Research on ACEI of Low-Molecular-Weight Peptides from Hirudo nipponia Whitman
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Pretreatment and Extraction Method
2.3. Separation Method
2.4. Orbitrap LC–MS Analysis
2.5. Database Search and Peptide Identification
2.6. Activity Prediction
2.7. ACE Inhibition and DPP 4 Inhibition Assay
2.8. ACE Inhibitory Peptide Screening
2.9. Molecular Docking Study
3. Results and Discussion
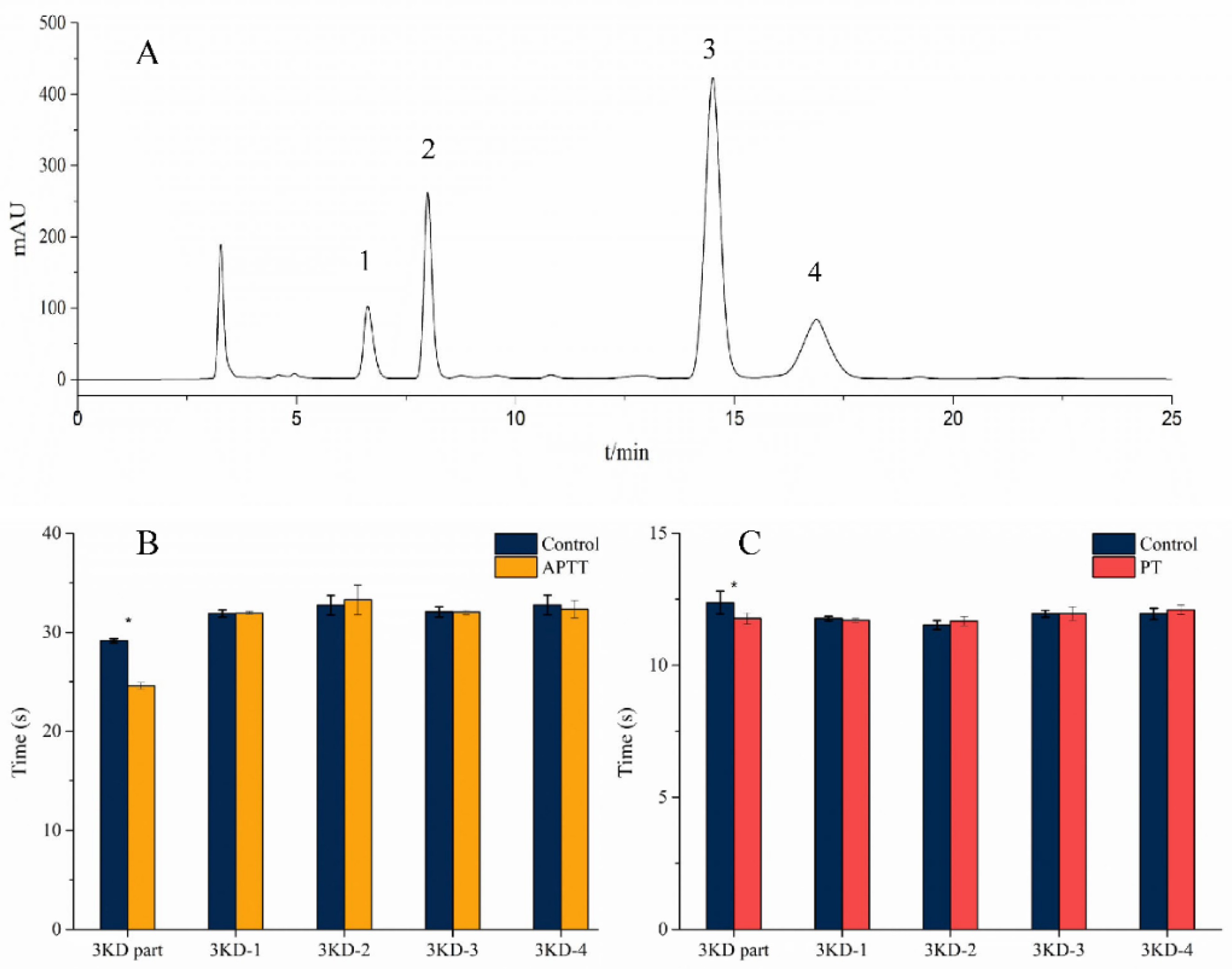
3.1. Isolation and Purification
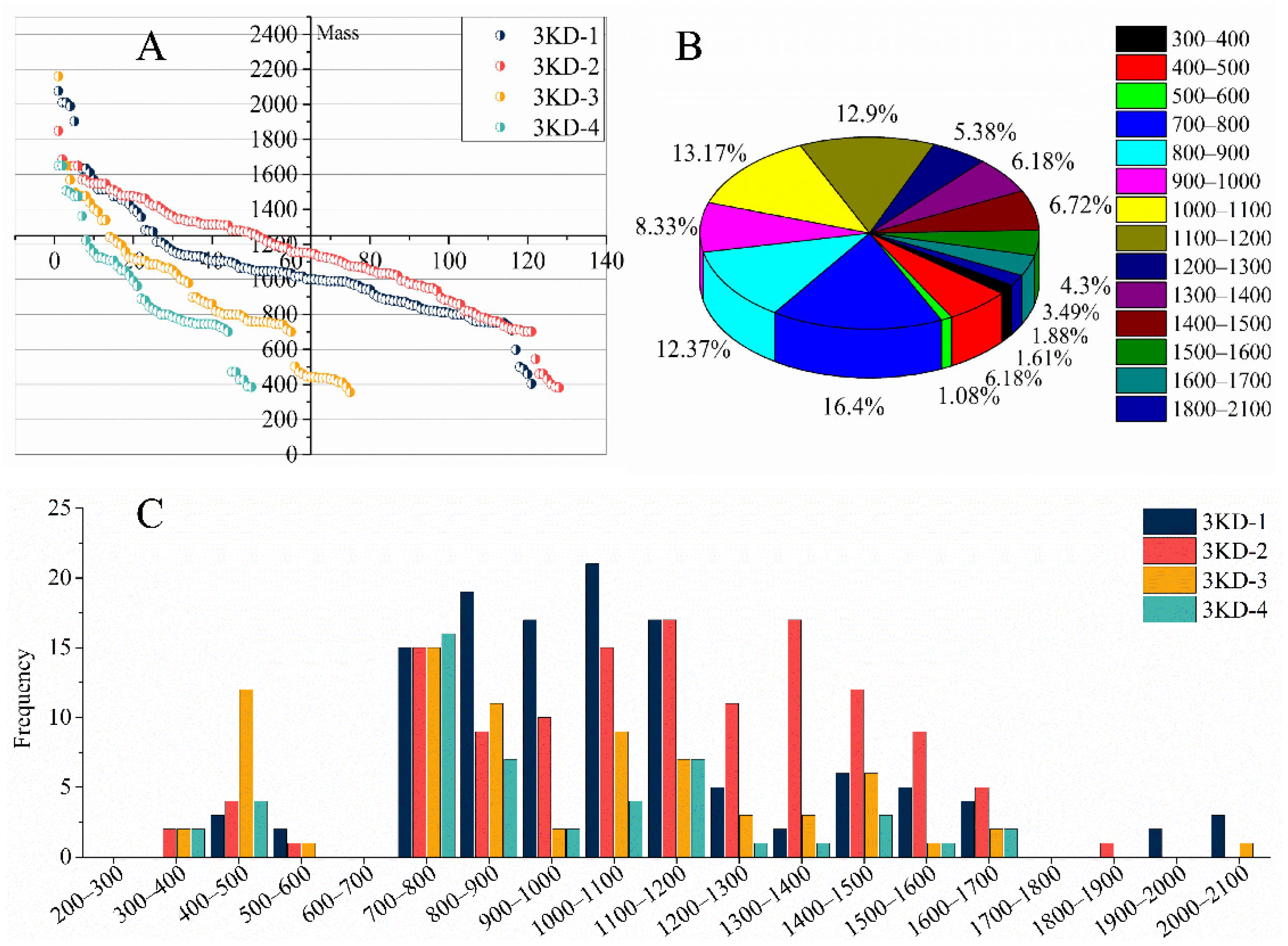
3.2. Peptide Sequencing and Identification Results
3.3. Activity Prediction Result
3.4. Activity Determination
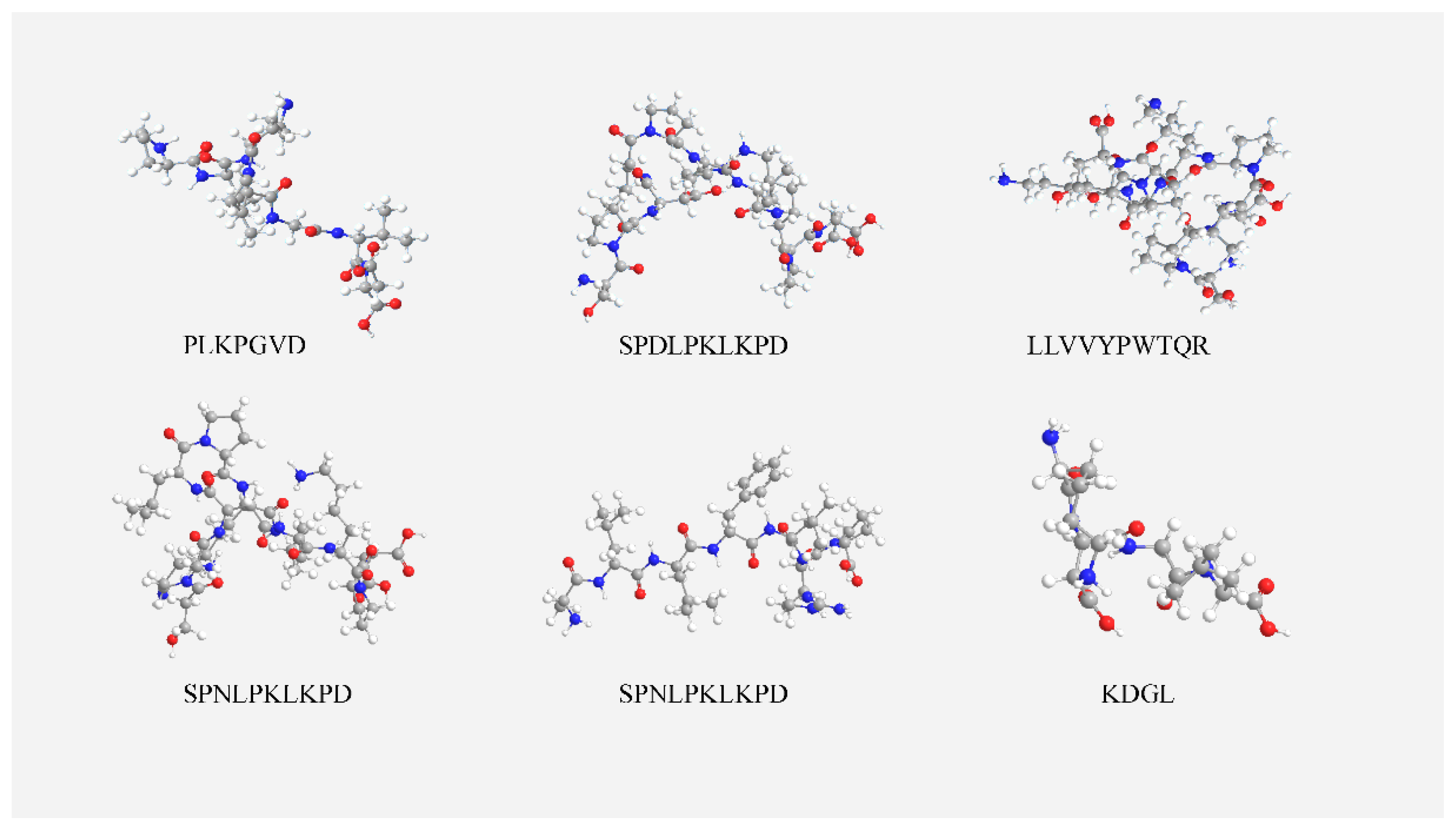
3.5. ACE Inhibitory Peptide Screening Results
3.6. Molecular Docking Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume I, 85p. [Google Scholar]
- Junren, C.; Xiaofang, X.; Huiqiong, Z.; Gangmin, L.; Yanpeng, Y.; Xiaoyu, C.; Yuqing, G.; Yanan, L.; Yue, Z.; Fu, P.; et al. Pharmacological Activities and Mechanisms of Hirudin and Its Derivatives—A Review. Front. Pharmacol. 2021, 12, 660757. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Ren, J.X.; Wang, J.J.; Ding, L.S.; Zhao, J.J.; Liu, S.Y.; Gao, H.M. Chinese Medicinal Leech: Ethnopharmacology, Phytochemistry, and Pharmacological Activities. Evid. Based Complement. Alternat. Med. 2016, 2016, 7895935. [Google Scholar] [CrossRef]
- Iwama, R.E.; Tessler, M.; Siddall, M.E.; Kvist, S. The Origin and Evolution of Antistasin-like Proteins in Leeches (Hirudinida, Clitellata). Genome Biol Evol. 2021, 13, evaa242. [Google Scholar] [CrossRef]
- Tang, X.; Chen, M.; Duan, Z.; Mwangi, J.; Li, P.; Lai, R. Isolation and Characterization of Poecistasin, an Anti-Thrombotic Antistasin-Type Serine Protease Inhibitor from Leech Poecilobdella manillensis. Toxins 2018, 10, 429. [Google Scholar] [CrossRef]
