Dwarf Kiwi (Actinidia arguta Miq.), a Source of Antioxidants for a Healthy and Sustainable Diet
Abstract
:1. Introduction
2. Results and Discussion
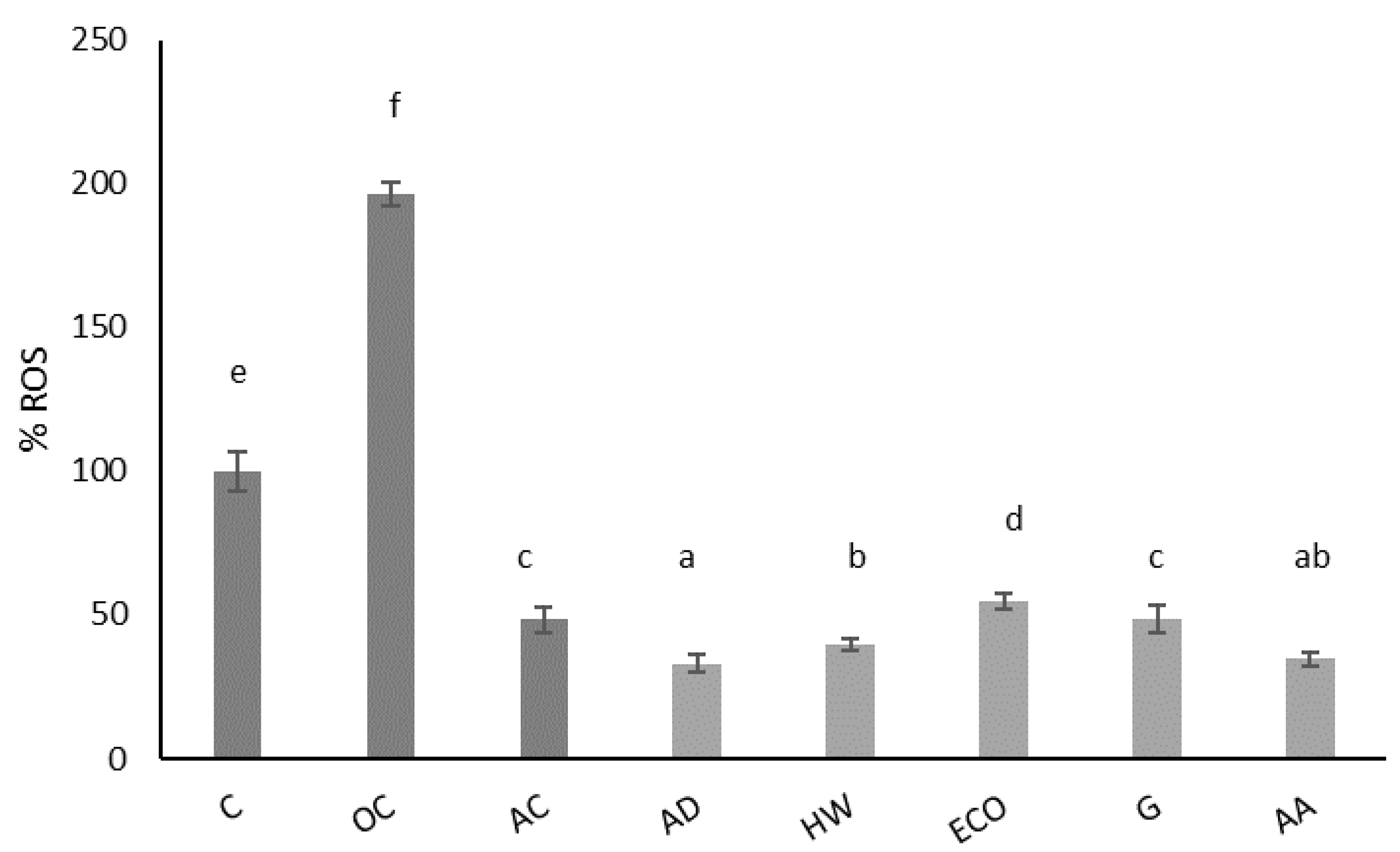
2.1. Antioxidant Properties
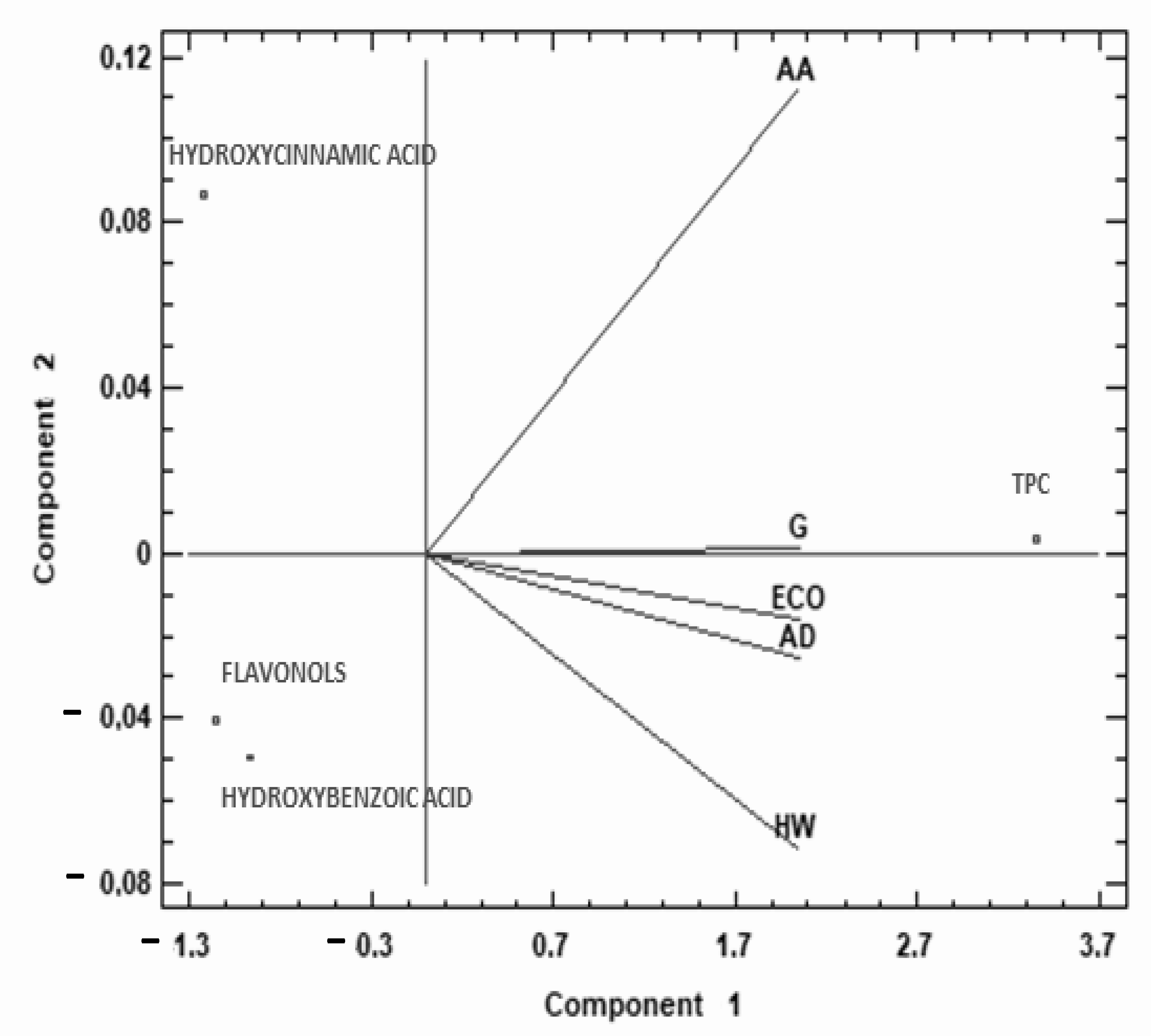
2.2. Phenolic Compounds
3. Materials and Methods
3.1. Raw Material
3.2. Chemicals
3.3. Antioxidant Properties
3.3.1. Overall Antioxidant Capacity—Extraction-Dependent Methods
- 2,2′-Azinobis-(3-Ethylbenzothiazoline 6-Sulfonic acid) (ABTS) Assay
- Oxygen Radical Absorbance Capacity (ORAC) Assay
3.3.2. Overall Antioxidant Capacity—Direct Methods: QUENCHER
- Folin–Ciocalteu Assay by QUENCHER method
- 1,1-diphenyl-2-picryl-hydrazl (DPPH) Assay by QUENCHER method
- 2,4,6-tripyridyl-s-triazine (FRAP) Assay by QUENCHER method
3.3.3. Intracellular Reactive Oxygen Species (ROS)
3.4. Phenolic Compounds
3.4.1. Total Polyphenols: Fast Blue (TPC) Assay by QUENCHER Method
3.4.2. Flavonols by QUENCHER Methodology
3.4.3. Hydroxycinnamic Acids by QUENCHER Methodology
3.4.4. Hydroxybenzoic Acids by QUENCHER Methodology
