Polyphenols from Dichrostachys cinerea Fruits Anti-Inflammatory, Analgesic, and Antioxidant Capacity in Freund’s Adjuvant-Induced Arthritic Rat Model
Abstract
:1. Introduction
2. Results
2.1. Total Polyphenol Content and In Vitro Antioxidant Capacity
2.2. Liquid Chromatography–Diode Array Detection–Electro-Spray Ionization Mass Spectrometry (HPLC-DAD-ESI MS) Analysis
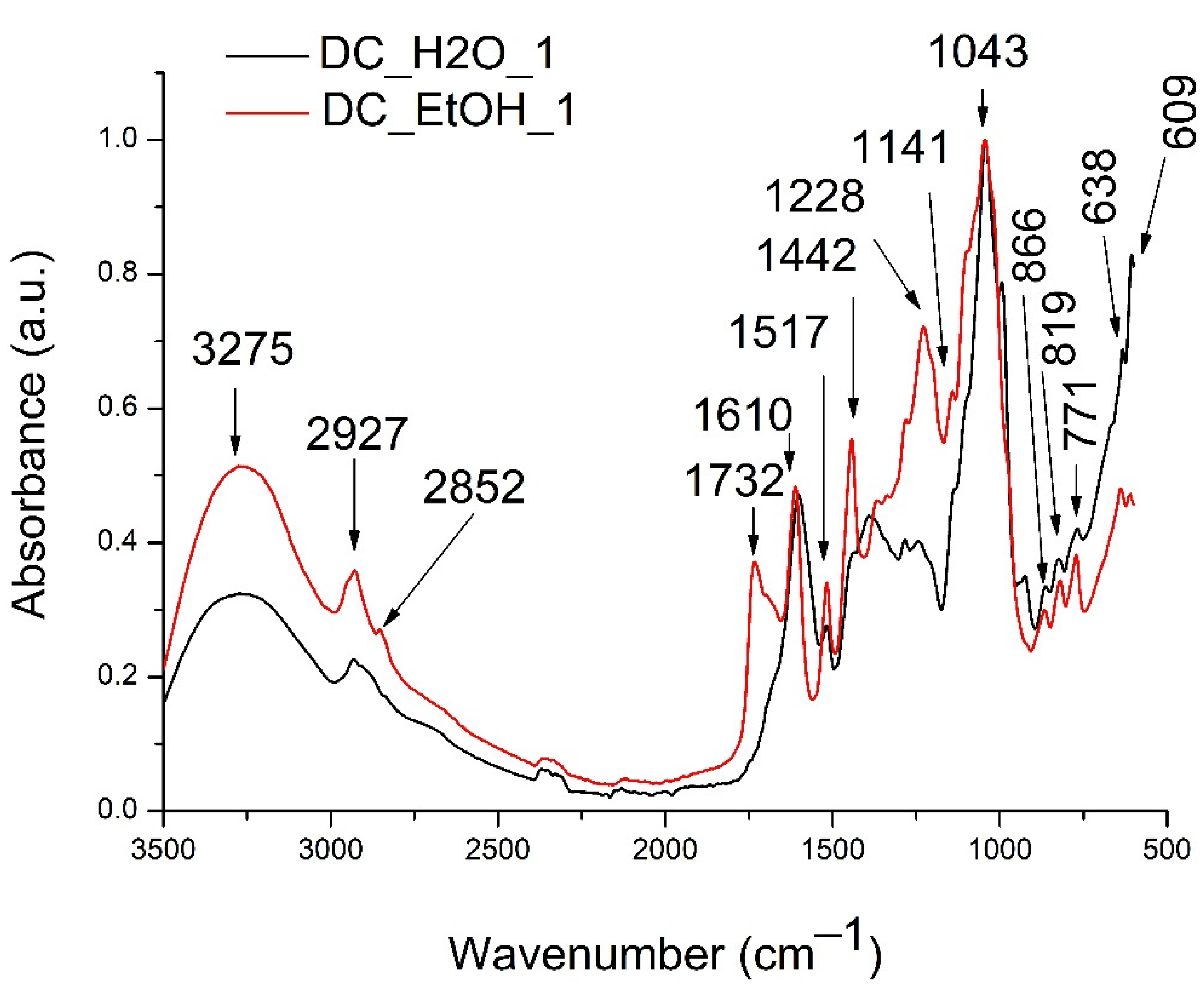
2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4. In-Tube Extraction Dynamic Headspace Gas-Chromatography–Mass Spectrometry (ITEX/GC-MS) Analysis
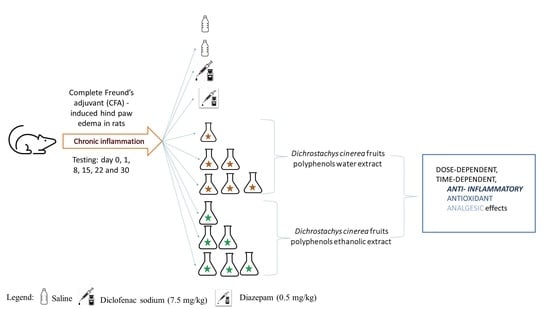
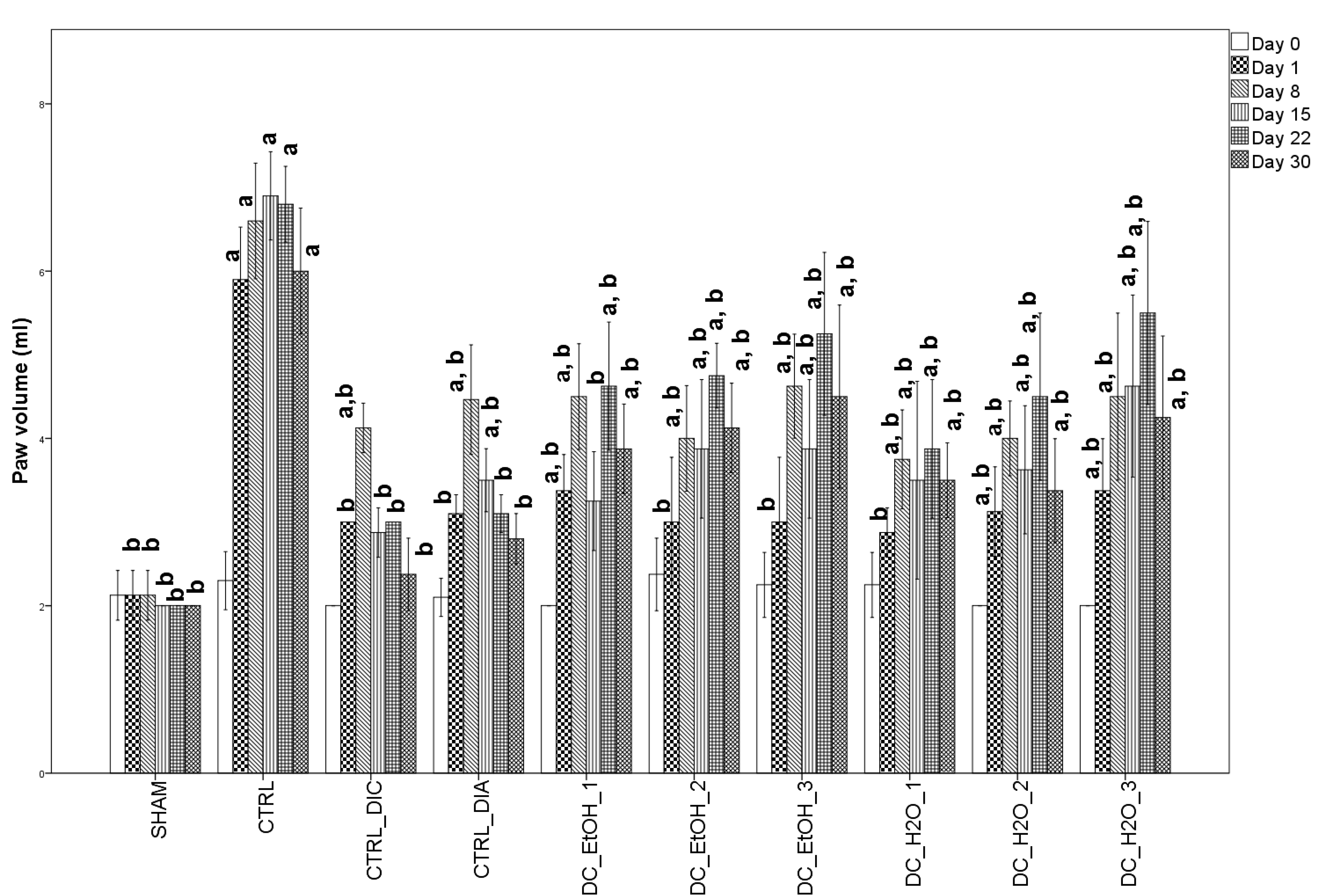
2.5. Anti-Inflammatory Effect of Dichrostachys Cinerea Fruit Extracts on Rats Paw Edema
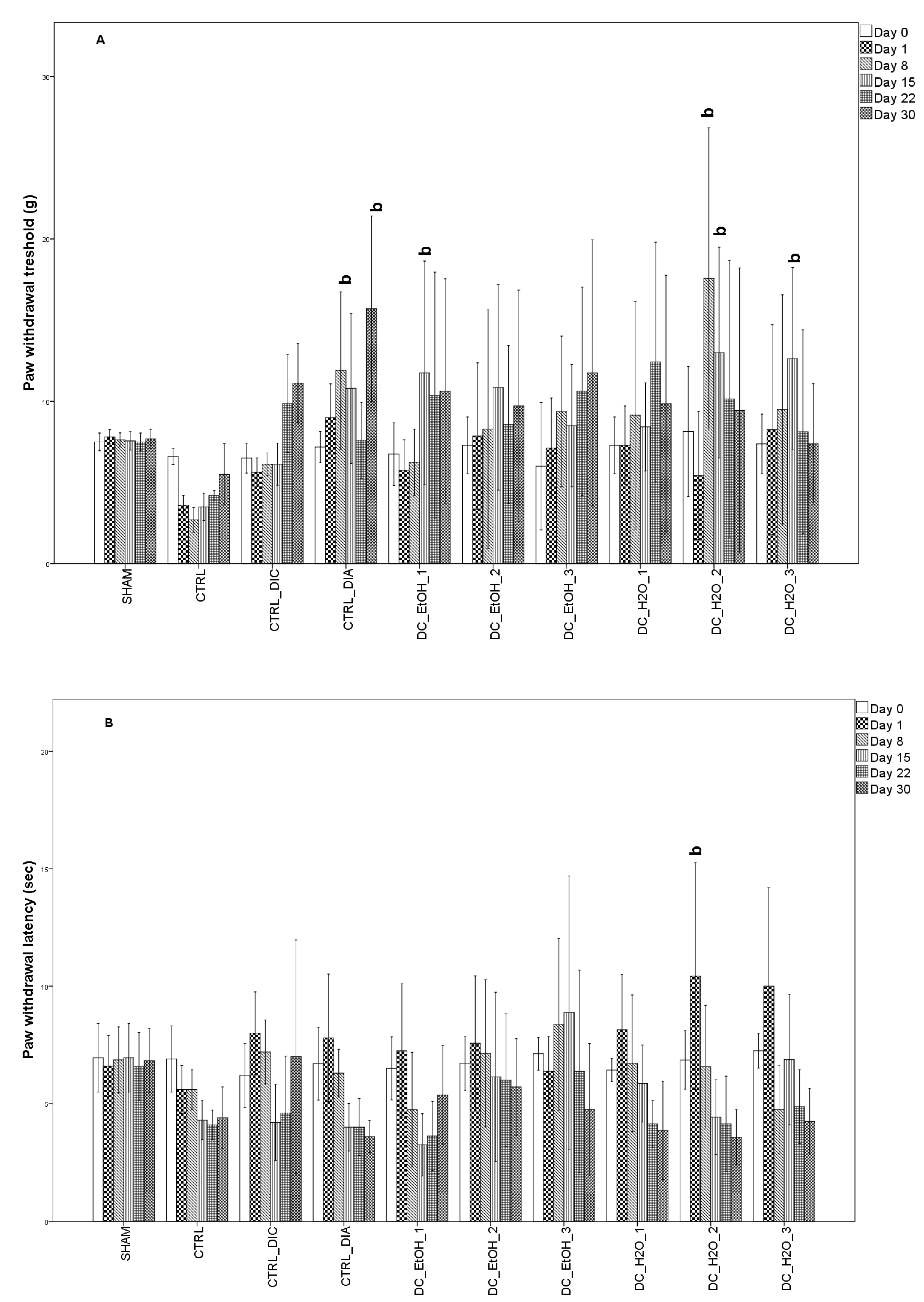
2.6. Antinociceptive Effect of Dichrostachys Cinerea Fruit Extracts
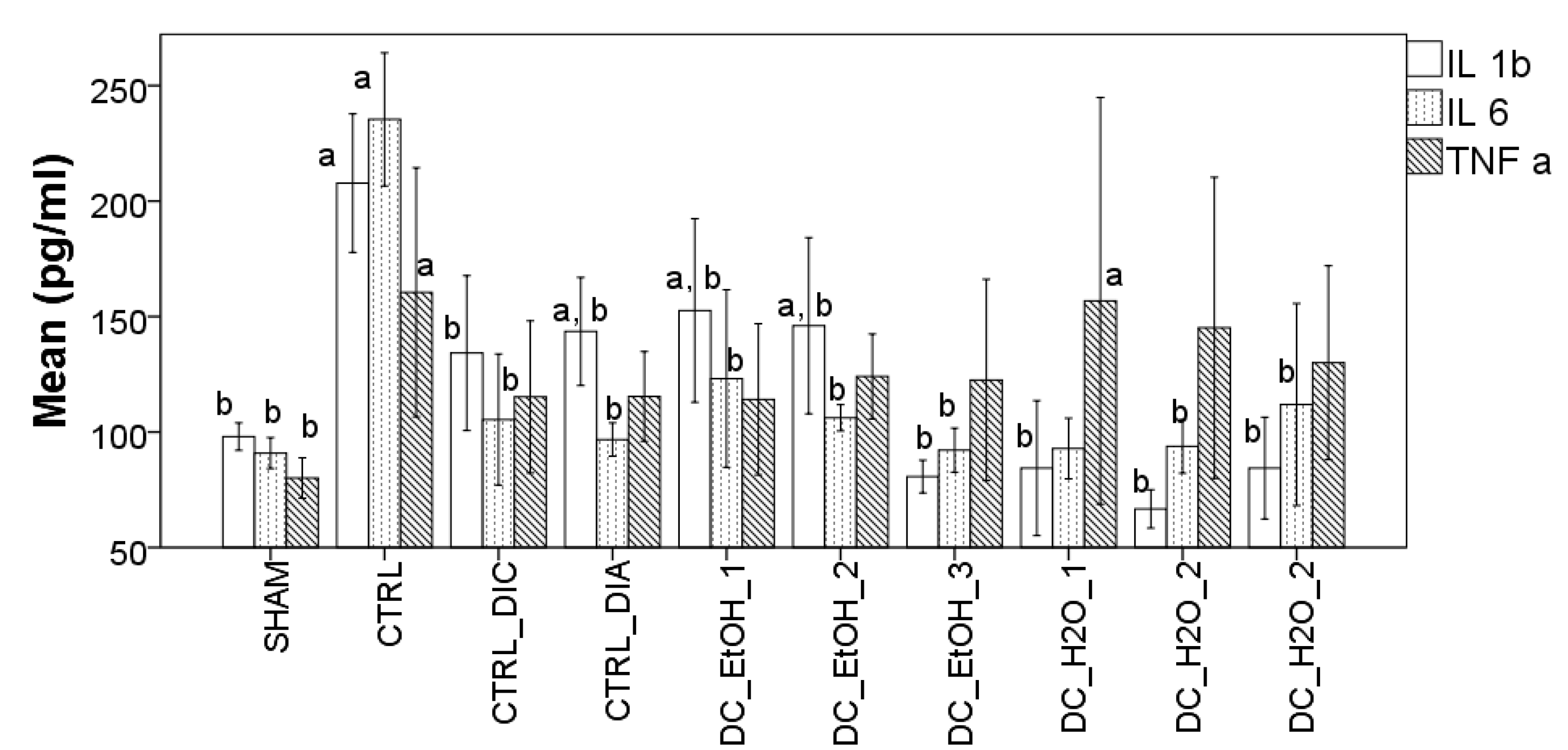
2.7. Effect of Dichrostachys Cinerea Fruit Extracts on the Production of Pro-Inflammatory Cytokines
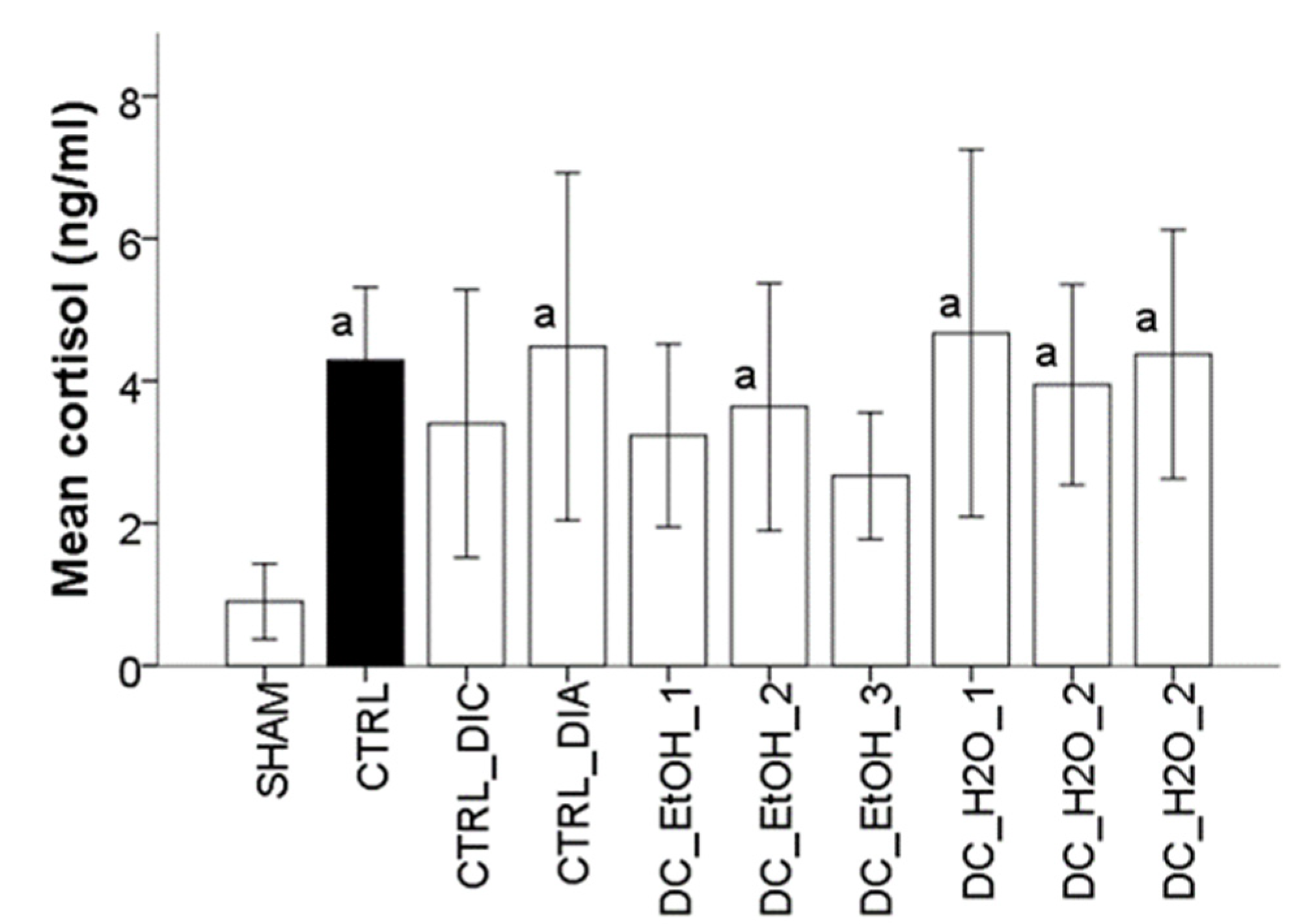
2.8. Effect of Dichrostachys Cinerea Fruit Extracts on the Production of Cortisol
2.9. Effect of Dichrostachys Cinerea Fruit Extracts on Oxidative Stress Parameters
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Fruits Ethanolic and Water Extraction
4.4. Total Polyphenol Content (TPC)
4.5. DPPH Antioxidant Capacity Test
4.6. Fruits Ethanolic and Water Extracts Analysis
4.6.1. Liquid Chromatography–Diode Array Detection–Electro-Spray Ionization Mass Spectrometry (HPLC-DAD-ESI MS)
4.6.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.6.3. In-Tube Extraction Dynamic Headspace Gas-Chromatography–Mass Spectrometry (ITEX/GC-MS) Qualitative Analysis of Volatile Compounds
4.7. Animals
4.7.1. Experimental Design
4.7.2. Arthritis Assessment
4.7.3. Edema Assessment
4.7.4. Pain Threshold Assessment
4.7.5. Thermal Hyperalgesia Assessment
4.7.6. Blood Samples
4.7.7. ELISA Analysis
4.7.8. Oxidative Stress Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dowman, B.; Campbell, R.M.; Zgaga, L.; Adeloye, D.; Chan, K.Y. Estimating the burden of rheumatoid arthritis in Africa: A systematic analysis. J. Glob. Health 2012, 2, 020406. [Google Scholar] [CrossRef]
- Gibofsky, A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: A synopsis. Am. J. Manag. Care 2014, 20, S128–S135. [Google Scholar]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, v3–v11. [Google Scholar] [CrossRef]