- Zheng, G.L.; Wu, Y.S. The effect of leech micropowder on blood vWF and GMP-140 levels in patients with essential hypertension. J. Shandong Univ. 2006, 44, 617. [Google Scholar] [CrossRef]
- Li, S.J. Application of Leech in Type 2 Diabetes—Analysis of 48 Cases. Gansu J. Tradit. Chin. Med. 2005, 18, 436–437. [Google Scholar] [CrossRef]
- Shu, Y.; Cao, X.Y.; Chen, J. Preparation and antagonistic effect of ACE inhibitory peptide from cashew. J. Sci. Food Agric. 2019, 99, 6822–6832. [Google Scholar] [CrossRef]
- Turner, J.M.; Kodali, R. Should Angiotensin-Converting Enzyme Inhibitors ever Be Used for the Management of Hypertension? Curr. Cardiol. Rep. 2020, 22, 95. [Google Scholar] [CrossRef]
- Li, R.; Zeng, X.; Yang, M.; Feng, J.; Xu, X.; Bao, L.; Ye, T.; Wang, X.; Xue, B.; Huang, Y. Antidiabetic DPP-4 Inhibitors Reprogram Tumor Microenvironment That Facilitates Murine Breast Cancer Metastasis through Interaction with Cancer Cells via a ROS-NF-кB-NLRP3 Axis. Front. Oncol. 2021, 11, 728047. [Google Scholar] [CrossRef]
- Kubo, A.; Hidaka, T.; Nakayama, M.; Sasaki, Y.; Takagi, M.; Suzuki, H.; Suzuki, Y. Protective effects of DPP-4 inhibitor on podocyte injury in glomerular diseases. BMC Nephrol. 2020, 21, 402. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, P.; Huang, G.; Yang, L.; Zhou, Z. Dipeptidyl peptidase-4(DPP-4) inhibitors: Promising new agents for autoimmune diabetes. Clin. Exp. Med. 2018, 18, 473–480. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Ontiveros, N.; López-Teros, V.; Vergara-Jiménez, M.J.; Islas-Rubio, A.R.; Cárdenas-Torres, F.I.; Cuevas-Rodríguez, E.O.; Reyes-Moreno, C.; Granda-Restrepo, D.M.; Lopera-Cardona, S.; Ramírez-Torres, G.I.; et al. Amaranth-hydrolyzate enriched cookies reduce the systolic blood pressure in spontaneously hypertensive rats. J. Funct. Foods 2020, 64, 103613. [Google Scholar] [CrossRef]