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Guroo, I.; Wani, S.A.; Wani, S.M.; Ahmad, M.; Mir, S.A.; Masoodi, F.A. A review of production and processing of kiwifruit. Int. J. Food Process. Technol. 2017, 8, 10. [Google Scholar]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar]
- Nishiyama, I.; Yamashita, Y.; Yamanaka, M.; Shimohashi, A.; Fukuda, T.; Oota, T. Varietal difference in vitamin C content in the fruit of kiwifruit and other Actinidia species. J. Agric. Food Chem. 2004, 52, 5472–5475. [Google Scholar]
- AESAN. Informe del Comité Científico de la Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) de Revisión y Actualización de las Recomendaciones Dietéticas para la Población Española (Informe nº AESAN-2020-005). Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/informes_comite/RECOMENDACIONES_DIETETICAS.pdf (accessed on 28 June 2022).
- FAO/WHO. Sustainable Healthy Diets—Guiding Principles, 1st ed.; FAO: Rome, Italy, 2019; pp. 5–13. [Google Scholar]
- European Commission. Available online: https://ec.europa.eu/food/horizontal-topics/farm-fork-strategy (accessed on 28 June 2022).
- FAO; OMS. Dietas Saludables Sostenibles—Principios Rectores. Roma. Available online: https://www.fao.org/documents/card/en/c/ca6640es (accessed on 28 June 2022). [CrossRef]
- Global Burden of Disease Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet 2019, 393, 1958–1972. [Google Scholar]
- Organización Mundial de la Salud (OMS). Alimentación Sana. Nota Descriptiva No. 394 (actualizada en Agosto 2018). Ginebra, Organización Mundial de la Salud. Available online: https://www.who.int/es/news-room/fact-sheets/detail/healthy-diet (accessed on 28 June 2022).
- HLPE. Las Pérdidas y el Desperdicio de Alimentos en el Contexto de Sistemas Alimentarios Sostenibles. Un Informe del Grupo de Alto Nivel de Expertos en Seguridad Alimentaria y Nutrición del Comité de Seguridad Alimentaria Mundial. Roma. Available online: http://www.fao.org/fileadmin/user_upload/hlpe/hlpe_documents/HLPE_Reports/HLPE-Report-8_ES.pdf (accessed on 28 June 2022).