- Sun, S.; Du, Y.; Li, S.; Gao, B.; Xia, R.; Cao, W.; Zhang, C.; Zhu, E. Anti-inflammatory activity of different isolated sites of Chloranthus serratus in complete Freund’s adjuvant-induced arthritic rats. Exp. Ther. Med. 2021, 22, 848. [Google Scholar] [CrossRef]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.; et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef]
- Koch, W. Dietary Polyphenols—Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Subramaniam, S.; Jaganathan, D. A Comprehensive review on Dichrostachys cinerea. J. Univ. Shanghai Sci. Technol. 2021, 23, 1298–1312. [Google Scholar] [CrossRef]
- Viol, D.I.; Chagonda, L.S.; Munodawafa, T.; Muchuweti, M.; Moyo, S.R.; Mericli, A.H. Antioxidant Activity and Total Phenolic Contents of some Traditional Medicinal Plants from Zimbabwe. J. Biol. Act. Prod. Nat. 2013, 3, 345–352. [Google Scholar] [CrossRef]
- Kambizi, L.; Afolayan, A.J. An ethnobotanical study of plants used for the treatment of sexually transmitted diseases (njovhera) in Guruve District, Zimbabwe. J. Ethnopharmacol. 2001, 77, 5–9. [Google Scholar] [CrossRef]
- Tamokou, J.d.D.; Chouna, J.R.; Fischer-Fodor, E.; Chereches, G.; Barbos, O.; Damian, G.; Benedec, D.; Duma, M.; Efouet, A.P.N.; Wabo, H.K.; et al. Anticancer and Antimicrobial Activities of Some Antioxidant-Rich Cameroonian Medicinal Plants. PLoS ONE 2013, 8, e55880. [Google Scholar] [CrossRef]
- Atsang, A.; Dzeufiet, D.; Foyet, H.; Dimo, T.; Kamtchouing, P. Analgesic and Anti-inflammatory Effect of the Aqueous Extract of Dichrostachys glomerata (Forssk.) Hutch Fruits. Eur. J. Med. Plants 2014, 4, 964–978. [Google Scholar] [CrossRef]
- Hassan, H.S.; Sule, I.M.; Musa, M.A.; Musa, Y.K.; Abubakar, S.M.; Hassan, S.A. Anti-inflammatory activity of crude saponin extracts from five Nigerian medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 250–255. [Google Scholar] [CrossRef]
- Susithra, E.; Jayakumari, S. Analgesic and anti-inflammatory activities of Dichrostachys cinerea (L.) Wight and Arn. Drug Invent. Today 2018, 10, 361–366. [Google Scholar]
- Abou Zeid, A.H.; Hifnawy, M.S.; Mohammed, R.S.; Sleem, A.A. Lipoidal contents, analgesic and antipyretic activities of the aerial parts of Dichrostachys cinerea L. J. Herbs Spices Med. Plants 2015, 21, 118–128. [Google Scholar] [CrossRef]
- Eisa, M.M.; Almagboul, A.Z.; Omer, M.E.; Elegami, A.A. Antibacterial activity of Dichrostachys cinerea. Fitoterapia 2000, 71, 324–327. [Google Scholar] [CrossRef]
