Antibody–Ferrocene Conjugates as a Platform for Electro-Chemical Detection of Low-Density Lipoprotein
Abstract
:1. Introduction
2. Results and Discussion
2.1. On-Bead Antibody Conjugation Using Amine-Reactive Ferrocene (NHS-Ferrocene)
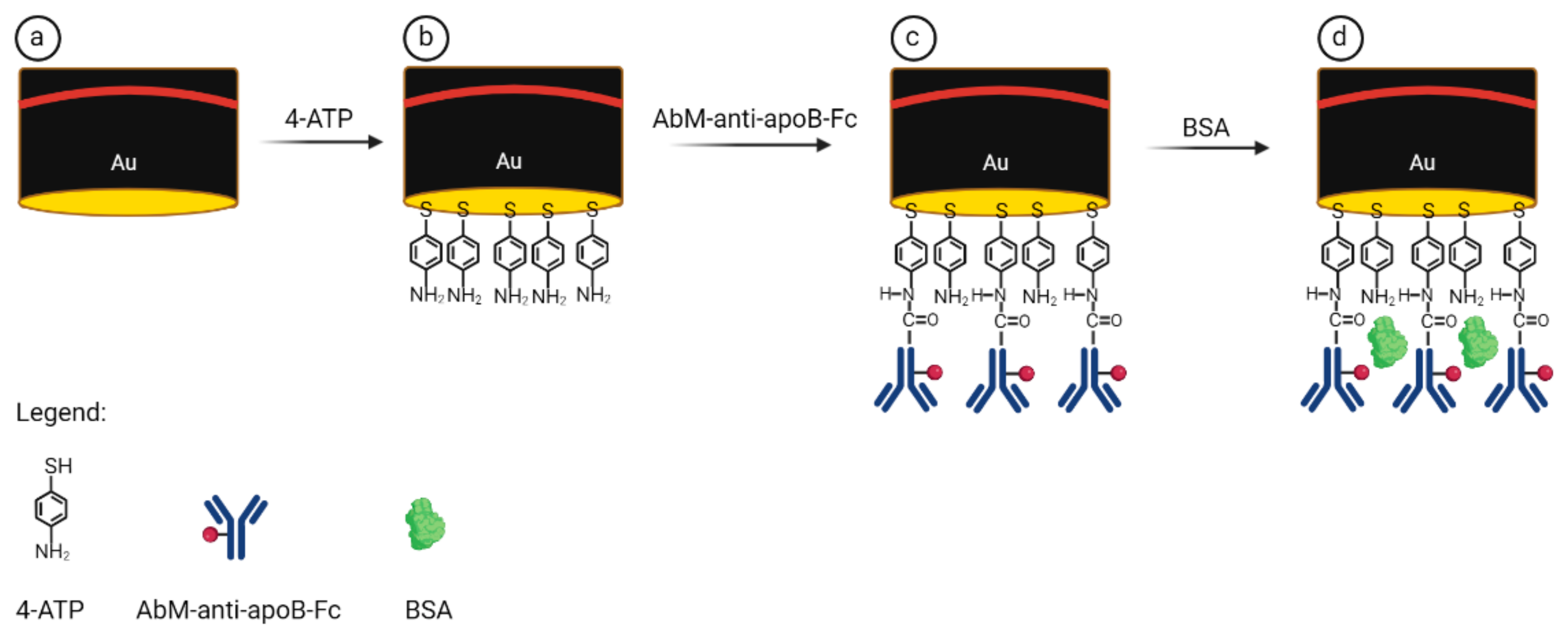
2.2. Preparation of Immunosensor Platform Based on Antibody–Ferrocene Conjugates
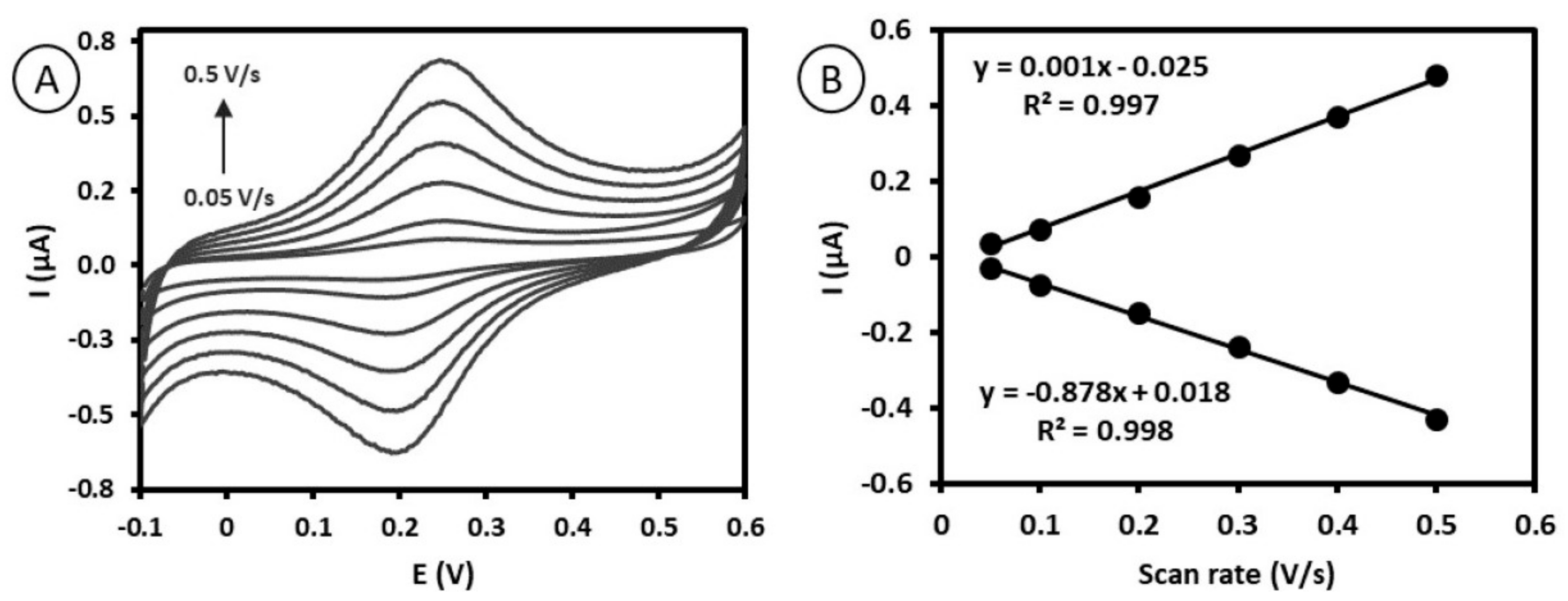
2.3. Electrochemical Characterization of Antibody–Ferrocene Conjugates Deposited on Gold Surface
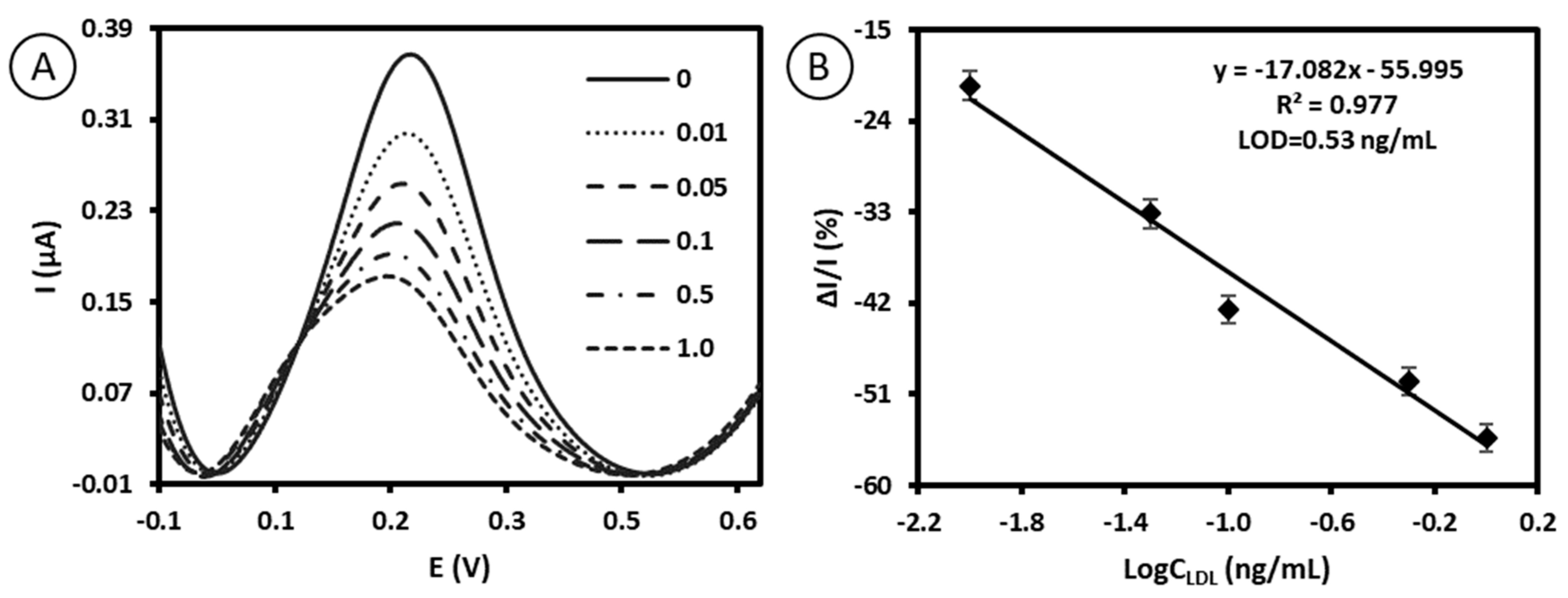
2.4. Quantitative Electrochemical Detection of LDL by Immunosensor Based on Antibody–Ferrocene Conjugates
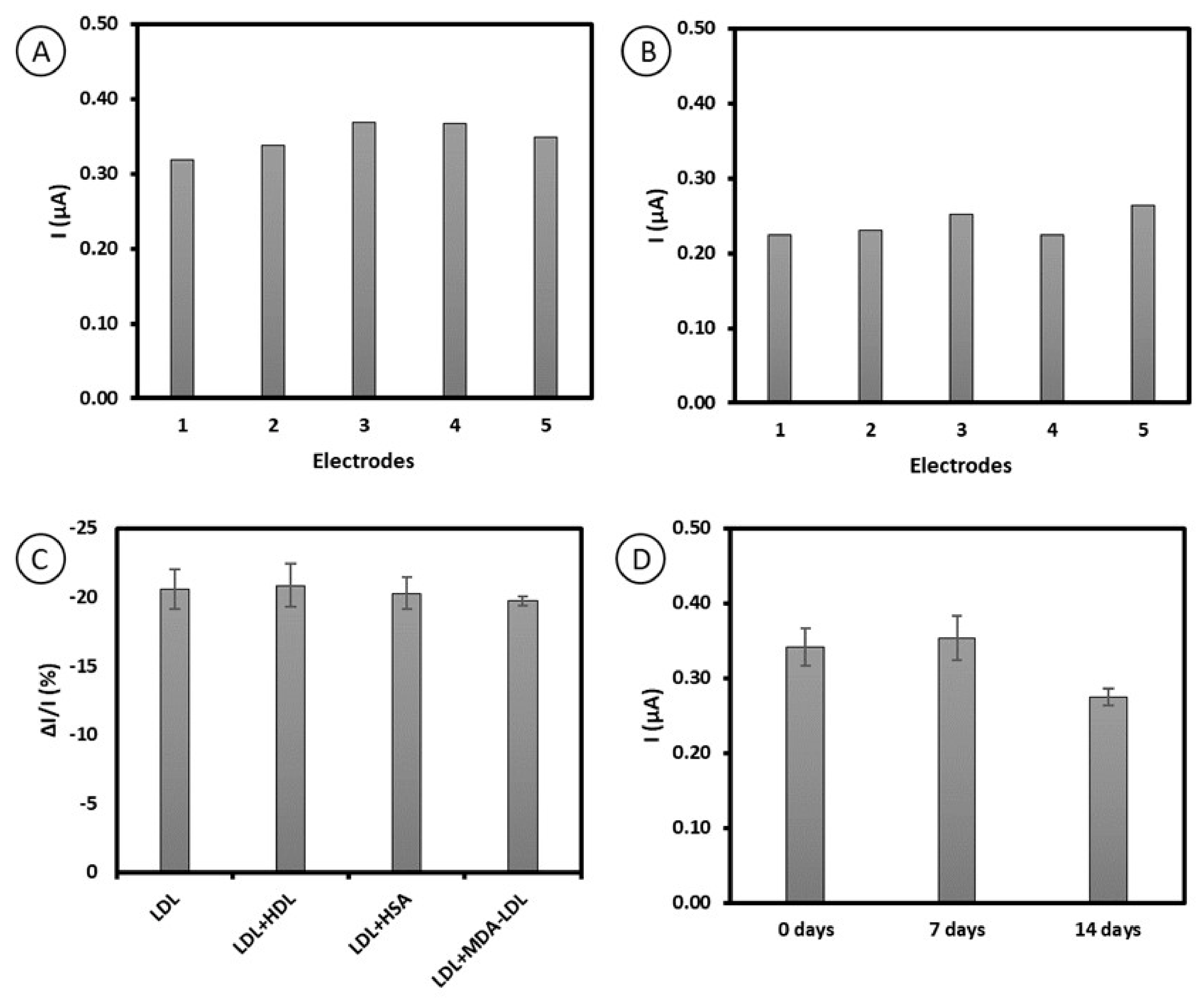
2.5. Reproducibility, Repeatability, Specificity, and Stability Studies of Immunosensor Based on Antibody–Ferrocene Conjugates
2.6. Application of Immunosensor Based on Antibody–Ferrocene Conjugates in Real Sample Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Equipment
3.3. Procedure of Ferrocene-NHS Conjugation to Antibody
3.4. Preparation of Immunosensor Platform Based on Conjugates of Antibodies with Ferrocene
3.5. Electrochemical Measurements of LDL
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Hevonoja, T.; Pentikäinen, M.O.; Hyvönen, M.T.; Kovanen, P.T.; Ala-Korpela, M. Structure of low density lipoprotein (LDL) particles: Basis for understanding molecular changes in modified LDL. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2000, 1488, 189–210. [Google Scholar] [CrossRef]
- Ec, M.-V.; Kk, R. Physiological Level of LDL Cholesterol: The Master Key a Nobel Dream Comes True; Cardiovascular Pharmacology; Walsh Medical Media: London, UK, 2016; Volume 6, p. 5. