Effects of Allyl Isothiocyanate on Oxidative and Inflammatory Stress in Type 2 Diabetic Rats
Abstract
:1. Introduction
2. Results
2.1. Effects of AITC on Fe, Cu, and Zn Content in the Liver, Kidney, and Spleen of Diabetic Rats
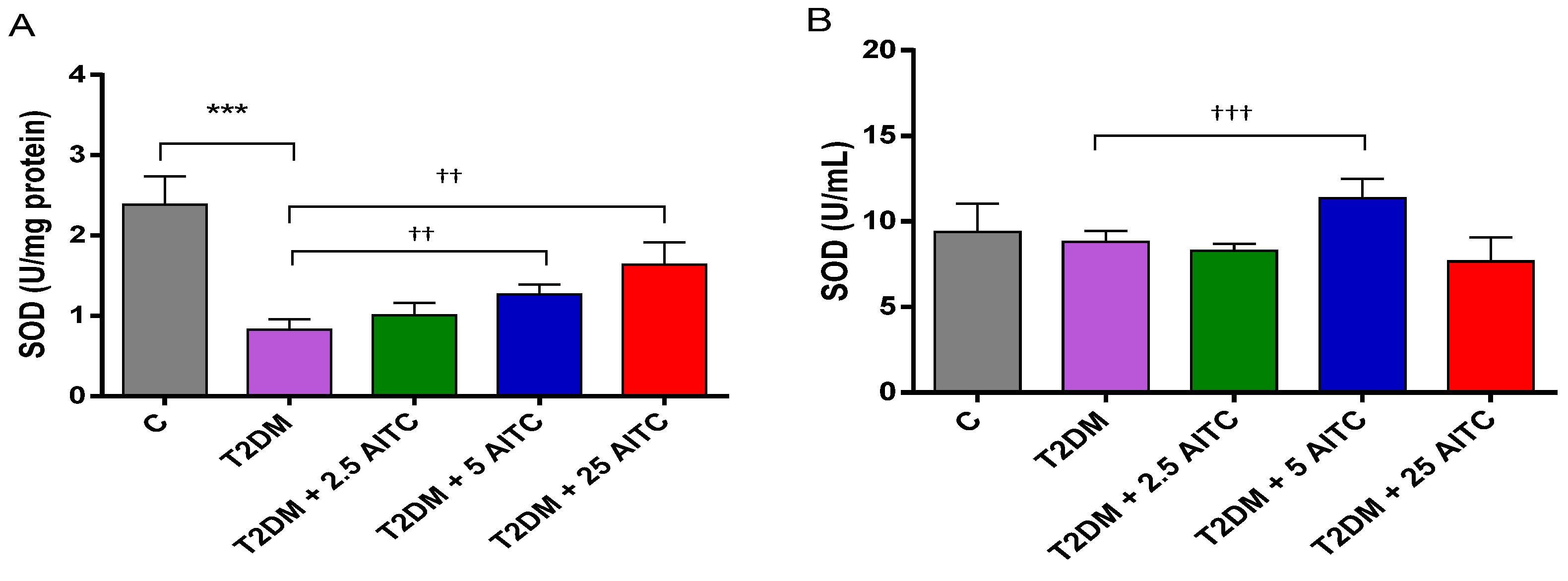
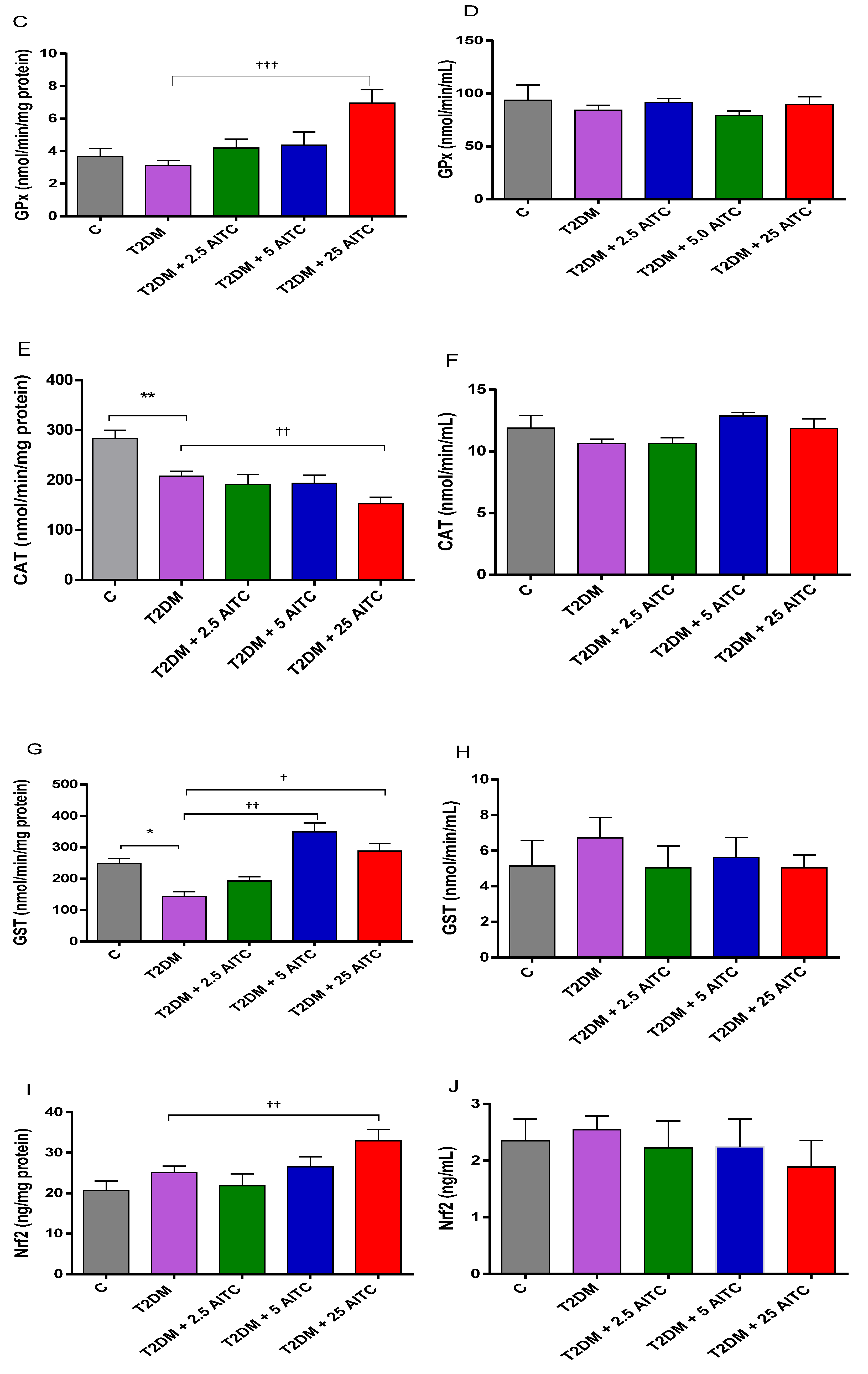
2.2. Effects of AITC on Antioxidant Enzyme Activities and Transcription Factor Content in Diabetic Rats
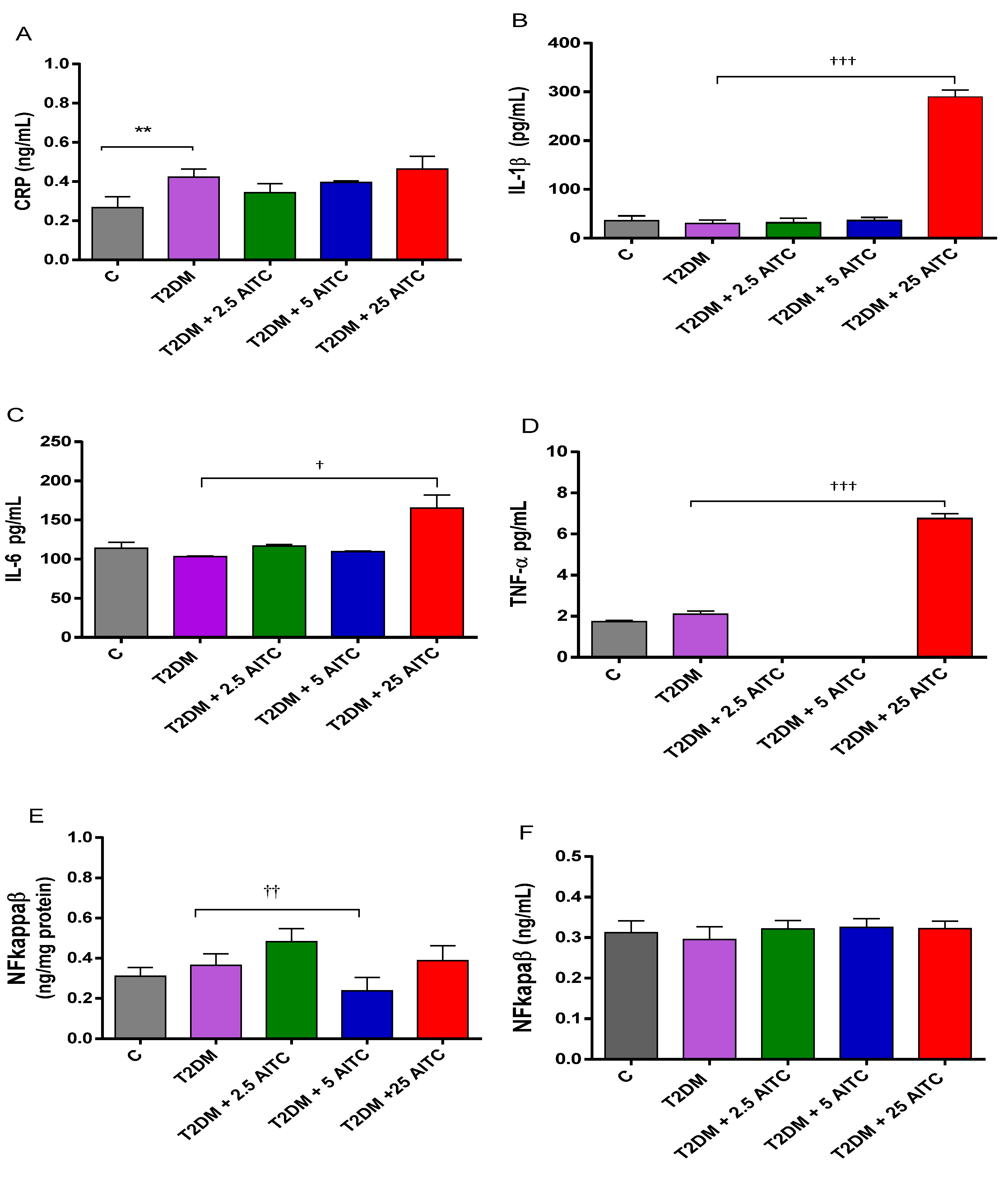
2.3. Effects of AITC on Inflammatory Markers in Diabetic Rats
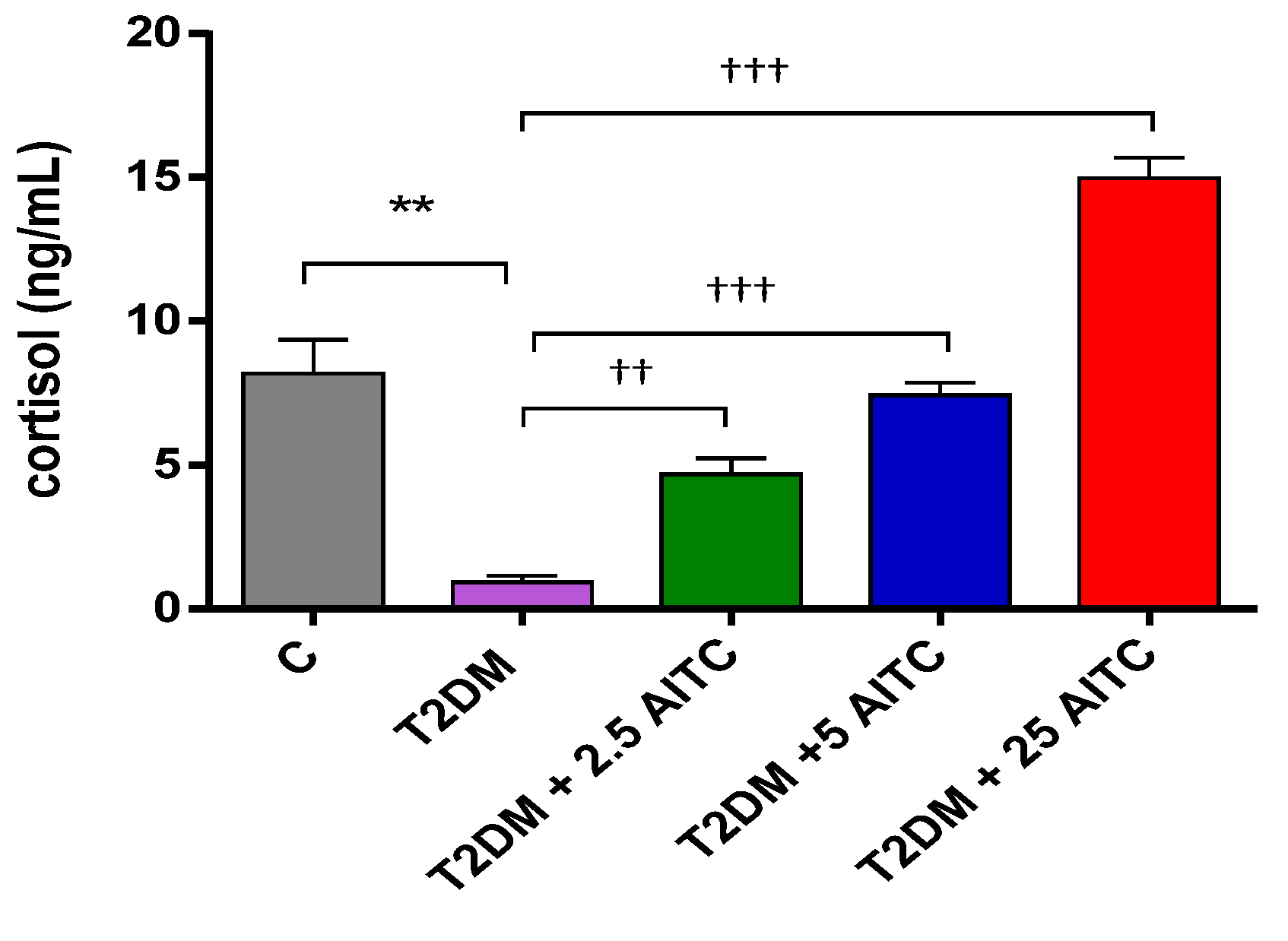
2.4. Effects of AITC on Cortisol Levels in Diabetic Rats
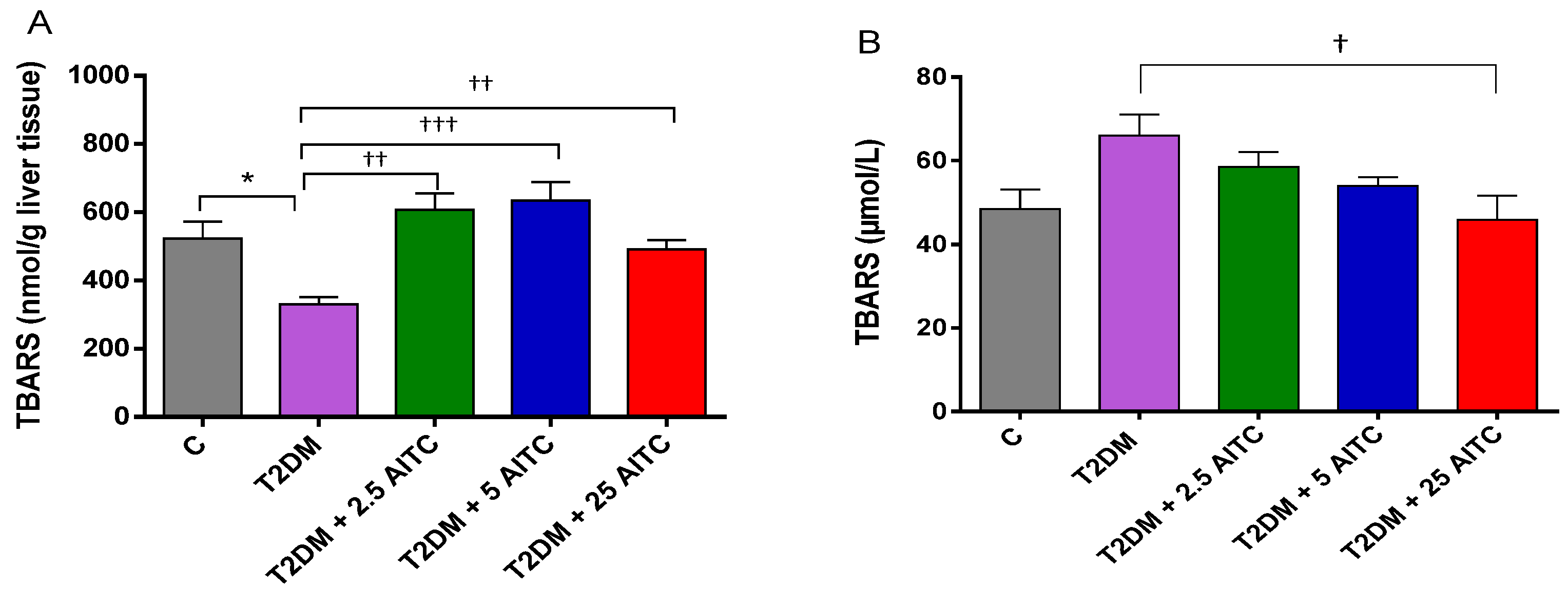
2.5. Effects of AITC on Lipid Peroxidation Marker in Diabetic Rats
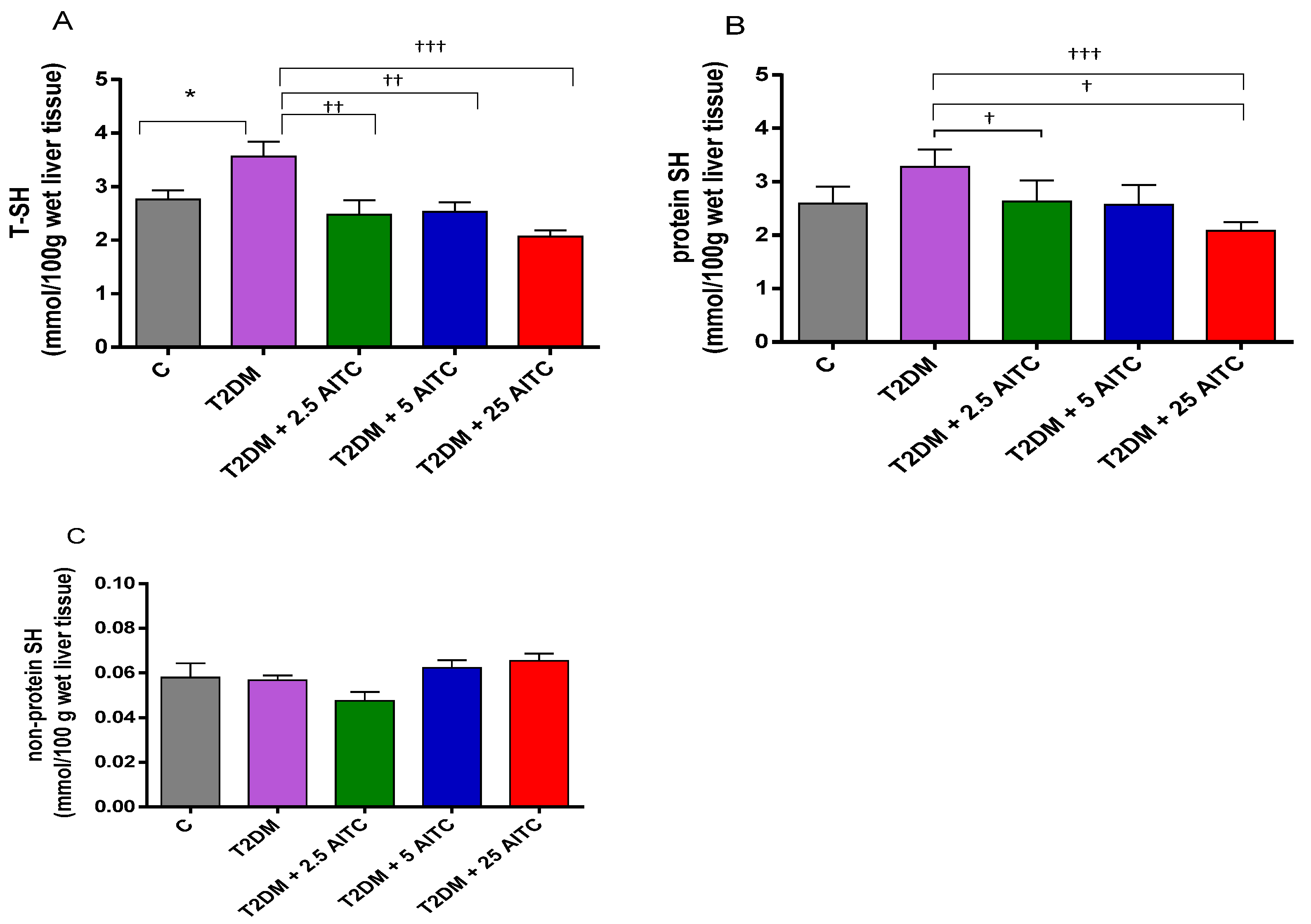
2.6. Effects of AITC on -SH Groups in Diabetic Rats
3. Discussion
4. Materials and Methods
4.1. STZ-Induced Experimental Diabetic Rats Fed a High-Fat Diet and Experimental Design
4.2. AITC Treatment
4.3. Data Collection
4.4. Trace Elements Determination in Tissues
4.5. Determination of Enzyme Activities
4.6. Determination of Proinflammatory Parameters
4.7. Measurements of Cortisol
4.8. Measurements of Lipid Peroxidation
4.9. Measurements of the Content of Total, Protein-Bound, and Non-Protein Sulfhydryl Groups
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ahmed, M.; Khan, K.R.; Ahmad, S.; Aati, H.Y.; Ovatlarnporn, C.; Rehman, M.S.; Javed, T.; Khursheed, A.; Ghalloo, B.A.; Dilshad, R.; et al. Comprehensive phytochemical profiling, biological activities, and molecular docking studies of Pleurospermum candollei: An insight into potential for natural products development. Molecules 2022, 27, 4113. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Farhan, A.; Asad, M.I.; Khan, M.R.; Hassan, S.S.; Haq, I.; Bungau, S. An extensive pharmacological evaluation of new anti-cancer triterpenoid (Nummularic Acid) from Ipomoea batatas through in vitro, in silico, and in vivo studies. Molecules 2022, 27, 2474. [Google Scholar] [CrossRef] [PubMed]
