Recent Advances in Photocatalytic Oxidation of Methane to Methanol
Abstract
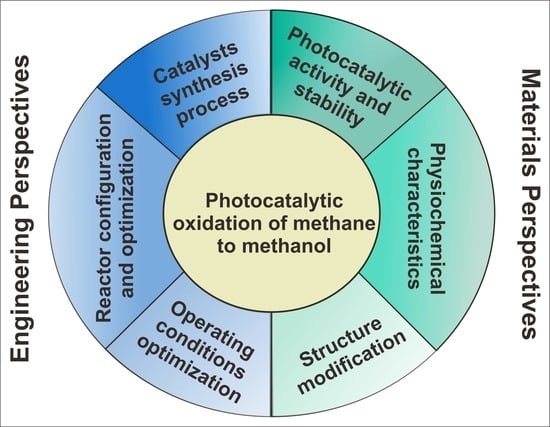
:1. Introduction
2. Heterogeneous Photocatalyst for Photo-Oxidation Methane to Methanol
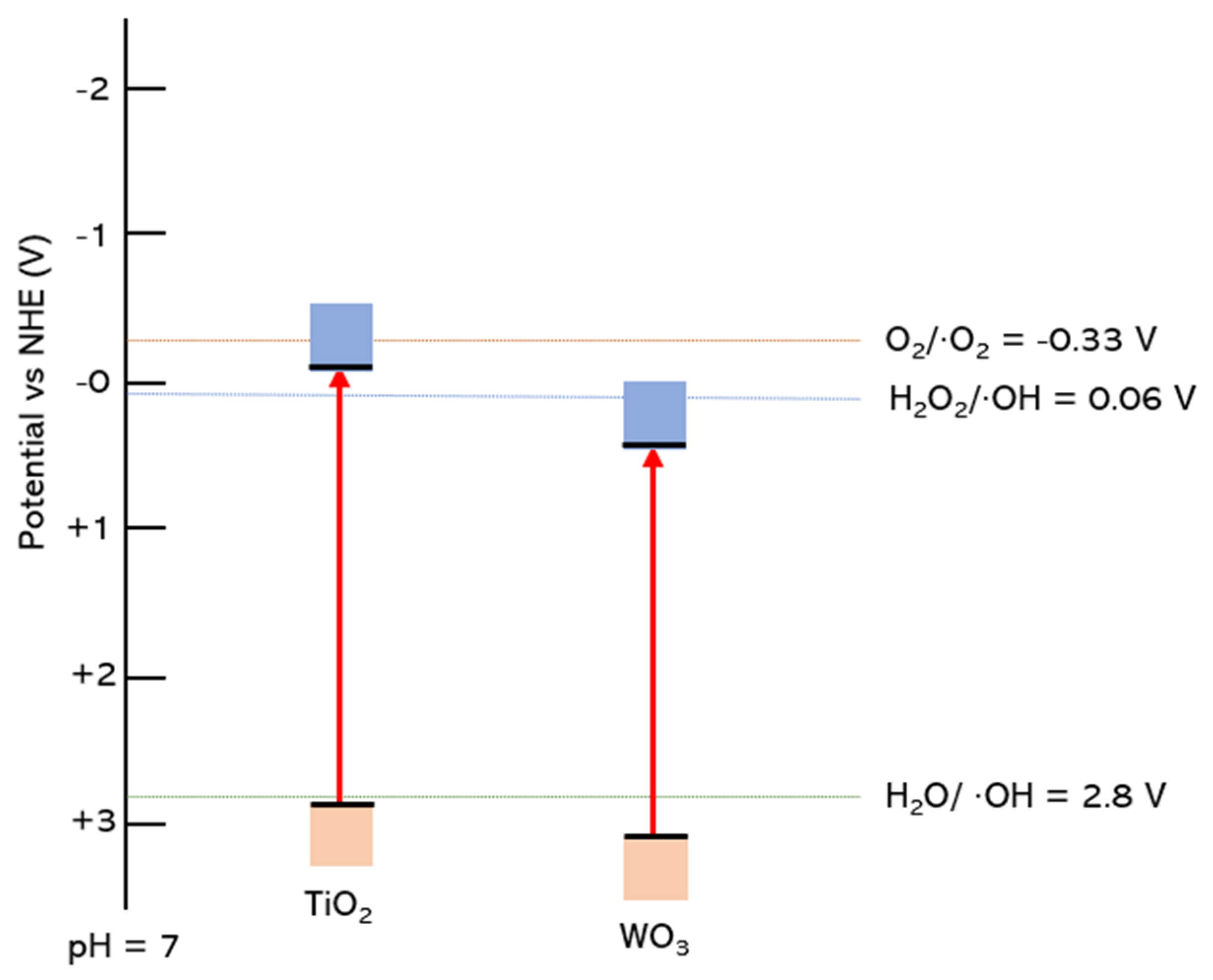
2.1. Thermodynamics for Methane to Methanol Conversion
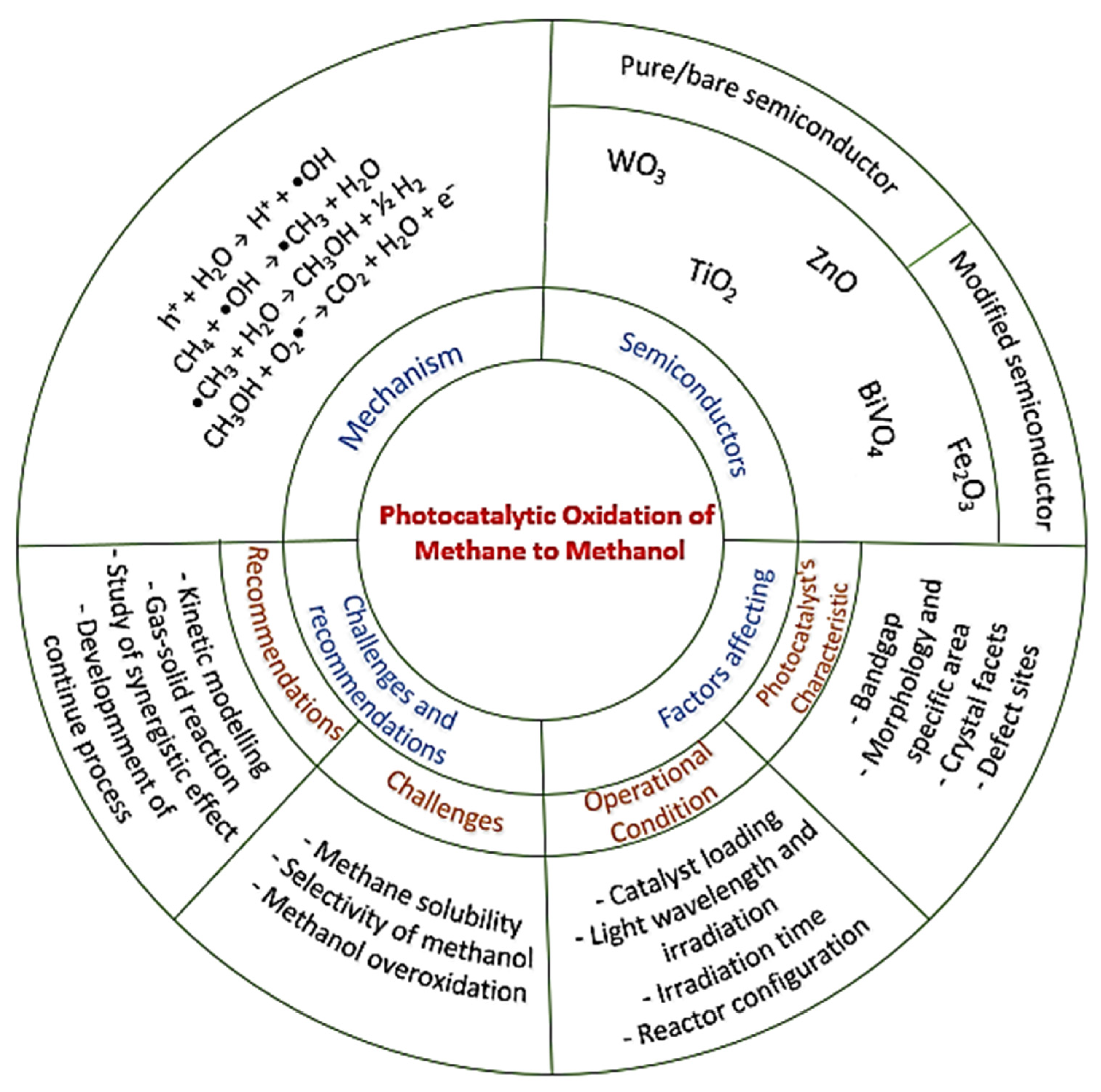
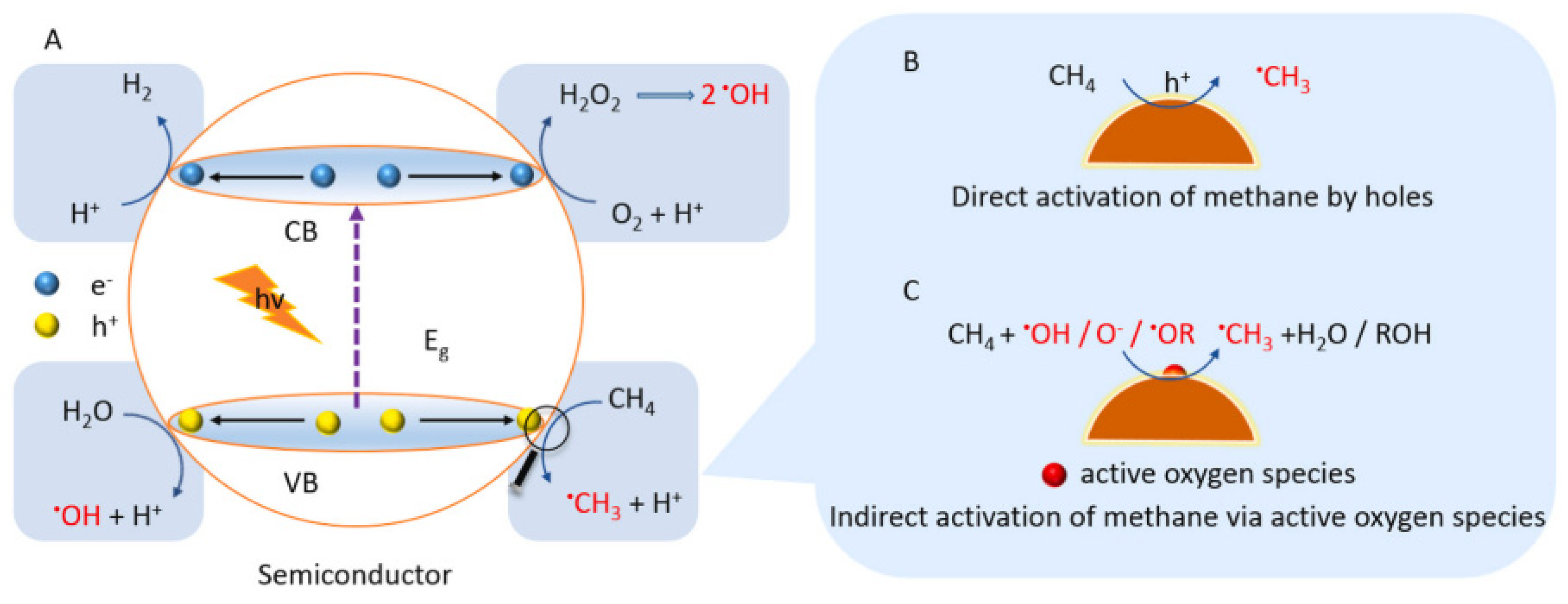