- Zalevsky, A.O.; Zlobin, A.S.; Gedzun, V.R.; Reshetnikov, R.V.; Lovat, M.L.; Malyshev, A.V.; Doronin, I.I.; Babkin, G.A.; Golovin, A.V. PeptoGrid-Rescoring Function for AutoDock Vina to Identify New Bioactive Molecules from Short Peptide Libraries. Molecules 2019, 24, 277. [Google Scholar] [CrossRef]
- Wüstenhagen, D.A.; Lukas, P.; Müller, C.; Aubele, S.A.; Hildebrandt, J.P.; Kubick, S. Cell-free synthesis of the hirudin variant 1 of the blood-sucking leech Hirudo medicinalis. Sci. Rep. 2020, 10, 19818. [Google Scholar] [CrossRef]
- Maleki, S.; Razavi, S.H. Pulses’ germination and fermentation: Two bioprocessing against hypertension by releasing ACE inhibitory peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 2876–2893. [Google Scholar] [CrossRef]
- Laghlam, D.; Jozwiak, M.; Nguyen, L.S. Renin-Angiotensin-Aldosterone System and Immunomodulation: A State-of-the-Art Review. Cells 2021, 10, 1767. [Google Scholar] [CrossRef]
- Gonzalez-Villalobos, R.A.; Shen, X.Z.; Bernstein, E.A.; Janjulia, T.; Taylor, B.; Giani, J.F.; Blackwell, W.-L.B.; Shah, K.H.; Shi, P.D.; Fuchs, S.; et al. BernsteinRediscovering ACE: Novel insights into the many roles of the angiotensin-converting enzyme. J. Mol. Med. 2013, 91, 1143–1154. [Google Scholar] [CrossRef][Green Version]
- Gallwitz, B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Lantz, I.; Glämsta, E.L.; Talbäck, L.; Nyberg, F. Hemorphins derived from hemoglobin have an inhibitory action on angiotensin converting enzyme activity. FEBS Lett. 1991, 287, 39–41. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of Angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship study of di- and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Recio, I.; Ramos, M. Preparation of ovine and caprine β-lactoglobulin hydrolysates with ACE-inhibitory activity. Int. Dairy J. 2002, 12, 805–812. [Google Scholar] [CrossRef]
