Abstract
G2-like (GLK) transcription factors contribute significantly and extensively in regulating chloroplast growth and development in plants. This study investigated the genome-wide identification, phylogenetic relationships, conserved motifs, promoter cis-elements, MCScanX, divergence times, and expression profile analysis of PeGLK genes in moso bamboo (Phyllostachys edulis). Overall, 78 putative PeGLKs (PeGLK1–PeGLK78) were identified and divided into 13 distinct subfamilies. Each subfamily contains members displaying similar gene structure and motif composition. By synteny analysis, 42 orthologous pairs and highly conserved microsynteny between regions of GLK genes across moso bamboo and maize were found. Furthermore, an analysis of the divergence times indicated that PeGLK genes had a duplication event around 15 million years ago (MYA) and a divergence happened around 38 MYA between PeGLK and ZmGLK. Tissue-specific expression analysis showed that PeGLK genes presented distinct expression profiles in various tissues, and many members were highly expressed in leaves. Additionally, several PeGLKs were significantly up-regulated under cold stress, osmotic stress, and MeJA and GA treatment, implying that they have a likelihood of affecting abiotic stress and phytohormone responses in plants. The results of this study provide a comprehensive understanding of the moso bamboo GLK gene family, as well as elucidating the potential functional characterization of PeGLK genes.
1. Introduction
Chloroplasts contain the green pigment chlorophyll, which can carry out photosynthesis and is thus essential in plant life processes [1,2,3]. Recent research has supported the idea that chloroplasts are derived from protoendosymbiosis events associated with these cyanobacteria [4,5,6]. Furthermore, photosynthetic organs are assembled under the coordinated regulation of chloroplasts and nucleus. Plastids function not only in photosynthesis and nutrient storage, but also in the compartmentalization of various metabolic intermediates [7]; for example, most amino acids, all fatty acids, purines and pyrimidine bases, terpenes and various pigments, and hormones are synthesized in plastids. In addition, proplastids in plant meristem cells (leaf sheaths in dark cotyledons) are converted to chloroplasts of mesophyll cells under light, while GLK genes participate in the regulation and control of chloroplast formation in transition and early maturity and are indispensable in the development of angiosperm chloroplasts [2,7,8,9,10].
GLK genes encode members of the GARP superfamily of nuclear transcription factors [11] defined by Golden2 in maize (Zea mays L.), Response regulator-B (ARR-B) proteins in Arabidopsis thaliana [12], and phosphate starvation response1 (PSR1) protein in Chlamydomonas. Most GLK proteins contain two conserved domains: an Myb-DNA binding domain (DBD, containing an HLH motif) and a C-terminal domain (containing a conserved GCT box) [13,14].
GLK genes promote the production of plant chloroplasts, and optimise photosynthesis under different biotic and abiotic environmental stress conditions [15,16]. For instance, AtGLK1 and AtGLK2 function in the formation and development of chloroplasts redundantly in Arabidopsis [17,18], and AtGLK1 OE increases resistance to Fusarium graminearum, a wide range of host pathogens that cause significant losses in cereal crops. In contrast, the glk1 glk2 double mutant provides resistance to Hyaloperonospora arabidopsidis (Hpa), implying a potential role of GLKs in plant defense and disease resistance [19]. In addition, GLK1 and GLK2 participate in chloroplast growth and development of C3 photosynthetic plants redundantly [15]. In tomato (Solanum lycopersicum), SlGLK2 OE enhances fruit photosynthesis and chloroplast development gene expression, resulting in elevated carbohydrates and carotenoids in ripe fruit, which can lead to strong resistance to cold, drought, heat and other abiotic stresses [20]. In maize, the spatial compartmentalization of ZmGLK1 transcripts and G2 might function as a specialization that was essential to the chloroplast development of mesophyll cells and distinct bundle sheath in plant tissues [14,17].
Moso bamboo (Phyllostachys edulis) is a rapidly growing forest product that is widely used to manufacture paper, art ware, and food resources, with multiple economic, ecological, and cultural values in China and many other countries, sees a possibility for gene identification and function analysis that will help with it contributing on an even wider spectrum with the completion of the genome sequence [6,20,21]. The GLK gene family, previously identified and described within certain plants, for example, maize [22], Arabidopsis [6], tomato [23], and tobacco [24]. However, there are scarcely any reports on the characteristics and functions of GLKs in Phyllostachys edulis. Thus, this study identified 78 putative PeGLK genes and conducted a systematic analysis, including phylogenetic relationships, gene and protein structures, conserved motifs, promoter cis-elements, chromosomal localization, MCScanX, evolutionary patterns, and tissue-specific expression. The expression patterns of PeGLKs in response to abiotic stress (cold and osmotic) and phytohormone (MeJA and GA) treatment were analyzed as well. These results are valuable insightful for further understanding of potential roles of the moso bamboo GLK gene family.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
For this experiment, moso bamboo (Phyllostachys edulis) seeds, which were provided by Gongcheng Yao Autonomous County, Guangxi Zhuang Autonomous Region, China, were germinated and grown under daylight conditions of 16 h light/8 h dark and maintained at 28 °C and 80% relative humidity in an artificial growth chamber for 100-day-old. The 100-day-old plants were subjected to cold stress, osmotic stress, and MeJA and GA treatments. Specifically, Phyllostachys edulis plants were placed in a 4 °C growth chamber and leaves were gathered under cold stress at 0, 1, 3, 6, 12, and 24 h, and under osmotic stress at 0, 1, 2, 3, 6, and 12 h after treatment with pure water containing 20% polyethylene glycol (PEG) 6000. For hormone treatments, a solution of 100 µM Methyl jasmonic (MeJA) and 300 mg/L gibberellic acid (GA) was sprayed onto seedlings based on the requirements, and seedlings were sampled randomly at 0, 1, 3, 6, and 12 h. Negative control plants were treated in a 28 °C artificial growth chamber and sprayed with distilled water.
2.2. Identification of PeGLK Genes
Phyllostachys edulis protein and nucleic acid sequences were acquired from the moso bamboo genome database (http://parrot.genomics.cn, accessed on 10 March 2022). Previously reported GLK protein sequences of Arabidopsis were used to inquire the Phyllostachys edulis protein database with BLASTP tools on 18 March 2022 (http://www.ncbi.nlm.nih.gov/tools/primer-blast/, accessed on 18 March 2022). Then, the redundant sequences were deleted according to the BLAST results of a ClustalW [25] alignment, and Pfam (http://pfam.xfam.org/, accessed on 25 March 2022) was used to identify putative PeGLK members in moso bamboo (Phyllostachys edulis) by analyzing conserved domains of these proteins. Detailed information of the PeGLK genes, including gene IDs, physical positions, gene and protein sequences, and coding sequences (CDSs), were retrieved from the bamboo database. The ExPASy online tool (http://www.expasy.ch/tools/pi_tool.html, accessed on 10 May 2022) was used to determine the physical parameters of PeGLK genes, including molecular weight (MW), isoelectric point (pI), and open reading frame length.
2.3. PeGLK Sequence Alignment and Phylogenetic Analysis
To identify the domain organization of 78 PeGLK proteins, multiple alignments of PeGLK domain-containing sequences were performed using ClustalW [25], and a phylogenetic tree based on the complete PeGLK sequences was constructed using the neighbor-joining method by MEGA 7.0 with 1000 bootstrap replications (https://megasoftware.net/, accessed on 10 April 2022). In addition, the combined phylogenetic tree of PeGLK and ZmGLK protein sequences was also generated with the N-J method.