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Chakraborty, M.; Singh, N. A Leap above Friedewald Formula for Calculation of Low-Density Lipoprotein-Cholesterol. J. Lab. Physicians 2015, 7, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors–A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Mahato, K.; Kumar, S.; Srivastava, A.; Maurya, P.K.; Singh, R.; Chandra, P. Chapter 14-Electrochemical Immunosensors: Fundamentals and Applications in Clinical Diagnostics. In Handbook of Immunoassay Technologies; Vashist, S.K., Luong, J.H.T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 359–414. [Google Scholar]
- Kondzior, M.; Grabowska, I. Antibody-Electroactive Probe Conjugates Based Electrochemical Immunosensors. Sensors 2020, 20, 2014. [Google Scholar] [CrossRef]
- Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Costa-García, A.; de la Escosura-Muñiz, A. Nanoparticles as Emerging Labels in Electrochemical Immunosensors. Sensors 2019, 19, 5137. [Google Scholar] [CrossRef]
- Feng, J.; Chu, C.; Ma, Z. Electrochemical Signal Substance for Multiplexed Immunosensing Interface Construction: A Mini Review. Molecules 2022, 27, 267. [Google Scholar] [CrossRef]
- Zhu, Q.; Chai, Y.; Yuan, R.; Zhuo, Y.; Han, J.; Li, Y.; Liao, N. Amperometric immunosensor for simultaneous detection of three analytes in one interface using dual functionalized graphene sheets integrated with redox-probes as tracer matrixes. Biosens. Bioelectron. 2013, 43, 440–445. [Google Scholar] [CrossRef]
- Jampasa, S.; Siangproh, W.; Laocharoensuk, R.; Vilaivan, T.; Chailapakul, O. Electrochemical detection of c-reactive protein based on anthraquinone-labeled antibody using a screen-printed graphene electrode. Talanta 2018, 183, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Prabhulkar, S.; Alwarappan, S.; Liu, G.; Li, C.-Z. Amperometric micro-immunosensor for the detection of tumor biomarker. Biosens. Bioelectron. 2009, 24, 3524–3530. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chen, X.; Li, X.; Feng, X.; Zhu, J.-J. Fabrication of a Label-Free Electrochemical Immunosensor of Low-Density Lipoprotein. J. Phys. Chem. B 2008, 112, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Matharu, Z.; Sumana, G.; Gupta, V.; Malhotra, B.D. Langmuir–Blodgett films of polyaniline for low density lipoprotein detection. Thin Solid Film. 2010, 519, 1110–1114. [Google Scholar] [CrossRef]
