Abstract
To search for efficient agricultural antifungal lead compounds, 39 Chimonanthus praecox derivatives were designed, synthesized, and evaluated for their antifungal activities. The structures of target compounds were fully characterized by 1H NMR, 13C NMR, and MS spectra. The preliminary bioassays revealed that some compounds exhibited excellent antifungal activities in vitro. For example, the minimum inhibitory concentration (MIC) of compound b15 against Phytophthora infestans was 1.95 µg mL−1, and the minimum inhibitory concentration (MIC) of compound b17 against Sclerotinia sclerotiorum was 1.95 µg mL−1. Therefore, compounds b15 and b17 were identified as the most promising candidates for further study.
1. Introduction
One billion tons of crops in the world are destroyed by diseases and insect pests every year according to statistics, resulting in a 20~30% reduction in crop production [1]. The traditional chemical pesticides play a great role in food protection, but they also lead to serious ecological and environmental problems. The development of new pesticides with active natural products as lead compounds can not only find analogues with better activity but also help the products meet the needs of environmental protection and national development requirements of carbon peak and carbon neutralization [2].
Chimonanthus praecox alkaloids (Figure 1) are widely distributed in plants, microorganisms, and marine organisms. Their biogenic source is considered to be tryptophan through multi-step transformation [3,4]. These natural products showed a variety of biological activities [3]: (-)-physistigmine is a cholinesterase inhibitor that is clinically used to treat Alzheimer’s disease, myasthenia gravis, postural hypotension, delayed gastric emptying, glaucoma, and other diseases [5]; (-)-folicanthine has antibacterial activity [6]; (-)-win-64745 is an excellent neurokinin antagonist [7]; (-)-asperdimin possess antiviral activity; and so on[8].
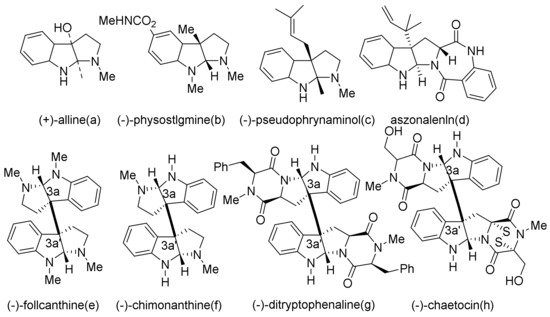
Figure 1.
Chimonanthus praecox alkaloids.
The research on the synthesis and activity of Chimonanthus praecox alkaloids focuses mainly on the research and development of medicine and less on the antifungal activity of agriculture. However, pesticide research and pharmaceutical research have been learning from each other, and they have many similarities. Pesticide researchers have found that Chimonanthus praecox has significant inhibitory activity against Watermelon fusariumwil, Fusarium oxysportium, Bipolaris maydis, Exserohilum turcium and Alternaria solanit [9,10,11,12,13]. Because of their broad spectrum of biological properties, a number of studies aimed at the synthesis and antimicrobial activity of Chimonanthus praecox alkaloids have been reported [14,15,16,17,18]. In the past few years, our research group has been committed to the synthesis of Chimonanthus praecox alkaloids and research on their activity against plant pathogens. The biological testing has shown that several of the synthesized compounds have exhibited diverse and promising bioactivities (Figure 2) [19,20,21,22,23,24]; for instance, compound i performed better against Verticillium dahlia compared with chlorothalonil, with the minimum inhibitory concentration (MIC) value of 7.81 µg ml−1, and compounds j and k revealed potent activity against acetylcholinesterase, with IC50 values of 0.01 and 0.1 ng ml−1, respectively [23]. These findings inspired us to further combine the structure of Chimonanthus praecox-based natural product with nicotine functional group so as to acquire potential agrochemical leads for plant disease control.
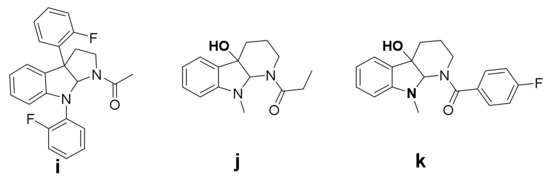
Figure 2.
Representative Chimonanthus praecox compounds found by our research group.
2. Results and Discussion
2.1. Chemistry
A series of Chimonanthus praecox analogues have been efficiently synthesized based on the methods developed in our previous work. The synthetic route to the Chimonanthus praecox analogues is shown in Scheme 1. Compound 1 was obtained using indole-3-acetonitrile as the starting material. Compound 1 reacted with an excess of R1Br or R2Br at the N-position and C-3 position using tetrahydrofuran (THF) as the solvent and sodium hydride(NaH) as a base to obtain compound 2 or 4, respectively. Compound 3 or 5 was synthesized from 2 or 4 using lithium aluminum hydride (LiAlH4) as the reducing agent. Then, the expected 39 compounds were obtained based on intermediate 3 or 5. The synthesized compounds were characterized by 1H NMR, 13C NMR, and MS. The spectra of all compounds are in the Supplementary Materials.
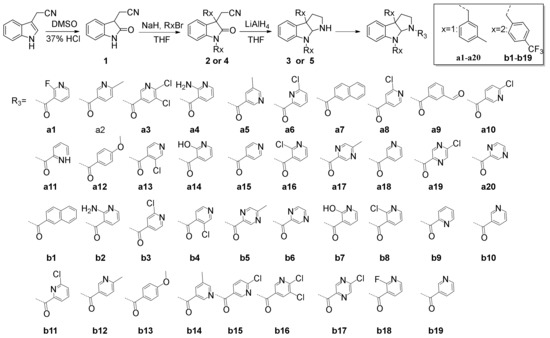
Scheme 1.
Synthesis of Chimonanthus praecox derivatives.
2.2. Antifungal Acitivity
The inhibitory effects of the Chimonanthus praecox analogues towards six plant pathogen fungi are outlined in Table 1. The MIC values were evaluated with Carbendazim and Amphotericin B as the positive controls to assay the activity of the prepared Chimonanthus praecox analogues against Sclerotinia sclerotiorum, Altenaria solani, Verticillium dahliae, Colletotrichum orbiculare, Cytospora juglandis, and Curvularia lunata. The preliminary bioassays showed that most of the synthesized compounds exhibited fungicidal activity. Compound b15 exhibited significant antifungal activity against A. solani, with a MIC value of 1.95 µg mL−1. Compound b17 showed the strongest antifungal activity against S. sclerotiorum, with a MIC value of 1.95 µg mL−1. Compounds b12, b13, and b17 exhibited significant antifungal activities against A. solani, with the same MIC value of 3.91 µg mL−1. Compounds a5 and b10 revealed improved activity against F. oxysporum compared with Carbendazim and Amphotericin B, with the same MIC value of 15.16 µg mL−1. Compounds b15, b16, b17, and b19 manifested much more activity against C. lunata than Carbendazim and Amphotericin B, all with the same MIC value of 15.16 µg mL−1. The activity of compounds a6, b8, and b10 was more potent than Carbendazim and Amphotericin B against A. solani, all with the same MIC value of 15.63 µg mL−1. The activity of compound b11 was more potent than Carbendazim and Amphotericin B against V. dahliae, with a MIC value of 15.63 µg mL−1. Compounds a2, a3, a10, b16, and b17 manifested much more activity against V. dahliae than Carbendazim and Amphotericin B, all with the same MIC value of 15.63 µg mL−1.

Table 1.
MIC of Chimonanthus praecox derivatives against 6 plant pathogens.
Although it is difficult to extract clear structure–activity relationships from the biological data, some conclusions can still be drawn (Figure 3). Firstly, the analysis of the relationship between structure and activity showed that compounds a6, a10, b4, b11, b15, b16, and b17 contained Cl atom, and the Cl atoms that were located at C-2, C-5, or C-6 positions showed excellent antifungal activities. Compound b16 contained two Cl atoms, and the antifungal activity was stronger than that of the target compound with one Cl. Secondly, when trifluoromethyl was introduced into the benzene ring of R3 group, the antifungal effect was significantly improved, and when the aromatic ring of R1 group was chloropyridine, the antifungal effect was significantly enhanced.
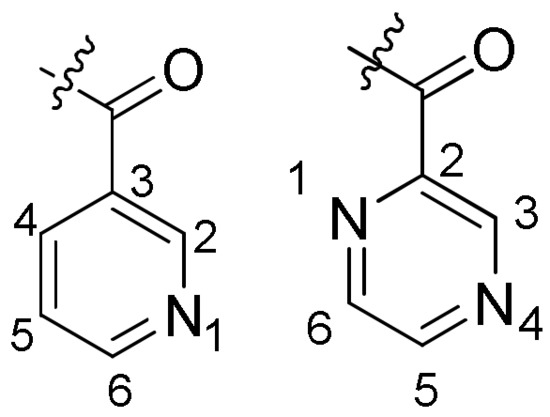
Figure 3.
Substituent R3.
3. Materials and Methods
3.1. Instruments and Chemicals
All reagents and solvents were reagent grade or purified according to standard methods before use. Analytical thin-layer chromatography (TLC) was performed with silica gel plates using silica gel 60 GF254 (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China). The 1H−NMR (400 MHz) and 13C−NMR (100 MHz) were obtained on an AM−500 FT−NMR spectrometer (Bruker Corporation, Fällanden, Switzerland) with CDCl3, acetone-d6, or DMSO-d6 as the solvent and TMS as the internal standard. MS were recorded under ESI conditions using a LCQ Fleet instrument (Thermo Fisher, Waltham, MA, USA).
3.2. Synthesis
The general synthetic methods for the compounds a1–a20 and b1–b19 are depicted in Scheme 1.
3.2.1. Synthesis of Compound 1
The 3-indole acetonitrile (2.0 g, 12.8 mmol) was dissolved in dimethyl sulfoxide (DMSO) (30 mL, 281.6 mmol). Then, 100 mL 37% HCl was added. The mixture was stirred at r.t. for 2 h. The reaction mixture was evaporated under reduced pressure to remove the solvent to obtain the white solid 1 without further purification (2.1 g, 93%).
