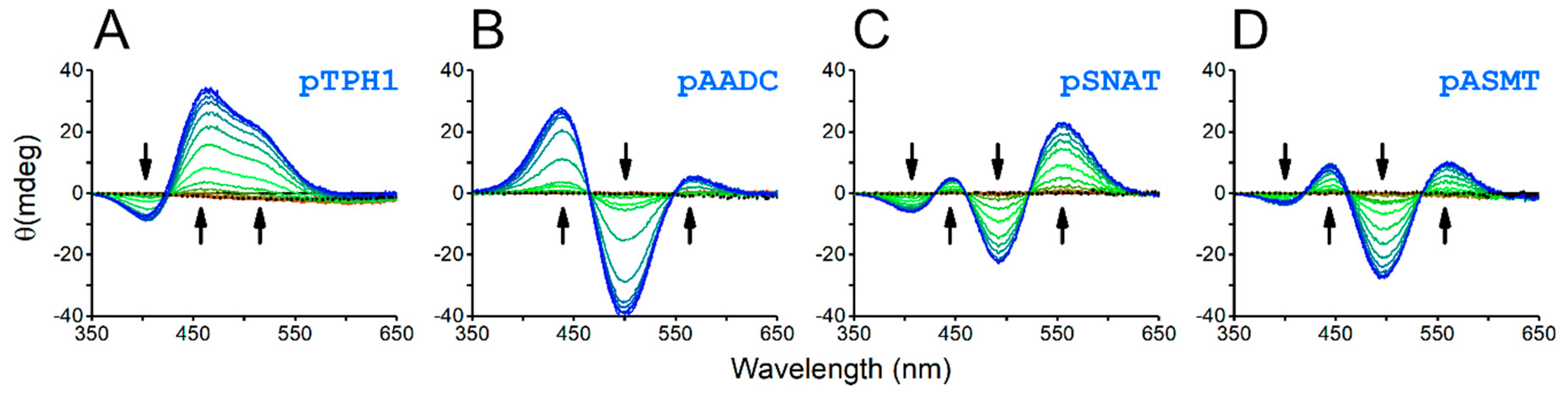
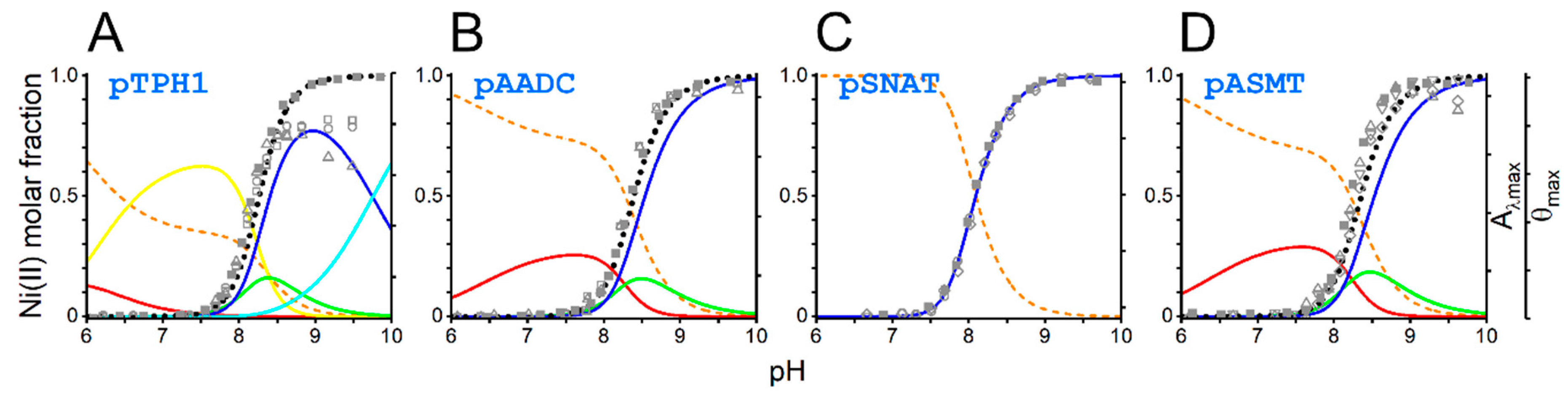
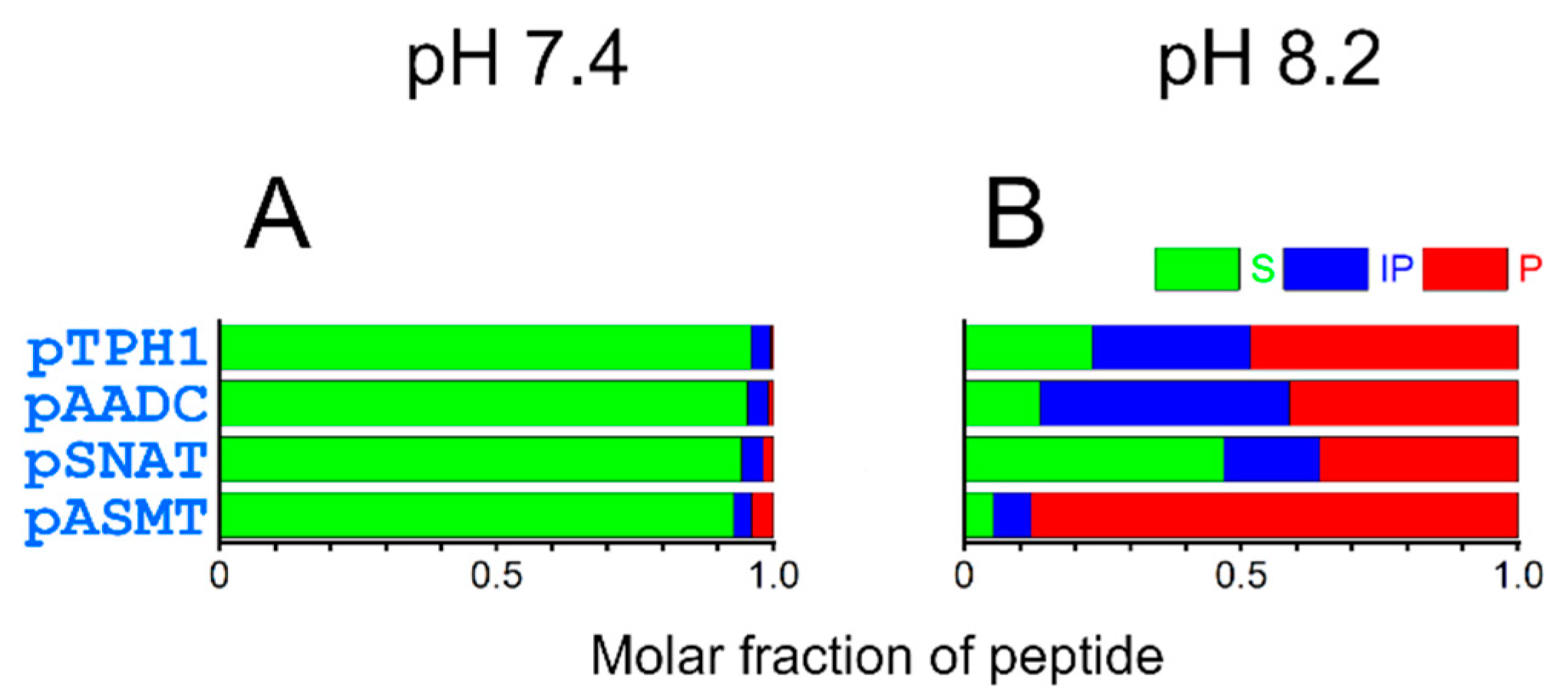
2. Results and Discussion
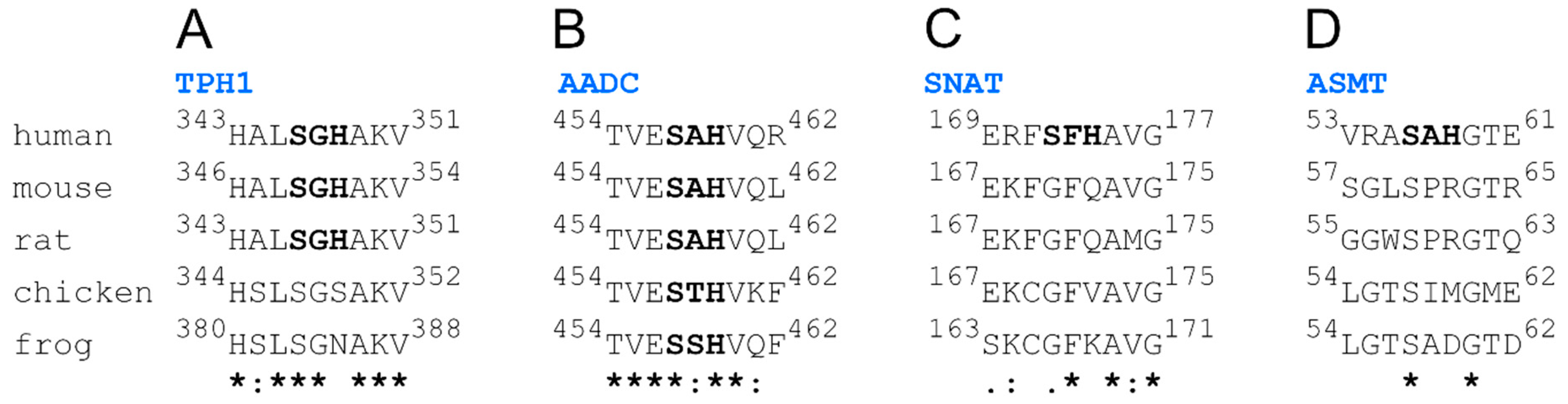
- TPH1: 267EPDTCHELL275, 343HALSGHAKV351,
- AADC: 454TVESAHVQR462,
- SNAT: 2STQSTHPLK10, 107ESLTLHRSG115, 169ERFSFHAVG177,
- ASMT: 53VRASAHGTE61.
3. Materials and Methods
3.1. Materials
3.2. Structural Analyses
3.3. Peptide Synthesis and Purification
3.4. UV-Vis and CD Spectroscopy
3.5. Potentiometry
3.6. Ni(II)-Assisted Peptide Bond Hydrolysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cameron, K.S.; Buchner, V.; Tchounwou, P.B. Exploring the Molecular Mechanisms of Nickel-Induced Genotoxicity and Carcinogenicity: A Literature Review. Rev. Environ. Health 2011, 26, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Costa, M. Elucidating the Mechanisms of Nickel Compound Uptake: A Review of Particulate and Nano-Nickel Endocytosis and Toxicity. Toxicol. Appl. Pharmacol. 2012, 260, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Ahlström, M.G.; Thyssen, J.P.; Menné, T.; Johansen, J.D. Prevalence of Nickel Allergy in Europe Following the EU Nickel Directive—A Review. Contact Dermat. 2017, 77, 193–200. [Google Scholar] [CrossRef]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel Allergy and Allergic Contact Dermatitis: A Clinical Review of Immunology, Epidemiology, Exposure, and Treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef]
- Alinaghi, F.; Bennike, N.H.; Egeberg, A.; Thyssen, J.P.; Johansen, J.D. Prevalence of Contact Allergy in the General Population: A Systematic Review and Meta-Analysis. Contact Dermat. 2019, 80, 77–85. [Google Scholar] [CrossRef]
- Wezynfeld, N.E.; Frączyk, T.; Bal, W. Metal Assisted Peptide Bond Hydrolysis: Chemistry, Biotechnology and Toxicological Implications. Coord. Chem. Rev. 2016, 327–328, 166–187. [Google Scholar] [CrossRef]
- Wilson, L.T.; Tipping, W.J.; Wetherill, C.; Henley, Z.; Faulds, K.; Graham, D.; Mackay, S.P.; Tomkinson, N.C.O. Mitokyne: A Ratiometric Raman Probe for Mitochondrial pH. Anal. Chem. 2021, 93, 12786–12792. [Google Scholar] [CrossRef]