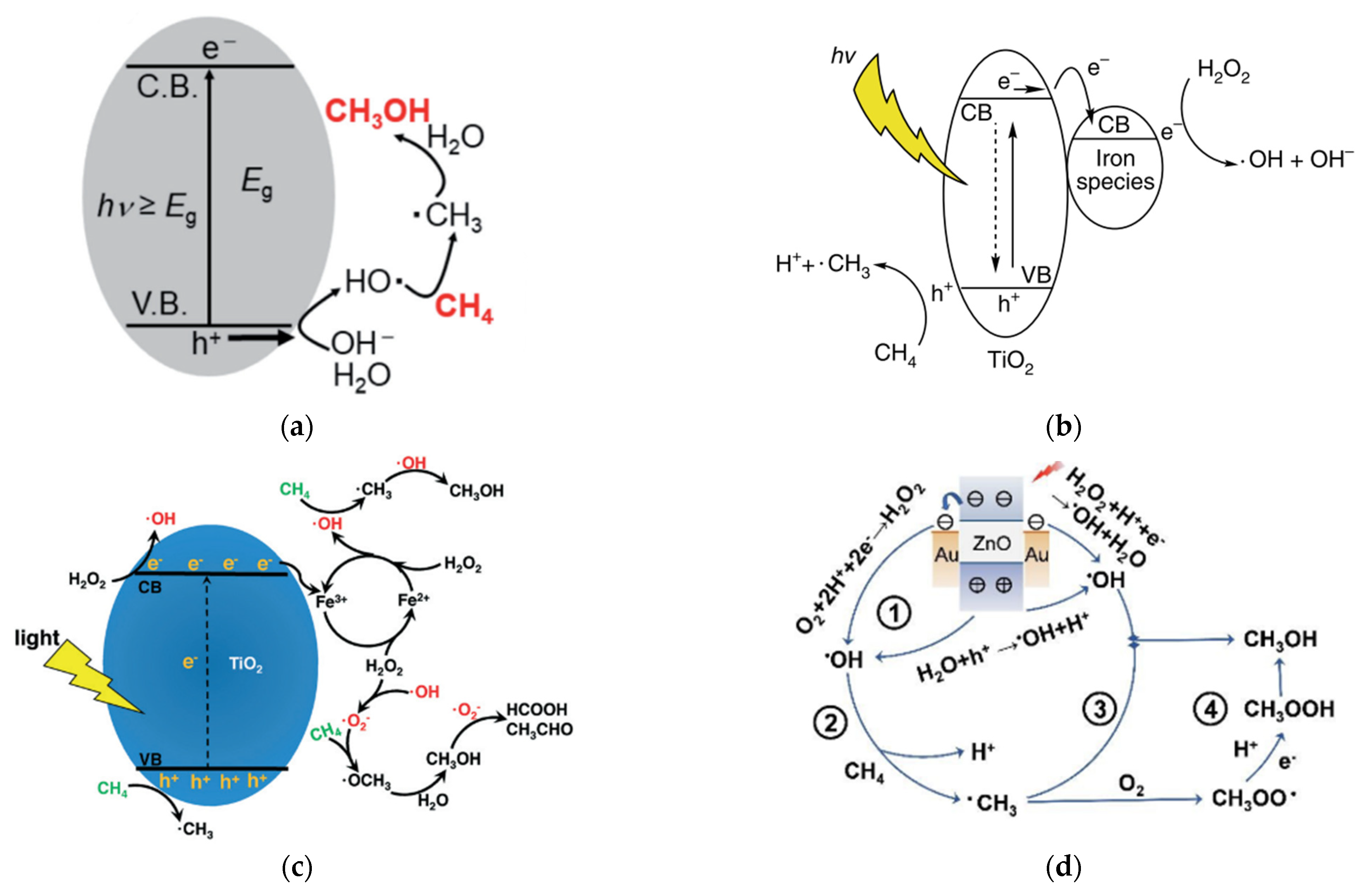
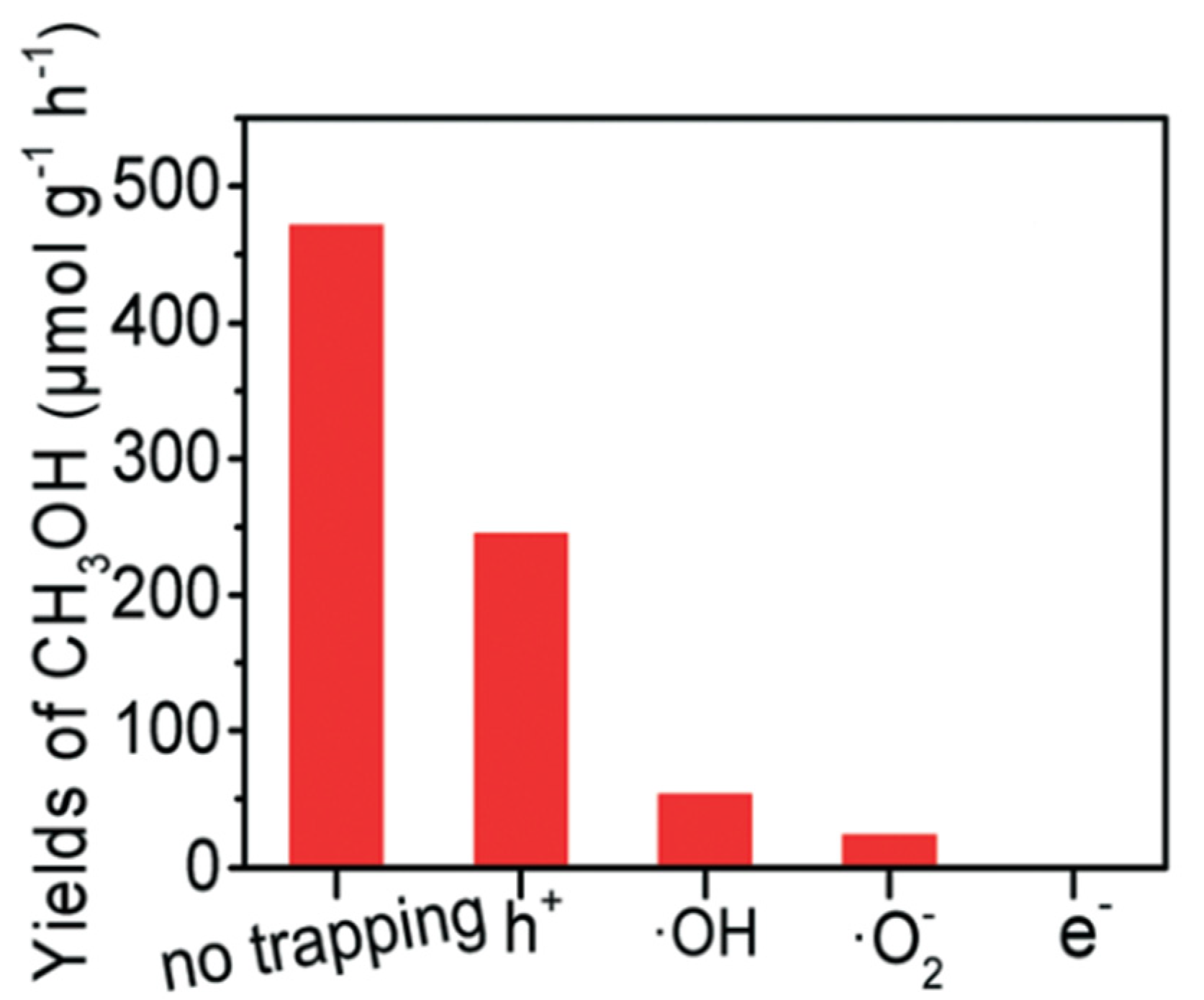
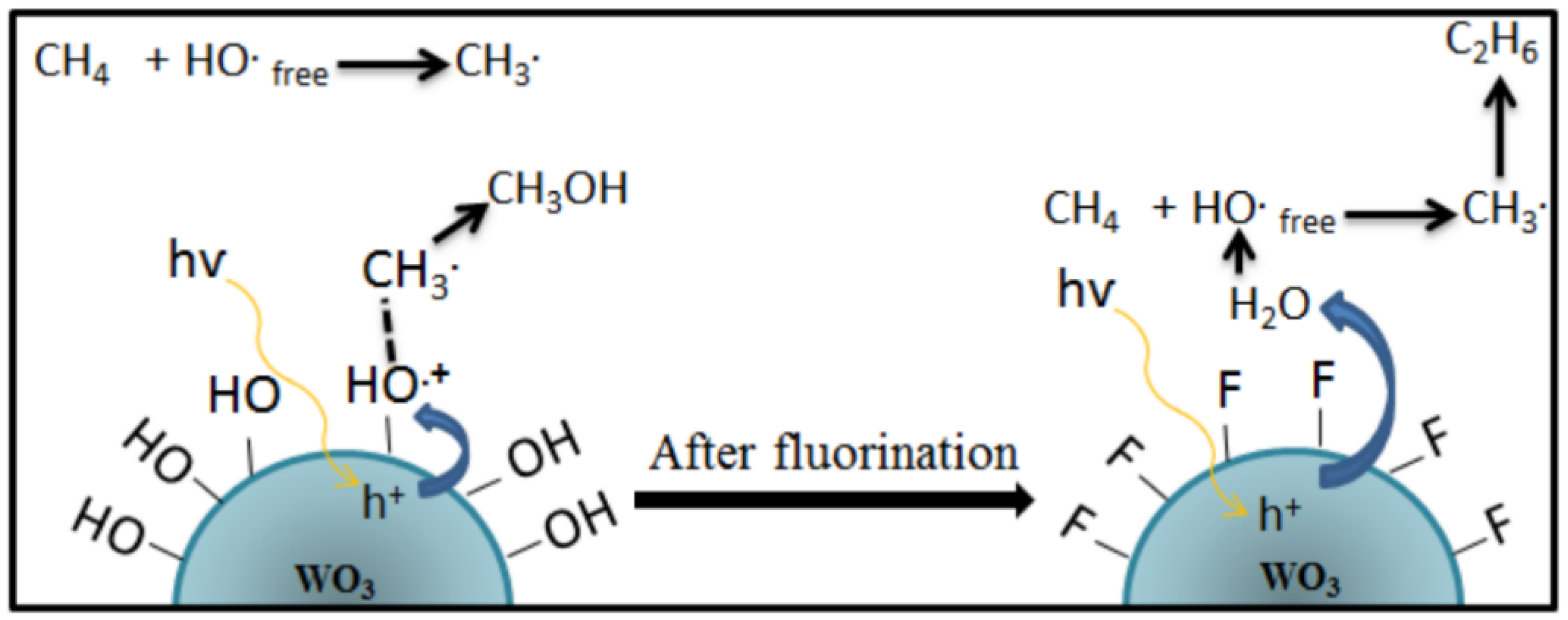
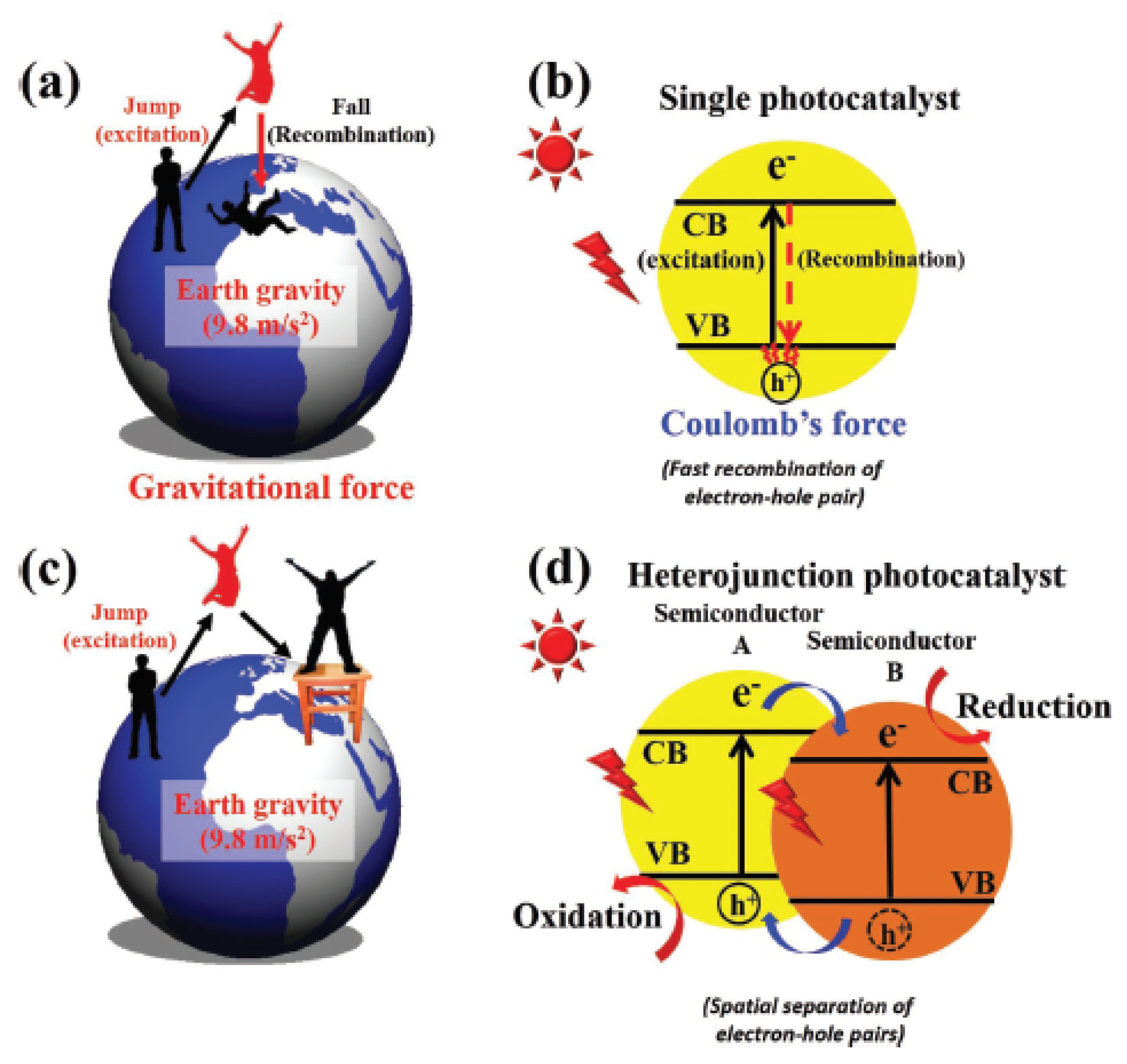
2.2. Proposed Mechanism of Photocatalytic Conversion of Methane to Methanol
- The formation of superoxide radicals (•O2−) produced via the reduction of O2 in CB of semiconductor [23], shown in Equations (12)–(14).
- 2.
