A Carabrane-Type Sesquiterpenolide Carabrone from Carpesium cernuum Inhibits SW1990 Pancreatic Cancer Cells by Inducing Ferroptosis
Abstract
:1. Introduction
2. Results
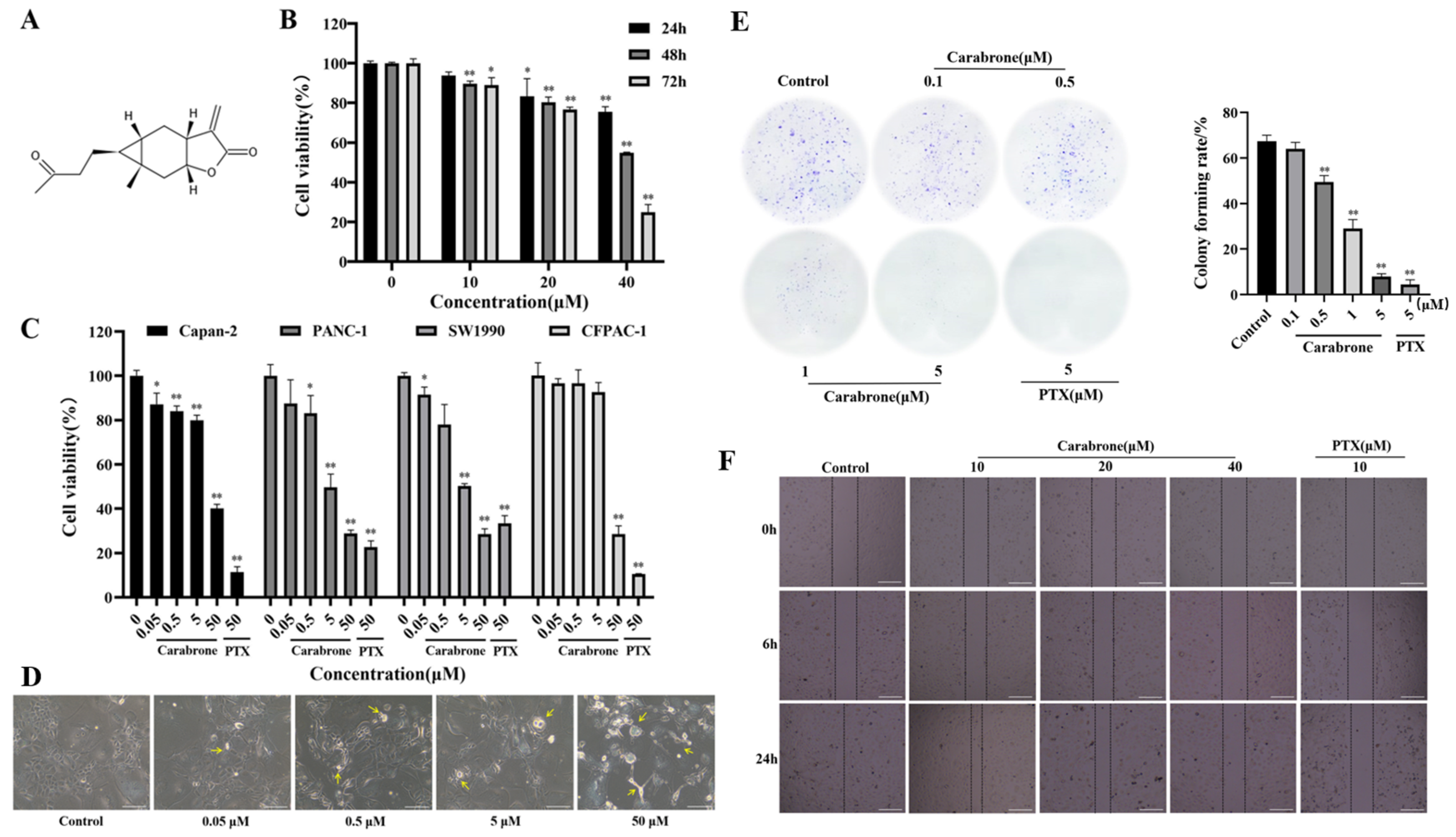
2.1. Effects of Carabrone on Viability and Migration in SW1990 Cells
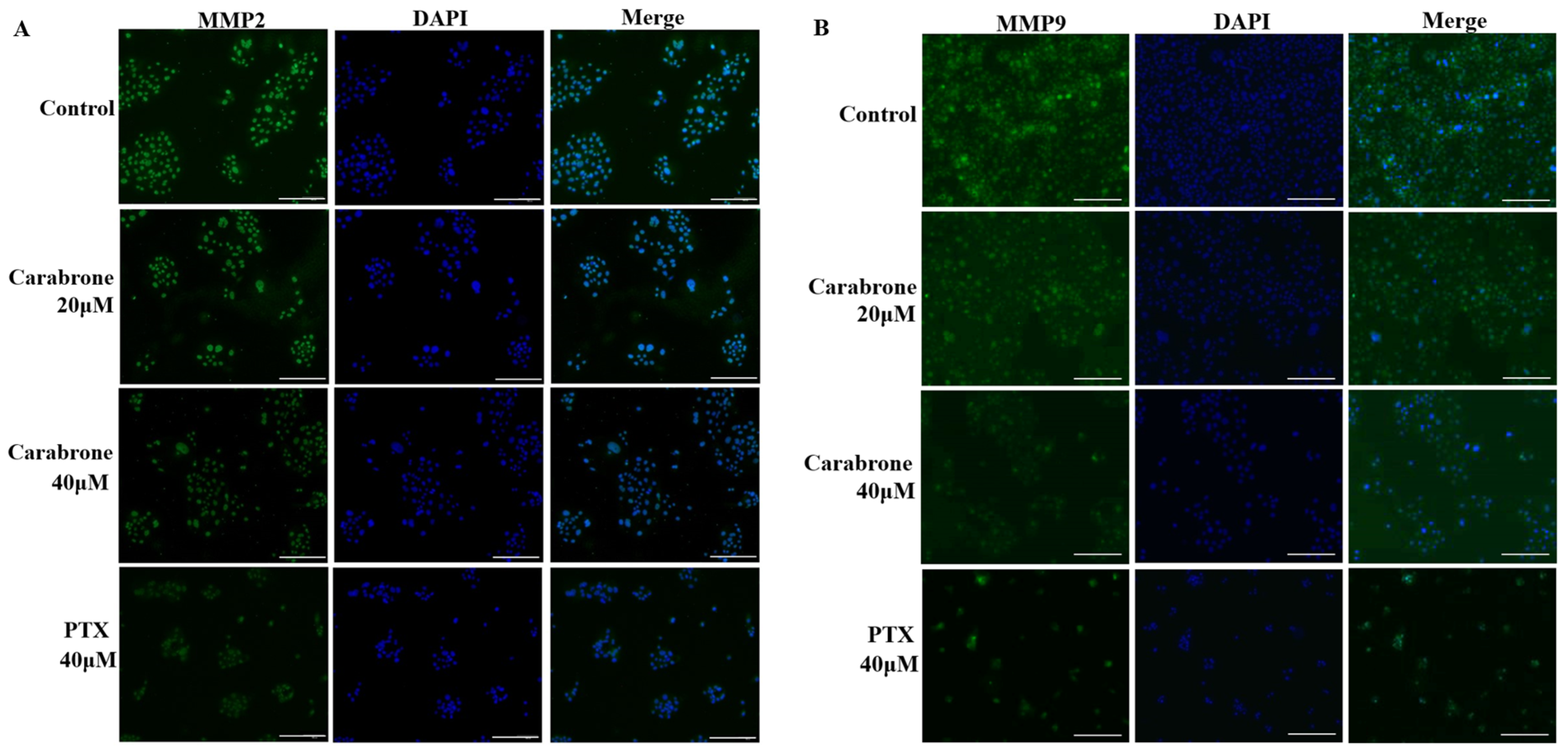
2.2. Effects of Carabrone on MMP2 and MMP9 Proteins in SW1990 Cells
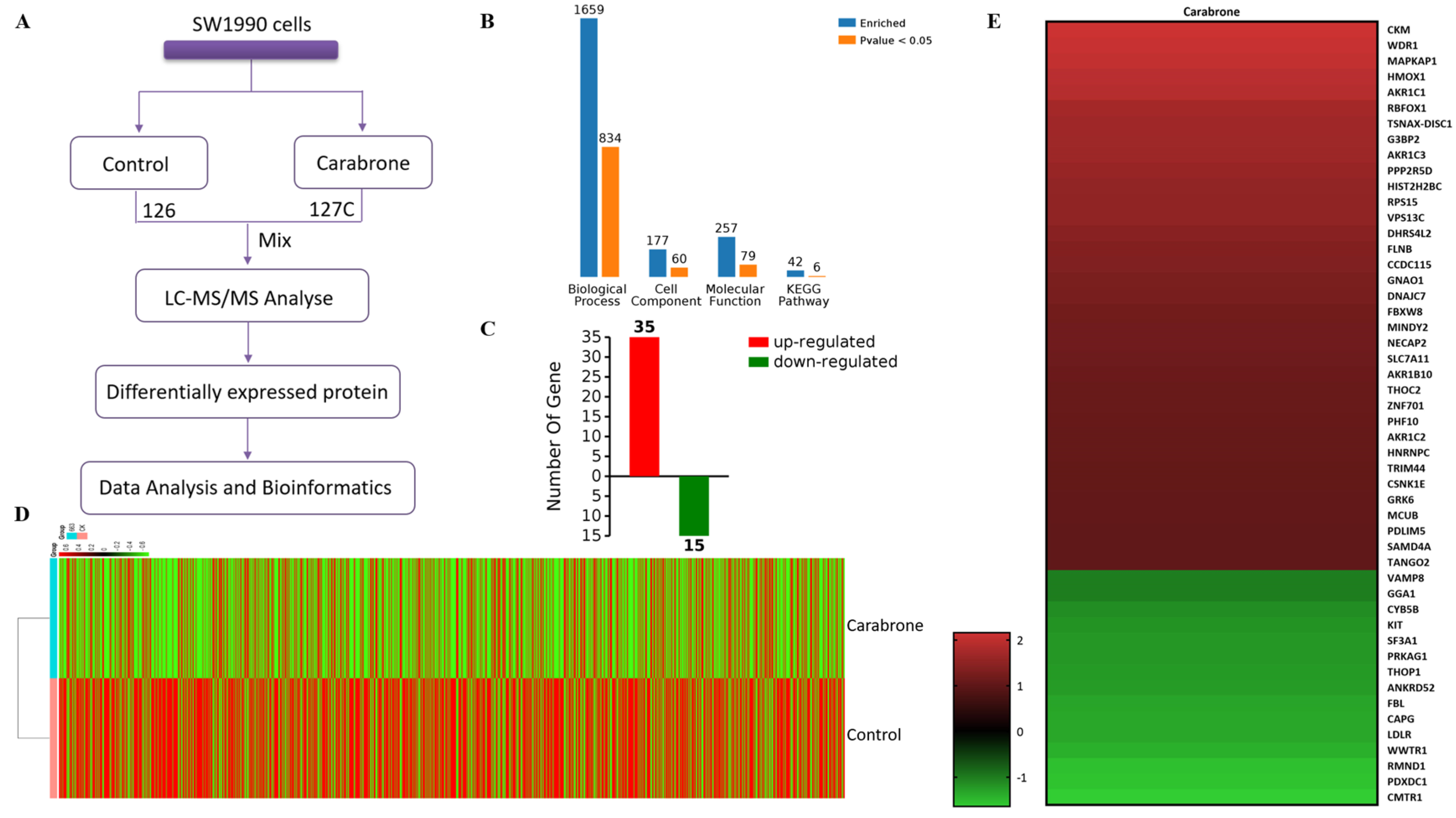
2.3. Proteomic Comparison of Carabrone-Treated SW1990 Cells with Control SW1990 Cells
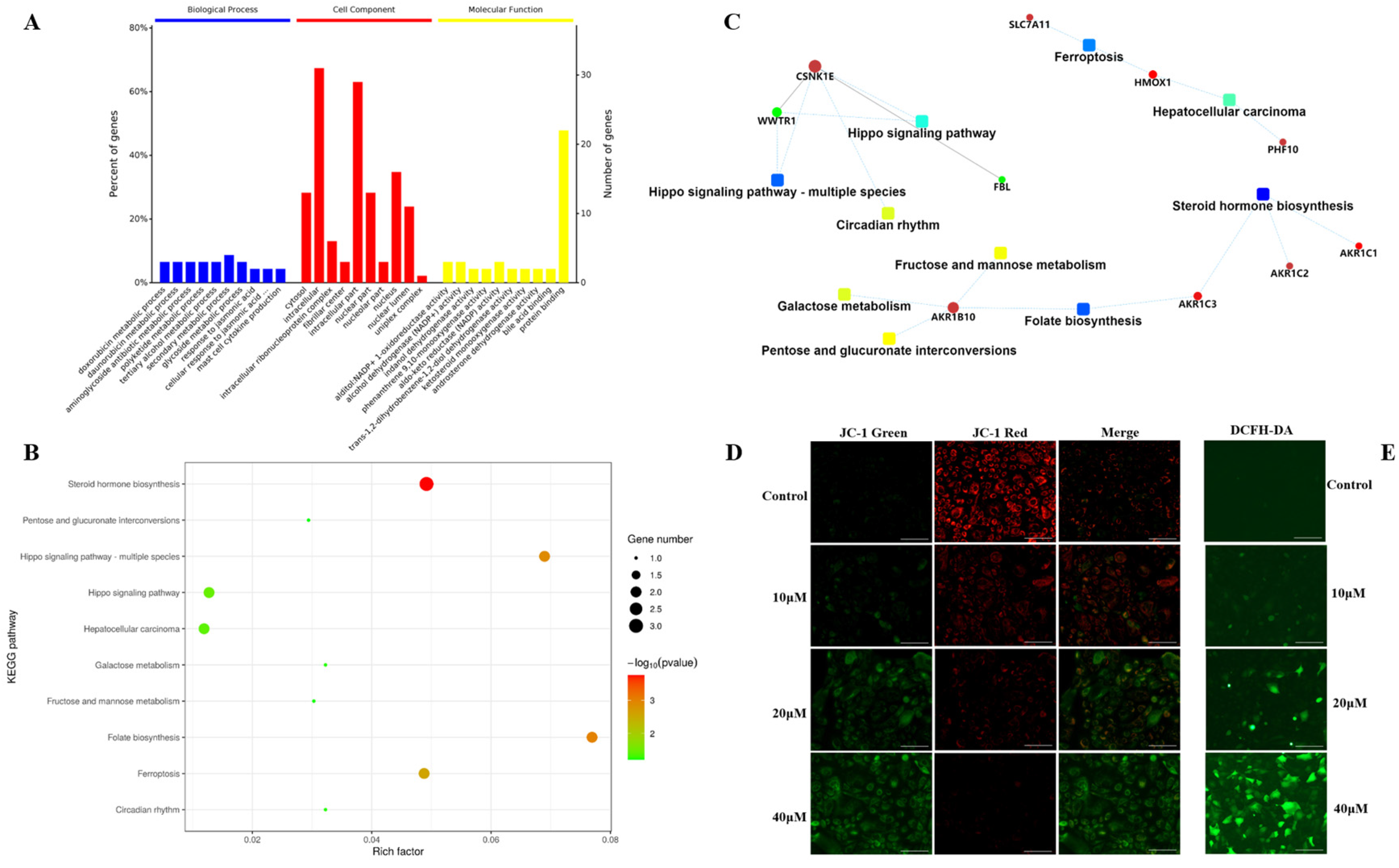
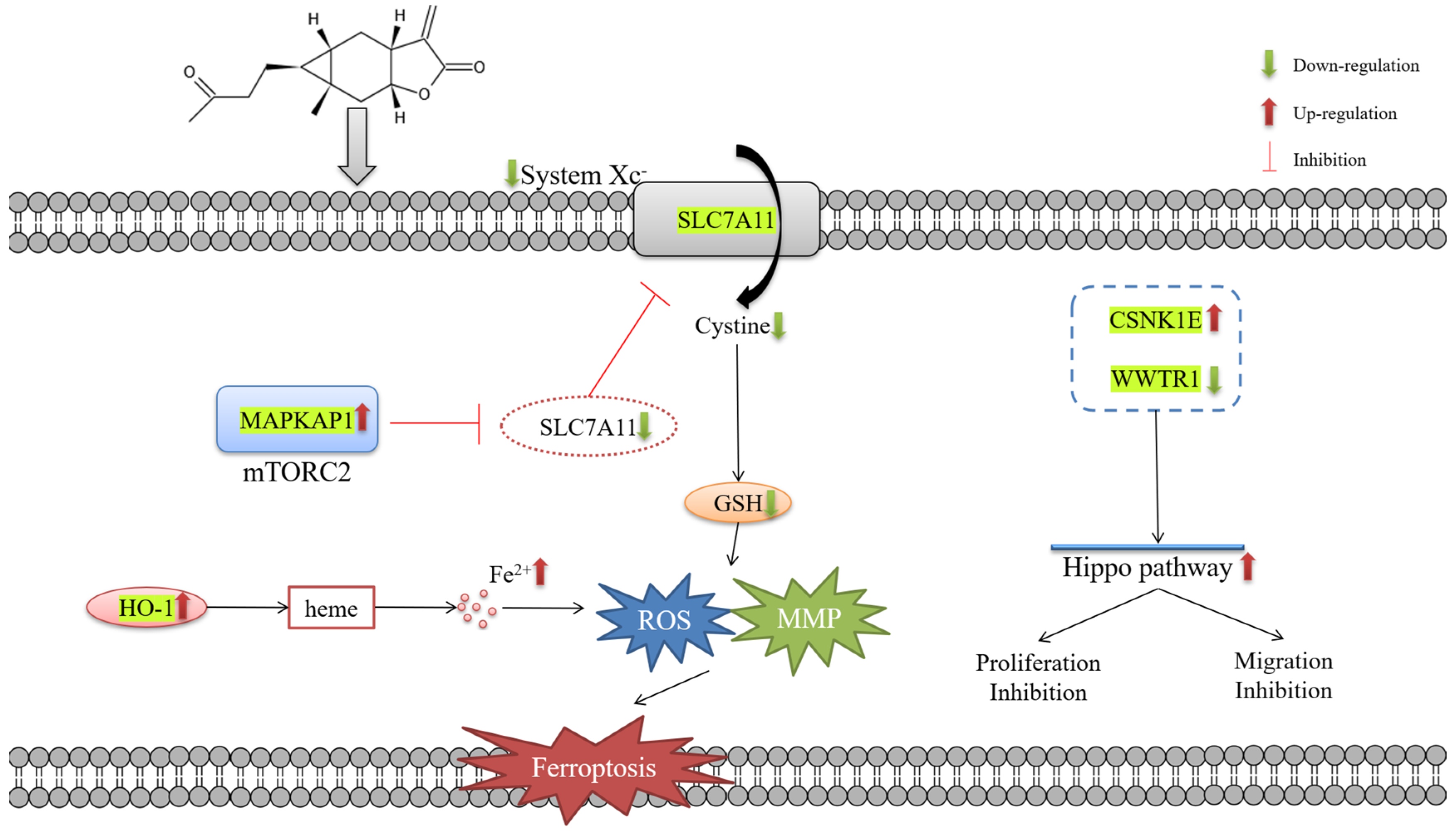
2.4. Functional Enrichment and Validation of Carabrone Regulatory Proteins
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Wound Healing Assay
4.5. Immunofluorescence
4.6. Colony Formation Assay
4.7. Intracellular ROS Detection Analysis
4.8. Protein Preparation and TMT Labeling
4.9. Nano LC-MS/MS Analysis
4.10. Bioinformatics Analysis and Data Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Park, Y.J.; Cheon, S.Y.; Lee, D.S.; Cominguez, D.C.; Zhang, Z.Y.; Lee, S.W.; An, H.J. Anti-Inflammatory and Antioxidant Effects of Carpesium cernuum L. Methanolic Extract in LPS-Stimulated RAW 264.7 Macrophages. Mediat. Inflamm. 2020, 2020, 3164239. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Song, J.R.; Yuan, D.B.; Rao, Q.; Jiang, K.H.; Feng, S.H.; Zhu, G.H.; Yan, C.; Li, Y.M.; Zhu, J.G. Incaspitolide A extracted from Carpesium cernuum induces apoptosis in vitro via the PI3K/AKT pathway in benign prostatic hyperplasia. Biosci. Rep. 2021, 41, BSR20210477. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.L.; Li, H.L.; Ma, C.M.; Wang, Y.H.; Tian, J.T.; Deng, L.L.; Wang, D.; Jing, X.S.; Luo, K.; Xing, W.L.; et al. Identification of Carpesium cernuum extract as a tumor migration inhibitor based on its biological response profiling in breast cancer cells. Phytomedicine 2019, 64, 153072. [Google Scholar] [CrossRef]
- Zhang, J.P.; Wang, G.W.; Tian, X.H.; Yang, Y.X.; Liu, Q.X.; Chen, L.P.; Chen, L.P.; Li, H.L.; Zhang, W.D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 163, 173–191. [Google Scholar] [CrossRef]
- Huang, Y.S.; Mao, J.X.; Zhang, L.; Guo, H.W.; Yan, C.; Chen, M. Antiprostate Cancer Activity of Ineupatolide Isolated from Carpesium cernuum L. Biomed Res. Int. 2021, 2021, 5515961. [Google Scholar] [CrossRef]
- Long, J.; Luo, G.P.; Xiao, Z.W.; Liu, Z.Q.; Guo, M.; Liu, L.; Liu, C.; Xu, J.; Gao, Y.T.; Zheng, Y.; et al. Cancer statistics: Current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer Lett. 2014, 346, 273–277. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Nosacka, R.L.; Delitto, A.E.; Delitto, D.; Patel, R.; Judge, S.M.; Trevino, J.G.; Judge, A.R. Distinct cachexia profiles in response to human pancreatic tumours in mouse limb and respiratory muscle. J. Cachexia Sarcopenia Muscle 2020, 11, 820–837. [Google Scholar] [CrossRef]
- Guerra, C.; Collado, M.; Navas, C.; Schuhmacher, A.J.; Hernández-Porras, I.; Cañamero, M.; Justo, M.R.; Serrano, M.; Barbacid, M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 2011, 19, 728–739. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Goldstein, D.; Korn, R.L.; Arena, F.; Moore, M.; Siena, S.; Teixeira, L.; Tabernero, J.; van Laethem, J.L.; Liu, H.; et al. Positron Emission Tomography Response Evaluation from a Randomized Phase III Trial of Weekly nab-Paclitaxel Plus Gemcitabine vs Gemcitabine Alone for Patients with Metastatic Adenocarcinoma of the Pancreas. Ann. Oncol. 2016, 27, 648–653. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, W.S.; Zhuo, Q.F.; Hu, Q.S.; Liu, M.Q.; Sun, Q.Q.; Zhang, Z.; Fan, G.X.; Xu, W.Y.; Ji, S.R.; et al. Ferroptosis: Final destination for cancer? Cell Prolif. 2020, 53, 12761. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Zhang, L.; Zheng, J.Z.; Sun, H.M.; Shao, C. Ferroptosis: Biochemistry and Biology in Cancers. Front. Oncol. 2021, 11, 579286. [Google Scholar] [CrossRef]