3.2.2. Synthesis of Compound 2 or 4
A round-bottom Schlenk flask was charged with NaH (0.7 g, 30 mmol), anhydrous THF (35 mL), 1-(bromomethyl)-3-(trifluoromethyl)benzene (2.1 g, 8.7 mmol) or 1-(bromomethyl)-4-methylbenzene (1.6 g, 8.7 mmol) and compound 1 (1.0 g, 5.8 mmol) respectively. The mixture was stirred at r.t. and the reaction progress was monitored by TLC until the reaction was complete (10 h~12 h). The solvent was then removed under vacuum, and the residue was extracted with ethyl acetate (3 × 30 mL) and purified by flash column chromatography (petroleum ether/EtOAc, 2:1) to produce the target product 2 (1.8 g, 63.6%) or product 4 (1.4 g, 64.5%).
3.2.3. Synthesis of Compound 3 or 5
To an oven-dried flask under nitrogen atmosphere were added LiAlH4 (0.6 g, 15.4 mmol), anhydrous THF (35 mL), and compound 2 (1.0 g, 2.1 mmol) or 4 (0.8 g, 2.1 mmol). The mixture was stirred at reflux for 6 h. The reaction progress was monitored by TLC until the reaction was complete (5~8 h). The solvent was then removed under vacuum, and the residue was extracted with ethyl acetate (3 × 30 mL) and purified by flash column chromatography (petroleum ether/EtOAc, 2:1) to produce the target product 3 (0.6 g, 61.4%) or 5 (0.5 g, 63.8%).
3.2.4. Synthesis of Chimonanthus praecox Derivatives a1–a20 and b1–b19
5-methylnicotinic acid (0.2 g, 1.3 mmol, 2 eq) and dichloro sulfoxide (0.3 mL, 4.3 mmol) were dissolved in 10 mL of dichloromethane (DCM). The resulting mixture was heated to reflux for 2 h. The solvent was then removed under vacuum, and the acyl chloride was obtained. A combination of 3a, 8-Bis(3-(trifluoromethyl)benzyl)-1, 2, 3, 3a, 8, 8a-hexahydrop-yrrole [2, 3-b]indole (0.3 g, 0.6 mmol, 1 eq) and triethylamine (0.2 mL, 1.1 mmol) was dissolved in 10 mL of dichloromethane (DCM). The prepared acyl chloride in 10 mL of DME was slowly added. The mixture was stirred at r.m. and monitored by TLC. When the reaction was complete, the solvent was removed under reduced pressure. The residue was washed with water and saturated brine. The organic layer was dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure, and the residue was purified by flash column chromatography to produce the target product a5 (0.2 g), which was yellow and oily (Yield 64.0%). For the procedure for the remaining Chimonanthus praecox derivatives, refer to the steps of compound a5.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrole[2,3-b]indol-1(2H)-yl)(2-fluoropyridin-3-yl)methanone(a1): White solid, Melting Point: 131–133 °C, 90% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.28 (d, J = 4.7 Hz, 1H), 7.85 (t, J = 8.3 Hz, 1H), 7.42–7.33 (m, 1H), 7.01 (dd, J = 15.9, 7.5 Hz, 8H), 6.87 (t, J = 8.0 Hz, 2H), 6.69 (dt, J = 14.7, 7.5 Hz, 1H), 6.15 (d, J = 7.9 Hz, 1H), 5.99 (s, 1H), 4.58 (d, J = 16.3 Hz, 1H), 4.49 (d, J = 16.3 Hz, 1H), 3.43–3.35 (m, 1H), 3.30–3.20 (m, 1H), 3.14 (d, J = 13.4 Hz, 1H), 2.96 (d, J = 13.4 Hz, 1H), 2.30–2.22 (m, 8H).13C NMR (100 MHz, Acetone-d6) δ 164.43, 160.65, 158.29, 151.44, 149.70 (CH, d, J = 13.0 Hz ), 149.55, 141.06, 136.98, 136.45, 136.38, 135.21, 132.56, 130.66, 129.51, 129.25, 129.00, 127.51, 123.99, 122.70, 122.65, 120.49 (d, J = 32.4 Hz ), 120.16, 117.97, 106.87, 83.17, 57.69, 49.98, 48.39, 44.69, 38.36, 21.00, 20.95. MS(ESI(+)) calcd for C32H30FN3O+ [M + H]+: 492.24; found: 492.24.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(6-methylpyridin-3-yl)methanone(a2): White solid, Melting Point: 153–155 °C, 89% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.48 (s, 1H), 7.76–7.54 (m, 1H), 7.23 (d, J = 8.0 Hz, 1H), 6.99 (dq, J = 8.9, 7.4 Hz, 9H), 6.85 (d, J = 7.8 Hz, 2H), 6.64 (t, J = 7.3 Hz, 1H), 6.18–6.03 (m, 2H), 4.57 (d, J = 8.9 Hz, 1H), 4.43 (d, J = 8.9 Hz, 1H), 3.36 (d, J = 9.0 Hz, 1H), 3.10 (d, J = 13.3 Hz, 1H), 2.94 (d, J = 13.3 Hz, 1H), 2.48 (s, 3H), 2.28 (s, 3H), 2.24–2.20 (m, 5H).13C NMR (100 MHz, Acetone-d6) δ 168.43, 160.77, 151.45, 148.66, 137.13, 136.38, 136.33, 136.00, 135.28, 135.23, 132.68, 130.69, 130.06, 129.47, 129.21, 128.95, 127.43, 123.99, 122.88, 117.76, 106.74, 83.08, 57.27, 49.75, 49.68, 44.68, 38.49, 24.28, 20.98, 20.92. MS(ESI(+)) calcd for C33H33N3O+ [M + H]+: 488.27; found: 488.27.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(5,6-dichloropyridin-3-yl)methanone(a3): White solid, Melting Point: 130–132 °C, 91%, 1H NMR (400 MHz, Acetone-d6), δ: 8.28 (d, J = 48.1 Hz, 1H), 7.92 (s, 1H), 7.25–6.58 (m, 10H), 6.35–5.54 (m, 2H), 4.76–4.08 (m, 2H), 3.10–3.01 (m, 71.2, 43.9 Hz, 5H), 2.28 (d, J = 14.9 Hz, 8H).13C NMR (100 MHz, Acetone) δ 165.73, 151.43, 150.02, 147.00, 138.67, 137.26, 136.55, 136.45, 135.25, 133.70, 132.62, 130.74, 130.31, 130.29, 129.77, 129.55, 129.31, 129.08, 127.51, 124.06, 117.92, 106.76, 83.36, 57.39, 49.53, 44.61, 38.56, 32.15, 23.14, 20.98, 20.94. MS(ESI(+)) calcd for C32H29Cl2N3O+ [M + H]+: 542.18; found: 542.18.
(2-aminopyridin-3-yl)(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)methanone (a4): White solid, Melting Point: 164–166 °C, 88% Yield, 1H NMR (400 MHz, Chloroform-d), δ: 7.95 (s, 1H), 7.25 (s, 1H), 6.90 (t, J = 9.8 Hz, 11H), 6.52 (d, J = 9.8 Hz, 2H), 6.10 (s, 2H), 4.34 (s, 2H), 2.97 (d, J = 6.5 Hz, 2H), 2.15 (d, J = 4.2 Hz, 10H), 1.21 (s, 1H).13C NMR (100 MHz, Chloroform-d) δ 169.00, 157.53, 150.74, 150.20, 137.13, 137.01, 136.21, 136.14, 136.04, 133.97, 131.54, 130.09, 129.03, 128.83, 128.68, 126.76, 123.49, 117.30, 113.09, 112.39, 106.28, 82.50, 56.62, 49.25, 44.43, 37.86, 21.17, 21.14. MS(ESI(+)) calcd for C32H32N4O+ [M + H]+: 489.26; found: 489.26.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(5-methylpyridin-3-yl)methanone (a5): White solid, Melting Point: 93–95 °C, 64% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.45 (d, J = 6.4 Hz, 2H), 7.59 (s, 1H), 7.13–6.88 (m, 10H), 6.69 (t, J = 7.3 Hz, 1H), 6.20 (d, J = 7.8 Hz, 1H), 6.11 (s, 1H), 4.64 (d, J = 6.3 Hz, 1H), 4.48 (d, J = 6.3 Hz, 1H), 3.63–3.52 (m, 1H), 3.39 (dd, J = 8.4, 9.5 Hz, 1H), 3.15 (d, J = 13.3 Hz, 1H), 2.99 (d, J = 13.3 Hz, 1H), 2.37–2.25 (m, 11H).13C NMR (100 MHz, Acetone-d6) δ 167.70, 151.32, 150.78, 145.57, 136.50, 135.72, 135.66, 135.05, 134.61, 132.71, 132.02, 131.82, 130.02, 128.81, 128.56, 128.29, 126.77, 123.31, 117.10, 106.03, 82.39, 70.34, 56.60, 48.98, 43.93, 37.78, 31.44, 22.43, 20.28, 17.26, 13.51. MS(ESI(+)) calcd for C33H33N3O+ [M+H]+: 488.27; found: 488.27.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(6-chloropyridin-2-yl)methanone (a6): White solid, Melting Point: 130–132 °C, 83% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 7.94 (t, J = 7.8 Hz, 1H), 7.68 (d, J = 7.6 Hz, 1H), 7.56–7.49 (m, 1H), 7.08–6.97 (m, 8H), 6.84 (s, 1H), 6.66 (t, J = 7.3 Hz, 1H), 6.14 (d, J = 7.8 Hz, 1H), 6.00 (s, 1H), 4.62 (d, J = 6.2 Hz, 1H), 4.49 (d, J = 6.2 Hz, 1H), 3.82–3.75 (m, 1H), 3.43 (d, J = 6.3 Hz, 1H), 3.09–2.88 (m, 3H), 2.26–2.12 (m, 8H).13C NMR (100 MHz, Acetone-d6) δ 165.65, 154.57, 150.61, 149.22, 140.30, 136.37, 135.77, 135.67, 134.55, 132.22, 130.20, 130.08, 128.84, 128.70, 128.63, 128.57, 128.26, 126.98, 125.70, 125.66, 123.24, 122.80, 117.25, 106.34, 82.75, 59.34, 56.36, 49.33, 48.68, 43.81, 37.32, 20.28. MS(ESI(+)) calcd for C32H30ClN3O+ [M + H]+: 508.22; found: 508.22.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(naphthalen-1-yl)methanone (a7): White solid, Melting Point: 147–149 °C, 95% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 7.93 (d, J = 8.3 Hz, 2H), 7.63–7.34 (m, 4H), 7.26 (d, J = 6.4 Hz, 1H), 7.10 (s, 7H), 7.00 (dd, J = 10.9, 7.9 Hz, 3H), 6.66 (t, J = 7.4 Hz, 1H), 6.30–6.20 (m, 2H), 4.73 (d, J = 6.3 Hz, 2H), 3.18–2.97 (m, 4H), 2.34 (d, J = 7.1 Hz, 6H), 2.18 (d, J = 6.9 Hz, 2H).13C NMR (100 MHz, Acetone-d6) δ 169.57, 151.58, 151.55, 137.35, 136.56, 136.46, 136.05, 135.30, 134.16, 133.14, 131.02, 130.95, 130.01, 129.65, 129.61, 129.39, 129.03, 129.01, 127.65, 127.54, 127.01, 125.87, 125.51, 124.54, 123.99, 117.88, 106.61, 100.70, 57.70, 50.04, 48.49, 44.51, 38.38, 21.03, 20.97. MS(ESI(+)) calcd for C37H34N2O+ [M + H]+: 523.27; found: 523.27.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(2-chloropyridin-4-yl)methanone (a8): Light yellow solid, Melting Point: 107–109 °C, 90% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.41 (dd, J = 4.9, 0.6 Hz, 1H), 7.30–7.25 (m, 2H), 7.01–6.92 (m, 9H), 6.86 (d, J = 8.0 Hz, 1H), 6.65 (td, J = 7.4, 0.8 Hz, 1H), 6.19 (d, J = 7.8 Hz, 1H), 6.00 (s, 1H), 4.51 (d, J = 6.4 Hz, 2H), 3.43 (d, J = 1.9 Hz, 1H), 3.29–3.15 (m, 1H), 3.11 (d, J = 13.4 Hz, 1H), 2.95 (d, J = 13.4 Hz, 1H), 2.29 (s, 2H), 2.28–2.18 (m, 6H).13C NMR (100 MHz, Acetone-d6) δ 166.69, 151.87, 151.34, 150.86, 148.04, 137.20, 136.52, 136.41, 135.19, 132.60, 130.68, 129.51, 129.27, 129.03, 127.46, 123.99, 122.64, 121.23, 117.91, 106.73, 83.40, 57.39, 49.71, 49.27, 44.53, 38.52, 20.99, 20.95. MS(ESI(+)) calcd for C32H30ClN3O+ [M + H]+: 508.22; found: 508.22.