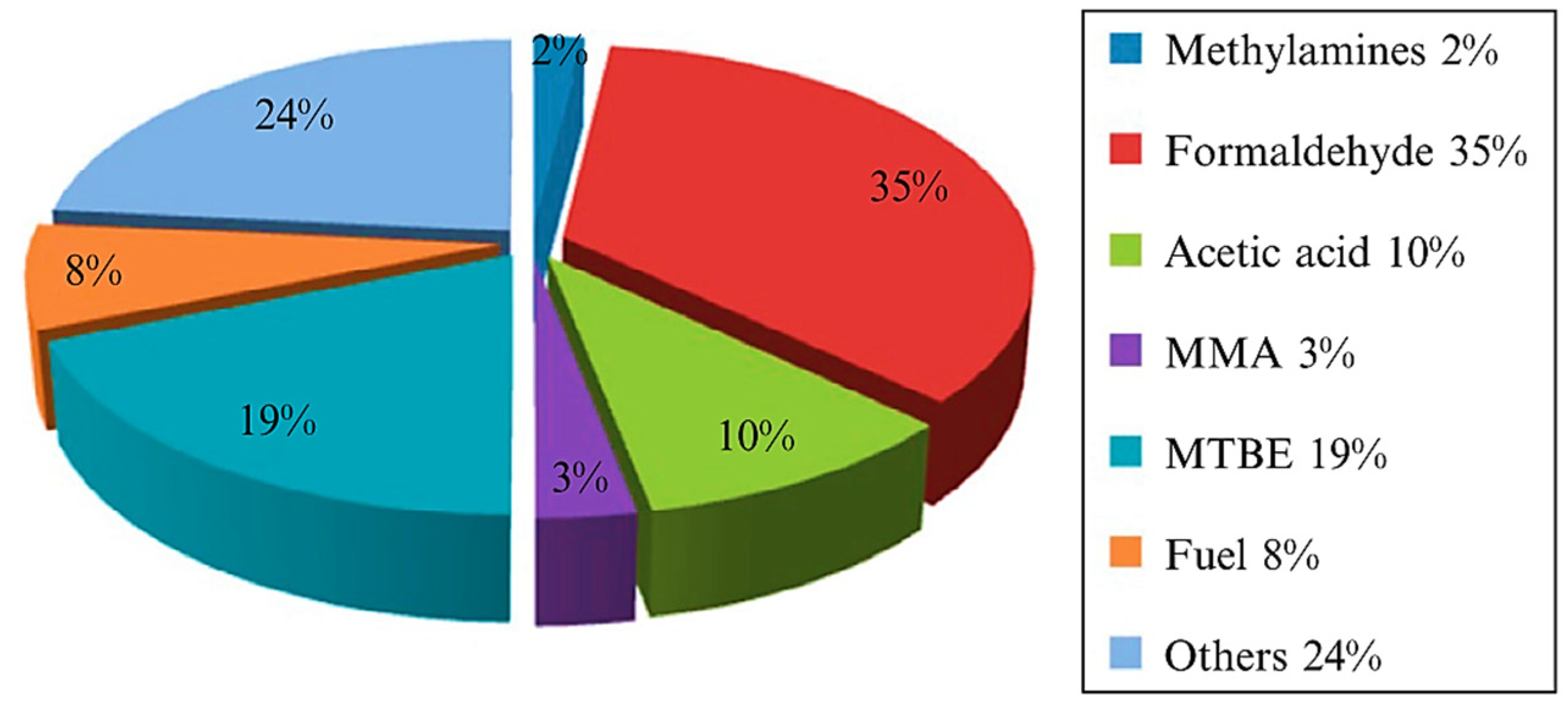
- A sufficient amount of methanol generation either competes with water to further react with a photogenerated hole in VB or direct interaction with •OH to produce side products such as formaldehyde [32], as shown in Equations (15)–(17)
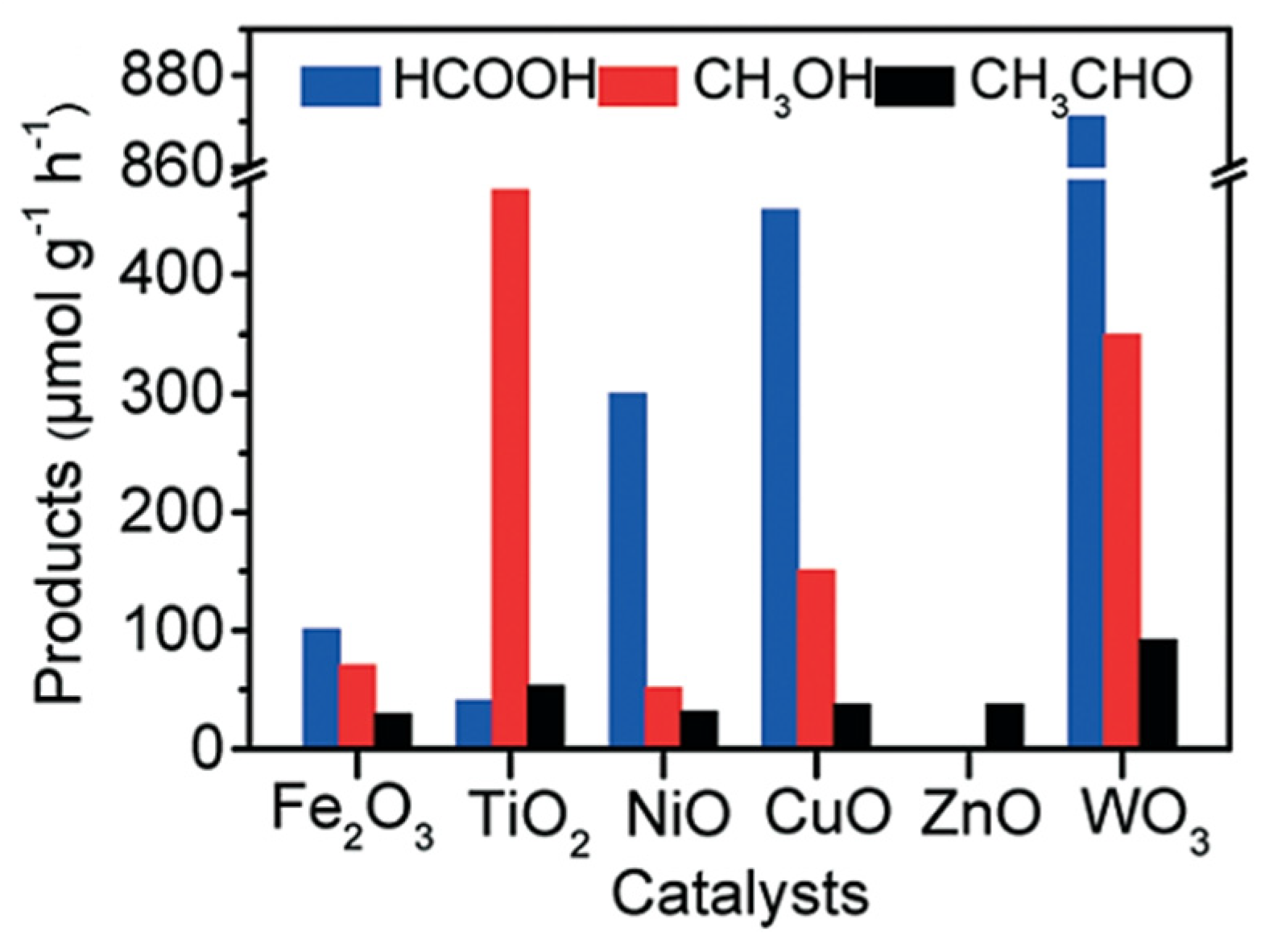
3. Selection of Materials for Photocatalytic Methane to Methanol Conversion
3.1. Pure Semiconductor
3.2. Modified Semiconductor
3.2.1. Metal/Non-Metal Doping
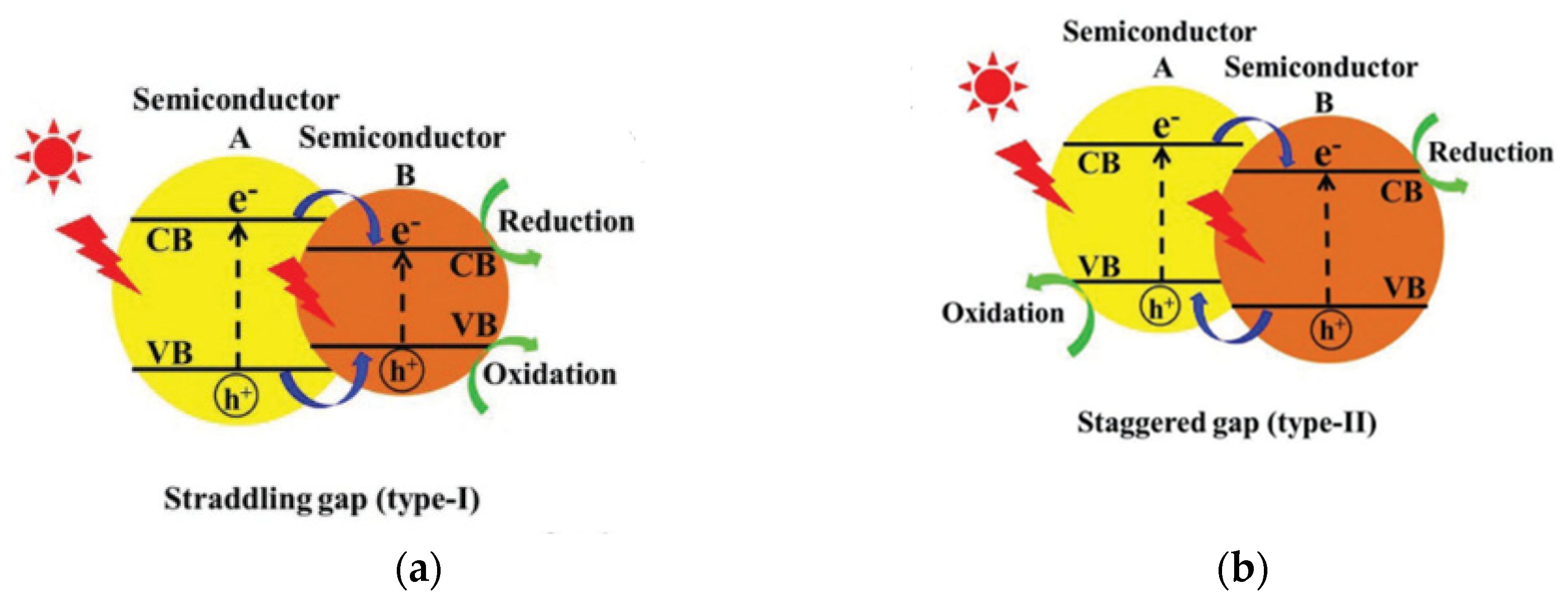
3.2.2. Heterojunction
3.2.3. Structure Modification
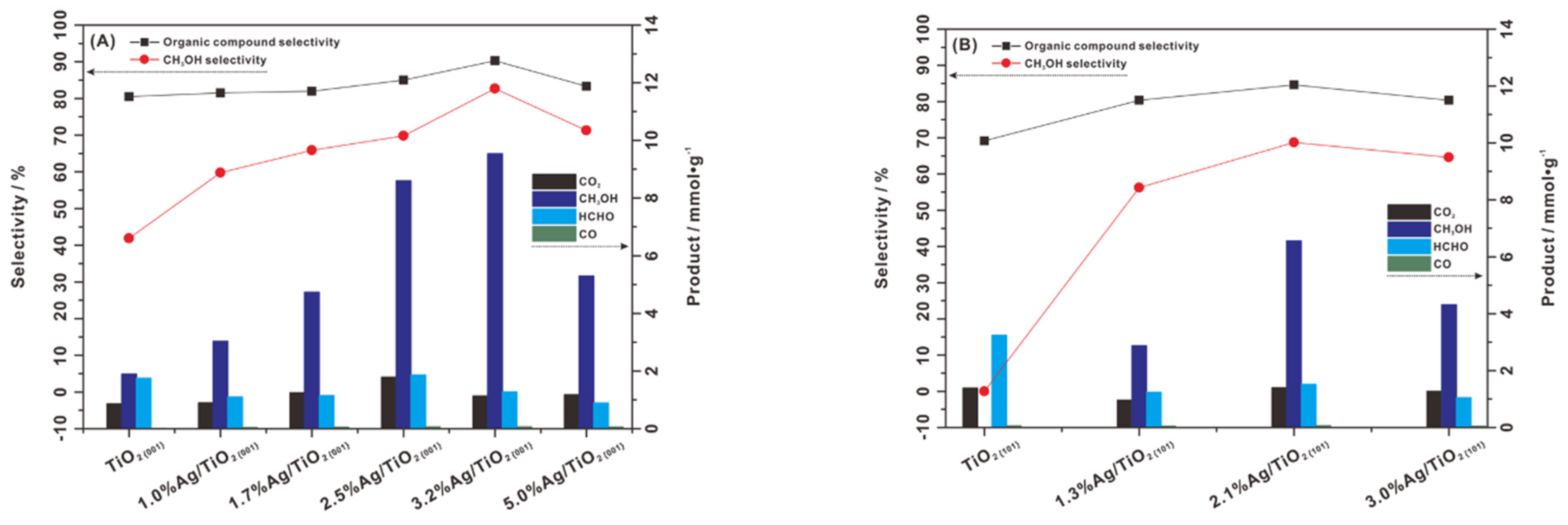
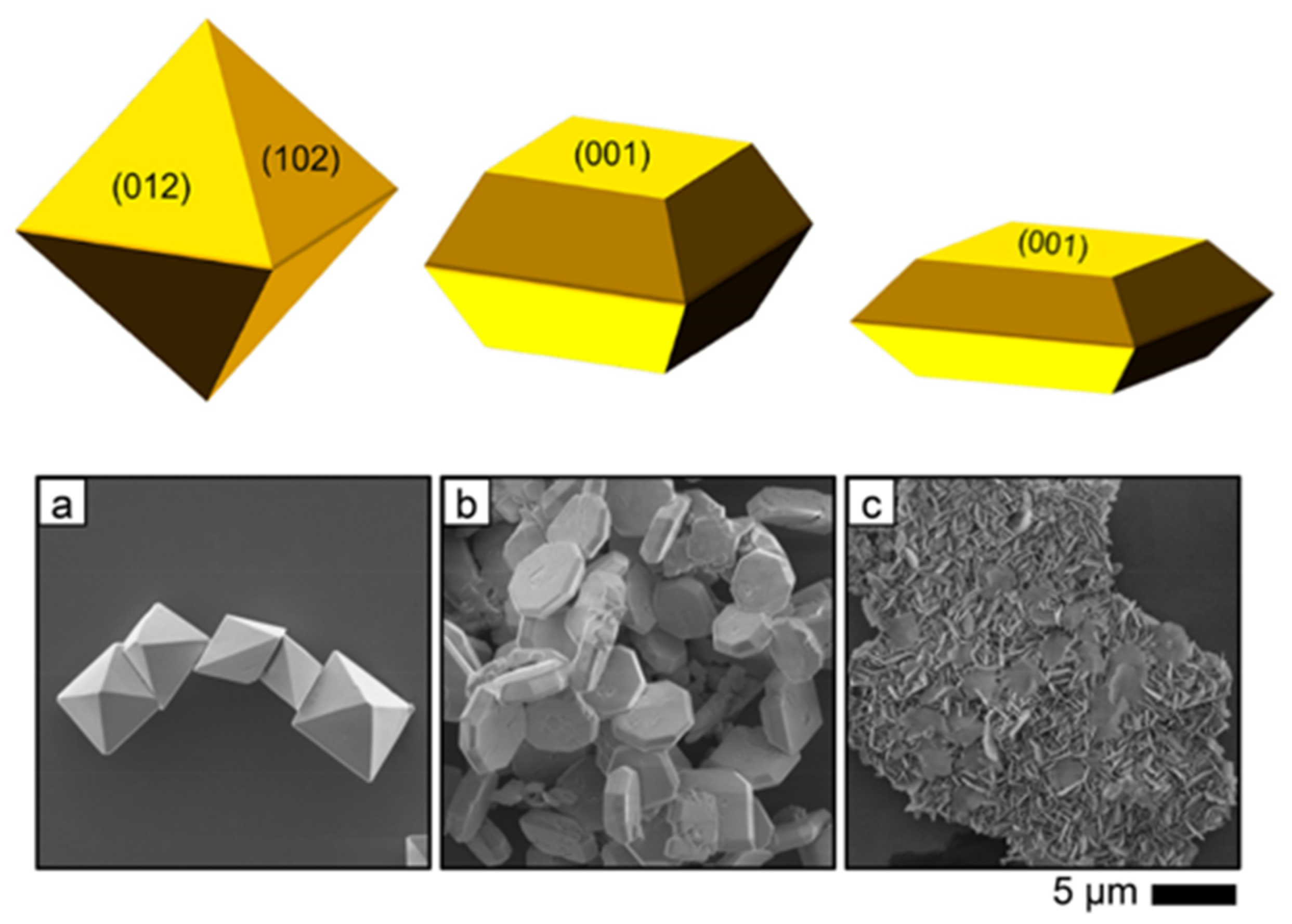
3.2.4. Crystal Facet Re-Arrangements
3.2.5. Electron Scavenger
4. Factors Affecting Photocatalytic Methane Oxidation to Methanol
4.1. Physiochemical Characteristics of Photocatalyst
4.1.1. Band-Gap
4.1.2. Morphology and Specific Surface Area
4.1.3. Crystal Facets
4.1.4. Defect Sites
4.2. Operational Condition
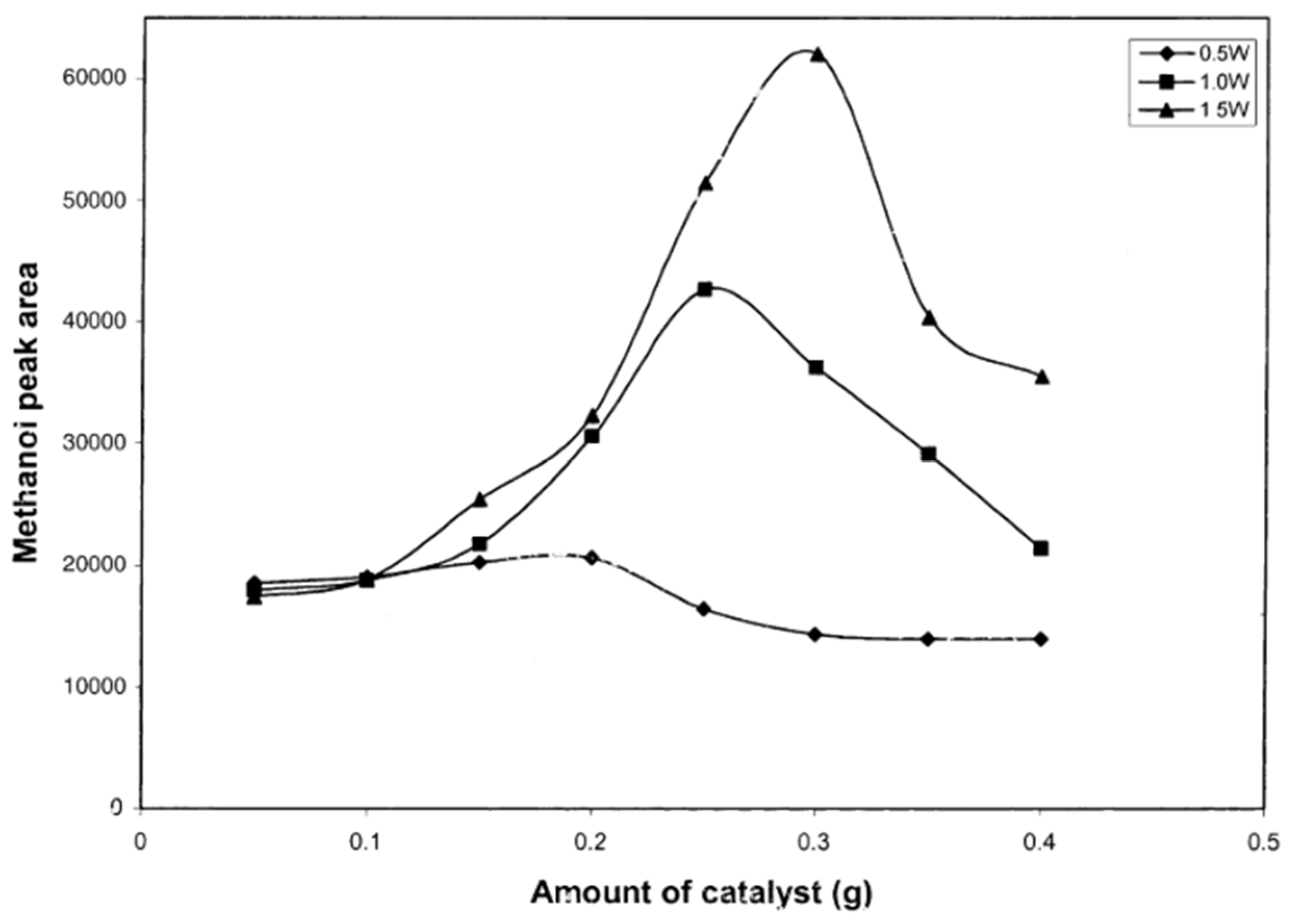
4.2.1. Catalyst Loading
4.2.2. Light Wavelength and Intensity
4.2.3. Irradiation Time
4.2.4. Reactor Configuration
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Environmental Protection Agency. Understanding Global Warming Potentials. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 5 June 2022).
- Song, H.; Meng, X.; Wang, Z.J.; Liu, H.; Ye, J. Solar-Energy-Mediated Methane Conversion. Joule 2019, 3, 1606–1636. [Google Scholar] [CrossRef]
- Holmen, A. Direct conversion of methane to fuels and chemicals. Catal. Today 2009, 142, 2–8. [Google Scholar] [CrossRef]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Methanol Production and Applications: An Overview. In Methanol; Elsevier B.V.: Amsterdam, The Netherlands, 2018; pp. 3–28. [Google Scholar] [CrossRef]
- Sharma, R.; Poelman, H.; Marin, G.B.; Galvita, V.V. Approaches for selective oxidation of methane to methanol. Catalysts 2020, 10, 194. [Google Scholar] [CrossRef]
- Lunsford, J.H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century. Catal. Today 2000, 63, 165–174. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Zu, Q. Recent Progress in Direct Partial Oxidation of Methane to Methanol. Catal. Surv. Asia 2012, 16, 53–61. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Direct conversion technologies of methane to methanol: An overview. Renew. Sustain. Energy Rev. 2016, 65, 250–261. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Muraro, P.C.L.; Mortari, S.R.; Vizzotto, B.S.; Chuy, G.; dos Santos, C.; Brum, L.F.W.; da Silva, W.L. Iron oxide nanocatalyst with titanium and silver nanoparticles: Synthesis, characterization and photocatalytic activity on the degradation of Rhodamine B dye. Sci. Rep. 2020, 10, 3055. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Saputera, W.H.; Amri, A.F.; Devianto, H.; Sasongko, D. Photocatalytic Degradation of Palm Oil Mill Effluent (POME) Waste Using BiVO4 Based Catalysts. Molecules 2021, 26, 6225. [Google Scholar] [CrossRef] [PubMed]
- Saputera, W.H.; Tahini, H.A.; Lovell, E.C.; Tan, T.H.; Rawal, A.; Aguey-Zinsou, K.-F.; Friedmann, D.; Smith, S.C.; Amal, R.; Scott, J. Cooperative defect-enriched SiO2 for oxygen activation and organic dehydrogenation. J. Catal. 2019, 376, 168–179. [Google Scholar] [CrossRef]
- Le, A.T.; Pung, S.Y.; Sreekantan, S.; Matsuda, A.; Huynh, D.P. Mechanisms of removal of heavy metal ions by ZnO particles. Heliyon 2019, 5, e01440. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Naidoo, D.B. Photocatalytic degradation of oily waste and phenol from a local South Africa oil refinery wastewater using response methodology. Sci. Rep. 2020, 10, 8850. [Google Scholar] [CrossRef] [PubMed]
- Yuliati, L.; Yoshida, H. Photocatalytic conversion of methane. Chem. Soc. Rev. 2008, 37, 1592–1602. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic hydrogen production from methane: A review on recent progress and prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Ogura, K.; Kataoka, M. Photochemical conversion of methane. J. Mol. Catal. 1988, 43, 371–379. [Google Scholar] [CrossRef]
- Noceti, R.P.; Taylor, C.E.; D’Este, J.R. Photocatalytic conversion of methane. Catal. Today 1997, 33, 199–204. [Google Scholar] [CrossRef]
- Lin, X.-Y.; Li, J.-Y.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Methane conversion over artificial photocatalysts. Catal. Commun. 2021, 159, 106346. [Google Scholar] [CrossRef]
- Gondal, M.A.; Hameed, A.; Yamani, Z.H.; Arfaj, A. Photocatalytic transformation of methane into methanol under UV laser irradiation over WO3, TiO2 and NiO catalysts. Chem. Phys. Lett. 2004, 392, 372–377. [Google Scholar] [CrossRef]
- Villa, K.; Murcia-López, S.; Andreu, T.; Morante, J.R. On the role of WO3 surface hydroxyl groups for the photocatalytic partial oxidation of methane to methanol. Catal. Commun. 2015, 58, 200–203. [Google Scholar] [CrossRef]
- Negishi, Y.; Watanabe, S.; Aoki, M.; Hossain, S.; Kurashige, W. Toward the Creation of Highly Active Photocatalysts That Convert Methane into Methanol Active Photocatalysts. Antimicrob. Agents Chemother. 2019, 32, 1854–1858. [Google Scholar] [CrossRef]
- Xie, J.; Jin, R.; Li, A.; Bi, Y.; Ruan, Q.; Deng, Y.; Zhang, Y.; Yao, S.; Sankar, G.; Ma, D.; et al. Highly selective oxidation of methane to methanol at ambient conditions by titanium dioxide-supported iron species. Nat. Catal. 2018, 1, 889–896. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, H.C.; Wang, J.S.; Wu, X.Y.; Wang, S.L. Synergistic photocatalysis-Fenton reaction for selective conversion of methane to methanol at room temperature. Catal. Sci. Technol. 2020, 10, 2329–2332. [Google Scholar] [CrossRef]
- Zhou, W.; Qiu, X.; Jiang, Y.; Fan, Y.; Wei, S.; Han, D.; Niu, L.; Tang, Z. Highly selective aerobic oxidation of methane to methanol over gold decorated zinc oxide: Via photocatalysis. J. Mater. Chem. A 2020, 8, 13277–13284. [Google Scholar] [CrossRef]