- Yang, W.H.; Ding, C.C.; Sun, T.N.; Rupprecht, G.; Lin, C.C.; Hsu, D.; Chi, J.T. The Hippo Pathway Effector TAZ Regulates Ferroptosis in Renal Cell Carcinoma. Cell Rep. 2019, 28, 2501–2508. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Su, Y.W.; Zhao, B.; Zhou, L.F.; Zhang, Z.Y.; Shen, Y.S.; Lv, H.H.; AlQudsy, L.H.H.; Shang, P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020, 483, 127–136. [Google Scholar] [CrossRef]
- Mehta, R.G.; Pezzuto, J.M. Discovery of cancer preventive agents from natural products: From plants to prevention. Curr. Oncol. Rep. 2002, 4, 478–486. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.M.; Zhao, R.; Wang, D.; Ma, Y.; Ai, L. Plant Natural Products: Promising Resources for Cancer Chemoprevention. Molecules 2021, 26, 933. [Google Scholar] [CrossRef]
- Yamaguchi, Y.K.; Kasukabe, T.S.; Kumakura, S.N. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 2018, 52, 1011–1022. [Google Scholar] [CrossRef]
- Eling, N.; Reuter, L.; Hazin, J.; Hamacher-Brady, A.; Brady, N.R. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2015, 2, 517–532. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.H.; Chen, P.; Liu, J.; Zhu, S.; Kroemer, G.; Klionsky, D.J.