2-(3a,8-bis(4-methylbenzyl)-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indole-1-carbonyl)benzaldehyde (a9): White solid, Melting Point: 97–99 °C, 92% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 7.58–7.49 (m, 4H), 7.08–6.97 (m, 7H), 6.73–6.54 (m, 4H), 6.21 (d, J = 7.8 Hz, 1H), 5.99 (s, 1H), 5.39 (s, 1H), 4.39 (d, J = 6.6 Hz, 3H), 3.57 (d, J = 9.5 Hz, 1H), 3.08–2.81 (m, 2H), 2.50–2.17 (m, 8H).13C NMR (100 MHz, Acetone-d6) δ 169.56, 151.55, 137.35, 136.56, 136.46, 136.05, 135.30, 134.16, 133.14, 130.95, 130.01, 129.65, 129.61, 129.39, 129.03, 129.01, 127.65, 127.54, 127.01, 125.87, 125.51, 124.54, 123.99, 117.88, 106.61, 57.70, 50.04, 48.49, 44.51, 38.38, 21.03, 20.97. MS(ESI(+)) calcd for C34H32N2O2 + [M + H]+: 501.25; found: 501.25.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(6-chloropyridin-3-yl)methanone (a10): White solid, Melting Point: 117–119 °C, 94% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.45 (d, J = 1.5 Hz, 1H), 7.88 (dd, J = 8.2, 1.9 Hz, 1H), 7.51 (d, J = 8.2 Hz, 1H), 7.09–6.87 (m, 10H), 6.69 (t, J = 7.3 Hz, 1H), 6.20 (d, J = 7.8 Hz, 1H), 6.09 (s, 1H), 4.62 (d, J = 16.3 Hz, 1H), 4.48 (d, J = 16.3 Hz, 1H), 3.60 (dd, J = 7.8, 4.5 Hz, 1H), 3.42 (dd, J = 18.4, 9.5 Hz, 1H), 3.15 (d, J = 13.2 Hz, 1H), 2.98 (d, J = 13.3 Hz, 1H), 2.30 (t, J = 11.0 Hz, 8H).13C NMR (100 MHz, Acetone-d6) δ 167.25, 152.95, 151.57, 149.61, 139.29, 137.27, 136.61, 136.52, 135.38, 132.77, 132.38, 130.85, 129.66, 129.40, 129.16, 127.59, 124.63, 124.17, 118.01, 106.92, 83.34, 71.19, 57.47, 49.81, 44.75, 38.56, 21.12, 21.05. MS(ESI(+)) calcd for C32H30ClN3O+ [M + H]+: 508.22; found: 508.22.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(pyridin-2-yl)methanone (a11): Light yellow solid, Melting Point: 120–122 °C, 90% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.55–8.45 (m, 1H), 7.92–7.86 (m, 1H), 7.73–7.68 (m, 1H), 7.49–7.41 (m, 1H), 7.06–6.96 (m, 7H), 6.87 (t, J = 8.5 Hz, 3H), 6.65 (dd, J = 7.4, 6.7 Hz, 1H), 6.16 (d, J = 7.8 Hz, 1H), 6.04 (s, 1H), 4.65 (d, J = 16.2 Hz, 1H), 4.50 (d, J = 16.2 Hz, 1H), 3.83 (ddd, J = 11.5, 6.1, 2.1 Hz, 1H), 3.42 (td, J = 11.3, 6.8 Hz, 1H), 3.09 (dd, J = 10.9, 5.7 Hz, 1H), 2.94 (dd, J = 13.5, 2.3 Hz, 1H), 2.30 (s, 3H), 2.26 (s, 3H), 2.22 (d, J = 3.0 Hz, 2H).13C NMR (100 MHz, Acetone-d6) δ 167.91, 155.13, 151.42, 148.67, 137.50, 137.15, 136.40, 136.30, 135.31, 133.00, 130.88, 130.75, 129.49, 129.32, 129.23, 128.87, 127.67, 126.76, 125.59, 125.32, 124.51, 123.89, 117.80, 106.92, 83.40, 57.04, 50.04, 49.30, 44.63, 38.26, 20.99, 20.94. MS(ESI(+)) calcd for C32H31N3O+ [M + H]+: 474.25; found: 474.25.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(4-methoxyphenyl)methanone (a12): Light yellow solid, Melting Point: 119–121 °C, 91% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 7.42 (s, 2H), 7.08–6.84 (m, 13H), 6.64 (t, J = 7.3 Hz, 1H), 6.14 (d, J = 7.7 Hz, 1H), 4.50 (dd, J = 80.9, 15.2 Hz, 2H), 3.82 (s, 3H), 3.69–3.23 (m, 2H), 3.10 (d, J = 13.3 Hz, 1H), 2.94 (d, J = 13.3 Hz, 1H), 2.28 (d, J = 16.9 Hz, 6H), 2.22–2.15 (m, 2H).13C NMR (100 MHz, Acetone-d6) δ 170.04, 161.91, 151.57, 137.14, 136.35, 136.29, 135.37, 132.90, 132.83, 132.80, 132.70, 130.74, 130.30, 129.46, 129.32, 129.22, 128.90, 127.48, 127.16, 123.98, 117.69, 113.90, 106.73, 83.02, 57.18, 55.54, 50.07, 49.59, 44.76, 38.51, 21.00, 20.94. MS(ESI(+)) calcd for C34H34N2O2+ [M + H]+: 503.27; found: 503.27.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(3-chloropyridin-4-yl)methanone (a13): White solid, Melting Point: 119–121 °C, 93% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.61 (s, 1H), 8.54 (d, J = 4.7 Hz, 1H), 7.11–6.88 (m, 11H), 6.69–6.62 (m, 1H), 6.18 (d, J = 7.6 Hz, 1H), 5.98 (s, 1H), 4.64 (d, J = 16.3 Hz, 1H), 4.51 (d, J = 16.3 Hz, 1H), 3.25–3.07 (m, 3H), 2.98 (d, J = 13.4 Hz, 1H), 2.29 (d, J = 9.1 Hz, 7H), 2.22–2.17 (m, 1H).13C NMR (100 MHz, Acetone-d6) δ 165.05, 151.39, 150.21, 149.17, 144.35, 136.99, 136.53, 136.45, 135.14, 132.65, 130.76, 129.60, 129.54, 129.32, 129.22, 129.04, 127.83, 127.60, 123.99, 122.49, 118.04, 106.78, 82.92, 57.87, 49.92, 47.87, 44.48, 38.58, 20.98. MS(ESI(+)) calcd for C32H30ClN3O+ [M + H]+: 508.22; found: 508.22.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(2-hydroxypyridin-3-yl)methanone (a14): Yellow oily, 83% Yield, 1H NMR (400 MHz, DMSO-d6), δ: 11.92 (d, J = 4.6 Hz, 1H), 7.46–7.35 (m, 2H), 7.06 (d, J = 7.1 Hz, 1H), 6.99–6.96 (m, 3H), 6.93–6.76 (m, 6H), 6.61 (d, J = 7.2 Hz, 1H), 6.26–6.16 (m, 1H), 6.02 (d, J = 7.8 Hz, 1H), 5.80 (s, 1H), 4.42 (d, J = 8.4 Hz, 2H), 3.07 (d, J = 13.4 Hz, 2H), 2.86 (d, J = 13.3 Hz, 1H), 2.25–2.20 (m, 7H), 2.10 (dd, J = 6.7, 2.5 Hz, 2H).13C NMR (100 MHz, DMSO-d6).δ 166.17, 158.73, 150.08, 140.48, 137.30, 135.77, 135.05, 134.26, 131.62, 131.21, 129.63, 129.55, 128.54, 128.36, 128.29, 128.16, 127.94, 126.50, 125.81, 123.58, 123.06, 116.61, 105.43, 104.50, 82.35, 81.42, 58.18, 56.33, 48.49, 46.81, 43.44, 20.57. MS(ESI(+)) calcd for C32H31N3O2+ [M + H]+: 490.25; found: 490.25.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(pyridin-4-yl)methanone (a15): Yellow oily, 92% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.62 (dd, J = 4.4, 1.5 Hz, 2H), 7.30 (dd, J = 4.4, 1.6 Hz, 2H), 7.09–6.96 (m, 9H), 6.87 (d, J = 8.0 Hz,2H), 6.66 (t, J = 7.3 Hz, 1H), 6.18 (d, J = 7.8 Hz, 1H), 6.03 (s, 1H), 4.60 (d, J = 16.3 Hz, 1H), 4.47 (d, J = 16.3 Hz, 1H), 3.35–3.27 (m, 1H), 3.12 (d, J = 13.3 Hz, 1H), 2.96 (d, J = 13.4 Hz, 1H), 2.30 (s, 3H), 2.26 (s, 3H), 2.24–2.19 (m, 2H).13C NMR (100 MHz, Acetone-d6) δ 168.03, 151.19, 150.47, 144.29, 136.92, 136.25, 136.16, 135.01, 132.44, 130.77, 130.48, 129.31, 129.04, 128.79, 127.27, 126.78, 123.79, 122.25, 121.86, 117.67, 106.60, 83.01, 57.18, 49.58, 49.22, 44.41, 38.20, 20.80, 20.73. MS(ESI(+)) calcd for C32H31N3O + [M + H]+: 474.25; found: 474.25.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(2-chloropyridin-3-yl)methanone (a16): Light yellow solid, Melting Point: 118–120 °C, 90% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.42 (dd, J = 4.7, 1.8 Hz, 1H), 7.46–7.39 (m, 1H), 7.31–6.92 (m, 10H), 6.90 (d, J = 8.0 Hz, 2H), 6.66 (t, J = 7.4 Hz, 1H), 6.18 (d, J = 6.4 Hz, 1H), 6.01 (s, 1H), 4.68 (d, J = 16.3 Hz, 1H), 4.53 (d, J = 16.3 Hz, 1H), 3.25 (dd, J = 9.8, 7.6 Hz, 1H), 3.16 (d, J = 13.3 Hz, 1H), 2.98 (d, J = 13.4 Hz, 1H), 2.31 (s, 3H), 2.29 (s, 3H), 2.26–2.15 (m, 2H).13C NMR (100 MHz, Acetone-d6)δ 165.56, 151.20, 150.58, 150.52, 146.77, 137.48, 136.81, 136.23, 136.16, 134.93, 133.57, 132.42, 130.52, 129.29, 129.07, 128.78, 127.35, 123.74), 123.53, 117.73, 106.48, 82.66, 57.63, 49.62, 47.77, 44.30, 38.36, 20.80, 20.76. MS(ESI(+)) calcd for C32H30ClN3O+ [M + H]+: 508.22; found: 508.22.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(5-methylpyrazin-2-yl)methanone (a17): Light yellow solid, Melting Point: 117–119 °C, 89% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.75 (d, J = 1.3 Hz, 1H), 8.45 (d, J = 0.8 Hz, 1H), 7.08–6.93 (m, 10H), 6.67–6.63 (m, 1H), 6.17 (d, J = 7.8 Hz, 1H), 4.62 (d, J = 16.1 Hz, 1H), 4.50 (d, J = 16.1 Hz, 1H), 3.47–3.37 (m, 1H), 3.08 (d, J = 13.4 Hz, 1H), 2.55 (s, 2H), 2.46 (s, 1H), 2.30–2.21 (m, 11H).13C NMR (100 MHz, Acetone-d6 ) δ 166.31, 155.96, 151.31, 147.20, 145.06, 142.71, 137.07, 136.45, 136.34, 135.22, 132.94, 130.89, 130.75, 129.49, 129.36, 129.24, 129.02, 128.92, 127.65, 126.34, 123.90, 117.91, 107.00, 83.48, 57.00, 50.10, 49.23, 44.53, 38.12, 21.48, 20.98, 20.94. MS(ESI(+)) calcd for C32H32N4O+ [M + H]+: 489.26; found: 489.26.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(pyridin-3-yl)methanone (a18): Light yellow solid, Melting Point: 92–94 °C, 93% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.62–8.59 (m, 2H), 7.77 (d, J = 7.8 Hz, 1H), 7.37 (dd, J = 7.7, 4.9 Hz, 1H), 7.24–6.90 (m, 10H), 6.87 (d, J = 7.8 Hz, 2H), 6.66 (t, J = 7.4 Hz, 1H), 6.18 (d, J = 7.8 Hz, 1H), 6.09 (s, 1H), 4.61 (d, J = 16.3 Hz, 1H), 4.47 (d, J = 16.3 Hz, 1H), 3.12 (d, J = 13.3 Hz, 1H), 2.98–2.89 (m, 1H), 2.30 (s, 3H), 2.26 (s, 3H), 2.24–2.20 (m, 2H).13C NMR (100 MHz, Acetone-d6)δ 168.17, 151.57, 151.40, 149.11, 137.10, 136.37, 136.30, 135.50, 135.22, 132.91, 132.62, 130.66, 129.47, 129.21, 128.96, 127.43, 123.98, 123.65, 117.78, 106.74, 83.15, 57.27, 49.68, 44.64, 38.47, 20.99, 20.93. MS(ESI(+)) calcd for C32H31N3O+ [M + H]+: 474.25; found: 474.25.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(5-chloropyrazin-2-yl)methanone (a19): Light yellow solid, Melting Point: 131–133 °C, 84% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.69 (d, J = 1.3 Hz, 1H), 8.66 (d, J = 1.3 Hz, 1H), 7.08 (dd, J = 7.3, 0.8 Hz, 1H), 7.01 (dt, J = 7.9, 4.6 Hz, 8H), 6.95 (dd, J = 10.0, 4.6 Hz, 1H), 6.85 (d, J = 7.9 Hz, 2H), 6.31–6.18 (m, 2H), 6.01 (s, 1H), 4.61 (d, J = 16.1 Hz, 1H), 4.50 (d, J = 16.2 Hz, 1H), 3.88–3.76 (m, 1H), 3.46–3.38 (m, 1H), 3.07–3.00 (m, 1H), 2.94 (d, J = 13.4 Hz, 1H), 2.29 (s, 3H), 2.26 (s, 3H).13C NMR (100 MHz, Acetone-d6) δ 165.16, 151.24, 150.35, 148.45, 145.69, 143.05, 137.00, 136.53, 136.39, 135.15, 132.88, 130.76, 129.52, 129.27, 128.98, 127.65, 126.16, 123.92, 118.04, 107.07, 83.52, 57.10, 50.12, 49.23, 44.45, 37.97, 20.97, 20.93. MS(ESI(+)) calcd for C31H29ClN4O+ [M + H]+: 509.21; found: 509.21.