- Atsang, A.; Dzeufiet, D.; Aboubakar, O.; Kamtchouing, P. Anti-inflammatory Effect of D. glomerata (Frossk) Hutch (Mimosaceae) methanol extract in CFA-induced rat edema. Int. J. Phytopharm. 2018, 9, 64–67. [Google Scholar]
- Bolleddu, R.; Venkatesh, S.; Rao, M.M.; Shyamsunder, R. Investigation of the pharmacognostical, phytochemical, and antioxidant studies of various fractions of Dichrostachys cinerea root. J. Nat. Sci. Med. 2019, 2, 141. [Google Scholar] [CrossRef]
- Kuate, D.; Etoundi, B.C.O.; Soukontoua, Y.B.; Ngondi, J.L.; Oben, J.E. Antioxidant characteristics of Dichrostachys glomerata spice extracts. CYTA J. Food 2010, 8, 23–37. [Google Scholar] [CrossRef]
- Chedea, V.S.; Echim, C.; Braicu, C.; Andjelkovic, M.; Verhe, R.; Socaciu, C. Composition in polyphenols and stability of the aqueous grape seed extract from the romanian variety “Merlot Recas”. J. Food Biochem. 2011, 35, 92–108. [Google Scholar] [CrossRef]
- Lu, Q.; Lv, S.; Peng, Y.; Zhu, C.; Pan, S. Characterization of phenolics and antioxidant abilities of red navel orange “Cara Cara” harvested from five regions of China. Int. J. Food Prop. 2018, 21, 1107–1116. [Google Scholar] [CrossRef]
- Zhang, L.; Tai, Y.; Wang, Y.; Meng, Q.; Yang, Y.; Zhang, S.; Yang, H.; Zhang, Z.; Li, D.; Wan, X. The proposed biosynthesis of procyanidins by the comparative chemical analysis of five Camellia species using LC-MS. Sci. Rep. 2017, 7, 46131. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Pascale, R.; Pappalardo, I.; Santarsiero, A.; Martelli, G.; Bianco, G. Characterization of Quercetin Derivatives in Crossing Combination of Habanero White and Capsicum annuum Peppers and of Anti-Inflammatory and Cytotoxic Activity. Separations 2021, 8, 90. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterization of phenolics by LC-UV/vis, LC-MS/MS and sugars by GC in Melicoccus bijugatus Jacq. ‘Montgomery’ fruits. Food Chem. 2008, 111, 1017. [Google Scholar] [CrossRef]
- Hasan, A.; Tahir, M.N. Flavonoids from the Leaves of Impatiens bicolor. Turk. J. Chem. 2005, 29, 65–70. [Google Scholar]
- Hanganu, D.; Vlase, L.; Olah, N. LC/MS analysis of isoflavones from Fabaceae species extracts. Farmacia 2010, 58, 177–183. [Google Scholar]
- Huang, B.; Ban, X.; He, J.; Tong, J.; Tian, J.; Wang, Y. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010, 120, 873–878. [Google Scholar] [CrossRef]