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D.L. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci. Adv. 2019, 5, 2238. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Chen, G.H.; Song, C.C.; Pantopoulos, K.; Wei, X.L.; Zheng, H.; Luo, Z. Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic. Biol. Med. 2022, 180, 95–107. [Google Scholar] [CrossRef]
- Gupta, S.; Pramanik, D.; Mukherjee, R.; Campbell, N.R.; Elumalai, S.; Wilde, R.F.; Hong, S.M.; Goggins, M.G.; Jesus-Acosta, A.D.; Laheru, D. Molecular Determinants of Retinoic Acid Sensitivity in Pancreatic Cancer. Clin. Cancer Res. 2011, 18, 280–289. [Google Scholar] [CrossRef]
- Waterhouse, M.; Risch, H.A.; Bosetti, C.; Anderson, K.E.; Petersen, G.M.; Bamlet, W.R.; Cotterchio, M.; Cleary, S.P.; Ibiebele, T.; Vecchia, C.L.; et al. Vitamin D and pancreatic cancer: A pooled analysis from the Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 2015, 26, 1776–1783. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, X.T.; Ma, Y.B.; Huang, X.Y.; Geng, C.A.; Li, T.Z.; Zhang, X.M.; Shen, C.; Su, L.H.; Gao, Z.; et al. Artemyrianolides A-S, Cytotoxic Sesquiterpenoids from Artemisia myriantha. J. Nat. Prod. 2020, 83, 2618–2630. [Google Scholar] [CrossRef]