- Gondal, M.A.; Hameed, A.; Suwaiyan, A. Photo-catalytic conversion of methane into methanol using visible laser. Appl. Catal. A 2003, 243, 165–174. [Google Scholar] [CrossRef]
- Villa, K.; Murcia-López, S.; Morante, J.R.; Andreu, T. An insight on the role of La in mesoporous WO3 for the photocatalytic conversion of methane into methanol. Appl. Catal. B Environ. 2016, 187, 30–36. [Google Scholar] [CrossRef]
- López-Martín, Á.; Caballero, A.; Colón, G. Photochemical methane partial oxidation to methanol assisted by H2O2. J. Photochem. Photobiol. A Chem. 2017, 349, 216–223. [Google Scholar] [CrossRef]
- Hameed, A.; Ismail, I.M.I.; Aslam, M.; Gondal, M.A. Photocatalytic conversion of methane into methanol: Performance of silver impregnated WO3. Appl. Catal. A Gen. 2014, 470, 327–335. [Google Scholar] [CrossRef]
- Augugliaro, V.; Palmisano, G.; Palmisano, L.; Soria, J. Heterogeneous photocatalysis and catalysis: An overview of their distinctive features. In Heterogeneous Photocatalysis; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 1–24. [Google Scholar]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Minerva Endocrinol. 2012, 37, 233–246. [Google Scholar] [CrossRef]
- Tian, Y.; Piao, L.; Chen, X. Research progress on the photocatalytic activation of methane to methanol. Green Chem. 2021, 23, 3526–3541. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nabi, G.; Rafique, M.; Khalid, N.R. Nanostructured-based WO3 photocatalysts: Recent development, activity enhancement, perspectives and applications for wastewater treatment. Int. J. Environ. Sci. Technol. 2017, 14, 2519–2542. [Google Scholar] [CrossRef]
- Villa, K.; Murcia-López, S.; Andreu, T.; Morante, J.R. Mesoporous WO3 photocatalyst for the partial oxidation of methane to methanol using electron scavengers. Appl. Catal. B Environ. 2015, 163, 150–155. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, W.; Xiong, F.; Yuan, Q.; Jin, Y.; Yang, J.; Huang, W. Active hydrogen species on TiO2 for photocatalytic H2 production. Phys. Chem. Chem. Phys. 2014, 16, 7051–7057. [Google Scholar] [CrossRef]
- Saputera, W.H.; Rizkiana, J.; Wulandari, W.; Sasongko, D. Role of defects on TiO2/SiO2 composites for boosting photocatalytic water splitting. RSC Adv. 2020, 10, 27713–27719. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Saputera, W.H.; Groenen, E.J.; Mul, G. A novel TiO2 composite for photocatalytic wastewater treatment. J. Catal. 2014, 310, 75–83. [Google Scholar] [CrossRef]
- Dubey, R.S.; Jadkar, S.R.; Bhorde, A.B. Synthesis and Characterization of Various Doped TiO2 Nanocrystals for Dye-Sensitized Solar Cells. ACS Omega 2021, 6, 3470–3482. [Google Scholar] [CrossRef]
- Nakata, K.; Ochiai, T.; Murakami, T.; Fujishima, A. Photoenergy conversion with TiO2 photocatalysis: New materials and recent applications. Electrochim. Acta 2012, 84, 103–111. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Tahir, M.B.; Rafique, M.; Fatima, N.; Israr, Z. Metal oxide- and metal sulfide-based nanomaterials as photocatalysts. In Nanotechnology and Photocatalysis for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–96. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Wu, X.; Ng, Y.H.; Saputera, W.H.; Wen, X.; Du, Y.; Dou, S.X.; Amal, R.; Scott, J. The Dependence of Bi2MoO6 Photocatalytic Water Oxidation Capability on Crystal Facet Engineering. ChemPhotoChem 2019, 3, 1246–1253. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Wang, Y.-F.; Li, Y.-Y.; Zhang, L.; Yao, H.-C.; Li, Z.-J. Enhanced photocatalytic CO2 reduction activity of Z-scheme CdS/BiVO4 nanocomposite with thinner BiVO4 nanosheets. J. CO2 Util. 2018, 28, 15–25. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Huang, W. Bismuth-based photocatalysts for solar energy conversion. J. Mater. Chem. A 2020, 8, 24307–24352. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconductor photocatalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef] [Green Version]
- Gharoy Ahangar, E.; Abbaspour-Fard, M.H.; Shahtahmassebi, N.; Khojastehpour, M.; Maddahi, P. Preparation and Characterization of PVA/ZnO Nanocomposite. J. Food Process. Preserv. 2015, 39, 1442–1451. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, K.; Mo, Y.; Huang, X. A novel ZnO nanoparticle blended polyvinylidene fluoride membrane for anti-irreversible fouling. J. Memb. Sci. 2012, 394–395, 184–192. [Google Scholar] [CrossRef]
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y. Oxidative degradation of acid orange 7 using Ag-doped zinc oxide thin films. J. Photochem. Photobiol. B Biol. 2012, 117, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Duan, L.; Wang, F.; Niu, H.; Guo, W.; Ali, A. Structure, luminescence and photocatalytic activity of Mg-doped ZnO nanoparticles prepared by auto combustion method. Mater. Sci. Semicond. Process. 2015, 29, 372–379. [Google Scholar] [CrossRef]
- Rajbongshi, B.M.; Ramchiary, A.; Samdarshi, S. Influence of N-doping on photocatalytic activity of ZnO nanoparticles under visible light irradiation. Mater. Lett. 2014, 134, 111–114. [Google Scholar] [CrossRef]
- Satriyatama, A.; Budi, I.D.M.; Iman, H.N.; Susilo, H.; Saputera, W.H. ZnO-Incorporated ZSM-5 for Photocatalytic CO2 Reduction into Solar Fuels under UV–Visible Light. Chem. Proc. 2022, 6, 1. [Google Scholar] [CrossRef]
- Suwanboon, S.; Amornpitoksuk, P.; Sukolrat, A.; Muensit, N. Optical and photocatalytic properties of La-doped ZnO nanoparticles prepared via precipitation and mechanical milling method. Ceram. Int. 2013, 39, 2811–2819. [Google Scholar] [CrossRef]