- Ding, S.; Dudley, E.; Plummer, S.; Tang, J.; Newton, R.P.; Brenton, A.G. Fingerprint profile of Ginkgo biloba nutritional supplements by LC/ESI-MS/MS. Phytochemistry 2008, 69, 1555–1564. [Google Scholar] [CrossRef]
- Venter, P.B.; Senekal, N.D.; Kemp, G.; Amra-Jordaan, M.; Khan, P.; Bonnet, S.L.; Van Der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 3: The chemical composition of wattle (Acacia mearnsii) bark extract. Phytochemistry 2012, 83, 153–167. [Google Scholar] [CrossRef]
- Danciu, C.; Zinuca Pavel, I.; Babuta, R.; Ersilia, A.; Suciu, O.; Pop, G.; Soica, C.; Dehelean, C.; Radulov, I. Total phenolic content, FTIR analysis, and antiproliferative evaluation of lupin seeds harvest from western Romania. Ann. Agric. Environ. Med. 2017, 24, 726–731. [Google Scholar] [CrossRef]
- Lavudi, H.N.; Kottapalli, S.; Goycoolea, F.M. Extraction and physicochemical characterization of galactomannans from Dichrostachys cinerea seeds. Food Hydrocoll. 2018, 82, 451–456. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Amin, H.H.H.; Aziz, S.B.; Sha, A.M.; Hassan, S.; Aziz, J.M.A.; Rahman, H.S. Structural Characterization, Antimicrobial Activity, and In Vitro Cytotoxicity Effect of Black Seed Oil. Evid. Based. Complement. Alternat. Med. 2019, 2019, 6515671. [Google Scholar] [CrossRef]
- Preserova, J.; Ranc, V.; Milde, D.; Kubistova, V.; Stavek, J. Study of phenolic profile and antioxidant activity in selected Moravian wines during winemaking process by FT-IR spectroscopy. J. Food Sci. Technol. 2015, 52, 6405–6414. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Zhihua, L.; Jiyong, S.; Zhai, X.; Wang, S.; Mariod, A.A. Rapid prediction of phenolic compounds and antioxidant activity of Sudanese honey using Raman and Fourier transform infrared (FT-IR) spectroscopy. Food Chem. 2017, 226, 202–211. [Google Scholar] [CrossRef]
- Lu, H.F.; Jiang, B.; Shen, Z.G.; Shen, J.B.; Peng, Q.F.; Cheng, C.G. Comparative leaf anatomy, FTIR discrimination and biogeographical analysis of Camellia section Tuberculata (Theaceae) with a discussion of its taxonomic treatments. Plant Syst. Evol. 2008, 274, 223–235. [Google Scholar] [CrossRef]
- Kato, K.; Nitta, M.; Mizuno, T. Infrared spectroscopy of some mannans. Agric. Biol. Chem. 1973, 37, 433–435. [Google Scholar]