- Aklima, J.; Onojima, T.; Kimura, S.; Umiuchi, K.; Shibata, T.; Kuraoka, Y.; Oie, Y.; Suganuma, Y.; Ohta, Y. Effects of Matrix pH on Spontaneous Transient Depolarization and Reactive Oxygen Species Production in Mitochondria. Front. Cell Dev. Biol. 2021, 9, 692776. [Google Scholar] [CrossRef]
- Lin, B.; Wei, Y.; Hao, Y.; E, S.; Shu, Y.; Wang, J. β-Naphthothiazolium-Based Ratiometric Fluorescent Probe with Ideal pKa for pH Imaging in Mitochondria of Living Cells. Talanta 2021, 232, 122475. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Li, X.; Ma, H. Mitochondria-Immobilized Near-Infrared Ratiometric Fluorescent pH Probe To Evaluate Cellular Mitophagy. Anal. Chem. 2019, 91, 11409–11416. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.D.; Kemp, W.J.M.; Luijten, P.R.; Petridou, N.; Klomp, D.W.J. SNR Optimized 31 P Functional MRS to Detect Mitochondrial and Extracellular pH Change during Visual Stimulation. NMR Biomed. 2019, 32, e4137. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Bing, T.; Zhang, N.; Shangguan, D. A Mitochondria-Targeted Ratiometric Fluorescent pH Probe. ACS Appl. Bio Mater. 2019, 2, 1368–1375. [Google Scholar] [CrossRef]
- Xu, S.; Zheng, G.; Zhou, K. Versatile Scaffold Applications Based on MoS2 Quantum Dots for Imaging Mitochondrial pH in Living Cells. Anal. Biochem. 2022, 640, 114545. [Google Scholar] [CrossRef]
- Żurawik, T.M.; Pomorski, A.; Belczyk-Ciesielska, A.; Goch, G.; Niedźwiedzka, K.; Kucharczyk, R.; Krężel, A.; Bal, W. Revisiting Mitochondrial pH with an Improved Algorithm for Calibration of the Ratiometric 5(6)-Carboxy-SNARF-1 Probe Reveals Anticooperative Reaction with H+ Ions and Warrants Further Studies of Organellar pH. PLoS ONE 2016, 11, e0161353. [Google Scholar] [CrossRef]
- Sigel, H.; Martin, R.B. Coordinating Properties of the Amide Bond. Stability and Structure of Metal Ion Complexes of Peptides and Related Ligands. Chem. Rev. 1982, 82, 385–426. [Google Scholar] [CrossRef]