- Kaneva, N.V.; Dimitrov, D.T.; Dushkin, C.D. Effect of nickel doping on the photocatalytic activity of ZnO thin films under UV and visible light. Appl. Surf. Sci. 2011, 257, 8113–8120. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Pan, X.; Cortie, D.; Huang, X.; Yi, Z. Photocatalytic oxidation of methane over silver decorated zinc oxide nanocatalysts. Nat. Commun. 2016, 7, 12273. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.; Roy, V.A.L. Recent progress in magnetic iron oxide-semiconductor composite nanomaterials as promising photocatalysts. Nanoscale 2015, 7, 38–58. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem.-Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Murcia-López, S.; Villa, K.; Andreu, T.; Morante, J.R. Partial oxidation of methane to methanol using bismuth-based photocatalysts. ACS Catal. 2014, 4, 3013–3019. [Google Scholar] [CrossRef]
- Song, H.; Meng, X.; Wang, S.; Zhou, W.; Wang, X.; Kako, T.; Ye, J. Direct and Selective Photocatalytic Oxidation of CH4 to Oxygenates with O2 on Cocatalysts/ZnO at Room Temperature in Water. J. Am. Chem. Soc. 2019, 141, 20507–20515. [Google Scholar] [CrossRef]
- Belousov, A.S.; Suleimanov, E.V. Application of metal-organic frameworks as an alternative to metal oxide-based photocatalysts for the production of industrially important organic chemicals. Green Chem. 2021, 23, 6172–6204. [Google Scholar] [CrossRef]
- Sher Shah, M.S.A.; Oh, C.; Park, H.; Hwang, Y.J.; Ma, M.; Park, J.H. Catalytic Oxidation of Methane to Oxygenated Products: Recent Advancements and Prospects for Electrocatalytic and Photocatalytic Conversion at Low Temperatures. Adv. Sci. 2020, 7, 1–24. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Li, Z.; Wang, Z.; Zeng, X.; Han, X.; Cheng, Y.; Sheveleva, A.M.; Zhang, Z.; Tuna, F.; Mcinnes, E.J.L.; et al. Direct photo-oxidation of methane to methanol over a mono-iron hydroxyl site. Nat. Mater. 2022, 21, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.; Ouyang, S.; Ye, J. Metal-organic frameworks for photocatalysis. Phys. Chem. Chem. Phys. 2016, 18, 7563–7572. [Google Scholar] [CrossRef]
- Huang, F.; Yan, A.; Zhao, H. Influences of Doping on Photocatalytic Properties of Photocatalyst; IntechOpen: London, UK, 2016; pp. 31–80. [Google Scholar]
- Shao, W.; Wang, H.; Zhang, X. Elemental doping for optimizing photocatalysis in semiconductors. Dalton Trans. 2018, 47, 12642–12646. [Google Scholar] [CrossRef]
- Taylor, C.E.; Noceti, R.P.; Joseph, R.; Este, D.; Martello, D.V. Photocatalytic Production of Methanol and Hydrogen fi’om Methane and Water. Stud. Surf. Sci. Catal. 1996, 101, 407–416. [Google Scholar]
- Taylor, C.E.; Noceti, R.P. New developments in the photocatalytic conversion of methane to methanol. Catal. Today 2000, 55, 259–267. [Google Scholar] [CrossRef]
- Liu, L.; Ji, Z.; Zou, W.; Gu, X.; Deng, Y.; Gao, F.; Tang, C.; Dong, L. In situ loading transition metal oxide clusters on TiO2 nanosheets as co-catalysts for exceptional high photoactivity. ACS Catal. 2013, 3, 2052–2061. [Google Scholar] [CrossRef]
- Yi, Y.; Tang, Z.; Wu, X.; Huang, A.; Luo, X.; Xu, G.Q.; Zhu, Y.; Wang, S.L. Photocatalytic oxidation of methane to methanol by tungsten trioxide-supported atomic gold at room temperature. Appl. Catal. B Environ. 2022, 306, 120919. [Google Scholar] [CrossRef]
- Umezawa, N.; Ye, J. Role of complex defects in photocatalytic activities of nitrogen-doped anatase TiO2. Phys. Chem. Chem. Phys. 2012, 14, 5924–5934. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Q.; Li, W.; Qiao, B.; Wang, S.L. Atomic-Scale Pd on 2D Titania Sheets for Selective Oxidation of Methane to Methanol. ACS Catal. 2021, 11, 14038–14046. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, W.; Cheng, B. Synthesis and enhanced photocatalytic activity of a hierarchical porous flowerlike p-n junction NiO/TiO2 photocatalyst. Chem.-Asian J. 2010, 5, 2499–2506. [Google Scholar] [CrossRef]
- Xie, W.; Li, R.; Xu, Q. Enhanced photocatalytic activity of Se-doped TiO2 under visible light irradiation. Sci. Rep. 2018, 8, 8752. [Google Scholar] [CrossRef]
- Li, L.; Krissanasaeranee, M.; Pattinson, S.W.; Stefik, M.; Wiesner, U.; Steiner, U.; Eder, D. Enhanced photocatalytic properties in well-ordered mesoporous WO3. Chem. Commun. 2010, 46, 7620–7622. [Google Scholar] [CrossRef]
- Tu, W.; Guo, W.; Hu, J.; He, H.; Li, H.; Li, Z.; Luo, W.; Zhou, Y.; Zou, Z. State-of-the-art advancements of crystal facet-exposed photocatalysts beyond TiO2: Design and dependent performance for solar energy conversion and environment applications. Mater. Today 2020, 33, 75–86. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B-Condens. Matter Mater. Phys. 2001, 63, 1554091–1554099. [Google Scholar] [CrossRef]
- Feng, N.; Lin, H.; Song, H.; Yang, L.; Tang, D.; Deng, F.; Ye, J. Efficient and selective photocatalytic CH4 conversion to CH3OH with O2 by controlling overoxidation on TiO2. Nat. Commun. 2021, 12, 4652. [Google Scholar] [CrossRef]
- Zhu, W.; Shen, M.; Fan, G.; Yang, A.; Meyer, J.R.; Ou, Y.; Yin, B.; Fortner, J.; Foston, M.; Li, Z.; et al. Facet-Dependent Enhancement in the Activity of Bismuth Vanadate Microcrystals for the Photocatalytic Conversion of Methane to Methanol. ACS Appl. Nano Mater. 2018, 1, 6683–6691. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, H.; Zong, X.; Xu, Q.; Ma, Y.; Li, G.; Li, C. Crystal facet dependence of water oxidation on BiVO4 sheets under visible light irradiation. Chem.-Eur. J. 2011, 17, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Murcia-López, S.; Villa, K.; Andreu, T.; Morante, J.R. Improved selectivity for partial oxidation of methane to methanol in the presence of nitrite ions and BiVO4 photocatalyst. Chem. Commun. 2015, 51, 7249–7252. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hao, J.; Wei, J.; Dai, J.; Li, Y. Visible-light-driven selective oxidation of methane to methanol on amorphous FeOOH coupled m-WO3. Fuel 2020, 266, 117104. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol-gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Zanatta, A.R. Revisiting the optical bandgap of semiconductors and the proposal of a unified methodology to its determination. Sci. Rep. 2019, 9, 11225. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B.; Ogawa, Y.; Nishimoto, S. Photocatalytic Activity of Amorphous−Anatase Mixture of Titanium(IV) Oxide Particles Suspended in Aqueous Solutions. J. Phys. Chem. B 1997, 101, 3746–3752. [Google Scholar] [CrossRef]
