Exploration of Antimicrobial Ingredients in Psoralea corylifolia L. Seed and Related Mechanism against Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Preparation
2.3. Chromatographic Separation and Mass Spectrometry Detection
2.4. Antibacterial Tests
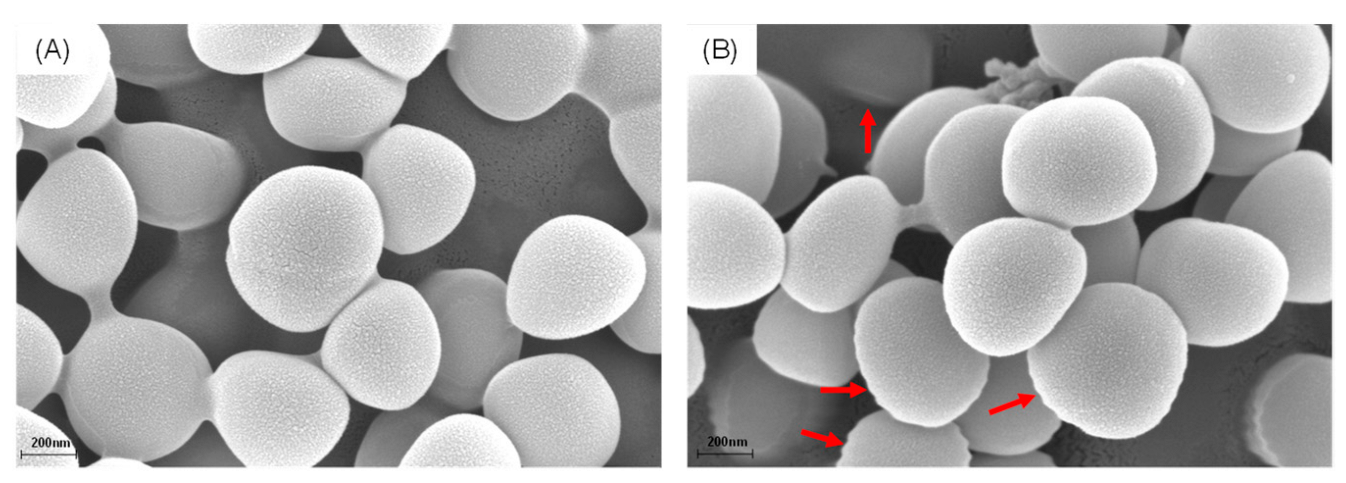
2.5. Scanning Electron Microscopy Analysis
2.6. Antibacterial Activity of the Mixtures of Compounds with Lipids
2.7. Membrane Integrity Assays
2.8. Membrane Fluidity Assays
2.9. Membrane Potential Assays
2.10. ATP Determination
2.11. ROS Measurement
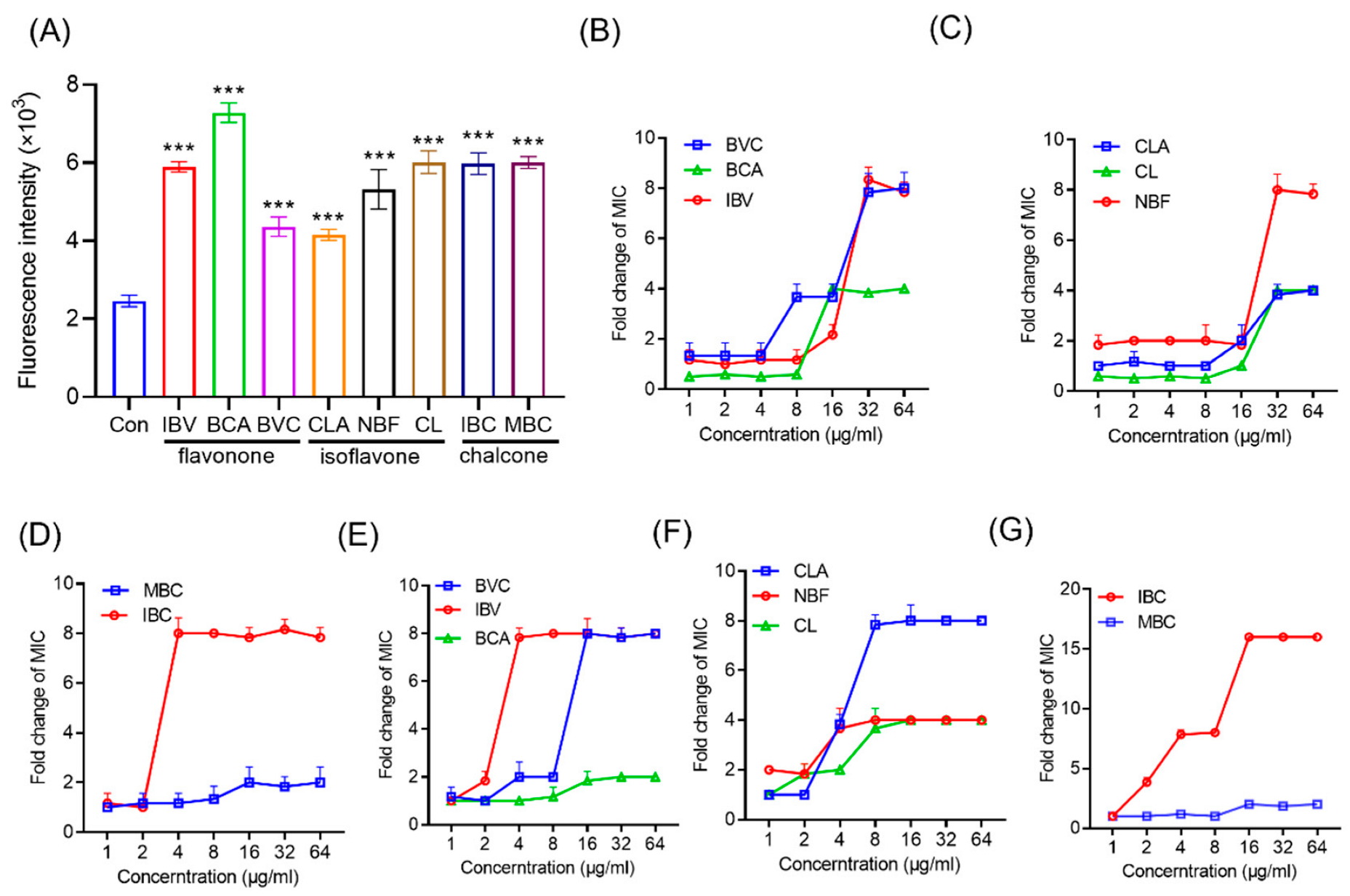
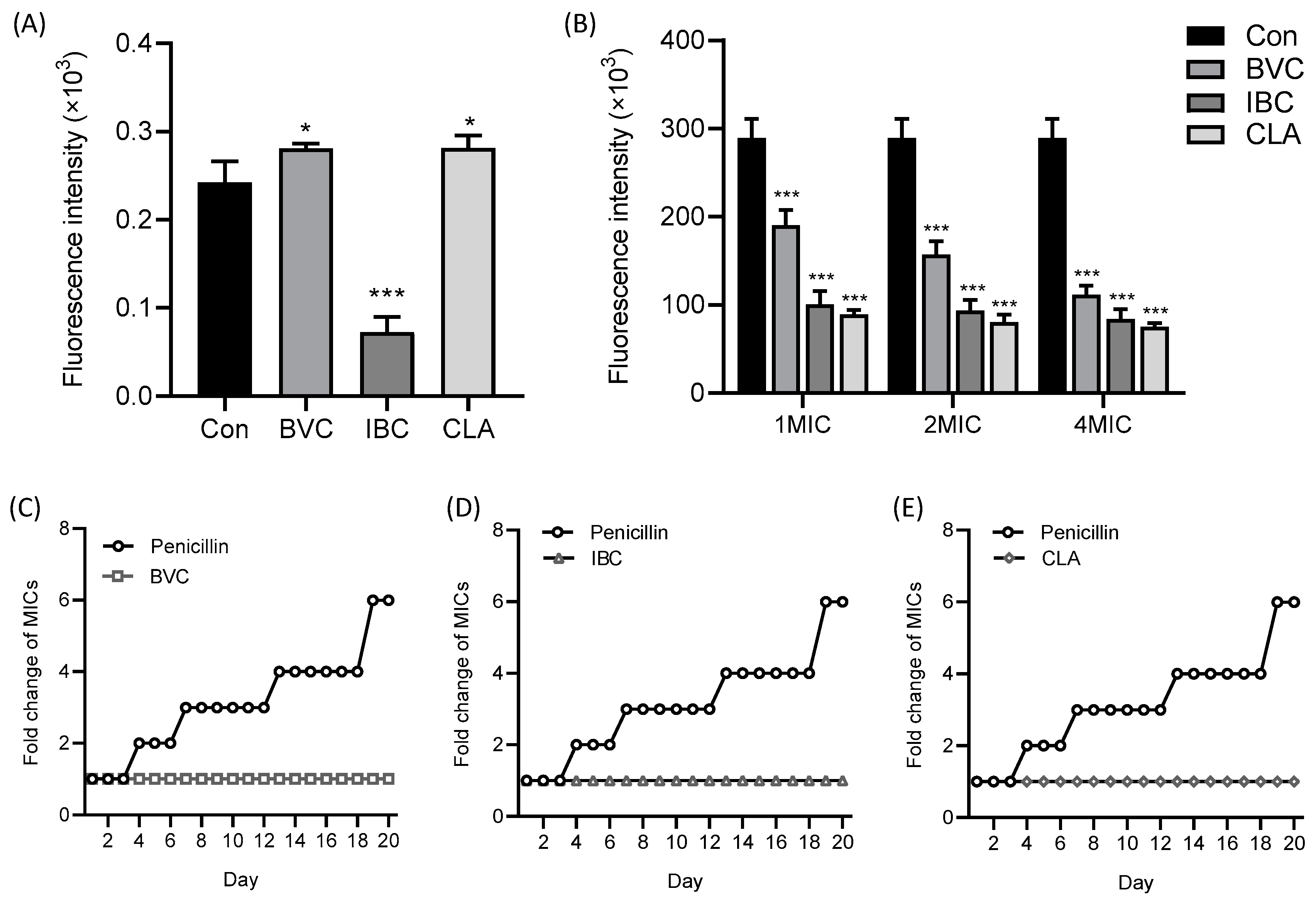
2.12. Resistance Development Studies
2.13. Isothermal Titration Calorimetry Assays
2.14. Statistical Data Analysis
3. Results and Discussion
3.1. Structural Characterizations of Reference Flavonoids
3.2. Identification and Isolation of Compounds in the Fractions of PCSs with Antibacterial Activity
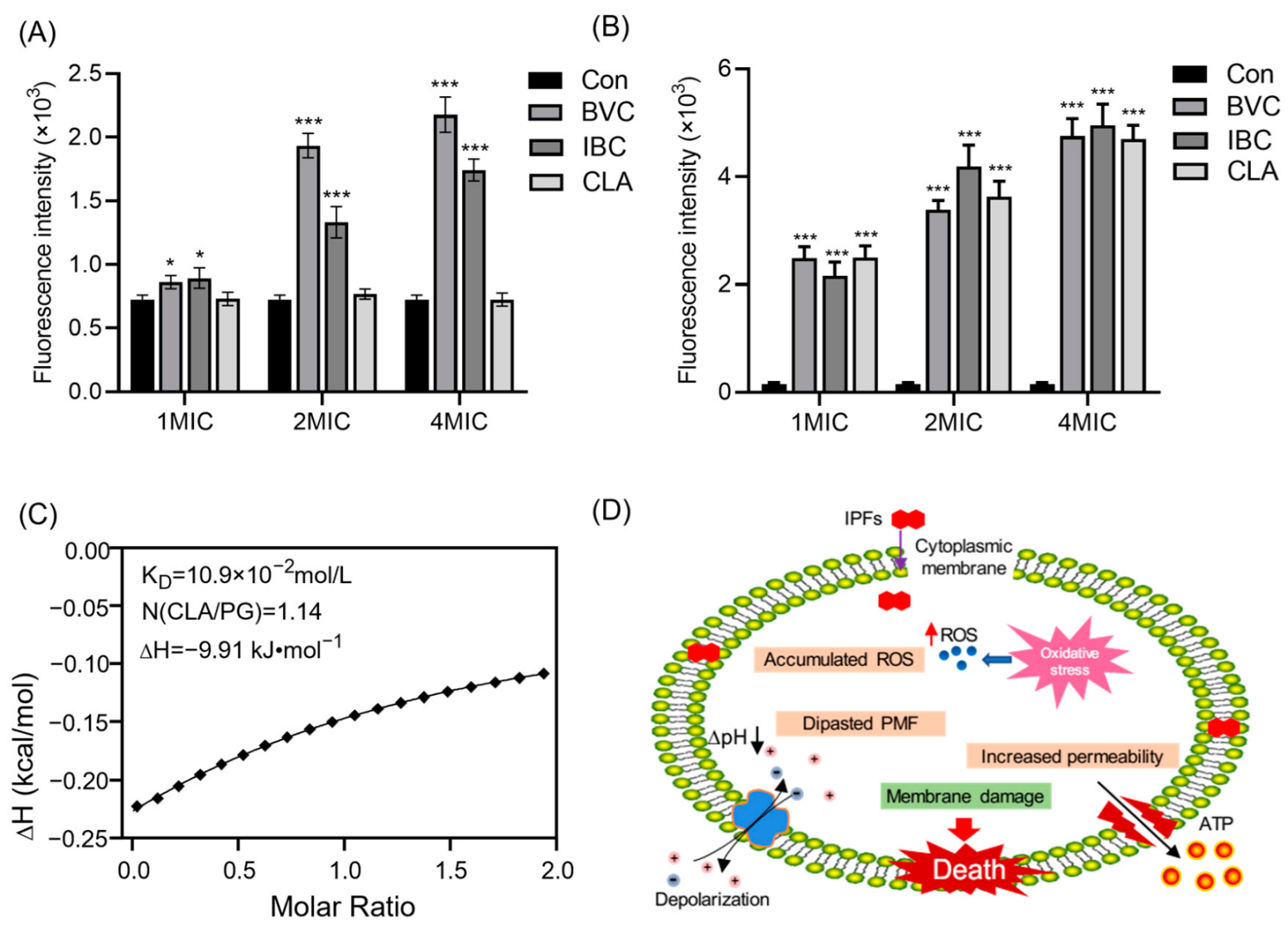
3.3. Effects of IPFs on MRSA Membrane
3.4. Mechanism of IPFs against MRSA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.; Bumah, V.V.; Niesman, I.R.; Cortez, P.; Enwemeka, C.S. Structural membrane changes induced by pulsed blue light on methicillin-resistant Staphylococcus aureus (MRSA). J. Photochem. Photobiol. B 2021, 216, 112150. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Xu, X.F.; Hou, W.; Meng, Y.; Huang, M.Y.; Lin, J.; Chen, W.M. Synthetic cajaninstilbene acid derivatives eradicate methicillin-resistant Staphylococcus aureus persisters and biofilms. Eur. J. Med. Chem. 2021, 224, 113691. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, Ł.; Moaddel, R. Comparison of analytical techniques for the identification of bioactive compounds from natural products. Nat. Prod. Rep. 2016, 33, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Cruz-Martins, N.; Nepovimova, E.; Oleksak, P.; Dhanjal, D.S.; Bhardwaj, S.; Singh, R.; Chopra, C.; Verma, R.; et al. Applications of Fruit Polyphenols and Their Functionalized Nanoparticles Against Foodborne Bacteria: A Mini Review. Molecules 2021, 26, 3447. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Silva, A.S.; Atanassova, M.; Sharma, R.; Nepovimova, E.; Musilek, K.; Sharma, R.; Alghuthaymi, M.A.; Dhanjal, D.S.; Nicoletti, M.; et al. Conifers Phytochemicals: A Valuable Forest with Therapeutic Potential. Molecules 2021, 26, 3005. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Dhanjal, D.S.; Sharma, A.; Nepovimova, E.; Kalia, A.; Thakur, S.; Bhardwaj, S.; Chopra, C.; Singh, R.; Verma, R.; et al. Conifer-Derived Metallic Nanoparticles: Green Synthesis and Biological Applications. Int. J. Mol. Sci. 2020, 21, 9028. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids Against Multidrug Resistant Pathogens. Adv. Sci. (Weinh.) 2021, 8, e2100749. [Google Scholar] [CrossRef]
- Wu, S.C.; Yang, Z.Q.; Liu, F.; Peng, W.J.; Qu, S.Q.; Li, Q.; Song, X.B.; Zhu, K.; Shen, J.Z. Antibacterial Effect and Mode of Action of Flavonoids From Licorice Against Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2019, 10, 2489. [Google Scholar] [CrossRef]
- Chen, X.; Xue, S.; Lin, Y.; Luo, J.; Kong, L. Immobilization of porcine pancreatic lipase onto a metal-organic framework, PPL@MOF: A new platform for efficient ligand discovery from natural herbs. Anal. Chim. Acta. 2020, 1099, 94–102. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Wang, Y.; Lu, J.; Chen, X. The Chemical Constituents and Bioactivities of Psoralea corylifolia Linn.: A Review. Am. J. Chin. Med. 2016, 44, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Khan, G.N.; Asad, M. Psoralea corylifolia L: Ethnobotanical, biological, and chemical aspects: A review. Phytother. Res. 2018, 32, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Li, H.N.; Wang, C.Y.; Wang, C.L.; Chou, C.H.; Leu, Y.L.; Chen, B.Y. Antimicrobial Effects and Mechanisms of Ethanol Extracts of Psoralea corylifolia Seeds Against Listeria monocytogenes and Methicillin-Resistant Staphylococcus aureus. Foodborne Pathog. Dis. 2019, 16, 573–580. [Google Scholar] [CrossRef]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J. Clin. Microbiol. 2021, 59, e0021321. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Zou, G.; Hari, T.P.A.; Wilt, I.K.; Zhu, W.; Galle, N.; Faizi, H.A.; Hendricks, G.L.; Tori, K.; Pan, W.; et al. A selective membrane-targeting repurposed antibiotic with activity against persistent methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2019, 116, 16529–16534. [Google Scholar] [CrossRef]
- Liang, M.; Ge, X.; Xua, H.; Ma, K.; Zhang, W.; Zan, Y.; Efferth, T.; Xue, Z.; Hua, X. Phytochemicals with activity against methicillin-resistant Staphylococcus aureus. Phytomedicine 2022, 100, 154073. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.; Vincken, J.P.; Bohin, M.C.; Kuijpers, T.F.; Verbruggen, M.A.; Gruppen, H. Identification of prenylated pterocarpans and other isoflavonoids in Rhizopus spp. elicited soya bean seedlings by electrospray ionisation mass spectrometry. Rapid. Commun. Mass. Spectrom. 2011, 25, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Buckett, L.; Schinko, S.; Urmann, C.; Riepl, H.; Rychlik, M. Stable Isotope Dilution Analysis of the Major Prenylated Flavonoids Found in Beer, Hop Tea, and Hops. Front. Nutr. 2020, 7, 619921. [Google Scholar] [CrossRef]
- He, M.; Wu, H.; Nie, J.; Yan, P.; Yang, T.B.; Yang, Z.Y.; Pei, R. Accurate recognition and feature qualify for flavonoid extracts from Liang-wai Gan Cao by liquid chromatography-high resolution-mass spectrometry and computational MS/MS fragmentation. J. Pharm. Biomed. Anal. 2017, 146, 37–47. [Google Scholar] [CrossRef]