2.4. Gene and Protein Structure Analysis
The exon–intron structures were obtained by contrasting the CDSs and relevant genomic DNA sequences of PeGLK genes from GSDS (http://gsds.cbi.pku.edu.cn/, accessed on 14 April 2022). Conserved motifs present in the PeGLK proteins were examined by the online MEME tool (http://meme-suite.org/tools/meme, accessed on 18 April 2022) [26]. The optimum width of motifs was limited to 5–50, and the maximal number of motifs was set to 10 residues. Then, we used Pfam tools to determine the motif annotations. The protein homology models were predicted by aligning GLK protein sequences with the HMM-HMM search in intensive mode on the Phyre2 website (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index, accessed on 26 April 2022) [27].
2.5. Chromosomal Location and Synteny Analysis
According to the position information in the genome annotation file (GFF file) of the moso bamboo (Phyllostachys edulis) genome database, 78 PeGLK genes were located on 23 moso bamboo chromosomes. MapInspect software (https://mybiosoftware.com/mapinspect-compare-display-linkage-maps.html, accessed on 3 May 2022) was then used to graphically depict these PeGLK genes. Possible segmental and tandem duplication events were investigated, and potential duplication events were identified by MCScanX software (http://chibba.pgml.uga.edu/mcscan2/#tm, accessed on 10 May 2022) [28].
To perform a collinearity analysis of PeGLK protein in Phyllostachys edulis, BLAST was used to compare the entire sequence, with a cutoff of truncated E-value of 1 × 10−20 [29]. The collinearity analysis of moso bamboo and maize also used the same truncated E-value. MCScanX software was used to produce collinearity blocks for the entire genome by calculating the BLASTP results [28].
2.6. Calculation of Ka/Ks Values
Ka and Ks values represent the numbers of nonsynonymous substitutions per nonsynonymous site and synonymous substitutions per synonymous site [30]. The KaKs Calculator 2.0 (https://ngdc.cncb.ac.cn/biocode/tools/BT000001, accessed on 25 May 2022) was used to calculate Ka and Ks values with the NY model, and the ratios were then calculated using DnaSP5 [31,32]. Additionally, the divergence time (T) was calculated using the formula T = Ks/2 λ (λ = 6.5 × 10−9) by transforming the duplication event date for each gene pair [33].
2.7. Putative Promoter Region Analysis of PeGLK Genes
The 2000 bp upstream sequences of PeGLK genes were selected to determine the cis-elements in the putative promoter regions. PlantCARE (http://www.dna.affrc.go.jp/PLACE/, accessed on 1 June 2022) was used to predict the putative cis-elements in the gene promoters [34,35]. Then, the cis-elements that responded to abiotic stress and phytohormone treatments were screened out.
2.8. PeGLK Expression Profiles, RNA Extraction, and qRT-PCR Analysis
According to the plant’s growth characteristics, four different tissues (leaf, root, stem, and rhizome) of a 100-day-old moso bamboo were used as samples. Then, the tissue-specific expression profile for each PeGLK gene was determined, taking the tonoplast intrinsic protein 41 (TIP41) gene as the internal reference standard, and the relative expression level of each gene was calculated via the 2−ΔΔCt method [36,37]. The heatmap of PeGLK gene expression was drawn using the Amazing Heatmap module in TBtools (https://github.com/CJ-Chen/TBtools/releases, accessed on 15 June 2022) for the four Phyllostachys edulis tissues [38].
According to the similarity with the reference gene in the phylogenetic tree, 36 genes were used for qRT-PCR. The 36 primers of PeGLK genes were designed by the NCBI Primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/, accessed on 25 June 2022) to amplify 150–300 bp PCR products (Table S4, Supplementary Materials). Total RNA was collected from samples of four different tissues (leaf, stem, rhizome, and root) using an RNA Easy Fast Plant Tissue Kit (Tiangen, Beijing, China) based on the manufacturer’s instructions. First-strand cDNA was synthesized using total RNA by an EasyPure Genomic DNA Kit (TransGen Biotech, Beijing, China) [39]. Reaction was performed by the Light Cycler 480 SYBR Green Master Mix (TaKaRa, Dalian, China) on a Bio-Rad CFX96, and the program was used as follows: 95 °C for 60 s, followed by 40 cycles of 95 °C for 10 s, and 60 °C for 30 s.
3. Results
3.1. Identification of PeGLK Genes in Phyllostachys edulis
To identify the moso bamboo GLK gene family members, previously reported Arabidopsis AtGLK1 (AT2G20570) and AtGLK2 (AT5G44190) protein sequences [17] were used as BLASTP queries to search for available protein databases in the moso bamboo genome bank. We identified 78 PeGLK candidate genes, most of which were used to confirm the presence of the Myb DNA-binding domain (DBD) and C-terminal domain (containing a conserved GCT box) using the Pfam database. Except for the conserved GLK Myb DNA-binding domain, the members of each subfamily have suspected functional diversity, represented by unique motifs. For instance, among groups 1–7, each has an Myb-CC-LHEQLE domain, which is a type of Myb-like domain. Group 10 has an REC-typeB-ARR-like domain (Figure 1). To discern the similarities between moso bamboo GLK domains, the domain sequences of 78 PeGLK proteins were blasted with the DNAMAN 8 platform (Figure S1). The results showed that PeGLKs were conserved in two regions of a putative Myb DNA-binding domain, with the HLH structure of the first helix containing the initial sequence, PELHRR, and the second helix containing NI/VASHLQ, which was consistent with the GLK members in Arabidopsis, maize [22], tobacco, and tomato [40].
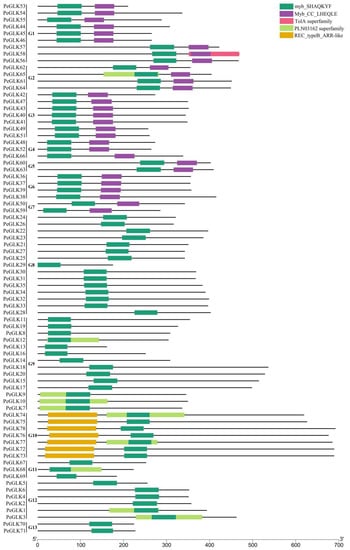
Figure 1.
Division of 78 PeGLK genes into 13 groups (G1–G13) based on predicted domain structures. Domains are represented by different colored boxes.
Basic information about PeGLK genes, such as the accession number, position, and physicochemical parameters, is presented in Table 1. The molecular weight (MW) varies from 18.08 to 75.00 kDa and the length of CDSs ranged from 486 to 2079 bp, and encoded sequences ranged from 161 to 692 aa. In addition, the theoretical isoelectric point (pI) ranged from 5.51 to 10.43.

Table 1.
Detailed information on 78 predicted PeGLK genes in Phyllostachys edulis. Last column lists gene ID, chromosomal locations, molecular weight (MW), protein isoelectric point (pI), and number of exons in each gene.