- Deli, M.; Ndjantou, E.B.; Ngatchic Metsagang, J.T.; Petit, J.; Njintang Yanou, N.; Scher, J. Successive grinding and sieving as a new tool to fractionate polyphenols and antioxidants of plants powders: Application to Boscia senegalensis seeds, Dichrostachys glomerata fruits, and Hibiscus sabdariffa calyx powders. Food Sci. Nutr. 2019, 7, 1795–1806. [Google Scholar] [CrossRef]
- Xia, L.; Xu, C.; Huang, K.; Lu, J.; Zhang, Y. Evaluation of phenolic compounds, antioxidant and antiproliferative activities of 31 grape cultivars with different genotypes. J. Food Biochem. 2019, 43, e12626. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols from Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 750508. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Socaciu, C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem. 2010, 121, 132–139. [Google Scholar] [CrossRef]
- Irié-N’guessan, A.G.; Kouakou, S.L.; Koua, K.B.D.; Etienne, K.Y.; Effo, K.E.; Djadji, A.T.L.; Diarrassouba, N.; Kouakou-Siransy, N.G. Anticonvulsant and Analgesic Assessment of Dichrostachys Cinerea Root Bark, an Ivorian Anti-Asthmatic Herbal, in Mice. J. Pharmacol. Clin. Res. 2018, 6, 555687. [Google Scholar]
- Dudhgaonkar, S.; Thyagarajan, A.; Sliva, D. Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. Int. Immunopharmacol. 2009, 9, 1272–1280. [Google Scholar] [CrossRef]
- Akramas, L.; Leonavičiene, L.; Vasiliauskas, A.; Bradunaite, R.; Vaitkiene, D.; Zabulyte, D.; Normantiene, T.; Lukošius, A.; Jonauskiene, I. Anti-inflammatory and anti-oxidative effects of herbal preparation EM 1201 in adjuvant arthritic rats. Medicina 2015, 51, 368–377. [Google Scholar] [CrossRef]
- De Almeida, M.; Da Rocha, B.A.; Francisco, C.R.L.; Miranda, C.G.; Santos, P.D.D.F.; De Araújo, P.H.H.; Sayer, C.; Leimann, F.V.; Gonçalves, O.H.; Bersani-Amado, C.A. Evaluation of the in vivo acute antiinflammatory response of curcumin-loaded nanoparticles. Food Funct. 2018, 9, 440–449. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef] [Green Version]
- Boarescu, I.; Boarescu, P.M.; Pop, R.M.; Bocșan, I.C.; Gheban, D.; Râjnoveanu, R.M.; Râjnoveanu, A.; Bulboacă, A.E.; Buzoianu, A.D.; Bolboacă, S.D. Curcumin Nanoparticles Enhance Antioxidant Efficacy of Diclofenac Sodium in Experimental Acute Inflammation. Biomedicines 2022, 10, 61. [Google Scholar] [CrossRef]