- Huneif, M.A.; Alqahtani, S.M.; Abdulwahab, A.; Almedhesh, S.A.; Mahnashi, M.H.; Riaz, M.; Rahman, N.U.; Jan, M.S.; Ullah, F.; Aasim, M.; et al. α-glucosidase, α-amylase and antioxidant evaluations of isolated bioactives from wild strawberry. Molecules 2022, 27, 3444. [Google Scholar] [CrossRef] [PubMed]
- White, J.R. A brief history of the development of diabetes medications. Diabetes Spectr. 2014, 27, 82–86. [Google Scholar] [CrossRef]
- Nakamura, T.; Abe-Kanoh, N.; Nakamura, Y. Physiological relevance of covalent protein modification by dietary isothiocyanates. J. Clin. Biochem. Nutr. 2018, 62, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Boesch-Saadatmandi, C.; Dose, J.; Schultheiss, G.; Rimbach, G. Anti-inflammatory potential of allyl-isothiocyanate –role of Nrf2, NF-κB and microRNA-155. J. Cell Mol. Med. 2012, 16, 836–843. [Google Scholar] [CrossRef]
- Gründemann, C.; Huber, R. Chemoprevention with isothiocyanates - From bench to bedside. Cancer Lett. 2018, 414, 26–33. [Google Scholar] [CrossRef]
- Caglayan, B.; Kilic, E.; Dalay, A.; Altunay, S.; Tuzcu, M.; Erten, F.; Orhan, C.; Gunal, M.Y.; Yulug, B.; Juturu, V.; et al. Allyl isothiocyanate attenuates oxidative stress and inflammation by modulating Nrf2/HO-1 and NF-κB pathways in traumatic brain injury in mice. Mol. Biol. Rep. 2019, 46, 241–250. [Google Scholar] [CrossRef]
- Chang, W.J.; Chen, B.H.; Inbaraj, B.S.; Chien, J.T. Preparation of allyl isothiocyanate nanoparticles, their anti-inflammatory activity towards RAW 264.7 macrophage cells and anti-proliferative effect on HT1376 bladder cancer cells. J. Sci. Food Agric. 2019, 99, 3106–3116. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, A.; Khan, I. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar]