- Ali, M.A.; Kamil Reza, K.; Srivastava, S.; Agrawal, V.V.; John, R.; Malhotra, B.D. Lipid–Lipid Interactions in Aminated Reduced Graphene Oxide Interface for Biosensing Application. Langmuir 2014, 30, 4192–4201. [Google Scholar] [CrossRef]
- Ali, M.A.; Singh, N.; Srivastava, S.; Agrawal, V.V.; John, R.; Onoda, M.; Malhotra, B.D. Chitosan-Modified Carbon Nanotubes-Based Platform for Low-Density Lipoprotein Detection. Appl. Biochem. Biotechnol. 2014, 174, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Srivastava, S.; Agrawal, V.V.; Willander, M.; John, R.; Malhotra, B.D. A biofunctionalized quantum dot–nickel oxide nanorod based smart platform for lipid detection. J. Mater. Chem. B 2016, 4, 2706–2714. [Google Scholar] [CrossRef]
- Ali, M.A.; Solanki, P.R.; Srivastava, S.; Singh, S.; Agrawal, V.V.; John, R.; Malhotra, B.D. Protein Functionalized Carbon Nanotubes-based Smart Lab-on-a-Chip. ACS Appl. Mater. Interfaces 2015, 7, 5837–5846. [Google Scholar] [CrossRef] [PubMed]
- Assaifan, A.K.; Alqahtani, F.A.; Alnamlah, S.; Almutairi, R.; Alkhammash, H.I. Detection and Real-Time Monitoring of LDL-Cholesterol by Redox-Free Impedimetric Biosensors. BioChip J. 2022, 16, 197–206. [Google Scholar] [CrossRef]
- Kaur, G.; Tomar, M.; Gupta, V. Realization of a label-free electrochemical immunosensor for detection of low density lipoprotein using NiO thin film. Biosens. Bioelectron. 2016, 80, 294–299. [Google Scholar] [CrossRef]