- Muhammad, I.; Takamatsu, S.; Mossa, J.S.; El-Feraly, F.S.; Walker, L.A.; Clark, A.M. Cytotoxic sesquiterpene lactones from Centaurothamnus maximus and Vicoa pentanema. Phytother. Res. 2003, 17, 168–173. [Google Scholar] [CrossRef]
- Xie, W.D.; Wang, X.R.; Ma, L.S.; Li, X.; Row, K.H. Sesquiterpenoids from Carpesium divaricatum and their cytotoxic activity. Fitoterapia 2012, 83, 1351–1355. [Google Scholar] [CrossRef]
- Slapak, E.J.; Duitman, J.W.; Tekin, C.S.; Bijlsma, M.F.; Spek, C.A. Matrix Metalloproteases in Pancreatic Ductal Adenocarcinoma: Key Drivers of Disease Progression? Biology 2020, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, J.P.; Carvalhais, V.; Ferreira, R.; Amado, F.; Vilanova, M.; Cerca, N.; Vitorino, R. Proteome signatures—How are they obtained and what do they teach us? Appl. Microbiol. Biot. 2015, 99, 7417–7431. [Google Scholar] [CrossRef]
- Lang, J.Y.; Zhao, X.; Wang, X.C.; Zhao, Y.; Li, Y.Y.; Zhao, R.F.; Chen, K.M.; Li, Y.; Han, X.X.; Zheng, X.W.; et al. Targeted Co-delivery of the Iron Chelator Deferoxamine and a HIF1α Inhibitor Impairs Pancreatic Tumor Growth. ACS Nano 2019, 13, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Hoang, K.N.L.; Anstee, J.E.; Arnold, J.N. The Diverse Roles of Heme Oxygenase-1 in Tumor Progression. Front. Immunol. 2021, 12, 658315. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2019, 20, 39. [Google Scholar] [CrossRef]