- Nie, J.; Xiao, L.; Zheng, L.; Du, Z.; Liu, D.; Zhou, J.; Xiang, J.; Hou, J.; Wang, X.; Fang, J. An integration of UPLC-DAD/ESI-Q-TOF MS, GC-MS, and PCA analysis for quality evaluation and identification of cultivars of Chrysanthemi Flos (Juhua). Phytomedicine 2019, 59, 152803. [Google Scholar] [CrossRef]
- Fang, S.; Qu, Q.; Zheng, Y.; Zhong, H.; Shan, C.; Wang, F.; Li, C.; Peng, G. Structural characterization and identification of flavonoid aglycones in three Glycyrrhiza species by liquid chromatography with photodiode array detection and quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2016, 39, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Maul, R.; Schebb, N.H.; Kulling, S.E. Application of LC and GC hyphenated with mass spectrometry as tool for characterization of unknown derivatives of isoflavonoids. Anal. Bioanal. Chem. 2008, 391, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Tsolmon, B.; Fang, Y.; Yang, T.; Guo, L.; He, K.; Li, G.Y.; Zhao, H. Structural identification and UPLC-ESI-QTOF-MS(2) analysis of flavonoids in the aquatic plant Landoltia punctata and their in vitro and in vivo antioxidant activities. Food Chem. 2021, 343, 128392. [Google Scholar] [CrossRef]
- Bodet, C.; Burucoa, C.; Rouillon, S.; Bellin, N.; Taddeo, V.A.; Fiorito, S.; Genovese, S.; Epifano, F. Antibacterial activities of oxyprenylated chalcones and napthtoquinone against Helicobacter pylori. Nat. Prod. Commun. 2014, 9, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Zeng, F.; Weng, Z.; Zheng, H.; Xu, M.; Liang, X.; Duan, J. Preparation and characterization of active oxidized starch films containing licorice residue extracts and its potential against methicillin-resistant S. aureus. Int. J. Biol. Macromol. 2021, 187, 858–866. [Google Scholar] [CrossRef]