- Miyoshi, S.; Kaneko, T.; Ishikawa, H.; Tanaka, H.; Maruyama, S. Production of bioactive peptides from corn endosperm proteins by some proteases. Ann N. Y. Acad. Sci. 1995, 750, 429–431. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Zhang, Y.; Zhang, R.; Zhang, Y. Novel Angiotensin-Converting Enzyme Inhibitory Peptides Identified from Walnut Glutelin-1 Hydrolysates: Molecular Interaction, Stability, and Antihypertensive Effects. Nutrients 2021, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Mirdamadi, S.; Safavi, M. Structural analysis of ACE-inhibitory peptide (VL-9) derived from Kluyveromyces marxianus protein hydrolysate. J. Mol. Struct. 2020, 1213, 128199. [Google Scholar] [CrossRef]
- Jao, C.; Huang, S.; Hsu, K. Angiotensin-I-converting enzyme inhibitory peptides: Inhibition mode, bioavailability, and antihypertensive effects. Biomedicine 2012, 2, 130–136. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. Research progress in structure-activity relationship of bioactive peptides. J. Med. Food. 2015, 18, 147–156. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zainal Abidin, N.; Zarei, M.; Tan, C.P.; Saari, N. Identification, structure-activity relationship and in silico molecular docking analyses of five novel angiotensin I-converting enzyme (ACE)-inhibitory peptides from stone fish (Actinopyga lecanora) hydrolysates. PLoS ONE 2019, 14, e0197644. [Google Scholar] [CrossRef]
- Jorge, R.R.; Gloria, D.O.; Luis, C.G.; David, B.A. Angiotensin I-converting enzyme inhibitory activity in peptide fractions from hard-to-cook bean hydrolysates. J. Biotechnol. 2010, 150, 309–310. [Google Scholar] [CrossRef]


| Peptide | Mass | Score | Accession | Species | Protein Sources |
|---|---|---|---|---|---|
| LVLGGVDVTGPHL | 1275.7186 | 30.02 | XP_009029636.1 | Helobdella robusta | Proteasome subunit beta |