3.2. Phylogenetic and Exon-Intron Structure Analysis of GLK Genes
From a phylogenetic tree formulated on the aligning of 137 sequences of GLK proteins from moso bamboo (78) and maize (59), the phylogenetic relationships of GLK proteins among different species were derived. The characteristics of ZmGLK genes from maize are listed in detail in Table S1. In this phylogenetic tree, GLK family members were classified into 16 groups based on evolutionary relationships and motif analysis, and poplar GLK family members were allocated into 13 groups (G1–G13, but not G14–G16). The numbers of PeGLKs in different groups were uneven: groups 1 to 13 contained 9, 4, 7, 5, 3, 4, 2, 15, 14, 7, 3, 6, and 2 genes, respectively (Figure 2).
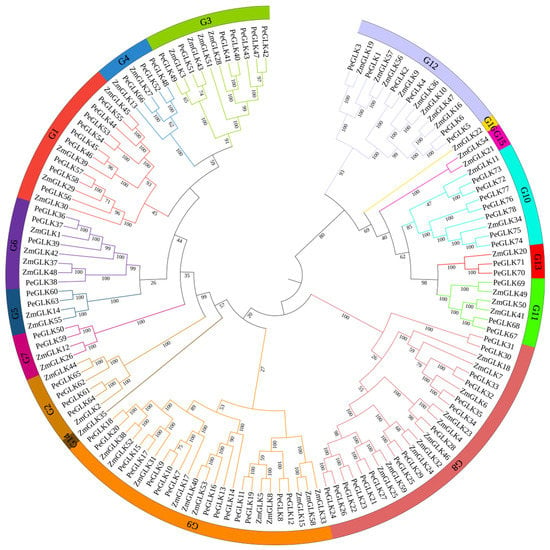
Figure 2.
Phylogenetic analysis of GLK proteins of moso bamboo and maize. Tree was constructed using neighbor-joining method with MEGA7.0. Each color represents one group in the branches, and 16 groups were found in total. The numbers at nodes indicate the bootstrap values per 1000 replicates determined by the neighbor-joining method.
To further explore the evolutionary relationships of PeGLK genes, a single phylogenetic tree was constructed using the moso bamboo PeGLK protein sequences. PeGLK proteins were also classified into 13 distinct subfamilies, in accordance with the phylogenetic tree constructed by moso bamboo and maize. The exon–intron predictions were identified by the online Gene Structure Display Server (GSDS) tool. As shown in Figure 3, the number of exons in different subfamilies varied from 1 to 12, and most PeGLK genes (91%) had five or more exons. In addition, most genomic sequences contained upstream and downstream sequences, except for PeGLK5, PeGLK13, PeGLK48, PeGLK50, PeGLK67, PeGLK68, PeGLK69, and PeGLK77, whereas PeGLK12 and PeGLK41 had only upstream sequences, and PeGLK19, PeGLK29, PeGLK36, PeGLK51, PeGLK70, and PeGLK74 had only downstream sequences. In general, the vast majority of putative paralogous had significant changes in structural organization and intron/exon numbers, except for three paralogous (PeGLK5/PeGLK6, PeGLK62/PeGLK65, PeGLK48/PeGLK67), which had the same intron/exon numbers but different intron lengths.
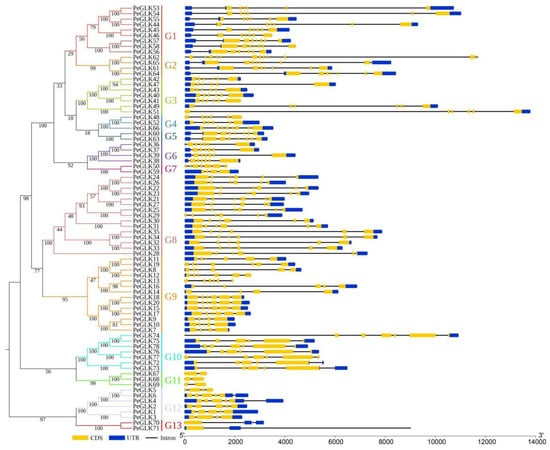
Figure 3.
Phylogenetic relationship and gene structures of PeGLKs. Left: Neighbor-joining (NJ) phylogenetic tree was constructed by MEGA 7.0 according to PeGLK protein sequences. Proteins in tree were divided into 13 distinct subfamilies, which are distinguished by different colors. Right: Exon/intron structures of PeGLK genes were generated by online GSDS. Yellow rectangles, gray lines, and blue rectangles represent exons, introns, and untranslated regions (UTRs), respectively. The numbers at nodes indicate the bootstrap values per 1000 replicates determined by the neighbor-joining method.
3.3. Identification of Conserved Sequence Motifs and Homology Modeling in PeGLK Genes
To evaluate the phylogenetic kinship, we examined the conserved motifs of 78 PeGLK proteins using the MEME software (Figure 4). In Table S2 the eight distinct motifs found are shown, with their specific characteristics noted. Functional annotation was made for six putative motifs with the Conserved Domain Database (CDD), in which motifs 1 and 2 were defined as Myb-SHAQKYF, motif 3 was Myb-CC-LHEQLE, and motifs 5, 6, and 8 were REC-type B-ARR-like. However, the remaining two putative motifs did not have functional annotations. Protein family members within the same group shared similar or identical components and spatial distributions, which implies that these proteins have similar functions. For instance, all of the PeGLK proteins were characterized by motif 1 in the Myb DNA-binding domain, with an HLH structure (the first helix contains initial sequence PELHRR and invariably comprises 14 amino acids, and the second helix contains an initial NI/VASHLQ motif. These helices are separated by a 22 amino acid loop.). Additionally, there was specificity within different subfamilies. For example, motif 5 was only present in subfamilies 1, 2, 3, 4, 5, 6, and 7, and motifs 5, 6, 8 only appeared in subfamily 10.
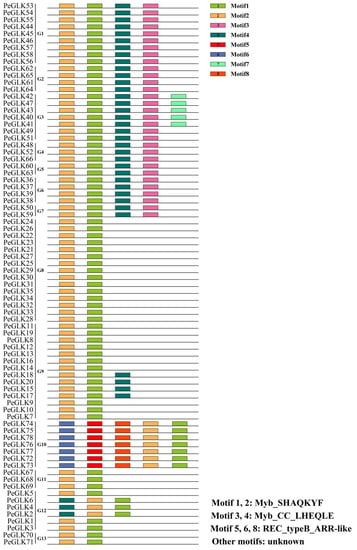
Figure 4.
Schematic representation of eight conserved motifs (1–8) in PeGLKs, ordered based on online MEME analysis. Lengths of motifs are displayed proportionally.
The structure and homology modeling of PeGLK proteins were determined by Phyre2, and alignment of the protein sequences with HMM-HMM search was conducted in intensive mode [41]. As a result, all 78 PeGLK proteins could be modeled with certainty. As shown in Figure 5, 10 PeGLKs (PeGLK5, PeGLK12, PeGLK13, PeGLK29, PeGLK49, PeGLK51, PeGLK53, PeGLK70, PeGLK71, and PeGLK74) had 100% of their predicted length modeled with >98% confidence.
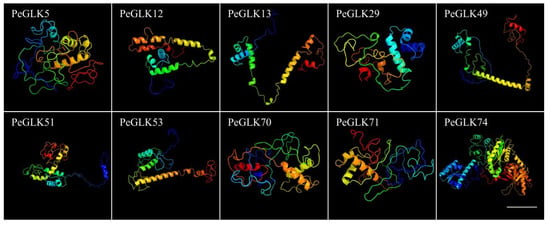
Figure 5.
Predicted structures of PeGLK proteins. Structures of 10 PeGLK proteins were predicted with >98% confidence. All colored lines represent α-helix, and the arrow with plane shape is β-sheet, which displayed only in PeGLK74. Bars: 20 nm.