- Krupta, T.; Latocha, P.; Liwinska, A. Changes of physicochemical quality, phenolics and vitamin C content in hardy kiwifruit (Actinidia arguta and its hybrid) during storage. Sci. Hortic. 2011, 130, 410–417. [Google Scholar] [CrossRef]
- Drzewiecki, J.; Latocha, P.; Leontowicz, H.; Leontowicz, M.; Park, Y.S.; Najman, K.; Weisz, M.; Gorinstein, S. Analytical Methods Applied to Characterization of Actinidia arguta, Actinidia deliciosa, and Actinidia eriantha Kiwi Fruit Cultivars. Food Anal. Methods 2016, 9, 1353–1366. [Google Scholar]
- Folkvord, F.; Naderer, B.; Coates, A.; Boyland, E. Promoting Fruit and Vegetable Consumption for Childhood Obesity Prevention. Nutrients 2021, 14, 157. [Google Scholar]
- Cömert, E.D.; Gökmen, V. Antioxidants bound to an insoluble food matrix: Their analysis, regeneration behavior, and physiological importance. Compr. Rev. Food Sci. 2011, 16, 382–399. [Google Scholar]
- Shahbaz, H.M.; Park, E.J.; Kim, G.R.; Akram, K.; Kwon, J.H. Assessment of antioxidant potential of pomegranate fruit by-products via a direct approach using a simple quencher method. J. AOAC Int. 2016, 99, 599–603. [Google Scholar]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar]
- Serpen, A.; Capuano, E.; Fogliano, V.; Gökmen, V. A new procedure to measure the antioxidant activity of insoluble food components. J. Agric. Food Chem. 2007, 55, 7676–7681. [Google Scholar]
- Del Pino-García, R.; García-Lomillo, J.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Adaptation and validation of QUick, Easy, New, CHEap, and Reproducible (QUENCHER) antioxidant capacity assays in model products obtained from residual wine pomace. J. Agric. Food. Chem. 2015, 63, 6922–6931. [Google Scholar]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The ‘QUENCHER’ approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar]
- Wang, Y.; Shan, T.; Yuan, Y.; Yue, T. Overall Quality Properties of Kiwifruit Treated by Cinnamaldehyde and Citral: Microbial, Antioxidant Capacity during Cold Storage. J. Food Sci. 2016, 81, 3043–3051. [Google Scholar]
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J. Food Process. Preserv. 2021, 45, e15588. [Google Scholar]
- Latocha, P.; Łata, B.; Stasiak, A. Phenolics, ascorbate and the antioxidant potential of kiwiberry vs. common kiwifruit: The effect of cultivar and tissue type. J. Funct. Foods 2015, 19, 155–163. [Google Scholar]