- Zhang, N.; Fan, Y.; Li, C.; Wang, Q.; Leksawasdi, N.; Li, F.; Wang, S. Cell permeability and nuclear DNA staining by propidium iodide in basidiomycetous yeasts. Appl. Microbiol. Biotechnol. 2018, 102, 4183–4191. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Mingeot-Leclercq, M.P.; Struelens, M.J.; Tulkens, P.M. The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends Pharmacol. Sci. 2008, 29, 124–134. [Google Scholar] [CrossRef]
- De Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; van der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef]
- Lin, S.; Li, H.; Tao, Y.; Liu, J.; Yuan, W.; Chen, Y.; Liu, Y.; Liu, S. In Vitro and in Vivo Evaluation of Membrane-Active Flavone Amphiphiles: Semisynthetic Kaempferol-Derived Antimicrobials against Drug-Resistant Gram-Positive Bacteria. J. Med. Chem. 2020, 63, 5797–5815. [Google Scholar] [CrossRef]
- García-Fernández, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Süss, B.; Geibel, S.; Markert, S.M.; Stigloher, C.; et al. Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance. Cell 2017, 171, 1354–1367.e1320. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Su, H.L.; Chou, C.C.; Hung, D.J.; Lin, S.H.; Pao, I.C.; Lin, J.H.; Huang, F.L.; Dong, R.X.; Lin, J.J. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials 2009, 30, 5979–5987. [Google Scholar] [CrossRef] [PubMed]
- Valliammai, A.; Selvaraj, A.; Muthuramalingam, P.; Priya, A.; Ramesh, M.; Pandian, S.K. Staphyloxanthin inhibitory potential of thymol impairs antioxidant fitness, enhances neutrophil mediated killing and alters membrane fluidity of methicillin resistant Staphylococcus aureus. Biomed. Pharm. 2021, 141, 111933. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Luo, M.; Fu, Y.J.; Zu, Y.G.; Wang, W.; Zhang, L.; Yao, L.P.; Zhao, C.J.; Sun, Y. Effect of corilagin on membrane permeability of Escherichia coli, Staphylococcus aureus and Candida albicans. Phytother. Res. 2013, 27, 1517–1523. [Google Scholar] [CrossRef]