- Rosa, F.T.; Freitas, E.C.; Deminice, R.; Jordão, A.A.; Marchini, J.S. Oxidative stress and inflammation in obesity after taurine supplementation: A double-blind, placebo-controlled study. Eur. J. Nutr. 2014, 53, 823–830. [Google Scholar] [CrossRef]
- de Souza, C.G.; da Motta, L.L.; de Assis, A.M.; Rech, A.; Bruch, R.; Klamt, F.; Souza, D.O. Sulforaphane ameliorates the insulin responsiveness and the lipid profile but does not alter the antioxidant response in diabetic rats. Food Funct. Apr. 2016, 7, 2060–2065. [Google Scholar] [CrossRef]
- Prawan, A.; Saw, C.L.L.; Khor, T.O.; Keum, Y.-S.; Yu, S.; Hu, L.; Kong, A.N. Anti-NF-κB and anti-inflammatory activities of synthetic isothiocyanates: Effect of chemical structures and cellular signaling. Chem. Biol. Interact. 2009, 179, 202–211. [Google Scholar] [CrossRef]
- Ugolini, L.; Righetti, L.; Carbone, K.; Paris, R.; Malaguti, L.; Di Francesco, A.; Micheli, L.; Paliotta, M.; Mari, M.; Lazzeri, L. Postharvest application of brassica meal-derived allyl-isothiocyanate to kiwifruit: Effect on fruit quality, nutraceutical parameters and physiological response. J. Food Sci. Technol. 2017, 54, 751–760. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Z.; Zhang, X.; Wu, H. Transcriptomic alterations in Sitophilus zeamais in response to allyl isothiocyanate fumigation. Pestic. Biochem. Physiol. 2017, 137, 62–70. [Google Scholar] [CrossRef]