- Wang, Y.; Zhao, C.; Li, J.; Liang, Y.; Yang, R.; Liu, J.; Ma, Z.; Wu, L. Evaluation of biochemical components and antioxidant capacity of different kiwifruit (Actinidia spp.) genotypes grown in China. Biotechnol. Biotechnol. Equip. 2018, 32, 558–565. [Google Scholar]
- Zhang, J.; Tian, J.; Gao, N.; Gong, E.S.; Xin, G.; Liu, C.; Si, X.; Sun, X.; Li, B. Assessment of the phytochemical profile and antioxidant activities of eight kiwi berry (Actinidia arguta (Siebold & Zuccarini) Miquel) varieties in China. Food Sci. Nutr. 2021, 20, 5616–5625. [Google Scholar]
- McGhie, T.K.; Ainge, G.D. Color in fruit of the genus Actinidia: Carotenoid and chlorophyll compositions. J. Agric. Food Chem. 2002, 50, 117–121. [Google Scholar] [PubMed]
- Montefiori, M.; McGhie, T.K.; Hallett, I.C.; Costa, G. Changes in pigments and plastid ultrastructure during ripening of green-fleshed and yellow-fleshed kiwifruit. Sci. Hortic. 2009, 119, 377–387. [Google Scholar]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, N.; Shu, C.; Cheng, S.; Sun, X.; Liu, C.; Xin, G.; Li, B.; Tian, J. Phenolics Profile and Antioxidant Activity Analysis of Kiwi Berry (Actinidia arguta) Flesh and Peel Extracts from Four Regions in China. Front. Plant Sci. 2021, 12, 689038. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Lee, S.G.; Kang, H.; Heo, H.J.; Cho, Y.S.; Kim, D.O. Antioxidant and anti-inflammatory effects of various cultivars of kiwi berry (Actinidia arguta) on lipopolysaccharide-stimulated RAW 264.7 cells. J. Microbiol. Biotechnol. 2016, 26, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Girard-Lalancette, K.; Pichette, A.; Legault, J. Sensitive cell-based assay using DCFH oxidation for the determination of pro-and antioxidant properties of compounds and mixtures: Analysis of fruit and vegetable juices. Food Chem. 2009, 115, 720–726. [Google Scholar] [CrossRef]
- Medina, M.B. Determination of the total phenolics in juices and superfruits by a novel chemical method. J. Funct. Foods 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Zuo, L.L.; Wang, Z.Y.; Fan, Z.L.; Tian, S.Q.; Liu, J.R. Evaluation of Antioxidant and Antiproliferative Properties of Three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) Extracts in Vitro. Int. J. Mol. Sci. 2012, 13, 5506–5518. [Google Scholar] [CrossRef]
- Chaves, V.C.; Calvete, E.; Reginatto, F.H. Quality properties and antioxidant activity of seven strawberry (Fragaria x ananassa duch) cultivars. Sci. Hortic. 2017, 225, 293–298. [Google Scholar] [CrossRef]