- Kaur, G.; Tomar, M.; Gupta, V. Nanostructured NiO-based reagentless biosensor for total cholesterol and low density lipoprotein detection. Anal. Bioanal. Chem. 2017, 409, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Godat, B.; Benink, H.; Urh, M. On-bead antibody-small molecule conjugation using high-capacity magnetic beads. J. Immunol. Methods 2015, 426, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Godat, B.; Zimprich, C.; Dwight, S.J.; Corona, C.; McDougall, M.; Urh, M. Homogeneous plate based antibody internalization assay using pH sensor fluorescent dye. J. Immunol. Methods 2016, 431, 11–21. [Google Scholar] [CrossRef]
- Flygare, J.A.; Pillow, T.H.; Aristoff, P. Antibody-Drug Conjugates for the Treatment of Cancer. Chem. Biol. Drug Des. 2013, 81, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Okochi, M.; Ohta, H.; Tanaka, T.; Matsunaga, T. Electrochemical probe for on-chip type flow immunoassay: Immunoglobulin G labeled with ferrocenecarboaldehyde. Biotechnol. Bioeng. 2005, 90, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Strachan, E.; Mallia, A.K.; Cox, J.M.; Antharavally, B.; Desai, S.; Sykaluk, L.; O’Sullivan, V.; Bell, P.A. Solid-phase biotinylation of antibodies. J. Mol. Recognit. 2004, 17, 268–276. [Google Scholar] [CrossRef]
- Lyon, R.P.; Meyer, D.L.; Setter, J.R.; Senter, P.D. Chapter six-Conjugation of Anticancer Drugs Through Endogenous Monoclonal Antibody Cysteine Residues. In Methods in Enzymology; Wittrup, K.D., Verdine, G.L., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 502, pp. 123–138. [Google Scholar]
- Gao, S.; Guisán, J.M.; Rocha-Martin, J. Oriented immobilization of antibodies onto sensing platforms-A critical review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef]
- Rudewicz-Kowalczyk, D.; Grabowska, I. Detection of Low Density Lipoprotein—Comparison of Electrochemical Immuno- and Aptasensor. Sensors 2021, 21, 7733. [Google Scholar] [CrossRef]
- Chauhan, R.; Solanki, P.R.; Singh, J.; Mukherjee, I.; Basu, T.; Malhotra, B.D. A novel electrochemical piezoelectric label free immunosensor for aflatoxin B1 detection in groundnut. Food Control 2015, 52, 60–70. [Google Scholar] [CrossRef]