- Øverby, A.; Stokland, R.A.; Ǻsberg, S.E.; Sporsheim, B.; Bones, A.M. Allyl isothiocyanate depletes glutathione and upregulates expression of glutathione S-transferases in Arabidopsis thaliana. Front. Plant. Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Leung, F.Y. Trace elements that act as antioxidants in parenteral micronutrition. J. Nutr. Biochem. 1998, 9, 304–307. [Google Scholar] [CrossRef]
- Król, E.; Jeszka-Skowron, M.; Krejpcio, Z.; Flaczyk, E.; Wójciak, R.W. The effects of supplementary mulberry leaf (Morus alba) extracts on the trace element status (Fe, Zn and Cu) in relation to diabetes management and antioxidant indices in diabetic rats. Biol. Trace Elem. Res. 2016, 174, 158–165. [Google Scholar] [CrossRef]
- Król, E.; Krejpcio, Z.; Chmurzynska, A. Folic acid and protein content in maternal diet and postnatal high-fat feeding affect the tissue levels of iron, zinc, and copper in the rat. Biol. Trace Elem. Res. 2011, 144, 885–893. [Google Scholar] [CrossRef]
- Salgueiro, M.J.; Krebs, N.; Zubillaga, M.B.; Weill, R.; Postaire, E.; Lysionek, A.E.; Caro, R.A.; de Paoli, T.; Hager, A.; Boccio, J. Zinc and Diabetes Mellitus. Is there a need of zinc supplementation in diabetes mellitus patients? Biol. Trace Elem. Res. 2011, 81, 215–228. [Google Scholar] [CrossRef]
- Guo, C.H.; Chen, P.C.; Yeh, M.S.; Hsiung, D.Y.; Wang, C.L. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin. Biochem. 2011, 44, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Okulicz, M.; Hertig, I.; Szkudelski, T. Differentiated effects of allyl isothiocyanate in diabetic rats: From toxic to beneficial action. Toxins 2022, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Kim, J.; Bang, H.; Moon, J.; Kim, G.O.; Shin, T. Hepatoprotective effects of allyl isothiocyanate against carbon tetrachloride-induced hepatotoxicity in rats. Chem. Biol. Interact. 2016, 254, 102–108. [Google Scholar] [CrossRef]