(3a,8-bis(4-methylbenzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(pyrazin-2-yl)methanone (a20): White solid, Melting Point: 105–107 °C, 91% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.86 (s, 1H), 8.67 (d, J = 2.5 Hz, 1H), 8.59–8.53 (m, 1H), 7.05–6.84 (m, 10H), 6.68–6.62 (m, 1H), 6.17 (t, J = 7.5 Hz, 1H), 6.03 (s, 1H), 4.56 (d, J = 16.2 Hz, 2H), 3.42 (td, J = 11.0, 7.7 Hz, 1H), 2.96–2.87 (m, 3H), 2.29 (s, 2H), 2.25 (t, J = 5.5 Hz, 6H).13C NMR (100 MHz, Acetone-d6) δ 165.97, 151.15, 150.03, 146.58, 146.30, 145.92, 143.18, 142.39, 136.90, 136.33, 136.22, 135.05, 132.77, 130.68, 130.60, 129.35, 129.10, 128.79, 127.50, 126.25, 123.76), 117.81, 106.87, 83.36, 56.88, 49.94, 49.01, 44.33, 37.93, 20.78, 20.74. MS(ESI(+)) calcd for C31H30N4O+ [M + H]+: 475.25; found: 475.25.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(naphthalen-1-yl)methanone (b1): White solid, Melting Point: 142–144 °C, 94% Yield, 1H NMR (400 MHz, DMSO- d6), δ: 7.97 (d, J = 8.2 Hz, 2H), 7.64–7.35 (m, 10H), 7.32–7.10 (m, 4H), 7.01 (t, J = 7.7 Hz, 1H), 6.70 (t, J = 7.3 Hz, 1H), 6.21–6.04 (m, 2H), 4.73 (d, J = 16.9 Hz, 1H), 4.56 (d, J = 16.9 Hz, 1H), 3.43–3.28 (m, 2H), 3.14–2.88 (m, 2H), 2.10 (d, J = 16.3 Hz, 2H).13C NMR (100 MHz, DMSO- d6) δ 169.21, 150.84, 141.38, 139.45, 134.87, 134.44, 133.45, 131.59 (q, J = 35.3 Hz), 130.88, 130.03, 129.85, 129.73, 129.66, 129.41, 129.28, 129.15, 128.92, 128.61, 127.51, 126.95, 126.76, 126.14, 126.08, 125.71, 124.82 (q, J = 268.2 Hz), 124.24, 124.16, 123.90, 123.56, 118.11, 106.06, 82.29, 57.28, 49.56, 48.31, 43.77, 38.22. MS(ESI(+)) calcd for C37H28F6N2O+ [M + H]+: 631.22; found: 631.22.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(naphthalen-1-yl)methanone (b2): White solid, Melting Point: 161–163 °C, 92% Yield, 1H NMR (400 MHz, DMSO- d6), δ: 7.98 (dd, J = 3.2, 1.3 Hz, 1H), 7.50 (t, J = 8.2 Hz, 2H), 7.37–7.30 (m, 3H), 7.25 (t, J = 7.7 Hz, 2H), 7.18 (d, J = 7.2 Hz, 1H), 7.08 (s, 1H), 7.00–6.90 (m, 2H), 6.69 (td, J = 7.4, 0.8 Hz, 1H), 6.49 (d, J = 0.5 Hz, 1H), 6.18 (s, 2H), 6.04–5.91 (m, 1H), 4.54–4.39 (m, 1H), 4.38–4.26 (m, 1H), 3.60–3.42 (m, 1H), 3.32 (d, J = 8.0 Hz, 2H), 3.13 (d, J = 13.1 Hz, 2H), 2.27–2.13 (m, 2H).13C NMR (100 MHz, DMSO- d6) δ 168.88, 157.22, 150.89, 150.27, 139.55, 136.94, 134.14, 131.15 (q, J = 35.3 Hz), 130.61, 129.69, 129.58, 129.38, 129.27, 129.16, 129.09, 128.96, 128.76, 126.47, 126.07, 126.00, 124.22 (q, J = 268.2 Hz), 123.79, 123.61, 123.38, 117.96, 111.88, 105.98, 82.23, 57.04, 49.00, 44.05, 38.71. MS(ESI(+)) calcd for C32H26F6N4O+ [M + H]+: 597.21; found: 597.21.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(2-chloropyridin-4-yl)methanone (b3): Yellow oily, 89% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.53 (d, J = 5.0 Hz, 1H), 7.62 (d, J = 7.7 Hz, 3H), 7.49 (dd, J = 9.5, 7.3 Hz, 3H), 7.39 (d, J = 7.7 Hz, 1H), 7.26 (dd, J = 7.8, 7.1 Hz, 2H), 7.12 (td, J = 7.7, 1.2 Hz, 1H), 6.84 (d, J = 3.9 Hz, 1H), 6.24 (d, J = 7.9 Hz, 1H), 6.06 (s, 1H), 4.68 (d, J = 16.8 Hz, 1H), 4.57 (d, J = 16.8 Hz, 1H), 3.71 (ddd, J = 10.9, 5.6, 2.8 Hz, 1H), 3.55–3.42 (m, 2H), 3.28 (d, J = 13.2 Hz, 1H), 2.48–2.45 (m, 1H), 2.20–2.11 (m, 1H), 1.46–1.26 (m, 2H).13C NMR (100 MHz, Acetone-d6) δ 166.93, 151.96, 151.39, 150.93, 147.75, 141.76, 139.70, 134.35, 131.47 (q, J = 35.3 Hz), 131.05, 130.73, 130.41, 130.32, 129.84, 129.49, 129.34, 127.08, 127.04, 126.51, 126.41, 124.37 (q, J = 268.2 Hz), 123.96, 122.68, 121.27, 118.61, 106.79, 83.58, 57.61, 49.94, 49.43, 44.80, 38.59. MS(ESI(+)) calcd for C32H24ClF6N3O+ [M + H]+: 616.16; found: 616.16.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(3-chloropyridin-4-yl)methanone (b4): Yellow oily, 85% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.64 (s, 1H), 8.56 (d, J = 4.8 Hz, 1H), 7.63–7.47 (m, 4H), 7.46–7.34 (m, 3H), 7.24–7.11 (m, 4H), 7.03 (td, J = 7.7, 1.2 Hz, 1H), 6.74 (d, J = 3.9 Hz, 1H), 6.17 (d, J = 7.9 Hz, 1H), 5.96 (s, 1H), 4.63 (s, 1H), 4.57 (d, J = 16.7 Hz, 1H), 3.40 (d, J = 13.3 Hz, 1H), 3.35–3.29 (m, 1H), 3.26–3.21 (m, 1H), 2.39–2.34 (m, 2H).13C NMR (100 MHz, Acetone-d6)δ 165.29, 151.40, 150.26, 149.20, 144.08, 141.62, 139.63, 134.47, 131.61 (q, J = 35.3 Hz), 131.18, 129.89, 129.50, 129.44, 127.79, 127.19, 127.15, 124.34 (q, J = 268.2 Hz), 124.16, 124.11, 124.07, 124.02, 122.45, 118.76, 118.21, 106.85, 83.09, 58.02, 50.13, 48.01, 44.59, 38.62. MS(ESI(+)) calcd for C32H24ClF6N3O+ [M + H]+: 616.16; found: 616.16.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(5-methylpyrazin-2-yl)methanone (b5): Yellow oily, 88% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.74 (d, J = 1.4 Hz, 1H), 8.50–8.47 (m, 1H), 7.55 (d, J = 8.4 Hz, 2H), 7.51–7.27 (m, 6H), 7.18–7.10 (m, 2H), 7.02 (dd, J = 5.7, 1.7 Hz, 1H), 6.77–6.72 (m, 1H), 6.11 (d, J = 7.9 Hz, 1H), 6.03 (s, 1H), 4.63 (d, J = 16.7 Hz, 1H), 4.47 (d, J = 16.7 Hz, 1H), 3.54–3.45 (m, 1H), 3.37 (d, J = 13.2 Hz, 1H), 3.20 (d, J = 13.2 Hz, 1H), 2.56 (s, 3H), 2.40–2.29 (m, 2H).13C NMR (100 MHz, Acetone-d6) δ 166.39, 156.15, 151.29, 146.90, 146.09, 145.02, 142.77, 141.71, 139.81, 134.41, 131.81 (q, J = 35.3 Hz), 131.10, 129.77, 129.38, 129.33, 127.13, 127.10, 124.30 (q, J = 268.2 Hz), 124.07, 124.03, 123.99, 123.92, 118.55, 106.84, 83.94, 57.12, 50.28, 49.34, 44.77, 38.54, 21.48. MS(ESI(+)) calcd for C32H26F6N4O+ [M + H]+: 597.21; found: 597.21.