- Du, X.F.; Zhang, L.L.; Zhang, D.Z.; Yang, L.; Fan, Y.Y.; Dong, S.P. Clinical significance of serum total oxidant/antioxidant status in patients with operable and advanced gastric cancer. Onco. Targets Ther. 2018, 11, 6767–6775. [Google Scholar] [CrossRef]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Leopold, N.; Bocsan, I.C.; Buzoianu, A.D. Characterization of Trametes versicolor: Medicinal Mushroom with Important Health Benefits. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 343–349. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Pop, R.M.; Bocsan, I.C.; Buzoianu, A.D.; Chedea, V.S.; Socaci, S.A.; Pecoraro, M.; Popolo, A. Evaluation of the Antioxidant Activity of Nigella sativa L. and Allium ursinum Extracts in a Cellular Model of Doxorubicin-Induced Cardiotoxicity. Molecules 2020, 25, 5259. [Google Scholar] [CrossRef]
- Szabo, A.; Bocsan, I.; Suciu, S.; Buzoianu, A. Comparative animal study of the antinociceptive efficacy of lamotrigine and gabapentin for the management of pain. Acta Physiol. Hung. 2015, 102, 363–371. [Google Scholar] [CrossRef]
- Conti, M.; Morand, P.C.; Levillain, P.; Lemonnier, A. Improved fluorometric determination of malonaldehyde. Clin. Chem. 1991, 37, 1273–1275. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide Biol. Chem. 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Hu, M.L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–11111. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal 2013, 7, 1374–1378. [Google Scholar] [CrossRef] [Green Version]
| Extracts | Total Polyphenols Content (mg GAE/g Dry Weight Fruits) | Radical Scavenging Capacity of DC Extracts by DPPH (Relative %) |
|---|---|---|
| DC_H2O_1 | 153.75 ± 7.16 | 38.29 ± 0.41 |
| DC_H2O_2 | 74.90 ± 4.66 | 23.60 ± 0.32 |
| DC_H2O_3 | 39.04 ± 3.11 | 13.19 ± 0.22 |
| DC_EtOH_1 | 207.19 ± 4.28 | 72.41 ± 0.50 |
| DC_EtOH_2 | 104.50 ± 7.06 | 39.53 ± 0.39 |
| DC_EtOH_3 | 52.68 ± 5.13 | 20.37 ± 0.45 |
| No | Rt (min) | Lambda Max (nm) | M/ [M+H]+ m/z | Tentative Identification | Extract Type DC_H2O_1 (W) DC_EtOH_1 (E) | Compound Class | References |
|---|---|---|---|---|---|---|---|
| 1 | 11.6 | 280 | 579 | Procyanidin dimer (C-C) | W, E | Proanthocyanidin | [20] |
| 2 | 12.9 | 280 | 291 | Catechin | W, E | Flavan-3-ol | [20] |
| 3 | 13.3 | 280 | 579 | Procyanidin dimer (C-EC) | W, E | Proanthocyanidin | [20] |
| 4 | 14.1 | 280 | 291 | Epicatechin | W, E | Flavan-3-ol | [20] |
| 5 | 14.4 | sh 240, 275, 340 | 565 | Apigenin-8-C-glucoside-2’-Oxyloside | W, E | Flavone glicoside | [21] |