- Kwon, M.Y.; Park, E.; Lee, S.J.; Chung, S.W. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 2015, 6, 24393–24403. [Google Scholar] [CrossRef]
- Lo, M.; Ling, V.; Wang, Y.Z.; Gout, P.W. The Xc− cystine/glutamate antiporter: A mediator of pancreatic cancer growth with a role in drug resistance. Br. J. Cancer 2008, 99, 464–472. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.B.; Kang, R.; Klionsky, D.J.; Tang, D.L. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Kuang, F.M.; Liu, J.; Tang, D.L.; Kang, R. Oxidative Damage and Antioxidant Defense in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 586578. [Google Scholar] [CrossRef]
- Wu, C.H.; Zhao, W.W.; Yu, J.; Li, S.J.; Lin, L.L.; Chen, X.P. Induction of ferroptosis and mitochondrial dysfunction by oxidative stress in PC12 cells. Sci. Rep. 2018, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.Y.; Zhang, H.; Zhao, B.; Zha, Z.Y.; Bai, F.; Pei, X.H.; Zhao, S.M.; Xiong, Y.; Guan, K.L. TAZ Promotes Cell Proliferation and Epithelial-Mesenchymal Transition and Is Inhibited by the Hippo Pathway. Mol. Cell Biol. 2008, 28, 2426–2436. [Google Scholar] [CrossRef]