- Tang, J.; Wu, Y.; McFarland, E.W.; Stucky, G.D. Synthesis and photocatalytic properties of highly crystalline and ordered mesoporous TiO2 thin films. Chem. Commun. 2004, 4, 1670–1671. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization of Porous Solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Rolison, D.R. Catalytic nanoarchitectures-The importance of nothing and the unimportance of periodicity. Science 2003, 299, 1698–1701. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Chen, C.; Jia, L.; Hou, B.; Li, D. Effects of macropores on reducing internal diffusion limitations in Fischer-Tropsch synthesis using a hierarchical cobalt catalyst. RSC Adv. 2017, 7, 9436–9445. [Google Scholar] [CrossRef]
- Wang, J.; Bian, Z.; Zhu, J.; Li, H. Ordered mesoporous TiO2 with exposed (001) facets and enhanced activity in photocatalytic selective oxidation of alcohols. J. Mater. Chem. A 2013, 1, 1296–1302. [Google Scholar] [CrossRef]
- Augustynski, J. The role of the surface intermediates in the photoelectrochemical behaviour of anatase and rutile TiO2. Electrochim. Acta 1993, 38, 43–46. [Google Scholar] [CrossRef]
- Han, C.; Wang, B.; Chunzhi, W.; Shen, S.; Zhang, X.; Sun, L.; Tian, Q.; Lei, Y.; Wang, Y. Ultrathin SiC Nanosheets with High Reduction Potential for Improved CH4 Generation from Photocatalytic Reduction of. ChemistrySelect 2019, 4, 2211–2217. [Google Scholar] [CrossRef]
- Wang, L.; Ha, M.N.; Liu, Z.; Zhao, Z. Mesoporous WO3 modified by Mo for enhancing reduction of CO2 to solar fuels under visible light and thermal conditions. Integr. Ferroelectr. 2016, 172, 97–108. [Google Scholar] [CrossRef]
- Ye, L.; Mao, J.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Synthesis of anatase TiO2 nanocrystals with {101}, {001} or {010} single facets of 90% level exposure and liquid-phase photocatalytic reduction and oxidation activity orders. J. Mater. Chem. A 2013, 1, 10532–10537. [Google Scholar] [CrossRef]
- Sun, X.; Shi, L.; Huang, H.; Song, X.; Ma, T. Surface engineered 2D materials for photocatalysis. Chem. Commun. 2020, 56, 11000–11013. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.M.; Cheng, H.M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef]
- Miao, J.; Liu, B. Anatase TiO2 microspheres with reactive {001} facets for improved photocatalytic activity. RSC Adv. 2013, 3, 1222–1226. [Google Scholar] [CrossRef]
- Phawa, C.; Prayoonpokarach, S.; Sinthiptharakoon, K.; Chakthranont, P.; Sangkhun, W.; Faungnawakij, K.; Butburee, T. Effects of Matching Facet Pairs of TiO2 on Photoelectrochemical Water Splitting Behaviors. ChemCatChem 2020, 12, 2116–2124. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Q.; Li, C.; Li, J.; Yang, J. Surface heterojunction between (001) and (101) facets of ultrafine anatase TiO2 nanocrystals for highly efficient photoreduction CO2 to CH4. Appl. Catal. B Environ. 2016, 198, 378–388. [Google Scholar] [CrossRef]
- Al Jitan, S.; Palmisano, G.; Garlisi, C. Synthesis and surface modification of TiO2-based photocatalysts for the conversion of CO2. Catalysts 2020, 10, 227. [Google Scholar] [CrossRef]
- Hu, Y.; Anpo, M.; Wei, C. Effect of the local structures of v-oxides in MCM-41 on the photocatalytic properties for the partial oxidation of methane to methanol. J. Photochem. Photobiol. A Chem. 2013, 264, 48–55. [Google Scholar] [CrossRef]
- Liu, N.; Chang, Y.; Feng, Y.; Cheng, Y.; Sun, X.; Jian, H.; Feng, Y.; Li, X.; Zhang, H. (101)-(001) Surface Heterojunction-Enhanced Antibacterial Activity of Titanium Dioxide Nanocrystals under Sunlight Irradiation. ACS Appl. Mater. Interfaces 2017, 9, 5907–5915. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.P.; Liu, G.; Yin, L.; Cheng, H.M. Crystal facet-dependent photocatalytic oxidation and reduction reactivity of monoclinic WO 3 for solar energy conversion. J. Mater. Chem. 2012, 22, 6746–6751. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced Photocatalytic CO2-Reduction Activity of Enhanced Photocatalytic CO2 -Reduction Activity of Anatase TiO2 by Co-exposed {001} and {101} Facets. J. Am. Chem. Soc. 2014, 136, 8839. [Google Scholar] [CrossRef]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yonga, S.T.; Mohamed, A.R. Highly Reactive {001} Facets of TiO2-Based Composites: Synthesis, Formation Mechanism and Characterizations. Nanoscale 2014, 6, 1946–2008. [Google Scholar] [CrossRef]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.M. Crystal facet engineering of semiconductor photocatalysts: Motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763–6783. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z-What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Sun, Z.; Bao, C.; Gao, T.; Hu, Y.H. The special route toward conversion of methane to methanol on a fluffy metal-free carbon nitride photocatalyst in the presence of H2O2. Int. J. Energy Res. 2020, 44, 2740–2753. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M. Recent advancements in engineering approach towards design of photo-reactors for selective photocatalytic CO2 reduction to renewable fuels. J. CO2 Util. 2019, 29, 205–239. [Google Scholar] [CrossRef]
- Yue, P.L. Introduction to the Modelling and Design of Photoreactors. Photoelectrochem. Photocatal. Photoreact. 1985, 527–547. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers. Manag. 2013, 76, 194–214. [Google Scholar] [CrossRef]