- Varma, G.Y.N.; Kummari, G.; Paik, P.; Kalle, A.M. Celecoxib potentiates antibiotic uptake by altering membrane potential and permeability in Staphylococcus aureus. J. Antimicrob. Chemother. 2019, 74, 3462–3472. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Q.; Wang, X.; Li, R.; Qiao, H.; Ma, P.; Sun, Q.; Zhang, H. Antibacterial activity of xanthan-oligosaccharide against Staphylococcus aureus via targeting biofilm and cell membrane. Int. J. Biol. Macromol. 2020, 153, 539–544. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Suwalsky, M.; Patiño-González, E.; Fandiño-Devia, E.; Jemioła-Rzemińska, M.; Strzałka, K. Interaction of the antimicrobial peptide ∆M3 with the Staphylococcus aureus membrane and molecular models. Biochim. Biophys. Acta. Biomembr. 2021, 1863, 183498. [Google Scholar] [CrossRef]
- Chusri, S.; Voravuthikunchai, S.P. Damage of staphylococcal cytoplasmic membrane by Quercus infectoria G. Olivier and its components. Lett. Appl. Microbiol. 2011, 52, 565–572. [Google Scholar] [CrossRef]
- Batista de Andrade Neto, J.; Pessoa de Farias Cabral, V.; Brito Nogueira, L.F.; Rocha da Silva, C.; Gurgel do Amaral Valente Sá, L.; Ramos da Silva, A.; Barbosa da Silva, W.M.; Silva, J.; Marinho, E.S.; Cavalcanti, B.C.; et al. Anti-MRSA activity of curcumin in planktonic cells and biofilms and determination of possible action mechanisms. Microb. Pathog. 2021, 155, 104892. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, E.; Wen, T.; Yan, X.; Han, M.; Bai, L.P.; Fu, X.; Liu, J.; Qin, S. Development of Membrane-Active Honokiol/Magnolol Amphiphiles as Potent Antibacterial Agents against Methicillin-Resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2021, 64, 12903–12916. [Google Scholar] [CrossRef] [PubMed]