- Zhu, C.; Chou, O.; Lee, F.Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Characterization of Phenolics in Rejected Kiwifruit and Their Antioxidant Potential. Processes 2021, 9, 781. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oki, T.; Nagai, S.; Yoshinaga, M.; Nishiba, Y.; Suda, I. Contribution of b-carotene to radical scavenging capacity varies among orange-fleshed sweet potato cultivars. Food Sci. Technol. Res. 2006, 12, 156–160. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-woodill, M.; Prior, R.L.; Laboratories, B.; Lane, T. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Bartolomé, B.; Gómez-Cordovés, C. Antioxidant properties of commercial grape juices and vinegars. Food Chem. 2005, 93, 325–330. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- López-Froilán, R.; Hernández-Ledesma, B.; Cámara, M.; Pérez-Rodríguez, M.L. Evaluation of the Antioxidant Potential of Mixed Fruit-Based Beverages: A New Insight on the Folin-Ciocalteu Method. Food Anal. Methods 2018, 11, 2897–2906. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1997, 28, 49–55. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Bakondi, E.; Gönczi, M.; Szabó, E.; Bai, P.; Pacher, P.; Gergely, P.; Kovács, L.; Hunyadi, J.; Szabó, C.; Csernoch, L.; et al. Role of intracellular calcium mobilization and cell-density-dependent signaling in oxidative-stress-induced cytotoxicity in HaCaT keratinocytes. J. Investig. Dermatol. 2003, 121, 88–95. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Aparicio García, N.; Velazquez Escobar, F.; San Andres, M.I.; Sanchez-Fortun, S.; Blanch, G.P.; Fernandez-Gomez, B.; Guisantes Batan, E.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Arias-Rico, J.; Cruz-Cansino, N.C.; Cámara-Hurtado, M.; López-Froilán, R.; Pérez-Rodríguez, M.L.; Sánchez-Mata, M.C.; Jaramillo-Morales, O.A.; Barrera-Gálvez, R.; Ramírez-Moreno, E. Study of Xoconostle (Opuntia spp.) Powder as Source of Dietary Fiber and Antioxidants. Foods 2020, 9, 403. [Google Scholar] [CrossRef]
- Pedro, A.C.; Pérez-Rodríguez, M.L.; Sánchez-Mata, M.C.; Bisinella, R.Z.; Soltovski de Oliveira, C.; Schnitzler, E.; Delinski Bet, C.; Maciel, G.M.; Haminiuk, G.W.I. Biological activities, chromatographic profile and thermal stability of organic and conventional goji berry. Food Meas. 2022, 16, 1263–1273. [Google Scholar] [CrossRef]
- Bonoli, M.; Verardo, V.; Marconi, E.; Caboni, M.F. Antioxidant phenols in Barley (Hordeum vulgare L.) flour: Comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J. Agric. Food Chem. 2004, 52, 5195–5200. [Google Scholar] [CrossRef] [PubMed]