| VLGGVDVTGPHL | 1162.6345 | 37.43 | XP_009029636.1 | Helobdella robusta | Proteasome subunit beta |
| YELPDGQVITIGNER | 1702.8525 | 30.36 | ESN97261.1 | Helobdella robusta | hypothetical protein HELRODRAFT_155129 |
| AGFAGDDAPR | 975.441 | 26.17 | ESN97261.1 | Helobdella robusta | hypothetical protein HELRODRAFT_155129 |
| DNIQGITKPAIR | 1324.7462 | 22.92 | ESO04417.1 | Helobdella robusta | Histone H4 |
| DSYVGDEAQSK | 1197.5149 | 24.02 | ESN97261.1 | Helobdella robusta | hypothetical protein HELRODRAFT_155129 |
| GYSFTTTAER | 1131.5197 | 23.87 | ESN97261.1 | Helobdella robusta | hypothetical protein HELRODRAFT_155129 |
| ISGLIYEETR | 1179.6135 | 22.73 | ESO04417.1 | Helobdella robusta | Histone H4 |
| NVINGGSHAGNKL | 1279.6633 | 23.26 | XP_009015226 | Helobdella robusta | Phosphopyruvate hydratase |
| SYELPDGQVITIGNER | 1789.8846 | 31.66 | ESN97261.1 | Helobdella robusta | hypothetical protein HELRODRAFT_155129 |
| TVTAM (+15.99) DVVYALK | 1325.6901 | 27.09 | ESO04417.1 | Helobdella robusta | Histone H4 |
| YSNRVVD | 851.4137 | 23.05 | ESO03354.1 | Helobdella robusta | Glyceraldehyde-3-phosphate dehydrogenase |
| YSNRVVDL | 964.4978 | 22.95 | ESO03354.1 | Helobdella robusta | Glyceraldehyde-3-phosphate dehydrogenase |
| ESTLHLVLR | 1066.6135 | 25.55 | XP_009027975.1 | Helobdella robusta | hypothetical protein HELRODRAFT_193870 |
| GYSFTTTAER | 1131.5197 | 23.66 | ESN97261.1 | Helobdella robusta | hypothetical protein HELRODRAFT_155129 |
| TVTAMDVVYALK | 1309.6952 | 35.92 | ESO04417.1 | Helobdella robusta | Histone H4 |
| PSIVGRPR | 880.5242 | 25.26 | ESN97261.1 | Helobdella robusta | hypothetical protein HELRODRAFT_155129 |
| PSLVGRPR | 880.5242 | 25.26 | ABC60435.1 | Hirudo medicinalis | cytoplasmic actin |
| Group | IC50 (mg/mL) | Equation | r Value |
|---|---|---|---|
| 3KD-1 | 0.8266 | y = 0.5950x − 1.7358 | 0.9976 |
| 3KD-2 | 0.2708 | y = 0.8372x − 2.0366 | 0.9951 |