| 6 | 14.8 | 280 | 731 | ECG-EC Procyanidin Dimer | W, E | Proanthocyanidin | [22] |
| 7 | 15.7 | 280 | 715 | EGC-EC Procyanidin Dimer | W, E | Proanthocyanidin | [22] |
| 8 | 16.4 | 280 | 443 | Catechin-gallate | W, E | Flavan-3-ol | [20] |
| 9 | 17.0 | 280 | 443 | Epicatechin-gallate | W | Flavan-3-ol | [20] |
| 10 | 17.7 | 260, 350 | 449 | Quercetin-rhamnoside | W, E | Flavone glycosides | [23] |
| 11 | 18.2 | 225, 300 | 535 | Resveratrol derivative | W, E | Stilbenoids | [24] |
| 12 | 19.5 | 235, 285, sh 310, 380 | 419 | Kaempferol 7-arabinoside | W, E | Flavone glicoside | [24,25] |
| 13 | 20.1 | 235, 280 sh 315 | 255 | Daidzein | E | Isoflavone | [26] |
| 14 | 20.8 | sh 210, 230, 285, 315 | 437 | Catechin rhamnoside | E | Flavan-3-ol glycoside | [27] |
| 15 | 22.9 | 240, 280 | 317 | (Iso)Rhamnetin | E | Flavonol | [28] |
| 16 | 24.7 | sh 310, 350 | 287 | Kaempferol | E | Flavonol | [28] |
| 17 | 28.8 | 280 | 1122 | Proanthocyanidin-tetramer | E | Proanthocyanidin | [29] |
| Compounds | Rt (min) | Concentration (% of Total Peak Area) |
|---|---|---|
| Ethanedioic acid, dimethyl ester | 4.723 | 16.19 |
| Butanedioic acid, dimethyl ester | 11.493 | 1.94 |
| Benzoic acid, methyl ester | 13.89 | 8.97 |
| Dimethyl malate | 14.944 | 72.89 |
| GROUPS | TOS | OSI | NOx | MDA | TIOLS |
|---|---|---|---|---|---|
| SHAM | 3.58 ± 0.76 b | 3.29 ± 0.70 b | 22.72 ± 3.58 b | 2.78 ± 1.08 b | 417.20 ± 31.29 b |
| CTRL | 8.16 ± 1.17 a | 7.51 ± 1.07 a | 52.71 ± 7.93 a | 5.37 ± 0.87 a | 275.75 ± 67.74 a |
| CTRL + DIC | 6.90 ± 0.83 a | 6.33 ± 0.77 a | 28.08 ± 3.41 b | 3.70 ± 0.15 | 318.60 ± 120.58 a |
| CTRL + DIA | 6.11 ± 1.36 a | 5.61 ± 1.25 a | 28.77 ± 4.46 a,b | 2.63 ± 1.52 b | 316.40 ± 53.85 a |
| DC_EtOH_1 | 6.08 ± 1.41 a | 5.59 ± 1.29 a | 29.91 ± 3.74 a,b | 3.51 ± 0.35 b | 339.00 ± 28.02 |
| DC_EtOH_2 | 6.59 ± 1.91 a | 6.05 ± 1.75 a | 26.74 ± 1.57 b | 3.15 ± 0.42 b | 360.00 ± 56.13 a |
| DC_EtOH_3 | 5.36 ± 0.80 a,b | 4.92 ± 0.74 a,b | 32.36 ± 4.26 a,b | 3.19 ± 1.33 b | 315.25 ± 68.14 a |
| DC_H2O_1 | 5.70 ± 1.29 a,b | 5.23 ± 1.19 a,b | 36.28 ± 5.56 a,b | 3.30 ± 1.37 b | 346.00 ± 70.29 a |
| DC_H2O_2 | 6.84 ± 0.60 a | 6.29 ± 0.55 a | 33.66 ± 3.83 a,b | 3.75 ± 0.36 b | 322.43 ± 44.03 a |
| DC_H2O_3 | 5.41 ± 1.89 a,b | 4.98 ± 1.74 a,b | 31.87 ± 6.14 a,b | 3.47 ± 0.54 b | 300.75 ± 47.89 a |
| Groups/Abbrev. | Administrated Substance/Dose | Route of Administration |
|---|---|---|