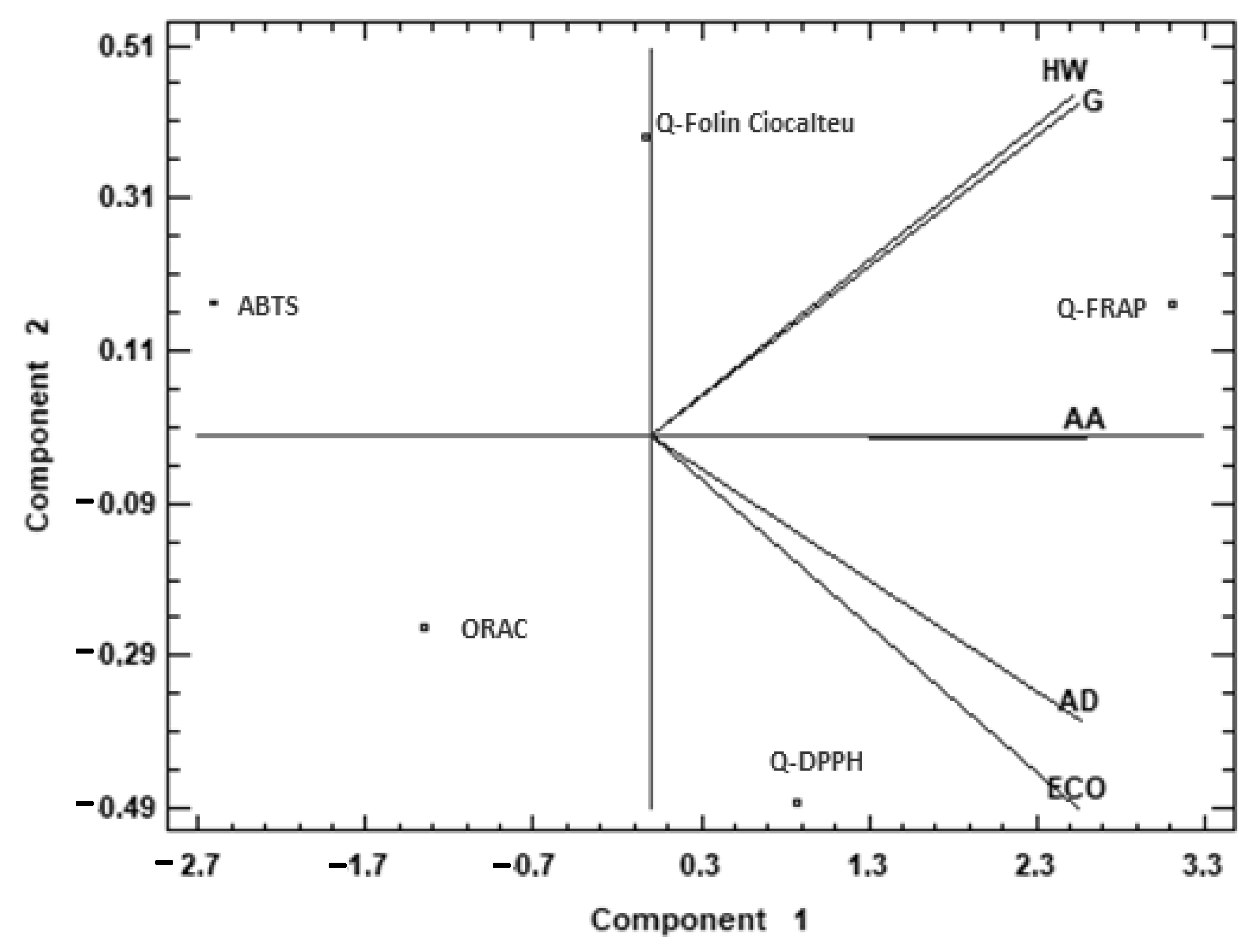

| Q-Folin–Ciocalteu (mg GAE/g) | Q-DPPH (mg TE/g) | Q-FRAP (mg TE/g) | ORAC (µg TE/g) | ABTS (mg GAE/g) | |
|---|---|---|---|---|---|
| AD | 4.09 ± 0.20 a | 6.41 ± 0.11 a | 9.28 ± 0.45 b | 3.94 ± 0.00 d | 1.45 ± 0.05 c |
| HW | 6.95 ± 0.35 b | 6.16 ± 0.20 a | 9.88 ± 0.20 b | 2.38 ± 0.00 a | 0.67 ± 0.02 a |
| ECO | 4.23 ± 0.22 a | 6.21 ± 0.35 a | 7.24 ± 0.31 a | 3.08 ± 0.00 b | 0.99 ± 0.02 b |
| G | 6.74 ± 0.56 b | 6.98 ± 0.62 b | 16.00 ± 0.78 c | 3.38 ± 0.00 c | 1.43 ± 0.11 c |
| AA | 10.82 ± 0.36 c | 16.31 ± 1.10 c | 26.24 ± 1.22 d | 4.75 ± 0.00 e | 1.73 ± 0.05 d |
| TPC (mg GAE/g) | Flavonols (mg QE/g) | Hydroxycinnamic Acid (mg FAE/g) | Hydroxybenzoic Acid (mg GAE/g) | |
|---|---|---|---|---|
| AD | 32.13 ± 2.10 b | 1.26 ± 0.03 c | 0.32 ± 0.01 a | 2.42 ± 0.21 a |
| HW | 23.02 ± 2.38 a | 1.44 ± 0.10 d | 0.34 ± 0.03 a | 2.34 ± 0.07 a |
| ECO | 29.34 ± 2.35 b | 1.28 ± 0.07 c | 0.60 ± 0.02 c | 2.41 ± 0.07 a |
| G | 31.30 ± 2.61 b | 0.85 ± 0.01 b | 0.49 ± 0.01 b | 2.28 ± 0.13 a |
| AA | 54.57 ± 4.60 c | 0.55 ± 0.02 a | 2.39 ± 0.02 d | 2.40 ± 0.19 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Herrera, P.; Maieves, H.A.; Vega, E.N.; Perez-Rodriguez, M.L.; Fernandez-Ruiz, V.; Iriondo-DeHond, A.; Castillo, M.D.d.; Sanchez-Mata, M.C. Dwarf Kiwi (Actinidia arguta Miq.), a Source of Antioxidants for a Healthy and Sustainable Diet. Molecules 2022, 27, 5495. https://doi.org/10.3390/molecules27175495
Garcia-Herrera P, Maieves HA, Vega EN, Perez-Rodriguez ML, Fernandez-Ruiz V, Iriondo-DeHond A, Castillo MDd, Sanchez-Mata MC. Dwarf Kiwi (Actinidia arguta Miq.), a Source of Antioxidants for a Healthy and Sustainable Diet. Molecules. 2022; 27(17):5495. https://doi.org/10.3390/molecules27175495
Chicago/Turabian StyleGarcia-Herrera, Patricia, Helayne A. Maieves, Erika N. Vega, María Luisa Perez-Rodriguez, Virginia Fernandez-Ruiz, Amaia Iriondo-DeHond, Maria Dolores del Castillo, and Maria Cortes Sanchez-Mata. 2022. "Dwarf Kiwi (Actinidia arguta Miq.), a Source of Antioxidants for a Healthy and Sustainable Diet" Molecules 27, no. 17: 5495. https://doi.org/10.3390/molecules27175495
APA StyleGarcia-Herrera, P., Maieves, H. A., Vega, E. N., Perez-Rodriguez, M. L., Fernandez-Ruiz, V., Iriondo-DeHond, A., Castillo, M. D. d., & Sanchez-Mata, M. C. (2022). Dwarf Kiwi (Actinidia arguta Miq.), a Source of Antioxidants for a Healthy and Sustainable Diet. Molecules, 27(17), 5495. https://doi.org/10.3390/molecules27175495