| 3KD-3 | 0.4432 | y = 0.944x − 2.4984 | 0.9938 |
| 3KD-4 | 0.1764 | y = 1.0675x − 2.3982 | 0.9972 |
| Leech extract | 0.0653 | y = 1.4515x − 2.6347 | 0.9971 |
| Number | Peptide | Mass | Score | ACE Inhibitor Peptide (ID 1) |
|---|---|---|---|---|
| 1 | PLKPGVD | 724.4119 | 0.45 | PL (7513), LKP (7549), GV (7608), PG (7625), KP (7810) |
| 2 | SPDLPKLKPD | 1108.6128 | 0.34 | DLP (7542), LKP (7549), KL (7693), KP (7810) |
| 3 | LLVVYPWTQR | 1273.7183 | 0.30 | LVVYPWTQR (3402), YPWTQR (3403), VY (3492), VYP (3505), YP (3666), TQ (7834), VVYPW (7750), VVYPWTQ (9277), LVVYPWTQ (9278) |
| 4 | SPNLPKLKPD | 1108.6128 | 0.19 | LKP (7549), KL (7693), KP (7810) |
| 5 | ALLFLRP | 828.5222 | 0.15 | LRP (3543), LF (3551), RP (7582), LLF (7807), LR (9213) |
| 6 | KDGL | 431.2380 | 0.14 | GL (7599), DG (7681), DGL (9056) |
| Number | Peptide | Binding Energy (kcal/mol) | Hydrogen Bond |
|---|---|---|---|
| 1 | PLKPGVD | −6.1 | 5 |
| 2 | SPDLPKLKPD | −6.3 | 5 |
| 3 | LLVVYPWTQR | −5.8 | 8 |
| 4 | SPNLPKLKPD | −5.8 | 6 |
| 5 | ALLFLRP | −7.2 | 6 |
| 6 | KDGL | −5.6 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Chen, K.; Chen, Y. Research on ACEI of Low-Molecular-Weight Peptides from Hirudo nipponia Whitman. Molecules 2022, 27, 5421. https://doi.org/10.3390/molecules27175421
Ding Z, Chen K, Chen Y. Research on ACEI of Low-Molecular-Weight Peptides from Hirudo nipponia Whitman. Molecules. 2022; 27(17):5421. https://doi.org/10.3390/molecules27175421
Chicago/Turabian StyleDing, Zhao, Keli Chen, and Yunzhong Chen. 2022. "Research on ACEI of Low-Molecular-Weight Peptides from Hirudo nipponia Whitman" Molecules 27, no. 17: 5421. https://doi.org/10.3390/molecules27175421
APA StyleDing, Z., Chen, K., & Chen, Y. (2022). Research on ACEI of Low-Molecular-Weight Peptides from Hirudo nipponia Whitman. Molecules, 27(17), 5421. https://doi.org/10.3390/molecules27175421