3.4. Physical Locations and Duplication Events of Pelf Genes in Phyllostachys edulis
The 78 PeGLKs were unevenly distributed on 23 moso bamboo chromosomes (Chr2-Chr24), except Chr1 (Figure 6). Chromosomes 6 and 8 contained eight PeGLK genes, the highest number; chromosomes 15, 17, and 21 had seven, and 20 had five, whereas there was only one PeGLK gene on chromosomes 2, 5, 12, 14, and 18. Interestingly, Chr13 was the longest chromosome with only four PeGLK genes. These results indicate that the chromosome length was not proportional to the number of genes. To explore the potential mechanism of the PeGLK gene family, the MCScanX program was used to investigate potential segmental and tandem duplication events. As shown in Figure 7A, 31 segmental duplicated pairs and one tandem duplicated pair of PeGLK genes were identified in a synteny map (Table 2). The 31 segmental duplicated pairs presented a biased distribution pattern, and no pairs were distributed on chromosome 1. These results suggest that moso bamboo PeGLKs may have arisen mainly from segmental duplication events.
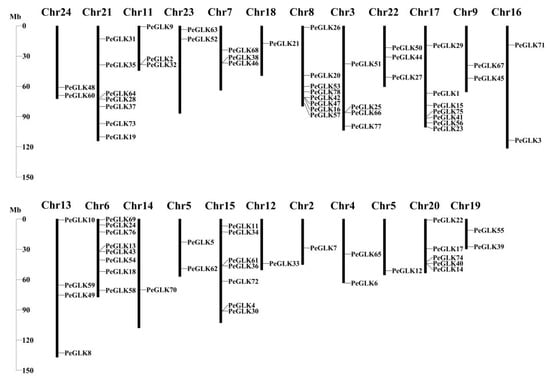
Figure 6.
Chromosomal locations of PeGLK genes in Phyllostachys edulis.
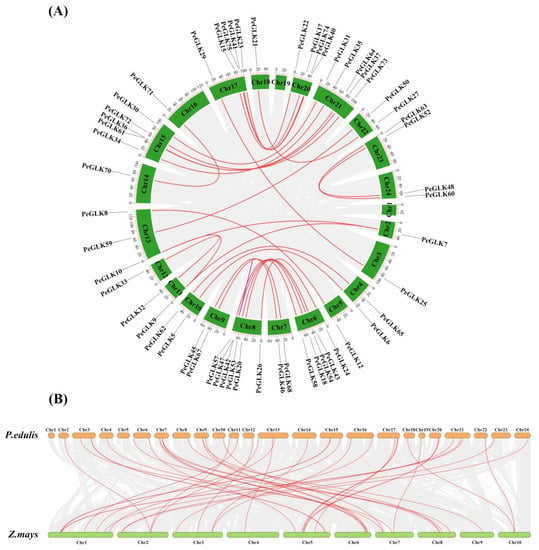
Figure 7.
Duplication events of GLK proteins. (A) Synteny of moso bamboo PeGLK genes. (B) Synteny of moso bamboo and maize GLK genes. All syntenic genes were located on a map; segmentally duplicated GLK gene pairs are linked by red lines and tandemly duplicated genes by purple lines.

Table 2.
Ka/Ks and divergence analysis of paralogous PeGLK genes in moso bamboo.
To get additional insight into PeGLK’s evolutionary orthologous relationships, a synteny map was plotted between moso bamboo and maize. As shown in Figure 7B, 42 orthologous pairs (Pe-Zm) of moso bamboo and maize were determined (Table 3). Across the two, highly conserved microsynteny was observed in the regions of PeGLK genes, especially in Pe3 and Zm5, Pe7 and Zm6, Pe15 and Zm1, Pe17 and Zm5, and Pe21 and Zm1, all with three synteny genes.

Table 3.
Ka/Ks and divergence analysis of orthologous GLK genes in moso bamboo and maize.
3.5. Evolutionary Patterns and Divergence Times of GLK Genes in Moso Bamboo and Maize
To explore the selective constrains for the duplicated PeGLK gene pairs, a comprehensive analysis of the Ka/Ks ratio and Ks values was conducted using full-length sequences to estimate divergence times. As shown in Figure 8 and Table 2, the distribution of calculated Ks values of paralogous pairs (Pe-Pe) showed an average value of ~0.2, indicating that PeGLK genes underwent a large-scale duplication event circa 15 MYA. A previous study estimated that the timing for whole-genome duplication of moso bamboo was 7-12 MYA [42], implying that large-scale duplication of PeGLK genes occurred earlier [33]. In addition, for the orthologous maize–moso bamboo pairs, the averaged Ks value was ~0.5 (Figure 8 and Table 3), indicating GLK genes differentiated 38 MYA. A comparison with a previous study revealed 42–52 divergence times between moso bamboo and maize, implying that GLK genes encountered gene evolution before the isolation of maize. In principle, Ka/Ks ratios greater than, equal to, and less than 1 indicate accelerated evolution with positive, neutral, and negative or stabilizing selection, respectively [26,43]. The Ka/Ks ratios of the Pe-Pe (Table 2) and Pe-Zm (Table 3) genomes were less than 1, which indicates that GLK genes experienced highly positive purifying selection among moso bamboo–maize and moso bamboo.
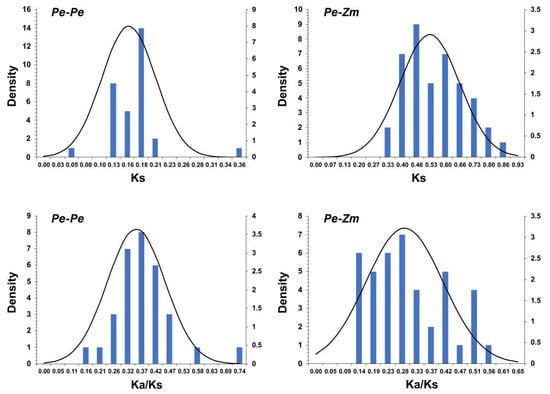
Figure 8.
Ks and Ka/Ks value distribution of PeGLK genes in paralogous gene pairs (Pe-Pe) of moso bamboo genome and orthologous gene pairs between moso bamboo and maize, viewed through frequency distribution of relative Ks and Ka/Ks modes.