- Waz, S.; Matouk, A.I. Cardioprotective effect of allyl isothiocyanate in a rat of doxorubicin acute toxicity. Toxicol. Mechan. Methods. 2022, 32, 194–203. [Google Scholar] [CrossRef]
- Manesh, C.; Kuttan, G. Effect of Naturally Occurring Isothiocyanates on the Immune System. Immunopharmacol. Immunotoxicol. 2003, 25, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Urig, S.; Koncarevic, S.; Wu, X.; Fischer, M.; Rahlfs, S.; Mersch-Sundermann, V.; Becker, K. Glutathione- and thioredoxin-related enzymes are modulated by sulfur-containing chemopreventive agents. Biol. Chem. 2007, 388, 1069–1081. [Google Scholar] [CrossRef]
- Kawakishi, S.; Kaneko, T. Interaction of oxidized glutathione with allyl isothiocyanate. Phytochemistry 1985, 24, 715–718. [Google Scholar] [CrossRef]
- Bertoti, R.; Vasas, G.; Gonda, S.; Nguyen, N.M.; Szoke, E.; Jakab, A.; Pocsi, I.; Emri, T. Glutathione protects Candida albicans against volatile horseradish oil. J. Basic Microbiol. 2016, 56, 1071–1079. [Google Scholar] [CrossRef]
- Ogunyinka, B.I.; Oyinloye, B.E.; Osunsanmi, F.O.; Kolanisi, U.; Opoku, A.R.; Kappo, A.P. Protein isolate from Parkia biglobosa seeds improves dyslipidaemia and cardiac oxidative Stress in streptozotocin-induced diabetic rats. Antioxidants 2019, 8, 481. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, T.; Qin, G.; Li, B.; Tian, S. Synergistic action of antioxidative systems contributes to the alleviation of senescence in kiwifruit. Postharvest Biol. Technol. 2016, 111, 15–24. [Google Scholar] [CrossRef]
- Łukaszewicz-Hussain, A.; Moniuszko-Jakoniuk, J. Liver catalase, glutathione peroxidase and reductase activity, reduced glutathione and hydrogen peroxide levels in acute intoxication with chlorfenvinphos, an organophosphate Insecticide. Pol. J. Environ. Stud. 2004, 13, 303–309. [Google Scholar]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Reece, J.M.; Deeney, J.T.; Andersen, M.E.; Corkey, B.E.; et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef]