- Braham, R.J.; Harris, A.T. A complete multi-scale simulation of light absorption within a fluidized bed photoreactor using integrated particle, fluid and photon behaviour models. Phys. Chem. Chem. Phys. 2013, 15, 12373–12385. [Google Scholar] [CrossRef]
- Taylor, C.E. Methane conversion via photocatalytic reactions. Catal. Today 2003, 84, 9–15. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R. Nanostructured Photocatalysts for the Production of Methanol from Methane and Water. ChemSusChem 2021, 14, 2023–2033. [Google Scholar] [CrossRef]
- Gondal, M.A.; Hameed, A.; Yamani, Z.H.; Suwaiyan, A. Laser induced photo-catalytic oxidation/splitting of water over α-Fe2O3, WO3, TiO2 and NiO catalysts: Activity comparison. Chem. Phys. Lett. 2004, 385, 111–115. [Google Scholar] [CrossRef]
- Bloh, J.Z. A holistic approach to model the kinetics of photocatalytic reactions. Front. Chem. 2019, 7, 128. [Google Scholar] [CrossRef]
- Mills, A.; O’Rourke, C.; Moore, K. Powder semiconductor photocatalysis in aqueous solution: An overview of kinetics-based reaction mechanisms. J. Photochem. Photobiol. A Chem. 2015, 310, 66–105. [Google Scholar] [CrossRef]



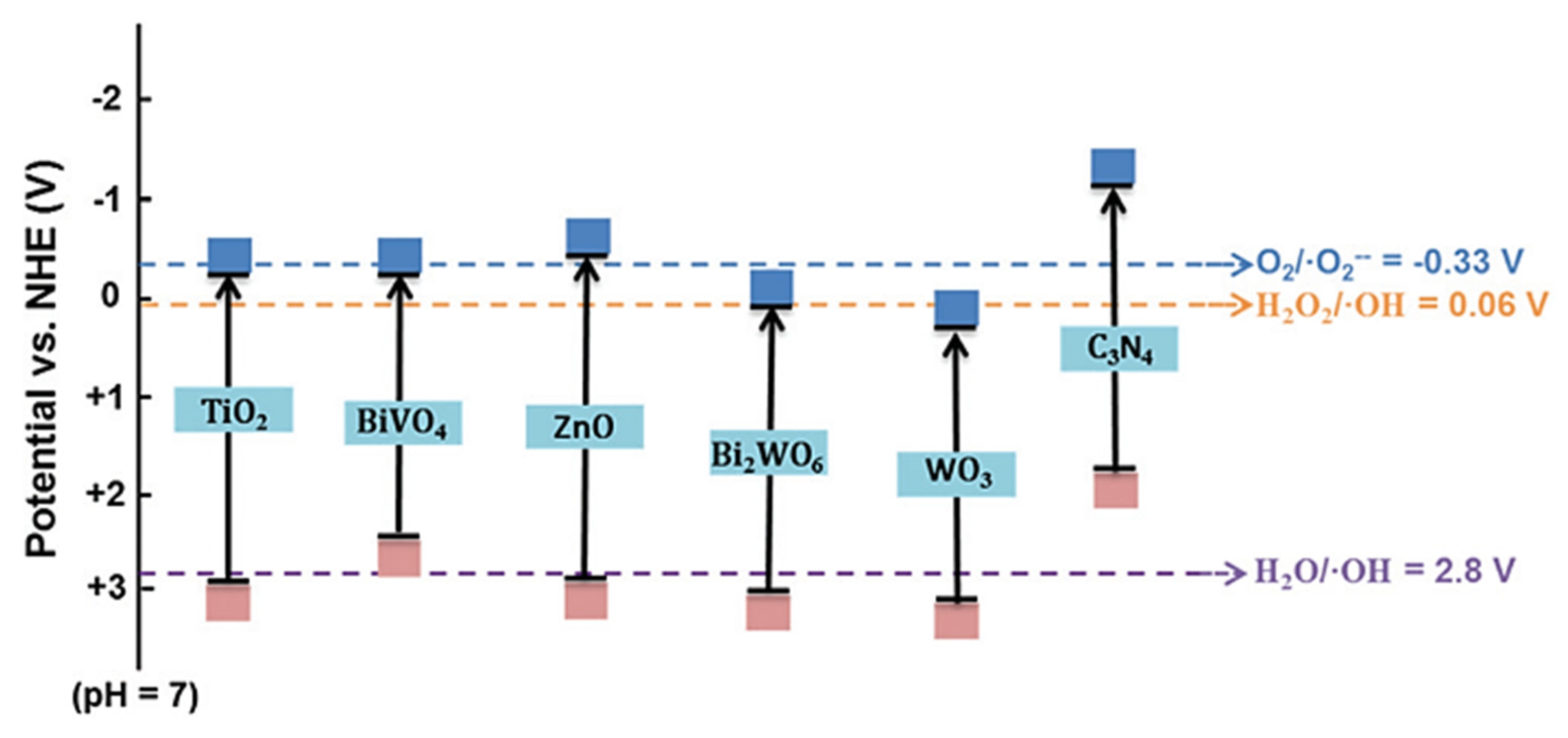

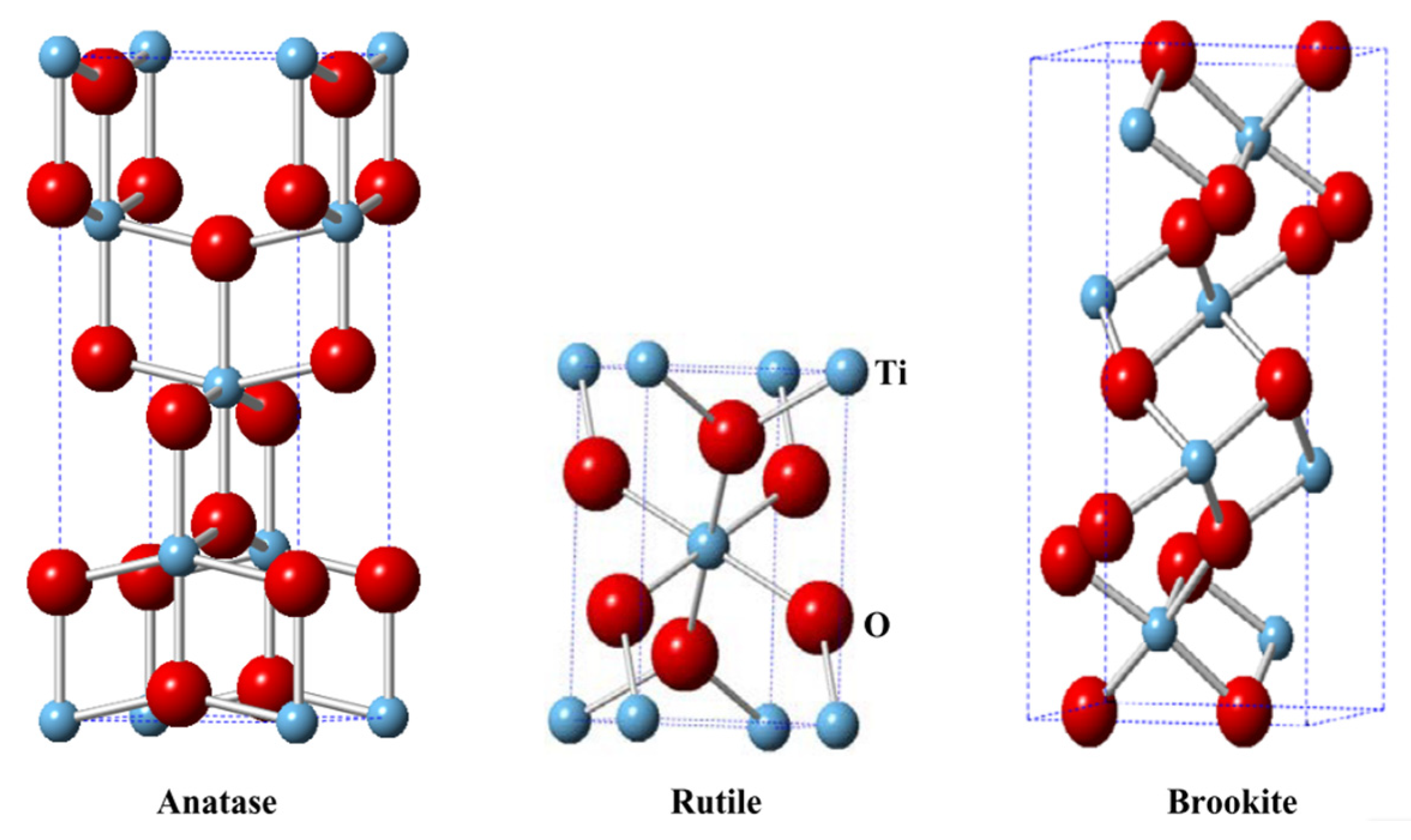











| No | Reaction | Chemical Reaction Equation | ΔG0 298 (kJ/mol) |
|---|---|---|---|
| 1 | Pyrolysis | 50.7 | |
| 2 | Non-oxidative coupling of methane (NOCM) | 68.6 | |
| 3 | Aromatization | 434 | |
| 4 | Total oxidation | −801 | |
| 5 | Oxidative coupling of methane (OCM) | −320 | |
| −288 | |||
| 6 | Partial oxidation of methane (POM) | −223 | |
| 7 | POM | −173 | |
| 8 | POM | −104 | |
| 9 | Methane to methanol | 117 | |
| 10 | Steam reforming of methane (SRM) | 142 | |
| 11 | Water-gas shift reaction | −28.6 | |
| 12 | SRM + water-gas shift reaction | 114 | |
| 13 | Methane to acetic acid | 71.1 | |
| 14 | Methane to acetone | 115 | |
| 15 | CO2 (dry) reforming of methane (DRM) | 171 | |
| 16 | Methane to amino acids | 204 |
| Temperature (K) | |
|---|---|
| 273 | 108 |
| 500 | 132 |
| 750 | 158 |
| 1000 | 183 |
| 1250 | 207 |
| 1500 | 232 |
| No. | Photocatalyst | Synthesis Method | Photocatalyst Loading | Reactant | Light Source | Operating Condition | Reactor | Methane Conversion (X); Methanol Yield (Y); Methanol Electivity (S) | Remarks | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | La/WO3 | Sintering | - | H2O/electron transfer | Mercury Lamp (222 ≤ λ ≤ 1367 nm, 46% Visible light) | P: 1 Atm T: 94 °C | Quartz photochemical reaction vessel (Immerse illumination) | X: 4% Y: n/a S: n/a | Sintered doped materials contained larger crystallites with smoother edges H2O2 improved the production of methanol | [21] |
| 2 | La/WO3 | - | H2O/H2O2/ electron transfer | X: 10% Y: n/a S: n/a | ||||||
| 3 | WO3 | Heating H2WO4, 300 °C | 0.05–0.4 g | H2O/H2O2/Fe3+ | Visible laser (514 nm, 0.5 W) | P: 1 Atm T: RT | Pyrex cell (Side illumination) | X: n/a Y: 6.15 mg/L S: n/a | XPS: W:O = 1:3 H2O2 decreased the production of methanol Fe3+ optimized the production yield | [29] |
| 4 | WO3 | X: n/a Y: 17.33 mg/L S: n/a | ||||||||
| 5 | WO3 | X: n/a Y: 32.36 mg/L S: n/a | ||||||||
| 6 | WO3 | - | 5 g/L | H2O | UV laser beam (355 nm) | P: 1 Atm T: RT | Glass cell (Side illumination) | X: 29% Y: n/a S: n/a | Methanol degradation occurs from electron donation to valance band holes in competition with water (WO3) or superoxide radical oxidation (TiO2) | [23] |
| 7 | TiO2 (rutile) | - | X: 21% Y: n/a S: n/a | |||||||
| 8 | NiO | - | X: 20% Y: n/a S: n/a | |||||||
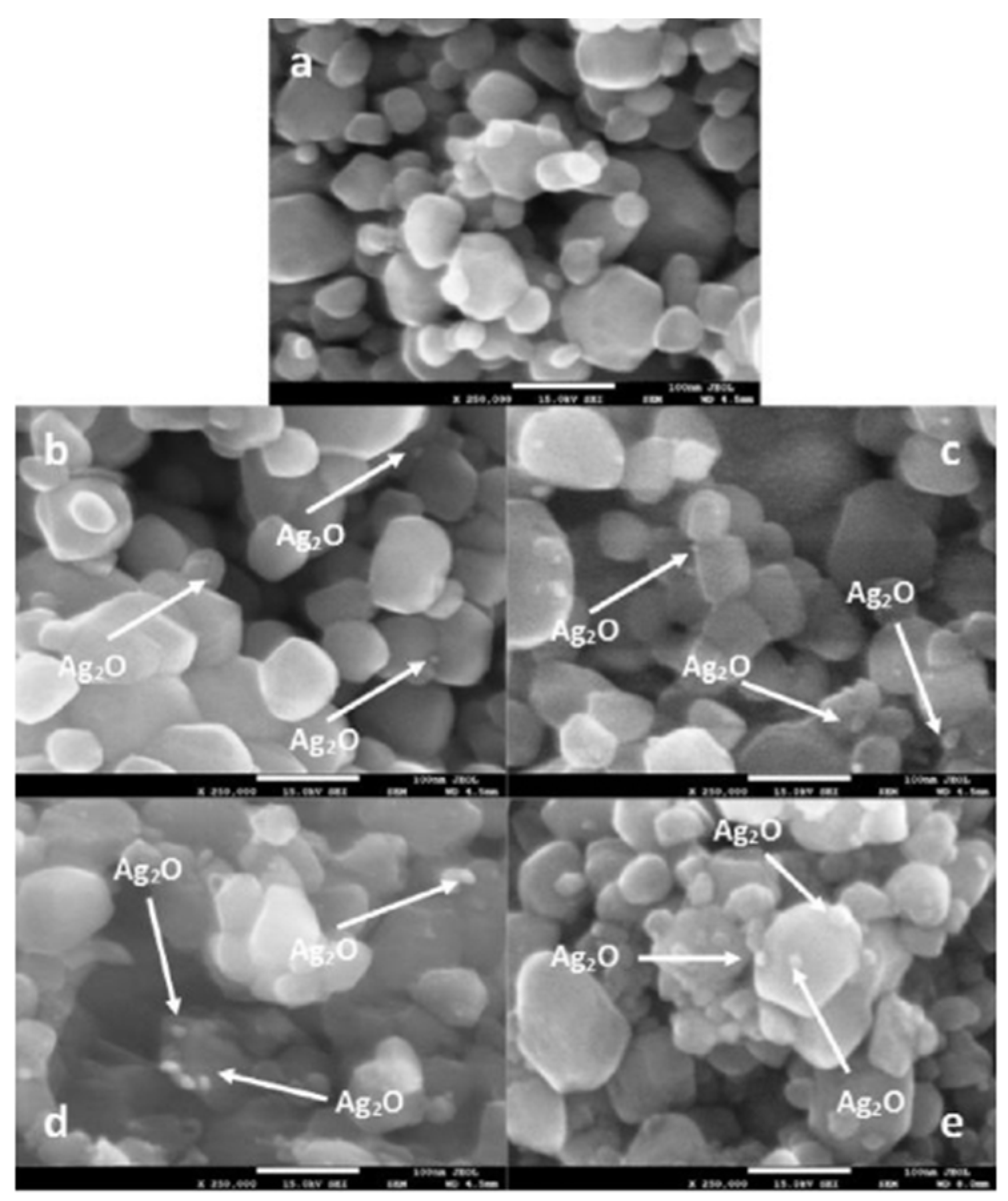
| 9 | Ag/WO3 | Impregnation | ~4 g/L | H2O | 355 nm laser foton | T: RT | A self-fabricated photocatalytic reactor (Side illumination) | X: n/a Y: 12.2 µmol min−1 S: n/a | The increasing loading of Ag+ ions shifted the band gaps in the longer wavelength region FE-SEM: 20–40 nm diameter XPS: the more Ag+ ion impregnated will exist Ag2O on surface | [32] |
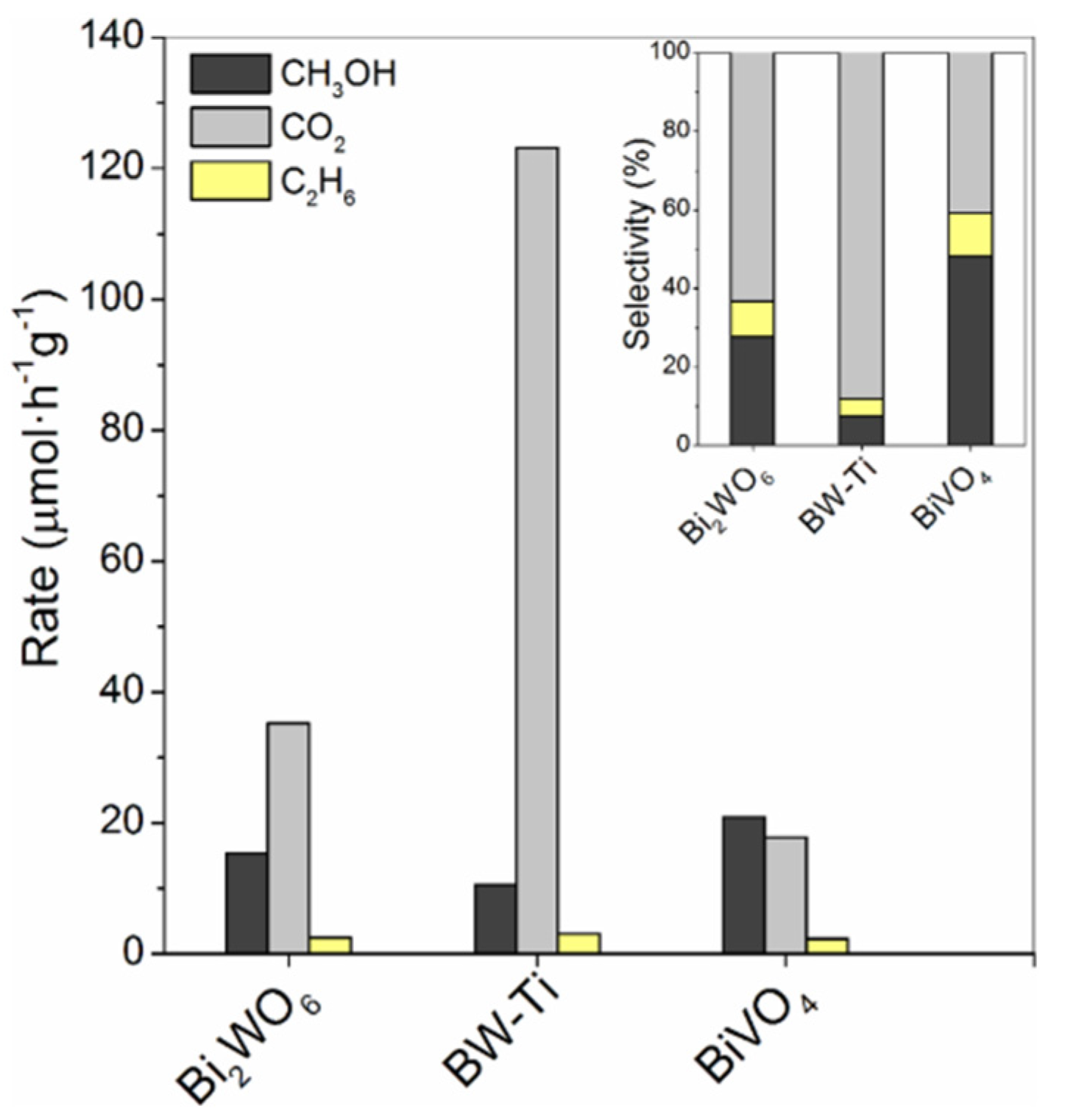
| 10 | Bi2WO6 | Hydrothermal | 1 g/L | H2O/FeCl3/H2SO4 | Mercury lamp (450 W, λ ≥ 185 nm) | P: 1 Atm T: 55 °C | A commercial photochemical reactor—Ace Glass (Immersed illumination) | X: n/a Y: 18 μmol·g−1·h−1 S: n/a | BET: BiWO6 (30 m2 g−1), BW-Ti (41 m2 g−1), BiVO4 (2 m2 g−1) Crystallite size: BiWO6 (8 nm), BW-Ti (10 nm), BiVO4 (28 nm) BiVO4 showed the most selective due to moderate oxidation potential from its band edge | [65] |
| 11 | BiVO4 | X: n/a Y: 21 μmol·g−1·h−1 S: n/a | ||||||||
| 12 | Bi2WO6/TiO2−P25 | X: n/a Y: 15 μmol·g−1·h−1 S: n/a | ||||||||
| 13 | BiVO4 | Hydrothermal | 1 g/L | H2O | Mercury lamp (450 W, λ ≥ 185 nm) | P: 1 Atm T: 55 °C | A commercial photochemical reactor—Ace Glass (Immersed illumination) | X: n/a Y: 6 µmol h−1 S: 50% | Nitrite ion acted as •OH scavenger to prevent oxidation of CH3OH | [88] |
| 14 | BiVO4 + NO2− | H2O/NO | X: n/a Y: 3 µmol h−1 S: 90% | |||||||
| 15 | WO3 | Hard template | 10 g/L | H2O | Quartz Hg-vapor lamp (λ ≥ 185 nm) | P: 1 Atm T: 55 °C | A commercial photochemical reactor—Ace Glass (Immersed illumination) | X: n/a Y: 14.5 µmol h−1 S: 22.0% | Methanol was produced by the reaction of hydroxyl radical in the surface | [24] |
| 16 | F/WO3 | H2O/HF | X: n/a Y: 8.5 µmol h−1 S: 17.9% | |||||||
| 17 | WO3 | Hard template | 10 g/L | H2O/H2O2 | Quartz Hg-vapor lamp (λ ≥ 185 nm) | P: 1 Atm T: 55 °C | A commercial photochemical reactor—Ace Glass (Immersed illumination) | X: n/a Y: 9 µmol h−1 S: n/a | XRD: mWO3 surface area was 151 m2 g−1 Fe3+ and Cu2+ significantly improved the generation of methanol by capturing the photogenerated electrons Ag+ aggravated the selectivity of methanol and deposited it on the surface | [37] |
| 18 | Mesopore WO3/Fe3+ | H2O | X: n/a Y: 55.5 μmol·g−1·h−1 S: n/a | |||||||