- Sonuç, M.N.; Sezgintürk, M.K. Ultrasensitive electrochemical detection of cancer associated biomarker HER3 based on anti-HER3 biosensor. Talanta 2014, 120, 355–361. [Google Scholar] [CrossRef]
- Cebula, Z.; Żołędowska, S.; Dziąbowska, K.; Skwarecka, M.; Malinowska, N.; Białobrzeska, W.; Czaczyk, E.; Siuzdak, K.; Sawczak, M.; Bogdanowicz, R.; et al. Detection of the Plant Pathogen Pseudomonas Syringae pv. Lachrymans on Antibody-Modified Gold Electrodes by Electrochemical Impedance Spectroscopy. Sensors 2019, 19, 5411. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, B.; Karst, U. Ferrocene-based derivatization in analytical chemistry. Anal. Bioanal. Chem. 2008, 390, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.-H.; Haswell, S.J.; Greenman, J.; Wadhawan, J. Voltammetric Immunoassay for the Detection of Protein Biomarkers. Electroanalysis 2012, 24, 264–272. [Google Scholar] [CrossRef]
- Eckermann, A.L.; Feld, D.J.; Shaw, J.A.; Meade, T.J. Electrochemistry of redox-active self-assembled monolayers. Coord. Chem. Rev. 2010, 254, 1769–1802. [Google Scholar] [CrossRef]
- Swartz, M.E.; Krull, I.S. Handbook of Analytical Validation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Le, H.T.N.; Kim, D.; Phan, L.M.T.; Cho, S. Ultrasensitive capacitance sensor to detect amyloid-beta 1-40 in human serum using supramolecular recognition of β-CD/RGO/ITO micro-disk electrode. Talanta 2022, 237, 122907. [Google Scholar] [CrossRef]
- Takamura, T.A.; Tsuchiya, T.; Oda, M.; Watanabe, M.; Saito, R.; Sato-Ishida, R.; Akao, H.; Kawai, Y.; Kitayama, M.; Kajinami, K. Circulating malondialdehyde-modified low-density lipoprotein (MDA-LDL) as a novel predictor of clinical outcome after endovascular therapy in patients with peripheral artery disease (PAD). Atherosclerosis 2017, 263, 192–197. [Google Scholar] [CrossRef]