- Sahin, N.; Orhan, C.; Erten, F.; Tuzcu, M.; Defo Deeh, P.B.; Ozercan, I.H.; Juturu, V.; Kazim, S. Effects of allyl isothiocyanate on insulin resistance, oxidative stress status, and transcription factors in high-fat diet/streptozotocin-induced type 2 diabetes mellitus in rats. J. Biochem. Mol. Toxicol. 2019, 33, e22328. [Google Scholar] [CrossRef]
- Jakubikova, J.; Sedlak, J.; Bod’o, J.; Bao, Y. Effect of isothiocyanates on nuclear accumulation of NF-kappaB, Nrf2, and thioredoxin in caco-2 cells. J. Agric. Food Chem. 2006, 54, 1656–1662. [Google Scholar] [CrossRef]
- Fourquet, S.; Guerois, R.; Biard, D.; Toledano, M.B. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 2010, 285, 8463–8471. [Google Scholar] [CrossRef]
- Flaherty, R.L.; Owen, M.; Fagan-Murphy, A.; Intabli, H.; Healy, D.; Patel, A.; Allen, M.C.; Patel, B.A.; Flint, M.S. Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer. Breast Cancer Res. 2017, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, Z.; Cao, J.; Cui, H.; Ma, Z. Cold stress increases reactive oxygen species formation via TRPA1 activation in A549 cells. Cell Stress Chaperones 2016, 21, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Esteve, M. Mechanisms underlying biological effects of cruciferous glucosinolate-derived isothiocyanates/indoles: A Focus on metabolic syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, C.G.; Rhee, D.K.; Um, S.H.; Pyo, S. Sinigrin inhibits production of inflammatory mediators by suppressing NF-κB/MAPK pathways or NLRP3 inflammasome activation in macrophages. Int. Immunopharmacol. 2017, 45, 163–173. [Google Scholar] [CrossRef]