3.6. PeGLK Expression Levels in Different Tissues
Tissue-specific gene expression profiles provide critical clues for exploring gene function. In order to characterize the expression levels of PeGLKs, we analyzed the transcription levels of 36 PeGLK genes selected as representatives of each subfamily in four different tissues (leaf, stem, rhizome, and root) of moso bamboo (Table S3). As shown in Figure 9, the vast majority of PeGLK genes (except for PeGLK6, PeGLK7, PeGLK52, PeGLK61, PeGLK63, PeGLK68, PeGLK71) in the leaf, nine genes (PeGLK47, PeGLK51, PeGLK52, PeGLK60, PeGLK63, PeGLK65, PeGLK66, PeGLK69, PeGLK71) in stem, four genes (PeGLK44, PeGLK52, PeGLK53, PeGLK68) in rhizome, and 12 genes (PeGLK6, PeGLK7, PeGLK20, PeGLK21, PeGLK28, PeGLK36, PeGLK37, PeGLK39, PeGLK53, PeGLK61, PeGLK72, PeGLK78) in root presented high expression levels. Interestingly, the expression level of most PeGLKs was significantly higher in leaf than in the other tissues. We also defined that some genes showed a tissue-specific expression pattern. For example, PeGLK20, PeGLK21, PeGLK28, PeGLK36, PeGLK37, PeGLK39, PeGLK72, and PeGLK78 displayed high expression levels in leaves and roots, but low levels in stems and rhizomes. PeGLK52 exhibited high expression levels in stems and rhizomes, but low levels in leaves and roots. PeGLK47, PeGLK51, PeGLK60, PeGLK65, PeGLK66, and PeGLK67 presented high expression levels in leaves and stems, but low levels in rhizomes and roots. PeGLK44 and PeGLK70 displayed high expression levels in leaves and rhizomes, but low levels in stems and roots. In addition, only PeGLK63 and PeGLK67 were prominently expressed in stems, and only PeGLK68 was prominently expressed in rhizomes, whereas PeGLK6, PeGLK7, PeGLK21, PeGLK36, PeGLK61, and PeGLK78 were relatively higher in roots, PeGLK63 and PeGLK71 in stems, and PeGLK52 and PeGLK68 in rhizomes than in the other tissues. Among our previously identified paralogous genes, five pairs (PeGLK60 and PeGLK63, PeGLK62 and PeGLK65, PeGLK67 and PeGLK68, PeGLK70 and PeGLK72) shared the same or similar expression patterns in the four tissues, revealing the same evolutionary fate of duplicated genes. Above all, PeGLKs displayed diverse expression profiles in different tissues, which provides insight into the role of PeGLKs in multiple growth and development of Phyllostachys edulis.
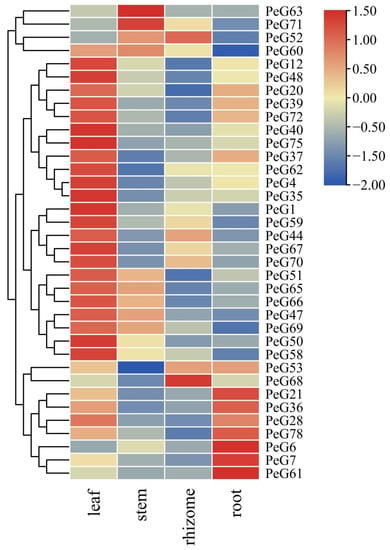
Figure 9.
Expression profiles of PeGLK genes in different tissues. Samples were from leaf, steam, rhizome, and root. Expression level of each PeGLK gene can be estimated based on scale on right. Red, yellow and blue indicate high, medium and low levels of gene expression, respectively.
3.7. Expression Profiles of GLK Genes under Abiotic Stress and Phytohormone Treatment
To determine the cis-regulatory elements in the putative promoter regions, we used PlantCARE to detect the 2000 bp upstream sequences of PeGLK genes [44]. Results portray that many cis-regulatory elements corresponding to LTRE (cold-responsive element), DRE (drought-responsive element), MeJA, and GA were detected, indicating that PeGLKs participate in plant development and stress responses (Figure 10).

Figure 10.
cis-Acting elements related to LTRE, DRE, MeJA, and GA in promoter regions of PeGLKs. Left: Different colors represent numbers of four cis-acting elements of PeGLKs. Right: Four CREs were predicted; each is displayed in a different color.
Several GLK genes have been reported to respond to cold and osmotic stresses in maize [22], tobacco [45], and tomato [24]. To investigate whether the expression of PeGLK genes was affected by abiotic stress and phytohormone treatment, we detected the dynamic expression levels of 13 genes (PeGLK1, PeGLK7, PeGLK21, PeGLK36, PeGLK40, PeGLK48, PeGLK50, PeGLK53, PeGLK60, PeGLK61, PeGLK67, PeGLK70, and PeGLK72) as representatives of each subfamily under cold and osmotic stress and MeJA and GA treatment (Figure 11 and Figure 12, and Table S5).
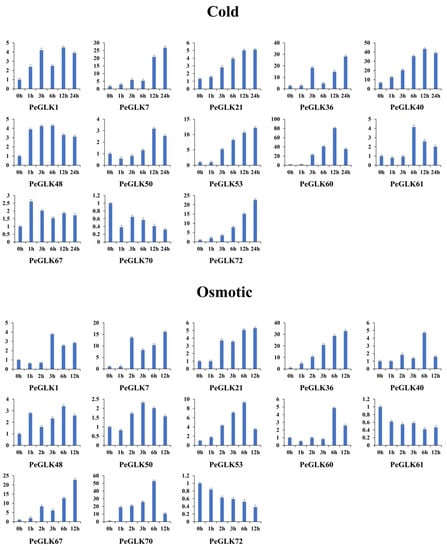
Figure 11.
Expression patterns of 13 representative PeGLK genes in response to abiotic stress treatments. X-axis and Y-axis indicate time points after cold and osmotic treatments, and relative expression levels are standardized to reference gene TIP41.
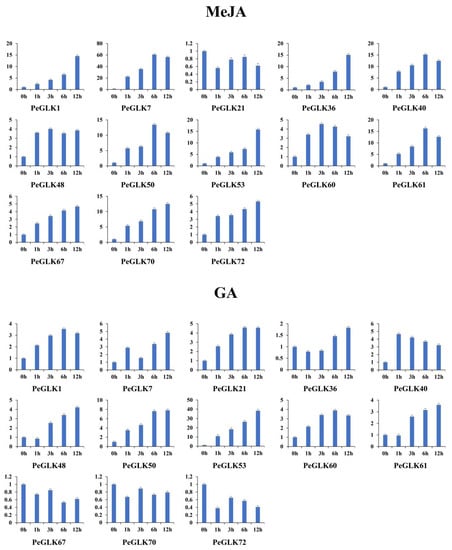
Figure 12.
Expression patterns of 13 representative PeGLK genes in response to phytohormone treatments. X-axis and Y-axis indicate time points after MeJA and GA treatments, and relative expression levels are standardized to reference gene TIP41.
In the cold stress treatment, eight genes (PeGLK1, PeGLK21, PeGLK36, PeGLK40, PeGLK53, PeGLK60, PeGLK67, and PeGLK72) were obviously up-regulated compared with 0 h. For example, the expression of two genes (PeGLK40 and PeGLK60) peaked at 12 h, and PeGLK60 was the most highly expressed (>80-fold) after 12 h of treatment. Four genes (PeGLK7, PeGLK36, PeGLK53, and PeGLK72) gradually increased over time and peaked at 24 h. Seven genes (PeGLK1, PeGLK21, PeGLK48, PeGLK50, PeGLK61, PeGLK67, and PeGLK70) showed slight (<5-fold) changes in response to cold stress treatment.
In the osmotic stress treatment, five genes (PeGLK7, PeGLK36, PeGLK53, PeGLK67, and PeGLK70) were obviously up-regulated compared with 0 h. For instance, two genes (PeGLK53 and PeGLK70) gradually accumulated and peaked at 6 h, especially PeGLK70, which showed an expression level more than 50-fold higher at 6 h, whereas the expression levels of PeGLK7, PeGLK36, and PeGLK67 presented parabolic trends and were highest at 12 h.