| Substrate | Redox | Electrochemical Method | Limit of Detection ng/mL | Ref. |
|---|---|---|---|---|
| AuNPs-AgCl@PANI | in solution: [Fe(CN)6]3−/4− | EIS | 0.34 × 10−3 | [15] |
| CNT−NiO | in solution [Fe(CN)6]3−/4− | EIS | 6.3 × 103 | [20] |
| CNT-CH/ITO | in solution [Fe(CN)6]3−/4− | EIS | 1.25 × 105 | [18] |
| CysCdS-nNiO/ITO | in solution [Fe(CN)6]3−/4− | CV | 500 | [19] |
| NH2-rGO/ITO | in solution [Fe(CN)6]3−/4− | EIS | 5.0 × 104 | [17] |
| PANI–SA LB | in solution [Fe(CN)6]3−/4− | EIS | - | [16] |
| Au/4-ATP/AbM/BSA | in solution: [Fe(CN)6]3−/4− | SWV | 0.31 | [31] |
| NiO | on surface NiO | DPV | 52.5 × 103 | [22,23] |
| Au/4-ATP/AbM-Fc/BSA | on surface ferrocene conjugates | SWV | 0.53 | Present work |
| Spiked CLDL (ng/mL) | Measured CLDL (ng/mL) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|
| 0.01 | 0.0105 | 105.0 | 3.50 |
| 0.02 | 0.0195 | 97.5 | 2.02 |
| 0.03 | 0.0290 | 96.7 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudewicz-Kowalczyk, D.; Grabowska, I. Antibody–Ferrocene Conjugates as a Platform for Electro-Chemical Detection of Low-Density Lipoprotein. Molecules 2022, 27, 5492. https://doi.org/10.3390/molecules27175492
Rudewicz-Kowalczyk D, Grabowska I. Antibody–Ferrocene Conjugates as a Platform for Electro-Chemical Detection of Low-Density Lipoprotein. Molecules. 2022; 27(17):5492. https://doi.org/10.3390/molecules27175492
Chicago/Turabian StyleRudewicz-Kowalczyk, Daria, and Iwona Grabowska. 2022. "Antibody–Ferrocene Conjugates as a Platform for Electro-Chemical Detection of Low-Density Lipoprotein" Molecules 27, no. 17: 5492. https://doi.org/10.3390/molecules27175492
APA StyleRudewicz-Kowalczyk, D., & Grabowska, I. (2022). Antibody–Ferrocene Conjugates as a Platform for Electro-Chemical Detection of Low-Density Lipoprotein. Molecules, 27(17), 5492. https://doi.org/10.3390/molecules27175492