- Kopera, E.; Krȩżel, A.; Protas, A.M.; Belczyk, A.; Bonna, A.; Wysłouch-Cieszyńska, A.; Poznański, J.; Bal, W. Sequence-Specific Ni(II)-Dependent Peptide Bond Hydrolysis for Protein Engineering: Reaction Conditions and Molecular Mechanism. Inorg. Chem. 2010, 49, 6636–6645. [Google Scholar] [CrossRef] [PubMed]
- Karaczyn, A.A.; Bal, W.; North, S.L.; Bare, R.M.; Hoang, V.M.; Fisher, R.J.; Kasprzak, K.S. The Octapeptidic End of the C-Terminal Tail of Histone H2A Is Cleaved off in Cells Exposed to Carcinogenic Nickel(II). Chem. Res. Toxicol. 2003, 16, 1555–1559. [Google Scholar] [CrossRef]
- Wezynfeld, N.E.; Bossak, K.; Goch, W.; Bonna, A.; Bal, W.; Frączyk, T. Human Annexins A1, A2, and A8 as Potential Molecular Targets for Ni(II) Ions. Chem. Res. Toxicol. 2014, 27, 1996–2009. [Google Scholar] [CrossRef]
- Wezynfeld, N.E.; Bonna, A.; Bal, W.; Frączyk, T. Ni(II) Ions Cleave and Inactivate Human Alpha-1 Antitrypsin Hydrolytically, Implicating Nickel Exposure as a Contributing Factor in Pathologies Related to Antitrypsin Deficiency. Metallomics 2015, 7, 596–604. [Google Scholar] [CrossRef]
- Frączyk, T.; Bonna, A.; Stefaniak, E.; Wezynfeld, N.E.; Bal, W. Peptide Bond Cleavage by Ni(II) Ions within the Nuclear Localization Signal Sequence. Chem. Biodivers. 2020, 17, e1900652. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S.; Manucha, W.; Chuffa, L.G.D.A.; Zuccari, D.A.P.D.C. Melatonin and Pathological Cell Interactions: Mitochondrial Glucose Processing in Cancer Cells. Int. J. Mol. Sci. 2021, 22, 12494. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules 2021, 26, 4105. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.; Qin, L.; Reiter, R. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef]
- Mu, Q.; Najafi, M. Modulation of the Tumor Microenvironment (TME) by Melatonin. Eur. J. Pharmacol. 2021, 907, 174365. [Google Scholar] [CrossRef]
- Radogna, F.; Diederich, M.; Ghibelli, L. Melatonin: A Pleiotropic Molecule Regulating Inflammation. Biochem. Pharmacol. 2010, 80, 1844–1852. [Google Scholar] [CrossRef]