In the MeJA treatment, four genes (PeGLK1, PeGLK36, PeGLK53, and PeGLK70) showed the highest expression at 12 h, and then a gradually increasing trend. The expression levels of four genes (PeGLK7, PeGLK40, PeGLK50, and PeGLK61) were highest at 6 h, especially PeGLK7, which presented more than 60-fold higher expression at 6 h. In the GA treatment, two genes (PeGLK50 and PeGLK53) were up-regulated, and PeGLK53 exhibited an expression level more than 35-fold higher at 12 h.
In total, 46.2, 38.5, 61.5, and 15.4% of genes, including six cold stress-related genes, five osmotic stress-related genes, eight MeJA-related genes, and two GA-related genes, were differently expressed; the detailed gene lists are shown in Figure S2. However, only the expression of PeGLK53 showed significant changes in response to the two abiotic stresses (Figure 11) and two phytohormone treatments (Figure 12). Additionally, the above results coincide with the promoter analysis of PeGLK members, indicating the widespread presence of several osmotic, cold, MeJA, and GA responsive cis-elements. Overall, these results imply that some PeGLK genes have potential functions in response to abiotic stress and phytohormone treatments.
4. Discussion
The function of GLK genes was first analyzed in maize and GLK genes were served as transcription factors. The GLK gene sequences were only found in photosynthetic eukaryotes such as green algae and higher plants, not present in the genome of cyanobacteria, which indicated that GLK genes were involved in the formation and development of chloroplast [45]. Members of the GLK gene family were characteristic groups in nuclear coding genes, and general feature of organisms with GLK genes were associated with chloroplasts and chlorophyll. Chloroplasts convert light energy into chemical energy and play a significant role in plant growth and development; thus, the study of GLK genes has become an important prospect of investigation [46].
4.1. GLKs in Phyllostachys edulis
The GLK transcription factor, which is a member of the newly classified GARP superfamily [14], plays an essential role in the formation and development of chloroplasts [11,47]. As reported in previous studies, detailed features and functions of GLK genes have been uncovered in Arabidopsis [6], maize [22], tobacco [24], and tomato [23]. However, little was known about the members of the moso bamboo GLK family until now. Here, 78 putative PeGLK genes were identified, which is 22 more than maize (59), and there are 30 and 42 times as many genes as tomato (54) and sorghum (45), respectively. The higher number of PeGLK genes was in accord with the genome duplication event that occurred in Phyllostachys edulis [33,48]. According to the phylogenetic analysis of grouping with maize sequences, the predicted moso bamboo GLK family members were divided into 13 groups, G1-G13. However, there were no orthologous genes in maize G14 and G15, which suggests divergence between maize and moso bamboo. In addition, the PeGLKs belonging to the same subfamilies displayed high similarity based on their domain structures and number of exons/introns, and these identical results indicate that the PeGLK groupings are relatively reliable.
4.2. Physical Locations and Divergence of GLKs in Moso Bamboo and Maize
According to the chromosome location analysis, PeGLKs were extensively and unevenly spread across 31 chromosomes of Phyllostachys edulis, which may be because of insertions, deletions, duplications, and reversions. Previous reports have shown that gene duplication is an important means of gene expansion in the process of plant genome evolution and is also essential for organisms to adapt to various environments during development and growth [49,50]. Thus, we performed genomic collinearity analysis, and found that the moso bamboo genome has 32 pairs of duplicated PeGLK genes, including 31 segmental and one tandem duplicated pair. Segmental duplication was obviously the major method of expansion of the moso bamboo GLK gene family. Segmental duplication has been shown to be more universal than tandem replication and to play a significant role in long-term evolution [22,51,52,53]. Otherwise, syntheny analysis of the moso bamboo genome with maize sequenced plant genomes indicated significant collinearity of family members between bamboo and monocot maize, which is in accord with the evolutionary relationship between dicotyledons and monocotyledons.
To get more insight into the Phyllostachys edulis macroevolutionary profile and divergence times, Ks and Ka for paralogous and orthologous gene pairs were estimated and Ks and Ka/Ks values were calculated for each gene pair. The Ks values showed that Phyllostachys edulis had a large-scale duplication event about 15 MYA, and the divergence time for orthologous gene pairs (Pe-Zm) was approximate 38 MYA. It has also been shown that a whole genome duplication event occurred in Phyllostachys edulis 7-12 MYA and the divergence time between moso bamboo and maize was 35-42 MYA [33]. Compared with the previous reports, our results suggest that the moso bamboo GLK gene family underwent an earlier large-scale duplication event and diversified earlier than maize. In general, the Ka/Ks ratio was available to determine the selective pressure of choice acts on the coding sequences [37]. In the current study, the Ka/Ks ratios for Pe-Pe and Pe-Zm gene pairs were both <1, which imply that the homologous PeGLK gene pairs experienced strong purifying selection during the evolutionary process [26,43].
4.3. Potential Functions of PeGLKs in Moso Bamboo Development and Stress Responses
GLK genes in maize play different roles in the growth and development of chloroplasts in mesophyll cells and bundle sheaths, and they also guide chloroplast development into monomorphic form in Arabidopsis [14,17]. Here, we examined the expression levels of 36 putative PeGLK genes in four different tissues of Phyllostachys edulis and found that great majority genes exhibited high expression levels in the leaf. In view of the important function of chloroplasts in the conversion of light energy into chemical energy in plant growth and development [54], these results imply that PeGLK might be involved in the development of chloroplasts, and the high expression level in leaves might satisfy the active metabolism or gene activity. Moreover, ZmGLK47 was reported to have high expression in all maize tissues, related to the formation and development of chloroplasts [22]. PeGLK67, as an ortholog to ZmGLK47 in Phyllostachys edulis, exhibits the same protein structure and expression patterns.
Plant genomes include various stress-related genes which enable them to respond to diverse environmental conditions [54]. Additionally, the cis-elements of promoter regions largely determine the stress-response gene expression profiles that help plants adapt to adverse conditions, and are also closely linked with multiple stimulus-response genes [55,56]. In view of the function of cis-elements in abiotic stress resistance, we examined the cis-elements of PeGLKs and discovered a high number of DRE, LTRE, MeJA, and GA responsive sequences in PeGLK promoters, which indicates the significant role of PeGLKs in stress responses [22]. Plants produce many signaling molecules in response to abiotic stresses and phytohormone, treatments such as low temperature, drought, salinity, ABA, MeJA, GA, and SA. To date, an association of plant GLK with abiotic and phytohormone stress responses has been discovered in many species [6,22,23,24]. Thus, we examined the expression of 13 selected PeGLK genes in response to cold, osmotic, MeJA, and GA stress in Phyllostachys edulis. Previous studies have shown that orthologous genes in different species were conservative in their gene functions, and paralogous genes present different functions owing to gene duplication [57]. Compared with previous studies, we found that the expression of PeGLK36 and its ortholog in maize, ZmGLK1, exhibited similar patterns in their cold and osmotic stress responses [22]. Conversely, the expression of PeGLK67 and its ortholog in maize, ZmGLK50, showed opposite patterns, indicating that PeGLKs may have lost or acquired functions in the process of evolution. Additionally, previous reports have also shown that AtGLK1 may be involved in the SA and JA signaling pathway in disease prevention [19], and OsGLK1 is related to resistance to pathogen invasion [58]. Further research on PeGLKs might provide more insight into stress-resistant and disease-resistant Phyllostachys edulis varieties.