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(pyrazin-2-yl)methanone (b6): Yellow oily, 86% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.59–8.54 (m, 1H), 7.89 (t, J = 7.7 Hz, 1H), 7.70 (d, J = 7.8 Hz, 1H), 7.57–7.40 (m, 6H), 7.36–7.29 (m, 2H), 7.22–7.14 (m, 2H), 7.02–6.98 (m, 1H), 6.77 (d, J = 7.3 Hz, 1H), 6.10 (d, J = 7.8 Hz, 1H), 6.03 (s, 1H), 4.64 (d, J = 16.6 Hz, 1H), 4.50 (d, J = 16.6 Hz, 1H), 3.53–3.44 (m, 1H), 3.37 (d, J = 13.1 Hz, 1H), 3.21 (d, J = 13.2 Hz, 1H), 2.38–2.28 (m, 2H).13C NMR (100 MHz, Acetone-d6) δ 167.79, 154.62, 151.20, 148.52, 141.58, 139.69, 137.36, 134.23, 131.70 (q, J = 35.3 Hz), 130.96, 129.57, 129.14, 126.96, 126.92, 125.69, 125.53, 125.39, 124.32 (q, J = 268.2 Hz), 124.09, 123.72, 118.26, 106.59, 83.63, 56.95, 50.03, 49.22, 44.65, 38.46. MS(ESI(+)) calcd for C31H24F6N4O+ [M + H]+: 583.19; found: 583.19.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(2-hydroxypyridin-3-yl)methanone (b7): White solid, Melting Point: 109–111 °C, 83% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 11.17 (s, 1H), 7.57–7.47 (m, 6H), 7.38 (dd, J = 7.8, 4.4 Hz, 2H), 7.32–7.26 (m, 2H), 7.19–7.15 (m, 3H), 6.99–6.95 (m, 1H), 6.71 (dd, J = 9.5, 5.3 Hz, 1H), 6.28 (t, J = 6.7 Hz, 1H), 6.04 (d, J = 8.0 Hz, 1H), 5.89 (s, 1H), 4.59 (d, J = 16.7 Hz, 1H), 4.47 (d, J = 16.8 Hz, 1H), 3.40–3.35 (m, 1H), 3.16 (d, J = 13.2 Hz, 1H), 2.32–2.25 (m, 2H).13C NMR (100 MHz, Acetone-d6)δ 167.20, 160.00, 151.53, 142.95, 141.83, 141.63, 140.51, 139.91, 137.75, 137.60, 134.52, 134.38, 131.71 (q, J = 35.3 Hz), 131.36, 129.81, 129.26, 127.09, 127.05, 124.23 (q, J = 268.2 Hz), 124.02, 123.82, 118.25, 106.58, 105.52, 83.14, 57.72, 50.08, 47.97, 44.91, 38.87. MS(ESI(+)) calcd for C32H25F6N3O2+ [M + H]+: 598.19; found: 598.19.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(2-chloropyridin-3-yl)methanone (b8): Yellow oily, 94% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.45 (dd, J = 4.8, 1.9 Hz, 1H), 7.64 (d, J = 4.8 Hz, 1H), 7.57 (d, J = 9.9 Hz, 3H), 7.54–7.37 (m, 4H), 7.34 (d, J = 7.7 Hz, 1H), 7.25–7.13 (m, 3H), 7.03 (td, J = 7.7, 1.1 Hz, 1H), 6.75 (t, J = 7.4 Hz, 1H), 6.17 (d, J = 7.9 Hz, 1H), 5.96 (s, 1H), 4.66 (d, J = 16.7 Hz, 1H), 4.59 (d, J = 16.7 Hz, 1H), 3.39 (t, J = 2.5 Hz, 1H), 3.22 (d, J = 7.4 Hz, 2H), 2.39–2.34 (m, 2H).13C NMR (100 MHz, Acetone-d6 )δ 166.06, 151.47, 150.91, 146.99, 141.71, 139.68, 137.75, 134.48, 133.59, 131.66 (q, J = 35.3 Hz), 131.21, 130.44, 130.39, 130.07, 129.87, 129.47, 129.44, 127.20, 127.16, 124.34 (q, J = 268.2 Hz), 124.08, 124.04, 124.00, 123.81, 118.69, 106.80, 83.08, 58.03, 50.08, 48.15, 44.66, 38.63. MS(ESI(+)) calcd for C32H24ClF6N3O+ [M + H]+: 616.16; found: 616.16.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(pyridin-2-yl)methanone (b9): Yellow oily, 92% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.56–8.51 (m, 1H), 7.92–7.86 (m, 1H), 7.69 (dd, J = 7.9, 2.8 Hz, 1H), 7.57–7.54 (m, 2H), 7.50–7.31 (m, 6H), 7.24 (d, J = 6.2 Hz, 1H), 7.17 (d, J = 5.0 Hz, 2H), 7.02–6.99 (m, 1H), 6.77–6.71 (m, 1H), 6.11 (t, J = 8.2 Hz, 1H), 6.03 (d, J = 3.9 Hz, 1H), 4.67–4.61 (m, 1H), 4.50 (d, J = 16.7 Hz, 1H), 4.03–3.96 (m, 1H), 3.37 (d, J = 13.2 Hz, 1H), 3.20 (dd, J = 13.3, 2.6 Hz, 1H), 2.37–2.28 (m, 2H).13C NMR (100 MHz, Acetone-d6) δ 167.98, 154.78, 151.37, 148.70, 141.75, 139.86, 137.54, 134.40, 131.87 (q, J = 35.3 Hz), 131.13, 129.75, 129.32, 129.30, 127.13, 127.09, 125.87, 125.72, 125.58, 124.51 (q, J = 268.2 Hz), 124.26, 124.12, 124.09, 123.90, 118.44, 106.77, 83.80, 57.13, 50.20, 49.41, 44.83, 38.64. MS(ESI(+)) calcd for C32H25F6N3O+ [M + H]+: 582.20; found: 582.20.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(pyridin-3-yl)methanone (b10): Yellow oily, 95% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.67–8.60 (m, 2H), 7.83 (d, J = 7.9 Hz, 1H), 7.59–7.24 (m, 8H), 7.23–7.11 (m, 3H), 7.03 (td, J = 7.6, 1.0 Hz, 1H), 6.75 (t, J = 7.4 Hz, 1H), 6.14 (d, J = 7.9 Hz, 1H), 6.05 (s, 1H), 4.60 (d, J = 16.8 Hz, 1H), 4.50 (d, J = 16.8 Hz, 1H), 3.72–3.62 (m, 1H), 3.38 (d, J = 13.2 Hz, 1H), 3.20 (d, J = 13.2 Hz, 1H), 2.42–2.30 (m, 2H).13C NMR (100 MHz, Acetone-d6)δ 168.21, 151.56, 151.26, 148.94, 141.58, 139.61, 135.36, 134.18, 132.50, 131.39 (q, J = 35.3 Hz), 130.83, 130.50, 129.61, 129.25, 129.12, 126.89, 126.86, 126.32, 126.23, 125.06, 124.17 (q, J = 268.2 Hz), 123.78, 123.74, 123.52, 118.30, 106.55, 83.22, 57.28, 49.69, 49.62, 44.67, 38.48. MS(ESI(+)) calcd for C32H25F6N3O+ [M + H]+: 582.20; found: 582.20.