- Mirza-Aghazadeh-Attari, M.; Reiter, R.J.; Rikhtegar, R.; Jalili, J.; Hajalioghli, P.; Mihanfar, A.; Majidinia, M.; Yousefi, B. Melatonin: An Atypical Hormone with Major Functions in the Regulation of Angiogenesis. IUBMB Life 2020, 72, 1560–1584. [Google Scholar] [CrossRef]
- Zhu, Y.; Costa, M. Metals and Molecular Carcinogenesis. Carcinogenesis 2020, 41, 1161–1172. [Google Scholar] [CrossRef]
- Davidson, T.L.; Chen, H.; di Toro, D.M.; D’Angelo, G.; Costa, M. Soluble Nickel Inhibits HIF-Prolyl-Hydroxylases Creating Persistent Hypoxic Signaling in A549 Cells. Mol. Carcinog. 2006, 45, 479–489. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.-K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, Mitochondria, and the Skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.; Kleszczyński, K.; Semak, I.; Janjetovic, Z.; Sweatman, T.; Skobowiat, C.; Steketee, J.D.; Lin, Z.; Postlethwaite, A.; et al. Characterization of Serotonin and N-acetylserotonin Systems in the Human Epidermis and Skin Cells. J. Pineal Res. 2020, 68, e12626. [Google Scholar] [CrossRef]
- Millán-Plano, S.; Piedrafita, E.; Miana-Mena, F.J.; Fuentes-Broto, L.; Martínez-Ballarín, E.; López-Pingarrón, L.; Sáenz, M.A.; García, J.J. Melatonin and Structurally-Related Compounds Protect Synaptosomal Membranes from Free Radical Damage. Int. J. Mol. Sci. 2010, 11, 312–328. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 1 August 2022).
- AlphaFold Protein Structure Database. Available online: https://alphafold.ebi.ac.uk/ (accessed on 1 August 2022).
- Mylonas, M.; Krężel, A.; Plakatouras, J.C.; Hadjiliadis, N.; Bal, W. The Binding of Ni(II) Ions to Terminally Blocked Hexapeptides Derived from the Metal Binding -ESHH- Motif of Histone H2A. J. Chem. Soc. Dalton Trans. 2002, 4296–4306. [Google Scholar] [CrossRef]
- Krezel, A.; Mylonas, M.; Kopera, E.; Bal, W. Sequence-Specific Ni(II)-Dependent Peptide Bond Hydrolysis in a Peptide Containing Threonine and Histidine Residues. Acta Biochim. Pol. 2006, 53, 721–727. [Google Scholar] [PubMed]