| SHAM | Normal saline solution | p.o. |
| Control (CTRL) | Normal saline solution + CFA | p.o. |
| Positive control | ||
| (CTRL_DIC) | Diclofenac sodium (7.5 mg/kg) + CFA | i.p. |
| Positive control | ||
| (CTRL_DIA) | Diazepam (0.5 mg/kg) + CFA | i.p. |
| Treatment | ||
| (DC_EtOH_1) | DC_EtOH_1 (0.5 mL/100 g out of conc 1–207 mg GAE /g) + CFA | p.o. |
| Treatment | ||
| (DC_EtOH_2) | DC_EtOH_2 + (0.5 mL/100 g out of conc 2–105 mg GAE/g) + CFA | p.o. |
| Treatment | ||
| (DC_EtOH_3) | DC_EtOH_3 + (0.5 mL/100 g out of conc 3–53 mg GAE/g) + CFA | p.o. |
| Treatment | ||
| (DC_H2O_1) | DC_H2O_1 (0.5 mL/100 g out of conc 1–154 mg GAE/g) + CFA | p.o. |
| Treatment | ||
| (DC_H2O_2) | DC_H2O_2 (0.5 mL/100 g out of conc 1–75 mg GAE/g) + CFA | p.o. |
| Treatment | ||
| (DC_H2O_3) | DC_H2O_3 (0.5 mL/100 g out of conc 1–39 mg GAE/g) + CFA | p.o. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiki, G.A.à.; Pop, R.M.; Sabin, O.; Bocsan, I.C.; Chedea, V.S.; Socaci, S.A.; Pârvu, A.E.; Finsia, E.; Francis, T.; Mathieu, Z.; et al. Polyphenols from Dichrostachys cinerea Fruits Anti-Inflammatory, Analgesic, and Antioxidant Capacity in Freund’s Adjuvant-Induced Arthritic Rat Model. Molecules 2022, 27, 5445. https://doi.org/10.3390/molecules27175445
Kiki GAà, Pop RM, Sabin O, Bocsan IC, Chedea VS, Socaci SA, Pârvu AE, Finsia E, Francis T, Mathieu Z, et al. Polyphenols from Dichrostachys cinerea Fruits Anti-Inflammatory, Analgesic, and Antioxidant Capacity in Freund’s Adjuvant-Induced Arthritic Rat Model. Molecules. 2022; 27(17):5445. https://doi.org/10.3390/molecules27175445
Chicago/Turabian StyleKiki, Gisèle Atsang à, Raluca Maria Pop, Octavia Sabin, Ioana Corina Bocsan, Veronica Sanda Chedea, Sonia Ancuța Socaci, Alina Elena Pârvu, Egre Finsia, Takvou Francis, Zramah Mathieu, and et al. 2022. "Polyphenols from Dichrostachys cinerea Fruits Anti-Inflammatory, Analgesic, and Antioxidant Capacity in Freund’s Adjuvant-Induced Arthritic Rat Model" Molecules 27, no. 17: 5445. https://doi.org/10.3390/molecules27175445
APA StyleKiki, G. A. à., Pop, R. M., Sabin, O., Bocsan, I. C., Chedea, V. S., Socaci, S. A., Pârvu, A. E., Finsia, E., Francis, T., Mathieu, Z., & Buzoianu, A. D. (2022). Polyphenols from Dichrostachys cinerea Fruits Anti-Inflammatory, Analgesic, and Antioxidant Capacity in Freund’s Adjuvant-Induced Arthritic Rat Model. Molecules, 27(17), 5445. https://doi.org/10.3390/molecules27175445