3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(6-chloropyridin-2-yl)methanone (b11): Yellow oily, 87%, 1H NMR (400 MHz, Acetone-d6), δ: 7.96 (t, J = 7.8 Hz, 1H), 7.70–7.67 (m, 1H), 7.56 (d, J = 7.7 Hz, 3H), 7.51–7.38 (m, 4H), 7.32 (t, J = 6.8 Hz, 1H), 7.24 (d, J = 8.3 Hz, 1H), 7.20–7.15 (m, 2H), 7.02 (dd, J = 7.8, 4.2 Hz, 1H), 6.74 (dd, J = 9.1, 3.9 Hz, 1H), 6.13–6.10 (m, 1H), 6.01 (d, J = 5.1 Hz, 1H), 4.63 (d, J = 16.7 Hz, 1H), 4.48 (d, J = 16.7 Hz, 1H), 3.54–3.46 (m, 1H), 3.36 (d, J = 13.2 Hz, 1H), 3.20 (d, J = 13.2 Hz, 1H), 2.41–2.30 (m, 2H).13C NMR (100 MHz, Acetone-d6)δ 166.37, 154.91, 151.28, 149.97, 141.70, 140.98, 139.80, 134.39, 131.78 (q, J = 35.3 Hz), 131.10, 129.77, 129.59, 129.40, 129.31, 127.13, 127.09, 126.47, 124.29 (q, J = 268.2 Hz), 124.07, 123.93, 123.46, 118.57, 106.88, 83.97, 57.16, 50.25, 49.47, 44.76, 38.47. MS(ESI(+)) calcd for C32H24ClF6N3O+ [M + H]+: 616.16; found: 616.16.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(6-methylpyridin-3-yl)methanone (b12): White solid, Melting Point: 117–119 °C, 89% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.53 (s, 1H), 7.72 (dd, J = 8.0, 1.9 Hz, 1H), 7.57–7.29 (m, 7H), 7.27–7.12 (m, 4H), 7.05–7.00 (m, 1H), 6.75 (t, J = 7.4 Hz, 1H), 6.12 (d, J = 7.9 Hz, 1H), 6.05 (s, 1H), 4.58 (d, J = 16.8 Hz, 1H), 4.49 (d, J = 16.8 Hz, 1H), 3.47 (td, J = 11.1, 6.0 Hz, 1H), 3.37 (d, J = 13.2 Hz, 1H), 3.20 (d, J = 13.2 Hz, 1H), 2.50 (s, 3H), 2.35–2.31 (m, 2H).13C NMR (100 MHz, Acetone-d6) δ 168.61, 160.96, 151.46, 148.65, 141.77, 139.82, 136.01, 134.37, 131.60 (q, J = 35.3 Hz), 131.02, 130.68, 130.37, 130.27, 129.79, 129.43, 129.30, 127.09, 127.05, 126.51, 126.42, 124.35 (q, J = 268.2 Hz), 123.96, 123.92, 123.72, 122.92, 118.45, 106.72, 83.37, 57.42, 49.86, 44.87, 38.67, 24.28. MS(ESI(+)) calcd for C33H27F6N3O+ [M + H]+: 596.21; found: 596.21.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(4-methoxyphenyl)methanone(b13): Yellow oily, 90% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 7.54 (t, J = 6.5 Hz, 2H), 7.51–7.34 (m, 6H), 7.31 (d, J = 7.6 Hz, 1H), 7.22–7.15 (m, 2H), 7.10 (d, J = 6.0 Hz, 1H), 7.04–7.00 (m, 1H), 6.93 (d, J = 8.5 Hz, 2H), 6.74 (td, J = 7.5, 0.8 Hz, 1H), 6.09 (d, J = 7.0 Hz, 2H), 4.52 (dd, J = 2.3, 0.7 Hz, 2H), 3.82 (s, 3H), 3.71 (d, J = 0.6 Hz, 1H), 3.36 (d, J = 11.2 Hz, 1H), 3.19 (d, J = 13.2 Hz, 1H), 2.32 (d, J = 6.4 Hz, 2H).13C NMR (100 MHz, Acetone-d6)δ 170.21, 162.02, 151.52, 141.76, 139.91, 134.39, 131.73 (q, J = 35.3 Hz), 131.04, 130.67, 130.31, 129.95, 129.77, 129.36, 129.28, 128.95, 127.10, 127.07, 126.53, 126.44, 124.33 (q, J = 268.2 Hz), 123.97, 123.93, 123.89, 123.85, 118.34, 113.91, 106.66, 83.21, 57.28, 55.54, 50.18, 49.68, 44.91, 38.73. MS(ESI(+)) calcd for C34H28F6N2O2+ [M + H]+: 611.21; found: 611.21.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(5-methylpyridin-3-yl)methanone (b14): Yellow oily, 93% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.49–8.41 (m, 2H), 7.67–7.35 (m, 7H), 7.32 (d, J = 7.7 Hz, 1H), 7.22–7.14 (m, 3H), 7.03 (td, J = 7.7, 1.1 Hz, 1H), 6.75 (t, J = 7.4 Hz, 1H), 6.14 (d, J = 7.9 Hz, 1H), 6.05 (s, 1H), 4.60 (d, J = 16.8 Hz, 1H), 4.49 (d, J = 16.8 Hz, 1H), 3.45 (td, J = 11.0, 6.3 Hz, 1H), 3.38 (d, J = 13.2 Hz, 1H), 3.20 (d, J = 13.2 Hz, 1H), 2.37–2.31 (m, 5H).13C NMR (100 MHz, Acetone-d6)δ 168.54, 152.14, 151.45, 146.25, 141.83, 139.82, 135.69, 134.38, 133.44, 132.24, 131.61 (q, J = 35.3 Hz), 131.07, 129.82, 129.44, 129.32, 127.10, 127.06, 126.53, 126.43, 124.36 (q, J = 268.2 Hz), 123.93, 118.47, 106.70, 83.38, 57.47, 49.89, 49.79, 44.87, 38.67, 17.94. MS(ESI(+)) calcd for C33H27F6N3O+ [M + H]+: 596.21; found: 596.21.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(6-chloropyridin-3-yl)methanone (b15): Yellow oily, 94% Yield, 1H NMR (400 MHz, DMSO- d6), δ: 8.47 (d, J = 2.0 Hz, 1H), 7.94 (dd, J = 8.3, 2.4 Hz, 1H), 7.63–7.50 (m, 3H), 7.47 (s, 1H), 7.40 (td, J = 7.7, 3.7 Hz, 2H), 7.29 (d, J = 7.7 Hz, 1H), 7.23 (d, J = 6.7 Hz, 1H), 7.12 (s, 1H), 7.08–6.98 (m, 2H), 6.75 (t, J = 7.3 Hz, 1H), 6.12 (d, J = 7.8 Hz, 1H), 5.93 (s, 1H), 4.46–4.35 (m, 2H), 3.57 (dd, J = 10.6, 6.6 Hz, 1H), 3.38 (d, J = 7.9 Hz, 2H), 3.14 (d, J = 13.2 Hz, 1H), 2.28 (dt, J = 11.8, 7.0 Hz, 2H).13C NMR (100 MHz, DMSO- d6) δ 166.74, 152.13, 150.76, 149.08, 141.11, 139.37, 139.28, 134.13, 131.40 (q, J = 35.3 Hz), 131.15, 130.67, 129.72, 129.59, 129.28, 129.16, 128.77, 126.50, 126.06, 126.00, 124.53 (q, J = 268.2 Hz), 124.18, 123.81, 123.60, 123.50, 118.08, 106.19, 82.63, 56.92, 49.33, 49.18, 44.04, 38.37. MS(ESI(+)) calcd for C32H24ClF6N3O+ [M + H]+: 616.16; found: 616.16.