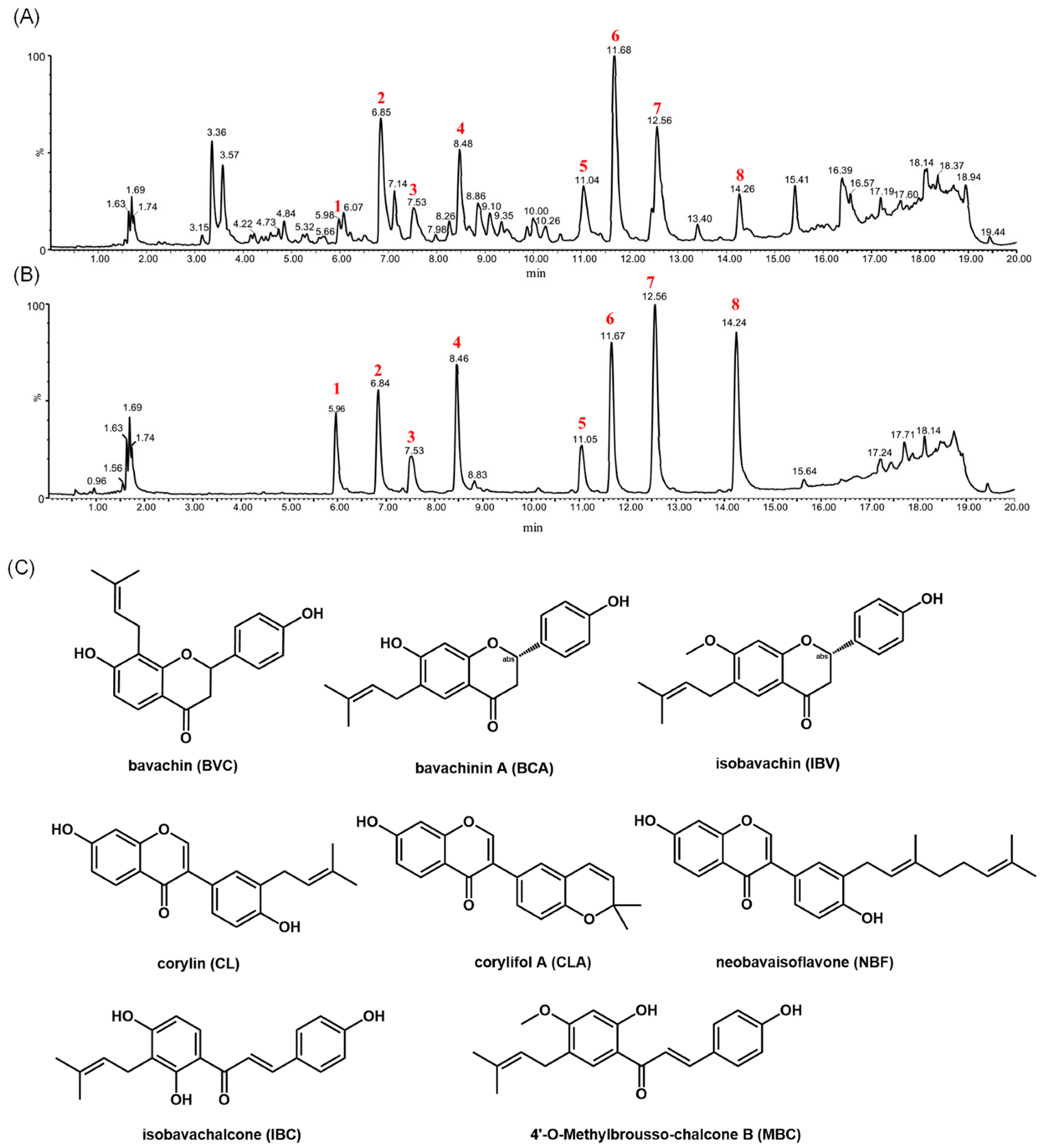
| No. | Name | Formula | [M + H] | Typical Fragment Ions (Da) | Typical Losses |
|---|---|---|---|---|---|
| 1 | Licochalcone D | C21H22O5 | 355.1557 | 313.1306, 299.0807, 193.0301, 189.0760, 133.0130 | 42 Da, 56 Da |
| 2 | Licochalcone B | C16H14O5 | 287.0707 | 193.0251, 121.0025 | |
| 3 | Glabrone | C20H16O5 | 337.0972 | 295.0490, 283.0486, 267.0648, 239.0527 | 28 Da, 42 Da, 54 Da, 70 Da |
| 4 | Licoisoflavone B | C20H16O6 | 353.0926 | 311.0442, 299.0442, 283.0490, 255.0520, 153.0030 | 42 Da, 54 Da, 70 Da |
| 5 | 6-prenylnaringenin | C20H20O5 | 341.1390 | 285.0764, 221.0791, 165.0158 | 56 Da |
| Strains | EE | EOE | Fr. A | Fr. B | Fr. C | Fr. D | Oxacillin |
|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | 125 | 62.5 | 31.2 | 125 | 250 | 250 | 31.2 |
| Staphylococcus epidermidis | 125 | 62.5 | 31.2 | 125 | 250 | 250 | 31.2 |
| MRSA | 125 | 62.5 | 31.2 | 125 | 250 | 250 | 31.2 |
| Shigella dysenteriae | NA | NA | NA | NA | NA | NA | NA |
| Pseudomonas aeruginosa | NA | NA | NA | NA | NA | NA | NA |
| Escherichia coli | NA | NA | NA | NA | NA | NA | NA |
| No. | Name | Formula | [M + H] | Typical Fragment Ions (Da) | Typical Losses (Da) |
|---|---|---|---|---|---|
| 1 | Isobavachin | C20H20O4 | 325.1321 | 269.0677, 205.0691 149.0081 | 56 |
| 2 | Neobavaisoflavone | C20H18O4 | 323.1176 | 267.0520, 255.0521 239.0563, 137.0080 | 56 |
| 3 | Bavachin | C20H20O4 | 325.1312 | 269.0690, 205.0693 149.0079 | 56 |
| 4 | Corylin | C20H16O4 | 321.1025 | 279.0534, 267.0527 251.0566, 137.0078 | 42, 54, 70 |
| 5 | Isobavachalcone | C20H20O4 | 325.1332 | 269.0688, 205.0731 149.0081 | 56 |
| 6 | Bavachinin A | C21H22O4 | 339.1493 | 283.0838, 219.0871 151.0236, 119.0341 | 56 |
| 7 | Corylifol A | C25H26O4 | 391.1815 | 323.1172, 267.0531 239.0565, 137.0084 | 56 |