- Kanai, F.; Marignani, P.A.; Sarbassova, D.; Yagi, R.; Hall, R.A.; Dniwitz, M.; Hisaminato, A.; Fujiwara, T.; Ito, Y.; Cantley, L.C.; et al. TAZ: A novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000, 19, 6778–6791. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.-D.; Zhang, Y.; Ma, J.-Y.; Sang, C.-Y.; Yang, J.-L. A Carabrane-Type Sesquiterpenolide Carabrone from Carpesium cernuum Inhibits SW1990 Pancreatic Cancer Cells by Inducing Ferroptosis. Molecules 2022, 27, 5841. https://doi.org/10.3390/molecules27185841
Zheng Y-D, Zhang Y, Ma J-Y, Sang C-Y, Yang J-L. A Carabrane-Type Sesquiterpenolide Carabrone from Carpesium cernuum Inhibits SW1990 Pancreatic Cancer Cells by Inducing Ferroptosis. Molecules. 2022; 27(18):5841. https://doi.org/10.3390/molecules27185841
Chicago/Turabian StyleZheng, Yi-Dan, Ying Zhang, Jun-Yi Ma, Chun-Yan Sang, and Jun-Li Yang. 2022. "A Carabrane-Type Sesquiterpenolide Carabrone from Carpesium cernuum Inhibits SW1990 Pancreatic Cancer Cells by Inducing Ferroptosis" Molecules 27, no. 18: 5841. https://doi.org/10.3390/molecules27185841
APA StyleZheng, Y.-D., Zhang, Y., Ma, J.-Y., Sang, C.-Y., & Yang, J.-L. (2022). A Carabrane-Type Sesquiterpenolide Carabrone from Carpesium cernuum Inhibits SW1990 Pancreatic Cancer Cells by Inducing Ferroptosis. Molecules, 27(18), 5841. https://doi.org/10.3390/molecules27185841