- Karavelas, T.; Malandrinos, G.; Hadjiliadis, N.; Mlynarz, P.; Kozlowski, H.; Barsam, M.; Butler, I. Coordination Properties of Cu(II) and Ni(II) Ions towards the C-Terminal Peptide Fragment -TYTEHA- of Histone H4. Dalton Trans. 2008, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Wezynfeld, N.E.; Frączyk, T.; Bonna, A.; Bal, W. Peptide Bond Cleavage in the Presence of Ni-Containing Particles. Metallomics 2020, 12, 649–653. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhou, C.; Lu, Y.; Mao, L.; Xi, Y.; Mei, X.; Wang, X.; Zhang, L.; Yu, Z.; Zhou, Z. Melatonin Antagonizes Nickel-Induced Aerobic Glycolysis by Blocking ROS-Mediated HIF-1α/MiR210/ISCU Axis Activation. Oxid. Med. Cell. Longev. 2020, 2020, 5406284. [Google Scholar] [CrossRef]
- Xu, S.-C.; He, M.-D.; Zhong, M.; Zhang, Y.-W.; Wang, Y.; Yang, L.; Yang, J.; Yu, Z.-P.; Zhou, Z. Melatonin Protects against Nickel-Induced Neurotoxicity in Vitro by Reducing Oxidative Stress and Maintaining Mitochondrial Function. J. Pineal Res. 2010, 49, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-C.; He, M.-D.; Lu, Y.-H.; Li, L.; Zhong, M.; Zhang, Y.-W.; Wang, Y.; Yu, Z.-P.; Zhou, Z. Nickel Exposure Induces Oxidative Damage to Mitochondrial DNA in Neuro2a Cells: The Neuroprotective Roles of Melatonin. J. Pineal Res. 2011, 51, 426–433. [Google Scholar] [CrossRef]
- Hahn, M. Receptor Surface Models. 1. Definition and Construction. J. Med. Chem. 1995, 38, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; White, P. (Eds.) Basic Procedures. In Fmoc Solid Phase Peptide Synthesis: A Practical Approach; Oxford University Press: Oxford, UK, 2000; pp. 41–76. [Google Scholar]
- Irving, H.; Miles, M.G.; Pettit, L.D. A Study of Some Problems in Determining the Stoicheiometric Proton Dissociation Constants of Complexes by Potentiometric Titrations Using a Glass Electrode. Anal. Chim. Acta 1967, 38, 475–488. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. SUPERQUAD: An Improved General Program for Computation of Formation Constants from Potentiometric Data. J. Chem. Soc. Dalton Trans. 1985, 1195–1200. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of Equilibria in Solution. Determination of Equilibrium Constants with the HYPERQUAD Suite of Programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]