- Mazumder, A.; Dwivedi, A.; du Plessis, J. Sinigrin and its therapeutic benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Venkatesan, R.; Kim, S.Y. Neuroprotective and anti-inflammatory activities of allyl isothiocyanate through attenuation of JNK/NK-κB/TNF-α signalling. Int. J. Mol. Sci. 2017, 18, 1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajakumar, T.; Pugalendhi, P.; Jayaganesh, R.; Ananthakrishan, D.; Gunasekaran, K. Effect of allyl isothiocyanate on NFκβ-signaling in 7,12-dimethylbenz(a)anthracene and N-methyl-N-nitrosourea-induced mammary carcinogenesis. Breast Cancer 2018, 25, 50–59. [Google Scholar] [CrossRef]
- Li, C.-X.; Gao, J.G.; Wan, X.Y.; Chen, Y.; Xu, C.-F.; Feng, Z.M.; Zeng, H.; Lin, Y.M.; Ma, H.; Xu, P.; et al. Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J. Gastroenterol. 2019, 25, 5120–5133. [Google Scholar] [CrossRef] [PubMed]
- Król, E.; Bogdański, P.; Suliburska, J.; Krejpcio, Z. The relationship between dietary, serum and hair levels of minerals (Fe, Zn, Cu) and glucose metabolism indices in obese type 2 diabetic patients. Biol. Trace Elem. Res. 2019, 189, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Bulska, E.; Ruszczyńska, A. Analytical techniques for trace element determination. Physical. Sci. Rev. 2017, 2, 20178002. [Google Scholar]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Elman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
| C | T2DM | T2DM + 2.5 AITC | T2DM + 5 AITC | T2DM + 25 AITC | |
|---|---|---|---|---|---|
| Liver | |||||
| Fe2+ | 373.30 ± 23.66 | 472.90 ± 20.80 ** | 400.10 ± 12.06 † | 549.50 ± 11.72 † | 439.50 ± 12.94 |
| Cu2+ | 8.35 ± 0.61 | 5.63 ± 0.53 ** | 4.70 ± 0.32 | 6.01 ± 0.58 | 6.15 ± 0.50 |
| Zn2+ | 86.14 ± 6.29 | 78.08 ± 2.73 | 64.13 ± 2.71 † | 81.35 ± 2.37 | 85.11 ± 6.27 |