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(5,6-dichloropyridin-3-yl)methanone (b16): Yellow oily, Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.39 (d, J = 1.9 Hz, 1H), 8.00 (d, J = 1.9 Hz, 1H), 7.59–7.32 (m, 6H), 7.28 (d, J = 7.7 Hz, 1H), 7.18–7.10 (m, 3H), 7.04–6.98 (m, 1H), 6.73 (t, J = 7.4 Hz, 1H), 6.14 (d, J = 7.9 Hz, 1H), 5.99 (s, 1H), 4.55 (d, J = 16.8 Hz, 1H), 4.46 (d, J = 16.8 Hz, 1H), 3.76–3.70 (m, 1H), 3.35 (d, J = 13.2 Hz, 1H), 3.16 (d, J = 13.2 Hz, 1H), 2.38–2.28 (m, 2H).13C NMR (100 MHz, Acetone-d6)δ 165.74, 151.21, 149.98, 146.79, 141.55, 139.51, 138.46, 134.15, 133.19, 131.27 (q, J = 35.3 Hz), 130.86, 130.55, 130.51, 130.17, 130.11, 129.65, 129.30, 129.15, 127.39, 126.88, 126.85, 124.18 (q, J = 268.2 Hz), 123.80, 123.77, 118.39, 106.60, 83.36, 57.36, 49.66, 49.49, 44.63, 38.43. MS(ESI(+)) calcd for C32H23Cl2F6N3O+ [M + H]+: 650.12; found: 650.12.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(5-chloropyrazin-2-yl)methanone (b17): Yellow oily, 83% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.76–8.60 (m, 2H), 7.57–7.47 (m, 3H), 7.47–7.33 (m, 3H), 7.31 (d, J = 7.5 Hz, 1H), 7.24–7.15 (m, 3H), 7.05–7.00 (m, 1H), 6.75 (td, J = 7.4, 0.7 Hz, 1H), 6.13 (d, J = 7.8 Hz, 1H), 6.02 (s, 1H), 4.63 (d, J = 16.7 Hz, 1H), 4.47 (d, J = 16.7 Hz, 1H), 3.50 (dt, J = 11.6, 3.6 Hz, 1H), 3.39–3.33 (m, 1H), 3.23–3.18 (m, 1H), 2.36 (dd, J = 12.1, 5.7 Hz, 2H).13C NMR (100 MHz, Acetone-d6) δ 165.24, 151.21, 150.49, 148.14, 145.66, 143.10, 141.62, 139.72, 134.40, 131.71 (q, J = 35.3 Hz), 131.09, 129.80, 129.44, 129.35, 127.13, 127.09, 124.31 (q, J = 268.2 Hz), 124.03, 124.00, 123.96, 123.93, 118.66, 106.89, 84.01, 57.20, 50.28, 49.35, 44.70, 38.42. MS(ESI(+)) calcd for C31H23ClF6N4O+ [M + H]+: 617.15; found: 617.15.
(3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(2-fluoropyridin-3-yl)methanone (b18): Yellow oily, 88% Yield, 1H NMR (400 MHz, Acetone-d6), δ: 8.30 (d, J = 4.9 Hz, 1H), 7.96–7.83 (m, 1H), 7.54 (dd, J = 14.4, 7.1 Hz, 3H), 7.44–7.37 (m, 3H), 7.36–7.06 (m, 5H), 7.02 (dd, J = 14.0, 6.4 Hz, 1H), 6.75 (t, J = 7.4 Hz, 1H), 6.11 (d, J = 7.9 Hz, 1H), 5.96 (s, 1H), 4.62 (d, J = 16.7 Hz, 1H), 4.46 (d, J = 16.7 Hz, 1H), 3.60–3.50 (m, 1H), 3.42–3.37 (m, 1H), 3.25–3.16 (m, 1H), 2.44–2.32 (m, 2H).13C NMR (100 MHz, Acetone-d6) δ 164.57, 160.66, 158.30, 151.45, 149.88, 149.73(CH, d, J = 13.0 Hz ), 141.64, 141.14, 141.10, 139.73, 134.36, 131.53 (q, J = 35.3 Hz), 131.01, 129.81, 129.48, 129.34, 127.08, 127.04, 124.36 (q, J = 268.2 Hz), 124.00, 122.74, 122.70, 120.24(d, J = 31.9 Hz ), 119.91, 118.67, 106.86, 83.43, 57.85, 50.23, 48.50, 44.84, 38.42. MS(ESI(+)) calcd for C32H24F7N3O+ [M + H]+: 600.19; found: 600.19.
3a,8-bis(3-(trifluoromethyl)benzyl)-3,3a,8,8a-tetrahydropyrrolo[2,3-b]indol-1(2H)-yl)(pyridin-4-yl)methanone(b19): Yellow oily, 90% Yield, 1H NMR (400 MHz, DMSO- d6), δ: 8.64 (dd, J = 4.5, 1.4 Hz, 2H), 7.53 (d, J = 7.7 Hz, 2H), 7.45 (s, 1H), 7.40–7.33 (m, 4H), 7.27 (d, J = 7.6 Hz, 1H), 7.20 (d, J = 7.3 Hz, 1H), 7.10 (s, 1H), 7.00 (d, J = 6.9 Hz, 2H), 6.72 (t, J = 7.4 Hz, 1H), 6.08 (d, J = 7.8 Hz, 1H), 5.88 (s, 1H), 4.44 (d, J = 16.9 Hz, 2H), 3.45 (dd, J = 10.5, 6.2 Hz, 1H), 3.29 (d, J = 5.4 Hz, 2H), 3.13 (d, J = 13.1 Hz, 1H), 2.29–2.19 (m, 2H).13C NMR (100 MHz, DMSO- d6)δ 167.84, 150.74, 150.46, 143.56, 141.13, 139.39, 134.15, 131.17 (q, J = 35.3 Hz), 130.68, 129.75, 129.61, 129.30, 129.17, 129.09, 128.78, 126.51, 126.08, 126.01, 124.18 (q, J = 268.2 Hz), 123.83, 123.63, 123.44, 123.31, 121.83, 118.11, 106.19, 82.65, 56.98, 49.28, 49.12, 43.98, 38.33. MS(ESI(+)) calcd for C32H25F6N3O+ [M + H]+: 582.20; found: 582.20.
3.3. Biological Activity
The antifungal activity of Chimonanthus praecox analogues was measured according to the previously reported method [17,18].
The six phytopathogenic fungi (Verticillium dahliae-ACCC 36211; Colletotrichum orbiculare-SAUM 0321; Cytospora juglandis-ACCC36357; Curvularia lunata-SAUM 1373; Sclerotinia sclerotiorum-ACCC34236; Altenaria solani-SAUM 1275) were provided by the School of Environmental and Chemical Engineering, Jiangsu University of Science and Technology. The antifungal concentrations were 250, 125, 62.5, 32.3, 15.63, 7.8, 3.9, and 1.95 µg mL−1, respectively. The antifungal test plates were incubated aerobically at 28 °C for 48 h. The MICs were examined. All tests were performed in triplicate and repeated if the results differed.