| 8 | 4′-O-Methylbrousso-chalcone B | C21H22O4 | 339.1479 | 283.0828, 271.0841 219.0872, 151.0241 | 56 |
| Compound | MIC (μg/mL) | ||
|---|---|---|---|
| S. aureus | S. epidermidis | MRSA | |
| Isobavachin (IBV) | 15.6 | 7.80 | 7.80 |
| Neobavaisoflavone (NBF) | 15.6 | 7.80 | 7.80 |
| Bavachin (BVC) | 7.80 | 15.6 | 7.80 |
| Corylin (CL) | 15.6 | 15.6 | 15.6 |
| Isobavachalcone (IBC) | 15.6 | 7.80 | 7.80 |
| Bavachinin A (BCA) | 31.2 | 31.2 | 31.2 |
| Corylifol A (CLA) | 3.90 | 3.90 | 3.90 |
| 4′-O-Methylbrousso- chalcone B(MBC) | 31.2 | 31.2 | 31.2 |
| Daidzein | NA | NA | NA |
| Naringenin | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Tang, Z.; Wang, M.; Shi, J.; Lin, Y.; Sun, T.; Zou, Z.; Weng, Z. Exploration of Antimicrobial Ingredients in Psoralea corylifolia L. Seed and Related Mechanism against Methicillin-Resistant Staphylococcus aureus. Molecules 2022, 27, 6952. https://doi.org/10.3390/molecules27206952
Sun L, Tang Z, Wang M, Shi J, Lin Y, Sun T, Zou Z, Weng Z. Exploration of Antimicrobial Ingredients in Psoralea corylifolia L. Seed and Related Mechanism against Methicillin-Resistant Staphylococcus aureus. Molecules. 2022; 27(20):6952. https://doi.org/10.3390/molecules27206952
Chicago/Turabian StyleSun, Liqiong, Zhijuan Tang, Minxin Wang, Jun Shi, Yajuan Lin, Tiefeng Sun, Zhilu Zou, and Zebin Weng. 2022. "Exploration of Antimicrobial Ingredients in Psoralea corylifolia L. Seed and Related Mechanism against Methicillin-Resistant Staphylococcus aureus" Molecules 27, no. 20: 6952. https://doi.org/10.3390/molecules27206952
APA StyleSun, L., Tang, Z., Wang, M., Shi, J., Lin, Y., Sun, T., Zou, Z., & Weng, Z. (2022). Exploration of Antimicrobial Ingredients in Psoralea corylifolia L. Seed and Related Mechanism against Methicillin-Resistant Staphylococcus aureus. Molecules, 27(20), 6952. https://doi.org/10.3390/molecules27206952