| Logβ | ||||
|---|---|---|---|---|
| pTPH1 | pAADC | pSNAT | pASMT | |
| HL | 10.25(1) | 6.68(1) | 6.67(1) | 6.68(1) |
| H2L | 16.95(1) | 11.07(1) | 11.03(1) | 11.01(1) |
| H3L | 22.94(1) | |||
| NiH2L | 19.77(5) c | |||
| NiHL | 14.02(2) d | |||
| NiL | 2.73(2) c | 2.83(1) c | ||
| NiH−1L | −2.77(4) e | |||
| NiH−2L | −10.68(2) f | −13.82(2) e | −13.65(1) e | |
| NiH−3L | −20.43(2) f | −21.85(1) f | −20.95(1) f | −21.74(1) f |
| pH50% of 4N | Molar Fraction at pH 7.4 | Molar Fraction at pH 8.2 | |
|---|---|---|---|
| pTPH1 | 8.4 | 0.002 | 0.28 |
| pAADC | 8.5 | 0.001 | 0.17 |
| pSNAT | 8.1 | 0.014 | 0.62 |
| pASMT | 8.5 | 0.001 | 0.18 |
| pH 7.4 | pH 8.2 | |||
|---|---|---|---|---|
| k1 [s−1 × 10−6] | k2 [s−1 × 10−6] | k1 [s−1 × 10−6] | k2 [s−1 × 10−6] | |
| pTPH1 | 0.72(4) | 5.4(7) | 18.5(1) | 19.4(1) |
| pAADC | 1.19(7) | 3.5(4) | 31.9(2) | 10.7(1) |
| pSNAT | 0.79(3) | 6.5(6) | 8.6(4) | 27(1) |
| pASMT | 0.96(9) | 28(9) | 55(4) | 40(5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wezynfeld, N.E.; Bonna, A.M.; Płonka, D.; Bal, W.; Frączyk, T. Ni(II) Ions May Target the Entire Melatonin Biosynthesis Pathway—A Plausible Mechanism of Nickel Toxicity. Molecules 2022, 27, 5582. https://doi.org/10.3390/molecules27175582
Wezynfeld NE, Bonna AM, Płonka D, Bal W, Frączyk T. Ni(II) Ions May Target the Entire Melatonin Biosynthesis Pathway—A Plausible Mechanism of Nickel Toxicity. Molecules. 2022; 27(17):5582. https://doi.org/10.3390/molecules27175582
Chicago/Turabian StyleWezynfeld, Nina E., Arkadiusz M. Bonna, Dawid Płonka, Wojciech Bal, and Tomasz Frączyk. 2022. "Ni(II) Ions May Target the Entire Melatonin Biosynthesis Pathway—A Plausible Mechanism of Nickel Toxicity" Molecules 27, no. 17: 5582. https://doi.org/10.3390/molecules27175582
APA StyleWezynfeld, N. E., Bonna, A. M., Płonka, D., Bal, W., & Frączyk, T. (2022). Ni(II) Ions May Target the Entire Melatonin Biosynthesis Pathway—A Plausible Mechanism of Nickel Toxicity. Molecules, 27(17), 5582. https://doi.org/10.3390/molecules27175582