4. Conclusions
In summary, a total of 39 Chimonanthus praecox alkaloids (a1~a20 and b1~b19) were synthesized from indole-3-acetonitrile via alkylation reaction, reduction reaction, and acylation reaction. Their antifungal activities were studied, and the relationship between the antifungal activity of the target compounds and their structures was discussed. Compound b15 showed the best inhibitory effect on A. solani, and its minimum inhibitory concentration (MIC) value is 1.95 µg mL−1; compound b17 displayed the best effect on S. sclerotiorum, and its minimum inhibitory concentration (MIC) value is 1.95 µg mL−1. These results will lay a foundation for subsequent research and development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27175570/s1, Figure S1: 1H-NMR spectroscopic data of compound a1; Figure S2: 13C-NMR spectroscopic data of compound a1; Figure S3: 1H-NMR spectroscopic data of compound a2; Figure S4: 13C-NMR spectroscopic data of compound a2; Figure S5: 1H-NMR spectroscopic data of compound a3; Figure S6: 13C-NMR spectroscopic data of compound a3; Figure S7: 1H-NMR spectroscopic data of compound a4; Figure S8: 13C-NMR spectroscopic data of compound a4; Figure S9: 1H-NMR spectroscopic data of compound a5; Figure S10: 13C-NMR spectroscopic data of compound a5; Figure S11: 1H-NMR spectroscopic data of compound a6; Figure S12: 13C-NMR spectroscopic data of compound a6; Figure S13: 1H-NMR spectroscopic data of compound a7; Figure S14: 13C-NMR spectroscopic data of compound a7; Figure S15: 1H-NMR spectroscopic data of compound a8;Figure S16: 13C-NMR spectroscopic data of compound a8; Figure S17: 1H-NMR spectroscopic data of compound a9; Figure S18: 13C-NMR spectroscopic data of compound a9; Figure S19: 1H-NMR spectroscopic data of compound a10; Figure S20: 13C-NMR spectroscopic data of compound a10; Figure S21: 1H-NMR spectroscopic data of compound a11; Figure S22: 13C-NMR spectroscopic data of compound a11; Figure S23: 1H-NMR spectroscopic data of compound a12; Figure S24: 13C-NMR spectroscopic data of compound a12; Figure S25: 1H-NMR spectroscopic data of compound a13; Figure S26: 13C-NMR spectroscopic data of compound a13; Figure S27: 1H-NMR spectroscopic data of compound a14; Figure S28: 13C-NMR spectroscopic data of compound a14; Figure S29: 1H-NMR spectroscopic data of compound a15; Figure S30: 13C-NMR spectroscopic data of compound a15; Figure S31: 1H-NMR spectroscopic data of compound a16; Figure S32: 13C-NMR spectroscopic data of compound a16; Figure S33: 1H-NMR spectroscopic data of compound a17; Figure S34: 13C-NMR spectroscopic data of compound a17; Figure S35: 1H-NMR spectroscopic data of compound a18; Figure S36: 13C-NMR spectroscopic data of compound a18; Figure S37: 1H-NMR spectroscopic data of compound a19; Figure S38: 13C-NMR spectroscopic data of compound a19; Figure S39: 1H-NMR spectroscopic data of compound a20; Figure S40: 13C-NMR spectroscopic data of compound a20; Figure S41: 1H-NMR spectroscopic data of compound b1; Figure S42: 13C-NMR spectroscopic data of compound b1; Figure S43: 1H-NMR spectroscopic data of compound b2; Figure S44: 13C-NMR spectroscopic data of compound b2; Figure S45: 1H-NMR spectroscopic data of compound b3; Figure S46: 13C-NMR spectroscopic data of compound b3; Figure S47: 1H-NMR spectroscopic data of compound b4; Figure S48: 13C-NMR spectroscopic data of compound b4; Figure S49: 1H-NMR spectroscopic data of compound b5; Figure S50: 13C-NMR spectroscopic data of compound b5; Figure S51: 1H-NMR spectroscopic data of compound b6; Figure S52: 13C-NMR spectroscopic data of compound b6; Figure S53: 1H-NMR spectroscopic data of compound b7; Figure S54: 13C-NMR spectroscopic data of compound b7; Figure S55: 1H-NMR spectroscopic data of compound b8; Figure S56: 13C-NMR spectroscopic data of compound b8; Figure S57: 1H-NMR spectroscopic data of compound b9; Figure S58: 13C-NMR spectroscopic data of compound b9; Figure S59: 1H-NMR spectroscopic data of compound b10; Figure S60: 13C-NMR spectroscopic data of compound b10; Figure S61: 1H-NMR spectroscopic data of compound b11; Figure S62: 13C-NMR spectroscopic data of compound b11; Figure S63: 1H-NMR spectroscopic data of compound b12; Figure S64: 13C-NMR spectroscopic data of compound b12; Figure S65: 1H-NMR spectroscopic data of compound b13; Figure S66: 13C-NMR spectroscopic data of compound b13; Figure S67: 1H-NMR spectroscopic data of compound b14; Figure S68: 13C-NMR spectroscopic data of compound b14; Figure S69: 1H-NMR spectroscopic data of compound b15; Figure S70: 13C-NMR spectroscopic data of compound b15; Figure S71: 1H-NMR spectroscopic data of compound b16; Figure S72: 13C-NMR spectroscopic data of compound b16; Figure S73: 1H-NMR spectroscopic data of compound b17; Figure S74: 13C-NMR spectroscopic data of compound b17; Figure S75: 1H-NMR spectroscopic data of compound b18; Figure S76: 13C-NMR spectroscopic data of compound b18; Figure S77: 1H-NMR spectroscopic data of compound b19; Figure S78: 13C-NMR spectroscopic data of compound b19.
Author Contributions
S.Z. designed research; Y.W. and J.C. performed research; Y.Y. and Y.Z. performed statistical analysis; Y.Y. wrote the paper; R.Z. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32202073) and the Development Program (Modern Agriculture) of Zhenjiang City (NY2018002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or the Supplementary Materials file.
Acknowledgments
The manuscript is approved by all authors for publication.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Qiao, J.M.; Yu, D.Y.; Wang, Q.F.; Liu, Y.P. Diverse effects of crop distribution and climate change on crop production in the agro-pastoral transitional zone of China. Front. Earth Sci. 2018, 12, 408–419. [Google Scholar] [CrossRef]
- Lewis, K. Recover the lost art of drug discovery. Nature 2012, 485, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sanchis, P.; Savina, S.A.; Albericio, F.; Álvarez, M. Structure, Bioactivity and Synthesis of Natural Products with Hexahydropyrrolo[2,3-b]indole. Chem. Eur. J. 2011, 17, 1388–1408. [Google Scholar] [CrossRef] [PubMed]
- Crich, D.; Banerjee, A. Chemistry of the Hexahydropyrrolo[2,3-b]indoles: Configuration, Conformation, Reactivity, and Applications in Synthesis. Acc. Chem. Res. 2007, 40, 151–161. [Google Scholar] [CrossRef]
- Saadi, R.; Bohnenberger, K. Physostigmine for antimuscarinic toxicity. J. Emerg. Nurs. 2020, 46, 126–128. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.C.; Yang, P.F.; Kou, X.; Ren, Z.H.; Guan, Z.H. Palladium-Catalyzed Enantioselective Heck Carbonylation with a Monodentate Phosphoramidite Ligand: Asymmetric Synthesis of (+)-Physostigmine, (+)-Physovenine, and (+)-Folicanthine. Angew. Chem. Int. Ed. 2020, 59, 12199–12205. [Google Scholar] [CrossRef]
- Sun, D.Q.; Xing, C.Y.; Wang, X.Q.; Su, Z.Q.; Li, C.Z. Highly efficient and stereocontrolled oxidative coupling of tetrahydropyrroloindoles: Synthesis of chimonanthines, (+)-WIN 64821 and (+)-WIN 64745. Org. Chem. Front. 2014, 1, 956–960. [Google Scholar] [CrossRef]
- Perez-Balado, C.; Rodriguez-Grana, P.; de Lera, A.R. Stereocontrolled and Versatile Total Synthesis of Bispyrrolidinoindoline Diketopiperazine Alkaloids: Structural Revision of the Fungal Isolate (+)-Asperdimin. Chem. Eur. J. 2009, 15, 9928–9937. [Google Scholar] [CrossRef]
- Zhang, J.W.; Gao, J.M.; Xu, T.; Zhang, X.C.; Ma, Y.T.; Jarussophon, S.; Konishi, Y. Antifungal activity of alkaloids from the seeds of Chimonanthus praecox. Chem. Biodivers. 2009, 6, 838–845. [Google Scholar] [CrossRef]
- Zhang, J.W.; Yang, C.P.; Pan, Z.; Wu, W.J. Antibiotic constituent in Chimonanthus praecox seeds. Xibei Zhiwu Xuebao 2005, 25, 2068–2071. [Google Scholar]
- Shi, L.J.; Yang, S.X.; Bi, J.L.; Yin, G.F.; Wang, Y.H. Chemical constituents from the branches and leaves of Chimonanthus praecox and their antiviral activity. Tianran Chanwu Yanjiu Yu Kaifa 2012, 24, 1335–1338. [Google Scholar]
- Joubouhi, C.; Tamokou, J.-D.-D.; Ngnokam, D.; Voutquenne-Nazabadioko, L.; Kuiate, J.-R. Iridoids from Canthium subcordatum iso-butanol fraction with potent biological activities. BMC Complementary Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamokou, J.D.D.; Mbaveng, A.T.; Kuete, V. Chapter 8—Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa; Kuete, V., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 207–237. [Google Scholar]
- Li, Y.; Wang, H.; Ali, S.; Xia, X.; Liang, Y. Iodine-mediated regioselective C2-amination of indoles and a concise total synthesis of (+/−)-folicanthine. Chem. Commun. 2012, 48, 2343–2345. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Movassaghi, M. Biogenetically-Inspired Total Synthesis of Epidithiodiketopiperazines and Related Alkaloids. Acc. Chem. Res. 2015, 48, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Liang, K.; Pan, R.; Zhang, H.; Xia, C. Total Synthesis of (+)-Chimonanthine, (+)-Folicanthine, and (-)-Calycanthine. J. Org. Chem. 2015, 80, 10309–10316. [Google Scholar] [CrossRef]
- Peng, Y.; Luo, L.; Yan, C.S.; Zhang, J.J.; Wang, Y.W. Ni-Catalyzed Reductive Homocoupling of Unactivated Alkyl Bromides at Room Temperature and Its Synthetic Application. J. Org. Chem. 2013, 78, 10960–10967. [Google Scholar] [CrossRef]
- Ishikawa, H.; Takayama, H.; Aimi, N. Dimerization of indole derivatives with hypervalent iodines(III): A new entry for the concise total synthesis of rac- and meso-chimonanthines. Tetrahedron Lett. 2002, 43, 5637–5639. [Google Scholar] [CrossRef]
- Zheng, S.; Gu, Y.; Li, L.; Zhu, R.; Cai, X.; Bai, H.; Zhang, J. Synthesis and fungicidal activity of tryptophan analogues—The unexpected calycanthaceous alkaloid derivatives. Nat. Prod. Res. 2017, 31, 1142–1149. [Google Scholar] [CrossRef]
- Zheng, S.; Zhou, X.; Xu, S.; Zhu, R.; Bai, H.; Zhang, J. Synthesis and Antimicrobial Characterization of Half-Calycanthaceous Alkaloid Derivatives. Molecules 2016, 21, 1207. [Google Scholar] [CrossRef]
- Zheng, S.; Li, L.; Wang, Y.; Zhu, R.; Baia, H.; Zhang, J. Synthesis and Antimicrobial Activity of Calycanthaceous Alkaloid Analogues. Nat. Prod. Commun. 2016, 11, 1429–1432. [Google Scholar] [CrossRef]
- Zheng, S.; Zhu, R.; Zhou, X.; Chen, L.; Bai, H.; Zhang, J. Synthesis and biological evaluation of calycanthaceous alkaloid analogs. Bioorgan. Med. Chem. 2019, 27, 115088. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhu, R.; Tang, B.; Chen, L.; Bai, H.; Zhang, J. Synthesis and biological evaluations of a series of calycanthaceous analogues as antifungal agents. Nat. Prod. Res. 2019, 35, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zheng, S.J.; Tan, Y.; Chen, X.Y.; Bai, H.J.; Zhu, R.; Gao, Y.H. Synthesis and Antimicrobial Evaluation of Calycanthaceous Alkaloid Derivatives. Chem. Nat. Compd. 2021, 57, 899–902. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).