| Cu2+/Zn2+ | 0.1126 ± 0.01 | 0.07457 ± 0.01 | 0.07336 ± 0.004 | 0.07422 ± 0.01 | 0.07957± 0.01 |
| Kidney | |||||
| Fe2+ | 220.10 ± 32.95 | 184.00 ± 12.81 | 232.20 ± 26.66 | 238.30 ± 10.03 | 229.70 ± 36.73 |
| Cu2+ | 26.63 ± 1.69 | 25.61 ± 2.39 | 23.26 ± 3.32 | 20.74 ± 0.88 | 18.90 ± 1.43 |
| Zn2+ | 99.61 ± 6.53 | 117.10 ± 6.90 | 115.20 ± 8.38 | 95.04 ± 3.17 | 93.14 ± 4.18 |
| Cu2+/Zn2+ | 0.2743 ± 0.02 | 0.2154 ± 0.01 | 0.1984 ± 0.02 | 0.2209 ± 0.01 | 0.2057 ± 0.02 |
| Spleen | |||||
| Fe2+ | 2919 ± 230.50 | 2161 ± 255.80 | 2393 ± 400.70 | 2557± 214.00 | 2594 ± 368.30 |
| Cu2+ | 6.621 ± 0.47 | 7.476 ± 0.54 | 4.929 ± 0.23 †† | 5.063 ± 0.38 † | 7.199 ± 0.72 |
| Zn2+ | 73.75 ± 2.64 | 75.36 ± 2.95 | 86.34 ± 7.47 | 88.95 ± 4.35 | 84.12 ± 5.97 |
| Cu2+/Zn2+ | 0.09004 ± 0.01 | 0.1013 ± 0.01 | 0.06034 ± 0.01 | 0.05649 ± 0.003 †† | 0.09156 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okulicz, M.; Hertig, I.; Król, E.; Szkudelski, T. Effects of Allyl Isothiocyanate on Oxidative and Inflammatory Stress in Type 2 Diabetic Rats. Molecules 2022, 27, 5568. https://doi.org/10.3390/molecules27175568
Okulicz M, Hertig I, Król E, Szkudelski T. Effects of Allyl Isothiocyanate on Oxidative and Inflammatory Stress in Type 2 Diabetic Rats. Molecules. 2022; 27(17):5568. https://doi.org/10.3390/molecules27175568
Chicago/Turabian StyleOkulicz, Monika, Iwona Hertig, Ewelina Król, and Tomasz Szkudelski. 2022. "Effects of Allyl Isothiocyanate on Oxidative and Inflammatory Stress in Type 2 Diabetic Rats" Molecules 27, no. 17: 5568. https://doi.org/10.3390/molecules27175568
APA StyleOkulicz, M., Hertig, I., Król, E., & Szkudelski, T. (2022). Effects of Allyl Isothiocyanate on Oxidative and Inflammatory Stress in Type 2 Diabetic Rats. Molecules, 27(17), 5568. https://doi.org/10.3390/molecules27175568