5. Conclusions
In this study, 78 moso bamboo GLK family genes were identified and could be divided into 11 subfamilies. Members of the same subfamily displayed similar gene structures and motif compositions, implying that PeGLK genes were conserved in the process of evolution. Furthermore, 78 PeGLK genes were unevenly distributed across the 23 moso bamboo chromosomes. Evolutionary analysis showed that segmental duplication was the main reason for the expansion of moso bamboo GLK gene family. The expression profiles of moso bamboo GLK genes indicated that PeGLKs play significant roles in different tissues of moso bamboo growth and development, and PeGLKs was highly expressed in leaf than in other tissues. The expression patterns of PeGLK genes under cold stress, osmotic stress, and MeJA and GA treatments offer a different viewpoint about the function of moso bamboo GLKs in responses to different abiotic stress and phytohormones treatments. Taken together, these results may be helpful to select suitable candidate genes for further cloning and provide valuable information for functional analysis of PeGLK genes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27175491/s1, Figure S1: Multiple sequence alignment of the moso bamboo GLK conserved domain. Identical residues are shown in black shadow and similar residues in pink; Figure S2: Percentage of PeGLKs response to abiotic stress and hormone treatments. Up and Down represent up and down regulated after cold stress, osmotic stress, MeJA, and GA treatments, respectively. No represents no expression change after treatments; Table S1: Detailed information about ZmGLK genes in maize; Table S2: The MEME motif sequences and lengths of PeGLK proteins in moso bamboo; Table S3: The microarray data of 36 PeGLK genes in Phyllostachys edulis; Table S4: The primers of qRT-PCR of 37 PeGLKs and PeTIP41; Table S5: Expression level of PeGLK genes in response to cold stress, osmotic stress, MeJA and GA treatments.
Author Contributions
Conceptualization, R.W. (Ruihua Wu); methodology, L.G.; software, L.G.; validation, L.G., R.W. (Ruoyu Wang); formal analysis, R.W. (Ruoyu Wang); investigation, R.W. (Ruihua Wu); resources, R.W. (Ruihua Wu) and Q.Z.; data curation, L.G.; writing—original draft preparation, R.W. (Ruihua Wu); writing—review and editing, R.W. (Ruihua Wu); visualization, H.Y.; supervision, H.Y.; project administration, R.W. (Ruihua Wu); funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Nature Science Foundation of China (Grant No. 32070548), the National Key Research and Development Program 570 of China (Grant No. 2018 YFD0600101), the National Natural Science Foundation of China (Grant No. 31270737), and Beijing Forestry University Excellent experimenter cultivation project (BJFUSY20210908).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Yixin Liu, from college of landscape architecture and art, the Northwest A & F University, XianYang, China, for providing technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds MeJA, GA, PEG are available from the authors.
Abbreviations
| CDD | conserved domain database |
| DBD | Myb DNA-binding domain |
| DRE | drought-responsive element |
| GA | gibberellic acid |
| GLK | Golden2-like |
| LTRE | cold-responsive element |
| MeJA | methyl jasmonate |
| MW | molecular weight |
| MYA | million years ago |
| PEG | polyethylene glycol 6000 |
| pI | isoelectric point |
| SA | silica acid |
| TIP41 | tonoplast intrinsic protein 41 |
References
- Eberhard, S.; Finazzi, G.; Wollman, F.A. The dynamics of photosynthesis. Annu. Rev. Genet. 2008, 42, 463–515. [Google Scholar] [CrossRef]
- Jarvis, P.; López-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 2013, 14, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Li, P.X.; Zou, L.J.; Tan, W.R.; Zheng, T.; Zhang, D.W. GOLDEN2-LIKE transcription factors coordinate the tolerance to Cucumber mosaic virus in Arabidopsis. Biochem. Biophys. Res. Commun. 2016, 477, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.F.S.; Mayor, R.; Kashef, J. Cadherin-11 mediates contact inhibition of locomotion during xenopus neural crest cell migration. PLoS ONE 2013, 8, e85717. [Google Scholar]
- Parente, D.J.; Swint-Kruse, L. Multiple co-evolutionary networks are supported by the common tertiary scaffold of the LacI/GalR proteins. PLoS ONE 2013, 8, e84398. [Google Scholar] [CrossRef]
- Waters, M.T.; Wang, P.; Korkaric, M.; Capper, R.G.; Saunders, N.J.; Langdale, J.A. GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 2009, 21, 1109–1128. [Google Scholar] [CrossRef]
- Moreira, D.; Le Guyader, H.; Philippe, H. The origin of red algae and the evolution of chloroplasts. Nature 2000, 405, 69–72. [Google Scholar] [CrossRef]
- Lopez-Juez, E.; Pyke, K.A. Plastids unleashed: Their development and their integration in plant development. Int. J. Dev. Biol. 2005, 49, 557–577. [Google Scholar] [CrossRef]
- Park, J.; Werley, C.A.; Venkatachalam, V.; Kralj, J.M.; Dib-Hajj, S.D.; Waxman, S.G.; Cohen, A.E. Screening fluorescent voltage indicators with spontaneously spiking HEK cells. PLoS ONE 2013, 8, e85221. [Google Scholar] [CrossRef]
- Neuhaus, H.E.; Emes, M.J. Nonphotosynthetic metabsolism in plastid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 111–140. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.Z.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef]
- Imamura, A.; Hanaki, N.; Nakamura, A.; Suzuki, T.; Taniguchi, M.; Kiba, T.; Ueguchi, C.; Sugiyama, T.; Mizuno, T. Compilation and characterization of Arabiopsis thaliana response regulators implicated in His-Asp phosphorelay signal transduction. Plant Cell Physiol. 1999, 40, 733–742. [Google Scholar] [CrossRef]
- Hosoda, K.; Imamura, A.; Katoh, E.; Hatta, T.; Tachiki, M.; Yamada, H.; Mizuno, T.; Yamazaki, T. Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. Plant Cell 2002, 14, 2015–2029. [Google Scholar] [CrossRef]
- Rossini, L.; Cribb, L.; Martin, D.J.; Langdale, J.A. The maize Golden2 gene defines a novel class of transcriptional regulators in plants. Plant Cell 2001, 13, 1231–1244. [Google Scholar] [CrossRef]
- Waters, M.T.; Moylan, E.C.; Langdale, J.A. GLK transcription factors regulate chloroplast development in a cell-autonomous manner. Plant J. 2008, 56, 432–444. [Google Scholar] [CrossRef]
- Xiao, Y.; You, S.; Kong, W.; Tang, Q.; Bai, W.; Cai, Y.; Zheng, H.; Wang, C.; Jiang, L.; Wang, C.; et al. A GARP transcription factor anther dehiscence defected 1 (OsADD1) regulates rice anther dehiscence. Plant Mol. Biol. 2019, 101, 403–414. [Google Scholar] [CrossRef]
- Fitter, D.W.; Martin, D.J.; Copley, M.J.; Scotland, R.W.; Langdale, J.A. GLK gene pairs regulate chloroplast development in diverse plant species. Plant J. 2002, 31, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, Y.; Moylan, E.C.; Langdale, J.A. A conserved transcription factor mediates nuclear control of organelle biogenesis in anciently diverged land plants. Plant Cell 2005, 17, 1894–1907. [Google Scholar] [CrossRef]