| 19 | Mesopore WO3/Cu2+ | H2O | X: n/a Y: 45 μmol·g−1·h−1 S: n/a | |||||||
| 20 | Mesopore WO3 | H2O | X: n/a Y: 25 μmol·g−1·h−1 S: n/a | |||||||
| 21 | Mesopore WO3/Ag+ | H2O | X: n/a Y: 15 μmol·g−1·h−1 S: n/a | |||||||
| 22 | Mesopore WO3/H2O2 | H2O | X: n/a Y: 20 μmol·g−1·h−1 S: n/a | |||||||
| 23 | TiO2 | - | ~1 g/L | H2O/H2O2 | Xenon lamp with 710 nm short-pass filter (300 W, 185 nm ≤ λ ≤ 710 nm) | P: 1 Atm T: RT | Custom-made reactor (Immerse illuminated) | X:10.5% Y: 290 μmol·g−1·h−1 S: n/a | XRD: Anatase crystal TiO2 BET: Surface area of TiO2 (52.38 m2 g−1), Au/TiO2 (42.26 m2 g−1), PdOx/TiO2 (47.49 m2 g−1), PtO/TiO2 (51.83 m2 g−1), Cu2O/TiO2 (45.63 m2 g−1), FeOx/TiO2 (45.52 m2 g−1) | [26] |
| 24 | Au/TiO2 | Facile impregnation | X:7.5% Y: 170 μmol·g−1·h−1 S: n/a | |||||||
| 25 | PdOx/TiO2 | Facile impregnation | X: 8.5% Y: 0 S: n/a | |||||||
| 26 | PtO/TiO2 | Facile impregnation | X: 7.5% Y: 0 S: n/a | |||||||
| 27 | Cu2O/TiO2 | Facile impregnation | X: 10.5% Y: ~10 μmol·g−1·h−1 S: n/a | |||||||
| 28 | FeOx/TiO2 | Facile impregnation | X: 15% Y: 1000 μmol·g−1·h−1 S: n/a | |||||||
| 29 | Bipyramid BiVO4 | Hydrothermal synthesis | 1 g/L | H2O | Xenon arc lamp (350 W, 200 nm ≤ λ ≤ 800 nm) | T: 65 °C | Custom quartz reaction vessel (Bottom illuminated) | X: 0.96% Y: 111.9 μmol·g−1·h−1 S: 85% | XRD: monoclinic scheelite structure of BiVO4 BET: Surface area of bipyramid (3.2 m2 g−1), thick platelet (3.6 m2 g−1), thin platelet (3.6 m2 g−1) | [86] |
| 30 | Thick platelet BiVO4 | X: 0.72% Y: 79.2 µmol h−1 g−1 S: 85.7% | ||||||||
| 31 | Thin platelet BiVO4 | X: 0.87% Y: 65.7 µmol h−1 g−1 S: 58.2% | ||||||||
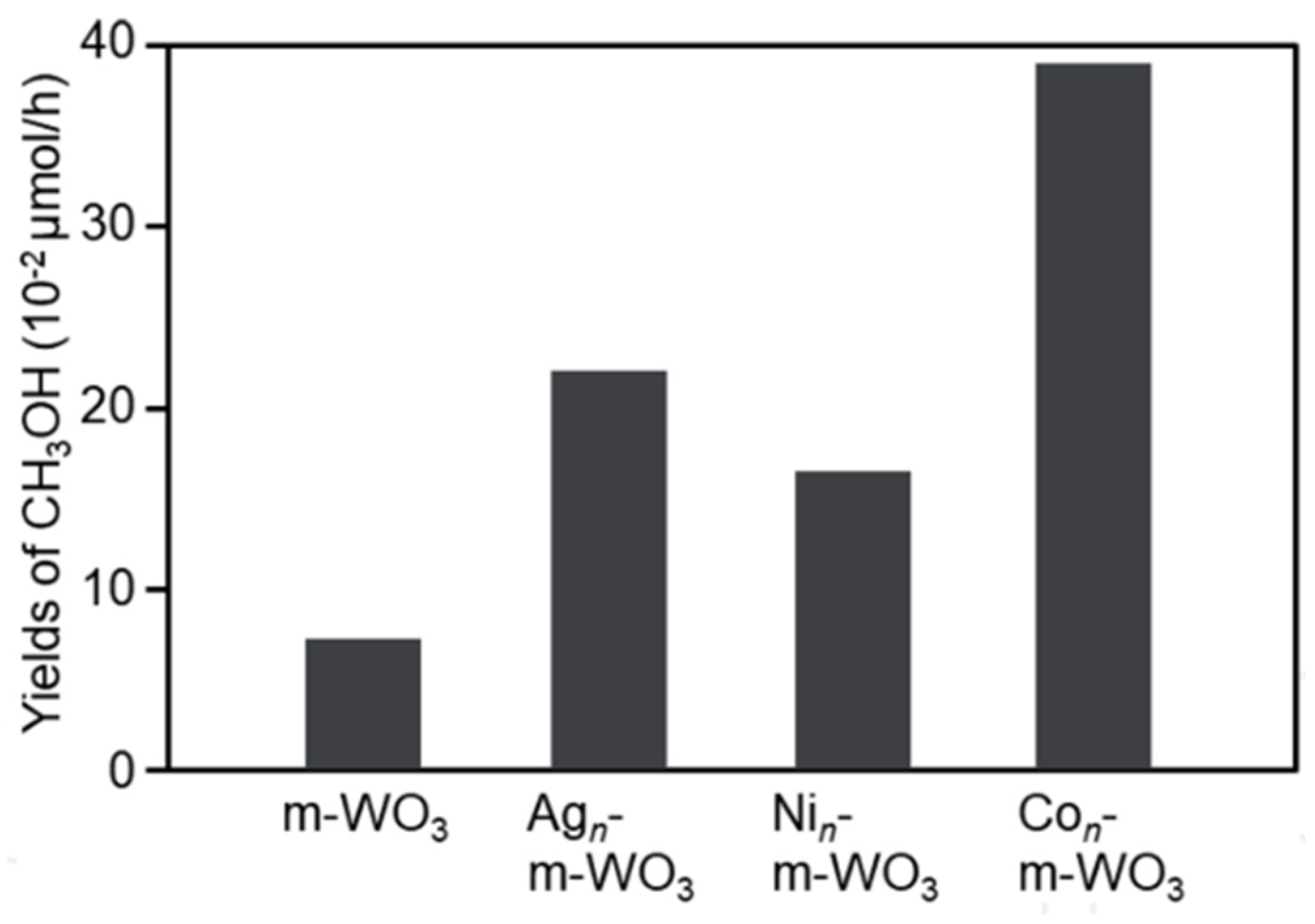
| 32 | m-WO3 | Hard template method | 1 g/L | H2O | Mercury lamp (400 W, λ ≥ 185 nm) | T: 55–60 °C | Quartz glass (Immerse illuminated) | X: n/a Y: 0.08 µmol h−1 S: n/a | It is essential to choose an appropriate co-catalyst for photocatalyst to improve the photocatalytic activity Particle size 1 nm in this study was considered not optimal for promoting the reaction | [25] |
| 33 | Ag/m-WO3 | X: n/a Y: 0.22 µmol h−1 S: n/a | ||||||||
| 34 | Ni/m-WO3 | X: n/a Y: 0.17 µmol h−1 S: n/a | ||||||||
| 35 | Co/m-WO3 | X: n/a Y: 0.39 µmol h−1 S: n/a | ||||||||
| 36 | m-TiO2 | X: n/a Y: 0.02 µmol h−1 S: n/a | ||||||||
| 37 | Ag/m-TiO2 | X: n/a Y: 0.12 µmol h−1 S: n/a | ||||||||
| 38 | Ni/TiO2 | X: n/a Y: 0.14 µmol h−1 S: n/a | ||||||||
| 39 | Co/m-TiO2 | X: n/a Y: 0.95 µmol h−1 S: n/a | ||||||||
| 40 | Amorphous FeOOH/m-WO3 | Hard template method | ~1 g/L | H2O2 | Visible Light (400 nm ≤ λ≤ 700 nm) | P: 1 Atm T: RT | Quartz window (Top illuminated) | X: n/a Y: 211 μmol·g−1 h−1 S: 91.0% | XPS: iron species on the surface are primarily amorphous FeOOH FE-SEM: WO3 nanocrystals with an average size of 13 nm | [89] |
| 41 | Fe2O3 | - | 1 g/L | H2O/H2O2/FeCl2 | Xenon lamp (300 W, 300 nm ≤ λ ≤ 2000 nm) | T: 30 °C P: 3 MPa | Autoclave (Top illuminated) | X: 0.15% Y: 100 μmol·g−1·h−1 S: 35% | An appropriate ratio of Fenton reagents (Fe2+ and H2O2) could contribute to the conversion of CH4 to selectively generate CH3OH | [27] |
| 42 | TiO2/Fe | - | X: 0.39% Y: 470 μmol·g−1·h−1 S: 84% | |||||||
| 43 | NiO/Fe | - | X: 0.27% Y: 40 μmol·g−1·h−1 S: 14% | |||||||
| 44 | CeO2/Fe | - | X: 0.43% Y: 23% S: 150 μmol·g−1·h−1 | |||||||
| 45 | ZnO/Fe | - | X: 0.05% Y: 0 S: 0 | |||||||
| 46 | WO3/Fe | - | X: 0.89% Y: 27% S: 350 μmol·g−1·h−1 | |||||||
| 47 | Ag/TiO2 | Hydrothermal method | 0.1 g/L | H2O/O2 | Xenon lamp (300 W, 300 nm ≤ λ ≤ 2000 nm) | P: 2 MPa T: 25 °C | Batch reactor (Top illuminated) | X: 0.31% Y: 4.8 mmol g−1 h−1 S: 80% | Oxygen vacancy in [001] TiO2 provided a distinct intermediate and reaction pathway that improved the selectivity of methanol | [85] |
| 48 | ZnO | NaBH4 reduction method | 0.1 g/L | H2O/O2 | Xenon lamp (300 W, 300 nm ≤ λ ≤ 2000 nm) | P: 2 MPa T: 25 °C | Batch reactor (Top illuminated) | X: n/a Y: 0 S: n/a | Controlled activation of O2 over cocatalysts produced mild reactive oxygen species, •OOH radicals that are important in selective oxidation of methanol | [66] |
| 49 | Pt/ZnO | X: n/a Y: 85.3 µmol S: n/a | ||||||||
| 50 | Pd/ZnO | X: n/a Y: 108.2 µmol S: n/a | ||||||||
| 51 | Au/ZnO | X: n/a Y: 58.6 µmol S: n/a | ||||||||
| 52 | Ag/ZnO | X: n/a Y: 23.0 µmol S: n/a | ||||||||
| 53 | Au/AgO | NaBH4 reduction method | 1 g/L | H2O/O2 | Xenon lamp (300 W, 300 nm ≤ λ ≤ 2000 nm) | P: 15 bar T: 30 °C | Batch reactor (Top illuminated) | X: n/a Y: 1371 µmol g−1 S: 99.1% | CH3OH can be produced from the combination of •CH3 with either O2 or •OH The low-intensity density of UV light could avoid the overoxidation of methanol | [28] |
| Light Position | Advantages | Disadvantages |
|---|---|---|
| Top illuminated |
|
|
| Side illuminated |
|
|
| Immerse illuminated |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuniar, G.; Saputera, W.H.; Sasongko, D.; Mukti, R.R.; Rizkiana, J.; Devianto, H. Recent Advances in Photocatalytic Oxidation of Methane to Methanol. Molecules 2022, 27, 5496. https://doi.org/10.3390/molecules27175496
Yuniar G, Saputera WH, Sasongko D, Mukti RR, Rizkiana J, Devianto H. Recent Advances in Photocatalytic Oxidation of Methane to Methanol. Molecules. 2022; 27(17):5496. https://doi.org/10.3390/molecules27175496
Chicago/Turabian StyleYuniar, Gita, Wibawa Hendra Saputera, Dwiwahju Sasongko, Rino R. Mukti, Jenny Rizkiana, and Hary Devianto. 2022. "Recent Advances in Photocatalytic Oxidation of Methane to Methanol" Molecules 27, no. 17: 5496. https://doi.org/10.3390/molecules27175496
APA StyleYuniar, G., Saputera, W. H., Sasongko, D., Mukti, R. R., Rizkiana, J., & Devianto, H. (2022). Recent Advances in Photocatalytic Oxidation of Methane to Methanol. Molecules, 27(17), 5496. https://doi.org/10.3390/molecules27175496