- Murmu, J.; Wilton, M.; Allard, G.; Pandeya, R.; Desveaux, D.; Singh, J.; Subramaniam, R. Arabidopsis GOLDEN2-LIKE (GLK) transcription factors activate jasmonic acid (JA)-dependent disease susceptibility to the biotrophic pathogen Hyaloperonospora arabidopsidis, as well as JA-independent plant immunity against the necrotrophic pathogen Botrytis cinerea. Mol. Plant Pathol. 2013, 15, 174–184. [Google Scholar]
- Powell, A.L.T.; Nguyen, C.V.; Hill, T.; Cheng, K.L.; Figueroa-Balderas, R.; Aktas, H.; Ashrafi, H.; Pons, C.; Fernández-Muñoz, R.; Vicente, A.; et al. Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 2012, 336, 1711–1715. [Google Scholar] [CrossRef]
- Hall, L.N.; Rossini, L.; Cribb, L.; Langdale, J.A. GOLDEN 2: A novel transcriptional regulator of cellular differentiation in the maize Leaf. Plant Cell 1998, 10, 925–936. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.; Han, G.; Zhou, L.; Ali, A.; Zhu, S.; Li, X. Molecular evolution and genetic variation of G2-like transcription factor genes in maize. PLoS ONE 2016, 11, e0161763. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhao, S.; Liu, J.; Zhao, H.; Sun, X.; Wu, T.; Pei, T.; Wang, Y.; Liu, Q.; Yang, H.; et al. Genome-wide identification of tomato Golden 2-like transcription factors and abiotic stress related members screening. BMC Plant Biol. 2022, 22, 82. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, B.; Gu, G.; Yuan, J.; Yang, X.; Yang, J.; Xie, X. Genome-wide analysis of the G2-like transcription factor genes and their expression in different senescence stages of tobacco (Nicotiana Tabacum L.). Front. Genet. 2021, 12, 787. [Google Scholar] [CrossRef]
- Walther, D.; Brunnemann, R.; Selbig, J. The regulatory code for transcriptional response diversity and its relation to genome structural properties in A. thaliana. PLoS Genet. 2007, 3, e11. [Google Scholar] [CrossRef]
- Cannon, S.; Mitra, A.; Baumgarten, A.; Young, N.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 10, 4. [Google Scholar]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Lugli, G.A.; Tarracchini, C.; Alessandri, G.; Milani, C.; Mancabelli, L.; Turroni, F.; Neuzil-Bunesova, V.; Ruiz, L.; Margolles, A.; Ventura, M. Decoding the genomic variability among members of the Bifidobacterium dentium species. Microorganisms 2020, 8, 1720. [Google Scholar] [CrossRef]
- Carlini, D.; Stephan, W. In vivo introduction of unpreferred synonymous codons into the drosophila Adh gene results in reduced levels of ADH protein. Genetics 2003, 163, 239–243. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Rozas, J. DNA sequence polymorphism analysis using DnaSP. Methods Mol. Biol. 2009, 537, 337–350. [Google Scholar]
- Peng, Z.; Lu, Y.; Li, L.; Zhao, Q.; Feng, Q.; Gao, Z.; Lu, H.; Hu, T.; Yao, N.; Liu, K.; et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat. Genet. 2013, 45, 456–461. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Lescot, M. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- He, Y.; Mao, S.; Gao, Y.; Zhu, L.; Wu, D.; Cui, Y.; Li, J.; Qian, W. Genome-wide identification and expression analysis of WRKY transcription factors under multiple stresses in Brassica napus. PLoS ONE 2016, 11, e0157558. [Google Scholar] [CrossRef]
- Wu, S.; Wu, M.; Dong, Q.; Jiang, H.; Cai, R.; Xiang, Y. Genome-wide identification, classification and expression analysis of the PHD-finger protein family in Populus trichocarpa. Gene 2016, 575, 75–89. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Fan, C.; Ma, J.; Guo, Q.; Li, X.; Wang, H.; Lu, M. Selection of reference genes for quantitative real-time PCR in bamboo (Phyllostachys edulis). PLoS ONE 2013, 8, e56573. [Google Scholar]
- Liu, H.L.; Wu, M.; Li, F.; Gao, Y.M.; Chen, F.; Xiang, Y. TCP transcription factors in moso bamboo (Phyllostachys edulis): Genome-wide identification and expression analysis. Front. Plant Sci. 2018, 9, 1263. [Google Scholar] [CrossRef]
- Soding, J. Protein homology detection by HMM-HMM comparison. Bioinformatics 2004, 21, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.H.; Karlowski, W.M.; Pan, R.; Tzeng, Y.H.; Mayer, K.F.X.; Li, W.H. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 2004, 16, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, R.S.; Lin, H.N.; Childs, K.L.; Hansey, C.N.; Buell, C.R. Genome-wide atlas of transcription during maize development. Plant J. 2011, 66, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, H. Chloroplast ultrastructure in plants. New Phytol. 2019, 223, 565–574. [Google Scholar] [CrossRef] [PubMed]
- La Marca, M.; Beffy, P.; Pugliese, A.; Longo, V. Fermented wheat powder induces the antioxidant and detoxifying system in primary rat hepatocytes. PLoS ONE 2013, 8, e83538. [Google Scholar] [CrossRef]
- Opanowicz, M.; Vain, P.; Draper, J.; Parker, D.; Doonan, J.H. Brachypodiumdistachyon: Making hay with a wild grass. Trends Plant Sci. 2008, 13, 172–177. [Google Scholar] [CrossRef]
- Gu, Z.; Steinmetz, L.M.; Gu, X.; Scharfe, C.; Davis, R.W.; Li, W.H. Role of duplicate genes in genetic robustness against null mutations. Nature 2003, 421, 63–66. [Google Scholar] [CrossRef]
- Bowers, J.E.; Chapman, B.A.; Rong, J.; Paterson, A.H. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 2003, 422, 433–438. [Google Scholar] [CrossRef]
- Nasim, J.; Malviya, N.; Kumar, R.; Yadav, D. Genome-wide bioinformatics analysis of dof transcription factor gene family of chickpea and its comparative phylogenetic assessment with Arabidopsis and Rice. Plant Syst. Evol. 2016, 302, 1009–1026. [Google Scholar] [CrossRef]
- Song, H.; Wang, P.; Lin, J.Y.; Zhao, C.; Bi, Y.; Wang, X. Genome-wide identification and characterization of WRKY gene family in peanut. Front. Plant Sci. 2016, 7, 9. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Chen, D.; Liu, H.; Zhu, D.; Xiang, Y. Genome-wide identification and expression analysis of the IQD gene family in moso bamboo (Phyllostachys edulis). Sci. Rep. 2016, 6, 24520. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Amerik, A.Y.; Hochstrasser, M. Mechanism and function of deubiquitinating enzymes. BBA-Mol. Cell Res. 2004, 1695, 189–207. [Google Scholar] [CrossRef] [Green Version]
- March, E.; Farrona, S. Plant deubiquitinases and their role in the control of gene expression through modification of histones. Front. Plant Sci. 2018, 8, 02274. [Google Scholar] [CrossRef]
- Gabaldón, T.; Koonin, E.V. Functional and evolutionary implications of gene orthology. Nat. Rev. Genet. 2013, 14, 360–366. [Google Scholar] [CrossRef]
- Nakamura, H.; Muramatsu, M.; Hakata, M.; Ueno, O.; Nagamura, Y.; Hirochika, H.; Takano, M.; Ichikawa, H. Ectopic overexpression of the transcription factor OsGLK1 induces chloroplast development in non-green rice cells. Plant Cell Physiol. 2009, 50, 1933–1949. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).